Introduction
Cochlear hair cells, as the mechanoreceptors of the
inner ear, are essential to auditory and vestibular function, the
loss of which ultimately leads to permanent sensory deficits in
mammals (1). Hearing loss, a very
frequent sensory disorder in humans, is mainly attributable to
cochlear hair cell damage caused by hazardous factors containing
ototoxic pharmaceutical agents, excessive noise, aging and genetic
disorders. Oxidative stress and high levels of reactive oxygen
species (ROS) have an involvement with drug- and noise-induced, and
age-related hearing injury, while cisplatin, aminoglycosides and
continuous noise can result in high level of ROS production in
cochlear hair cells, thereby inducing cell apoptosis (2). Cochlear hair cells do not
spontaneously regenerate following loss or damage, due to the
limitation of regenerative capacity of vestibular organs (3). Therefore, it will be of great
significance to fathom out the pathways and molecular regulators
involved in the pathogenesis of ROS-related hair cell cytotoxicity
for the advancement of therapies toward functional restoration.
MicroRNAs (miRNAs/miRs), an important class of small
non-coding RNAs, bind to target mRNAs and subsequently inhibit
protein expression through mRNA degradation or translational
inhibition (4). miRNAs play
pivotal roles in various important biological processes as well as
in the development and progression of various human diseases, where
one miRNA can exert impacts on multiple target genes, and multiple
miRNAs in turn can also synergistically act on one target gene
(5,6). Several research studies have
manifested that miR-122-5p is associated with many diseases,
especially tumors or cancers, including colorectal cancer,
melanoma, gastric cancer, lung cancer and cervical cancer (7-12).
In addition, plasma miRNA-122-5p has been identified as a potential
biomarker for liver injury among chronic hepatitis B (CHB) patients
with persistently normal alanine aminotransferase (PNALT) levels
(13), and transient ischemic
attack in rats (14). Zhou et
al (15) demonstrated that
miRNA-122-5p promotes the proliferation and DNA synthesis and
represses the early apoptosis of human spermatogonial stem cells
via targeting CBL and competing with lncRNA CASC7. Peng et
al (16) reported that lncRNA
XIST relieves hypoxia-induced injury in H9c2 cardiomyocytes via
targeting the miR-122-5p/FOXP2 axis. Furthermore, many miRNAs also
play vital roles in the development of cochlea inner ear hair cells
and may be pivotal regulators in the process of hearing loss
(17-21).
Wang et al (2) demonstrated
that tert-butyl hydroperoxide (t-BHP) promotes the production of
ROS, and miR-122-5p expression was significantly downregulated in
House Ear Institute-Organ of Corti 1 (HEI-OC1) cells. miR-122-5p
was found to inhibit cell apoptosis and facilitate tumor
progression by directly targeting forkhead box O3 (FOXO3) in
α-fetoprotein (AFP)-producing gastric cancer (AFPGC) (7). Moreover, FOXO3 expression was found
to be increased in the inner ear hair cells during cisplatin
treatment in vitro (22).
Therefore, we hypothesized that miR-122-5p can
directly target FOXO3 to regulate the viability and apoptosis of
cochlear hair cells under oxidative stress condition. The oxidative
damage model was established in HEI-OC1 cells to elucidate the role
and mechanism of miR-122-5p, hoping to provide more treatment
options for hearing disorders. Our present study demonstrated that
miR-122-5p overexpression attenuated the
H2O2-induced damage in mouse cochlear hair
cells by directly regulating FOXO3.
Materials and methods
Cell culture
The House Ear Institute-Organ of Corti 1 (HEI-OC1)
cell line was obtained from the Medical Experimental Center of
Guangzhou Red Cross Hospital (China). High-glucose Dulbecco's
modified Eagle's medium (DMEM) (30-2002, American Type Culture
Collection, Beijing, China) supplemented with 10% fetal bovine
serum (FBS; C0257, Beyotime Institute of Biotechnology) was used to
culture the HEI-OC1 cells at 33˚C in a humidified incubator with 5%
CO2.
Cell transfection
The miR-122-5p mimic (M;
5'-UGGAGUGACAAUGGUGUUUG-3'), mimic control (MC;
5'-UUCUCCGAACGUGUCACGUTT-3') and FOXO3 lentivirus were obtained by
transfection of 293T cells (ab266546, Abcam) with pPACKH1
Lentivector Packaging Kit (US SBI Co.). The FOXO3 overexpression
vector was constructed by cloning the cDNA of mouse FOXO3 into the
pPACKH1 lentivector. The HEI-OC1 cells were placed into a 24-well
plate at the density of 1x105 cells/well. The density of
the cells during lentiviral transfection was ~2x105
cells/well. The next day, the original medium was replaced by 2 ml
of fresh medium containing 6 µg/ml polybrene, followed by the
addition of an appropriate amount of the viral suspension.
Subsequently, the membrane was incubated at 37˚C. After 4 h,
another 2 ml of fresh medium was supplemented to dilute the
polybrene. Following continuous culture for 24 h, the
virus-containing medium was substituted with fresh medium, which
was then used to continuously culture the cells. Four days later,
the infected cells were collected by trypsinization and replated on
a new 100-mm dish. While cell confluence reached about 30%, the
culture medium was replaced by fresh medium containing 1 µg/µl
puromycin for colony selection of stable transfected cells. The
medium was changed every 3 day, and colonies were chosen and
expanded for subsequent experiments after section. A blank vector
lentivirus was used as a negative control (NC).
Oxidative stress exposure and
grouping
HEI-OC1 cells were exposed to 50 µM hydrogen
peroxide (H2O2) for 1 h post transfection to
mimic oxidative stress condition, as previously reported (10). To unveil the effect of miR-122-5p
in HEI-OC1 cells under oxidative stress, HEI-OC1 cells were
assigned into four groups: Blank, Model, Model+MC and Model+M
groups. Similarly, in order to explore the roles of miR-122-5p and
FOXO3 in HEI-OC1 cells under oxidative stress, HEI-OC1 cells were
divided into seven groups: Blank, Model, Model+MC, Model+M,
Model+NC, Model+FOXO3 and Model+M+FOXO3 groups. The specific
treatment of cells in the various groups was shown as follows. In
the Blank group, HEI-OC1 cells were only incubated with medium; in
the Model group, HEI-OC1 cells were exposed to 50 µM
H2O2 for 1 h; in the Model+MC group, HEI-OC1
cells were exposed to 50 µM H2O2 for 1 h and
then transfected with the mimic control; in the Model+M group,
HEI-OC1 cells were exposed to 50 µM H2O2 for
1 h and then transfected with miR-122-5p mimic; in the Model+NC
group, HEI-OC1 cells were exposed to 50 µM
H2O2 for 1 h and then transfected with the
blank vector lentivirus; in the Model+FOXO3 group: HEI-OC1 cells
were exposed to 50 µM H2O2 for 1 h and then
transfected with the FOXO3 lentivirus; in the Model+M+FOXO3 group:
HEI-OC1 cells were exposed to 50 µM H2O2 for
1 h and then co-transfected with the miR-122-5p mimic and FOXO3
lentivirus.
Dual luciferase reporter assays
TargetScan V7.2 (www.targetscan.org/vert_72/) was used to explore the
targets of miR-122-5p. HEI-OC1 cells (1x105) were seeded
to a 24-well plate and then incubated overnight. Next, pcDNA3.1
FOXO3-wild-type (WT; 5'-TGAAGGCCTCGGTCACACTCCA-3') or pcDNA3.1
FOXO3-mutant (MUT; 5'-TGCAAGGCCTCGGTCGACAAGTTG-3') and miR-122-5p M
(5'-TGGAGTGTGACAATGGTGTTTG-3'; HMI1002, MilliporeSigma, USA) were
co-transfected into 293T cells (12022001, MilliporeSigma, USA) with
Lipofectamine 2000 (11668019, Thermo Fisher Scientific, Inc.).
Subsequently, 100 µl transfected cells were placed
in a 96-well plate and then added together with 100 µl Dual-Lumi™
firefly luciferase assay reagent or 100 µl Dual-Lumi™
Renilla luciferase assay working solution that was derived
from the dual luciferase reporter gene assay kit (RG088M, Beyotime
Institute of Biotechnology). After being mixed well, the cells were
incubated at room temperature (~25˚C) for 10 min. Finally, cell
luciferase was determined with the dual luciferase reporter assay
system (Promega Corp.). In this research, dual luciferase reporter
assays were performed three times.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
After being exposed to 50 µM
H2O2 for 1 h post transfection, HEI-OC1 cells
were collected for RNA extraction using the RNAeasy kit (R0027;
Beyotime Institute of Biotechnology) and the miRNA was extracted by
the RNAeasy kit (R0028; Beyotime Institute of Biotechnology). RNA
was detected using a UV spectrophotometer (DR6000; Hash) and then
reversed by the reverse transcription kit (D7168L; Beyotime
Institute of Biotechnology) for cDNA synthesis. Finally, cDNA, as a
template, was amplified using a real-time fluorescence quantitative
PCR instrument (ABI 7500; Thermo Fisher Scientific, Inc.). The
conditions of amplification are listed as follows: pre-denaturation
at 95˚C for 10 sec, followed by 30 cycles of denaturation at 95˚C
for 5 sec and 60˚C for 25 sec, and an elongation at 70˚C for 30
min. The forward and reverse primers for the miR-122-5p sequence,
according to Primer3Plus (http://www.primer3plus.com/cgi-bin/dev/primer3plus.cgi),
were 5'-TGTGACAATGGTGTTTGGTCG-3' and 5'-TGTCGTGGAGTCGGCAATTG-3';
FOXO3 forward primer was 5'-TCACGCACCAATTCTAACGC-3', and the
universal primer was 5'-CACGGCTTGCTTACTGAAGG-3'. U6 (forward primer
5'-CTCGCTTCGGCAGCACA-3' and reverse primer
5'-AACGCTTCACGAATTTGCGT-3') was used as the reference gene, and the
2-ΔΔCq method was utilized to calculate the expression
level (23).
Western blot (WB) analysis
Cells (1x106-1x107) were taken
from each group as samples, and then washed with phosphate-buffered
saline (PBS; C0221A; Beyotime Institute of Biotechnology). Next,
the cell samples were added with 0.5 ml total protein extraction
reagent to extract the total protein. Based on the instructions of
the total protein extraction kit (W034-1-1; Nanjing Jiancheng
Bioengineering Institute, http://www.njjcbio.com), the protein concentrations
were determined. Subsequently, the proteins (20 µg) were loaded and
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). After the proteins were transferred to
the nitrocellulose membrane and polyvinylidene fluoride (PVDF)
membrane, the membranes were cultured in blocking solution (P0023B,
100 ml; Beyotime Institute of Biotechnology) at room temperature
for 1 h. After that, anti-Bax (SAB3500343, 23 kDa, 1:1,000;
Sigma-Adrich; Merck KGaA), anti-caspase 3 (ab49822, 17 kDa,
1:1,000, Abcam), Bcl-2 (ab182858, 26 kDa, 1:800, Abcam),
anti-caspase-9 (SAB4503334, 46 kDa, 1:1,000, Sigma-Adrich; Merck
KGaA) and anti-FOXO3 (ab23683, 90 kDa, 1:500, Abcam) antibodies
were added to the membranes followed by incubation for 1 h. Later,
the membranes were removed and washed three times with
Tris-buffered saline Tween (TBST; P0231; Beyotime Institute of
Biotechnology) at room temperature. To bind the primary antibody,
the membranes were supplemented with horseradish peroxidase
(HRP)-labeled secondary antibody (A0201, Beyotime Institute of
Biotechnology) and then incubated at room temperature for 1 h.
Finally, the membranes were rinsed with TBST (P0231; Beyotime
Institute of Biotechnology) again. Finally, bands were visualized
using enhanced chemiluminescence (ECL), and then quantified with
Image Lab 4.1 software (Bio-Rad Laboratories, Inc.). During this
process, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
utilized as an internal reference. The raw data of all western blot
analyses have been provided in the supplementary materials.
Cell viability assay
After being transfected, cells (2x103
cells/well) were seeded into a 96-well plate and maintained at 37˚C
in 100 µl of culture medium. After transfection for 24, 48, 72 and
96 h, the cells in each well were supplemented with 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide
(MTT, 5 mg/ml, 30 µl; M2128; Sigma-Adrich; Merck KGaA). Then, after
removing the medium, 100 µl of dimethyl sulfoxide (DMSO; D2650;
Sigma-Adrich; Merck KGaA) was added to solubilize the crystals, and
the absorbance was measured at 450 nm. The cell viability assay was
independently performed at least three times.
Flow cytometry
Firstly, the cells were resuspended in 400 µl of
binding buffer (1X) at a concentration of 1x106
cells/ml. After the addition of 5 µl Annexin V-FITC (APOAF-20TST;
Sigma-Adrich; Merck KGaA), the cells were cultivated at room
temperature for 15 min in the dark, followed by continuous
incubation with 10 µl of propidium iodide (PI; P4170; Sigma-Adrich;
Merck KGaA) for 5 min. Finally, the fluorescence intensity of cells
in each group was measured by flow cytometry (Fortessa X-20;
Bio-Rad Laboratories, Inc. USA).
Enzyme-linked immunosorbent assay
(ELISA)
Diluted cell samples (100 µl) were transferred to a
96-well plate and incubated at room temperature for 2.5 h. After
being rinsed 4 times with 1X washing buffer, each well of the plate
was added together with 100 µl of prepared biotin conjugate,
followed by 1 h of incubation at room temperature with gentle
shaking. After rinsing 4 times with 1X washing buffer, the plate
was supplemented with 100 µl of the prepared streptavidin-HRP
solution, and incubated at room temperature for 45 min with gentle
shaking. Following that, the solution was discarded, the plate was
rinsed with 1X washing buffer for another 4 times, and each well
was added with 100 µl of TMB substrate. Subsequently, the plate was
incubated at room temperature for 30 min in the dark with gentle
shaking. Afterwards, 50 µl of stop solution was placed into each
well, and the side of the plate was tapped to mix the solution
well. Finally, 200 µl of supernatant was collected and then added
to the 96-well plate, subsequent to which the absorbance was
measured at 532 nm using a microplate reader (Z742711-1EA;
Sigma-Adrich; Merck KGaA). Lipid peroxidation assay kit (MDA;
A0031-2) and superoxide dismutase assay kit (SOD; A001-3-2) applied
in the whole processes were purchased from Nanjing Jiancheng
Bioengineering Institute.
Confocal laser scanning microscopy
analysis
After exposure to 50 µM H2O2
for 1 h, the HEI-OC1 cells were cultured using the ROS Assay Kit
(S0033S; Beyotime Institute of Biotechnology), added together with
an appropriate volume of diluted 2',7'-dichlorodihydrofluorescein
diacetate (DCFH-DA; 1:1,000), and then inoculated to a 6-well
plate. Then cells were separately dyed by Mito-SOX Red (C1049-50
µg; Beyotime Institute of Biotechnology) staining with a final
concentration of 4 µmol/l at 37˚C for 10 min in the dark or
CM-H2DCFDA (S0033S; Beyotime Institute of Biotechnology)
staining at a final concentration of 5 µmol/l at 37˚C for 30 min in
the dark. After washing three times with PBS (C0221A; Beyotime
Institute of Biotechnology), the cells were captured by laser
confocal microscope (LSM800; Zeiss) at different excitation
wavelengths (510 nm/488 nm) and different emission wavelengths
(580/515 nm), and fluorescence images were captured.
Flow cytometry to detect the
mitochondrial membrane potential level
The mitochondrial membrane potential level was
measured with the mitochondrial membrane potential assay kit (JC-1;
C2006; Beyotime Institute of Biotechnology). Concretely, the cells
were collected and then seeded to each cell of a 6-well plate, with
the culture medium aspirated. Following being washed once with PBS
(C0221A; Beyotime Institute of Biotechnology), the cells were added
together with 1 ml of fresh cell culture medium which contained
serum and phenol red. Subsequently, 1 ml JC-1 staining working
solution was added and mixed well to cultivate the cells in an
incubator at 37˚C for 20 min. During the incubation period, an
appropriate amount of JC-1 staining buffer (1X) was prepared
according to the ratio of 4 ml distilled water per 1 ml JC-1
staining buffer (5X), and placed in an ice bath. After incubation
at 37˚C, the supernatant was aspirated and the cells were washed
twice with JC-1 staining buffer (1X). Finally, the cells were
supplemented with 2 ml of cell culture medium containing serum and
phenol red and observed using a flow cytometer (Fortessa X-20;
Bio-Rad Laboratories, Inc.).
Statistical analyses
SPSS version 17.0 (SPSS Inc.) was adopted to analyze
the statistical data from this research, and these data are
represented as the mean ± standard deviation (SD). The Student's
t-test or one way analysis of variance (one-way ANOVA) with post
hoc Tukey test was utilized to gauge the results for two or
multiple groups. P<0.05 was considered as indicative of a
statistically significant difference.
Results
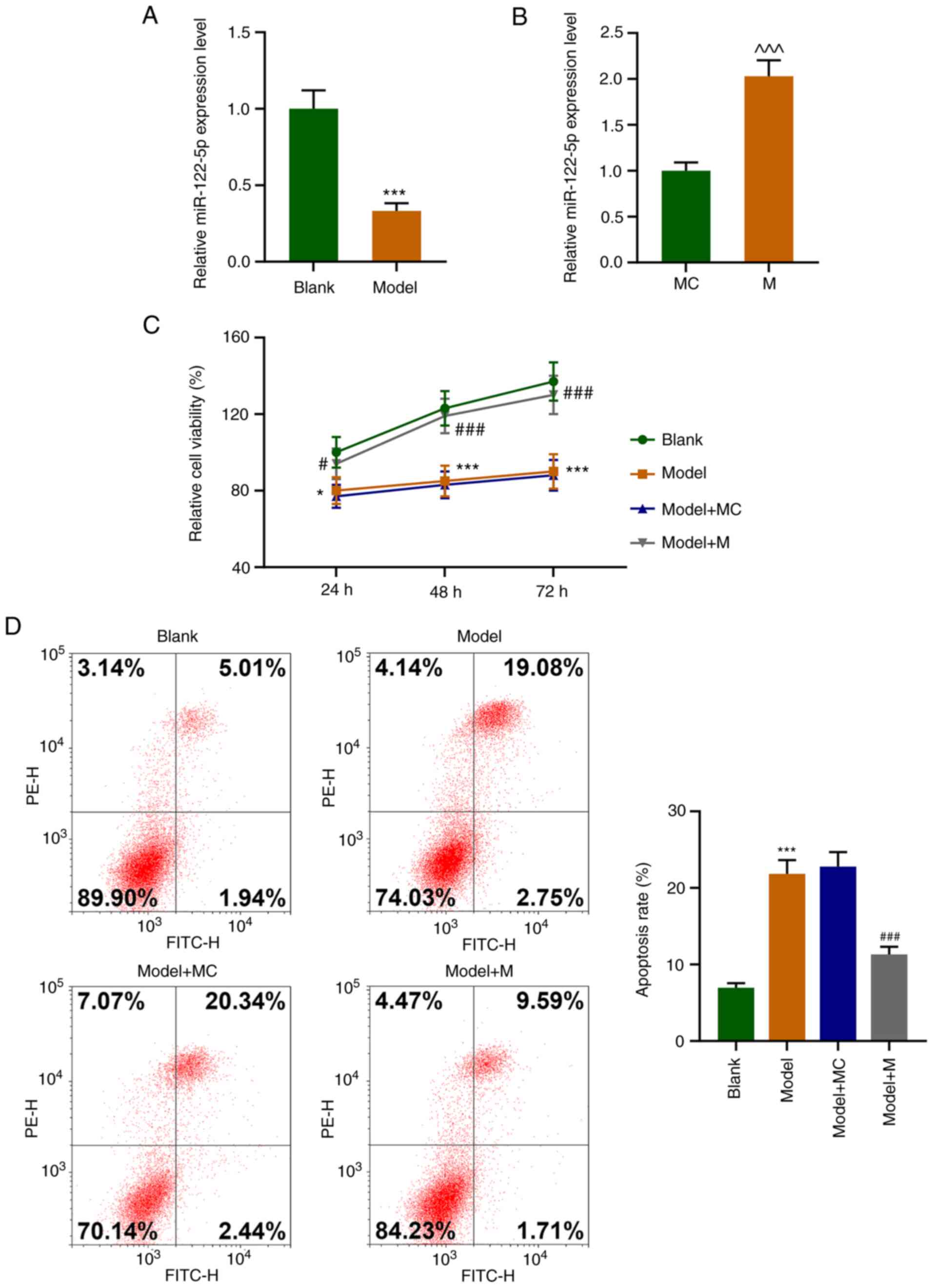
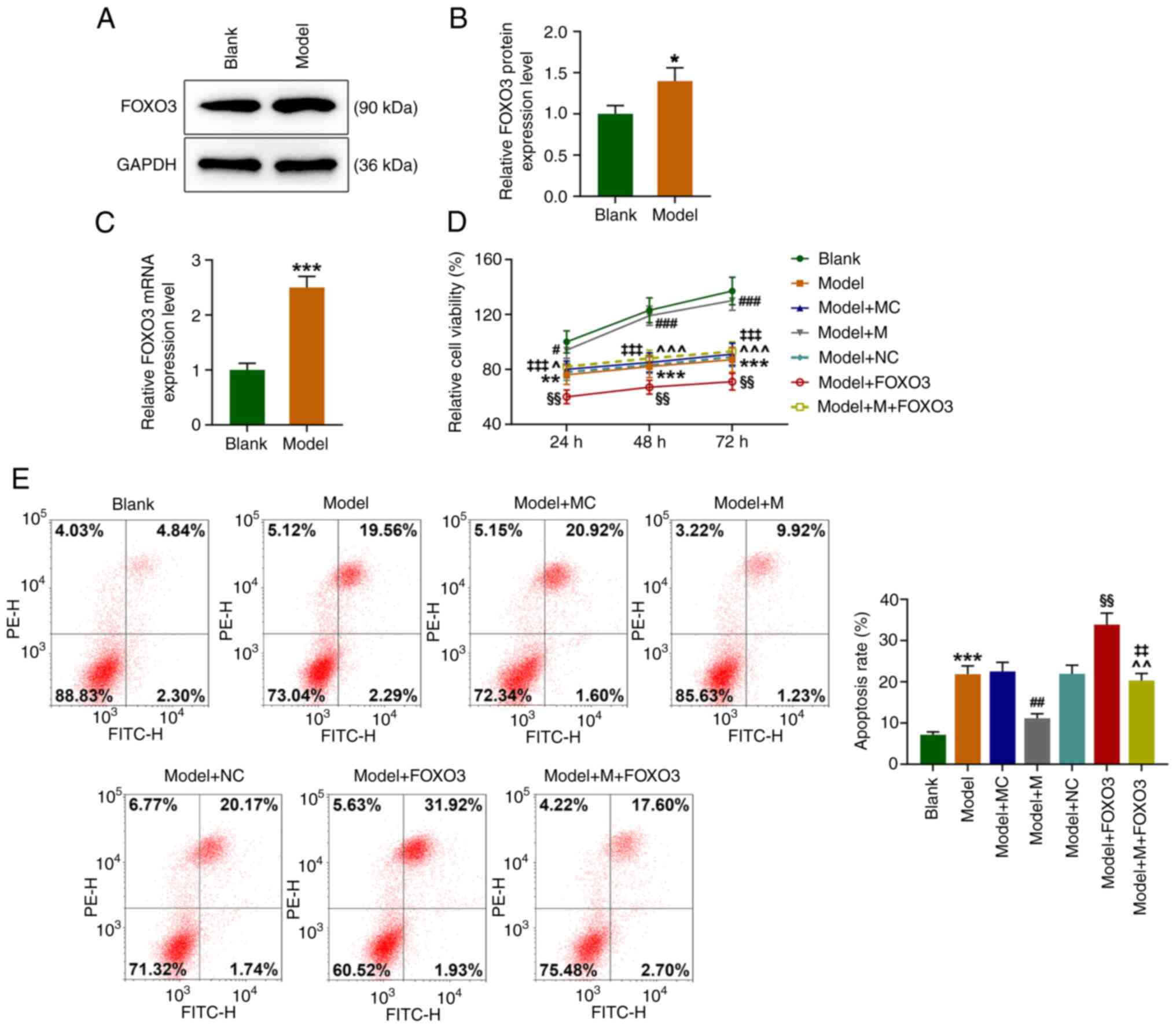
miR-122-5p promotes the viability but
inhibits the apoptosis of H2O2-induced
HEI-OC1 cells
HEI-OC1 cells were exposed to 50 µM
H2O2 for 1 h (Model group), and subsequently,
the expression level of miR-122-5p was observed to be significantly
decreased relative to that of the Blank group (Fig. 1A, P<0.001). As shown in Fig. 1B, miR-122-5p mimic (M) transfection
significantly increased the level of miR-122-5p in the HEI-OC1
cells (P<0.001). Compared with that of the Blank group, the cell
viability of the Model group showed a significant decrease at 24,
48 and 72 h (Fig. 1C, P<0.05).
In comparison with that of the Model+mimic control (MC) group, the
cell viability of the Model+M group was significantly elevated at
24, 48 and 72 h (Fig. 1C,
P<0.05). Additionally, the Model group exhibited a significant
increase in the apoptosis rate as compared with Blank group, while
the Model+M group had a significantly lower apoptosis rate than the
Model+MC group (Fig. 1D,
P<0.001). These findings indicated that oxidative stress reduced
the cell viability but promoted cell apoptosis. Moreover, high
expression of miR-122-5p reversed the effects of the oxidative
stress on the viability and apoptosis of the HEI-OC1 cells.
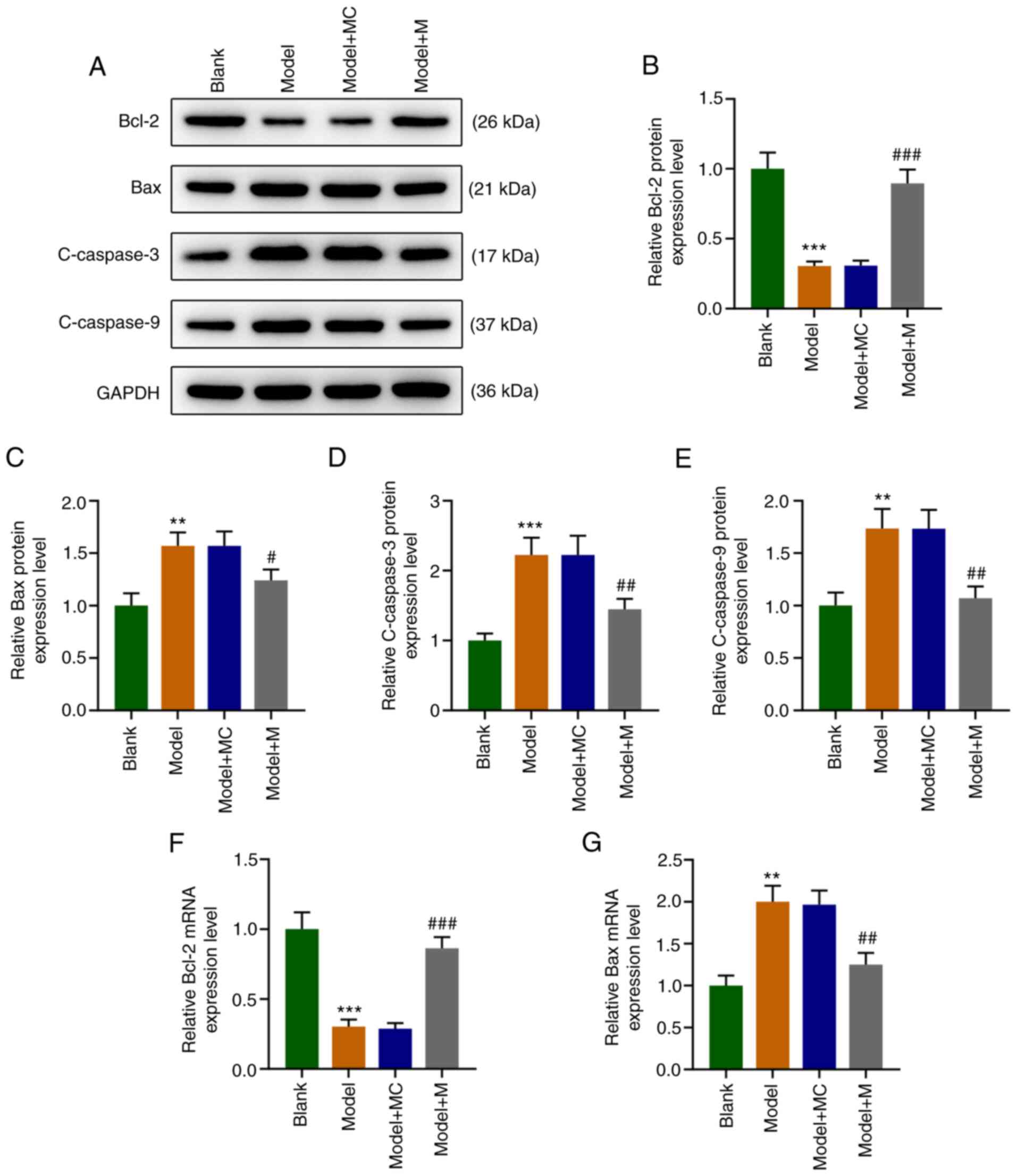
High expression of miR-122-5p
partially offsets the effect of H2O2 on the
expression levels of apoptosis-related proteins in HEI-OC1
cells
The results revealed that compared with the Blank
group, the protein expression levels of Bax, cleaved (C)-caspase-3,
and C-caspase-9 in the Model group were significantly upregulated
(Fig. 2A, C-E, P<0.01) as well as the RNA level
of Bax (Fig. 2G, P<0.01), while
that of Bcl-2 was downregulated at both the protein (Fig. 2A and B, P<0.001) and RNA level (Fig. 2F, P<0.001). Compared with the
Model+MC group, the expression levels of Bax, C-caspase-3, and
C-caspase-9 were significantly downregulated (Fig. 2A, C-E and G, P<0.05), while the Bcl-2 level was
significantly upregulated in the Model+M group (Fig. 2A and B, P<0.001) and also at the RNA level
(Fig. 2F, P<0.001). These
findings demonstrated that oxidative stress regulated the
expression levels of apoptosis-related proteins (Bax, C-caspase-3,
C-caspase-9 and Bcl-2), and high expression of miR-122-5p can
partially counteract the trend.
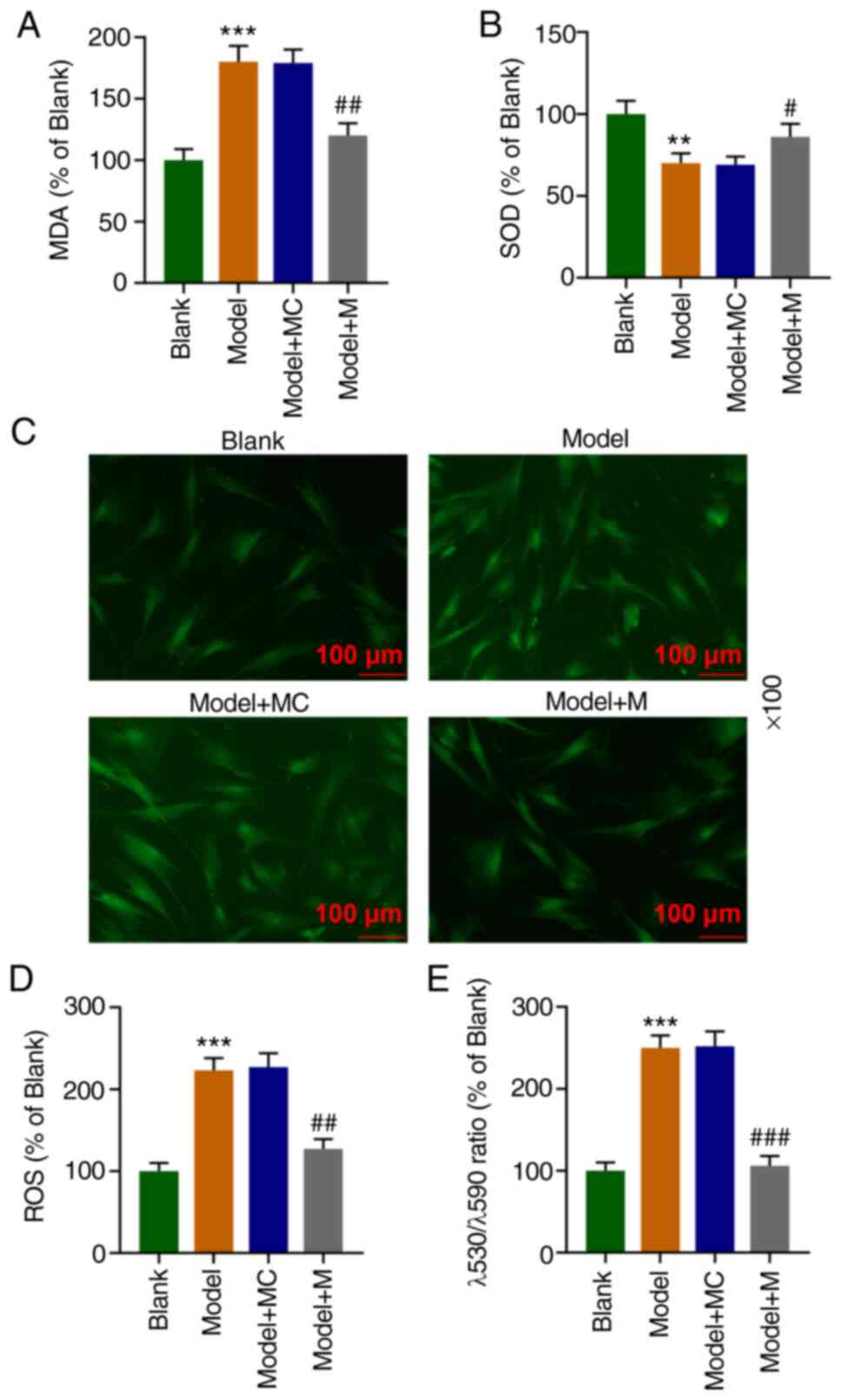
High expression of miR-122-5p reduces
the levels of ROS and MDA and mitochondrial depolarization, but
increases the SOD level under H2O2
condition
The MDA content was significantly elevated (Fig. 3A, P<0.001) while the SOD content
was significantly reduced in the Model group (Fig. 3B, P<0.01) as compared with these
findings in the Blank group. However, the MDA content was
significantly decreased (Fig. 3A,
P<0.01) while the SOD content was significantly increased in the
Model+M group (Fig. 3B,
P<0.05), relative to the Model+MC group. According to DCFH-DA
fluorescence detection of the ROS level in the HEI-OC1 cells,
compared with the Blank group, the ROS level was significantly
increased in the Model group (Fig.
3C and D, P<0.001); while
compared to the Model + MC group, the ROS level was significantly
reduced in the Model+M group (Fig.
3C and D, P<0.01).
Mitochondrial membrane potential analysis indicated that the Model
group had an significantly increased λ530/λ590 ratio relative to
Blank group (P<0.001), while the Model+M group exhibited a
statistically decreased λ530/λ590 ratio (P<0.001), when compared
with that of the Model+MC group (Fig.
3E). The results above suggest that the high expression of
miR-122-5p can partially reverse the oxidative damage of HEI-OC1
cells.
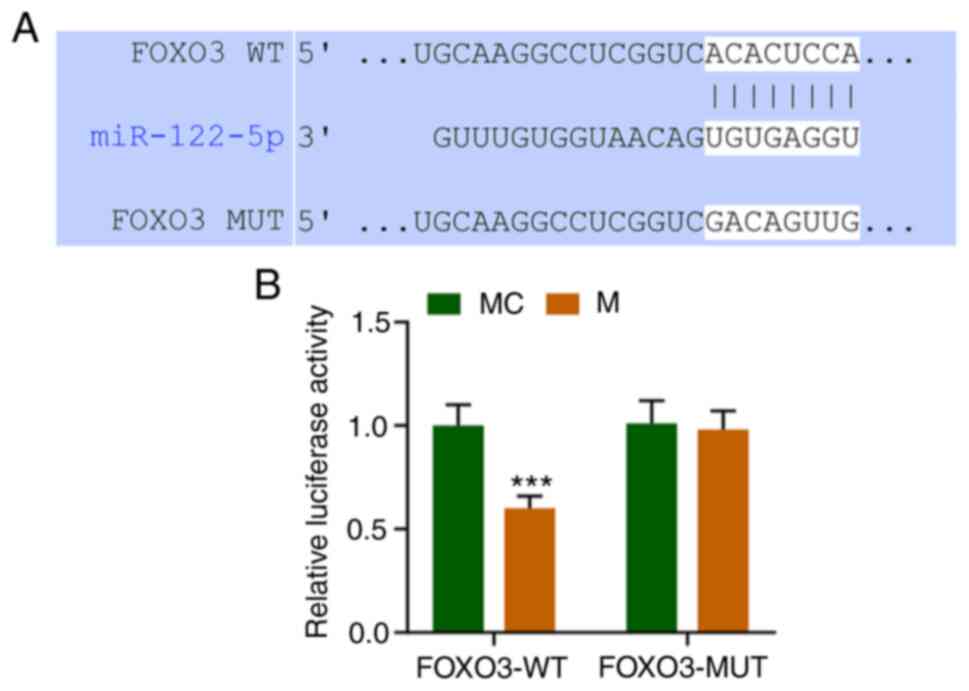
miR-122-5p directly targets FOXO3
TargetScan V7.2 (www.targetscan.org/vert_72/) predicted that miR-122-5p
targets FOXO3 (Fig. 4A). The dual
luciferase results showed that FOXO3-WT (wild-type) decreased
luciferase activity in the M group compared to MC group (Fig. 4B, P<0.001), while FOXO3-MUT
(mutated) had no significant changes. This demonstrated that
miR-122-5p can directly target FOXO the 3'UTR (untranslated region)
sequences (Fig. 4).
FOXO3 overexpression reverses the
effects of miR-122-5p mimic on viability and apoptosis of
H2O2-induced HEI-OC1 cells
WB and RT-qPCR assays demonstrated that the FOXO3
expression level was significantly increased in the Model group
(Fig. 5A-C, P<0.05) after
HEI-OC1 cells were treated with H2O2,
compared with that in the Blank group. HEI-OC1 cell viability was
determined by MTT assay, as depicted in Fig. 5D. At 24, 48, and 72 h after HEI-OC1
cells were transfected, the cell viability in the Model group was
significantly lower than that of the Blank group (P<0.05); the
cell viability in the Model+M group was significantly increased as
compared with Model+MC group (P<0.001); the cell viability in
the Model+FOXO3 group was significantly decreased compared with the
Model+NC group (P<0.01), which was offset by miR-122-5p mimic
(P<0.01). Flow cytometry was utilized to evaluate the apoptosis
rate, as delineated in Fig. 5E. In
the Model group, there was a significant increase in the apoptosis
rate in comparison with the Blank group (P<0.001). In addition,
the Model+M group exhibited a significant decrease in the apoptosis
rate (P<0.01) as compared with Model+MC group. And compared with
the Model+NC group, the apoptosis rate of cells in the Model+FOXO3
group was significantly increased (P<0.01). Moreover, the
apoptosis rate of cells of the Model+M+FOXO3 group was notably
lower than that in the Model+FOXO3 group (P<0.01). Moreover, in
comparison with the Model+M group, the apoptosis rate of cells in
the Model+M+FOXO3 group was dramatically elevated (P<0.01).
These above-mentioned findings indicated that FOXO3 overexpression
overturned the effects of miR-122-5p mimic on viability and
apoptosis of H2O2-induced HEI-OC1 cells.
FOXO3 overexpression reverses the
effect of the miR-122-5p mimic on the expression levels of
apoptosis-related molecules in H2O2-induced
HEI-OC1 cells
Compared to the Blank group, the level of Bcl-2 was
significantly decreased [at both the protein (Fig. 6A and B) and mRNA level (Fig. 6F), P<0.001], while the protein
levels of Bax (and at the mRNA level as shown in Fig. 6G, P<0.01), C-caspase-3, and
C-caspase-9 were significantly elevated in the Model group
(Fig. 6A, C-E, P<0.001). As compared with
Model+MC group, the expression levels of Bax (at the protein and
mRNA levels), C-caspase-3 and C-caspase-9 in the Model+M group were
significantly downregulated (Fig.
6A, C-E and G, P<0.01), whereas that of Bcl-2 was
significantly upregulated [at the protein level as shown in
Fig. A and B, P<0.001; at the mRNA level as shown
in Fig. 6F, P<0.001). Relative
to the Model+NC group, the level of Bcl-2 (at the protein level as
shown in Fig. 6A and B, P<0.05; at the mRNA level as shown
in Fig. 6F, P<0.05) was
significantly decreased, while levels of Bax (at both the protein
and mRNA levels), C-caspase-3, C-caspase-9 were significantly
elevated in the Model+FOXO3 group (Fig. 6A, C-E and G, P<0.01). In contrast with the
Model+FOXO3 group, the level of Bcl-2 was increased (both at the
protein as shown in Fig. A and
B; and the mRNA level as shown in
Fig. 6F, P<0.05), while levels
of Bax (at both the protein and mRNA level), C-caspase-3, and
C-caspase-9 were significantly decreased in the Model+M+FOXO3 group
(Fig. 6A, C-E and G, P<0.01). These data above indicated
that FOXO3 overexpression counteracted the effects of miR-122-5p
mimic on the expression levels of apoptosis-related proteins in
H2O2-induced HEI-OC1 cells.
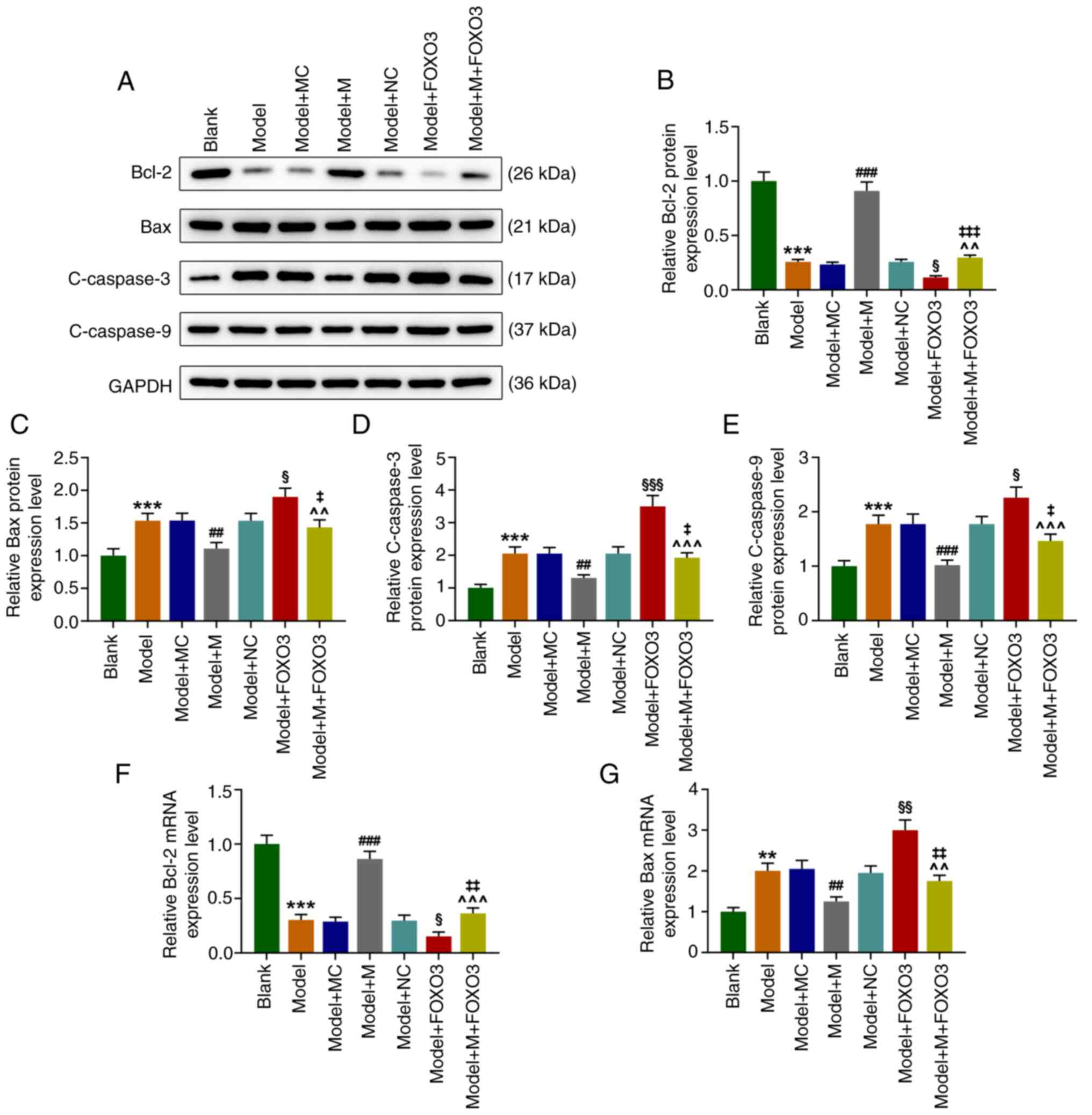 | Figure 6FOXO3 overexpression reverses the
effect of miR-122-5p mimic on the expression levels of
apoptosis-related proteins in H2O2-induced
HEI-OC1 cells. (A) HEI-OC1 cells were transfected with miR-122-5p
mimic (M), mimic control (MC), FOXO3 overexpression vector or
negative control (NC), and the expression levels of Bcl-2, Bax,
cleaved-caspase-3 and C-caspase-9 were detected by western blot
(WB) analysis in HEI-OC1 cells after oxidative damage of
H2O2. (B) The protein expression level of
Bcl-2 was quantified by WB analysis in HEI-OC1 cells after
oxidative damage of H2O2. (C) The protein
expression level of Bax was measured by WB analysis in HEI-OC1
cells after oxidative damage of H2O2. (D) The
protein expression level of C-caspase-3 in
H2O2-induced HEI-OC1 cells was tested by WB
analysis. (E) The expression level of C-caspase-9 in
H2O2-induced HEI-OC1 cells was assessed by WB
analysis. (F) The mRNA expression level of Bcl-2 in
H2O2-induced HEI-OC1 cells was quantified by
RT-qPCR. (G) The mRNA expression level of Bax in
H2O2-induced HEI-OC1 cells was determined by
RT-qPCR. **P<0.01 and ***P<0.001 vs.
the Blank group; ##P<0.01 and
###P<0.001 vs. the Model+MC group;
^^P<0.01 and ^^^P<0.001 vs. the Model+M
group; §P<0.05, §§P<0.01 and and
§§§P<0.001 vs. the Model+NC group;
‡P<0.05, ‡‡P<0.01 and
‡‡‡P<0.001 vs. the Model + FOXO3. Model, cells
exposed to 50 µM H2O2 for 1 h; M, miR-122-5p
mimic; MC, mimic control; NC, negative control; FOXO3, forkhead box
O3 overexpression vector; C-, cleaved. |
FOXO3 overexpression overturns the
effect of miR-122-5p mimic on the levels of ROS, MDA, and SOD, and
mitochondrial membrane depolarization in
H2O2-induced HEI-OC1 cells
As compared with the Model+NC group, the levels of
MDA (Fig. 7A), ROS (Fig. 7D) and λ530/λ590 ratio (Fig. 7E) were significantly increased in
the Model+FOXO3 group (P<0.05), while the level of SOD (Fig. 7B) was significantly depleted
(Fig. 7B, P<0.05). In
comparison with the Model+FOXO3 group, the levels of MDA, ROS,
together with λ530/λ590 ratio were significantly reduced in the
Model+M+FOXO3 group (P<0.01), whereas the level of SOD was
significantly elevated (Fig. 7A-D,
P<0.01). These data suggested that FOXO3 overexpression
neutralized the effects of miR-122-5p mimic on the levels of ROS,
MDA, and SOD, and mitochondrial depolarization in
H2O2-induced HEI-OC1 cells.
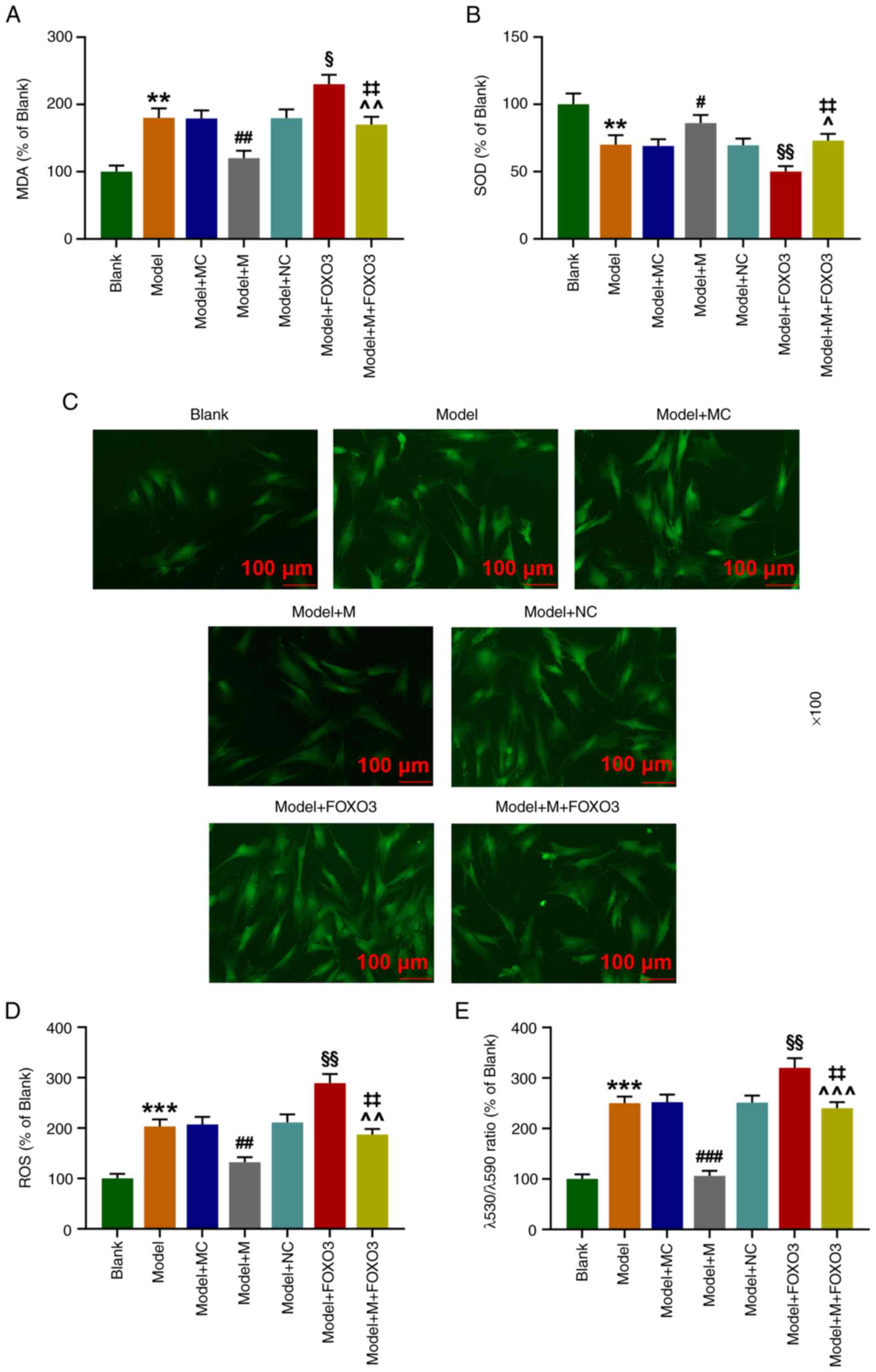 | Figure 7FOXO3 overexpression reverses the
effects of miR-122-5p mimic on the levels of ROS, MDA, and SOD, and
mitochondrial depolarization in H2O2-induced
HEI-OC1 cells. (A) The content of MDA in transfected HEI-OC1 cells
after oxidative damage of H2O2 was assessed
by ELISA. (B) The content of SOD in transfected HEI-OC1 cells after
oxidative damage of H2O2 was evaluated by
ELISA. (C and D) ROS content of transfected HEI-OC1 cells after
oxidative damage of H2O2 was detected by
laser scanning confocal microscopy. (E) Cell membrane potential
level of transfected HEI-OC1 cells after oxidative damage of
H2O2 was determined by flow cytometry.
**P<0.01 and ***P<0.001 vs. the Blank
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. the Model+MC group;
^P<0.05, ^^P<0.01 and
^^^P<0.001 vs. the Model+M group;
§P<0.05 and §§P<0.01 vs. the Model+NC
group; ‡‡P<0.01 vs. the Model + FOXO3. Model, cells
exposed to 50 µM H2O2 for 1 h; M, miR-122-5p
mimic; MC, mimic control; NC, negative control; FOXO3, forkhead box
O3 overexpression vector. |
Discussion
In order to elucidate the regulatory mechanism of
miR-122-5p on cochlear hair cells under oxidative stress, we
established an oxidative stress model by exposing HEI-OC1 cells to
50 µM H2O2 for 1 h. The results of the
present study were utilized to analyze the underlying role of
miR-122-5p as a potential target for hearing loss treatment.
Under oxidative stress, reactive oxygen species
(ROS) is a well-documented factor in noise-induced hearing loss. In
several prior research studies, noise was found to activate AMPKα
in outer hair cells (OHCs) through formation of ROS, and noise
exposure-induced OHC death was mediated by a ROS/AMPKα-dependent
pathway (24-26).
High level of ROS is associated with hearing loss and hair cell
death (2,27-29),
which can cause changes in the expression levels of related
proteins (30). Excess ROS
overwhelms the redox balance and skews cell metabolism toward the
activation of intrinsic apoptosis, which are regulated by the
combined actions of pro- and anti-apoptotic members of the Bcl-2
family (31-34).
It has been well established that the anti-apoptotic protein Bcl-2
can prevent the release of cytochrome c and reduce the
activation of caspase-9 and caspase-3, thus inhibiting
caspase-3-dependent apoptosis (30,35).
Thus, the degree of oxidative stress can be identified in cells by
assessing the expression levels of apoptotic proteins. Apoptosis,
MDA production, SOD expression and changes in mitochondrial
membrane potential can all be exploited to assess oxidation
reactions (36,37). The results in this study signified
that under oxidative stress, the cell viability was weakened and
apoptosis was enhanced. With regard to the expression levels of
apoptosis-related proteins, the Bcl-2 level was decreased, while
those of Bax, cleaved (C)-caspase-3, and C-caspase-9 were elevated
in HEI-OC1 cells. Additionally, the levels of ROS, MDA and the
mitochondrial membrane potential were increased, yet the SOD level
was reduced in the HEI-OC1 cells. Seminal miRNA-122 has been
manifested to be negatively correlated with oxidative stress, and
apoptotic markers (Bax, Bcl-2) in infertile men with varicocele
(38). In the present study, the
miR-122-5p level was found to be decreased in
H2O2-induced HEI-OC1 cells, and miR-122-5p
mimic was able to partially offset the effect of
H2O2 on the cell viability and apoptosis,
mitochondrial membrane potential levels, as well as apoptosis- and
oxidative-related molecules in the HEI-OC1 cells. Taken together,
miR-122-5p can attenuate H2O2-induced
oxidative damage in HEI-OC1 cells.
In order to elucidate the possible mechanism of
miR-122-5p on cochlear hair cells under oxidative stress, we gained
access to the TargetScan V7.2 website to predict the targeting
relationship between miR-122-5p and FOXO3. Forkhead box O3 (FOXO3)
belongs to the forkhead box O (FOX) family (FKHRL1) that has a
common structural motif, namely the ‘forkhead box’ or ‘winged
helix’ domain that is responsible for binding to chromatin DNA in
the nucleus of cells (39). FOXO
proteins act as nuclear transcription factors that mediate the
inhibitory action of insulin or insulin-like growth factor (IGF-1)
on key functions in diverse pathways including cell metabolism,
proliferation, differentiation, oxidative stress, cell survival and
senescence, autophagy and aging in mammals (39). FOXO3 has important significance in
the process of oxidative stress. In the process of self-eating due
to oxidative stress, cytoplasmic STAT3 constitutively inhibits
autophagy by sequestering EIF2AK2 as well as by interacting with
other autophagy-related signaling molecules such as FOXO1 and
FOXO3(40). Increasing evidence
demonstrates that multiple miRNAs can also synergistically act on
FOXO3a, thus playing important roles in the development and
progression of various human diseases (41-43).
Additionally, overexpression of miR-182 represses the intrinsic
apoptotic pathway by inhibiting the translation of FOXO3a,
protecting cochlear hair cells from cisplatin-induced apoptosis in
the inner ear (44).
Gentamicin-induced cochlear hair cell ototoxicity, including
oxidative stress and apoptosis, could be attenuated by mouse inner
ear stem cells (IESCs) through the miR-182-5p/FOXO3 axis (45).
In the present study, it was found that the FOXO3
level was increased in H2O2-induced HEI-OC1
cells, and FOXO3 overexpression could further promote the effects
of H2O2 on the viability and apoptosis,
mitochondrial membrane potential levels, as well as apoptosis- and
oxidative-related molecules in HEI-OC1 cells. In addition, the
present findings also indicated that FOXO3 overexpression can
partially offset the effect of high expression of miR-122-5p in
H2O2-induced HEI-OC1 cells. Despite these
achievements, our research still had some shortcomings. Only in
vitro experiments, no in vivo experiments were
conducted. The research also lacked a morphological basis. These
should be explored in the further study. The above results
illustrated that miR-122-5p can attenuate the
H2O2-induced damage in mouse cochlear hair
cells by targeting FOXO3.
In conclusion, the oxidative stress damage of hair
cells caused by H2O2 can be alleviated by
inhibiting the expression of FOXO3 or promoting the expression of
miR-122-5p, providing a new perspective and scientific basis for
the effective treatment of hearing impairment or loss.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
JC made substantial contributions to conception and
design of the study. JQ and JL were responsible for the data
acquisition, data analysis and interpretation and confirm the
authenticity of all the raw data. JJ performed the drafting of the
article and critically revised it for important intellectual
content. All authors read and approved the final manuscript. All
authors agree to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of the
work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Goutman JD, Elgoyhen AB and Gómez-Casati
ME: Cochlear hair cells: The sound-sensing machines. FEBS Lett.
589:3354–3361. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Z, Liu Y, Han N, Chen X, Yu W, Zhang
W and Zou F: Profiles of oxidative stress-related microRNA and mRNA
expression in auditory cells. Brain Res. 1346:14–25.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Burns JC and Stone JS: Development and
regeneration of vestibular hair cells in mammals. Semin Cell Dev
Biol. 65:96–105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vishnoi A and Rani S: MiRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yao Q, Chen Y and Zhou X: The roles of
microRNAs in epigenetic regulation. Curr Opin Chem Biol. 51:11–17.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maruyama S, Furuya S, Shiraishi K, Shimizu
H, Saito R, Akaike H, Hosomura N, Kawaguchi Y, Amemiya H, Kawaida
H, et al: Inhibition of apoptosis by miR-122-5p in
α-fetoprotein-producing gastric cancer. Oncol Rep. 41:2595–2600.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang W, Dong L, Zhao B, Lu J and Zhao Y:
E-cadherin is downregulated by microenvironmental changes in
pancreatic cancer and induces EMT. Oncol Rep. 40:1641–1649.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Byrnes CC, Jia W, Alshamrani AA, Kuppa SS
and Murph MM: miR-122-5p expression and secretion in melanoma cells
is amplified by the LPAR3 SH3-binding domain to regulate Wnt1. Mol
Cancer Res. 17:299–309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou
Y, Huang Q, Chen S, Zhang Z, Xu Y, et al: Activation of
miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell
apoptosis: Implications for age-related hearing loss. Neurobiol
Aging. 36:1692–1701. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Meng L, Chen Z, Jiang Z, Huang T, Hu J,
Luo P, Zhang H, Huang M, Huang L, Chen Y, et al: MiR-122-5p
suppresses the proliferation, migration, and invasion of gastric
cancer cells by targeting LYN. Acta Biochim Biophys Sin (Shanghai).
52:49–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ding FN, Gao BH, Wu X, Gong CW, Wang WQ
and Bio SM: miR-122-5p modulates the radiosensitivity of cervical
cancer cells by regulating cell division cycle 25A (CDC25A). FEBS
Open Bio. 9:1869–1879. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cheng JL, Zhao H, Yang SG, Chen EM, Chen
WQ and Li LJ: Plasma miRNA-122-5p and miRNA-151a-3p identified as
potential biomarkers for liver injury among CHB patients with
PNALT. Hepatol Int. 12:277–287. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li DB, Liu JL, Wang W, Luo XM, Zhou X, Li
JP, Cao XL, Long XH, Chen JG and Qin C: Plasma exosomal
miRNA-122-5p and miR-300-3p as potential markers for transient
ischaemic attack in rats. Front Aging Neurosci.
10(24)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou F, Chen W, Cui Y, Liu B, Yuan Q, Li Z
and He Z: miRNA-122-5p stimulates the proliferation and DNA
synthesis and inhibits the early apoptosis of human spermatogonial
stem cells by targeting CBL and competing with lncRNA CASC7. Aging
(Albany NY). 12:25528–25546. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Peng H, Luo Y and Ying Y: lncRNA XIST
attenuates hypoxia-induced H9c2 cardiomyocyte injury by targeting
the miR-122-5p/FOXP2 axis. Mol Cell Probes.
50(101500)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rao L, Meng FL, Fang R, Cai CY and Zhao
XL: Molecular mechanism of microRNA in regulating cochlear hair
cell development. Yi Chuan. 41:994–1008. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
18
|
Xiong H, Chen S, Lai L, Yang H, Xu Y, Pang
J, Su Z, Lin H and Zheng Y: Modulation of miR-34a/SIRT1 signaling
protects cochlear hair cells against oxidative stress and delays
age-related hearing loss through coordinated regulation of
mitophagy and mitochondrial biogenesis. Neurobiol Aging. 79:30–42.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang JY, Xia Y, Yang CC and Wang Z:
Analysis of microRNA regulatory network in cochlear hair cells with
oxidative stress injury. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 51:751–755. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
20
|
Li Y, Tang XL, Yu F, Li HJ and Yuan W:
Expression and regulatory effect of miR-30b on dynamin in cochlear
hair cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 49:347–351.
2018.PubMed/NCBI(In Chinese).
|
|
21
|
Zhou W, Du J, Jiang D, Wang X, Chen K,
Tang H, Zhang X, Cao H, Zong L, Dong C and Jiang H: microRNA-183 is
involved in the differentiation and regeneration of Notch
signaling-prohibited hair cells from mouse cochlea. Mol Med Rep.
18:1253–1262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y, Li A, Wu J, He Y, Yu H, Chai R and
Li H: MiR-182-5p protects inner ear hair cells from
cisplatin-induced apoptosis by inhibiting FOXO3a. Cell Death Dis.
7(e2362)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsuchihashi NA, Hayashi K, Dan K, Goto F,
Nomura Y, Fujioka M, Kanzaki S, Komune S and Ogawa K: Autophagy
through 4EBP1 and AMPK regulates oxidative stress-induced premature
senescence in auditory cells. Oncotarget. 6:3644–3655.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu F, Xiong H and Sha S: Noise-induced
loss of sensory hair cells is mediated by ROS/AMPKα pathway. Redox
Biol. 29(101406)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Henderson D, Mcfadden SL, Liu CC, Hight N
and Zheng XY: The role of antioxidants in protection from impulse
noise. Ann N Y Acad Sci. 884:368–380. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ohlemiller KK, Wright JS and Dugan LL:
Early elevation of cochlear reactive oxygen species following noise
exposure. Audiol Neurootol. 4:229–236. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guthrie OW: Aminoglycoside induced
ototoxicity. Toxicology. 249:91–96. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Henderson D, Bielefeld EC, Harris KC and
Hu BH: The role of oxidative stress in noise-induced hearing loss.
Ear Hear. 27:1–19. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sugahara K, Rubel EW and Cunningham LL:
JNK signaling in neomycin-induced vestibular hair cell death. Hear
Res. 221:128–135. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu X, Liu W, Fan Z, Qian F, Zhang D, Han
Y, Xu L, Sun G, Qi J, Zhang S, et al: c-Myb knockdown increases the
neomycin-induced damage to hair-cell-like HEI-OC1 cells in vitro.
Sci Rep. 7(41094)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xie J, Talaska AE and Schacht J: New
developments in aminoglycoside therapy and ototoxicity. Hear Res.
281:28–37. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mangiardi DA, McLaughlin-Williamson K, May
KE, Messana EP, Mountain DC and Cotanche DA: Progression of hair
cell ejection and molecular markers of apoptosis in the avian
cochlea following gentamicin treatment. J Comp Neurol. 475:1–18.
2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Coffin AB, Rubel EW and Raible DW: Bax,
Bcl2, and p53 differentially regulate neomycin- and
gentamicin-induced hair cell death in the zebrafish lateral line. J
Assoc Res Otolaryngol. 14:645–659. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fetoni AR, Paciello F, Rolesi R, Paludetti
G and Troiani D: Targeting dysregulation of redox homeostasis in
noise-induced hearing loss: Oxidative stress and ROS signaling.
Free Radic Biol Med. 135:46–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang Z, Yu J, Wu J, Qi F, Wang H, Wang Z
and Xu Z: Scutellarin protects cardiomyocyte ischemia-reperfusion
injury by reducing apoptosis and oxidative stress. Life Sci.
157:200–207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wu X, Li X, Song Y, Li H, Bai X, Liu W,
Han Y, Xu L, Li J, Zhang D, et al: Allicin protects auditory hair
cells and spiral ganglion neurons from cisplatin-Induced apoptosis.
Neuropharmacology. 116:429–440. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mostafa T, Rashed LA, Nabil NI, Osman I,
Mostafa R and Farag M: Seminal miRNA relationship with apoptotic
markers and oxidative stress in infertile men with varicocele.
Biomed Res Int. 2016(4302754)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation, and
transformation. Cell. 117:421–426. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
You L, Wang Z, Li H, Shou J, Jing Z, Xie
J, Sui X, Pan H and Han W: The role of STAT3 in autophagy.
Autophagy. 11:729–739. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang N, Zhang Q and Bi XJ: MiRNA-96
accelerates the malignant progression of ovarian cancer via
targeting FOXO3a. Eur Rev Med Pharmacol Sci. 24:65–73.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang J, Xu H, Gong L and Liu L:
MicroRNA-132 protects H9c2 cells against oxygen and glucose
deprivation-evoked injury by targeting FOXO3A. J Cell Physiol.
235:176–184. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yao RD, Li HL, Liu Y and Sun LT: MiRNA-1
promotes pyroptosis of cardiomyocytes and release of inflammatory
factors by downregulating the expression level of PIK3R1 through
the FoxO3a pathway. Eur Rev Med Pharmacol Sci. 24:11243–11250.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ran X, Li Y, Chen G, Fu S, He D, Huang B,
Wei L, Lin Y, Guo Y and Hu G: Farrerol ameliorates TNBS-induced
colonic inflammation by inhibiting ERK1/2, JNK1/2, and NF-κB
signaling pathway. Int J Mol Sci. 19(2037)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lai R, Cai C, Wu W, Hu P and Wang Q:
Exosomes derived from mouse inner ear stem cells attenuate
gentamicin-induced ototoxicity in vitro through the
miR-182-5p/FOXO3 axis. J Tissue Eng Regen Med. 14:1149–1156.
2020.PubMed/NCBI View Article : Google Scholar
|