Introduction
Acute myocardial infarction (AMI) is a major cause
of disability and mortality worldwide (1). Timely revascularization is the most
effective approach for reducing cardiomyocyte death, although the
incidence of complications following reperfusion therapy remains
high (2). Among the postoperative
complications, cardiac insufficiency affects the prognosis of
patients and their quality of life (3). Following AMI, overactivation of the
sympathetic nervous system and the renin-angiotensin-aldosterone
system (RAAS) may cause ventricular remodeling, which is the main
pathological event associated with cardiac insufficiency (4).
In recent years, although the application of
conventional heart failure treatment following myocardial
infarction has reduced the mortality of patients to a certain
extent, the incidence of cardiac insufficiency following AMI
remains high (5). In 2014, the
Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor
(ARNI) with Angiotensin-Converting-Enzyme Inhibitor (ACEI) to
Determine Impact on Global Mortality and Morbidity in Heart Failure
Trial indicated that, compared with enalapril, the cardiovascular
mortality, heart failure rehospitalization and all-cause mortality
rates of patients with chronic heart failure were all decreased
following the administration of sacubitril/valsartan (LCZ 696)
(6). Sacubitril/valsartan is a
first-in-class ARNI that simultaneously suppresses RAAS activation
by blocking angiotensin II type 1 (AT1) receptors and enhances
vasoactive peptides, such as natriuretic peptides, by inhibiting
neprilysin, the enzyme responsible for their degradation (7). Notably, compared with ACEI or AT1
receptor blockers (ARB), sacubitril/valsartan may modulate the
neurohormonal axis by inhibiting angiotensin receptors and
neprilysin, and could thus improve the neurohormonal balance more
than by blocking the RAAS alone (8). Sacubitril/valsartan is as well
tolerated by patients as ACEI or ARB, with the most common side
effect being hypotension (9).
Furthermore, a series of studies have revealed that treatment with
sacubitril/valsartan may lead to enhanced clinical benefits for
patients with chronic heart failure (10,11).
Considering the mechanism of sacubitril/valsartan, it may also have
a protective effect on patients with AMI by inhibiting RAAS
activation. However, the clinical benefits of using
sacubitril/valsartan in patients with AMI after revascularization
remain controversial. Therefore, the aim of the present study was
to conduct a systematic review to provide further evidence in
support of the clinical application of sacubitril/valsartan in
patients with AMI.
Materials and methods
Literature inclusion and exclusion
criteria
The inclusion criteria were defined according to the
Population, Intervention, Comparison, Outcome and Study design tool
(12): i) Population, patients
with AMI after coronary revascularization, including percutaneous
transluminal coronary intervention (PCI), coronary artery bypass
grafting (CABG) or thrombolysis, were included; ii) intervention,
the sacubitril/valsartan group was administered
sacubitril/valsartan on the basis of conventional treatment
strategies; iii) comparison, the control group was treated with
ACEI or ARB on the basis of conventional treatment strategies; iv)
outcome, the main outcomes were major adverse cardiovascular and
cerebrovascular events (MACCEs; including cardiac death, myocardial
infarction, severe arrhythmia, stroke, rehospitalization for
congestive heart failure and repeated revascularization),
readmission rate, adverse events, incidence of acute heart failure
(AHF) and incidence of hypotension, whereas the secondary outcomes
were N-terminal pro B-type natriuretic peptide (NT-proBNP)/BNP,
left ventricular ejection fraction (LVEF) and soluble suppression
of tumorigenesis-2 (sST2); and v) study design, randomized
controlled trials (RCTs) were included.
The exclusion criteria (in terms of the
publications) were as follows: Republished studies, studies with no
available data, studies in which the full text was not available,
and studies written in a language other than English.
Literature retrieval strategy
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library
(https://www.cochranelibrary.com/),
Embase (https://www.embase.com/) and Web of
Sciences (https://www.webofscience.com/wos/alldb/basic-search)
databases, and the ClinicalTrials.gov website (https://www.clinicaltrials.gov/) were searched for AMI
and sacubitril/valsartan through the combination of medical subject
headings (MeSHs) and entry terms. The literature search start date
was not restricted, and the search end date was July 2021. A manual
search of cross-references was also conducted based on the original
articles. The following search strategy was used for PubMed and
modified to suit other databases (the detailed retrieval strategy
of other databases is outlined in the Supplementary Data): Search I, myocardial
infarction (MeSH terms); search II, (Infarction, Myocardial
Title/Abstract) OR [Infarctions, Myocardial (Title/Abstract)] OR
[Myocardial Infarctions (Title/Abstract)] OR [Cardiovascular Stroke
(Title/Abstract)] OR [Cardiovascular Strokes (Title/Abstract)] OR
[Stroke, Cardiovascular (Title/Abstract)] OR [Strokes,
Cardiovascular (Title/Abstract)] OR [Myocardial Infarct
(Title/Abstract)] OR [Infarct, Myocardial (Title/Abstract)] OR
[Infarcts, Myocardial (Title/Abstract)] OR [Myocardial Infarcts
(Title/Abstract)] OR [Heart Attack (Title/Abstract)] OR [Heart
Attacks (Title/Abstract]; search III, sacubitril and valsartan
sodium hydrate drug combination (MeSH terms); search IV [sacubitril
valsartan sodium hydrate (Title/Abstract)] OR [sacubitril-valsartan
sodium hydrate drug combination (Title/Abstract)] OR [trisodium
(3-(1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-3'-methyl-2'-(pentanoyl(2'-(tetrazol-5-ylate)biphenyl-4'-ylmethyl)amino)butyrate
hemipentahydrate (Title/Abstract)] OR [sacubitril (Title/Abstract)
AND valsartan drug combination (Title/Abstract)] OR [sacubitril
valsartan drug combination (Title/Abstract)] OR
[sacubitril-valsartan (Title/Abstract)] OR
[3-(1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-3'-methyl-2'-(pentanoyl(2'-(tetrazol-5-ylate)biphenyl-4'-ylmethyl)amino)butyrate
(Title/Abstract)] OR [sacubitril (Title/Abstract) AND valsartan
sodium anhydrous drug combination (Title/Abstract)] OR [sacubitril
valsartan sodium anhydrous (Title/Abstract)] OR
[sacubitril-valsartan sodium anhydrous drug combination
(Title/Abstract)] OR [LCZ 696 (Title/Abstract)] OR [LCZ696
(Title/Abstract)] OR [LCZ-696 (Title/Abstract)] OR [Entresto
(Title/Abstract)] OR [sacubitril/valsartan (Title/Abstract)];
search V, search I OR search II; search VI, search III OR search
IV; and search VII, search V AND search VI.
Literature screening and data
extraction
Two researchers (SSL and BY) independently searched
and screened the literature according to the inclusion and
exclusion criteria. Any potential disagreements were resolved by
discussion until either a consensus was reached, or a third author
(BW or ZXF) was consulted. The extracted information included the
basic information of the study in question and the original
research data of the outcomes. The data that could not be directly
extracted were obtained either by data transformation or by
contacting the authors.
Literature quality assessment
The Cochrane collaboration bias risk assessment tool
recommended by the Cochrane handbook (13) was used to assess the risk of bias
in the included literature. A number of characteristics were
evaluated, including random sequence generation (selection bias),
allocation concealment (selection bias), blinding of participants
and personnel (performance bias), incomplete outcome data
(attrition bias), selective reporting (reporting bias), and other
biases.
Statistical methods
Statistical analysis of the data was performed using
Review Manager 5.3 (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman)
and STATA 14 software (https://www.stata.com/stata14/). Odds ratio (OR) was
used as the effect measure for dichotomous data. The effect measure
used for continuous data was the mean differences; either the mean
difference (MD) or the standardized mean difference (SMD) when the
data were measured based on the different measurement methods. All
effect indicators were calculated with 95% confidence intervals
(CIs). Statistical heterogeneity was assessed using the
χ2 test according to the I2 and P-values.
Notably, I2>50% or P<0.05 was taken to indicate a
significant level of heterogeneity among the studies, so, in this
case, the effect indicators were combined using the randomized
effects model (REM). If included studies were completely
independent of each other, REM was used. Sensitivity analysis was
conducted to verify the stability of the model by single study
elimination method in STATA 14 software, exploring the possible
source of heterogeneity. Publication bias was assessed using funnel
plots for meta-analysis and quantified using the Egger method,
although it must be mentioned that the test power of this method is
limited when only a few studies are included. P<0.05 was
considered to indicate a statistically significant difference.
Results
Literature search results
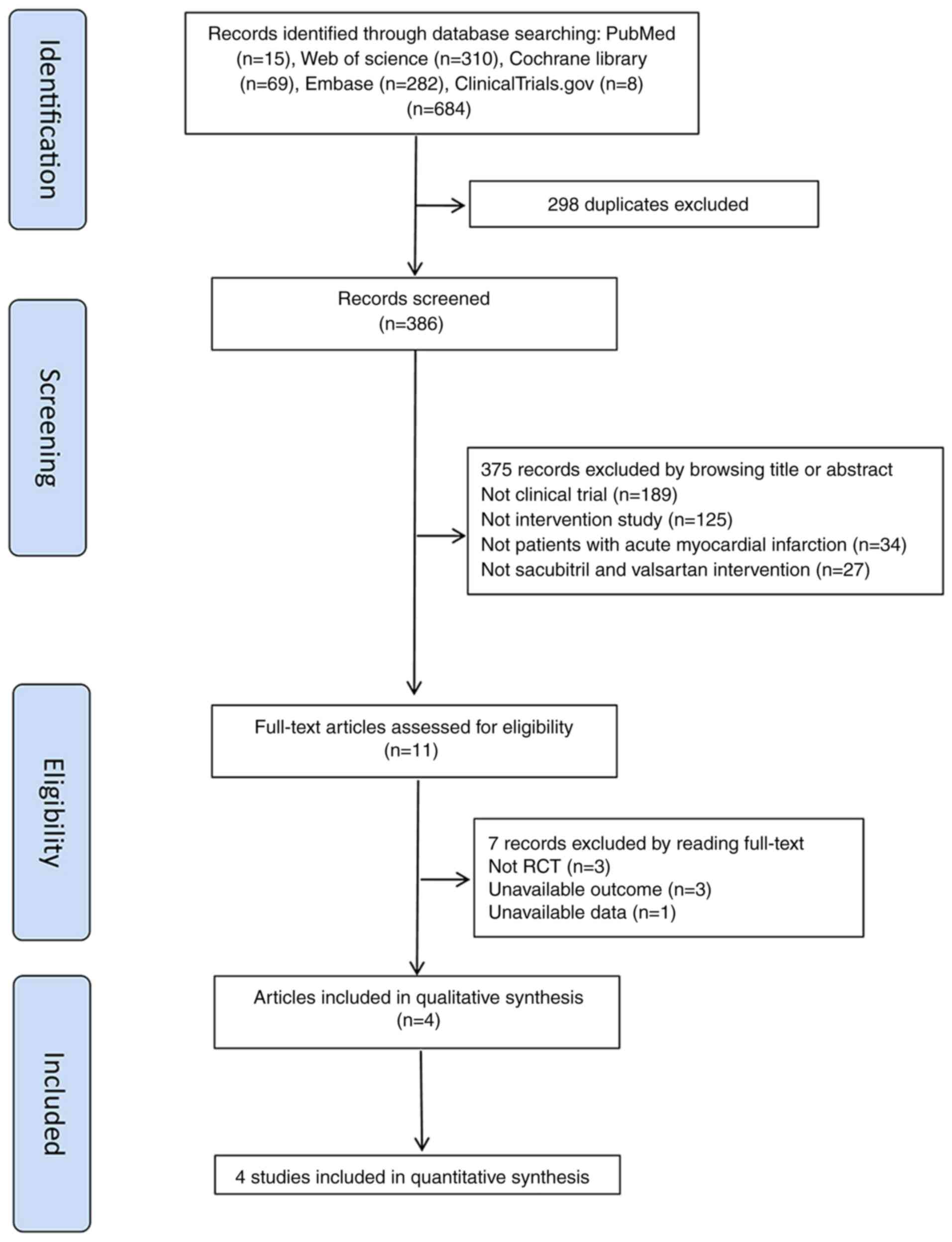
A total of 684 articles were obtained by searching
the databases, and a total of 386 articles were retrieved after
removing duplicates. By reading the titles and abstracts, 375
articles were initially excluded according to the inclusion and
exclusion criteria (189 were found not to be clinical trials, 125
were not intervention studies, 34 were not dealing with patients
with AMI, and 27 articles were not concerned with sacubitril and
valsartan interventions). A total of 11 articles were subsequently
investigated, and seven of them were excluded upon reading their
full text. For the seven excluded articles, three were not RCTs,
three articles had unavailable outcomes and one was without
available data. Ultimately, four studies were included in the
meta-analysis (14-17)
(Fig. 1).
Basic characteristics of the included
literature
A total of four studies were included. The basic
information of the included studies is shown in Table I. The total sample size of 586
patients was included, involving cases from China, Egypt and the
UK. The four studies comprised two prospective single-center RCTs
and two prospective multicenter RCTs. Regarding the type of AMI
involved, three studies assessed ST-elevation myocardial infarction
(STEMI) that was treated with PCI, whereas the remaining study
assessed STEMI and non-STEMI (NSTEMI) that was treated with PCI,
thrombolysis or CABG. The intervention used for all experimental
groups was sacubitril and valsartan, although the time between the
onset of AMI and the intervention varied, including early
administration of sacubitril/valsartan and treatment with
sacubitril/valsartan over several months following PCI. Regarding
the dose of sacubitril/valsartan, Zhang et al (14) decided on dose titration and
medication changes according to patient condition. Wang et
al (15) decided on a starting
dose of 24/26 or 49/51 mg sacubitril/valsartan (two times per day
for 2 weeks). At the end of the run-in period (2 weeks),
uptitration of sacubitril/valsartan, if tolerated by the patient,
was allowed. Rezq et al (16) administered sacubitril/valsartan
orally twice daily at a dose of 50 mg and increased to 100 mg twice
daily after 2 weeks if tolerated. Docherty et al (17) administered sacubitril/valsartan
twice daily at a dose of 24/26, 49/51 or 97/103 mg depending on
renal function, blood pressure and ACEI or ARB dose at
randomization. The treatments used in the control groups included
perindopril, enalapril, ramipril and valsartan, and the follow-up
period was 6 or 12 months.
 | Table ICharacteristics of the included
studies. |
Table I
Characteristics of the included
studies.
| | Sex, M/F | Mean age ± SD,
years | | Intervention | |
|---|
| First author,
year | Country | Research | Characteristics | Sample size, T/C | T | C | T | C | Type of AMI | AMI treatment | T | C | Dosage of
Sal/Val | Time between AMI and
intervention | Follow-up,
months | (Refs.) |
|---|
| Zhang, et al
2021 | China | Prospective
single-center RCT | Not double-
blinded | 79/77 | 59/20 | 55/22 | 60.30±11.70 | 60.00±10.90 | All STEMI | All PCI | Sal/Val + RBT | Perindopril +
RBT | According to
patient condition | Early
administration of Sal/Val within 24 h after PCI | 6 | (14) |
| Wang, et al
2021 | China | Prospective
single-center RCT | Blinded | 68/69 | 52/16 | 54/15 | 59.13±7.15 | 60.56±7.62 | All STEMI | All PCI | Sal/Val + RBT | Enalapril +
RBT | 24/26 or 49/51 mg
bid and then up titration | When hemodynamic
stabilization reached after PCI | 6 | (15) |
| Rezq, et al
2021 | Egypt | Prospective
multicenter RCT | Double-
blinded | 100/100 | 86/14 | 88/12 | 52.00±9.20 | 57.00±11.60 | All STEMI | All PCI | Sal/Val + RBT | Ramipril + RBT | 50 or 100 mg
bid | After PCI | 6 | (16) |
| Docherty, et
al 2021 | UK | Prospective
multicenter RCT | Double-
blinded | 47/46 | 42/5 | 43/3 | 61.80±10.60 | 57.00±11.60 | 90 STEMI and 30
NSTEMI | 86 PCI, one
thrombolysis and three CABG | Sal/Val + RBT | Valsartan +
RBT | 24/26, 49/51 and
97/103 mg bid | >3 months after
PCI | 12 | (17) |
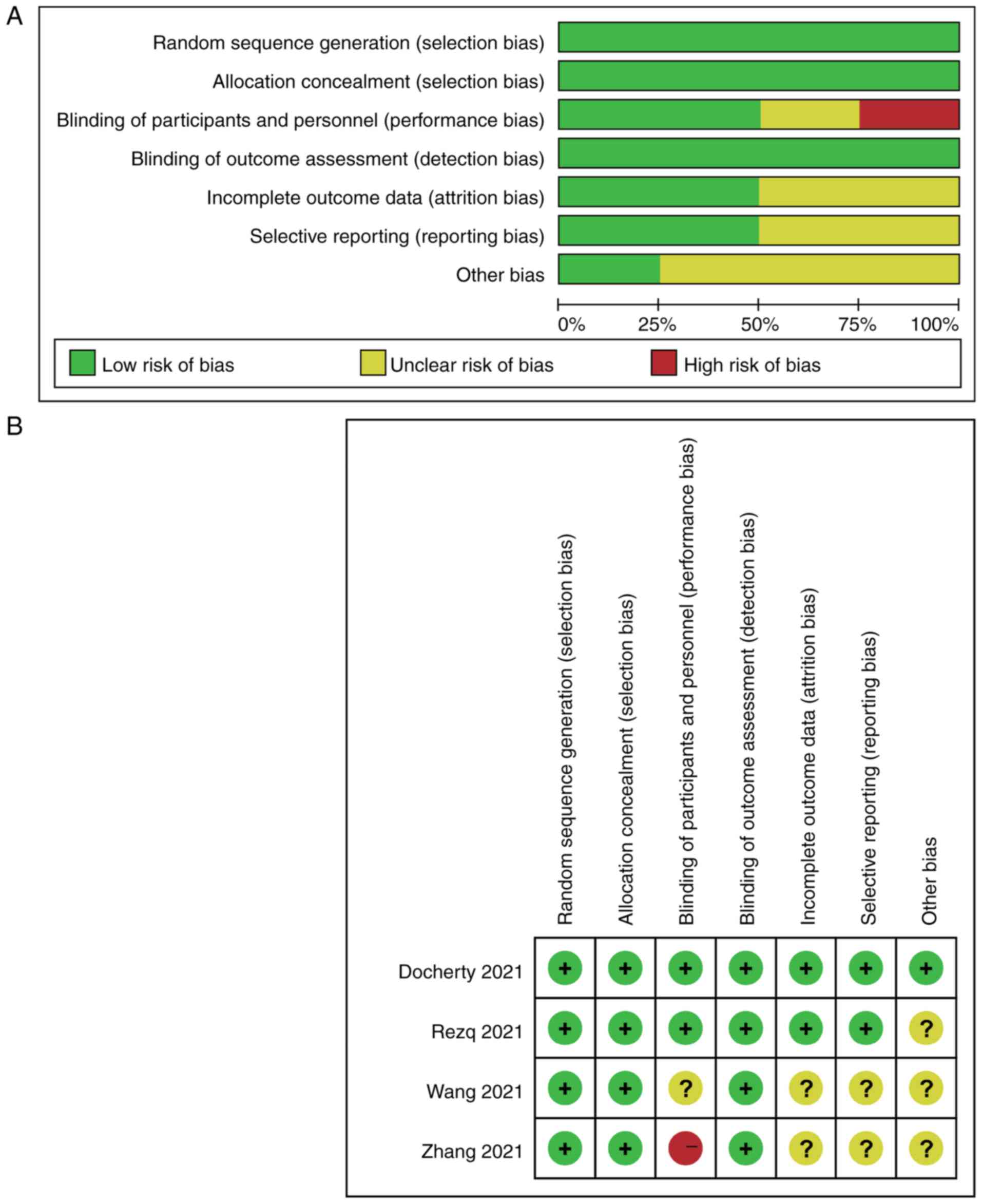
Quality assessment of the included studies is shown
in Fig. 2. All of the studies had
a low risk of bias for random sequence generation, allocation
concealment and blinding of outcome assessment. With regard to
blinding of participants and personnel, in the study by Zhang et
al (14), the staff knew the
patient grouping and medication changes were performed according to
the patients' condition; therefore, the study was ‘not
double-blinded’ and was considered to have high risk of bias. In
addition, Wang et al (15)
described the study as ‘blinded’; however, it was not possible to
determine whether the study was double-blinded or not. By contrast,
the other two studies were double-blinded with low risk. In
addition, there was unclear risk of bias for incomplete outcome
data, selective reporting and other bias in Zhang et al and
Wang et al (14,15)
Overall analysis
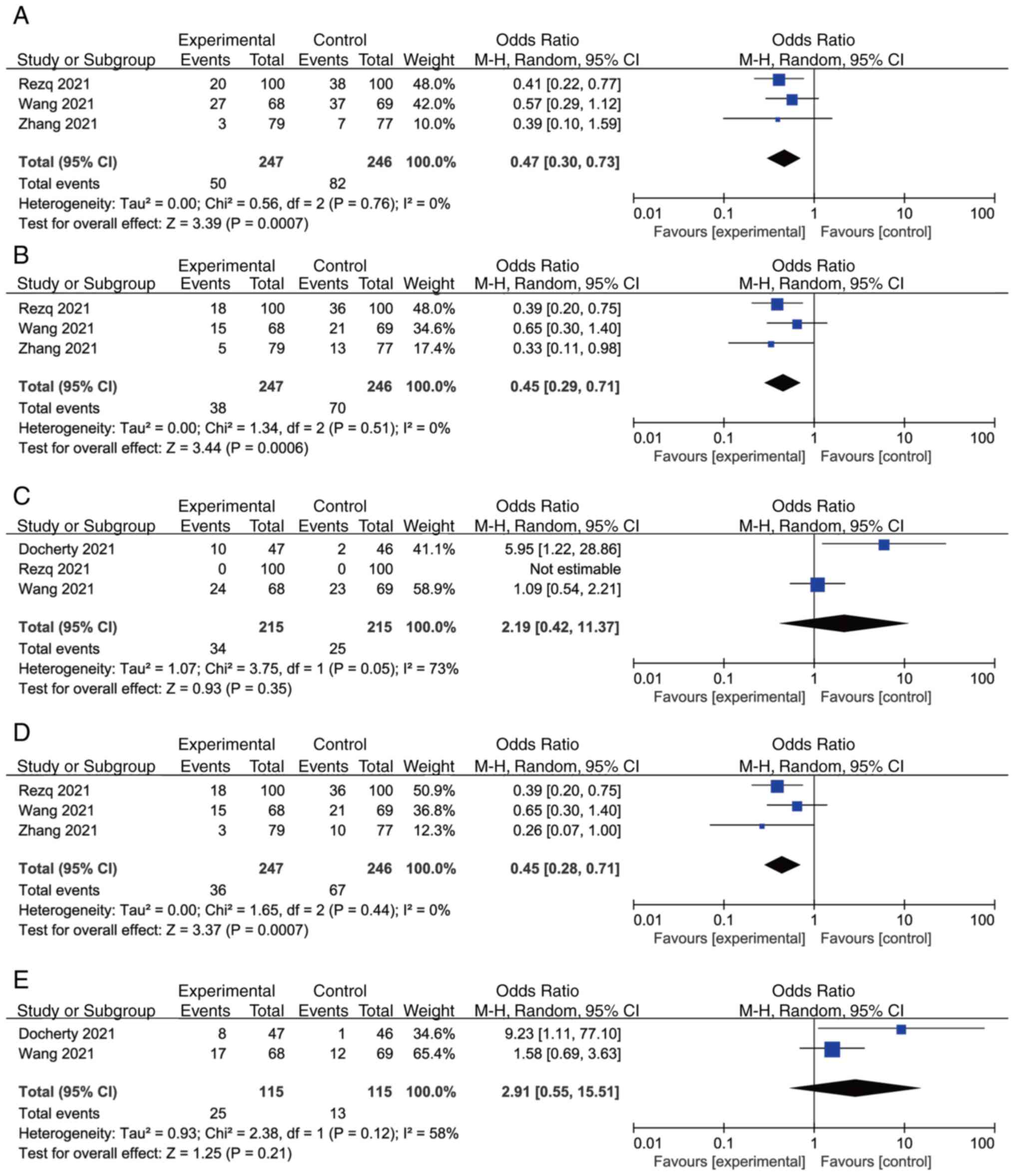
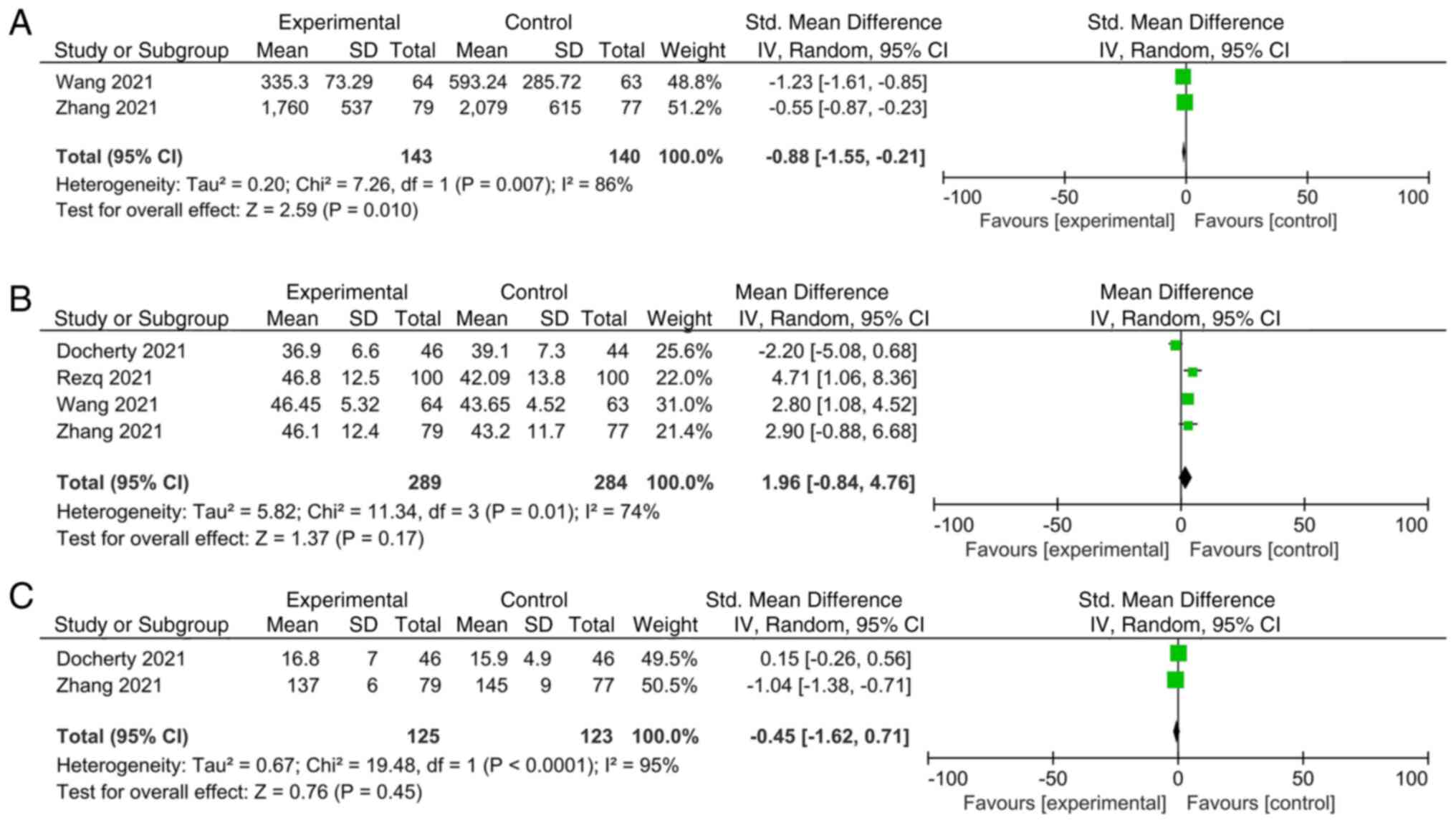
An overall analysis for the primary outcomes is
presented in Fig. 3, whereas that
for the secondary outcomes is provided in Fig. 4. The results of the meta-analysis
revealed a significant reduction in MACCEs (OR, 0.47; 95% CI,
0.30-0.73; P=0.0007, Fig. 3A),
readmission (OR, 0.45; 95% CI, 0.29-0.71; P=0.0006, Fig. 3B), incidence of AHF (OR, 0.45; 95%
CI, 0.28-0.71; P=0.0007, Fig. 3D)
and NT-proBNP [SMD, -0.88; 95% CI, -(1.55-0.21); P=0.01, Fig. 4A] in the sacubitril/valsartan group
compared with that in the control group, and a REM was used to pool
these data. No significant differences were identified in
hypotension (OR, 2.91; 95% CI, 0.55-15.51; P=0.21, Fig. 3E), adverse events (OR, 2.19; 95%
CI, 0.42-11.37; P=0.35, Fig. 3C),
LVEF (MD, 1.96; 95% CI, -0.84-4.76; P=0.17, Fig. 4B) and sST2 (SMD, -0.45; 95% CI,
-1.62-0.71; P=0.45, Fig. 4C) with
the REM. There was a significant statistical heterogeneity when the
effect sizes of adverse events were combined (I2, 73%;
individual I2 values: NT-proBNP, 86%; LVEF, 74% and
sST2, 95%, Fig. 4). Sensitivity
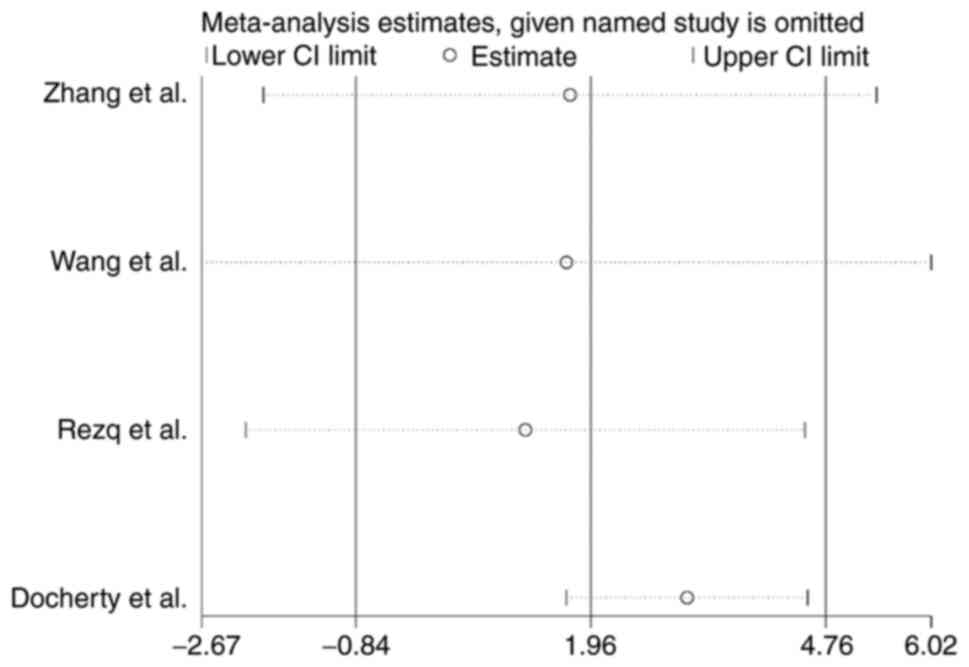
analysis of LVEF was performed and indicated a significant
elevation in LVEF (OR, 3.11; 95% CI, 1.67-4.55; P<0.0001,
Fig. 5) in the
sacubitril/valsartan group compared with that in the control group
after exclusion of Docherty et al (17). This result indicated that Docherty
et al (17) may be the
source of heterogeneity (Fig.
5).
Discussion
Following AMI, disordered ventricular muscle
contraction, activation of the RAAS and ventricular remodeling may
lead to cardiac insufficiency, or even pump failure (18). At present, the drugs that are
recommended by guidelines have a low effect on inhibiting excessive
activation of the nonendocrine system in the mechanism of heart
failure following AMI and are unable to bring about the rapid
rectification of hemodynamic disorders (19,20).
Notably, the overall therapeutic effects of treatments often do not
meet clinical expectations. Therefore, choosing an effective drug
treatment after reperfusion therapy is essential in terms of
improving functional recovery and prognosis. Sacubitril/valsartan
has fulfilled an important role in the treatment of chronic heart
failure in the clinic (21). It
has been recommended by the 2016 European Chronic Heart Failure
Guidelines, the US Heart Failure Management Guidelines and the 2018
Chinese Heart Failure Guidelines for the treatment of chronic heart
failure (22,23). However, at present there is no
consensus on the application of sacubitril/valsartan in heart
failure following AMI. The application of sacubitril/valsartan has
been trialed in animal experiments and in clinical trials of AMI,
and this has achieved impressive results that are continually
expanding the clinically applicable scope of
sacubitril/valsartan.
As the first dual-effect compound preparation of an
enkephalinase inhibitor and ARB, sacubitril and valsartan have been
reported to exert a dual role in neuroendocrine system activity
(24). Valsartan not only exerts
its effects by blocking angiotensin receptors to relax blood
vessels, but also acts as an antagonist of aldosterone, producing
diuresis and sodium excretion, resulting in a net reduction of
water and sodium retention (25).
As an enkephalinase inhibitor, sacubitril can block enkephalinase
activity and reduce the degradation of BNP (26). Sacubitril not only can strengthen
the activity of BNP, expand blood vessels, discharge natriuretic
and diuresis, but it may also reduce the role of pro-fibrotic
signal transduction markers in heart failure.
Recently published clinical studies have revealed
that early application of sacubitril/valsartan following emergency
PCI in patients with AMI can effectively improve left ventricular
remodeling, reduce the occurrence of cardiac insufficiency and
adverse cardiovascular events, and reduce the rehospitalization
rate (14-17).
In addition, a meta-analysis performed by Zhao et al
(27) also indicated that early
initiation of Sacubitril/Valsartan in patients after AMI was
reasonable, but more data are required to support this. Of note,
the results of the present study revealed a significant reduction
in MACCEs, readmission and incidence of AHF, without there being
any statistically significant differences in adverse events noted
between the sacubitril/valsartan group and the control group. In
addition, no significant differences in LVEF were identified
between the two groups of this meta-analysis, a finding that is
inconsistent with previous research results on chronic heart
failure (28). This difference may
be associated with the length of follow-up time. Considering the
influence of follow-up time and the number of included studies,
further big-data RCTs are required to verify these findings. The
Prospective ARNI vs. ACE Inhibitor Trial to Determine Superiority
in Reducing Heart Failure Events After MI study (29) aimed to evaluate the efficacy of
sacubitril/valsartan in patients with left ventricular systolic
dysfunction after AMI compared with ramipril, and the impact that
this therapy may have on the composite end-points of cardiovascular
death and heart failure hospitalization. The results of this study
should provide new evidence for the treatment of heart failure
following AMI.
The present study has a number of limitations.
First, the number of included studies was only four and the study
sample size was small. Hence, although there were no significant
differences in hypotension between the two groups, it must be
emphasized that attention should be paid to changes in blood
pressure considering that this is the most common side effect of
sacubitril/valsartan. Furthermore, certain studies did not provide
blinding of participants. In addition, certain articles did not
specify what the specific medications for the conventional/control
treatment were. The heterogeneity in the meta-analysis of the
primary outcome, adverse events, was significant. The differences
in study design, including differences in patient age, severity of
MI, comorbidities, the dose of sacubitril/valsartan and the
specific medications for conventional treatment, may have resulted
in heterogeneity in the meta-analysis of adverse events. However,
the type of adverse event may be slightly different, which may
cause the heterogeneity. Rezq et al (16) defined adverse events as symptomatic
hypotension, significant hyperkalemia, worsening renal function or
angioedema. Docherty et al (17) regarded adverse events as serum
creatinine ≥2.5 mg/dl, serum potassium >5.5 mmol/l, symptomatic
hypotension with systolic blood pressure <90 mmHg, angioedema
and cough, whereas Wang et al (15) considered hypotension, cough, renal
impairment and hyperkalemia as adverse events. Sensitive analysis
revealed that Docherty et al (17) may be the source of heterogeneity,
which may be due to disparities compared with the other three
studies with regard to the types of AMI and AMI treatment. Docherty
et al (17) included STEMI
and NSTEMI, and conducted thrombolysis, PCI and CABG to treat AMI,
whereas the others only assessed STEMI and implemented PCI.
In conclusion, the present meta-analysis revealed
that sacubitril/valsartan may effectively reduce the incidence of
MACCEs, readmission and AHF in patients with AMI following
revascularization without any obvious adverse events. However,
given the limitations in the quality and quantity of the included
articles and the risk of bias, these findings need to be further
confirmed by big-data and high-quality prospective randomized
controlled studies in order to provide corroborating evidence.
Supplementary Material
Supplementary Data
Acknowledgements
The authors would like to thank Dr Chaojun Yang, Dr
Jun Yang and Dr Jian Yang (Department of Cardiology, Three Gorges
University, Yichang, China) for editing the English text of a draft
of this manuscript and for the registration in PROSPERO.
Funding
Funding: No funding was received.
Availability of data and materials
The present meta-analysis was performed, and has
been reported, according to the guidelines of Preferred Reporting
Items for Systematic Reviews and Meta-Analyses
(CRD42021269433,https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=269433).
The datasets used and/or analyzed during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
BY and ZXF conceived and designed the current study,
defined the content of the research, conducted literature search,
performed statistical analysis and prepared and edited the
manuscript. SSL is the guarantor of study integrity, designed the
current study, defined the content of the research and reviewed the
manuscript. BW conducted the literature search, acquired data and
performed statistical analysis. BY and ZXF confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bauersachs R, Zeymer U, Brière JB, Marre
C, Bowrin K and Huelsebeck M: Burden of coronary artery disease and
peripheral artery disease: A literature review. Cardiovasc Ther.
2019(8295054)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tripolt NJ, Kolesnik E, Pferschy PN,
Verheyen N, Ablasser K, Sailer S, Alber H, Berger R, Kaulfersch C,
Leitner K, et al: Impact of EMpagliflozin on cardiac function and
biomarkers of heart failure in patients with acute MYocardial
infarction-the EMMY trial. Am Heart J. 221:39–47. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fontes-Carvalho R, Azevedo AI, Sampaio F,
Teixeira M, Bettencourt N, Campos L, Gonçalves FR, Ribeiro VG,
Azevedo A and Leite-Moreira A: The effect of exercise training on
diastolic and systolic function after acute myocardial infarction:
A randomized study. Medicine (Baltimore). 94(e1450)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miyazaki S, Kasai T, Miyauchi K, Miyazaki
T, Akimoto Y, Takagi A, Aihara K, Kawamura M, Suwa S, Kojima S, et
al: Changes of matrix metalloproteinase-9 level is associated with
left ventricular remodeling following acute myocardial infarction
among patients treated with trandolapril, valsartan or both. Circ
J. 74:1158–1164. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Her AY, Choi BG, Rha SW, Kim YH, Choi CU
and Jeong MH: The impact of angiotensin-converting-enzyme
inhibitors versus angiotensin receptor blockers on 3-year clinical
outcomes in patients with acute myocardial infarction without
hypertension. PLoS One. 15(e0242314)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McMurray JJ, Packer M, Desai AS, Gong J,
Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg
K, et al: Baseline characteristics and treatment of patients in
prospective comparison of ARNI with ACEI to determine impact on
global mortality and morbidity in heart failure trial
(PARADIGM-HF). Eur J Heart Fail. 16:817–825. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Sauer AJ, Cole R, Jensen BC, Pal J, Sharma
N, Yehya A and Vader J: Practical guidance on the use of
sacubitril/valsartan for heart failure. Heart Fail Rev. 24:167–176.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khder Y, Shi V, McMurray JJV and Lefkowitz
MP: Sacubitril/valsartan (LCZ696) in heart failure. Handb Exp
Pharmacol. 243:133–165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Singh JSS, Burrell LM, Cherif M, Squire
IB, Clark AL and Lang CC: Sacubitril/valsartan: Beyond natriuretic
peptides. Heart. 103:1569–1577. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Böhm M, Young R, Jhund PS, Solomon SD,
Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K,
et al: Systolic blood pressure, cardiovascular outcomes and
efficacy and safety of sacubitril/valsartan (LCZ696) in patients
with chronic heart failure and reduced ejection fraction: Results
from PARADIGM-HF. Eur Heart J. 38:1132–1143. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kang H, Zhang J, Zhang X, Qin G, Wang K,
Deng Z, Fang Y and Chen G: Effects of sacubitril/valsartan in
patients with heart failure and chronic kidney disease: A
meta-analysis. Eur J Pharmacol. 884(173444)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Methley AM, Campbell S, Chew-Graham C,
McNally R and Cheraghi-Sohi S: PICO, PICOS and SPIDER: A comparison
study of specificity and sensitivity in three search tools for
qualitative systematic reviews. BMC Health Serv Res.
14(579)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zeng X, Zhang Y, Kwong JS, Zhang C, Li S,
Sun F, Niu Y and Du L: The methodological quality assessment tools
for preclinical and clinical studies, systematic review and
meta-analysis, and clinical practice guideline: A systematic
review. J Evid Based Med. 8:2–10. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Y, Wu Y, Zhang K, Ke Z, Hu P and Jin
D: Benefits of early administration of Sacubitril/Valsartan in
patients with ST-elevation myocardial infarction after primary
percutaneous coronary intervention. Coron Artery Dis. 32:427–431.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang H and Fu X: Effects of
sacubitril/valsartan on ventricular remodeling in patents with left
ventricular systolic dysfunction following acute anterior wall
myocardial infarction. Coron Artery Dis. 32:418–426.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rezq A, Saad M and El Nozahi M: Comparison
of the efficacy and safety of sacubitril/valsartan versus ramipril
in patients with ST-segment elevation myocardial infarction. Am J
Cardiol. 143:7–13. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Docherty KF, Campbell RT, Brooksbank KJM,
Dreisbach JG, Forsyth P, Godeseth RL, Hopkins T, Jackson AM, Lee
MMY, McConnachie A, et al: Effect of neprilysin inhibition on left
ventricular remodeling in patients with asymptomatic left
ventricular systolic dysfunction late after myocardial infarction.
Circulation. 144:199–209. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sawhney JP: Angiotensine converting enzyme
inhibitors in acute myocardial infarction-a review. Indian Heart J.
63:71–78. 2011.PubMed/NCBI
|
|
19
|
Ibanez B, James S, Agewall S, Antunes MJ,
Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA,
Halvorsen S, et al: 2017 ESC guidelines for the management of acute
myocardial infarction in patients presenting with ST-segment
elevation: The task force for the management of acute myocardial
infarction in patients presenting with ST-segment elevation of the
European society of cardiology (ESC). Eur Heart J. 39:119–177.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cespón-Fernández M, Raposeiras-Roubín S,
Abu-Assi E, Manzano-Fernández S, Flores-Blanco P, Barreiro-Pardal
C, Castiñeira-Busto M, Muñoz-Pousa I, López-Rodríguez E,
Caneiro-Queija B, et al: Renin-angiotensin system blockade and risk
of heart failure after myocardial infarction based on left
ventricular ejection fraction: A retrospective cohort study. Am J
Cardiovasc Drugs. 19:487–495. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nguyen E, Weeda ER and White CM: A review
of new pharmacologic treatments for patients with chronic heart
failure with reduced ejection fraction. J Clin Pharmacol.
56:936–947. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC guidelines for the diagnosis and
treatment of acute and chronic heart failure: The task force for
the diagnosis and treatment of acute and chronic heart failure of
the European society of cardiology (ESC). Developed with the
special contribution of the heart failure association (HFA) of the
ESC. Eur J Heart Fail. 18:891–975. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American college of cardiology
foundation/American heart association task force on practice
guidelines. J Am Coll Cardiol. 62:e147–e239. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ferrari R, Cardoso J, Fonseca MC, Aguiar
C, Moreira JI, Fucili A and Rapezzi C: ‘Italian-Portuguese Action
on Heart Failure’ Group. ARNIs: Balancing ‘the good and the bad’ of
neuroendocrine response to HF. Clin Res Cardiol. 109:599–610.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ardiana F and Suciati and Indrayanto G:
Valsartan. Profiles Drug Subst Excip Relat Methodol. 40:431–493.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Drugs and Lactation Database (LactMed)
[Internet]. Bethesda (MD): National Library of Medicine (US);
2006-. Sacubitril, 2019.
|
|
27
|
Zhao J, Zeng Y and Shen X: Efficacy and
safety of early initiation of sacubitril/valsartan in patients
after acute myocardial infarction: A meta-analysis. Clin Cardiol.
44:1354–1359. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tsutsui H, Momomura S, Saito Y, Ito H,
Yamamoto K, Ohishi T, Okino N and Guo W: Efficacy and safety of
sacubitril/valsartan (LCZ696) in Japanese patients with chronic
heart failure and reduced ejection fraction: Rationale for and
design of the randomized, double-blind PARALLEL-HF study. J
Cardiol. 70:225–231. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jering KS, Claggett B, Pfeffer MA, Granger
C, Køber L, Lewis EF, Maggioni AP, Mann D, McMurray JJV, Rouleau
JL, et al: Prospective ARNI vs ACE inhibitor trial to determine
superiority in reducing heart failure events after myocardial
infarction (PARADISE-MI): Design and baseline characteristics. Eur
J Heart Fail. 23:1040–1048. 2021.PubMed/NCBI View Article : Google Scholar
|