Introduction
Coronary artery disease (CAD) is a common health
problem and is the main cause of mortality globally (1). CAD is predominantly established
following myocardial ischemia/reperfusion (I/R) injury, which
refers to myocardial dysfunction and injury following the
reperfusion of previously viable ischemic cardiac tissues (2). It has previously been reported that
myocardial I/R injury disrupts endothelial barrier function,
enhances endothelial permeability and contributes to cellular
swelling, which results in microthrombosis and microvascular
obstruction, and therefore blocks blood supply to the heart
(3,4). Furthermore, myocardial I/R injury
involves numerous pathophysiological responses, including calcium
overload, oxygen radical production, endothelial dysfunction,
immune response, mitochondrial dysfunction, cardiomyocyte apoptosis
and autophagy and platelet aggregation (5-7).
Sufentanil is a lipophilic opioid agonist that is
selective for µ-opioid receptors and can therefore exert analgesic
and sedative effects (8). Compared
with fentanyl and remifentanil, sufentanil not only reduces the
incidence of postoperative nausea and vomiting, but also maintains
stable hemodynamics (9,10). As a derivative of fentanyl,
sufentanil is widely used for the control of tracheal
intubation-induced cardiovascular responses due to its significant
µ-receptor affinity (11).
Moreover, the continuous administration of sufentanil exhibits
cardioprotective effects on the human myocardium against
hypoxia/reoxygenation (H/R) in vitro (12). Furthermore, sufentanil has been
reported to activate the PI3K/Akt signaling pathway (13).
Phosphatidylinositol-3-kinase (PI3K) and protein
kinase B (Akt) serve as the two most vital proteins in the PI3K/Akt
signaling pathway (14). As an
intracellular phosphatidylinositol kinase, PI3K serves a vital role
in various aspects of cardiology, including cell survival,
myocardial hypertrophy and myocardial contractility (15,16).
Being a serine/threonine tyrosine kinase, Akt acts as a downstream
effector molecule of PI3K and promotes numerous biological
mechanisms, such as cell proliferation and apoptosis (17,18).
Furthermore, the PI3K/Akt signaling pathway acts as a crucial
cardioprotective contributor against myocardial infarction and I/R
injury (19).
In the present study, the oxygen-glucose
deprivation/reoxygenation (OGD/R) model was established to simulate
myocardial I/R injury in vitro. The aims of the present
study were to investigate the efficacy of sufentanil in myocardial
I/R injury as well as to explore its relationship with the PI3K/Akt
signaling pathway.
Materials and methods
Cell culture and treatment
Immortalized human cardiac microvascular endothelial
cells (HCMECs), without mycoplasma contamination, were purchased
from ScienCell Research Laboratories, Inc. HCMECs were cultured in
DMEM (Wisent, Inc.), which was supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37˚C in a humidified incubator with 5%
CO2.
Establishment of the OGD/R model
The OGD/R model was established to simulate
myocardial I/R injury in vitro. HCMECs were cultured in
glucose-free DMEM for 2, 4, 6 or 8 h and were then incubated at
37˚C in hypoxic conditions (1% O2, 5% CO2 and
94% N2). Subsequently, the cells were added to normal
DMEM with continuous reoxygenation (21% O2, 5%
CO2 and 74% N2). During the process of
reoxygenation, sufentanil at different concentrations (5, 10 and 20
µM) was administered to the HCMECs. To explore the relationship
between sufentanil and the PI3K/Akt signaling pathway, LY294002 (10
µM; Sigma-Aldrich; Merck KGaA), an inhibitor of the PI3K/Akt
signaling pathway, was used to treat HCMECs for 1 h prior to
sufentanil treatment.
Cell counting kit-8 (CCK-8) assay
To assess the effects of sufentanil on HCMEC
viability and OGD/R-induced HCMECs a CCK-8 assay was performed at
37˚C. Following OGD/R induction, 10 µl CCK-8 reagent per well was
added to the HCMECs seeded into a 96-well plate at a density of
1x105 cells/well for 3 h. A wavelength of 450 nm was
used to determine the absorbance using a microplate reader (Bio-Rad
Laboratories, Inc.).
Lactate dehydrogenase (LDH) activity
assay
LDH activity was analyzed to assess cell death. A
loss of plasma membrane integrity is demonstrated by the release of
LDH. Therefore, 100-µl cell supernatant was added to 100 µl
cytotoxicity detection reagent (cat. no. C0016; Beyotime Institute
of Biotechnology) in a 96-well plate. Colorimetric analysis of
sodium pyruvate reduction in the presence of oxycodone was used to
determine LDH activity as previously described (20).
TUNEL assay
A TUNEL kit (cat. no. C1086; Beyotime Institute of
Biotechnology) was used to assess the effects of sufentanil on the
apoptosis of OGD/R-induced HCMECs. Briefly, the cells were fixed
using 4% paraformaldehyde for 15 min, after which the cells were
permeabilized using 0.25% Triton X-100 for 20 min at room
temperature. Subsequently, the cells were stained using TUNEL
reagent for 1 h and with 1 µg/ml DAPI (cat. no. C1005; Beyotime
Institute of Biotechnology) in the dark. In total, 10 fields were
randomly selected and observed using a fluorescent microscope
(magnification, x200; Olympus Corporation).
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) and protein
concentration was determined using a BCA Protein Assay kit (cat.
no. P0012S; Beyotime Institute of Biotechnology). An equal amount
of protein (30 µg) was loaded into each lane and separated on a 10%
gel using SDS-PAGE and separated proteins were subsequently
transferred onto a PVDF membrane. Membranes were blocked with 5%
non-fat milk for 2 h at room temperature and were then incubated
with primary antibodies against the following: Bcl-2 (1:1,000; cat.
no. ab32124), Bax (1:1,000; cat. no. ab32503), cleaved
(c)-caspase-3 (1:500; cat. no. ab32042), cytochrome c
(1:5,000; cat. no. ab133504), Zonula occludens-1 (ZO-1; 1:1,000;
cat. no. ab216880), Occludin (1:1,000; cat. no. ab216327), vascular
endothelial (VE)-cadherin (1:2,000; cat. no. ab33168), Claudin-5
(1:1,000; cat. no. ab131259), phosphorylated (p)-PI3K (1:1,000;
cat. no. ab278545), p-Akt (1:1,000; cat. no. ab38449), PI3K
(1:1,000; cat. no. ab32089), Akt (1:500; cat. no. ab8805) or GAPDH
(1:2,500; cat. no. ab9485; all from Abcam) at 4˚C overnight.
Following the primary incubation, cells were incubated with an
HRP-conjugated goat anti-rabbit secondary antibody (1:5,000; cat.
no. ab6759; Abcam) at room temperature for 2 h. Protein bands were
visualized using ECL (MilliporeSigma) and subsequently analyzed
with ImageJ software v1.8.0 (National Institutes of Health).
In vitro permeability assay
Using the In Vitro Permeability Assay kit
(MilliporeSigma), the effects of sufentanil on endothelial barrier
function in OGD/R-induced HCMECs were investigated. Briefly, in
order to form a tight monolayer, 5x103 HCMECs were
inoculated onto collagen-coated inserts and cultured for 72 h.
After treating cells for 1 h, OGD/R induction was performed. Each
receiver plate contained 500 ml glucose-free DMEM. To each insert
150 ml 2.5% FITC-dextran (40 kDa) solution was added for 20 min at
37˚C in the dark. The extent of endothelial permeability was
quantified using the fluorescence intensity of the bottom plate,
which had been permeated by FITC-dextran. Cells were observed using
wavelengths of 485 nm (excitation) and 535 nm (emission) using a
fluorescence spectrophotometer (Tecan Group, Ltd.) as previously
described (21).
Immunofluorescence
HCMECs were cultured as a confluent monolayer on
glass slides. Cells were fixed with 2% paraformaldehyde in PBS at
4˚C for 3 h and permeabilized in 0.5% Triton X-100 in 1X PBS at
room temperature for 1 h. Subsequently, blocking was performed at
37˚C using 1% BSA (Beijing Solarbio Science & Technology Co.,
Ltd.) in 0.2% Triton X-100 for 30 min. Then, cells were incubated
with rabbit anti-VE-cadherin antibody (1:200; cat. no. ab33168;
Abcam) overnight at 4˚C. Cells were rinsed in PBS three times and
were subsequently incubated with a goat anti-rabbit IgG Alexa Fluor
555-conjugated secondary antibody (1:100; cat. no. A27017;
Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 1 h and 1
mg/ml DAPI. A confocal microscope was used to examine the
slides.
Statistical analysis
Data were analyzed using GraphPad Prism 8.0 software
(GraphPad Software, Inc.). All data are presented as the mean ± SD.
A one-way ANOVA was used to statistically compare differences among
more than two groups, followed by Tukey's post-hoc test. An
unpaired Student's t-test was used to compare means between two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sufentanil enhances the viability of
OGD/R-induced HCMECs
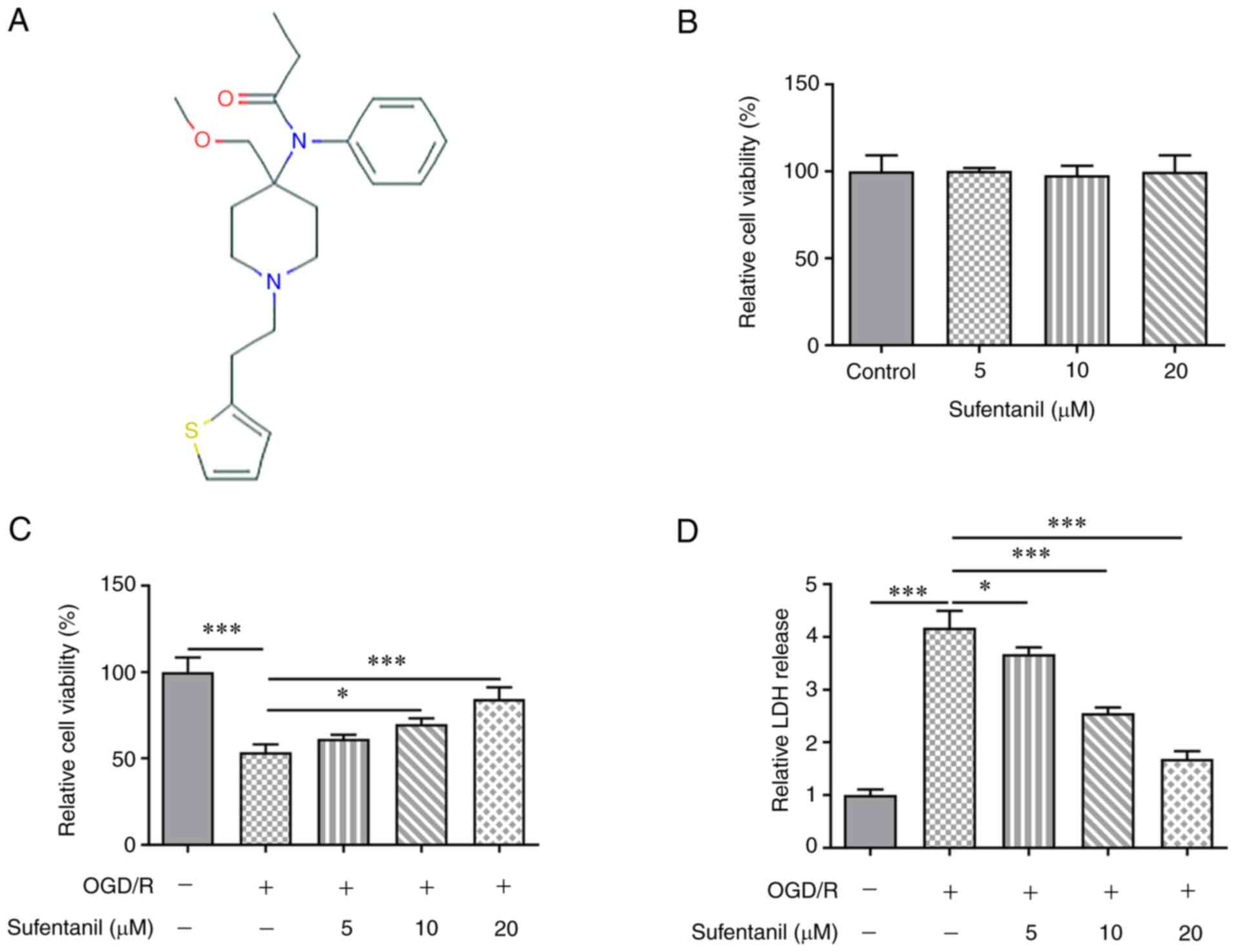
The chemical structure of sufentanil is presented in
Fig. 1A. Sufentanil was
demonstrated to have no significant effect on the viability of
HCMECs (Fig. 1B). Compared with
the control group, OGD/R induction significantly decreased HCMEC
viability, which was partially enhanced by sufentanil treatment
(Fig. 1C). Furthermore, it was
determined that sufentanil exhibited promotive effects on the
viability of OGD/R-induced HCMECs in a dose-dependent manner. OGD/R
induction increased LDH activity, which was then gradually
decreased by sufentanil treatment (Fig. 1D). These results indicated the
inhibitory effects of sufentanil on LDH activity in OGD/R-induced
HCMECs.
Sufentanil inhibits the apoptosis of
OGD/R-induced HCMECs
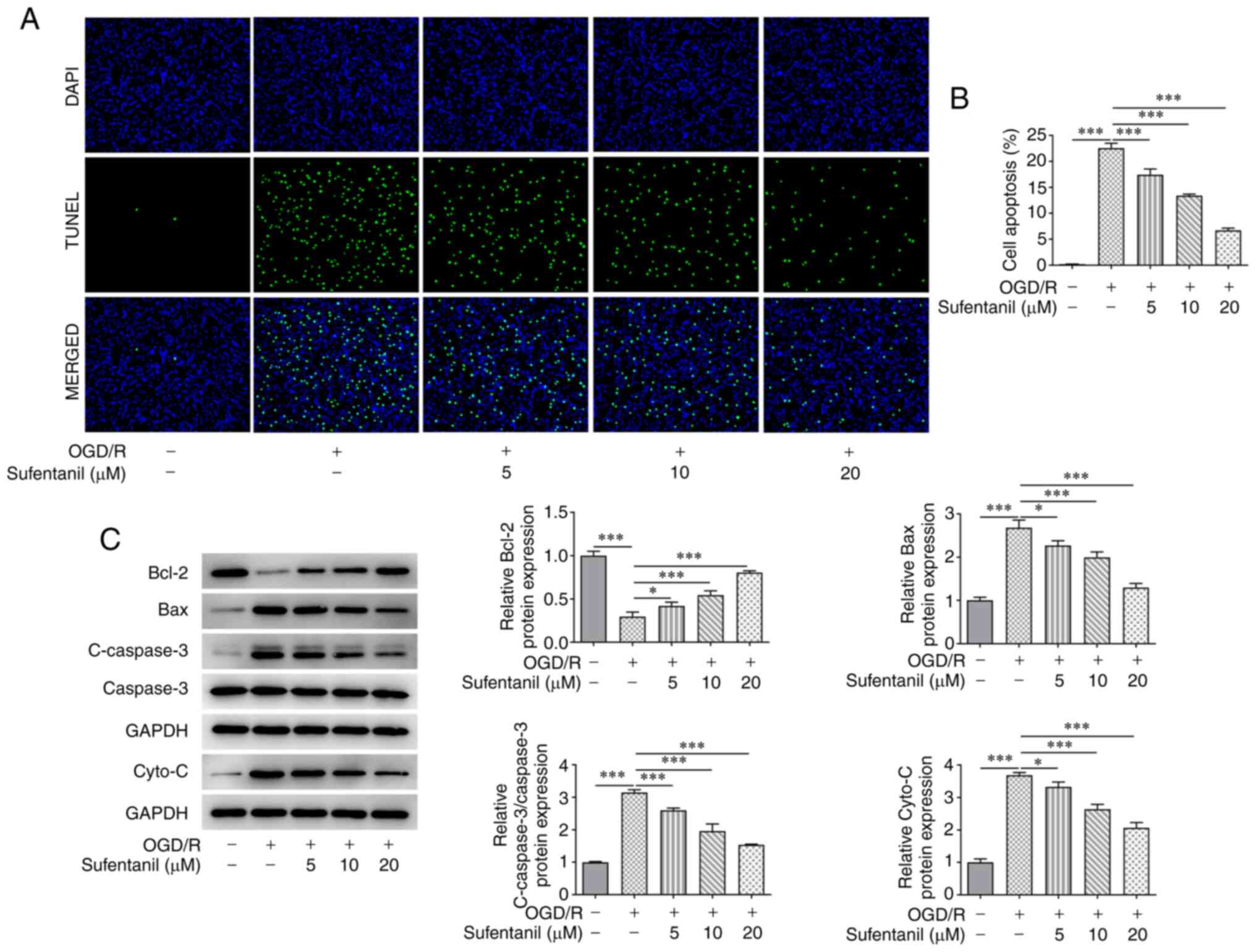
Compared with the control group, HCMEC apoptosis was
significantly increased by OGD/R induction. However, the enhanced
apoptosis in OGD/R-induced HCMECs was suppressed by sufentanil
treatment in a dose-dependent manner. Furthermore, 20 µM sufentanil
had optimal suppressive effects on the apoptosis of HCMECs with
OGD/R induction (Fig. 2A and
B). Moreover, OGD/R induction
downregulated Bcl-2 protein expression levels but upregulated the
protein expression levels of Bax, c-caspase-3 and cytochrome
c. However, sufentanil treatment reversed the effects of
OGD/R induction on these proteins. This was demonstrated by the
upregulated Bcl-2 protein expression levels, as well as the
downregulated protein expression levels of Bax, c-caspase-3 and
cytochrome c in the OGD/R + 5 µM, OGD/R + 10 µM and OGD/R +
20 µM groups (Fig. 2C).
Sufentanil reduces the improved cell
permeability of OGD/R-induced HCMECs and upregulates the protein
expression levels of tight junction proteins
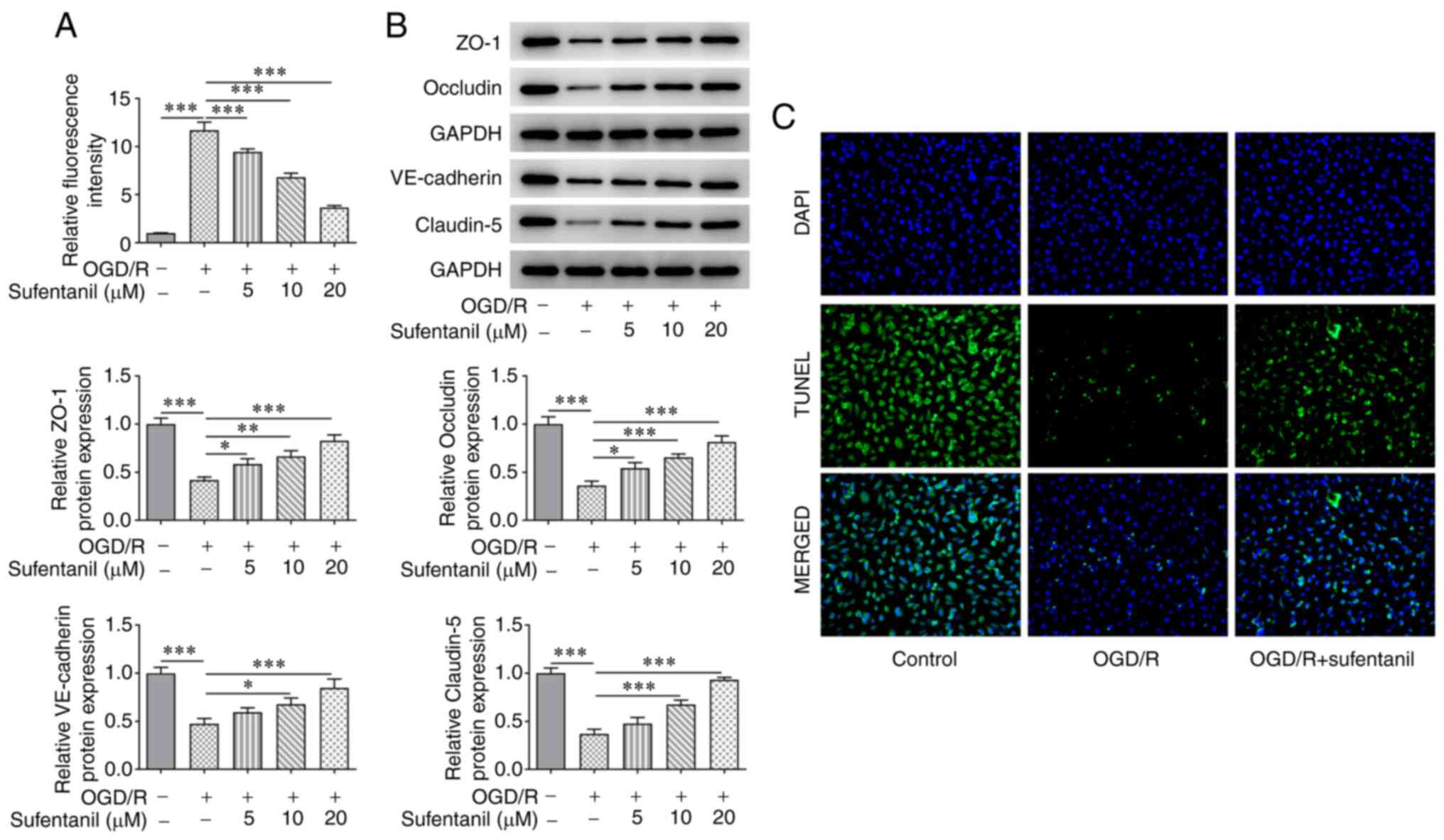
The results demonstrated that OGD/R induction
significantly enhanced the relative fluorescence intensity of
HCMECs, which was subsequently reduced following sufentanil
treatment (Fig. 3A). In addition,
OGD/R induction contributed to decreased protein expression levels
of ZO-1, Occludin, VE-cadherin and Claudin-5. However, sufentanil
exhibited the opposite effect, whereby ZO-1, Occludin, VE-cadherin
and Claudin-5 protein expression levels were increased in the OGD/R
+ 5 µM, OGD/R + 10 µM and OGD/R + 20 µM groups, compared with the
OGD/R group (Fig. 3B).
Furthermore, the decreased VE-cadherin protein expression levels in
OGD/R-induced HCMECs were subsequently increased by sufentanil
treatment, which suggested that sufentanil treatment had a
promotive effect on tight junction proteins (Fig. 3C). Based on the aforementioned
results, the dose of 20 µM sufentanil was selected for the
following experiments.
LY294002 reverses the protective
effects of sufentanil on OGD/R-induced HCMEC apoptosis
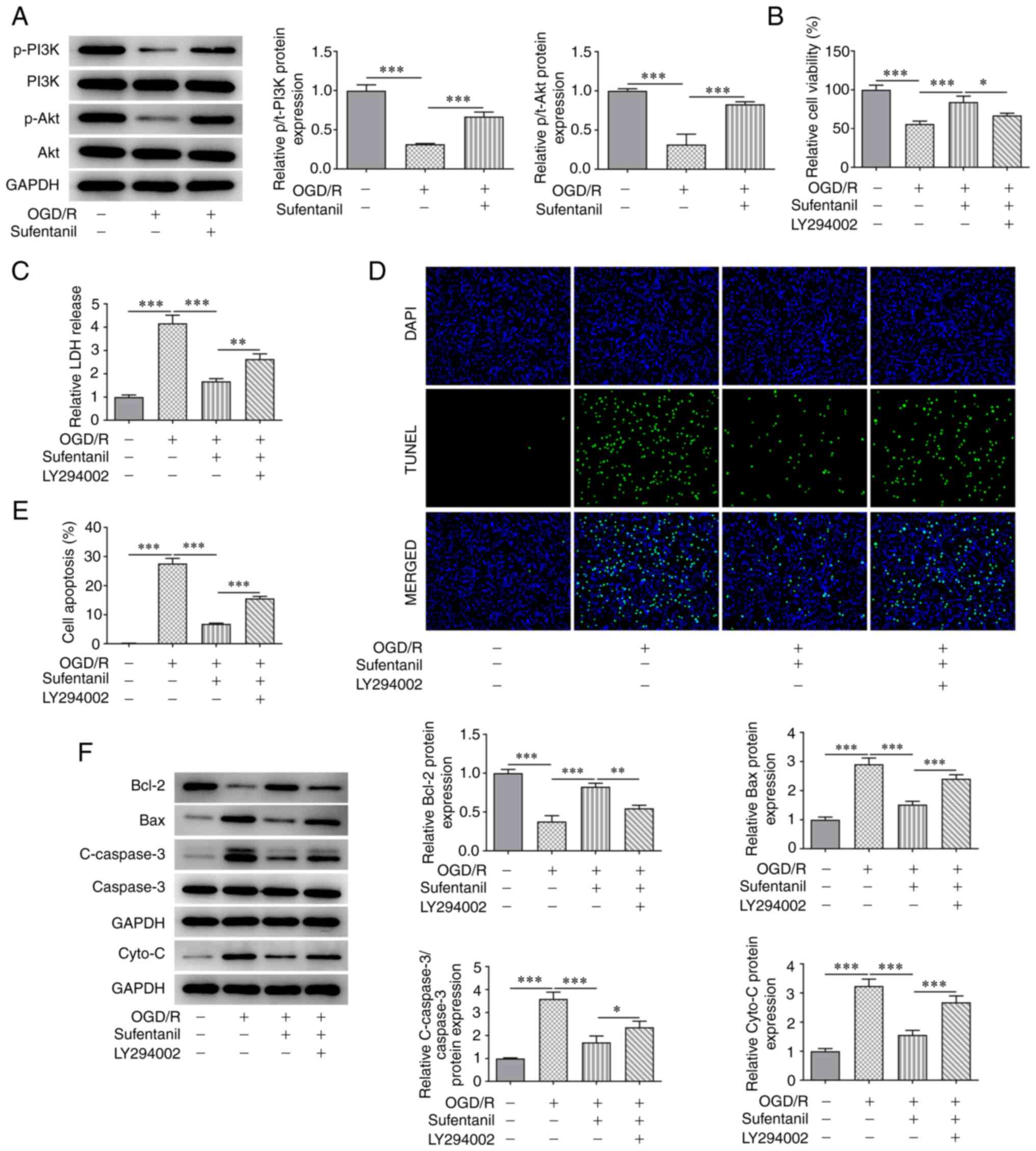
The results demonstrated OGD/R induction reduced the
protein expression levels of p-PI3K and p-Akt compared with the
control group. However, this was reversed by sufentanil treatment,
as demonstrated by the increased protein expression levels of
p-PI3K and p-Akt in the OGD/R + sufentanil group (Fig. 4A). Moreover, the decreased cell
viability in HCMECs caused by OGD/R induction was improved
following sufentanil treatment. These results indicated that
sufentanil promoted the viability of OGD/R-induced HCMECs. However,
LY294002, an inhibitor of the PI3K/Akt signaling pathway, partially
abolished the protective effects of sufentanil, which was
demonstrated by the decreased viability in the LY294002 + OGD/R +
sufentanil group compared with the OGD/R + sufentanil group
(Fig. 4B). Moreover, LDH activity
was significantly enhanced by OGD/R induction compared with the
control group. However, compared with the OGD/R + sufentanil group,
LDH activity was increased by LY294002 treatment (Fig. 4C).
The results also demonstrated that the decreased
apoptotic rate in OGD/R-induced HCMECs, as a result of sufentanil
treatment, was increased by LY294002 treatment compared with the
OGD/R + sufentanil group (Fig. 4D
and E). Moreover, sufentanil
upregulated Bcl-2 protein expression levels but downregulated the
protein expression levels of Bax, c-caspase-3 and cytochrome
c compared with the OGD/R group. However, LY294002 had the
opposite effect on the expression levels of these proteins, which
was demonstrated by the decreased Bcl-2, as well as increased Bax,
c-caspase-3 and cytochrome c protein expression levels in
the LY294002 + OGD/R + sufentanil group, compared with the OGD/R +
sufentanil group (Fig. 4F).
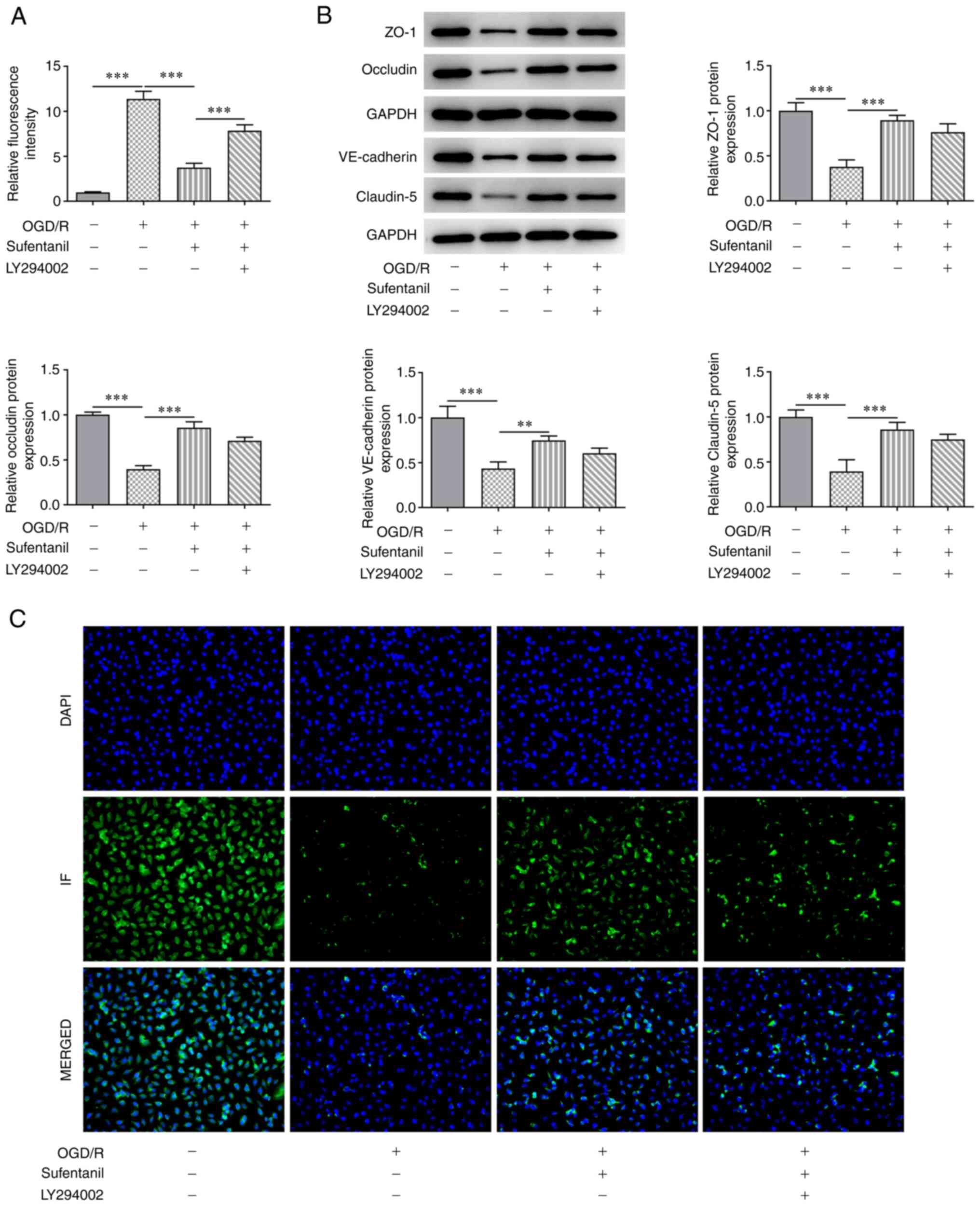
LY294002 reverses the protective
effects of sufentanil on the cell permeability and tight junction
proteins of OGD/R-induced HCMECs
The results demonstrated that the enhanced
fluorescence intensity caused by OGD/R induction was significantly
reduced following sufentanil treatment compared with the OGD/R
group (Fig. 5A). However, compared
with the OGD/R + sufentanil group, LY294002 reversed the inhibitory
effects of sufentanil, which was demonstrated by the increased
fluorescence intensity in the LY294002 + OGD/R + sufentanil group.
Furthermore, the upregulated protein expression levels of ZO-1,
Occludin, VE-cadherin and Claudin-5 in OGD/R-induced HCMECs with
sufentanil treatment, were partially reduced by LY294002
administration. These results suggested that LY294002 may reverse
the protective effects of sufentanil on endothelial barrier
function of OGD/R-induced HCMECs (Fig.
5B). Moreover, LY294002 treatment had the opposite effect on
VE-cadherin protein expression levels, which was demonstrated by
decreased expression of VE-cadherin in the LY294002 + OGD/R +
sufentanil group compared with the OGD/R + sufentanil group
(Fig. 5C).
Discussion
In the present study, it was demonstrated that
sufentanil had no significant influence on the viability of HCMECs
but promoted the viability of OGD/R-induced HCMECs in a
dose-dependent manner. To determine the effects of sufentanil on
OGD/R-induced HCMECs, a series of cellular experiments were
performed. The results demonstrated that sufentanil inhibited the
apoptosis and cell permeability of OGD/R-induced HCMECs, but
enhanced the protein expression levels of the tight junction
protein VE-cadherin. Furthermore, the protein expression levels of
p-PI3K and p-Akt in OGD/R-induced HCMECs were significantly
upregulated by sufentanil treatment, which revealed that sufentanil
could potentially activate the PI3K/Akt signaling pathway. To
further investigate the relationship among sufentanil, myocardial
I/R injury and the PI3K/Akt signaling pathway, LY294002, an
inhibitor of the PI3K/Akt signaling pathway, was used to treat
HCMECs. The results demonstrated that LY294002 partially abolished
the protective effects of sufentanil on the apoptosis, cell
permeability and on tight junction protein expression of
OGD/R-induced HCMECs.
Being a selective µ-opioid receptor agonist,
sufentanil is commonly used in the clinic and exerts a protective
effect on myocardial I/R injury (22). For example, sufentanil protects
against myocardial I/R injury in rats via activation of the ERK1/2
signaling pathway (23). Moreover,
sufentanil has been reported to reduce myocardial infarct size,
preserve phosphorylation of connexin 43 and confer cardioprotective
effects (24). Previous studies
have demonstrated that microvascular dysfunction in cardiac I/R
injury is typically characterized by inflammation, reduced
microvascular flow, and impaired angiogenesis and self-repairing
capacity (25-28).
Among these, endothelial apoptosis is closely associated with the
initiation of angiogenesis (29).
In the present study, it was demonstrated that an increased
apoptotic rate in OGD/R-induced HCMECs was suppressed by sufentanil
in a dose-dependent manner. Furthermore, sufentanil treatment
increased Bcl-2 protein expression levels but decreased the protein
expression levels of Bax, c-caspase-3 and cytochrome c in
OGD/R-induced HCMECs, compared with cells without sufentanil
treatment. These results suggested that sufentanil potentially
inhibits apoptosis in myocardial I/R injury.
The insufficient secretion of endothelium-derived
diastolic factor and the excessive secretion of endothelin-1 in
myocardial microvascular endothelial cells results in increased
cell permeability (30). ZO-1,
VE-cadherin and Claudin-5, which are important mediators of
endothelial adherence junctions, serve critical roles in
maintaining the blood-brain barrier balance in ischemic stroke
(31,32). In the present study, it was
determined that the increased fluorescence intensity of
OGD/R-induced HCMECs was markedly decreased in a dose-dependent
manner following treatment with sufentanil. Furthermore, sufentanil
treatment enhanced the protein expression levels of ZO-1, Occludin,
VE-cadherin and Claudin-5 in OGD/R-induced HCMECs. These results
indicated that sufentanil may have had a suppressive effect on the
enhanced cell permeability of OGD/R-induced HCMECs. Furthermore,
the decreased protein expression levels of the tight junction
protein VE-cadherin, caused by OGD/R induction, were also improved
following sufentanil treatment.
It has previously been reported that the activation
of the PI3K/Akt signaling pathway contributes to the inhibition of
apoptosis and endothelial dysfunction of H/R-induced HCMECs
(33). Moreover, an increasing
number of studies have demonstrated that the regulation of the
PI3K/Akt signaling pathway alleviates apoptosis and endothelial
dysfunction in myocardial I/R injury (34,35),
and sufentanil activates the PI3K/Akt signaling pathway (36). In the present study, it was
demonstrated that the decreased protein expression levels of p-PI3K
and p-Akt in OGD/R-induced HCMECs were significantly upregulated by
sufentanil treatment. These results suggested that sufentanil may
activate the PI3K/Akt signaling pathway, which is consistent with
the results from a previous study (36). However, further experiments
performed in the present study demonstrated that LY294002, an
inhibitor of the PI3K/Akt signaling pathway, reversed the
protective effects of sufentanil on apoptosis, cell permeability
and tight junction proteins in OGD/R-induced HCMECs. Therefore,
these results indicated that sufentanil may ameliorate
OGD/R-induced endothelial barrier dysfunction in HCMECs via
activating the PI3K/Akt signaling pathway.
In conclusion, the present study highlighted the
potential therapeutic application of sufentanil in myocardial I/R
injury via the activation of the PI3K/Akt signaling pathway.
However, further studies are needed to examine the effects of
sufentanil on myocardial I/R injury in vivo.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW and XZ designed the study, drafted and revised
the manuscript. LW, CG and XZ analyzed the data and searched the
literature. LW and CG performed the experiments. All authors read
and approved the final manuscript. LW and CG confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Romero-Corral A, Montori VM, Somers VK,
Korinek J, Thomas RJ, Allison TG, Mookadam F and Lopez-Jimenez F:
Association of bodyweight with total mortality and with
cardiovascular events in coronary artery disease: A systematic
review of cohort studies. Lancet. 368:666–678. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang N, Song G, Yang Y, Yuan W and Qi M:
Inactivated Lactobacillus promotes protection against myocardial
ischemia-reperfusion injury through NF-κB pathway. Biosci Rep.
37(BSR20171025)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang J, Toan S and Zhou H: New insights
into the role of mitochondria in cardiac microvascular
ischemia/reperfusion injury. Angiogenesis. 23:299–314.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gao XM, Su Y, Moore S, Han LP, Kiriazis H,
Lu Q, Zhao WB, Ruze A, Fang BB, Duan MJ and Du XJ: Relaxin
mitigates microvascular damage and inflammation following cardiac
ischemia-reperfusion. Basic Res Cardiol. 114(30)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Beckman JS, Beckman TW, Chen J, Marshall
PA and Freeman BA: Apparent hydroxyl radical production by
peroxynitrite: Implications for endothelial injury from nitric
oxide and superoxide. Proc Natl Acad Sci USA. 87:1620–1624.
1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Loke KE, McConnell PI, Tuzman JM, Shesely
EG, Smith CJ, Stackpole CJ, Thompson CI, Kaley G, Wolin MS and
Hintze TH: Endogenous endothelial nitric oxide synthase-derived
nitric oxide is a physiological regulator of myocardial oxygen
consumption. Circ Res. 84:840–845. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Radomski MW, Palmer RM and Moncada S:
Endogenous nitric oxide inhibits human platelet adhesion to
vascular endothelium. Lancet. 2:1057–1058. 1987.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reardon CE, Kane-Gill SL, Smithburger PL
and Dasta JF: Sufentanil sublingual tablet: A new option for acute
pain management. Ann Pharmacother. 53:1220–1226. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim DK, Yoon SH, Kim JY, Oh CH, Jung JK
and Kim J: Comparison of the effects of sufentanil and fentanyl
intravenous patient controlled analgesia after lumbar fusion. J
Korean Neurosurg Soc. 60:54–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ohnesorge H, Alpes A, Baron R and
Gierthmühlen J: Influence of intraoperative remifentanil and
sufentanil on sensory perception: A randomized trial. Curr Med Res
Opin. 32:1797–1805. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang Y, Teng X and Zhu J: Sufentanil
blunts the myocardial stress induced by tracheal intubation in
older adult patients with coronary heart disease better than
equipotent fentanyl. Ann Palliat Med. 9:3909–3914. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lemoine S, Zhu L, Massetti M, Gérard JL
and Hanouz JL: Continuous administration of remifentanil and
sufentanil induces cardioprotection in human myocardium, in vitro.
Acta Anaesthesiol Scand. 55:758–764. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu X, Jing G, Bai J and Yuan H: Effect of
sufentanil preconditioning on myocardial P-Akt expression in rats
during myocardial ischemia-reperfusion. Nan Fang Yi Ke Da Xue Xue
Bao. 34:335–340. 2014.PubMed/NCBI(In Chinese).
|
|
14
|
Wei J, Gou Z, Wen Y, Luo Q and Huang Z:
Marine compounds targeting the PI3K/Akt signaling pathway in cancer
therapy. Biomed Pharmacother. 129(110484)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Y, Xia J, Jiang N, Xian Y, Ju H, Wei Y
and Zhang X: Corin protects H2O2-induced
apoptosis through PI3K/AKT and NF-κB pathway in cardiomyocytes.
Biomed Pharmacother. 97:594–599. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fruman DA, Chiu H, Hopkins BD, Bagrodia S,
Cantley LC and Abraham RT: The PI3K pathway in human disease. Cell.
170:605–635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hay N: The Akt-mTOR tango and its
relevance to cancer. Cancer Cell. 8:179–183. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Chen E, Chen C, Niu Z, Gan L, Wang Q, Li
M, Cai X, Gao R, Katakam S, Chen H, et al: Poly(I:C)
preconditioning protects the heart against myocardial
ischemia/reperfusion injury through TLR3/PI3K/Akt-dependent
pathway. Signal Transduct Target Ther. 5(216)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jacob B, Kloss N, Böhle S, Kirschberg J,
Zippelius T, Heinecke M, Matziolis G and Röhner E: Tranexamic acid
is toxic on human chondrocytes, in vitro. J Orthop. 20:1–5.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qi K, Yang Y, Geng Y, Cui H, Li X, Jin C,
Chen G, Tian X and Meng X: Tongxinluo attenuates
oxygen-glucose-serum deprivation/restoration-induced endothelial
barrier breakdown via peroxisome proliferator activated
receptor-α/angiopoietin-like 4 pathway in high
glucose-incubated human cardiac microvascular endothelial cells.
Medicine (Baltimore). 99(e21821)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen QL, Gu EW, Zhang L, Cao YY, Zhu Y and
Fang WP: Diabetes mellitus abrogates the cardioprotection of
sufentanil against ischaemia/reperfusion injury by altering
glycogen synthase kinase-3β. Acta Anaesthesiol Scand.
57:236–242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tao H, Nuo M and Min S: Sufentanil
protects the rat myocardium against ischemia-reperfusion injury via
activation of the ERK1/2 pathway. Cytotechnology. 70:169–176.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu Y, Gu EW, Zhu Y, Zhang L, Liu XQ and
Fang WP: Sufentanil limits the myocardial infarct size by
preservation of the phosphorylated connexin 43. Int
Immunopharmacol. 13:341–346. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu H, Kalogeris T and Korthuis RJ:
Reactive species-induced microvascular dysfunction in
ischemia/reperfusion. Free Radic Biol Med. 135:182–197.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Müller-Werdan U, Prondzinsky R and Werdan
K: Effect of inflammatory mediators on cardiovascular function.
Curr Opin Crit Care. 22:453–463. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ito H: Etiology and clinical implications
of microvascular dysfunction in patients with acute myocardial
infarction. Int Heart J. 55:185–189. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ríos-Navarro C, Hueso L, Miñana G, Núñez
J, Ruiz-Saurí A, Sanz MJ, Cànoves J, Chorro FJ, Piqueras L and Bodí
V: Coronary serum obtained after myocardial infarction induces
angiogenesis and microvascular obstruction repair. Role of
hypoxia-inducible factor-1A. Rev Esp Cardiol (Engl Ed). 71:440–449.
2018.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
29
|
Zhu T, Yao Q, Wang W, Yao H and Chao J:
iNOS induces vascular endothelial cell migration and apoptosis via
autophagy in ischemia/reperfusion injury. Cell Physiol Biochem.
38:1575–1588. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Filep JG, Sirois MG, Foldes-Filep E,
Rousseau A, Plante GE, Fournier A, Yano M and Sirois P: Enhancement
by endothelin-1 of microvascular permeability via the activation of
ETA receptors. Br J Pharmacol. 109:880–886. 1993.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tornavaca O, Chia M, Dufton N, Almagro LO,
Conway DE, Randi AM, Schwartz MA, Matter K and Balda MS: ZO-1
controls endothelial adherens junctions, cell-cell tension,
angiogenesis, and barrier formation. J Cell Biol. 208:821–838.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Giannotta M, Trani M and Dejana E:
VE-cadherin and endothelial adherens junctions: Active guardians of
vascular integrity. Dev Cell. 26:441–454. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xie L, Wu Y, Fan Z, Liu Y and Zeng J:
Astragalus polysaccharide protects human cardiac microvascular
endothelial cells from hypoxia/reoxygenation injury: The role of
PI3K/AKT, Bax/Bcl-2 and caspase-3. Mol Med Rep. 14:904–910.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xing X, Guo S, Zhang G, Liu Y, Bi S, Wang
X and Lu Q: miR-26a-5p protects against myocardial
ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT
signaling pathway. Braz J Med Biol Res. 53(e9106)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yu P, Ma S, Dai X and Cao F: Elabela
alleviates myocardial ischemia reperfusion-induced apoptosis,
fibrosis and mitochondrial dysfunction through PI3K/AKT signaling.
Am J Transl Res. 12:4467–4477. 2020.PubMed/NCBI
|
|
36
|
Wu QL, Shen T, Ma H and Wang JK:
Sufentanil postconditioning protects the myocardium from
ischemia-reperfusion via PI3K/Akt-GSK-3β pathway. J Surg
Res. 178:563–570. 2012.PubMed/NCBI View Article : Google Scholar
|