Introduction
Esophageal cancer is a common type digestive system
malignancy and currently ranks sixth in terms of the rate of
cancer-associated mortality worldwide (1). Despite progress in the development of
novel treatment strategies, the 5-year survival rate of esophageal
cancer remains <20% (2).
Esophageal cancer in the early stages is confined to the mucosa or
superficial submucosa and accounts ~20% of all types of esophageal
cancers (3). Surgical resection
has long been the standard treatment method for early esophageal
cancer (4). However, the high
incidence of complications (such as gastroesophageal reflux and
respiratory failure) as a result of this technique renders it
unattractive, due to its severe negative effects on the quality of
life of patients (4).
Nevertheless, the development of gastroscopy has greatly improved
the quality of life of patients with early esophageal cancer.
As advancements in endoscopic technology are made
continuously, endoscopic resection is becoming the standard,
minimally invasive treatment procedure for early esophageal cancer
(5-7).
It has been found to shorten the length of hospital stay and reduce
the incidence of complications without affecting the quality of
life, compared with those after esophagectomy (5-7).
However, similar to traditional esophagectomy,
endoscopic resection also has the risk of a positive margin
(positive margin refers to the presence of atypical cells at the
lateral or deep resection margins). Positive margin after the
endoscopic resection of early esophageal carcinoma has direct
implications on the choice of treatment and disease prognosis
(8). To date, previous studies of
patients with early esophageal cancer (9-11)
after undergoing endoscopic submucosal dissection (ESD) have
produced sporadic results based on a small number of cases. Amongst
the available reports on positive margins, there are no reports of
multicenter, large-sample trials with long-term follow-up
periods.
Therefore, to explore methods of improving the
prognosis of patients after the endoscopic resection of esophageal
cancer, the present study investigated the potential risk factors
for positive margins by comprehensively analyzing clinical and
pathological data.
Materials and methods
Patients
The present study retrospectively analyzed the
clinical, endoscopic and pathological data of patients with
esophageal mucosal lesions treated with endoscopic resection in the
Fourth Hospital of Hebei Medical University (Shijiazhuang, China)
from January 2011 to December 2020.
The inclusion criteria of the lesions were as
follows: i) Complete resection of lesions; ii) pathological
diagnosis after endoscopic treatment was atypical cells (cells that
looked different and function differently than normal) [low-grade
intraepithelial neoplasia (LIN), high-grade intraepithelial
neoplasia (HIN) or invasive cancer]; and iii) patients provided
informed consent. The exclusion criterion was patients who were
pathologically diagnosed with adenocarcinoma or inflammation after
endoscopic treatment. Of note, intraepithelial neoplasia was
defined as the neoplastic change before epithelial invasion, which
can be divided further into ‘low-grade’ and ‘high-grade’ according
to whether the structural and cytological abnormalities observed
occupy the upper part of the epithelium (12).
A total of 369 patients (381 lesions) received
endoscopic resection due to early esophageal carcinoma. Of these,
236 were males and 133 were females with a mean age of 63.5±9.9
years. The main clinical symptoms were retropharyngeal asphyxia and
retrosternal pain. No other symptoms or findings could be observed
during physical examination. All patients and their relatives were
aware of the risks and benefits of endoscopic resection, following
which written informed consent was obtained from all participants.
The present study was approved by the Ethics committee of the
Fourth Hospital of Hebei Medical University (Shijiazhuang,
China).
Endoscopic treatment and pathological
examination
Under the guidance of endoscopy, two treatment
methods were used to remove the tumor: Endoscopic mucosal resection
(EMR) and ESD.
Endoscopy-guided surgery
The type of endoscopic treatment performed in the
present study was by professionally trained endoscopists using a
single-channel endoscope (Olympus H260; Olympus Corporation). Argon
plasma coagulation (APC; APC probes for flexible endoscope; Erbe
Elektromedizin GmbH) was used to mark ~5 mm outside the boundary of
the lesion. After fully marking the border, an
epinephrine-containing hypertonic saline solution was injected into
the submucosa for submucosal lifting. A circumferential mucosal
incision was then created around the marking spots. In cases where
the EMR technique was used, asymmetrical polypectomy snare (MTW
Endoskopie) resection was initiated. For ESD, the submucosal layer
was dissected using the IT-knife2 (KD-611L; Olympus Corporation).
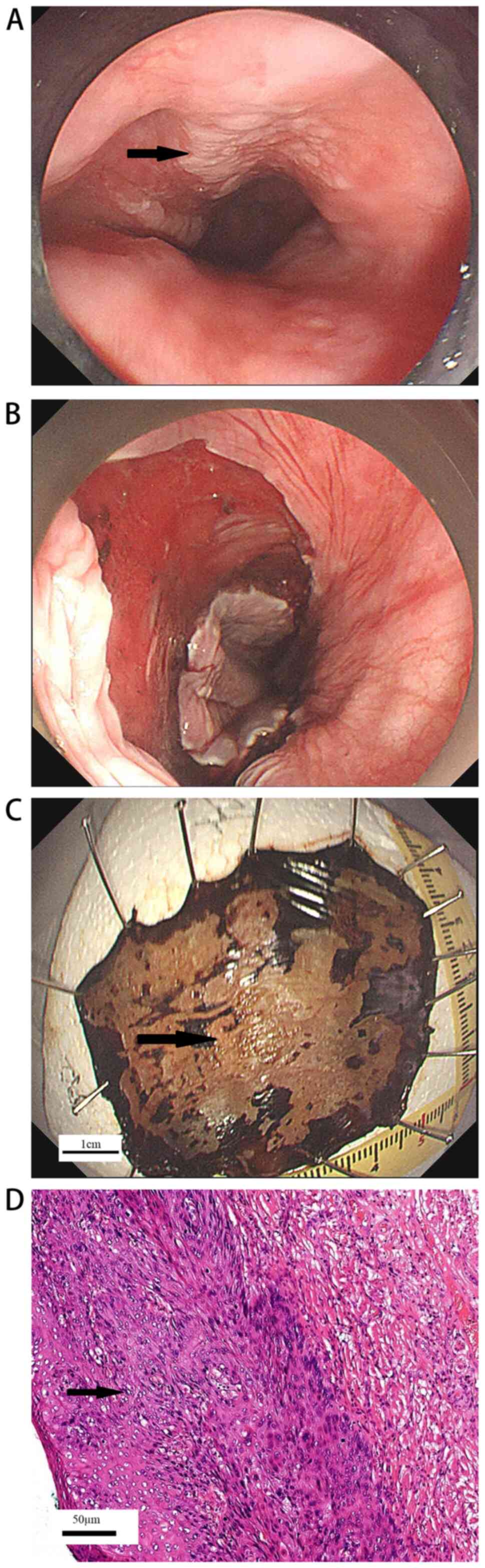
After the lesions were resected, the wounds were closed with APC,
hot biopsy forceps coagulation or using titanium clips according to
the wound conditions (Fig. 1A and
B). The lesion was completely
removed en bloc (Fig. 1C).
The pathologist then evaluated tumor involvement in the lateral or
deep resection margin (Fig.
1D).
Post-operative pathological
examination
The resected specimens were processed and fixed
immediately, before the lesion size was measured. After fixation
with 10% formalin (20-25˚C for 12-24 h), the specimens were sent
for pathological examination. The samples were cut into continuous
sections at 2-mm intervals from top to bottom and embedded in
paraffin. In total, three sections were prepared from each tissue
and then stained with hematoxylin and eosin using the Ventana HE
600 automatic staining machine (Roche Diagnostics; 20-25˚C). An
experienced pathologist (Dr Yao Liu, Department of Pathology,
Fourth Hospital of Hebei Medical University) then used a light
microscope (DM1000; Leica Microsystems GmbH; magnification, x200)
to evaluate tumor involvement in the lateral or deep resection
margin to avoid misjudgment. When examining the tissue sections for
pathological evaluation, all sections were observed from the top of
the tissue to identify the nature of the lesion, the degree of
tumor differentiation, whether the vertical/transverse edge of the
tumor was positive and whether there was vascular infiltration. A
positive resection margin as defined as the presence of atypical
cells (LIN, HIN or invasive cancer) at the lateral or deep
resection margin.
Statistical analysis
Sex, age, lesion location, tumor diameter, depth of
invasion, endoscopic treatment, endoscopic ultrasonography (EUS)
before resection, working experience of the endoscopist and degree
of tumor differentiation were all analyzed as potential risk
factors.
Continuous data are presented as the mean ± standard
deviation. χ2 and nonparametric Mann-Whitney U tests
were used for statistical analysis. Univariate and multivariate
logistic regression models were used to examine the association
between the variables and positive margins risk factors. P<0.05
was considered to indicate a statistically significant
significance. The SPSS vl9.0 (IBM Corp.) was used for data
processing.
Results
General data
The present study was a retrospective analysis of
the data collected from 369 patients with early esophageal cancer
who met the inclusion criteria. Among all study participants, 236
were male and 133 were female with a ratio of 1.8:1. The average
age was 63.5±9.9 (range, 31-85) years.
Residues at the resection margin and
follow-up data
In total, 73 patients had positive margin, such that
the positive rate was 19.2%, where 64 (16.8%) were positive for
lateral margins and nine (2.4%) were positive for vertical margins.
In addition, it was found that the residual rates of EMR and ESD
were 26.9 and 16.7%, respectively. Only 7.5% (23/308) poorly
differentiated lesions were diagnosed in negative margins. By
contrast, the proportion of positive margin specimens was 28.8%
(21/73). There was no vascular invasion or submucosal lymphatic
metastasis. After communicating with the patients and their
families, 29 patients asked for regular follow-up, while others
with positive margins received another ESD or EMR and were followed
up 1 month after operation. During the follow-up period, endoscopy
was performed 3, 6 and 12 months after endoscopic resection, before
being performed every year thereafter. Chest CT was also performed
6 and 12 months after endoscopic resection followed by once a year
thereafter as a precaution for distant metastasis. Follow up was
halted 5 years after endoscopic resection. If tumor cells were
found at the edge of the deep resection, additional esophagectomy
or esophageal radiotherapy and chemotherapy would be performed.
These treatment options were selected according to the situation of
each patient and the wishes of patients and their families. In the
present study, 65 cases of residual tumors were successfully
followed up for 2-60 months, with an average follow-up time of
28.1±10.1 months. The follow-up rate was 89%. However, eight
patients were lost to follow-up. During the follow-up, five
patients succumbed to cardiovascular and cerebrovascular diseases,
whilst the other 60 patients remained alive during the follow-up
period.
Comparison of groups with or without
residual margins
A number of associated factors can affect the
residual margin, including sex, age, lesion location, tumor
diameter, depth of invasion, endoscopic treatment, EUS before
resection, working experience of the endoscopist and degree of
tumor differentiation (12).
Univariate and multivariate analyses were performed to determine if
any of these factors can affect the residual margin after
endoscopic resection. As shown in Table I, there was no significant
difference in sex, age, lesion location or working experience of
endoscopists in the univariate analysis of incision margins. By
contrast, tumor diameter, endoscopic treatment, depth of invasion,
EUS before resection and the degree of tumor differentiation were
all risk factors for residual margins. To exclude any confounding
factors, multivariate logistic regression analysis (Table II) was performed. Only the depth
of tumor invasion, degree of differentiation and EUS before
resection were regarded to be risk factors for residual
margins.
 | Table IComparison of the groups with and
without post-endoscopic resection residues at resection
margins. |
Table I
Comparison of the groups with and
without post-endoscopic resection residues at resection
margins.
| Parameter | Residues at resection
margin (n=73) | No Residues at
resection margin (n=308) | P-value |
|---|
| Age (years) | 64.8±8.9 | 60.9±11.4 | 0.891 |
| Sex | | | 0.435 |
|
Female | 23 | 112 | |
|
Male | 50 | 196 | |
| Location | | | 0.136 |
|
Upper | 3 | 23 | |
|
Middle | 42 | 201 | |
|
Lower | 28 | 84 | |
| Tumor diameter | | | <0.001 |
|
≤1 cm | 2 | 29 | |
|
1.1-3
cm | 28 | 190 | |
|
>3
cm | 43 | 89 | |
| Endoscopic
treatment methods | | | 0.03 |
|
Endoscopic
mucosal resection | 25 | 68 | |
|
Endoscopic
submucosal dissection | 48 | 240 | |
| Endoscopic
ultrasonography evaluation before resection | | | <0.001 |
|
Yes | 42 | 257 | |
|
No | 31 | 51 | |
| Working
experience | | | 0.673 |
|
≥5
years | 45 | 198 | |
|
<5
years | 28 | 110 | |
| Depth of
invasion | | | <0.001 |
|
Intramucosal
cancer (M1) | 30 | 208 | |
|
Lamina
propria (M2) | 11 | 33 | |
|
Muscularis
mucosa (M3) | 9 | 45 | |
| Shallow and deep
submucosal layer (SM1-2) | 23 | 22 | |
|
Differentiation | | | <0.001 |
|
Well | 23 | 233 | |
|
Moderate | 29 | 52 | |
|
Poor | 21 | 23 | |
 | Table IIResults of multivariate logistic
regression analysis. |
Table II
Results of multivariate logistic
regression analysis.
| Parameter | P-value | Odds ratio | 95% Confidence
interval |
|---|
| Age | 0.820 | 1.051 | 0.686-1.610 |
| Sex | 0.437 | 0.763 | 0.385-1.510 |
| Maximum diameter of
resected specimen | 0.39 | 1.325 | 0.698-2.515 |
| Location | 0.663 | 1.187 | 0.549-2.564 |
| Endoscopic
resection procedures | 0.526 | 0.774 | 0.350-1.710 |
| Depth of tumor
invasion | <0.001 | 2.182 | 1.704-2.795 |
| Work
experience | 0.155 | 0.624 | 0.325-1.196 |
| Degree of tumor
differentiation | 0.018 | 0.451 | 0.166-1.224 |
| Endoscopic
ultrasonography before resection | <0.001 | 35.826 | 7.400-173.454 |
Discussion
Applications of EMR and ESD are becoming
increasingly common for the treatment of early esophageal cancer
(7). Endoscopic resection is able
to not only preserve the integrity of the esophagus, but also
circumvent the considerable risk of morbidity and mortality
associated with esophageal resection (13). Previous studies have reported that
although the effect of endoscopic resection on early esophageal
cancer is equivalent to that of surgical resection, a lower
incidence of complications and superior quality of life was
associated with endoscopic resection (5,7).
Several cohort studies (14-17)
have recommended the use of EMR or ESD for T1a esophageal tumors,
including highly atypical hyperplasia, adenocarcinoma or squamous
cell carcinoma, which are limited to the superficial mucosa and do
not extend to the muscular mucosa. In addition, Manner et al
(18) have evaluated the efficacy
and safety of endoscopic resection in selected cases with mucosal
myometrial infiltration and upper third submucosal involvement.
These studies further verified the application of endoscopic
resection in early esophageal cancer.
After the endoscopic resection of early esophageal
cancer, the positive rate of resection edge varies greatly
according to the literature, ranging 1.7-22% (9-11).
In the present study, observation of atypical cells (including LIN,
HIN or invasive cancer) at the resection margin was used as the
criteria, where the positive rate of the resection margin was
19.2%. If only invasive cancer was considered, the positive rate of
the resection edge then decreases to 5.4%. Previous studies have
found that ESD has a higher en bloc resection and complete
resection rates compared with those following traditional EMR
(19-21).
This finding is consistent with results from the present study,
which found that the residual rates of EMR and ESD were 26.9 and
16.7%, respectively. However, based on logistic regression
analysis, endoscopic resection was not found to be an independent
risk factor, which is consistent with the results of Sgourakis
et al (22). This finding
may be associated with the results of retrospective analysis and
not to the results of randomized controlled trials.
Over recent years, an increasing number of
gastroenterologists in China consider ESD to be the optimal choice
for the treatment of early esophageal cancer (23,24).
They believe that with continuous advancements in ESD, the
incidence of serious complications, such as perforation and
bleeding, can be controlled to negligible levels (23-25).
These studies further confirm that ESD is safe and effective in the
treatment of early esophageal cancer.
Isomoto et al (26) previously reported that tumor size
had no significant association with curative resection. However,
the tumor size was significantly associated with segmental
resection, where the cure resection rate was significantly lower
compared with that of whole resection (26,27).
In the present study, univariate analysis revealed that the maximum
diameter of the primary tumor was associated with residual margin,
but not in the multivariate analysis, which may be due to the
nonrandomized endoscopic treatment methods (EMR and ESD) in the
present retrospective analysis. In addition, the present study
found that preoperative EUS examination and depth of tumor invasion
are independent risk factors for residual resection margin.
Previous studies have shown that EUS is the optimal noninvasive
tool for T1 esophageal cancer, with a sensitivity of 85% and a
specificity of 87% (28,29). This finding was also illustrated in
previous studies of gastrointestinal neuroendocrine tumors
(30,31) and is consistent with results from
the present study.
Preoperative evaluation of the depth of tumor
invasion can minimize the residual edge of deep resection (32). Lugol iodine staining, NBI
amplification and EUS can all be used to evaluate lesion size and
depth of tumor invasion (33,34).
Through the above methods, it is possible to reduce the residual
margin in the process of endoscopic resection. Furthermore,
unnecessary resection should be avoided to prevent esophageal
stenosis.
The degree of tumor differentiation has also been
previously established to be an independent risk factor (31,35-37).
However, no similar reports exist regarding esophageal cancer.
Poorly differentiated tumors are typically more likely to invade
the vascular system and lymph nodes earlier or cause deep
infiltration (38). In the present
study, only 23 (7.5%) poorly differentiated lesions were diagnosed
in 308 specimens with negative margins. By contrast, the proportion
of positive margin specimens (21 poorly differentiated,
representing 28.8% of 73 specimens) was significantly higher
compared with that of negative margin specimens. In a previous
study with gastric cancer, in the high-risk category, the benefit
of surgery appears to be positive, since the cancer-specific
survival rate in the salvage surgery group was higher compared that
in the follow-up group (39).
These data suggest that if biopsy indicates poorly differentiated
tumors and if the patient's physical conditions allow, more
aggressive treatment strategies should be recommended. Due to the
limited follow-up time, no corresponding data were available and
these patients require continuous close attention.
It remains unclear whether surgery and other medical
interventions should be actively performed for patients with
residual margins after endoscopic resection. For the designation of
subsequent treatment plans, the patient's age, complications and
willingness should also be considered. In this group of data,
amongst the nine patients with positive deep margins, six patients
received surgical treatment, two patients received radiotherapy and
chemotherapy, whereas one patient was closely observed. No
recurrence was found during the follow-up. Among the 64 patients
with positive lateral margins, one patient received radiotherapy,
one patient received chemotherapy, six patients received surgical
treatment, 20 patients received additional EMR or ESD, eight
patients were lost to follow-up and the remaining 28 patients were
closely observed. During the follow-up period, five patients
succumbed to cardiovascular and cerebrovascular diseases whereas no
recurrence was found in other patients. In the present study, a
preliminary prognostic analysis of patients with positive margins
after endoscopic resection of early esophageal cancer was
performed. A more detailed prognostic analysis needs to be verified
by a multicenter study with a longer term of follow-up.
The present study has a number of limitations. The
present study is only a retrospective single-center study, not a
randomized controlled study. Only one pathologist participated in
the analysis of the tissues. Therefore, there may be potential
selection bias in the present study. Prospective clinical trials
may be conducted in the future to further verify these results.
In conclusion, the present study analyzed the risk
factors associated with the residual margin and the prognosis of
patients. According to the results of data analysis, it is highly
recommended to apply EUS for evaluating the depth of tumor
invasion, determining the depth of lesion invasion and the
indication of endoscopic resection before operation, all of which
can effectively prevent a residual margin. If biopsy indicates a
poorly differentiated tumor, more aggressive treatment strategies
may be required to prevent recurrence after endoscopic
resection.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The Key topics of
medical science research of Hebei Provincial Health Commission
(grant no. 20190765).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF and BQL designed the study. YF and SG confirm the
authenticity of all the raw data. YF and WW collected clinical and
pathological data of patients. SG and YF analyzed the data. BQL and
WW contributed to the interpretation of results. All authors
critically reviewed the manuscript, and read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients and their families agreed to
participate in the present study and signed an informed consent
form. The study was approved by the Ethics Committee of the Fourth
Hospital of Hebei Medical University (Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang L, Zhou Y, Cheng C, Cui HY, Cheng L,
Kong PZ, Wang JQ, Li Y, Chen WL, Song B, et al: Genomic analyses
reveal mutational signatures and frequently altered genes in
esophageal squamous cell carcinoma. Am J Hum Genet. 96:597–611.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Njei B, McCarty TR and Birk JW: Trends in
esophageal cancer survival in United States adults from 1973 to
2009: A SEER database analysis. J Gastroenterol Hepatol.
31:1141–1146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Naveed M and Kubiliun N: Endoscopic
treatment of early-stage esophageal cancer. Curr Oncol Rep.
20(71)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wani S, Early D, Edmundowicz S and Sharma
P: Management of high-grade dysplasia and intramucosal
adenocarcinoma in Barrett's esophagus. Clin Gastroenterol Hepatol.
10:704–711. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Neuhaus H: Endoscopic submucosal
dissection in the upper gastrointestinal tract: Present and future
view of Europe. Dig Endosc. 21 (Suppl 1):S4–S6. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chennat J, Konda VJ, Ross AS, de Tejada
AH, Noffsinger A, Hart J, Lin S, Ferguson MK, Posner MC and Waxman
I: Complete Barrett's eradication endoscopic mucosal resection: an
effective treatment modality for high-grade dysplasia and
intramucosal carcinoma-an American single-center experience. Am J
Gastroenterol. 104:2684–2692. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Oyama T, Tomori A, Hotta K, Morita S,
Kominato K, Tanaka M and Miyata Y: Endoscopic submucosal dissection
of early esophageal cancer. Clin Gastroenterol Hepatol. 3 (Suppl
1):S67–S70. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mariette C, Fabre S, Balon JM, Finzi L and
Triboulet JP: Factors predictive of complete resection of operable
esophageal cancer: review of 746 patients. Gastroenterol Clin Biol.
26:454–462. 2002.PubMed/NCBI
|
|
9
|
Fujishiro M, Yahagi N, Kakushima N,
Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y,
Oka M, et al: Endoscopic submucosal dissection of esophageal
squamous cell neoplasms. Clin Gastroenterol Hepatol. 4:688–694.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Repici A, Hassan C, Carlino A, Pagano N,
Zullo A, Rando G, Strangio G, Romeo F, Nicita R, Rosati R and
Malesci A: Endoscopic submucosal dissection in patients with early
esophageal squamous cell carcinoma: Results from a prospective
Western series. Gastrointest Endosc. 71:715–721. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ono S, Fujishiro M, Niimi K, Goto O,
Kodashima S, Yamamichi N and Omata M: Long-term outcomes of
endoscopic submucosal dissection for superficial esophageal
squamous cell neoplasms. Gastrointest Endosc. 70:860–886.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hamilton SR and Aaltonen LA: World Health
Organization classification of tumours: Pathology and genetics of
tumours of the digestive system. IARC Press, Lyon, pp11-36,
2000.
|
|
13
|
Birkmeyer JD, Siewers AE, Finlayson EV,
Stukel TA, Lucas FL, Batista I, Welch HG and Wennberg DE: Hospital
volume and surgical mortality in the United States. N Engl J Med.
346:1128–1137. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ell C, May A, Pech O, Gossner L, Guenter
E, Behrens A, Nachbar L, Huijsmans J, Vieth M and Stolte M:
Curative endoscopic resection of early esophageal adenocarcinomas
(Barrett's cancer). Gastrointest Endosc. 65:3–10. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pech O, Behrens A, May A, Nachbar L,
Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M,
et al: Long-term results and risk factor analysis for recurrence
after curative endoscopic therapy in 349 patients with high-grade
intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's
oesophagus. Gut. 57:1200–1206. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pech O, Gossner L, May A, Vieth M, Stolte
M and Ell C: Endoscopic resection of superficial esophageal
squamous-cell carcinomas: Western experience. Am J Gastroenterol.
99:1226–1232. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stahl M, Budach W, Meyer HJ and Cervantes
A: Esophageal cancer: Clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 21 (Suppl 5):v46–v49.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Manner H, May A, Pech O, Gossner L,
Rabenstein T, Günter E, Vieth M, Stolte M and Ell C: Early
Barrett's carcinoma with ‘low-risk’ submucosal invasion: Long-term
results of endoscopic resection with a curative intent. Am J
Gastroenterol. 103:2589–2597. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hazama H, Tanaka M, Kakushima N, Yabuuchi
Y, Yoshida M, Kawata N, Takizawa K, Ito S, Imai K, Hotta K, et al:
Predictors of technical difficulty during endoscopic submucosal
dissection of superficial esophageal cancer. Surg Endosc.
33:2909–2915. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Plum PS, Hölscher AH, Pacheco Godoy K,
Schmidt H, Berlth F, Chon SH, Alakus H and Bollschweiler E:
Prognosis of patients with superficial T1 esophageal cancer who
underwent endoscopic resection before esophagectomy-A propensity
score-matched comparison. Surg Endosc. 32:3972–3980.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang HY, Zeng X, Bai SY, Pu K, Zheng Y, Ji
R, Guo QH, Guan QL, Wang YP and Zhou YN: The safety and efficacy of
endoscopic submucosal dissection for treating early oesophageal
carcinoma: A meta-analysis. Ann R Coll Surg Engl. 102:702–711.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sgourakis G, Gockel I and Lang H:
Endoscopic and surgical resection of T1a/T1b esophageal neoplasms:
A systematic review. World J Gastroenterol. 19:1424–1437.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qi ZP, Chen T, Li B, Ren Z, Yao LQ, Shi Q,
Cai SL, Zhong YS and Zhou PH: Endoscopic submucosal dissection for
early esophageal cancer in elderly patients with relative
indications for endoscopic treatment. Endoscopy. 50:839–845.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu Y, Zhang H, Zhou B, Han SY and Zhang
YR: Clinical efficacy of endoscopic submucosal dissection in the
treatment of early esophageal cancer and precancerous lesions. J
Cancer Res Ther. 14:52–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yip HC and Chiu PW: Endoscopic diagnosis
and management of early squamous cell carcinoma of esophagus. J
Thorac Dis. 9 (Suppl 8):S689–S696. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Isomoto H, Shikuwa S, Yamaguchi N, Fukuda
E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J and Kohno
S: Endoscopic submucosal dissection for early gastric cancer: A
large-scale feasibility study. Gut. 58:331–336. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY,
Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, et al: Therapeutic
outcomes in 1000 cases of endoscopic submucosal dissection for
early gastric neoplasms: Korean ESD study group multicenter study.
Gastrointest Endosc. 69:1228–1235. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Thosani N, Singh H, Kapadia A, Ochi N, Lee
JH, Ajani J, Swisher SG, Hofstetter WL, Guha S and Bhutani MS:
Diagnostic accuracy of EUS in differentiating mucosal versus
submucosal invasion of superficial esophageal cancers: A systematic
review and meta-analysis. Gastrointest Endosc. 75:242–253.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang J, Luo GY, Liang RB, Zeng TS, Long H,
Fu LH, Xu GL, Yang MZ, Li S, Zhang LJ, et al: Efficacy of
endoscopic ultrasonography for determining clinical T category for
esophageal squamous cell carcinoma: Data from 1434 surgical cases.
Ann Surg Oncol. 25:2075–2082. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ishii N, Horiki N, Itoh T, Maruyama M,
Matsuda M, Setoyama T, Suzuki S, Uchida S, Uemura M, Iizuka Y, et
al: Endoscopic submucosal dissection and preoperative assessment
with endoscopic ultrasonogra-phy for the treatment of rectal
carcinoid tumors. Surg Endosc. 24:1413–1419. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou FR, Huang LY and Wu CR: Endoscopic
mucosal resection for rectal carcinoids under micro-probe
ultrasound guidance. World J Gastroenterol. 19:2555–2559.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lee TH, Cho JY, Chang YW, Kim JO, Lee JS,
Cho WY, Kim HG, Kim WJ, Park YS and Jin SY: Appropriate indications
for endoscopic submucosal dissection of early gastric cancer
according to tumor size and histologic type. Gastrointest Endosc.
71:920–926. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fujishiro M, Kodashima S, Goto O, Ono S,
Niimi K, Yamamichi N, Oka M, Ichinose M and Omata M: Endoscopic
submucosal dissection for esophageal squamous cell neoplasms. Dig
Endosc. 21:109–115. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yoshida T, Inoue H, Usui S, Satodate H,
Fukami N and Kudo SE: Narrow-band imaging system with magnifying
endoscopy for superficial esophageal lesions. Gastrointest Endosc.
59:288–295. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kakushima N, Ono H, Tanaka M, Takizawa K,
Yamaguchi Y and Matsubayashi H: Factors related to lateral margin
positivity for cancer in gastric specimens of endoscopic submucosal
dissection. Dig Endosc. 23:227–232. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wen J, Linghu EQ, Yang YS, Liu QS, Yang J
and Lu ZS: Associated risk factor analysis for positive resection
margins after endoscopic submucosal dissection in early-stage
gastric cancer. J BUON. 20:421–427. 2015.PubMed/NCBI
|
|
37
|
Hwang JJ, Park KJ, Park YS, Lee HS, Yoon
H, Shin CM, Kim N and Lee DH: A scoring system for patients with a
tumor-positive lateral resection margin after endoscopic resection
of early gastric cancer. Surg Endosc. 30:2751–2758. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Oyama T, Inoue H, Arima M, Momma K, Omori
T, Ishihara R, Hirasawa D, Takeuchi M, Tomori A and Goda K:
Prediction of the invasion depth of superficial squamous cell
carcinoma based on microvessel morphology: Magnifying endoscopic
classification of the Japan esophageal society. Esophagus.
14:105–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hatta W, Gotoda T, Oyama T, Kawata N,
Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M and Esaki
M: , et al: Is the eCura system useful for selecting
patients who require radical surgery after noncurative endoscopic
submucosal dissection for early gastric cancer? A comparative
study. Gastric Cancer. 21:481–489. 2018.PubMed/NCBI View Article : Google Scholar
|