Introduction
Ovarian carcinomas are heterogeneous with few
identified causative genetic mutations (1); they also exhibit genomic aberrations
associated with altered gene expression that also has been reported
in breast cancer (2). Paired box 2
(PAX2) protein expression is upregulated in certain types of
ovarian cancer, including endometrioid ovarian cancer, as well as
clear cell, mucinous and serous carcinoma (3). However, its role in promoting tumor
progression is not yet clear. PAX2 serves important roles in
embryogenesis, driving epithelial differentiation during urogenital
tract development (4). PAX2 is
also hypothesized to induce cell elongation and tubule formation in
different types of organs, such as the inner ear, neural tube and
genital tract, during embryonic development (5). In our previous study, it was
demonstrated that PAX2 is crucial for maintaining epithelial cell
differentiation in adult reproductive tissue (6). In the kidney, PAX2 serves an
important role in the development of the collecting duct, where it
induces branching morphogenesis (7,8).
This was confirmed by decreased ability to form branched collecting
ducts in PAX2 mutant compared with wild-type mice (8). Despite its well-established roles in
normal tissue, the contribution of PAX2 to initiation and
progression of ovarian cancer remains unclear. High expression
levels of PAX2 in ovarian cancer cells enhance tumorigenesis in
animals and are associated with shorter survival time in humans
(9).
Angiogenesis is required to establish blood supply
necessary for tumor growth. Anti-angiogenic therapeutic approaches
have been used widely to inhibit neovascularization, but the
results have been unsatisfactory in invasive and aggressive types
of cancer, such as melanoma and glioblastoma (10,11).
The growth of tumors, even in the presence of angiogenesis
inhibiting drugs, indicates that tumor cells may have other means
of promoting/obtaining blood supply (12). Anti-angiogenic therapies, such as
anti-VEGF antibodies, target only endothelial cell proliferation,
whereas cancerous cells may proliferate and form de novo
vascular channels connected to endothelial-lined vasculature
(13).
In 1999, Maniotis et al (14) observed aggressive melanoma cells
forming a blood vessel-like pattern in 3D culture. When the cells
were injected into mice, they formed tumors that contained
vessel-like structures. This formation of vessel-like structures
was termed ‘vasculogenic mimicry’ (VM) (14). Red blood cells are detected inside
vessels, and the cells lining these vessels are negative for
endothelial markers CD31 and CD34(15). Tumor cells are organized in a
pattern supported by remodeling of the extracellular matrix, as
determined by periodic acid-Schiff (PAS) staining to label
carbohydrates in the remodeled extracellular matrix (16).
In previous studies, CD31-negative/PAS-positive
vessels formed by tumor cells demonstrated VM in in vitro
and in vivo experiments (17-19).
Attempts have been made to determine the molecular mechanisms
underlying VM formation (20). In
aggressive breast cancer cells, cyclooxygenase-2 (COX2) induces VM
formation in 3D cultures and knockdown of COX2 decreases VM
formation (21). Moreover, the
ovarian cancer cell line SKOV3 has been reported to form VM in
vitro; targeting CD147 in these cells causes significant
downregulation of VM formation (22). CD147 is an inducer of extracellular
matrix metalloproteinase (MMP)-2(23). Overexpression of MMP has been
previously linked with VM incidence in the ovarian cancer cell
lines SKOV3 and OVCAR3(24), where
MMP-2 expression contributes to extracellular matrix remodeling
(25). Furthermore, expression of
MMP-9 has been detected in different ovarian cancer cell lines
(26), and the role of MMP-9 in VM
is associated with cancer progression (27).
Ovarian cancer cell lines have been reported to form
VM in Matrigel culture and in xenograft mouse models (25,28).
The vascular channels formed by SKOV3 and OVCAR3 ovarian cancer
cells are matrix-enriched and endothelial cell-independent, and
cells lining the vascular channels are positive for cytokeratin
(25). Blood cells have been
detected inside vascular channels formed in vivo (14,16). At present, the mechanism by
which ovarian cancer cells promote VM is unknown. However, certain
ovarian cancer cell lines express endothelial markers de
novo and it has been hypothesized that cancer cells
differentiate into endothelial cells to promote angiogenesis
(13). The SKOV3 cell line has the
ability to form VM in 3D culture; this may be due to weak
expression of the endothelial marker CD31, which is expressed on
the cell surface in monolayer cultures and in VM formed by cells
(29).
Neovascularization mechanisms are key for ovarian
cancer growth and dissemination to other organs (13). Our previous study found that
upregulated expression of the angiogenesis biomarker CD31 may
underlie the increased rate of cancer progression in a mouse model
of ovarian cancer associated with high PAX2 expression (30). As these appeared to be similar to
what has been previously reported for SKOV3 cells showing VM
(22,29), the present study aimed to
investigate the effects of PAX2 expression in epithelial cells and
their ability to form branching structures when placed in 3D
cultures.
Materials and methods
Human samples
Formalin-fixed paraffin-embedded (FFPE) human
ovarian cancer tissue blocks were selected randomly from the cancer
registry at King Fahad Specialist Hospital (Dammam, Saudi Arabia).
The protocol was approved by the registered Institutional Review
Board (IRB) affiliated with King Fahad Specialist Hospital-Dammam
(approval no. IRB# ONC0340). The need for informed consent was
waived by the IRB as the samples were archived for >10 years.
Cancer tissues were assessed by independent pathologists to confirm
the presence of tumor cells in the tissues before doing further
experiments. The tested samples included 10 serous, four
endometroid, two clear, two granulosa, one mucinous and one germ
cell tumor. All 20 samples were collected from the Pathology
Department and stained for PAX2 and CD31 antibodies, and PAS
staining, as described in below sections, regardless of
histological type. PAS staining was used to label the extracellular
matrix (ECM) formed by cancer cells. The clinicopathological data
including histological type, tumor status, stage and grade were
collected from patient files (Table
I). The age range for the 20 patients with ovarian cancer was
42-60 years old.
 | Table IClinicopathological characteristics
of patients and immunohistochemical characteristics of ovarian
cancer samples. |
Table I
Clinicopathological characteristics
of patients and immunohistochemical characteristics of ovarian
cancer samples.
| Patient sample
no. | Type | Stage | Grade | Recurrent | Metastatic | Patient tumor
status | CD31 | PAX2 |
PAS+/CD31- |
|---|
| 1 | Serous | N/A | 3 | No | Yes | Tumor-free | High | Yes | High |
| 2 | Serous | N/A | 3 | No | Yes | Not tumor-free | High | No | Low |
| 3 | Serous | I | 3 | No | No | Not tumor-free | High | No | Low |
| 4 | Serous | IV | 3 | Yes | Yes | Not tumor-free | High | Yes | High |
| 5 | Serous | IV | 3 | Yes | Yes | Not tumor-free | Low | Yes | High |
| 6 | Serous | IV | 3 | Yes | yes | Not tumor-free | Low | Yes | Low |
| 7 | Serous | III | 3 | No | No | Tumor-free | Low | No | Low |
| 8 | Serous | IV | 3 | Yes | Yes | Not tumor-free | Low | No | High |
| 9 | Serous | III | 3 | Yes | Yes | Not tumor-free | High | No | Low |
| 10 | Serous | N/A | 3 | No | Yes | Not tumor-free | High | No | Low |
| 11 | Endometrioid | IV | 3 | Yes | Yes | Not tumor-free | Low | Yes | High |
| 12 | Endometrioid | III | 3 | Yes | Yes | Not tumor-free | High | No | High |
| 13 | Endometrioid | III | 2 | Yes | No | Tumor-free | Low | Yes | Low |
| 14 | Endometrioid | II | 1 | Yes | No | N/A | High | Yes | Low |
| 15 | Clear cell | IV | 3 | Yes | Yes | Not tumor-free | High | No | High |
| 16 | Clear cell | N/A | N/A | No | No | Tumor-free | High | Yes | High |
| 17 | Granulosa cell | III | 3 | Yes | Yes | Not tumor-free | High | Yes | High |
| 18 | Granulosa cell | III | N/A | Yes | Yes | Not tumor-free | High | No | High |
| 19 | Mucinous cell | IV | 2 | No | No | Not tumor-free | High | No | Low |
| 20 | Germ cell | III | 3 | Yes | Yes | N/A | High | Yes | High |
Cell lines
Normal mouse ovarian surface epithelial (MOSE) cells
(M1102) were obtained from our previous study (9,30).
Briefly, MOSE cells were isolated from the ovarian surface of
6-week-old FVB/N mice and cultured in DMEM (Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
PAA Laboratories GmbH; GE Healthcare) as described previously
(30). RM ovarian cancer cells
were obtained from our previous reported study (31). Briefly, RM ovarian cancer cells
were derived from immortalized MOSE cells that were previously
transduced with K-Ras (KRASG12D) and
c-Myc and maintained in DMEM + 10% FBS (31).
SKOV3 human ovarian cancer cells were maintained in
DMEM + 10% FBS. Immortalized HUVECs were obtained as a gift from Dr
Christina Addison (Ottawa Hospital Research Institute; Ontario,
Canada). HUVECs were maintained in EGM-2 media (Lonza Group, Ltd.)
supplemented with 1.0 ng/ml epidermal growth factor and 10% FBS.
All cell lines were incubated at 37˚C and 5% CO2.
The M1102 and RM cells were previously modified to
overexpress PAX2 (6,30) and those stably expressing cell
lines were used in the present study. Briefly, lentiviral vectors
were constructed by co-transfection of vector plasmids with
packaging plasmid and the ecotropic envelope expression plasmid
into 293T cells as described previously (32). M1102 and RM cells were infected
with the lentiviral vector WPI (negative control) or lentiviral
vector WPI with PAX2 gene to generate the cell lines M1102-WPI,
M1102-PAX2, RM-WPI and RM-PAX2, as described previously (30). In that same study, the generation
of PAX2 gene knockout cells was also described. The integrated
proviruses harbor loxP sites that were excised by Cre-mediated
recombination which was achieved by treating the cells with
adenovirus expressing Cre recombinase (AdCre; Vector Development
Laboratory) to form M1102-PAX2-AdCre cells and RM-PAX2-AdCre cells,
as described previously (30).
Western blotting
The cellular proteins for M1102, M1102-WPI,
M1102-PAX2 and M1102-PAX2-AdCre cells were extracted to detect PAX2
expressions using western blots as described previously (6). Briefly, cellular proteins were
extracted using M-PER mammalian protein extraction reagent (Thermo
Fisher Scientific, Inc.). A total of 40 µg of the extracted
proteins were loaded for protein separation using 4-12%
polyacrylamide gel (Thermo Fisher Scientific, Inc.), and then
transferred on nitrocellulose membrane (Thermo Fisher Scientific,
Inc.). The membrane was blocked using 5% non-fat milk at 4˚C for 1
h. The transferred proteins were incubated with anti-mouse PAX2
(1:20,000; cat. no. sc-130387; Santa Cruz Biotechnology, Inc.) for
1 h at room temperature, then incubated with anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:10,000, cat. no.
12-349; Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. The
immune reactivity was detected and analyzed using chemiluminescence
detection kit (Clarity Western ECL Substrate; Bio-Rad Laboratories,
Inc.). The protein signals were documented on the western blot
membrane using FluorChem imaging system (FluorChem FC2; Alpha
Innotech Corporation). The protein band densities were analyzed
using ImageJ software (version 1.53a; NIH).
Animals
Animal experiments were not performed in this study.
All tumor samples were obtained from tissue preserved from our
previous animal experiments (9,30),
which were performed in accordance with the guidelines of the
Canadian Council on Animal Care and were approved by the University
of Ottawa Animal Care Committee (approval no. ME-256). In that
study, five female CB17 SCID mice (age, 8 weeks; weight, 17-18 g;
Charles River, Montreal, Quebec) were injected in the peritoneal
cavity with 1x107 RM cells with or without PAX2
expression, suspended in 500 µl PBS. Animals were housed in
standard rodent cages with a 12-h light/dark cycle and provided
with free access to food and water. The temperature was maintained
at 20-23˚C with relative humidity of 55±15%. Animals were monitored
daily for disease progression and health status, noting any signs
of distress or loss of wellbeing, including weight gain/loss,
decreased activity, labored respiration and/or decreased ability to
ambulate. Mice were euthanized when they reached a humane endpoint,
such as weight gain of 15% compared with age-matched controls or
abdominal distention owing to tumor and ascites formation. All
animals were euthanized using CO2 with a 50% volume
displacement rate, followed by cervical dislocation. Tumor volume
and total tumor mass were not assessed in the previous study
(26). The solid tumor nodules
were resected, fixed in 10% formalin overnight at room temperature,
transferred to 70% ethanol and paraffin-embedded. The embedded
tissues were retrieved from storage and used for
immunohistochemistry analysis in the present study.
Matrigel (3D) culture
Eight-well chamber slides (Thermo Fisher Scientific,
Inc.) were covered with 60 µl cold growth factor-reduced Matrigel
(Trevigen, Inc.) and incubated at 37˚C for 30 min to polymerize the
Matrigel. Each of M1102, RM, SKOV3 or HUVECs were seeded with assay
medium [Mammary Epithelial Cell Growth Medium (MEGM™)
supplemented with 5% FBS, 1.0 ng/ml epidermal growth factor and 0.5
ng/ml of each of insulin, hydrocortisone, cholera toxin and
gentamicin]. All reagents were obtained from Lonza Group, Ltd.
Different concentrations of the cells (4x104,
8x104, 16x104, 20x104) were plated
on Matrigel as described previously (33). The cells were covered with assay
media containing 4% Matrigel and incubated at 37˚C for 48 h. RM
cells were similarly plated; however, serum-free DMEM (Thermo
Fisher Scientific, Inc.) was used in place of MEGM™. The
formation of vascular channels was quantified manually by counting
all tubule branches in each well using a light microscope at 100X
magnification.
Immunofluorescence analysis
Immunofluorescence analysis was performed on HUVEC,
M1102-PAX2 and SKOV3 cells grown in Matrigel. The cells were fixed
with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 20 min and
permeabilized with 1:1 methanol and acetone for 15 min, both at
4˚C. After blocking for 1 h at room temperature in 5% goat serum
(Sigma-Aldrich; Merck KGaA), cells were probed with rabbit
anti-CD31 (1:100; cat. no. ab222783; Abcam) for 1 h at room
temperature. Alexa Fluor goat anti-rabbit IgG (1:500, cat. no.
A32731; Thermo Fisher Scientific, Inc.) was used as the secondary
antibody for 1 h at room temperature. The cells were mounted on
Matrigel slides with VectaShield hard set mounting medium
containing DAPI (Vector Laboratories, Inc.), and immunofluorescence
was visualized at 100X magnification using an inverted fluorescence
microscope (Axioskop 2 MOT plus) and AxioVision LE software
(version 4.8.2; Carl Zeiss AG).
Immunohistochemistry
Immunohistochemistry analysis was performed on both
human and animal ovarian cancer tissues. The tumor tissues were
collected either from experimental animals that were injected with
ovarian cancer cells or from patients that had been diagnosed with
ovarian cancer, as described above. The FFPE tissues were cut into
4-µm sections, de-paraffinized and rehydrated in decreasing ethanol
gradient. The tissue was heated for 10 min in a microwave in
antigen unmasking solution for antigen retrieval (Vector
Laboratories, Inc.). The endogenous peroxidase was neutralized in
the tissues using Novocastra Peroxidase Block, 3% hydrogen peroxide
(cat. no. RE7157; Leica Microsystems, Inc.). Tissue sections were
blocked with Dako protein block, serum-free (Dako; Agilent
Technologies, Inc.) for 1 h at room temperature. The animal tissue
sections were incubated overnight at 4˚C with rabbit anti-CD31
antibody (1:100, cat. no. ab182981; Abcam) or with rabbit anti-PAX2
(1:1,000; cat. no. 71-6000; Thermo Fisher Scientific, Inc.). After
washing with PBS, the animal sections were incubated with
pre-diluted Dako Envision system-horseradish peroxidase (HRP)
labeled polymer anti-rabbit antibodies (cat no. K4002; Dako;
Agilent Technologies, Inc.) for 30 min at room temperature. The
immunoreactivity was detected using DAB chromogen (50:1,000; cat.
no. 8059; Cell Signaling Technology, Inc.) for 5 min at room
temperature and then counterstained using hematoxylin (American
Master Tech Scientific, Inc.) for 1 min at room temperature. The
human tissue sections were incubated for 1 h at room temperature
with mouse anti-CD31 antibody (1:500; cat. no. NCL-L-CD31-607,
Leica Biosystems, Inc.) or 1 h with rabbit polyclonal anti-PAX2
antibody (1:200; cat. no. 71-6000, Thermo Fisher Scientific, Inc.).
After washing with PBS, human tissues were incubated with
pre-diluted anti-mouse secondary antibody IgG-HRP conjugated (cat
no. RE7140-CE; Leica Microsystems, Inc.) for 30 min at room
temperature or goat anti-rabbit-HRP (1:500; cat. no. MP02794;
Thermo Fisher Scientific, Inc.) followed by Novolink DAB chromogen
(1:500; cat. no. NCL-L-CD31-60; Leica Microsystems, Inc.) for 5 min
at room temperature using Novolink Polymer Detection system
according to the manufacturer's instructions. The human sections
were counterstained with hematoxylin for 1 min at room
temperature.
Images were investigated using light microscopy,
captured using the Aperio ScanScope system and analyzed using
Aperio ImageScope software (version 12.3; Leica Microsystems,
Inc.). Protein expression was quantified as positive pixels using
the Aperio Positive Pixel Count Algorithm software (version 12.3;
Leica Microsystems, Inc). The scanned tissues were carefully
screened using ImageScope software to determine PAX2 expression. A
minimum of 10 fields of view were investigated for PAX2 expression
and four random fields were selected and images captured for
analysis at 200X magnification and selected to analyze the vascular
channel formation.
PAS
PAS staining was used on sections of paraffin-fixed
tissues that were prepared as aforementioned or 3D cultures to
stain vascular-like structures. For paraffin-fixed tissue, the
fixed tissue was cut, de-paraffinized using xylene, rehydrated
using graded ethanol and then heated on 95˚C for antigen retrieval
using antigen unmasking solution (Vector Laboratories, Inc.). For
vascular channels formed by MOSE and RM cells in 3D culture, the
8-well chamber slides were washed with PBS, fixed with 2%
paraformaldehyde for 15 min at room temperature and then washed
with PBS three times. PAS staining was performed using PAS kit
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol for both FFPE sections and 3D culture. Periodic acid (250
µl/well or section) was added at room temperature for 5 min, and
then washed with PBS 3-5 times for 5 min each. Schiff stain (250
µl/well or section) was added at room temperature for 15 min and
washed three times for 5 min each. All washing was performed using
PBS (500 µl/well or section). All washing steps for the wells were
performed using a pipette to drop the solution on top of the
Matrigel. For the double staining of PAS and antibody in FFPE
tissues, PAS staining was performed following DAB and hematoxylin
staining for the immunohistochemistry analysis.
Evaluation of the staining
All stained and scanned human tissues were analyzed
using light microscopy (magnification, 200X). The positive index
was applied to evaluate PAX2, CD31 and PAS staining. For PAX2, ≥30%
of positively nuclear staining out of the total stained tissue was
considered as PAX2-positive. For evaluating the stained vessels,
the staining evaluation was performed based on assessing all
stained vessels in all inspected fields in the tissues. If >50%
of the vessels of the inspected fields were stained with CD31
and/or PAS, the evaluations were considered as CD31high
and/or PAShigh; while <50% were considered as
CD31low and/or PASlow. The evaluation of VM
after the dual staining of CD31 and PAS was observed in each field
by detecting vessels that were CD31-/PAS+.
Detecting >30% of the vessels that were
CD31-/PAS+ was considered as VM-positive
tissues and <30% was considered as negative.
Statistical analysis
All experiments were performed at least three times.
Image analysis was performed using Aperio Positive Pixel Count
Algorithm (version 12.3; Leica Microsystems, Inc.) and ImageJ
(National Institutes of Health). The quantification of PAX2, CD31
and PAS expression levels were determined based on positive pixel
counts relative to tissue area. Statistical analysis was performed
using GraphPad Prism (versions 7 and 9; GraphPad Software, Inc.).
An unpaired Student's t-test (for two groups) or one way ANOVA with
post hoc Tukey's test (for >2 groups) was used to determine
statistical significance. Data are presented as the mean ± standard
error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
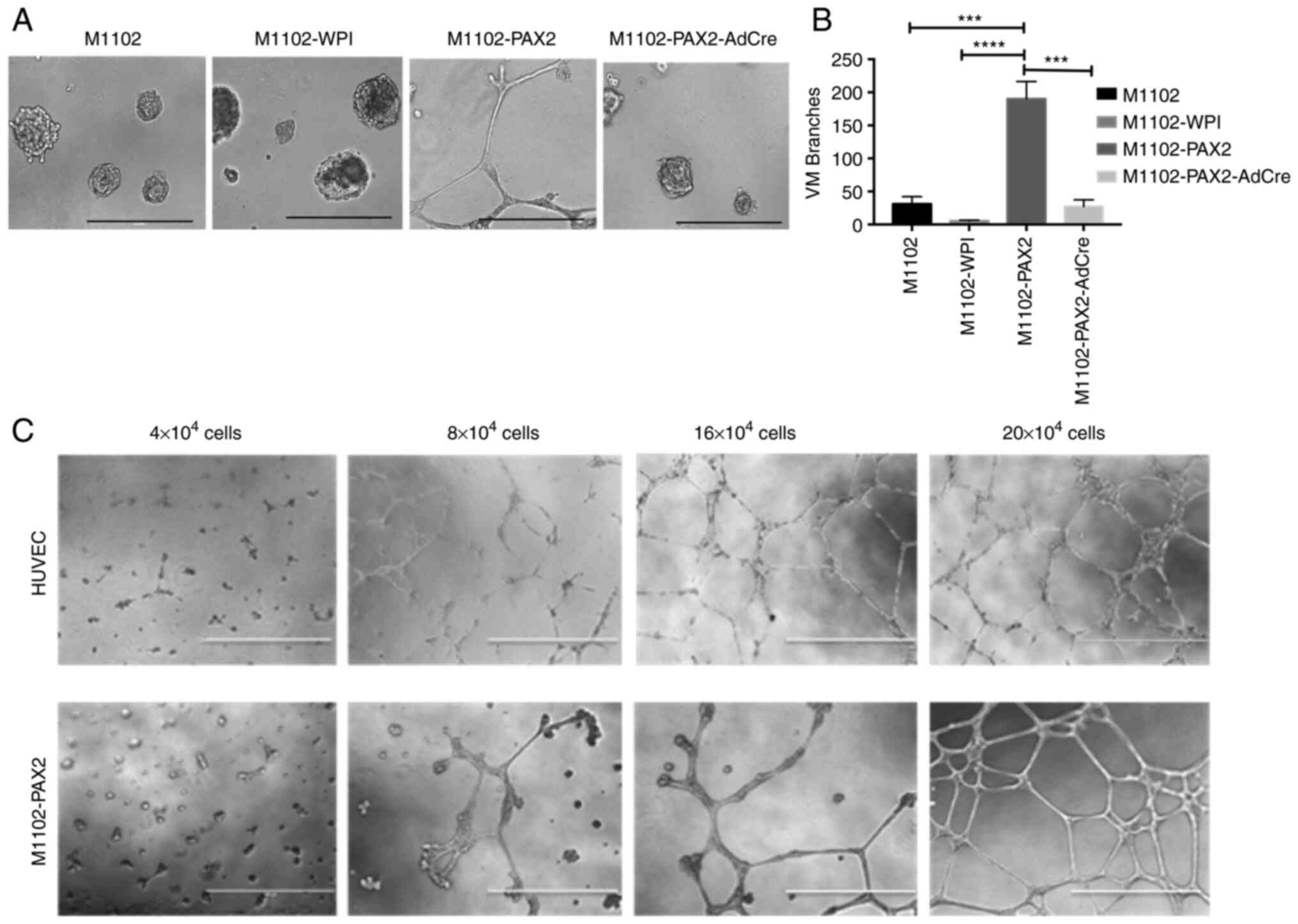
PAX2 induces vascular-like channels in
MOSE cells in vitro
The overexpression and knockout of PAX2 in M1102
cells was confirmed using western blotting (Fig. S1), as reported in our previous
study (6). To determine whether
PAX2 induces formation of vascular-like channels by normal
epithelial cells, M1102-PAX2 cells were plated in 3D cultures using
Matrigel. M1102-PAX2 cells exhibited branching and elongation to
form vascular-like channels that mimicked vascular channels formed
by endothelial cells (Fig. 1). By
contrast, parental and vector control M1102 cells did not form
vascular-like channels, and knockout of Pax2 by AdCre decreased
formation of vascular-like channels relative to their
PAX2-expressing counterparts (Fig.
1A). The statistical analysis of tubule formation showed a
significant increase in the number of tubular branches formed by
M1102-PAX2 compared with M1102, M1102-WPI or M1102-PAX2-AdCre
(Fig. 1B). Formation of
vascular-like channels was associated with cell density, in which
the higher the number of plated cells, the more organized the
branched vascular channels were after 48 h (Fig. 1C and Data S1); HUVECs were used as a positive
control.
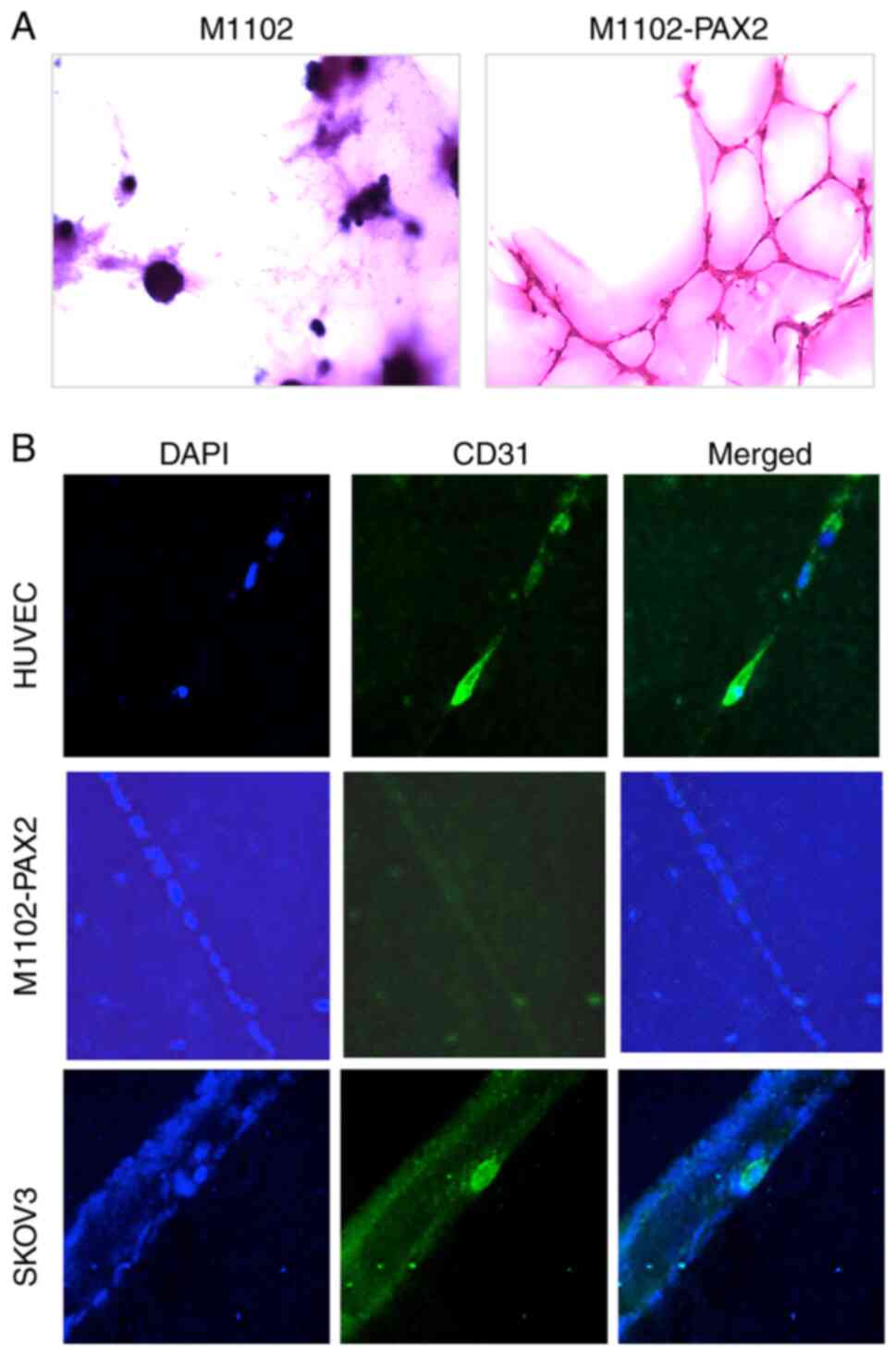
PAX2 induces endothelial
cell-independent vascular-like channels
To determine whether PAX2 induced the
differentiation of epithelial to endothelial cells and the
formation of vascular-like channels, the formed channels were
stained with PAS to label ECM formed by M1102-PAX2 cells. The
glycogen in the ECM reacts with PAS, which stains tissue dark
purple. Oxidized glycogens (aldehydes) are detected by the PAS
stain, which results in a pink color in the tissue (34). The vascular-like channels formed by
M1102-PAX2 cells were PAS-positive, which indicated the presence of
ECM supporting vascular-like channels, whereas spheres formed by
control M1102 cells were PAS-negative (Fig. 2A). Furthermore, vascular-like
channels formed by M1102-PAX2 cells were CD31-negative, unlike
HUVEC cells, suggesting that PAX2 did not induce ovarian epithelial
differentiation to endothelial cells but did induce formation of
vascular-like channels. HUVEC cells were used as a positive control
for endothelial cells expressing CD31. SKOV3 cells were included as
a positive control for assessment of vascular channel formation, as
it has been previously reported that they do not express CD31
normally (25) but form vascular
channels in 3D culture (22).
SKOV3 cells readily formed vascular-like channels and cells within
those channels differentiated and expressed CD31 (Fig. 2B).
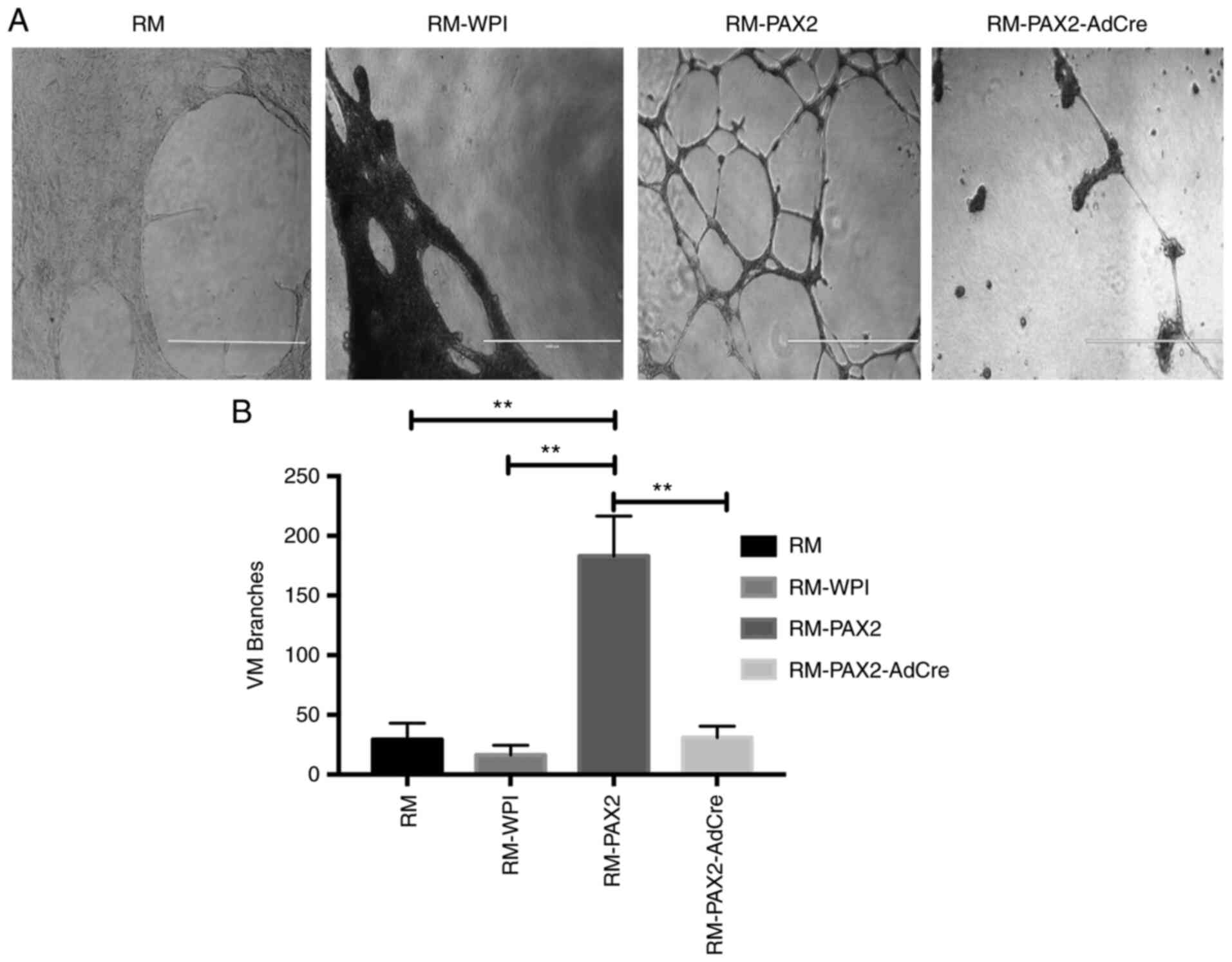
PAX2 enhances vascular-like channel
formation in RM ovarian cancer cells in vitro
To determine whether PAX2 enhanced vascular channel
formation by cancer cells, tumors derived from the RM mouse ovarian
cancer cell line was used. To determine whether RM cells that
overexpressed PAX2 induced vascular-like channel formation, the
ability of these cells to elongate and form branched channels in
Matrigel was assessed. Overexpression of PAX2 in RM cells
significantly enhanced formation of vascular-like channels in
Matrigel compared with RM and RM-WPI control cells, which did not
form vascular-like channels in Matrigel. Moreover, RM-PAX2-AdCre
cells that had PAX2 knocked out exhibited significantly decreased
formation of vascular-like channels compared with RM-PAX2 (Fig. 3A and B).
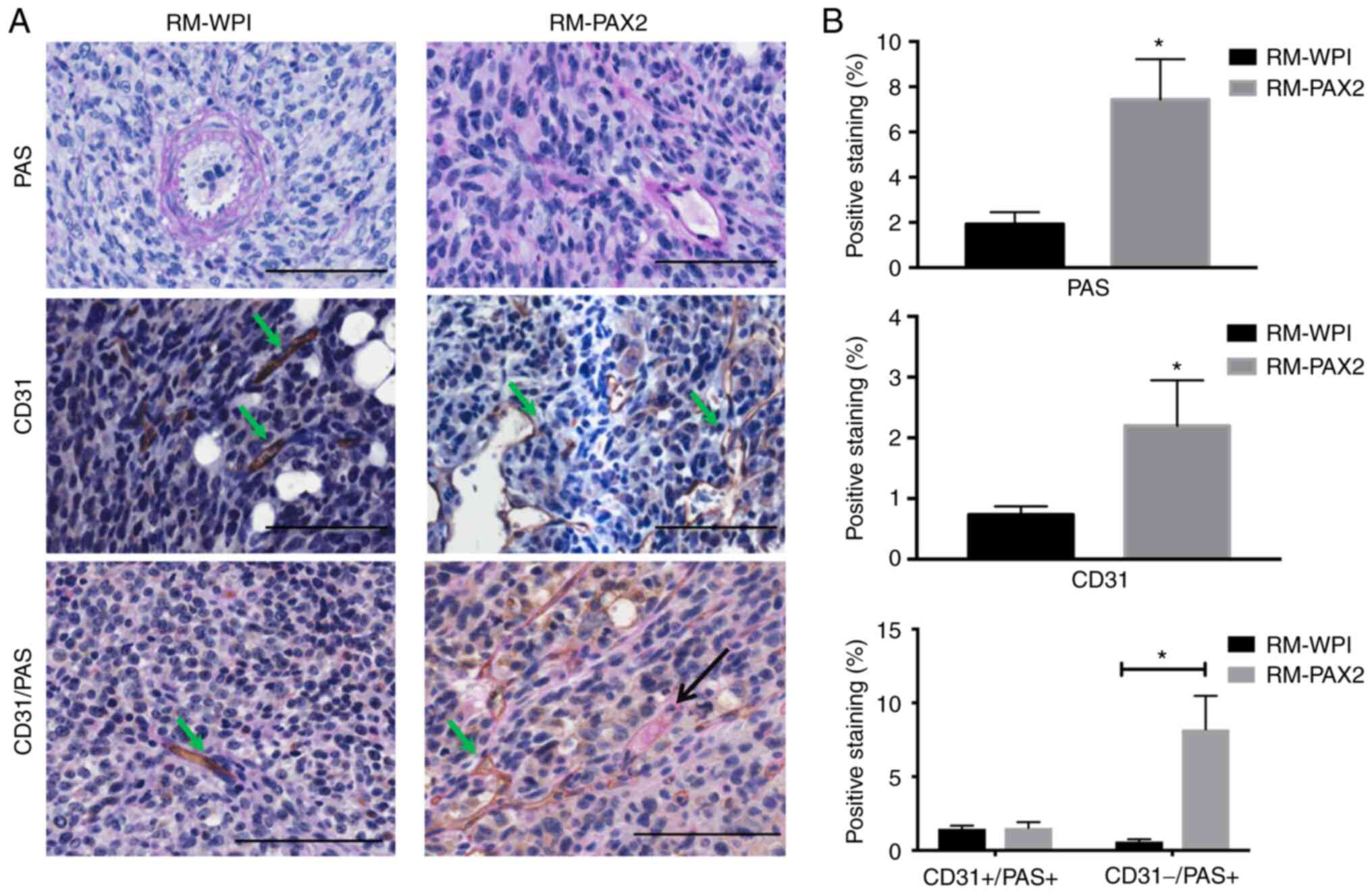
PAX2 enhances vascular-like channel
formation in tumors formed by RM cells in vivo
To determine the role of PAX2 in inducing tumor
progression in a model of ovarian cancer, RM-WPI or RM-PAX2 cells
were injected into immunocompromised mice. Both RM-WPI and RM-PAX2
cells developed tumors in injected mice. RM-PAX2 tumors exhibited
small vascular-like channels that were PAS-positive, whereas RM-WPI
tumors lacked small vascular-like channels and PAS staining labeled
large blood vessels (Fig. 4A).
RM-PAX2 tumors also exhibited a higher density of CD31-stained
vessels compared with RM-WPI tumors (Fig. 4A), suggesting that PAX2 enhanced
angiogenesis in these ovarian cancer cells. To determine if RM-PAX2
tumors induced de novo vessel formation, PAS/CD31 double
staining was performed. RM-PAX2 tumors possessed both types of
vascular channels; those formed by endothelial cells that were PAS-
and CD31-positive, whereas small vascular channels that were not
formed by endothelial cells were PAS-positive but CD31-negative
(Fig. 4A and B). Since VM is PAS-positive/CD31-negative
(12), these results suggested
that PAX2 may have enhanced tumor progression by inducing
vasculogenesis.
To confirm whether cancer cells expressing PAX2
formed vascular-like channels, tumors derived from RM-WPI and
RM-PAX2 cells were stained for PAX2, PAS and CD31. Tumors arising
from RM-PAX2 cells exhibited vascular channels lined with cells
expressing PAX2 and contained red blood cells (Fig. 5A). Double staining showed vessels
lined with PAX2-expressing cells stained positive for PAS and
contained red blood cells, indicative of a blood vessel. The
sections that exhibited PAX2 expression contained both
CD31-positive/PAS-positive vessels, indicating regular blood
vessels and CD31-negative/PAS-positive vessels, suggesting
vasculogenesis in these tumors (Fig.
5A and B). However, RM-WPI
tumors were negative for PAX2, as expected and exhibited
CD31-positive/PAS-positive vessels, indicating regular blood
vessels that were formed by endothelial cells. These observations
indicated that PAX2 contributed to formation of vascular channels
in ovarian tumors.
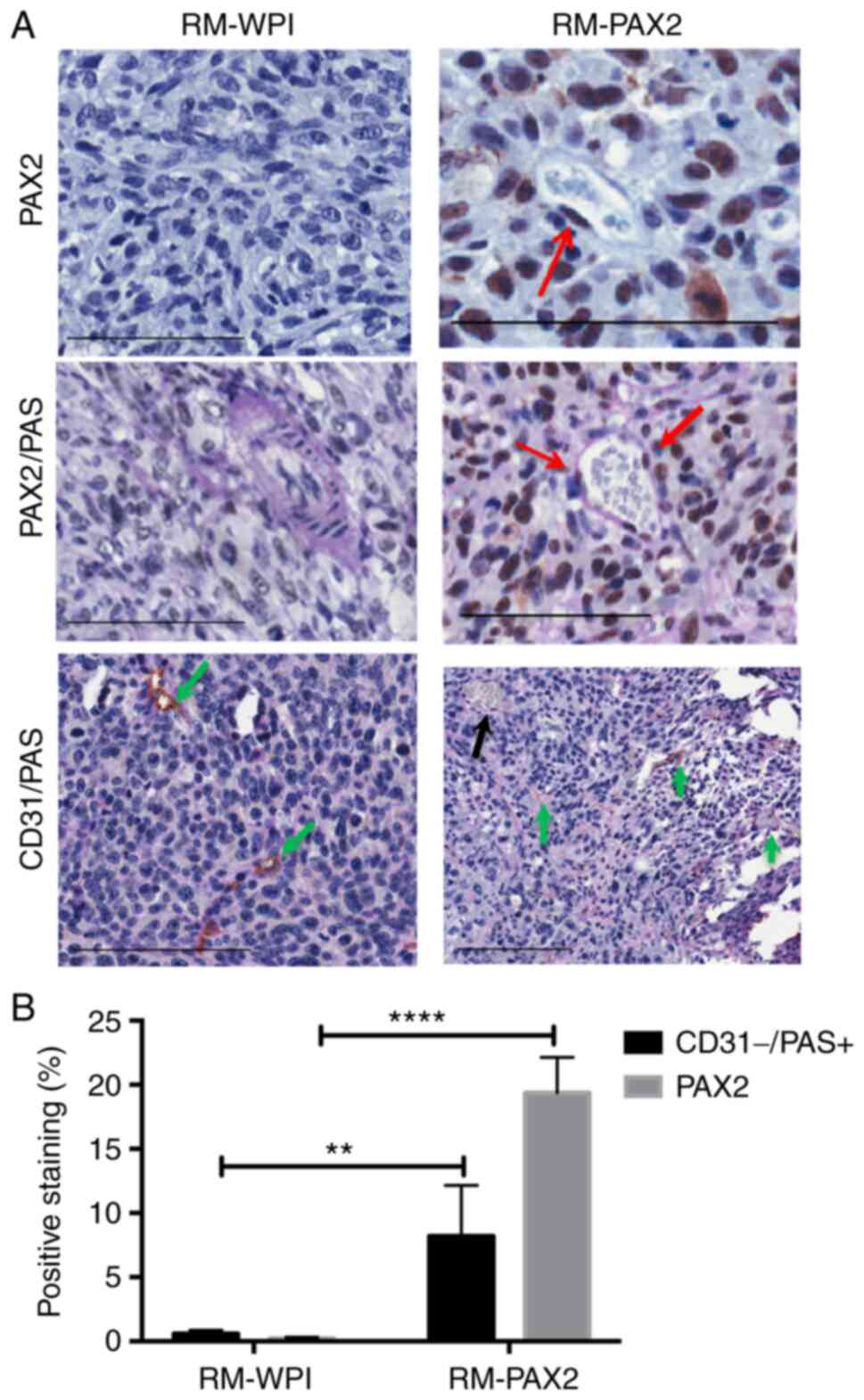 | Figure 5PAX2 is expressed in the cells lining
vascular-like channels and is associated with VM. (A) PAX2 is
expressed in the nucleus of RM-PAX2 tumors (red arrows), whereas
RM-WPI (transfected with empty vector) tissue was PAX2-negative
(upper panels). RM-WPI possessed PAS-positive (pink) vascular
channels only in large vessels (middle panels). In RM-PAX2 tumors,
vessels were stained with PAS, with some cells lining channels
positive for PAX2 (red arrows). The lower panels show the double
staining for CD31 and PAS on PAX2-negative tumor sections (RM-WPI)
and PAX2-positive tumor sections (RM-PAX2). RM-WPI showed
CD31+/PAS+ staining, indicating regular blood
vessels (green arrows). In RM-PAX2 tumors,
CD31+/PAS+ staining indicated blood vessels
(green arrows) and CD31-/PAS+ staining
indicated VM formation (black arrow). Scale bar, 100 µm. (B)
Quantification of immunohistochemical detection of PAX2 and
CD31-/PAS+ showed an increase in PAX2 and
CD31-/PAS+ staining in RM-PAX2 compared with
RM-WPI tumors, indicating that expression of PAX2 was associated
with increased VM. Image analysis was performed using Aperio
Positive Pixel Count Algorithm software. Analysis was performed
using unpaired Student's t-test for three different fields of the
tumor section. **P<0.01, ****P<0.0001.
PAS, periodic acid-Schiff; PAX2, paired box 2; VM, vasculogenic
mimicry. |
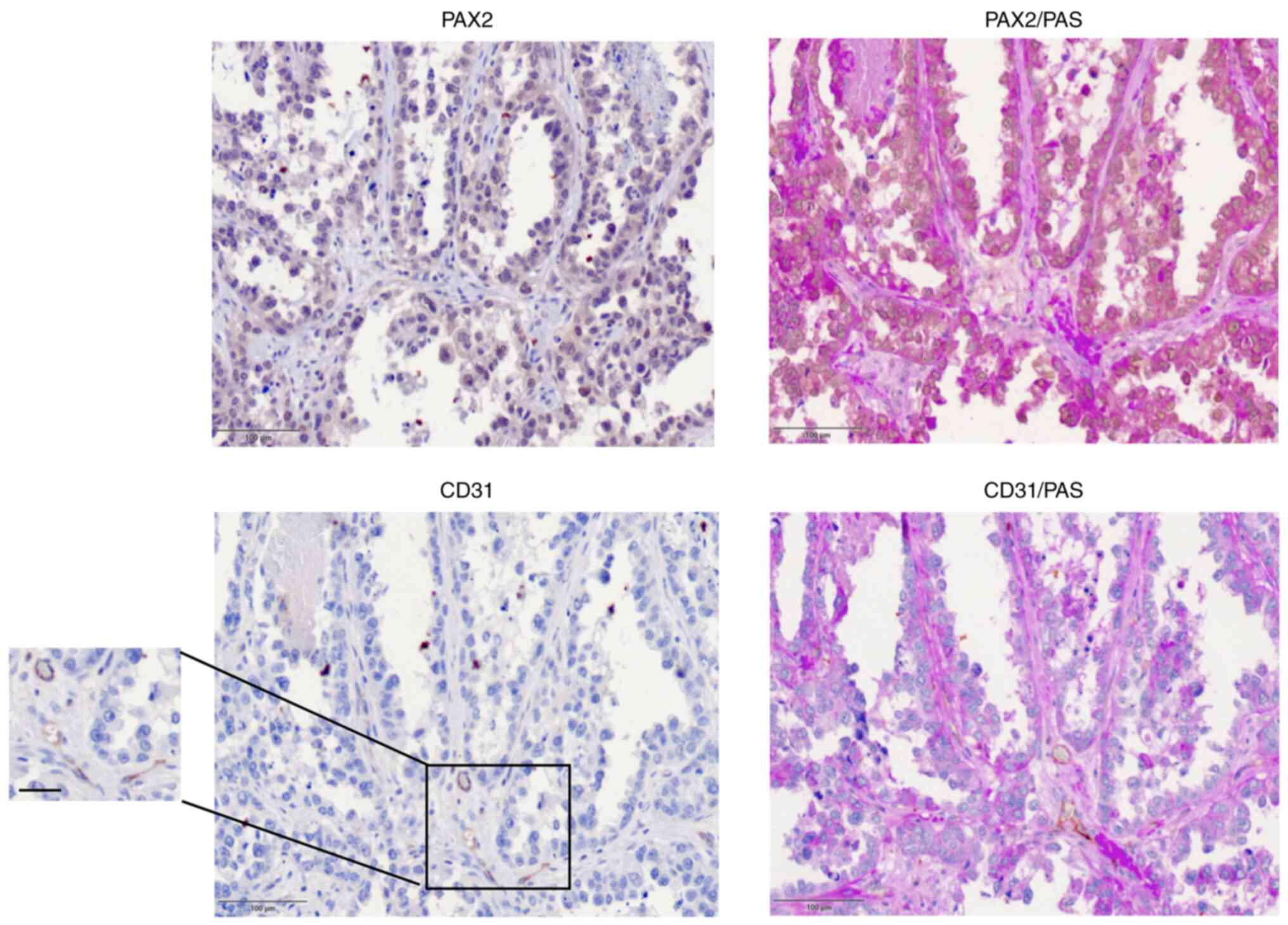
PAX2 is associated with tubular-like
structures in human ovarian cancer
To investigate whether PAX2 expression was
associated with specific histological and tissue structures, the
histological subtypes of human ovarian cancer tissue were
investigated. PAX2 immunohistochemical staining showed the presence
of cells that expressed nuclear PAX2 in certain ovarian cancer
samples (Figs. 6 and S2).
A total of four (40%) serous carcinoma tissue
samples expressed nuclear PAX2 (Table
II). However, three samples that expressed PAX2 showed high PAS
staining and low CD31 expressions, indicative of vasculogenic
activity in these tissues. The serous carcinoma samples that showed
high vasculogenic activity were not associated with specific
ovarian cancer grade or stage. Furthermore, three cases (75%) of
endometrioid carcinoma expressed PAX2. However, only one sample
showed high PAX2 expression, high PAS staining and low CD31
expression. Only one sample of each of the other ovarian cancer
histological types (clear, granulosa and germ cell tumors)
exhibited vasculogenic activity.
 | Table IINumber of ovarian cancer samples
stained positive for PAX2, PAS and CD31, to show the vasculogenic
activity of each histological type of ovarian cancer. |
Table II
Number of ovarian cancer samples
stained positive for PAX2, PAS and CD31, to show the vasculogenic
activity of each histological type of ovarian cancer.
| Histological
type | Total, n | PAX2+, n
(%) |
PAX2+/PAShigh,
n |
PAX+/PASlow, n |
PAX2-/PAShigh,
n | CD31low,
n |
|---|
| Serous
carcinoma | 10 | 4(40) | 3 | 0 | 1 | 3 |
| Endometroid
carcinoma | 4 | 3(75) | 1 | 2 | 1 | 2 |
| Clear cell | 2 | 1(50) | 1 | 1 | 0 | 0 |
| Granulosa cell | 2 | 1(50) | 1 | 0 | 1 | 0 |
| Mucinous cell | 1 | 0 (0) | 0 | 0 | 0 | 0 |
| Germ cell
tumor | 1 | 1(100) | 1 | 0 | 0 | 0 |
Serous ovarian carcinoma cells expressing PAX2
exhibited different morphological structures compared with
endometrioid ovarian cancer. Serous ovarian carcinoma that
expressed PAX2 showed tubular- or papillary-like structures
(Fig. 6), whereas in endometrioid
ovarian cancer tissue, PAX2 expression was associated with solid
and unbranched masses that possessed CD31-negative blood vessels
(Fig. S2). Moreover, serous tumor
cells that exhibited high levels of PAX2 were associated with low
levels of CD31, indicating a decreased number of blood vessels
(Fig. 6). CD31/PAS staining showed
similar results. CD31 expression was notably lower compared with
PAS staining in PAX2-positive human tumor tissue (Fig. 6). By contrast, PAX2-negative tumor
tissue was associated with high levels of CD31 expression in
endothelial cells that lined the blood vessels (Fig. S3). This indicated that
PAX2-negative tissues exhibited increased levels of angiogenesis
and lacked vasculogenesis (Fig.
S3). Unlike animal tumors that were generated using RM-PAX2
cancer cells and presented high angiogenesis activity, human
ovarian tumors had decreased angiogenesis by endothelial cells in
PAX2-positive tissues and induced de novo vascular
formation.
Discussion
The role of PAX2 in ovarian cancer is poorly
understood; however, previous studies suggested that expression of
PAX2 alone does not induce OSE cell transformation into cancerous
cells (9,30). However, the ability of PAX2 to
promote vascular-like channels suggests that tumors that express
PAX2 may be more aggressive owing to the development of
vascular-like channels that augment tumor blood supply in the
absence of angiogenesis (35).
Normal OSE cells do not express PAX2(30), whereas 61% of ovarian cancer cell
lines express PAX2(3), suggesting
a potential oncogenic role for PAX2 in cancer derived from ovarian
epithelium. The oncogenic role of PAX2 in ovarian cancer has been
investigated in terms of its ability to promote cell proliferation,
migration and tumor formation (9,30).
The present investigation of the ability of PAX2 to promote
epithelial differentiation of MOSE cells revealed that PAX2
expression in M1102 MOSE cells induced formation of vascular-like
structures in Matrigel, whereas the control groups did not form
these structures. The ability to form vessel-like structures is
associated with aggressive tumor cells, such as those present in
melanoma (14), and is known as
VM. Unlike MOSE cells, normal expression of PAX2 in mouse oviductal
epithelial cells does not allow for VM, but enhances the formation
of hollow lumen structures under the same 3D culture conditions
(6), resembling acini-like
structures that are formed by normal mammary epithelial cells
(33).
HUVEC endothelial cells commonly form vascular
networks in 3D culture, and this type of cellular organization has
been used as a standard measure of the potential for cellular
vasculogenesis (36). However, to
the best of our knowledge, no other normal cells have been reported
to possess this capability. Aggressive cancer cells such as
melanoma form de novo webs of vessels (14). In the present study, vascular-like
channels formed by M1102-PAX2 in Matrigel were morphologically
similar to vessels formed by HUVECs and VM networks formed by SKOV3
ovarian cancer cells, providing the first evidence of such capacity
in normal non-endothelial cells.
Vascular-like channels in M1102-PAX2 cells were
supported by remodeling of the ECM, which was positive for PAS and
negative for CD31. However, SKOV3 VM resulted in few cells
expressing CD31, which is consistent with a previous report
suggesting that cancer cells may differentiate into endothelial
cells de novo to induce angiogenesis (29). Thus, it was hypothesized that PAX2
could induce vascular-like channels in vitro in normal MOSE
cells. Vascular-like channel induction in M1102 cells by PAX2 was
not the result of stem cell differentiation, as our previous study
showed that PAX2 decreases stemness in MOSE cells (6). However, it was not possible to
investigate vessel formation in vivo with normal MOSE-PAX2
cells since these cells do not form tumors when injected into mice
(30).
RM mouse ovarian cancer cells are tumorigenic in
mice and overexpression of PAX2 results in increased rate of tumor
progression (30). To investigate
the potential role of PAX2 in VM during tumor progression, the
present study investigated RM cells in vitro and in
vivo. It was revealed that overexpression of PAX2 in RM cells
significantly increased formation of vascular-like channels in
vitro. In addition, RM-PAX2 was revealed to increase both VM
and angiogenesis in vivo, as determined by staining the
cancer tissues for CD31 and PAS. Some of the neo-vasculature was
determined to be CD31-negative and PAS-positive, indicating that
PAX2 induces cancer cells to form VM. Certain cells lining those
vessels expressed PAX2 in the nucleus, suggesting that cancer cells
that express PAX2 may contribute to formation of neo-vasculature in
the tumor.
The molecular mechanism by which PAX2 promotes
vascular channels has not yet been established, although it may be
mediated by COX2 expression. As PAX2 induces COX2 in RM cells
(30) and COX2 induces VM in
breast cancer (21), it is
hypothesized that PAX2-induced VM may be mediated by COX2
expression. Moreover, PAX2 may also regulate vascular channels
through MMP activity; the role of MMPs during morphogenesis, tissue
remodeling and VM has been widely reported (37,38).
In ovarian cancer, overexpression of MMPs is associated with ECM
remodeling (25), cancer
progression (26,27) and poor prognosis (39). Previous studies support the role of
MMPs in the formation of tubular-like structures and VM in cancer
cell lines (39,40). However, targeting the MMP-2 inducer
CD147 in SKOV3 cells resulted in significantly decreased formation
of CD31-negative/PAS-positive vessels (22). Whether PAX2 regulates MMP
expression in ovarian cancer cells, and whether this contributes to
the underlying mechanism involved in induction of vascular
channels, remains to be determined.
PAX2 has been reported to induce tubular branching
in the kidney and elongation of inner ear precursors (41), as well as serving a role in
fallopian tube morphogenesis (30), which may occur via a similar
mechanism to VM in ovarian cancer cells. To the best of our
knowledge, few studies have reported expression levels of PAX2 in
ovarian cancer. One study showed that PAX2 is upregulated in
papillary serous ovarian cancer (42). Another study showed a higher
percentage of low-grade serous cancer exhibits increased PAX2
expression compared with high-grade cancer (43). PAX2-positive cells have been
detected in non-serous ovarian cancer, including endometrioid
(33%), mucinous (17%) and clear cell carcinoma (35% of cases)
(3). In the present study, PAX2
was associated with the web-like structures in certain subtypes of
ovarian cancer such as serous and endometrioid carcinoma. The tumor
subtypes that exhibited high PAX2 and low CD31 expression were
associated with decreased angiogenesis. However, PAX2 expression
was associated with increased PAS staining, which was indicative of
the level of VM. These findings indicated that PAX2 may induce
formation of tubular structures or vascular-like channels in serous
cancer that is not associated with a high degree of angiogenesis.
However, PAX2 expression is associated with poorer prognosis in
patients with serous ovarian cancer (9). The p53 status is also significantly
associated with patient survival. Wild-type p53 is associated with
a significant decrease in progression-free survival rate (44), which may indicate that ovarian
tumors that have high PAX2 expression and wild-type P53 exhibit a
high degree of VM associated with more aggressive tumors.
To the best of our knowledge, the potential role of
PAX2 in the formation of vascular-like channels in ovarian cancer
is a novel finding. In the present study, overexpression of PAX2
promoted formation of tubular-like channels both in vitro
and in vivo. In established ovarian tumors, PAX2 may enhance
tumor progression and metastasis by VM, which allows increased
blood supply to the tumor. In a similar fashion, high expression
levels of PAX2 in renal tumor-derived endothelial cells are
associated with angiogenesis and VM that enhance tumor growth
(35). However, the role of PAX2
in cancer cells may be context dependent. Previous studies have
demonstrated the oncogenic role of PAX2 in ovarian, prostate and
melanoma cancer cells (9,30,45-47),
whereas other studies found that PAX2 exerts a tumor suppressive
role in different types of ovarian cancers (48,49).
Thus, the role of PAX2 in enhancing vascular-like structures may
depend on the specific cancer type and genetic profile of cancer
cells.
The present study had certain limitations. The M1102
normal epithelial cells and RM cancer ovarian cell line were
derived from mice. Human ovarian cancer cell lines derived from
specific histological subtypes of ovarian cancer should be used to
determine the capacity for vasculogenesis and involvement of PAX2
in each subtype. In addition, antibody staining of cells grown in
Matrigel was limited owing to the difficulties in performing this
experiment. However, staining vascular channels with other
antibodies including vascular endothelial cadherin, vascular
endothelial growth factor receptor and MMPs should be performed in
future to further characterize VM (50). Cancer tissue that exhibited
vasculogenesis expressed PAX2 in the nucleus. However, it was not
possible to directly assess whether PAX2 induced vasculogenic
activity in human ovarian cancer tissues and whether tubular-like
channels were formed by PAX2-positive cells. Although multiple
vascular-like structures that contained blood cells were found,
whether PAX2 induced cell elongation to form web-like structures or
vessel formation to supply blood remains to be confirmed.
Investigating PAX2 expression in different types of human ovarian
cancer cells using confocal and electron microscopy should also be
performed to determine if tubular structures are lined with cells
expressing PAX2 and form hollow structures that contain blood cells
in 3D culture and cancer tissue. In addition, molecular signaling
that mediates vasculogenesis in different types of ovarian cancer
cells was not assessed. The role of PAX2 downstream signaling and
genes involved in mediating PAX2 function in tubular structure
formation remain to be determined.
Supplementary Material
Live time-lapse video for 3D cultures
of M1102 ovarian epithelial cells overexpressing PAX2 and tagged
with GFP. Single and dissociated cells of M1102-PAX2 were plated in
3D cultures and subsequently rearranged and elongated to form
vascular-like channels within 48 h incubation. PAX2, paired box
2.
Western blotting and densitometric
analysis. Results demonstrate high expression of PAX2 in M1102-PAX2
(M1102 with PAX2 overexpression) compared with M1102, M1102-WPI
(parental cells infected with empty viral vector) and
M1102-PAX2-AdCre (M1102-PAX2 cells in which PAX2 was knocked out).
PAX2, paired box 2.
Human serous ovarian cancer tissue
sections showed tubule-like structures lined with cells expressing
PAX2 and basement membrane stained with PAS. Red arrow, vessels
positive for CD31 and PAS, indicating regular angiogenesis; black
arrow, CD31-negative blood vessels, indicating vasculogenesis and
VM. Scale bar, 200 (original) and 50 μm (magnified). PAX2, paired
box 2; PAS, periodic acid-Schiff.
PAX2 and CD31 immunohistochemistry and
PAS staining for the detection of blood vessels in human
endometrioid ovarian cancer tissue. Red arrow, vessels positive for
CD31 and PAS; black arrow, CD31-negative vessels. Scale bar, 200
μm. PAS, periodic acid-Schiff; PAX2, paired box 2.
Supplementary Data
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Canadian Institutes
of Health Research and King Fahad Specialist Hospital-Dammam.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KA and EMA made substantial contributions to the
conception and design of the current study. EMA and SA performed
the experiments and collected data. BCV, KG and SG made significant
contributions to data analysis and interpretation. BCV and KG
revised the manuscript for important intellectual content. KA and
BCV confirmed the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The protocols related to the animal experiments were
approved by the University of Ottawa Animal Care Committee
(approval no. ME-256). The protocols related to human tissues were
approved by the ethical committee that affiliated with King Fahad
Specialist Hospital-Dammam (approval no. IRB# ONC0340). The
informed consents for the patients were waived by IRB.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krzystyniak J, Ceppi L, Dizon DS and
Birrer MJ: Epithelial ovarian cancer: The molecular genetics of
epithelial ovarian cancer. Ann Oncol. 27 (Suppl 1):i4–i10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bogdanova N and Dörk T: Molecular genetics
of breast and ovarian cancer: Recent advances and clinical
implications. Balkan J Med Genet. 15 (Suppl):S75–S80.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Song H, Kwan SY, Izaguirre DI, Zu Z, Tsang
YT, Tung CS, King ER, Mok SC, Gershenson DM and Wong KK: PAX2
expression in ovarian cancer. Int J Mol Sci. 14:6090–6105.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eccles MR, He S, Legge M, Kumar R, Fox J,
Zhou C, French M and Tsai RW: PAX genes in development and disease:
The role of PAX2 in urogenital tract development. Int J Dev Biol.
46:535–544. 2002.PubMed/NCBI
|
|
5
|
Terzić J, Muller C, Gajović S and
Saraga-Babić M: Expression of PAX2 gene during human development.
Int J Dev Biol. 42:701–707. 1998.PubMed/NCBI
|
|
6
|
Alwosaibai K, Abedini A, Al-Hujaily EM,
Tang Y, Garson K, Collins O and Vanderhyden BC: PAX2 maintains the
differentiation of mouse oviductal epithelium and inhibits the
transition to a stem cell-like state. Oncotarget. 8:76881–76897.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Narlis M, Grote D, Gaitan Y, Boualia SK
and Bouchard M: Pax2 and pax8 regulate branching morphogenesis and
nephron differentiation in the developing kidney. J Am Soc Nephrol.
18:1121–1129. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mansouri A, Hallonet M and Gruss P: Pax
genes and their roles in cell differentiation and development. Curr
Opin Cell Biol. 8:851–857. 1996.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Feng Y, Tang Y, Mao Y, Liu Y, Yao D, Yang
L, Garson K, Vanderhyden BC and Wang Q: PAX2 promotes epithelial
ovarian cancer progression involving fatty acid metabolic
reprogramming. Int J Oncol. 56:697–708. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Piao Y, Liang J, Holmes L, Henry V, Sulman
E and de Groot JF: Acquired resistance to anti-VEGF therapy in
glioblastoma is associated with a mesenchymal transition. Clin
Cancer Res. 19:4392–4403. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Helfrich I, Scheffrahn I, Bartling S, Weis
J, von Felbert V, Middleton M, Kato M, Ergün S, Augustin HG and
Schadendorf D: Resistance to antiangiogenic therapy is directed by
vascular phenotype, vessel stabilization, and maturation in
malignant melanoma. J Exp Med. 207:491–503. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Folberg R, Hendrix MJ and Maniotis AJ:
Vasculogenic mimicry and tumor angiogenesis. Am J Pathol.
156:361–381. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tang HS, Feng YJ and Yao LQ: Angiogenesis,
vasculogenesis, and vasculogenic mimicry in ovarian cancer. Int J
Gynecol Cancer. 19:605–610. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
vasculogenic mimicry. Am J Pathol. 155:739–752. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Racordon D, Valdivia A, Mingo G, Erices R,
Aravena R, Santoro F, Bravo ML, Ramirez C, Gonzalez P, Sandoval A,
et al: Structural and functional identification of vasculogenic
mimicry in vitro. Sci Rep. 7(6985)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Valdivia A, Mingo G, Aldana V, Pinto MP,
Ramirez M, Retamal C, Gonzalez A, Nualart F, Corvalan AH and Owen
GI: Fact or fiction, it is time for a verdict on vasculogenic
mimicry? Front Oncol. 9(680)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Itzhaki O, Greenberg E, Shalmon B, Kubi A,
Treves AJ, Shapira-Frommer R, Avivi C, Ortenberg R, Ben-Ami E,
Schachter J, et al: Nicotinamide inhibits vasculogenic mimicry, an
alternative vascularization pathway observed in highly aggressive
melanoma. PLoS One. 8(e57160)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dong X, Sun B, Zhao X, Liu Z, Gu Q, Zhang
D, Zhao N, Wang J and Chi J: Expression of relative-protein of
hypoxia-inducible factor-1α in vasculogenesis of mouse embryo. J
Biol Res (Thessalon). 21(4)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Luo F, Yang K, Liu RL, Meng C, Dang RF and
Xu Y: Formation of vasculogenic mimicry in bone metastasis of
prostate cancer: Correlation with cell apoptosis and senescence
regulation pathways. Pathol Res Pract. 210:291–295. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu
D, Yu X and Tian Y: Advanced research on vasculogenic mimicry in
cancer. J Cell Mol Med. 19:315–326. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Basu GD, Liang WS, Stephan DA, Wegener LT,
Conley CR, Pockaj BA and Mukherjee P: A novel role for
cyclooxygenase-2 in regulating vascular channel formation by human
breast cancer cells. Breast Cancer Res. 8(R69)2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Millimaggi D, Mari M, D'Ascenzo S, Giusti
I, Pavan A and Dolo V: Vasculogenic mimicry of human ovarian cancer
cells: Role of CD147. Int J Oncol. 35:1423–1428. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
24
|
Sood AK, Fletcher MS, Coffin JE, Yang M,
Seftor EA, Gruman LM, Gershenson DM and Hendrix MJ: Functional role
of matrix metalloproteinases in ovarian tumor cell plasticity. Am J
Obstet Gynecol. 190:899–909. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sood AK, Seftor EA, Fletcher MS, Gardner
LM, Heidger PM, Buller RE, Seftor RE and Hendrix MJ: Molecular
determinants of ovarian cancer plasticity. Am J Pathol.
158:1279–1288. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Labrie M, Vladoiu MC, Grosset AA, Gaboury
L and St-Pierre Y: Expression and functions of galectin-7 in
ovarian cancer. Oncotarget. 5:7705–7721. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lin H, Pan JC, Zhang FM, Huang B, Chen X,
Zhuang JT, Wang H, Mo CQ, Wang DH and Qiu SP: Matrix
metalloproteinase-9 is required for vasculogenic mimicry by clear
cell renal carcinoma cells. Urol Oncol. 33:168.e9–e16.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ayala-Domínguez L, Olmedo-Nieva L,
Muñoz-Bello JO, Contreras-Paredes A, Manzo-Merino J,
Martínez-Ramírez I and Lizano M: Mechanisms of vasculogenic mimicry
in ovarian cancer. Front Oncol. 9(998)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Su M, Feng YJ, Yao LQ, Cheng MJ, Xu CJ,
Huang Y, Zhao YQ and Jiang H: Plasticity of ovarian cancer cell
SKOV3ip and vasculogenic mimicry in vivo. Int J Gynecol Cancer.
18:476–486. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Al-Hujaily EM, Tang Y, Yao DS, Carmona E,
Garson K and Vanderhyden BC: Divergent roles of PAX2 in the
etiology and progression of ovarian cancer. Cancer Prev Res
(Phila). 8:1163–1173. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yao DS, Li L, Garson K and Vanderhyden BC:
The mouse ovarian surface epithelium cells (MOSE) transformation
induced by c-myc/K-ras in. Zhonghua Zhong Liu Za Zhi. 28:881–885.
2006.PubMed/NCBI(In Chinese).
|
|
32
|
Kutner RH, Zhang XY and Reiser J:
Production, concentration and titration of pseudotyped HIV-1-based
lentiviral vectors. Nat Protoc. 4:495–505. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Debnath J, Muthuswamy SK and Brugge JS:
Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini
grown in three-dimensional basement membrane cultures. Methods.
30:256–268. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vigier S and Fülöp T: Exploring the
extracellular matrix to create biomaterials. In: Composition and
Function of the Extracellular Matrix in the Human Body [Internet].
Travascio F (ed). IntechOpen, Rijeka, 2016. https://doi.org/10.5772/62979.
|
|
35
|
Fonsato V, Buttiglieri S, Deregibus MC,
Puntorieri V, Bussolati B and Camussi G: Expression of Pax2 in
human renal tumor-derived endothelial cells sustains apoptosis
resistance and angiogenesis. Am J Pathol. 168:706–713.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ponce ML: Tube formation: An in vitro
matrigel angiogenesis assay. Methods Mol Biol. 467:183–188.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sivak JM, Mohan R, Rinehart WB, Xu PX,
Maas RL and Fini ME: Pax-6 expression and activity are induced in
the reepithelializing cornea and control activity of the
transcriptional promoter for matrix metalloproteinase gelatinase B.
Dev Biol. 222:41–54. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Marchenko GN, Marchenko ND and Strongin
AY: The structure and regulation of the human and mouse matrix
metalloproteinase-21 gene and protein. Biochem J. 372:503–515.
2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mariya T, Hirohashi Y, Torigoe T, Tabuchi
Y, Asano T, Saijo H, Kuroda T, Yasuda K, Mizuuchi M, Saito T and
Sato N: Matrix metalloproteinase-10 regulates stemness of ovarian
cancer stem-like cells by activation of canonical Wnt signaling and
can be a target of chemotherapy-resistant ovarian cancer.
Oncotarget. 7:26806–26822. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rodriguez JA, Orbe J, De Lizarrondo SM,
Calvayrac O, Rodriguez C, Martinez-Gonzalez J and Paramo JA:
Metalloproteinases and atherothrombosis: MMP-10 mediates vascular
remodeling promoted by inflammatory stimuli. Front Biosci.
13:2916–2921. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Christophorou NAD, Mende M, Lleras-Forero
L, Grocott T and Streit A: Pax2 coordinates epithelial
morphogenesis and cell fate in the inner ear. Dev Biol.
345:180–190. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tong GX, Chiriboga L, Hamele-Bena D and
Borczuk AC: Expression of PAX2 in papillary serous carcinoma of the
ovary: Immunohistochemical evidence of fallopian tube or secondary
Müllerian system origin? Mod Pathol. 20:856–863. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tung CS, Mok SC, Tsang YT, Zu Z, Song H,
Liu J, Deavers MT, Malpica A, Wolf JK, Lu KH, et al: PAX2
expression in low malignant potential ovarian tumors and low-grade
ovarian serous carcinomas. Mod Pathol. 22:1243–1250.
2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wong KK, Izaguirre DI, Kwan SY, King ER,
Deavers MT, Sood AK, Mok SC and Gershenson DM: Poor survival with
wild-type TP53 ovarian cancer? Gynecol Oncol. 130:565–569.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ueda T, Ito S, Shiraishi T, Kulkarni P,
Ueno A, Nakagawa H, Kimura Y, Hongo F, Kamoi K, Kawauchi A and Miki
T: Hyper-expression of PAX2 in human metastatic prostate tumors and
its role as a cancer promoter in an in vitro invasion model.
Prostate. 73:1403–1412. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee SB, Doberstein K, Baumgarten P,
Wieland A, Ungerer C, Bürger C, Hardt K, Boehncke WH, Pfeilschifter
J, Mihic-Probst D, et al: PAX2 regulates ADAM10 expression and
mediates anchorage-independent cell growth of melanoma cells. PLoS
One. 6(e22312)2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hardy LR, Salvi A and Burdette JE:
UnPAXing the divergent roles of PAX2 and PAX8 in high-grade serous
ovarian cancer. Cancers (Basel). 10(262)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Raffone A, Travaglino A, Saccone G,
Mascolo M, Insabato L, Mollo A, De Placido G and Zullo F: PAX2 in
endometrial carcinogenesis and in differential diagnosis of
endometrial hyperplasia: A systematic review and meta-analysis of
diagnostic accuracy. Acta Obstet Gynecol Scand. 98:287–299.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gruss P and Walther C: Pax in development.
Cell. 69:719–722. 1992.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Luo Q, Wang J, Zhao W, Peng Z, Liu X, Li
B, Zhang H, Shan B, Zhang C and Duan C: Vasculogenic mimicry in
carcinogenesis and clinical applications. J Hematol Oncol. 13:1–15.
2020.PubMed/NCBI View Article : Google Scholar
|