Introduction
Multiple myeloma (MM) is a malignant blood cancer
characterized by the proliferation of clonal plasma cells in the
bone marrow (1). As the second
most common hematological malignancy worldwide, MM accounts for
~10% of all hematological malignancies, and 1% of all cancers
(2,3). In the past few years, the development
of novel agents, including immunomodulatory drugs, proteasome
inhibitors, monoclonal antibodies and histone deacetylase
inhibitors have achieved great advances on improving the response
rate and survival time of patients with MM (4-6).
However, Since MM is still unable to be cured (5), novel therapeutic agents against MM
are still needed to be explored.
Toad venom, a dried product of toxic secretions of
Bufo bufo gargarizans Cantor or Bufo melanostictus
Schneider, is a traditional natural medicine widely used in China
that has been revealed to have cardiotonic and analgesic activities
(7). A recent study has confirmed
that toad venom is also a source of antitumor drugs, and contains
96 types of bufadienolide monomers and 23 types of indole alkaloids
(7). Resibufogenin (RBG) is an
active ingredient of toad venom that exhibits potential in the
treatment of diverse types of cancer, such as gastric carcinoma,
colorectal cancer and osteosarcoma (8). It also has been reported that RBG is
a detectable component of ‘cinobufotalin injection’, which is
permitted for clinical administration in the treatment of liver and
gastric cancer by the Chinese food and drug administration
(9).
Han et al (2021) demonstrated that RBG
inhibits the proliferative activity and induces the necrosis of
colorectal cancer cells (10).
Zhou et al (2019) revealed that RBG inhibits the
proliferation, migration and invasion of ovarian clear cell
carcinoma cells in vitro, as well as the growth of tumor
xenografts in vivo (11).
Guo et al (2020) revealed that RBG inhibits glycolysis and
cell proliferation and promotes the apoptosis of breast cancer
cells (12).
Epithelial-mesenchymal transition (EMT) also is a notable factor
contributing to the metastasis of MM (13,14).
Han et al (2018) demonstrated that RBG inhibits the liver
metastasis of colorectal cancer by repressing EMT (10). However, to the best of our
knowledge, the specific role of RBG in the cell proliferation,
invasion and EMT of MM is still unclear.
PI3K/AKT signaling pathway is well known as a
notable cellular pathway that plays an important regulatory role in
basic intracellular functions, such as cell proliferation,
survival, autophagy, motility and differentiation (15,16).
Because the PI3K/Akt pathway can be activated by diverse cytokines
stimulated by the interaction of MM cells with bone marrow
mesenchymal stem cells (multipotent adult stem cells), its blocking
has become a promising therapeutic strategy for MM (17,18).
For example, an Akt inhibitor, TAS-117, inhibits the growth in
addition to inducing the apoptosis and autophagy of MM cells
(19). Afuresertib, an
ATP-competitive Akt inhibitor, exhibits a favorable safety profile
and clinical activity against MM in a phase I clinical trial
(20). A pan-PI3K inhibitor,
BKM120, inhibits the survival of MM cells by inducing apoptosis and
G2/M arrest (21). In
addition, the blocking of the PI3K/Akt pathway is also closely
associated with the antitumor efficiency of numerous natural
traditional Chinese medicines in MM, such as silybin (22), plumbagin (23), triptolide (24) and icaritin (25). Furthermore, RBG can exert antitumor
effects by regulating the PI3K/Akt signaling pathway in multiple
types of cancer, such as ovarian clear cell carcinoma and gastric
carcinoma (11,26). Zhou et al (2019) revealed
that RBG inhibits ovarian clear cell carcinoma growth and cell
migration by downregulating the PI3K/AKT pathway (11). Lu et al (2018) suggested
that the anticancer effect of RBG is achieved through the
PI3K/AKT/GSK3β pathway (26).
However, the action mechanism of RBG involving the PI3K/Akt pathway
in MM has not been revealed.
In the present study, the antitumor potential of RBG
was first evaluated on the malignant characteristics of MM cells.
The action mechanism of RBG involving the PI3K/AKT signaling
pathway was further studied. Overall, this study may reveal a
promising therapeutic drug for MM.
Materials and methods
Cell treatments
A human MM cell line, RPMI8226, (American Type
Culture Collection) was cultured in Roswell Park Memorial Institute
(RPMI) 1640 Medium (HyClone; Cytiva) containing 10% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin at 37˚C with 5% CO2. Different
concentrations of RBG (2, 4 and 8 µM) were used to treat RPMI8226
cells for 12, 24 and 48 h at 37˚C. The doses for RBG treatment were
selected according to previous reports (10,12,27).
In addition, RPMI8226 cells also received the treatments of 8 µM
RBG combined with 50 ng/ml insulin-like growth factor 1 (IGF-1; an
activator of the PI3K/AKT signaling pathway) (14,28)
for 12, 24 and 48 h at 37˚C in feedback verification assays.
RPMI8226 cells without treatments were used as the control, and
cells treated with RBG + PBS was used as a control for treatment
with RBG + IGF-1.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 (Beyotime Institute of Biotechnology) was used
for the detection of cell viability. Simply, 100 µl cells
(2x104 cells/ml) were seeded into 96-well plates and
then treated with RBG and/or IGF-1 for 12, 24 and 48 h at 37˚C,
respectively. CCK-8 solution (10 µl) was subsequently added into
each well. After 2 h of incubation at 37˚C, the optical density at
450 nm was detected using a microplate reader (Wuxi Hiwell Diatek
Instruments Co., Ltd.). In addition, the IC50 value of
RBG was calculated at 48 h post treatment.
Flow cytometry
Flow cytometry was conducted to detect apoptosis
using an Apoptosis Detection Kit (cat. no. C1062S; Beyotime
Institute of Biotechnology). Briefly, cells (1x105
cells/ml) at 48 h post-treatment with RBG and/or IGF-1 were washed
with PBS three times and then suspended in 300 µl binding buffer.
After incubation with 5 µl Annexin V-Fluorescein isothiocyanate for
15 min at room temperature, cells were re-stained with 10 µl
propidium iodide (PI) for 10 min at room temperature. The apoptotic
ratio was measured on a flow cytometer (CytoFLEX S; Beckman
Coulter, Inc.) using Cell Quest software (version 5.1; BD
Biosciences).
Transwell assay
Cell migration and invasion were detected using
Transwell chambers. Cells at 48 h post-treatment with RBG and/or
IGF-1 were adjusted to 1x105/ml, and 200 µl cells were
added into the upper chamber (pre-coated with Matrigel and
air-dried naturally for invasion assay). The lower chamber was
added with RPMI 1640 containing 10% FBS. After 24 h of incubation
at 37˚C, cells in the lower chamber were washed with PBS, fixed
with methanol for 30 min at room temperature, and stained with
crystal violet for 20 min at room temperature. Cells were finally
counted under a microscope (DMi3000 B; Leica Microsystems GmbH) in
five randomly selected fields.
Western blotting
The protein expression of E-cadherin, N-cadherin,
Vimentin, AKT, phosphorylated (p)-AKT, PI3K and p-PI3K were
detected using western blotting. Total proteins were extracted by
lysing cells in RIPA lysis buffer (Beyotime Institute of
Biotechnology) and quantified using a BCA kit (Beyotime Institute
of Biotechnology). After separation using 10% SDS polyacrylamide
gel electrophoresis (50 µg protein per lane), the proteins were
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked with 5% non-fat milk for 1 h at room temperature and
incubated with the following specific primary antibodies:
Anti-E-cadherin (cat. no. ab133597; Abcam), -N-cadherin (cat. no.
ab76057; Abcam), -Vimentin (cat. no. ab137321; Abcam), -GAPHD (cat.
no. ab245355; Abcam), anti-AKT (cat. no. ab38449; Abcam), -PI3K
(cat. no. 4292; Cell Signaling Technology, Inc.), -p-AKT (cat. no.
4060; Cell Signaling Technology, Inc.) and -p-PI3K (cat. no.
AF3242; Affinity Biosciences Ltd.) (all 1:1,000) for 12 h at 4˚C.
Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:2,000; cat. no. ab205718; Abcam) for 1 h at 25˚C in the dark.
After visualization using ECL kit (Pierce; Thermo Fisher
Scientific, Inc.), images of the protein bands were captured using
a Gel Imaging System (Tanon 3500; Tanon Science and Technology Co.,
Ltd.). Gray analysis for protein bands was performed using the
ImageJ software (version 1.53r; National Institutes of Health) and
the protein expression was normalized to GAPDH.
Statistical analysis
The software of GraphPad Prism 7.0 (GraphPad
Software, Inc.) was used for statistical analysis. Each experiment
was performed in triplicate. Data are presented as the mean ±
standard deviation. Comparisons among different groups were
determined using one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
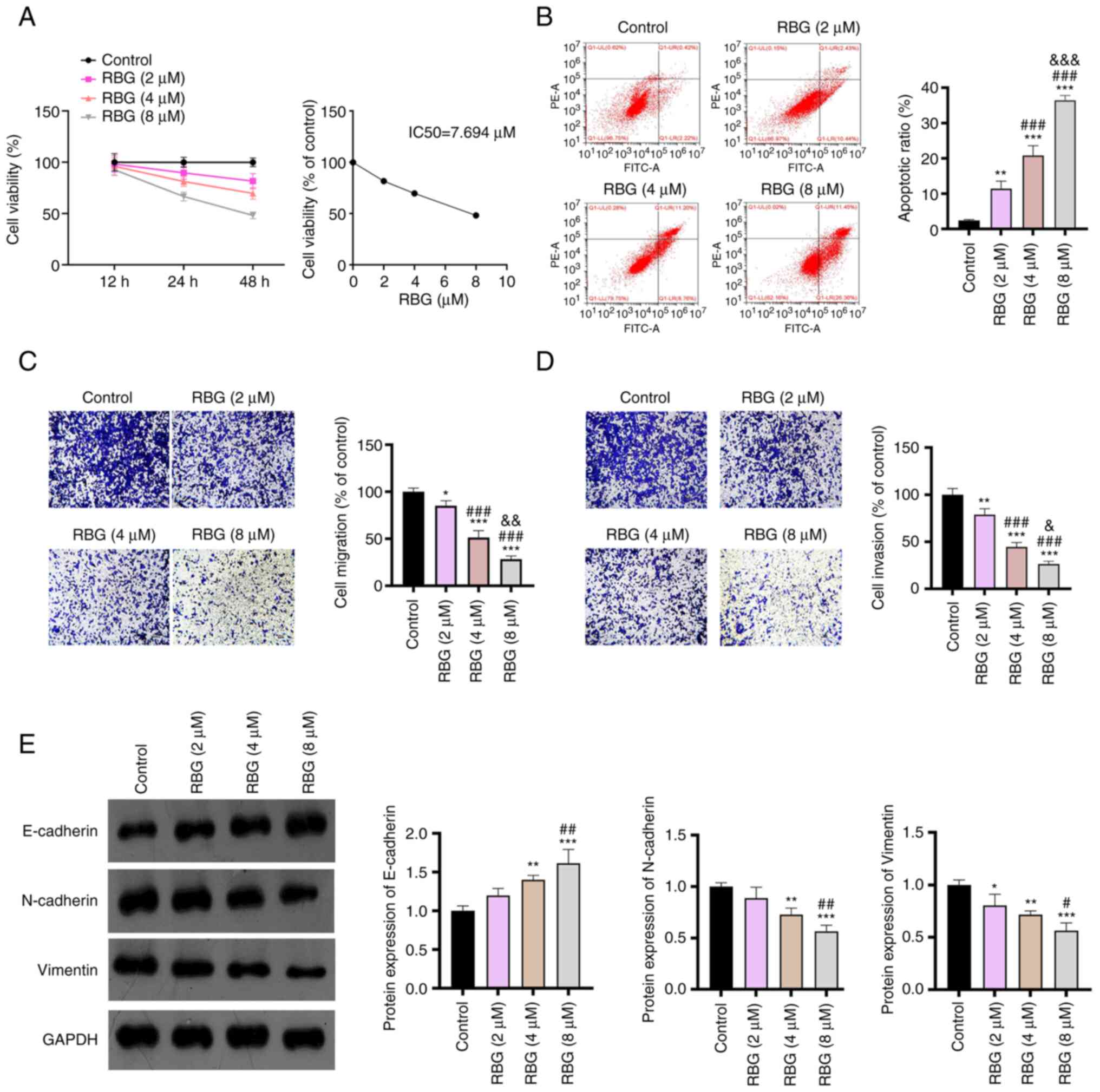
RBG inhibits the malignant
characteristics of MM cells
The function of RBG in MM was first evaluated in
RPMI8226 cells (a human MM cell line). As presented in Fig. 1A, the viability of RPMI8226 cells
treated with 8 µM RBG for 12 h was markedly lower compared with
that of the control. Both 24 and 48 h of RBG treatment could
markedly decrease the viability of RPMI8226 cells in a
dose-dependent manner. Meanwhile, the IC50 of RBG on
RPMI8226 cells was determined as 7.694 µM at 48 h treatment
(Fig. 1A). The time point of 48 h
was then used for subsequent functional experiments. Flow cytometry
demonstrated that RBG significantly promoted the apoptosis of
RPMI8226 cells with increasing concentrations in comparison to the
control group (P<0.01; Fig.
1B). In addition, the migration and invasion of RPMI8226 cells
were both significantly inhibited compared with the control by the
treatment of RBG in a dose-dependent manner (P<0.05; Fig. 1C and D). Western blotting further demonstrated
that 4 and 8 µM RBG significantly upregulated E-cadherin and
downregulated N-cadherin and Vimentin in RPMI8226 cells compared
with the control (P<0.001, Fig.
1E). These results indicated that RBG inhibits the
proliferation, migration and invasion of MM cells in a
dose-dependent manner.
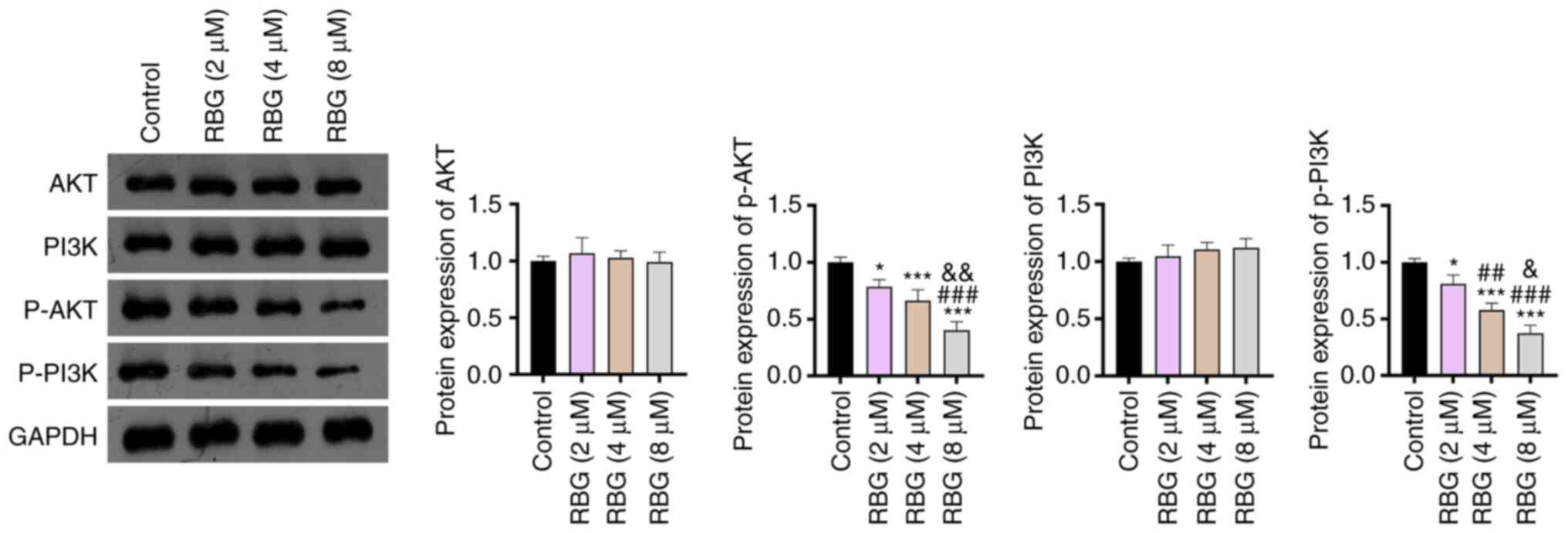
RBG blocks the PI3K/AKT signaling
pathway in MM cells
The action mechanism of RBG involving the PI3K/AKT
signaling pathway was subsequently analyzed in RPMI8226 cells.
Western blotting demonstrated that, compared with the control, RBG
significantly reduced the protein expression of p-AKT and p-PI3K in
RPMI8226 cells in a dose-dependent manner (P<0.05). The protein
expression of AKT and PI3K was not significantly changed by the
treatment of RBG in RPMI8226 cells (Fig. 2). These findings indicated that RBG
blocked the PI3K/AKT signaling pathway in MM cells.
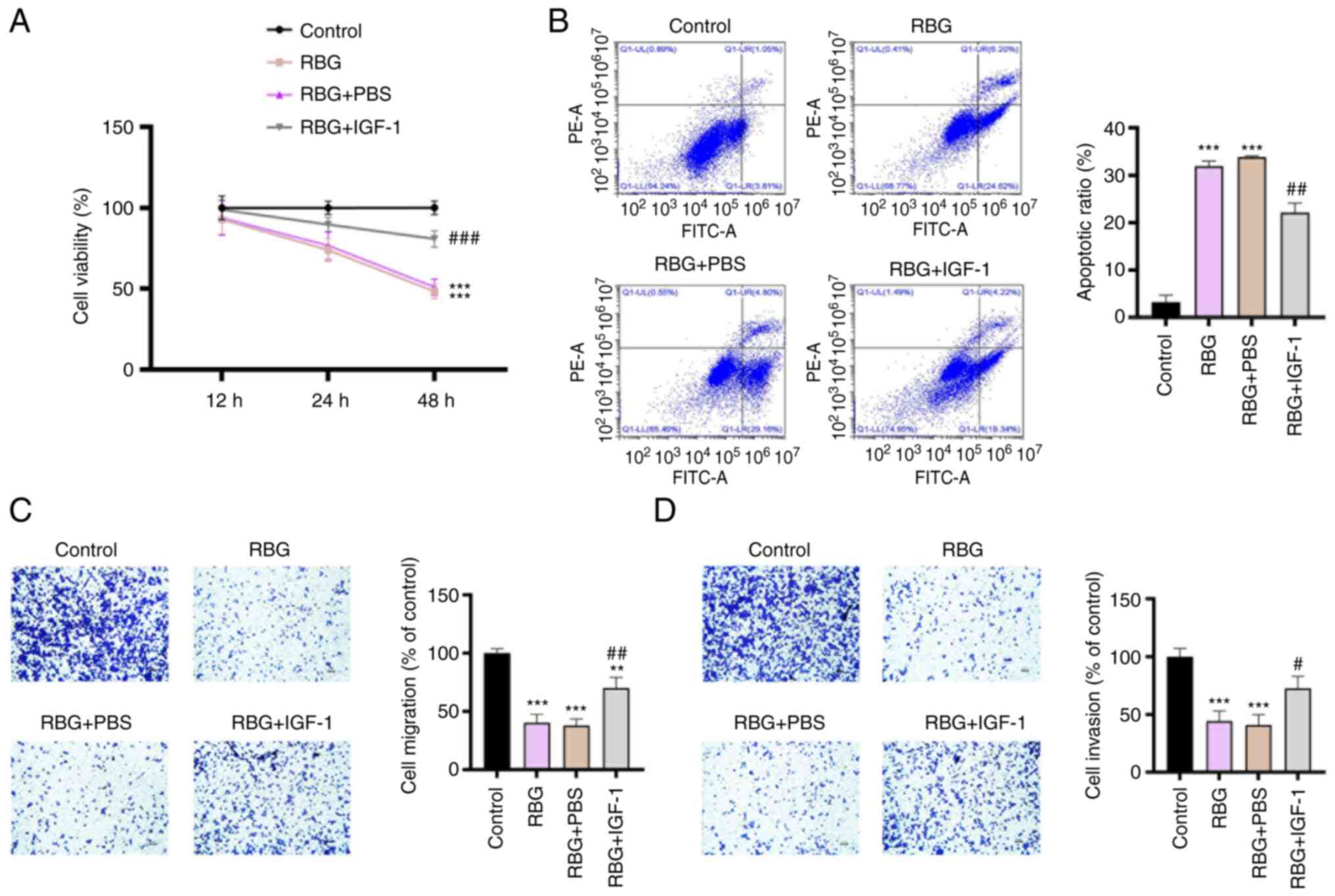
The activation of PI3K/AKT signaling
pathway weakens the antitumor effect of RBG in MM cells
In order to verify whether the antitumor effect of
RBG was associated with the blocking of PI3K/AKT signaling pathway,
IGF-1, an activator of the PI3K/AKT signaling pathway was used to
treat RPMI8226 cells. As presented in Fig. 3A, the intervention of IGF-1
significantly weakened the inhibiting effects of RBG on the
viability of RPMI8226 cells at 48 h post-treatment (P<0.001). By
contrast, IGF-1 significantly inhibited the promoting effect of RBG
on the apoptosis of RPMI8226 cells (P<0.01; Fig. 3B). The inhibiting effects of RBG on
the migration and invasion of RPMI8226 cells were also
significantly reversed by the intervention of IGF-1 (P<0.05;
Fig. 3C and D). In addition, EMT of MM cells was
evaluated by measuring the associated biomarkers (E-cadherin,
N-cadherin and Vimentin). The upregulation of E-cadherin and
downregulation of N-cadherin and Vimentin that were induced by RBG
were partially reversed by IGF-1 (P<0.01; Fig. 4). Western blotting further verified
that IGF-1 weakened RBG-induced blocking of the PI3K/AKT signaling
pathway, as evidenced by the increased levels of p-AKT/AKT and
p-PI3K/PI3K. (P<0.01; Fig. 4).
These results indicated that RBG exerts an antitumor effect on MM
by deactivating the PI3K/AKT signaling pathway.
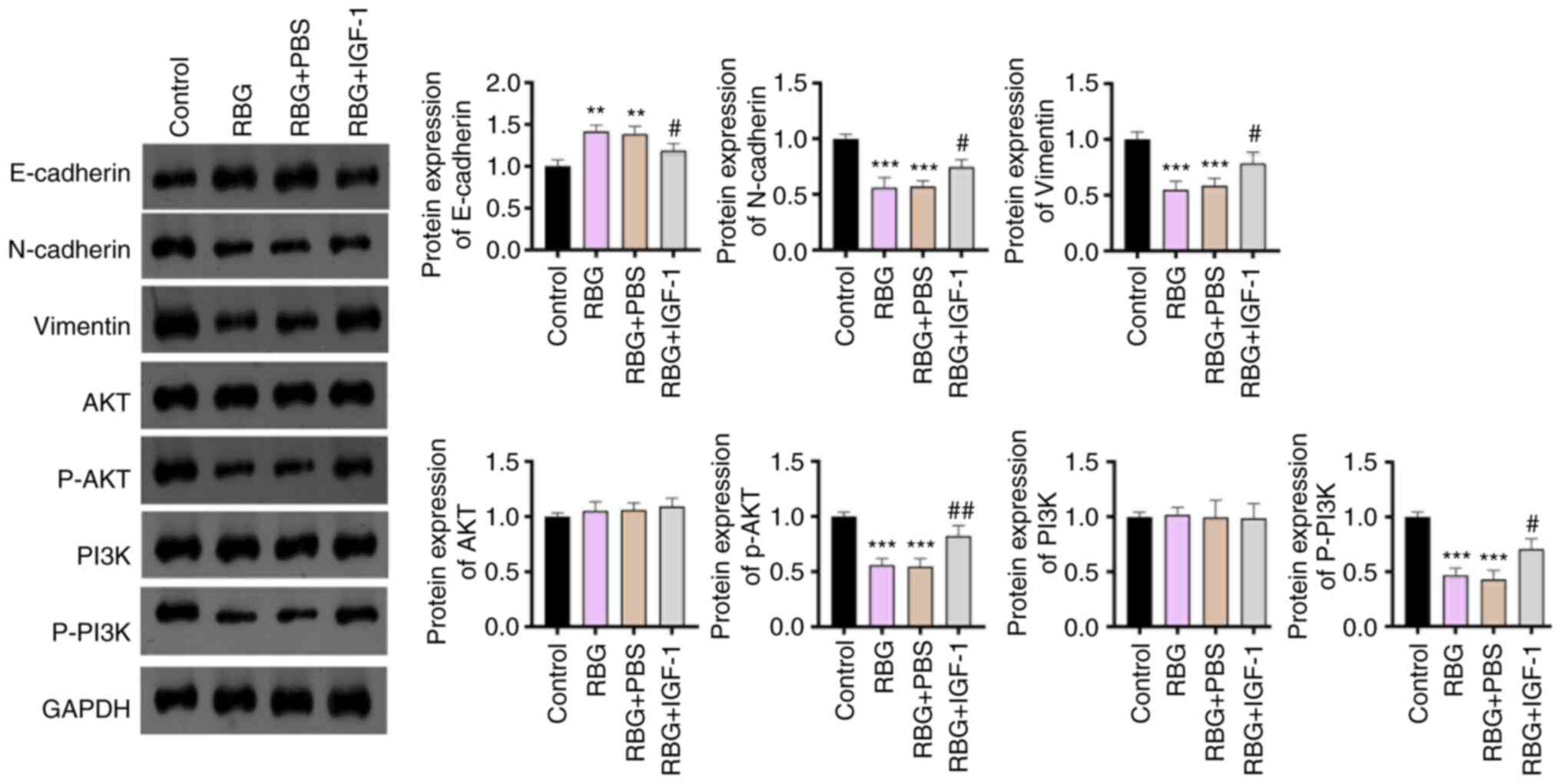 | Figure 4IGF-1 weakens the inhibiting effects
of RBG on the EMT and PI3K/AKT signaling pathway in MM cells.
RPMI8226 cells were treated with 8 µM RBG and 50 ng/ml IGF-1 (an
activator of the PI3K/AKT signaling pathway). The protein
expression of E-cadherin, N-cadherin, Vimentin, AKT, p-AKT, PI3K
and p-PI3K was detected by Western blot. **P<0.01,
***P<0.001 vs. Control; #P<0.05,
##P<0.01 vs. RBG. RBG, resibufogenin; IGF-1,
insulin-like growth factor 1; EMT, epithelial-mesenchymal
transition; MM, multiple myeloma; p, phosphorylated. |
Discussion
MM is a bone marrow-resident hematological
malignancy involving plasma cells (29). With the development of
immunomodulatory drugs, proteasome inhibitors and monoclonal
antibodies, improvements have been achieved in the survival of
patients with MM (5). However, MM
remains incurable and its prognosis remains unsatisfactory,
especially for elderly patients. Natural traditional Chinese
medicine is a promising source of potential antitumor drugs with
the advantages of having high efficiency and mild side effects
(30). RBG is a bufadienolide
isolated from toad venom that has been used to treat malignancies
for several decades in China (11). In the present study, the antitumor
ability of RBG against MM was preliminary revealed through the
assessment of MM cell proliferation, migration, invasion and EMT.
The underlying mechanism of RBG was revealed to be associated with
the blocking of the PI3K/AKT signaling pathway.
Toad venom is a product of toxic secretions,
containing a variety of active ingredients with antitumor activity,
such as bufalin, cinobufagin, arenobufagin and RBG (7). Previous studies have demonstrated
that bufalin and cinobufagin possess antitumor properties against
MM (31,32). Hence, the present study
hypothesized that RBG may also be used as a potential antitumor
drug for MM. The current study revealed that RBG significantly
inhibited the viability, migration and invasion, and promoted the
apoptosis of a MM cell line (RPMI8226 cells) in a dose-dependent
manner. These findings indicated that RBG was effective in
inhibiting the malignant characteristics of MM cells.
The antitumor effect of RBG in MM cells was
consistent with its role in a number of other types of cancer. For
example, RBG inhibits the proliferation, migration and invasion,
and induces the apoptosis of ovarian clear cell carcinoma cells
(11). In addition, RBG inhibits
the proliferation and promotes the apoptosis of gastric carcinoma
cells (26) and breast cancer
cells (12). Moreover, the present
study also demonstrated that RBG increased E-cadherin expression,
and decreased N-cadherin and Vimentin expression in RPMI8226 cells,
which are markers of EMT. These results indicated that RBG could
inhibit EMT in MM. As EMT confers enhanced tumor-initiating and
metastatic potential in cancer cells (33), the RBG-induced inhibition of EMT
may directly contribute to the treatment of MM. As aforementioned,
this provided evidence that RBG may be an effective antitumor drug
against MM.
The classic PI3K/Akt signaling pathway is an
important participant in tumorigenesis, which acts a key regulator
for cell proliferation, migration, adhesion, angiogenesis and drug
resistance (34). A previous study
determined that the PI3K/AKT signaling pathway is a promising
therapeutic target for MM (35).
Various inhibitors targeting this pathway have been developed for
the treatment of MM, such as TAS-117(19), Afuresertib (20), BKM120(21), BENC-511(36) and PIK-C98(37). In the present study, the potential
mechanism of RBG in MM involving the PI3K/Akt signaling pathway was
analyzed. The results revealed that RBG blocked the PI3K/AKT
signaling pathway in MM cells. It is hypothesized that RBG may
inhibit the malignant characteristics of MM cells by blocking the
PI3K/Akt signaling pathway. The present study's feedback assays
further verified this speculation, as evidenced by the fact that
the intervention of IGF-1 weakened the inhibiting effects of RBG on
the malignant characteristics of MM cells.
In conclusion, RBG is a potential therapeutic drug
against MM, which could inhibit cell viability, migration, invasion
and EMT, and promoted apoptosis in vitro. The blocking of
the PI3K/Akt signaling pathway is an underlying action mechanism of
RBG against MM. However, the present study is still limited to the
cellular level. The underlying mechanisms of RBG are not limited to
the PI3K/Akt signaling pathway. Further research on these
limitations is still needed.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Chinese Medicine Research
Fund Project of Zhejiang Province (grant no. 2021ZB092).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, ZH and SD substantially contributed to the
conception and the design of the study. YZ, ZH, KJ, CL, JX, HG, ZZ
and JS were responsible for the acquisition, analysis and
interpretation of the data. HG, ZZ, JS and SD confirm the
authenticity of all the raw data. YZ, ZH and JS contributed to
manuscript drafting and critical revisions of the intellectual
content. SD approved the final manuscript to be published and
obtained the funding. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rollig C, Knop S and Bornhauser M:
Multiple myeloma. Lancet. 385:2197–2208. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rashid N, Su Y, Gustavus Aranda J, Wu YL
and Han AK: Patterns and predictors of first-line therapy use among
newly diagnosed multiple myeloma patients ineligible for stem cell
transplant in an integrated healthcare system. Internet J Hematol.
10:1–8. 2014.
|
|
3
|
Rajkumar SV: Multiple myeloma: Every year
a new standard? Hematol Oncol. 37 (Suppl 1):S62–S65.
2019.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Kazandjian D: Multiple myeloma
epidemiology and survival: A unique malignancy. Semin Oncol.
43:676–681. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kehrer M, Koob S, Strauss A, Wirtz DC and
Schmolders J: Multiple Myeloma-current status in diagnostic testing
and therapy. Z Orthop Unfall. 155:575–586. 2017.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
6
|
Mimura N, Hideshima T and Anderson KC:
Novel therapeutic strategies for multiple myeloma. Exp Hematol.
43:732–741. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li FJ, Hu JH, Ren X, Zhou CM, Liu Q and
Zhang YQ: Toad venom: A comprehensive review of chemical
constituents, anticancer activities, and mechanisms. Arch Pharm
(Weinheim). 354(e2100060)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wei WL, An YL, Li ZW, Wang YY, Ji HJ, Hou
JJ, Wu WY and Guo DA: Simultaneous determination of resibufogenin
and its eight metabolites in rat plasma by LC-MS/MS for metabolic
profiles and pharmacokinetic study. Phytomedicine.
60(152971)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang LX, Zhao HY, Yuan SF, Ya-Jun LI, Bian
BL and Wang HJ: Determination of Total Bufadienolides in
Cinobufotalin Injection Using Ultraviolet Spectrophotometry. Chin J
Exp Tradit Med Form. 6:87–89. 2013.(In Chinese).
|
|
10
|
Han Q, Ma Y, Wang H, Dai Y, Chen C, Liu Y,
Jing L and Sun X: Resibufogenin suppresses colorectal cancer growth
and metastasis through RIP3-mediated necroptosis. J Transl Med.
16(201)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou G, Zhu Z, Li L and Ding J:
Resibufogenin inhibits ovarian clear cell carcinoma (OCCC) growth
in vivo, and migration of OCCC cells in vitro, by down-regulating
the PI3K/AKT and actin cytoskeleton signaling pathways. Am J Transl
Res. 11:6290–6303. 2019.PubMed/NCBI
|
|
12
|
Guo Y, Liang F, Zhao F and Zhao J:
Resibufogenin suppresses tumor growth and Warburg effect through
regulating miR-143-3p/HK2 axis in breast cancer. Mol Cell Biochem.
466:103–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Babaei G, Aziz SG and Jaghi NZZ: EMT,
cancer stem cells and autophagy; The three main axes of metastasis.
Biomed Pharmacother. 133(110909)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peng Y, Li F, Zhang P, Wang X, Shen Y,
Feng Y, Jia Y, Zhang R, Hu J and He A: IGF-1 promotes multiple
myeloma progression through PI3K/Akt-mediated
epithelial-mesenchymal transition. Life Sci.
249(117503)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jafari M, Ghadami E, Dadkhah T and
Akhavan-Niaki H: PI3k/AKT signaling pathway: Erythropoiesis and
beyond. J Cell Physiol. 234:2373–2385. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alzahrani AS: PI3K/Akt/mTOR inhibitors in
cancer: At the bench and bedside. Semin Cancer Biol. 59:125–132.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Keane NA, Glavey SV, Krawczyk J and
O'Dwyer M: AKT as a therapeutic target in multiple myeloma. Expert
Opin Ther Targets. 18:897–915. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Younes H, Leleu X, Hatjiharissi E, Moreau
AS, Hideshima T, Richardson P, Anderson KC and Ghobrial IM:
Targeting the phosphatidylinositol 3-kinase pathway in multiple
myeloma. Clin Cancer Res. 13:3771–3775. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mimura N, Hideshima T, Shimomura T, Suzuki
R, Ohguchi H, Rizq O, Kikuchi S, Yoshida Y, Cottini F, Jakubikova
J, et al: Selective and potent Akt inhibition triggers anti-myeloma
activities and enhances fatal endoplasmic reticulum stress induced
by proteasome inhibition. Cancer Res. 74:4458–4469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Spencer A, Yoon SS, Harrison SJ, Morris
SR, Smith DA, Brigandi RA, Gauvin J, Kumar R, Opalinska JB and Chen
C: The novel AKT inhibitor afuresertib shows favorable safety,
pharmacokinetics, and clinical activity in multiple myeloma. Blood.
124:2190–2195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Safaroghli-Azar A, Bashash D, Kazemi A,
Pourbagheri-Sigaroodi A and Momeny M: Anticancer effect of pan-PI3K
inhibitor on multiple myeloma cells: Shedding new light on the
mechanisms involved in BKM120 resistance. Eur J Pharmacol.
842:89–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Feng N, Luo J and Guo X: Silybin
suppresses cell proliferation and induces apoptosis of multiple
myeloma cells via the PI3K/Akt/mTOR signaling pathway. Mol Med Rep.
13:3243–3248. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu H, Dai X and Wang E: Plumbagin inhibits
cell proliferation and promotes apoptosis in multiple myeloma cells
through inhibition of the PI3K/Akt-mTOR pathway. Oncol Lett.
12:3614–3618. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang M, Huang J, Pan HZ and Jin J:
Triptolide overcomes dexamethasone resistance and enhanced
PS-341-induced apoptosis via PI3k/Akt/NF-kappaB pathways in human
multiple myeloma cells. Int J Mol Med. 22:489–496. 2008.PubMed/NCBI
|
|
25
|
Yang XJ, Xi YM and Li ZJ: Icaritin: A
novel natural candidate for hematological malignancies therapy.
Biomed Res Int. 2019(4860268)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu Z, Xu A, Yuan X, Chen K, Wang L and Guo
T: Anticancer effect of resibufogenin on gastric carcinoma cells
through the phosphoinositide 3-kinase/protein kinase B/glycogen
synthase kinase 3β signaling pathway. Oncol Lett. 16:3297–3302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang T, Jiang YX, Wu Y, Lu D, Huang R,
Wang LL, Wang SQ, Guan YY, Zhang H and Luan X: Resibufogenin
suppresses triple-negative breast cancer angiogenesis by blocking
VEGFR2-mediated signaling pathway. Front Pharmacol.
12(682735)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A,
Takahashi H, Wakasugi T, Funahashi H, Sato M and Takeyama H: IGF-1
mediates PTEN suppression and enhances cell invasion and
proliferation via activation of the IGF-1/PI3K/Akt signaling
pathway in pancreatic cancer cells. J Surg Res. 160:90–101.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Minnie SA and Hill GR: Immunotherapy of
multiple myeloma. J Clin Invest. 130:1565–1575. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu Y, Yang S, Wang K, Lu J, Bao X, Wang
R, Qiu Y, Wang T and Yu H: Cellular senescence and cancer: Focusing
on traditional Chinese medicine and natural products. Cell Prolif.
53(e12894)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang H, Cao Y, Wei W, Liu W, Lu SY, Chen
YB, Wang Y, Yan H and Wu YL: Targeting poly (ADP-ribose) polymerase
partially contributes to bufalin-induced cell death in multiple
myeloma cells. PLoS One. 8(e66130)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Baek SH, Kim C, Lee JH, Nam D, Lee J, Lee
SG, Chung WS, Jang HJ, Kim SH and Ahn KS: Cinobufagin exerts
anti-proliferative and pro-apoptotic effects through the modulation
ROS-mediated MAPKs signaling pathway. Immunopharmacol
Immunotoxicol. 37:265–273. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Harvey RD and Lonial S: PI3 kinase/AKT
pathway as a therapeutic target in multiple myeloma. Future Oncol.
3:639–647. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhu J, Wang M, Cao B, Hou T and Mao X:
Targeting the phosphatidylinositol 3-kinase/AKT pathway for the
treatment of multiple myeloma. Curr Med Chem. 21:3173–3187.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Han K, Xu X, Chen G, Zeng Y, Zhu J, Du X,
Zhang Z, Cao B, Liu Z and Mao X: Identification of a promising PI3K
inhibitor for the treatment of multiple myeloma through the
structural optimization. J Hematol Oncol. 7(9)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu J, Wang M, Yu Y, Qi H, Han K, Tang J,
Zhang Z, Zeng Y, Cao B, Qiao C, et al: A novel PI3K inhibitor
PIK-C98 displays potent preclinical activity against multiple
myeloma. Oncotarget. 6:185–195. 2015.PubMed/NCBI View Article : Google Scholar
|