Introduction
A recent and ongoing pandemic that originated from
Wuhan China, caused by a new β coronavirus termed severe acute
respiratory syndrome coronavirus-2 (SARS-CoV-2) results in a
disease termed coronavirus-19 disease (COVID-19) (1). COVID-19 presents with varied clinical
features ranging from asymptomatic course to acute respiratory
distress syndrome associated with high morbidity and mortality
(2,3). The majority of COVID-19 patients
(~80%) recover by their own in due course of time, but the rest
suffer from moderate to severe disease (4). To date, ~29 million people have been
infected with COVID-19 resulting in more than 5.4 million
mortalities (https://www.worldome-69 ters.info/coronavirus,
accessed on Jan, 02, 2022).
COVID-19 has been associated with age, blood group
type and ACE-2 gene polymorphism (5-7).
The severity of the disease has also been linked with some
comorbidities including hypertension, obesity and diabetes
(8,9). Angiotensin converting enzyme (ACE)
converts angiotensin (Ang) I to Ang II and breaks down bradykinin
which serves a role in the control of blood pressure (10). ACE2 converts Ang II into Ang
(1-7),
which is a vasodilatory peptide (11). The ACE2 gene is found on chromosome
Xp22(12). The ACE and ACE2 share
42% amino acid similarity as the ACE2 originates through
duplication of genes (12). The
ACE2 is a glycoprotein and consists of 805 amino acids (12). The N-terminal of ACE2 (catalytic
domain) is a signal peptide region containing an HEXXH zinc binding
metalloprotease motif (12,13).
The C-terminus of the ACE2 is the functional transmembrane domain
(12).
ACE2 is expressed in the respiratory system, renal
system, lungs, heart, blood vessels, testes, gastrointestinal tract
and central nervous system (12).
ACE and ACE2 gene variations are associated with different diseases
such as hypertension, cardiovascular disease (CVD) and diabetes
mellitus (14-16).
The ACE2 counterbalances the ACE to regulate the level of
circulating Ang II (15). Ang II
is the main effector of the classic RAS (15). RAS dysfunctions are associated with
pulmonary injury and acute respiratory distress syndrome caused by
a number of factors such as viral infections (17). Dysregulation of ACE2 expression is
associated with CVD in experimental models (15). In humans, the levels of ACE2 are
elevated in atherosclerosis and heart failure (15). The SARS-CoV-2 uses the spike
glycoprotein on its envelope to bind the ACE2 and enter the host
cells (18). It has been reported
that the binding between the spike glycoprotein of the novel
coronavirus (2019-nCoV) is stronger than the binding between the
ACE2 with the spike glycoprotein of the SARS virus (19). It is suggested that the ACE2 levels
correlate with SARS-CoV-2 infection susceptibility (13). Our recent work found a strong
association between ACE2 DD genotype and COVID-19 mortality and
also reported that two genotypes ACE2-CC and CT are associated with
COVID-19 severity (20).
microRNAs (miRNAs) are short non-coding RNA
molecules with 18-23 nucleotides and are involved in the regulation
of the expression of their target genes (21). They serve important roles in
differentiation, apoptosis, inflammation, diabetes, cardiovascular
disease and also in diagnosis and prognosis of various diseases
(22). The genome wide association
studies uncovered the association of different miRNA loci with
different diseases (23-26).
It has been reported that miR-196b inhibits the
hepatitis C virus (HCV) replication (27) and is gradually upregulated
following COVID-19 infection (28). In a report from Turkey, miR-1962a
rs3217927 SNP was found to be a very effective prognostic marker
for multiple myeloma (29) but to
the best of the authors' knowledge the role of miR-1962a rs3217927
SNP in COVID-19 has not been reported. The present study
investigated the association of ACE2 rs4343 G>A and miR-196a2
rs11614913 C>T gene variations with the COVID-19 disease
severity and mortality in a patient population from the Asir and
Tabuk regions of Saudi Arabia.
Materials and methods
Study population
The present collaborative and population-based
case-control study involved 117 COVID-19 patients and 200 healthy
controls. The blood specimens from 117 reverse transcription (RT)
PCR confirmed positive COVID-19 patients were collected from
different hospitals in Saudi Arabia (Bisha, Abha and Tabuk;
Table I). The patient group
included 85 males and 32 females with a male to female ratio of
2.66 and their ages ranged between 32 and 69 years. The recruitment
time for the patients was between January 15, 2021 and August 31,
2021. The ethical approvals were obtained from three local
institutional ethics committees of College of Medicine, University
of Bisha (Ref. no. UBCOM/H-06-BH-087(05/25), University of Tabuk
(Decision no. KAEK2020/4/4) and College of Medicine, King Khalid
University, Abha (Ref. no. H-06-B-091) in accordance with local
guidelines which complied in essence with the principles of the
Helsinki Declaration. Written informed consent was obtained before
the collection of blood samples from the patients.
 | Table IBaseline characteristics of the
COVID-19 patients. |
Table I
Baseline characteristics of the
COVID-19 patients.
| Patient
characteristics | n=117 | % |
|---|
| Age (years) | | |
|
>40 | 97 | 82.90 |
|
≤40 | 20 | 17.09 |
| Sex | | |
|
Male | 85 | 72.64 |
|
Female | 32 | 27.36 |
| CKD | | |
|
Yes | 11 | 9.40 |
|
No | 106 | 90.60 |
| T2DM | | |
|
Yes | 47 | 40.17 |
|
No | 70 | 59.83 |
| Oxygen
saturation | | |
|
<60 | 47 | 40.17 |
|
>80 | 70 | 59.83 |
| Hypertension | | |
|
Yes | 37 | 31.62 |
|
No | 80 | 68.37 |
| CAD | | |
|
Yes | 17 | 14.53 |
|
No | 100 | 85.47 |
| Duration in
hospital (days) | | |
|
>30 | 57 | 48.71 |
|
<30 | 60 | 51.29 |
| CRP | | |
|
<10
mg/l | 13 | 2.56 |
|
≥10
mg/l | 104 | 97.44 |
| ALT | | |
|
<36
U/l | 72 | 61.53 |
|
>36
U/l | 45 | 38.57 |
| AST | | |
|
<40
U/l | 69 | 58.97 |
|
>40
U/l | 48 | 41.3 |
| Steroids
therapy | | |
|
Yes | 77 | 65.81 |
|
No | 40 | 34.19 |
| Antiviral
therapy | | |
|
Yes | 79 | 67.52 |
|
No | 38 | 32.48 |
| Survival | | |
|
Yes | 43 | 36.75 |
|
No | 74 | 63.24 |
Data collection
A structured and bilingual (Arabic and English)
questionnaire was given to all study subjects before enrolling for
the present study. The subjects were interviewed for details of
epidemiological/demographic data, history of co-morbid conditions
such as cardiovascular diseases, type 2 diabetes mellitus (T2DM),
history of addiction particularly smoking and family history of any
other significant diseases.
Sample collection from COVID-19
patients
A lavender top (LT) tube containing EDTA was used
for the collection of 3 ml of peripheral blood from all the
COVID-19 patients. The blood specimens were immediately stored at
-20˚C until further analyses.
Sample collection from control
subjects
Written consent was obtained from healthy and age
matched controls and the purpose of their participation was
explained to them using a structured bilingual questionnaire. The
sample collection was timed in such a way that it coincided with
the routine blood draws of such subjects who reported to the
hospital for their routine health checkups. This group comprised of
RTPCR confirmed negative individuals who attended hospital for
general health checkups. As a matter of policy, RTPCR was conducted
on all those individuals who wanted to see a physician in the
outpatient departments during first wave of COVID-19 pandemic. 3 ml
peripheral blood samples were collected in LT tubes containing EDTA
and were immediately stored at -20˚C until further analyses.
Genomic DNA extraction
A commercial kit from Qiagen GmbH (DNeasy) was used
for DNA extraction according to the instructions provided by the
manufacturer. The extracted DNA from patients and control group was
dissolved in nuclease-free water and was stored at 4˚C further
analyses. NanoDrop (Thermo Fisher Scientific, Inc.) was used to
establish the quality and integrity of extracted DNA samples. The
ratio of optical density at 260 nm (OD260) and 280 nm
(OD280) was used to verify the purity of the DNA
samples. The OD260/OD280 ratios ranged from
1.83-1.99, thus confirming good quality DNA.
Genotyping of ACE2-rs4343 G>A and
miR-196a2 rs11614913 C>T
ACE2 rs4343 G>A and miR-196a2 rs11614913 C>T
genotyping was performed by using amplification refractory mutation
system (ARMS-PCR) on T100 Thermocycler from Bio-Rad Laboratories,
Inc. Primer3 software (version 4, https://primer3.ut.ee/) was used to design ARMS PCR
primers and the details are given in Table II.
 | Table IIARMS primer details. |
Table II
ARMS primer details.
| Direction | Sequence | Product size | Annealing
temperature |
|---|
| ARMS primers for
ACE2 rs4343 (2350A>G) |
|---|
| ACErs4343
FO |
5'-CTGAAATTCTCTGAGCTCCCCT-3' | 268 bp | 58˚C |
| ACErs4343
RO |
5'-GAAAATGAAGGGACCCAAGTGC-3' | | |
| ACErs4343
FIA |
5'-CTGACGAATGTGATGGCCCCA-3' | 190 bp | |
| ACErs4343
RIG |
5'-CATAACAGGTCTTCATATTTCCGGTAC-3' | 125 bp | |
| ARMS primers for
miR-196a2 rs11614913 C>T |
| miR-196a2 FO |
5'-ACCCCCTTCCCTTCTCCTCCAGATAGAT-3' | 297 bp | 61˚C |
| miR-196a2 RO |
5'-AAAGCAGGGTTCTCCAGACTTGTTCTGC-3' | | |
| miR-196a2 FI (T
allele): |
5'-AGTTTTGAACTCGGCAACAAGAAACGGT-3' | 199 bp | |
| miR-196a2 RI (C
allele) |
5'-GACGAAAACCGACTGATGTAACTCCGG-3' | 153 bp | |
Preparation of PCR cocktail
A 25 µl ARMS-PCR cocktail, containing 50 ng DNA was
prepared by adding 0.25 µl solution containing 25 pmol of Fo, Ro,
FI and RI primers respectively. 10 µl PCR master mix (DreamTaq
Green, Thermo Fisher Scientific, Inc.) was added and the final
volume of 25 µl was made by using nuclease-free double distilled
water.
Thermocycling conditions
The thermocycling conditions included a hot start at
95˚C for 8 min, followed by 40 amplification cycles at 95˚C for 35
sec, 60˚C for miR-196a2 rs11614913 C>T and 58˚C for ACE2
rs4343 (2350A>G) for 40 sec and 72˚C for 45 sec. This was
followed by an elongation step at 72˚C for 10 min and storage at
4˚C.
Gel electrophoresis for ACE2 rs4343
G>A
The PCR products of ACE2 rs4343 (2350A>G)
genotyping were separated by electrophoresis on 2% agarose and
visualized on a UV transilluminator. GelPilot 100 bp Plus ladder
(100) from Qiagen (cat. no. 239046) was used as a marker. Primers
Fo and Ro flank the exon of the ACE2 rs4343 (2350A>G)
gene and gave a band corresponding to 268 bp that acted as a
control for quality and quantity of DNA. Primers FI and Ro that
amplified T allele gave a band corresponding to 190 bp and primers
Fo and R1 gave a band corresponding to 125 bp from the allele G as
depicted in Fig. 1.
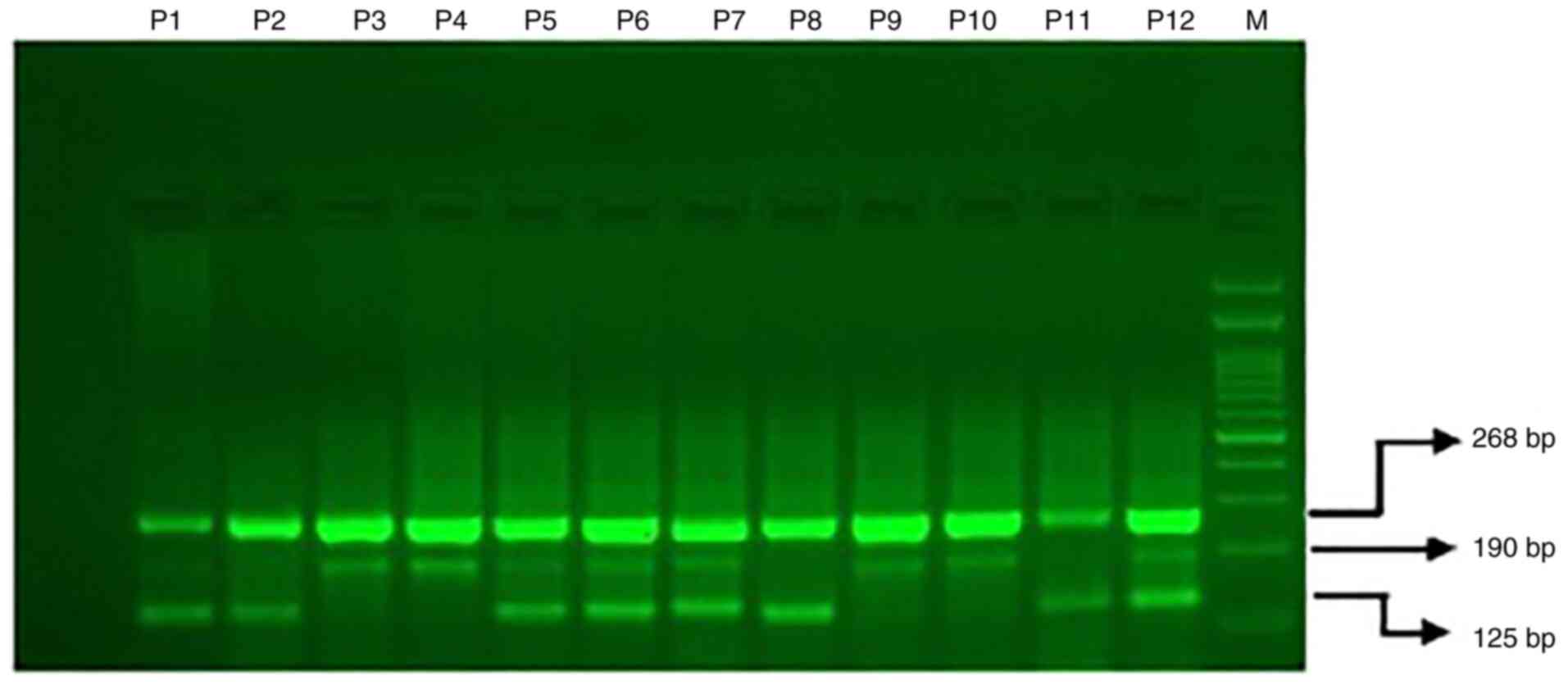 | Figure 1ACE2 rs4343 (2350A>G) genotyping
utilizing amplification refractory mutation system (ARMS-PCR) in
COVID-19 patients. M, 100 bp DNA ladder; P1, P2, P5, P6, P7 and
P12, heterozygous; P3, P4, P9 and P10, homozygous GG-(190 bp); P2,
P8 and P11, homozygous TT-(125 bp). |
Gel electrophoresis for miR-196a2
rs11614913 C>T
The ARMS-PCR products for miR-196a2 were
analyzed by electrophoresis on 2% agarose gel and visualized on a
UV transilluminator. Primers Fo and Ro flanked the exon of the
miR-196a2 rs11614913 C>T gene and gave a band corresponding to
297 bp that acted as a control for quality and quantity of DNA.
Primers F1 and Ro amplified T allele and generated a band
corresponding to 199 bp and primers Fo and R1 gave a band
corresponding to 153 bp from the C allele as depicted in Fig. 2.
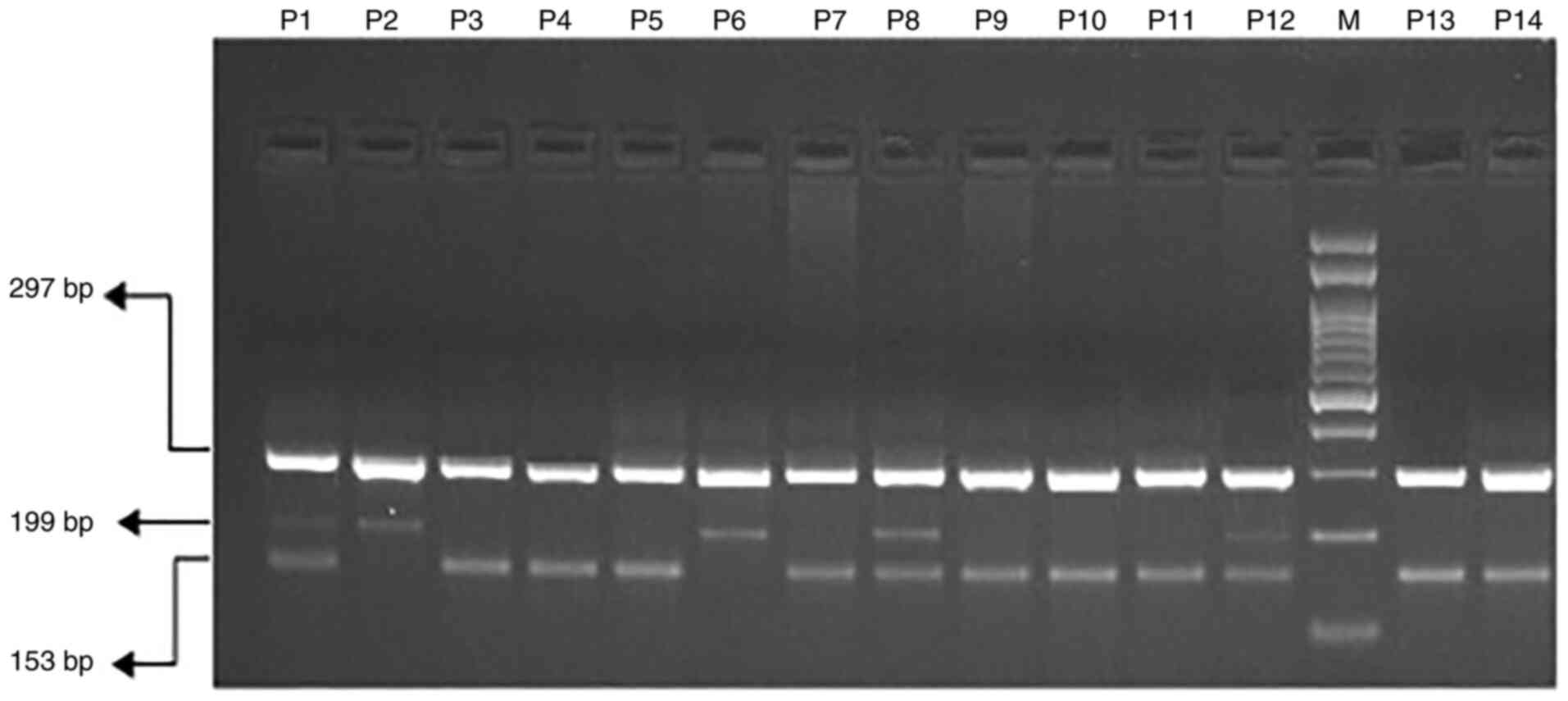 | Figure 2MicroRNA-196a2 rs11614913 C>T
genotyping utilizing amplification refractory mutation system
(ARMS-PCR) in COVID-19 patients. M, 100 bp DNA ladder; P1, P8 and
P12, heterozygous; P3, P4, P5, P7, P9, P10, P11, P13 and P14,
homozygous CC-(153 bp); P2 and P6, homozygous TT-(199 bp). |
Healthy controls For ACE2 rs4343
G>A gene polymorphism
The age matched and healthy control group comprised
103 subjects out of whom 70 (67%) were males and 33 (33%) were
females. The age distribution of the control group showed that 75
(72%) patients were >40 years and 28 (27%) were ≤40 years
old.
For miR-196a2 rs11614913 C>T gene
polymorphism
The miR-196a2 rs11614913 was studied in 200 age
matched healthy controls comprising 130 (65%) males and 70 (35%)
females. The age distribution of the control group showed that 146
(73%) were >40 years and 54 (27%) were ≤40 years old.
Statistical analysis
Deviations from Hardy-Weinberg disequilibrium (HWD)
were calculated by Chi-square (χ2) goodness-of-fit test.
Group differences were compared using Student's two-sample t-test
and one-way analysis of variance (ANOVA) with Tukey's post hoc test
for continuous variables and χ2 for categorical
variables. Differences in the ACE-rs4646994 I/D, and ACE2 rs4240157
C>T allele and genotype frequencies between groups were
evaluated using χ2 test. The associations between
ACE2-rs4646994 I/D, ACE2 rs4240157 C>T genotypes, miR-196a2
rs11614913 C>T and risk of Covid-19 patients were estimated by
computing the odds ratios (ORs) and risk ratios (RRs) with 95%
confidence intervals (CIs). Allele frequencies among patients as
well as controls were evaluated by using the χ2
Hardy-Weinberg equilibrium (HWE) test. All statistical analyses
were performed with SPSS 16.0 (SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic characteristics and
baseline features
The demographic features and the baseline
characteristics for 117 COVID-19 patients are given in Table I. Of the patients, 97 (82.90%) were
>40 years of age and 20 (17.10%) patients were ≤40 years old.
From the patients, 85 (72.64%) were male and 32 (27.36%) were
female. Regarding the co-morbidities, 47 (40.17%) were T2DM
patients, 37 (31.62%) had hypertension and 11(9.40%) had chronic
kidney disease. A total of 47 (40.17%) patients had low oxygen
saturation (<60 mm Hg) at the time of admission and 57 (48.71%)
patients stayed >30 days in hospital. In the COVID-19 patient
group, 79 (67.52%) patients received antiviral therapy whereas 77
(65.81%) received steroid therapy. Out of 117 COVID-19 patients, 43
(36.75%) patients succumbed and 74 (63.24%) survived and were
discharged from the hospital. As can be seen in Table I, out of 117 COVID-19 patients, 45
(38.57%) had elevated levels of alanine aminotransferase, 104
(97.44%) had high levels of C-reactive protein and 48 (41.3%) had
high levels of aspartate transaminase (AST).
Association of ACE2 rs4343 G>A SNP
between COVID-19 patients and controls
The present study found the frequency of ACE2 rs4343
G>A in compliance to the Hard-Weinberg equation (HWE) in all the
study subjects and randomly chose only 10% samples from control
group to analyze genotyping results, ensuring an accuracy rate of
more than 99%. The GG, GA and AA genotype frequencies were 48.71,
45.29 and 5.98% in COVID-19 patients respectively, whereas in
healthy controls GG, GA and AA genotype frequencies were 63.10,
29.12 and 7.76% respectively (Table
III). The difference in the distribution of ACE2 rs2323G>A
genotypes in COVID-19 patients and healthy controls was significant
(P<0.047). The frequency of G allele (fG) was also found to be
significantly higher in COVID-19 patients as compared with the
control group (0.71 vs. 0.29; Table
III).
 | Table IIIAssociation of ACE2 rs4343 G>A
gene variation in COVID-19 cases and controls. |
Table III
Association of ACE2 rs4343 G>A
gene variation in COVID-19 cases and controls.
| Subjects | n= | GG % | GA % | AA % | G | A | Degree of
freedom | χ2 | P-value |
|---|
| Cases | 117 | 57 (48.71) | 53 (45.29) | 7 (5.98) | 0.71 | 0.29 | 2 | 6.10 | 0.047 |
| Controls | 103 | 65 (63.10) | 30 (29.12) | 8 (7.76) | 0.78 | 0.22 | | | |
Association between ACE2 rs4343G>A
genotypes and COVID-19 severity
Table IV
summarizes the data on the association between ACE2 rs4343G>A
genotypes and risk to COVID-19. These data were obtained by using a
multivariate analysis model based on logistic regression such as
odds ratio (OR) and risk ratio (RR) with 95% confidence intervals
(CI). The results indicated that the COVID-19 disease severity
correlated significantly with ACE2 genotypes (GG vs. GA) in the
codominant model with OR 2.10 CI=1.13-3.56, RR=1.47 (1.05-2.05) and
P<0.016. A strong association was also observed between ACE2 GG
vs. ACE2 (GA+AA) genotype in dominant inheritance model that leads
to increased COVID-19 severity with OR=1.80, 95% CI=1.04-3.08,
RR=1.37 (1.01-1.85) and P<0.032 as depicted in Table IV. The A allele was not associated
with COVID-19 severity with an OR 1.39, 95% CI=0.90-2.15, RR=1.20
(0.93-1.54) and P-value=0.131 on making allelic comparisons. No
significance was observed between different alleles and COVID-19
severity in over dominant inheritance model. The results indicated
a potential dominant effect of ACE2-AA genotype but not A allele on
COVID-19 severity in the patients from Asir and Tabuk regions of
KSA. The results also showed that in case of overdominant
inheritance model, the ACE2 rs4343-GG+AA vs. GA genotype of
the ACE2 rs4343 G>A was not associated with
susceptibility to COVID-19 with OR=1.89 (1.29-1.90) and
P=0.170.
 | Table IVAssociation of the ACE2 rs4343 G>A
polymorphism with the COVID-19. |
Table IV
Association of the ACE2 rs4343 G>A
polymorphism with the COVID-19.
| Genotypes | Healthy controls
(n=103) | Covid-19 cases
(n=117) | OR (95% CI) | Risk Ratio
(RR) | P-value |
|---|
| Codominant | n | % | n | % | | | |
|
ACE2-GG | 65 | 63.10 | 57 | 48.71 | 1 (ref.) | 1 (ref.) | |
|
ACE2-GA | 30 | 29.12 | 53 | 45.29 | 2.10
(1.13-3.56) | 1.47
(1.05-2.05) | 0.016 |
|
ACE2-AA | 08 | 7.76 | 07 | 5.98 | 1.01
(0.34-2.92) | 1.01
(0.60-1.64) | 0.99 |
| Dominant | | | | | | | |
|
ACE2-GG | 65 | 63.10 | 57 | 48.71 | 1 (ref.) | 1 (ref.) | |
|
ACE2-(GA+AA) | 38 | 29.12 | 60 | 45.29 | 1.80
(1.04-3.08) | 1.37
(1.01-1.85) | 0.032 |
| Recessive | | | | | | | |
|
ACE2-(GG+GA) | 95 | 92.23 | 110 | 94.01 | 1 (ref.) | 1 (ref.) | |
|
ACE2-AA | 08 | 7.76 | 07 | 5.98 | 0.75
(0.26-2.16) | 0.86
(0.52-1.42) | 0.60 |
| Allele | | | | | | | |
|
ACE2-G | 160 | 81.63 | 167 | 71.36 | 1 (ref.) | 1 (ref.) | |
|
ACE2-A | 46 | 23.46 | 67 | 28.3 | 1.39
(0.90-2.15) | 1.20
(0.93-1.54) | 0.131 |
| Overdominant | | | | | | | |
|
ACE2-G/G-A/A | 73 | 26.16 | 64 | 21.47 | 1 (ref.) | 1 (ref.) | |
|
ACE2-G/A | 206 | 73.83 | 234 | 78.52 | 1.29
(0.88-1.90) | 1.13
(0.94-1.37) | 0.17 |
Association of ACE2 rs4343 G>A
genotypes with gender and comorbid conditions and COVID-19
severity
Table V summarizes
the statistical comparisons (P-values) of ACE2 rs4343 G>A
genotypes with comorbid conditions of COVID-19 patients and disease
severity. A multivariate analysis based on logistic regression such
as OD and RR with 95% CI was used to analyze these results. The
results showed that the there was a significant correlation between
the ACE2 rs4343 G>A genotypes with respect to the sex of the
COVID-19 patients (P<0.023) and COVID-19 patients having
coronary artery disease (P<0.0001). Similarly, a significant
correlation was also reported between ACE2 rs4343 G>A genotypes
and COVID-19 patients with oxygen saturation <60 mm Hg
(P<0.0009). The duration of hospital stays of COVID-19 patients
also correlated with ACE2 rs4343 G>A genotype distribution
(P<0.496) but a non-significant association was observed. As can
be seen in Table V, a
non-significant correlation was also observed between antiviral
therapy and ACE2 rs4343 G>A genotypes.
 | Table VAssociation of ACE2 rs4343 G>A
polymorphism with COVID-19 patient characteristics. |
Table V
Association of ACE2 rs4343 G>A
polymorphism with COVID-19 patient characteristics.
| Patient
characteristics | n=117 | GG | GA | AA | Degree of
freedom | χ2 | P-value |
|---|
| Age (years) | | | | | | | 0.44 | NS |
|
>40 | 97 | 46 | 44 | 07 | 1.64 | 2 | | |
|
≤40 | 20 | 11 | 09 | 00 | | | | |
| Sex | | | | | | | 0.023 | SG |
|
Male | 85 | 42 | 41 | 02 | 7.47 | 2 | | |
|
Female | 32 | 15 | 12 | 5 | | | | |
| T2DM | | | | | | | 0.61 | NS |
|
Yes | 47 | 23 | 20 | 04 | 0.97 | 2 | | |
|
No | 70 | 34 | 33 | 03 | | | | |
| CKD | | | | | | | 0.47 | NS |
|
Yes | 11 | 07 | 04 | 00 | 1.5 | 2 | | |
|
No | 106 | 50 | 49 | 07 | | | | |
| Hypertension | | | | | | | 0.11 | NS |
|
Yes | 37 | 14 | 21 | 02 | 4.37 | 2 | | |
|
No | 80 | 43 | 32 | 05 | | | | |
| CAD | | | | | | | | |
|
Yes | 17 | 06 | 05 | 06 | 30.41 | 2 | 0.0001 | SG |
|
No | 100 | 51 | 48 | 01 | | | | |
| Oxygen
saturation | | | | | | | 0.0009 | SG |
|
Yes | 47 | 15 | 29 | 03 | 9.24 | 2 | | |
|
No | 70 | 42 | 24 | 04 | | | | |
| Duration in
hospital (days) | | | | | | | 0.490 | NS |
|
>30 | 57 | 25 | 29 | 03 | 1.4 | 2 | | |
|
<30 | 60 | 32 | 24 | 04 | | | | |
| ALT | | | | | | | 0.82 | NS |
|
<36
U/l | 45 | 23 | 20 | 02 | 0.39 | 2 | | |
|
>36
U/l | 72 | 34 | 33 | 05 | | | | |
| CRP | | | | | | | | |
|
<10
mg/l | 13 | 51 | 47 | 06 | 0.09 | 2 | 0.956 | NS |
|
≥10
mg/l | 104 | 06 | 06 | 01 | | | | |
| AST | | | | | | | 0.014 | SG |
|
<40
U/l | 48 | 30 | 18 | 0 | 9.14 | 2 | | |
|
>40
U/l | 69 | 27 | 35 | 07 | | | | |
| Antiviral
therapy | | | | | | | 0.003 | SG |
|
Yes | 79 | 30 | 43 | 06 | 3.87 | 2 | | |
|
No | 38 | 27 | 10 | 01 | | | | |
| Steroids
therapy | | | | | | | 0.400 | NS |
|
Yes | 77 | 35 | 36 | 06 | 1.82 | 2 | | |
|
No | 40 | 22 | 17 | 01 | | | | |
| Survival | | | | | | | 0.004 | SG |
|
Yes | 43 | 14 | 28 | 1 | 11.6 | 2 | | |
|
No | 74 | 43 | 25 | 6 | | | | |
Correlation of ACE2 rs4343 G>A
genotypes with mortality of COVID-19 patients
In a co-dominant model, ACE2-DD genotype
heterozygosity showed a strong association with increased COVID-19
mortality with OR 3.44, 95% CI=1.53-7.72 and P=0.0028 as depicted
in Table VI. However, ACE2-AA
genotype (GG vs. AA) was not associated with COVID-19 mortality
with OR 0.51 95% CI=0.056-4.62 and P=0.55 as depicted in Table VI. In dominant inheritance model,
ACE2-GA+AA genotype (GG vs. GA+AA) was strongly associated with
increased COVID-19 mortality with OR 2.87, 95% CI=1.30 to 6.31 and
P<0.008. However, in recessive inheritance model, ACE2-genotype
(AA vs. GG+GA) was not associated with COVID-19 mortality with OR
3.7, 95% CI=0.43 to 31.86 and P<0.23. The A allele too did not
show any association with COVID-19 mortality, with an OR 1.60, 95%
CI=0.90-2.86 and P=0.108, on allelic comparisons. In overdominant
inheritance model, ACE2-genotype (GA vs. GG+AA) was strongly
associated with increased COVID-19 mortality with OR 1.89, 95%
CI=1.004 to 3.58 and P<0.040.
 | Table VIStatistical comparisons (P-values) of
ACE2 rs4343 G>A genotypes with mortality of COVID-19
patients. |
Table VI
Statistical comparisons (P-values) of
ACE2 rs4343 G>A genotypes with mortality of COVID-19
patients.
| Model | Genotype | Survival | Mortality | OR (95% CI) | P-value | |
|---|
| Codominant | | n=74 | n=43 | | | |
| | GG | 43 | 14 | 1 (ref.) | | |
| | GA | 25 | 28 | 3.44
(1.53-7.72) | 0.0028 | SG |
| | AA | 06 | 01 | 0.51
(0.056-4.62) | 0.55 | NS |
| Dominant | | | | | | |
| | GG | 43 | 14 | 1 (ref.) | | |
| | GA+AA | 31 | 29 | 2.87
(1.30-6.31) | 0.008 | SG |
| Recessive | | | | | | |
| | AA | 06 | 01 | 1 (ref.) | | |
| | GG+GA | 68 | 42 | 3.7
(0.43-31.86) | 0.23 | NS |
| Allele | | | | | | |
| | G | 111 | 56 | 1 (ref.) | | |
| | A | 37 | 30 | 1.60
(0.90-2.86) | 0.108 | NS |
| Overdominant | | | | | | |
| | GG+AA | 49 | 15 | 1 (ref.) | | |
| | GA | 148 | 86 | 1.89
(1.004-3.58) | 0.040 | SG |
Comparison of miR-196 rs11614913 C>
SNPs between COVID-19 patients and controls
As the miR-196 rs11614913 C>T frequency was in
agreement with HWE in all study subjects, only 10% samples were
chosen randomly to analyze the results from the control group.
As is evident in Table VII, the CC, CT and TT genotype
frequencies in COVID-19 patients were 76.92, 18.80 and 4.27%
respectively whereas in healthy controls CC, CT and TT genotype
frequencies were 60, 32 and 8% respectively. The distribution of
miR-196a2 rs11614913 C>T genotypes between COVID-19 patients and
healthy controls was significant (P<0.008). Moreover, the
frequency of C allele (fC) was found to be higher among COVID-19
patients than in control group (0.86 vs. 0.76; Table VII).
 | Table VIIAssociation of miR-196a2 rs11614913
C>T gene variation in COVID-19 cases and controls. |
Table VII
Association of miR-196a2 rs11614913
C>T gene variation in COVID-19 cases and controls.
| Subjects | n | CC | CT | TT | Degree of
freedom | χ2 | C | T | P-value |
|---|
| Cases | 117 | 90 (76.92%) | 22 (18.80%) | 05 (4.27%) | 2 | 9.48 | 0.86 | 0.14 | 0.008 |
| Controls | 200 | 120 (60%) | 64 (32%) | 16 (8%) | | | 0.76 | 0.24 | |
Potential association of miR-196a2
rs11614913 C>T genotypes with COVID-19
A multivariate analysis based on logistic regression
such as OD and RR with 95% CI was used to determine the association
between miR-196a rs11614913 C>T genotypes and risk to COVID-19
and the data are summarized in Table VIII. The results showed that the
CT genotype of the miR-196a2 rs11614913 was associated with
decreased susceptibility to COVID-19 with OR=0.452 (0.26-0.79),
RR=0.76 (0.64-0.91) and P=0.006. The T allele of the miR-196a2
rs11614913 was also associated with decreased susceptibility to
COVID-19 with OR=0.54 (0.35-84), RR=0.81 (0.71-0.92) and P=0.005
(Table VIII). The results
showed that in case of the overdominant model, the miR-196-CC+TT
vs. CT genotype of the miR-196a2 rs11614913 was associated with
decreased susceptibility to COVID-19 with OR=0.49 (0.28-0.85),
RR=0.79 (0.67-0.93) and P=0.0016.
 | Table VIIIAllele and genotype distribution of
miR-196a2 rs11614913 C>T polymorphism in the COVID-19
patients and control groups. |
Table VIII
Allele and genotype distribution of
miR-196a2 rs11614913 C>T polymorphism in the COVID-19
patients and control groups.
| Genotypes | Healthy
controls | Covid-19 cases | OR (95% CI) | Risk Ratio
(RR) | P-value |
|---|
| Codominant | (N=200) | (N=117) | | | |
| miR-196a2-CC | 120 | 90 | 1 (ref.) | 1 (ref.) | |
| miR-196a2-CT | 64 | 22 | 0.452
(0.26-0.79) | 0.76
(0.64-0.91) | 0.006 |
| miR-196a2-TT | 16 | 05 | 0.41
(0.14-1.17) | 0.75
(0.57-0.97) | 0.09 |
| Dominant | | | | | |
| miR-196-CC | 120 | 90 | 1 (ref.) | 1 (ref.) | |
| miR-196-CT+TT) | 80 | 27 | 0.45
(0.26-0.75) | 0.76
(0.65-0.89) | 0.001 |
| Recessive | | | | | |
|
miR-196-(CC+CT) | 184 | 112 | 1 (ref.) | 1 (ref.) | |
| miR-196-TT | 16 | 05 | 0.51
(0.18-1.43) | 0.81
(0.63-1.05) | 0.20 |
| Allele | | | | | |
| miR-196-C
allele | 304 | 202 | 1 (ref.) | 1 (ref.) | |
| miR-196-T
allele | 96 | 35 | 0.54 (0.35-84) | 0.81
(0.71-0.92) | 0.005 |
| Over dominant | | | | | |
| miR-196-CC+TT | 136 | 95 | 1 (ref.) | 1 (ref.) | |
| miR-196-CT | 64 | 22 | 0.49
(0.28-0.85) | 0.79
(0.67-0.93) | 0.0016 |
Association of miR-196a2 rs11614913
C>T genotypes with gender, comorbid conditions and COVID-19
severity
A multivariate analysis was used to elucidate the
association of miR-196a2 rs11614913 C>T genotypes with sex,
comorbid conditions and COVID-19 severity and the results are
summarized in Table IX. The
results indicated that there was a significant difference (P=0.006)
in rs11614913 genotype distribution between patients >40 years
old and patients ≤40 years old (Table
IX). The results also showed that there was a significant
difference (P=0.035) in rs11614913 genotype distribution between
male and female patients (Table
IX). The results showed that there were significant differences
in patients with hypertension and coronary artery disease (CAD)
compared with patients without hypertension and CAD (P=0.044 and
0.035, respectively; Table IX).
Results also indicated that there was a significant difference
(P=0.01) in patients with oxygen saturation <60 and those with
oxygen saturation >80. Furthermore, the results showed that
there was a significant difference (P=0.01) in rs11614913 genotype
distribution between the patients who survived from the COVID-19
and the patients who succumbed (Table
IX).
 | Table IXAllele and genotype distribution of
miR-196a rs11614913 C>T polymorphism in the COVID-19
patients. |
Table IX
Allele and genotype distribution of
miR-196a rs11614913 C>T polymorphism in the COVID-19
patients.
| Patient
characteristics | n=117 | CC | CT | TT | Degree of
freedom | χ2 | P-value |
|---|
| Age (years) | | | | | | | 0.006 |
|
>40 | 97 | 82 | 30 | 05 | 02 | 10.18 | |
|
≤40 | 20 | 12 | 06 | 02 | | | |
| Sex | | | | | | | 0.035 |
|
Male | 85 | 63 | 20 | 02 | 02 | 6.69 | |
|
Female | 32 | 27 | 02 | 03 | | | |
| T2DM | | | | | | | 0.16 |
|
Yes | 47 | 36 | 10 | 00 | 02 | 3.6 | |
|
No | 70 | 54 | 12 | 05 | | | |
| CKD | | | | | | | 0.017 |
|
Yes | 11 | 08 | 06 | 02 | 02 | 8.0 | |
|
No | 106 | 82 | 16 | 03 | | | |
| Hypertension | | | | | | | 0.044 |
|
Yes | 37 | 28 | 05 | 04 | 02 | 28 | |
|
No | 80 | 62 | 17 | 01 | | 62 | |
| CAD | | | | | | | 0.035 |
|
Yes | 17 | 15 | 00 | 02 | 02 | 6.68 | |
|
No | 100 | 75 | 22 | 03 | | | |
| Oxygen
saturation | | | | | | | 0.035 |
|
Yes | 47 | 33 | 14 | 00 | 02 | 8.86 | |
|
No | 70 | 57 | 08 | 05 | | | |
| Duration in
hospital (days) | | | | | | | 0.83 |
|
>30 | 57 | 44 | 10 | 03 | 02 | 0.35 | |
|
<30 | 60 | 46 | 12 | 02 | | | |
| ALT | | | | | | | 0.82 |
|
<36
U/l | 45 | 72 | 54 | 13 | 05 | 02 | |
|
>36
U/l | 72 | 45 | 36 | 09 | 00 | | |
| CRP | | | | | | | |
|
<0.8
mg/dl | 27 | 27 | 00 | 00 | 02 | 10.53 | 0.005 |
|
>0.8
mg/dl | 90 | 63 | 22 | 05 | | | |
| AST | | | | | | | 0.25 |
|
<40
U/l | 69 | 55 | 10 | 04 | 02 | 2.75 | |
|
>40
U/l | 48 | 35 | 12 | 01 | | | |
| Antiviral
therapy | | | | | | | 0.42 |
|
Yes | 79 | 58 | 17 | 04 | 02 | 1.7 | |
|
No | 38 | 32 | 05 | 01 | | | |
| Steroids
therapy | | | | | | | 0.733 |
|
Yes | 77 | 58 | 16 | 03 | 02 | 0.62 | |
|
No | 40 | 32 | 06 | 02 | | | |
| Survival | | | | | | | 0.010 |
|
Yes | 43 | 31 | 07 | 05 | 02 | 9.0 | |
|
No | 74 | 59 | 15 | 00 | | | |
Discussion
The diverse clinical manifestations of the
SARS-CoV-2 infection vary from no symptoms to severe disease (ICU
admission) and mortality in COVID-19 patients. The results of the
present study indicated that there was a significant difference in
the ACE2 rs4343 G>A genotype distribution between the patient
and the control groups (P<0.05; Table III). Results also showed that the
GA genotype of the rs4343 G>A was associated with increased
susceptibility to COVID-19(9)
(Table IV). rs4343 G>A
influences the activity and the levels of ACE and increases
susceptibility to hypertension, T2DM, obesity, renal disease, CVD
and autoimmune diseases (30). The
results of the present study are consistent with a recent study
that reported the association of the G allele with the SARS-CoV-2
severity in the presence or absence of metabolic and other
comorbidities (30). Furthermore,
it has been suggested that GG genotype of the rs4343 SNP is
associated with increased circulating ACE levels and its activity
(31,32). The increased activity and levels of
the ACE2 are reported to increase the susceptibility to
COVID-19(33). The results of the
present study seem to be in agreement with these studies (31-33)
as rs4343 GA genotype increases the activity and levels of
ACE2(30) which may increase the
susceptibility to COVID-19(9)
(Table IV). The results also
showed that there was a significant difference in ACE2 rs4343
G>A SNPs between male and female patients (P<0.023; Table V). This result is in agreement with
earlier studies that report higher expression of ACE2 in males
compared with females and the increased expression of ACE2 is
reported to promote the entry of SARS-CoV-2 (9,13).
It is suggested that the reduced expression of ACE2 in females
renders them less sensitive to severe adverse effects of
COVID-19(13). The results of the
present study also indicated that there were significant
differences (P<0.05) in the rs4343 G>A genotype distribution
between the patients with CAD and reduced oxygen saturation and
patients without CAD and with normal oxygen saturation (Table V). This result is in agreement with
a study that reports the association of the ACE2 rs4343 G>A with
dyslipidemia and severity of COVID-19(30). Moreover, the results of the present
study indicated that there was a significant difference (P<0.05)
of rs4343 G>A genotype distribution with elevated patient AST
levels (Table V). This result is
in agreement with an earlier study that reports the association of
SARS-COV-2 with liver dysfunction (34). As ACE2 is expressed in the hepatic
tissues (9), it is possible that
the rs4343 G>A SNP modulates the SARS-COV-2 infection and
increases the liver damage but this need further validation. The
results of the present study also indicated that there was no
significant difference (P>0.05) in rs4343 G>A genotype
distribution between diabetic and non-diabetic subjects (Table V). This result was rather
unexpected and was inconsistent with a study that reported diabetes
to increase the susceptibility to coronavirus infection since ACE2
is highly expressed in T2DM patients (9). This inconsistency may be because the
sample size used in the present study was relatively small (n=117).
In addition, the results showed that the genotype distribution of
rs4343 G>A was significantly different (P<0.05) between
patients who needed antiviral therapy and those who did not
(Table V). This result is in
agreement with Íñiguez et al (30), who report that the G allele of the
rs4343 increases the severity of COVID-19. The results of the
present study also showed that there was a significant difference
(P<0.05) in ACE2 rs4343 G>A genotype distribution between
patients who survived and those who did not (Tables V and VI), i.e. the G allele of the rs4343
increased the severity and mortality of COVID-19. Again, this
result is in agreement with Íñiguez et al (30), who demonstrate that the ACE2 rs4343
G allele increases the severity of COVID-19.
miRNAs serve important and diverse roles in cellular
physiology and pathology including immunity, development, apoptosis
and types of cancer (35-38).
miRNA gene variations are associated with various metabolic
diseases (25,26,39-41)
and have been demonstrated to influence the susceptibility to viral
infections and the clinical course of the viral disease (42,43).
miR-196 is found in the regions of homeobox clusters within the
vertebrates genome (44) and is
located in the 3'-untranslated region of the miR-196a2 precursor.
Polymorphism of miR-196 rs11614913 not only influences the
transcription level of mature miR-196a, but also has a biological
effect on target gene production (42).
The results of the present study indicated that the
miR-196a rs11614913 C>T genotype distribution was significantly
different (P<0.05) between patients and controls (Table VII). The results also showed that
the CT genotype and the T allele of the miR-196a rs11614913 C>T
were associated with the decreased risk to COVID-19 (Table VIII). It is reported that
miR-196 is among interferon-induced miRNAs and that miR-196
directly targets the CORE and NS5A coding region of genomic RNA of
the HCV and thereby suppresses the replication of the virus by ≤80%
(45). In addition, it has been
demonstrated that miR196 inhibits the expression of the HCV
(46) by repressing the expression
of the Bach-1 protein (46).
Bach-1 is an inhibitor of the anti-oxidative and anti-inflammatory
heme oxygenase 1 (HMOX1) (46,47).
miR-196 mimics significantly repress the expression of the protein
Bach1 and upregulate the gene expression of HMOX1 and thereby
inhibit the HCV expression (46).
In an experiment conducted in lung tissues of hamster, it was shown
that miR-196a is among five miRNAs that commonly bind to SARS-CoV,
MERS-CoV and SARS-CoV-2 viruses (48). It is reported that miR-196a is
gradually upregulated after SARS-CoV-2 infection (48). The present study hypothesized that
rs11614913 affected the immune response against SARS-CoV-2 and that
the T allele and CT genotype carriers became less susceptible to
the SARS-CoV-2 infection (Table
VIII). The results are in partial agreement with the result of
Tian et al (47), who
report that miR-196a-2 C>T (rs11614913) is probably associated
with reduced susceptibility of HBV and HCV-related HCC,
particularly in the Chinese population.
The results of the present study further showed that
the carriers of the CT genotype and the T allele of the miR-196a
rs11614913 in >40-year-old patients were at reduced risk to
SARS-CoV-2 infection (Table IX).
It also observed that the males who are carriers of the CT genotype
and T allele of the miR-196 rs11614913 were less susceptible to the
SARS-CoV-2 infection compared with females (Table IX). The results also showed that
miR-196 rs11614913 significantly (P>0.05) increased the risk to
SARS-CoV-2 infection in patients with hypertension and CAD
(Table IX). This result is
consistent with a study that indicated the association of miR-196
rs11614913 with CAD (41). The
results of the present study also suggested that 69% of the
patients who succumbed were miR-196 rs11614913 CC genotype carriers
(Table IX) suggesting that CC
genotype contributed to disease severity and mortality. Limitations
of the present study included the relatively small sample size.
Further studies with larger sample size and on different ethnic
populations are recommended.
Taken together, the present study examined the
association of the ACE2 rs4343 G>A and miR-196a rs11614913
C>T with the severity and mortality of SARS-CoV-2 infection in a
study population from Asir and Tabuk regions of Saudi Arabia. The
results clearly indicated that the GA genotype of the ACE2 rs4343
was associated with increased severity and mortality of COVID-19.
The results also showed that the CT genotype and T allele of the
miR-196a rs11614913 C>T were associated with decreased
susceptibility of COVID-19. More studies in different ethnic
populations and bigger sample sizes are necessary to further
investigate the roles of genetic alterations of ACE2 and miR-196a
in the molecular pathogenesis of SARS-CoV-2 and COVID-19.
Acknowledgements
The authors extend their appreciation to Dr Suhail
Ahmed of the English Department, University of Bisha, for language
review and editing and Dr Mohammed Jeelani of UBCOM for his
technical assistance.
Funding
Funding: The authors extend their appreciation to the Deputyship
for Research and Innovation, Ministry of Education in Saudi Arabia
for funding this research work through the project number
UB-47-1442.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon a reasonable
request.
Authors' contributions
All the authors were involved in the conception and
planning of the study. MMM, RM, MAAA, RF, MMSA and MA designed the
study. MAAA, BAA, AAB, MMSA and AMA were involved in the
recruitment of patients. BAA, AAB and AMA collected the patient
data and analyzed the clinical outcomes of patients with COVID-19.
MHA, RM and IE performed the experiments. MMM, RM and IE wrote the
initial draft. which was revised and edited by all the authors. MMM
and MAAA were involved in the acquisition of grants and project
administration. RM, MMM and IE confirm the authenticity of all raw
data. All the authors read and approved the final version of the
manuscript for publication and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The ethical approvals were obtained from three local
institutional ethics committees of College of Medicine, University
of Bisha (Ref. no. UBCOM/H-06-BH-087(05/25), University of Tabuk
(Decision No: KAEK2020/4/4) and College of Medicine, King Khalid
University, Abha (Ref. no. H-06-B-091) in accordance with local
guidelines which complied in essence with the principles of the
Helsinki Declaration. Written informed consent was obtained before
the collection of blood samples from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information (optional)
Prof. Mohammad Muzaffar Mir (corresponding author)
is currently working as Professor and Chairman, Medical
Biochemistry at College of Medicine, University of Bisha. He is
actively involved in biomedical research in molecular genetics,
signal transduction and cancer biology, in addition to his
commitment to teaching of medical students in a problem-based and
SPICES model curriculum.
References
|
1
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Z, Ye D, Wang M, Zhao M, Li D, Ye J,
Liu J, Xu Y, Zhang J, Pan W, et al: Clinical features of COVID-19
patients with different outcomes in Wuhan: A retrospective
observational study. Biomed Res Int. 2020(2138387)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alsofayan YM, Althunayyan SM, Khan AA,
Hakawi AM and Assiri AM: Clinical characteristics of COVID-19 in
Saudi Arabia: A national retrospective study. J Infect Public
Health. 13:920–925. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mahase E: Coronavirus covid-19 has killed
more people than SARS and MERS combined, despite lower case
fatality rate. BMJ. 368(m641)2020.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Chegni H, Pakravan N, Saadati M, Ghaffari
AD, Shirzad H and Hassan ZM: Is there a link between COVID-19
mortality with genus, age, ABO blood group type, and ACE2 gene
polymorphism? Iran J Public Health. 49:1582–1584. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zietz M, Zucker J and Tatonetti NP:
Associations between blood type and COVID-19 infection, intubation,
and death. Nat Commun. 11(5761)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Suryamohan K, Diwanji D, Stawiski EW,
Gupta R, Miersch S, Liu J, Chen C, Jiang YP, Fellouse FA,
Sathirapongsasuti JF, et al: Human ACE2 receptor polymorphisms and
altered susceptibility to SARS-CoV-2. Commun Biol.
4(475)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shah H, Khan MS, Dhurandhar NV and Hegde
V: The triumvirate: Why hypertension, obesity, and diabetes are
risk factors for adverse effects in patients with COVID-19. Acta
Diabetol. 58:831–843. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Soldo J, Heni M, Königsrainer A, Häring
HU, Birkenfeld AL and Peter A: Increased hepatic ACE2 expression in
NAFL and diabetes-a risk for COVID-19 patients? Diabetes Care.
43:e134–e136. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wong MK: Angiotensin converting enzymes,
subchapter 29D. In: Handbook of Hormones. Comparative Endocrinology
for Basic and Clinical Research. Takei Y, Ando H and Tsutsui K
(eds). Elsevier, pp263-265, e29D-1-e29D-4, 2016.
|
|
11
|
Clarke NE and Turner AJ:
Angiotensin-converting enzyme 2: The first decade. Int J Hypertens.
2012(307315)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Samavati L and Uhal BD: ACE2, Much more
than just a receptor for SARS-COV-2. Front Cell Infect Microbiol.
10(317)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Devaux CA, Rolain JM and Raoult D: ACE2
receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension,
multi-organ failure, and COVID-19 disease outcome. J Microbiol
Immunol Infect. 53:425–435. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elfaki I, Mir R, Duhier FMA, Alotaibi MA,
Alalawy AI, Barnawi J, Babakr AT, Mir MM, Altayeb F, Mirghani H and
Frah EA: Clinical implications of MiR128, angiotensin I converting
enzyme and vascular endothelial growth factor gene abnormalities
and their association with T2D. Curr Issues Mol Biol. 43:1859–1875.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Burrell LM, Harrap SB, Velkoska E and
Patel SK: The ACE2 gene: Its potential as a functional candidate
for cardiovascular disease. Clin Sci (Lond). 124:65–76.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Luo Y, Liu C, Guan T, Li Y, Lai Y, Li F,
Zhao H, Maimaiti T and Zeyaweiding A: Association of ACE2 genetic
polymorphisms with hypertension-related target organ damages in
south Xinjiang. Hypertens Res. 42:681–689. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sarzani R, Giulietti F, Di Pentima C,
Giordano P and Spannella F: Disequilibrium between the classic
renin-angiotensin system and its opposing arm in SARS-CoV-2-related
lung injury. Am J Physiol Lung Cell Mol Physiol. 319:L325–L336.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y,
Hou C, Wang H, Liu J, Yang D, et al: Role of angiotensin-converting
enzyme 2 (ACE2) in COVID-19. Crit Care. 24(422)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wrapp D, Wang N, Corbett KS, Goldsmith JA,
Hsieh CL, Abiona O, Graham BS and McLellan JS: Cryo-EM structure of
the 2019-nCoV spike in the prefusion conformation. Science.
367:1260–1263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mir MM, Mir R, Alghamdi MA, Alsayed BA,
Wani JI, Alharthi MH and Al-Shahrani AM: Strong association of
angiotensin converting enzyme-2 gene insertion/deletion
polymorphism with susceptibility to SARS-CoV-2, hypertension,
coronary artery disease and COVID-19 disease mortality. J Pers Med.
11(1098)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of MicroRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9(402)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shantikumar S, Caporali A and Emanueli C:
Role of microRNAs in diabetes and its cardiovascular complications.
Cardiovasc Res. 93:583–593. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mir R, Elfaki I, Khullar N, Waza AA, Jha
C, Mir MM, Nisa S, Mohammad B, Mir TA, Maqbool M, et al: Role of
selected miRNAs as diagnostic and prognostic biomarkers in
cardiovascular diseases, including coronary artery disease,
myocardial infarction and atherosclerosis. J Cardiovasc Dev Dis.
8(22)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bhat AA, Younes SN, Raza SS, Zarif L,
Nisar S, Ahmed I, Mir R, Kumar S, Sharawat SK, Hashem S, et al:
Role of non-coding RNA networks in leukemia progression, metastasis
and drug resistance. Mol Cancer. 19(57)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jha CK, Mir R, Elfaki I, Khullar N, Rehman
S, Javid J, Banu S and Chahal SM: Potential impact of MicroRNA-423
gene variability in coronary artery disease. Endocr Metab Immune
Disord Drug Targets. 19:67–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Elfaki I, Mir R, Mir MM, AbuDuhier FM,
Babakr AT and Barnawi J: Potential impact of MicroRNA gene
polymorphisms in the pathogenesis of diabetes and atherosclerotic
cardiovascular disease. J Pers Med. 9(51)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kałużna EM: MicroRNA-155 and
microRNA-196b: Promising biomarkers in hepatitis C virus infection?
Rev Med Virol. 24:169–185. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim D, Kim S, Park J, Chang HR, Chang J,
Ahn J, Park H, Park J, Son N, Kang G, et al: A high-resolution
temporal atlas of the SARS-CoV-2 translatome and transcriptome. Nat
Commun. 12(5120)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kirik MP, Pehlivan M, Nursal AF, Oyaci Y,
Pehlivan S and Serin I: The miRNA 196a2 rs11614913 variant has
prognostic impact on Turkish patients with multiple myeloma. BMC
Res Notes. 13(545)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Íñiguez M, Pérez-Matute P,
Villoslada-Blanco P, Recio-Fernandez E, Ezquerro-Pérez D, Alba J,
Ferreira-Laso ML and Oteo JA: ACE gene variants rise the risk of
severe COVID-19 in patients with hypertension, dyslipidemia or
diabetes: A spanish pilot study. Front Endocrinol (Lausanne).
12(688071)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schuler R, Osterhoff MA, Frahnow T,
Seltmann AC, Busjahn A, Kabisch S, Xu L, Mosig AS, Spranger J,
Möhlig M, et al: High-saturated-fat diet increases circulating
angiotensin-converting enzyme, which is enhanced by the rs4343
polymorphism defining persons at risk of nutrient-dependent
increases of blood pressure. J Am Heart Assoc.
6(e004465)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Firouzabadi N, Shafiei M, Bahramali E,
Ebrahimi SA, Bakhshandeh H and Tajik N: Association of
angiotensin-converting enzyme (ACE) gene polymorphism with elevated
serum ACE activity and major depression in an Iranian population.
Psychiatry Res. 200:336–342. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fagyas M, Kertész A, Siket IM, Bánhegyi V,
Kracskó B, Szegedi A, Szokol M, Vajda G, Rácz I, Gulyás H, et al:
Level of the SARS-CoV-2 receptor ACE2 activity is highly elevated
in old-aged patients with aortic stenosis: Implications for ACE2 as
a biomarker for the severity of COVID-19. Geroscience. 43:19–29.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yu D, Du Q, Yan S, Guo XG, He Y, Zhu G,
Zhao K and Ouyang S: Liver injury in COVID-19: Clinical features
and treatment management. Virol J. 18(121)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bensen JT, Graff M, Young KL, Sethupathy
P, Parker J, Pecot CV, Currin K, Haddad SA, Ruiz-Narváez EA, Haiman
CA, et al: A survey of microRNA single nucleotide polymorphisms
identifies novel breast cancer susceptibility loci in a
case-control, population-based study of African-American women.
Breast Cancer Res. 20(45)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pordzik J, Jakubik D, Jarosz-Popek J,
Wicik Z, Eyileten C, De Rosa S, Indolfi C, Siller-Matula JM, Czajka
P and Postula M: Significance of circulating microRNAs in diabetes
mellitus type 2 and platelet reactivity: Bioinformatic analysis and
review. Cardiovasc Diabetol. 18(113)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhou SS, Jin JP, Wang JQ, Zhang ZG,
Freedman JH, Zheng Y and Cai L: miRNAS in cardiovascular diseases:
Potential biomarkers, therapeutic targets and challenges. Acta
Pharmacol Sin. 39:1073–1084. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther.
1(15004)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gholami M, Asgarbeik S, Razi F, Esfahani
EN, Zoughi M, Vahidi A, Larijani B and Amoli MM: Association of
microRNA gene polymorphisms with type 2 diabetes mellitus: A
systematic review and meta-analysis. J Res Med Sci.
25(56)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ghafouri-Fard S, Gholipour M and Taheri M:
Role of MicroRNAs in the pathogenesis of coronary artery disease.
Front Cardiovasc Med. 8(632392)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sung JH, Kim SH, Yang WI, Kim WJ, Moon JY,
Kim IJ, Cha DH, Cho SY, Kim JO, Kim KA, et al: miRNA polymorphisms
(miR-146a, miR-149, miR-196a2 and miR-499) are associated with the
risk of coronary artery disease. Mol Med Rep. 14:2328–2342.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ellwanger JH, Zambra FMB, Guimarães RL and
Chies JA: MicroRNA-related polymorphisms in infectious
diseases-tiny changes with a huge impact on viral infections and
potential clinical applications. Front Immunol.
9(1316)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Drury RE, O'Connor D and Pollard AJ: The
clinical application of MicroRNAs in infectious disease. Front
Immunol. 8(1182)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen C, Zhang Y, Zhang L, Weakley SM and
Yao Q: MicroRNA-196: Critical roles and clinical applications in
development and cancer. J Cell Mol Med. 15:14–23. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gupta P, Cairns MJ and Saksena NK:
Regulation of gene expression by microRNA in HCV infection and
HCV-mediated hepatocellular carcinoma. Virol J.
11(64)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hou W, Tian Q, Zheng J and Bonkovsky HL:
MicroRNA-196 represses bach1 protein and hepatitis C virus gene
expression in human hepatoma cells expressing hepatitis C viral
proteins. Hepatology. 51:1494–1504. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tian T, Wang M, Zhu W, Dai ZM, Lin S, Yang
PT, Liu XH, Liu K, Zhu YY, Zheng Y, et al: MiR-146a and miR-196a-2
polymorphisms are associated with hepatitis virus-related
hepatocellular cancer risk: a meta-analysis. Aging (Albany NY).
9:381–392. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim WR, Park EG, Kang KW, Lee SM, Kim B
and Kim HS: Expression analyses of MicroRNAs in hamster lung
tissues infected by SARS-CoV-2. Mol Cells. 43:953–963.
2020.PubMed/NCBI View Article : Google Scholar
|
















