Introduction
Myocardial hypertrophy is an independent risk factor
for cardiovascular disease and may develop into heart failure
(1). Myocardial hypertrophy is
marked by increased myocyte volume, ventricular wall thickness and
myocardial contractility under early overload stress conditions
(2). Persistent myocardial
hypertrophy is often accompanied by severe myocardial interstitial
fibrosis, leading to dilated cardiomyopathy and even sudden death
(3). The current pharmacological
treatment for cardiac hypertrophy is based on anti-hypertension,
such as angiotensin-converting enzyme inhibitors, angiotensin II
receptor blockers, calcium antagonists and nitric oxide donors
(4). However, the toxic side
effects of the drugs used inhibit efficient treatment of myocardial
hypertrophy. Results of previous studies have demonstrated that
myocardial hypertrophy exhibits multiple damaging effects on the
heart, including apoptosis, cellular hypertrophy and fibrosis,
oxidative stress, and overproduction of inflammation (5-8).
Consequently, the reduction in the hypertrophic damage of the
cardiomyocytes is one of the most important ways to improve the
symptoms of heart disease.
F-box and WD repeat domain-containing protein 7
(FBW7), also known as FBXW7, is a member of the F-box protein
family (9). Previous studies have
revealed that FBW7 exerts a notable regulatory role in
cardiomyocytes. For example, microRNA (miR)-25 promotes
cardiomyocyte proliferation by targeting FBXW7(10). MiR-195-5p promotes cardiomyocyte
hypertrophy by targeting mitofusin 2 and FBXW7(11). In addition, the dysregulation of
FBW7 exerts a key role in the progression of hematological tumors
(12-14).
A previous study indicated that FBW7 expression is significantly
downregulated in Ang II-induced cardiac fibroblasts and that
miR-27b promotes cardiac fibrosis by targeting the FBW7/Snail
pathway (15). In addition, mice
lacking FBW7 have defective cardiovascular development (16). This evidence suggests that FBW7 may
have a notable regulatory role in cardiac development.
By contrast, mTOR is a serine/threonine kinase,
which performs a key role in the regulation of autophagy (17). It has been documented that FBW7
levels are reduced in high glucose-induced renal thylakoid cells,
whereas their overexpression increases cellular autophagy by
inhibiting the mTOR signaling pathway (18). Diabetic nephropathy is highly
correlated with cardiac hypertrophy (19). Ang II is the most important active
component of the renin-angiotensin system and can directly promote
the effects of cardiomyocyte hypertrophy (20). Based on these findings, the
presents study investigated the hypothesis that FBW7 may be
involved in Ang II-induced myocardial hypertrophic injury through
the mTOR-mediated autophagic pathway.
In the present study, the present study aimed to
explore the biological role of FBW7 and mechanism involved in Ang
II-induced myocardial hypertrophic injury. The expression levels of
FBW7 were explored in Ang II-treated H9C2 cells and the association
of this protein with mTOR was explored. Following FBW7
overexpression, the induction of apoptosis, hypertrophy, fibrosis,
inflammation and oxidative stress were investigated in Ang
II-induced H9C2 cells. The current study comprehensively described
the roles of FBW7 in Ang II-treated H9C2 cells, which provided a
novel therapeutical target in the treatment of myocardial
hypertrophy.
Materials and methods
Cell culture and treatment
The rat cardiomyocyte cell line H9C2 was purchased
from Procell Life Science & Technology Co., Ltd. The cells were
maintained in Dulbecco's modified Eagle's Medium containing 10%
fetal bovine serum and 100 U/ml penicillin with 100 mg/ml
streptomycin at 37˚C in the presence of 5% CO2 and 95%
air.
The establishment of an in vitro model of
cardiomyocyte hypertrophy was performed by culturing H9C2 cells in
a 6-well plate overnight. Following starvation, the cells were
incubated with serum-free medium for 4 h and Ang II (Sigma-Aldrich;
Merck KGaA) was used at the concentrations of 0.5, 1, 5 and 10 µM
to induce H9C2 cells for 24 h at 37˚C.
The autophagic pathway was examined by pre-treatment
of H9C2 cells with 10 µM 3-methyladenine (3-MA) for 3 h at 37˚C
prior to the establishment of an in vitro model of
myocardial hypertrophy.
Cell transfection
The FBW7 overexpression plasmid (Oe-FBW7) and its
negative control (Oe-NC) were obtained from Guangzhou RiboBio Co.,
Ltd. Oe-FBW7 consisted of a pcDNA3.1 vector containing the
full-length FBW7 cDNA sequence, with empty pcDNA3.1 as the negative
control (Oe-NC). These vectors were transfected into H9C2 cells
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) at a concentration of 50 ng/ml for 48 h at
37˚C. The overexpression transfection efficiency was determined
using reverse transcription-quantitative (RT-q)PCR and western
blotting 48 h post-transfection.
RT-qPCR
The extraction of total RNA was conducted from H9C2
cells by employing TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The reverse transcription of cDNA was carried out
using total RNA as a template by PrimeScript RT Master Mix obtained
from Takara (Takara Bio, Inc.) according to the manufacturer's
instructions. cDNA was amplified with qPCR using the SYBR Green I
dye detection kit (Takara Bio, Inc.) according to the instructions
provided by the manufacturer. The reaction was performed on a
Bio-Rad CFX96 system (Bio-Rad Laboratories, Inc.). The
amplification conditions for this reaction were as follows: 95˚C
For 30 sec, followed by 40 cycles of 60˚C for 30 sec and 72˚C for
30 sec. The primer sequences were designed by Sangon Biotech Co.,
Ltd. The primer sequences are listed as follows: FBW7 forward,
5'-TTGGCTTGGGACAACAGACT-3', and reverse,
5'-TGGAAGATAACCAGCCCGTG-3'; GAPDH forward,
5'-GTCGTGGAGTCTACTGGCGTCTTCA-3, and reverse,
5-TCGTGGTTCACACCCATCACAAACA-3'. The relative expression of FBW7 was
normalized to that of the expression of GAPDH by the
2-ΔΔCq method (21).
Western blotting
Whole-cell protein lysates were extracted from H9C2
cells with an ice-cold radioimmunoprecipitation (Beyotime Institute
of Biotechnology) buffer containing protease inhibitors and
phenylmethylsulfonyl fluoride. Subsequently, the protein
concentration levels were detected using the bicinchoninic acid
protein assay kit. Each protein sample (30 µg per lane) was
resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis using 15% SDS gels. The proteins were transferred
to PVDF membranes, which were subsequently blocked with 5% non-fat
milk and incubated at 4˚C overnight with the primary antibodies
against FBW7 (1:2,000; cat. no. s-5996R; BIOSS),
microtubule-associated proteins 1A/1B light chain 3B-II/I
(LC3B-II/I; 1:3,000; cat. no. ab51520; Abcam), p62 (1:2,000; cat.
no. FNab06086; Fine Test), Beclin-1 (1:2,000; cat. no. ab207612;
Abcam), p phosphorylated (p)-mTOR (1:10,000; cat. no. ab109268;
Abcam), mTOR (1:10,000; cat. no. ab134903; Abcam), Bcl-2 (1:1,000;
cat. no. ab32124; Abcam), Bax (1:10,000; cat. no. ab32503; Abcam),
cleaved caspase 3 (1:500; cat. no. ab32042; Abcam), beta-myosin
heavy chain (β-MHC; bs-4298R; cat. no. 1:2,000; BIOSS), brain
natriuretic peptide (BNP; 1:20,000; cat. no. ab92500; Abcam),
atrial natriuretic factor (ANF; 1:2,000; cat. no. ATA24633;
AtaGenix), α-smooth muscle actin (α-SMA; 1:1,000; cat. no. YT680;
Bjbalb), fibronectin (1:1,000; cat. no. ab268020; Abcam), vimentin
(1:1,000; cat. no. ab92547; Abcam), collagen I (1:10,000; cat. no.
ab34710; Abcam), collagen III (1:1,000; cat. no. ab184993; Abcam),
inducible nitric oxide synthase (iNOS; 1:1,000; cat. no. ab178945;
Abcam) and cyclooxygenase-2 (COX-2; 1:500; cat. no. 252153;
Signalway Antibody LLC) followed by the incubation with a goat
anti-rabbit IgG secondary antibody conjugated to HRP (1:2,000; cat.
no. ab6721; Abcam) for an additional 1 h at room temperature. The
protein bands were visualized using the enhanced chemiluminescence
kit (MilliporeSigma) and the ChemiDoc imaging system (Bio-Rad
Laboratories, Inc.), and were semi-quantified using ImageJ software
(version 1.48v; National Institutes of Health).
Cell viability assay
The cell viability of H9C2 cells was detected using
the Cell Counting Kit (CCK)-8 method. H9C2 cells were incubated in
96-well plates at a density of 5x103 cells/well at 37˚C
in the presence of 5% CO2 for 24 h. Subsequently, 10 µl
CCK-8 solution (Beyotime Institute of Biotechnology) was added into
the wells and incubated for 2 h according to the manufacturer's
instructions. The absorbance was detected at 450 nm using a
microplate reader (Molecular Devices, LLC).
Detection of apoptosis
Apoptosis was examined using the TUNEL assay.
Briefly, H9C2 cells were fixed in a 1% formaldehyde solution for 15
min at room temperature. Subsequently, these cells were mixed with
0.2% Triton X-100 and analyzed with the ApopTag® Plus
Fluorescein In Situ Apoptosis Detection kit (cat. no. S7111;
Millipore Sigma) containing TUNEL reagent, according to the
manufacturer's instructions. Subsequently, the cells were incubated
with DAPI at 37˚C for 2-3 min and mounted in an anti-fade reagent
(Beijing Solarbio Science & Technology Co., Ltd.). The stained
cells were observed in three random fields of view with the
application of a fluorescence microscope (Nikon TE200-U; Nikon
Corporation).
Cytoskeletal assay
H9C2 cells were incubated on slides with medium at
37˚C, in the presence of 5% CO2. When the cells were
cultured to 80-90% confluence, they were washed twice with PBS (pH
7.4). The cells were fixed with 4% formaldehyde for 10 min at room
temperature and washed twice with PBS for 10 min. Subsequently, the
cells were permeabilized with 0.5% Triton X-100 solution for 5 min
at room temperature. Following washing twice with PBS for 10 min,
200 µl rhodamine-labeled phalloidin solution (5 µg/ml;
Sigma-Aldrich; Merck KGaA) was incubated for 30 min with the cells
at room temperature in the dark. Following a second wash with PBS
twice for 5 min each time, the nuclei were re-stained with 200 µl
DAPI solution at 37˚C for 30 sec. Finally, the cell length was
observed after 24 h using a Smartproof 5 confocal microscope (Zeiss
AG).
Enzyme-linked immunosorbent assay
(ELISA)
H9C2 cells were treated with Ang II (0.5, 1, 5 and
10 µM) at 37˚C for 24 h and centrifuged at 2,000 x g for 5 min at
4˚C. The supernatants were collected for the determination of the
expression levels of the inflammatory cytokines TNF-α, IL-6 and
IL-1β. TNF-α (cat. no. ab236712), IL-6 (cat. no. ab234570) and
IL-1β (cat. no. ab255730) ELISA kits (all from Abcam) were used and
the protocol was performed as determined by the manufacturer's
instructions.
Measurement of superoxide dismutase
(SOD) activity and malondialdehyde (MDA) levels
The activity of SOD (cat. no. BC0170) and the MDA
(cat. no. BC0025) levels (markers of oxidative stress) were
assessed using the corresponding commercial kits (Beijing Solarbio
Science & Technology Co., Ltd.) according to the manufacturer's
instructions. The absorbance values were recorded at 560 and 532 nm
using a microplate reader for the determination of the SOD activity
and the MDA levels, respectively.
Statistical analysis
All data are presented as mean ± standard deviation
from at least three independent experiments. Statistical analysis
was conducted using SPSS 19.0 (SPSS, Inc.). Significant differences
were noted between multiple groups and the data were analyzed using
one-way analysis of variance followed by a Bonferroni post hoc
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
FBW7 expression is downregulated in
Ang II-induced H9C2 cells and its upregulation activates
mTOR-mediated autophagy
The protein and mRNA expression levels of FBW7 were
significantly reduced in H9C2 cells treated by Ang II at the
concentrations of 0.5, 1, 5 and 10 µM compared with those in the
control group (Fig. 1A and
B). FBW7 expression was the lowest
when the concentration of Ang II was 10 µM. Therefore, 10 µM Ang II
was selected as the concentration required for the induction
experiments. By transfecting Oe-FBW7 into H9C2 cells, the
expression levels of FBW7 were successfully significantly elevated
compared with those of the negative control (Fig. 1C and D). The expression levels of the
autophagy-related proteins LC3B-II and Beclin-1 were significantly
reduced in the Ang II group (vs. control), whereas they were
significantly increased in the Ang II+Oe-FBW7 group (vs. Oe-NC).
The expression levels of LC3B-I and p62 indicated the opposite
trends to the aforementioned two proteins in each group (Fig. 1E). In addition, western blotting
was used to detect a significantly increased expression of p-mTOR
in the Ang II group compared with that of the control group. A
significantly decreased expression of p-mTOR was noted in the Ang
II+Oe-FBW7 group in comparison with the Oe-NC group (Fig. 1F). The expression levels of mTOR
remained the same in each group. Therefore, FBW7 expression was
downregulated in Ang II-induced H9C2 cells and the upregulation of
FBW7 expression activated mTOR-mediated cellular autophagy.
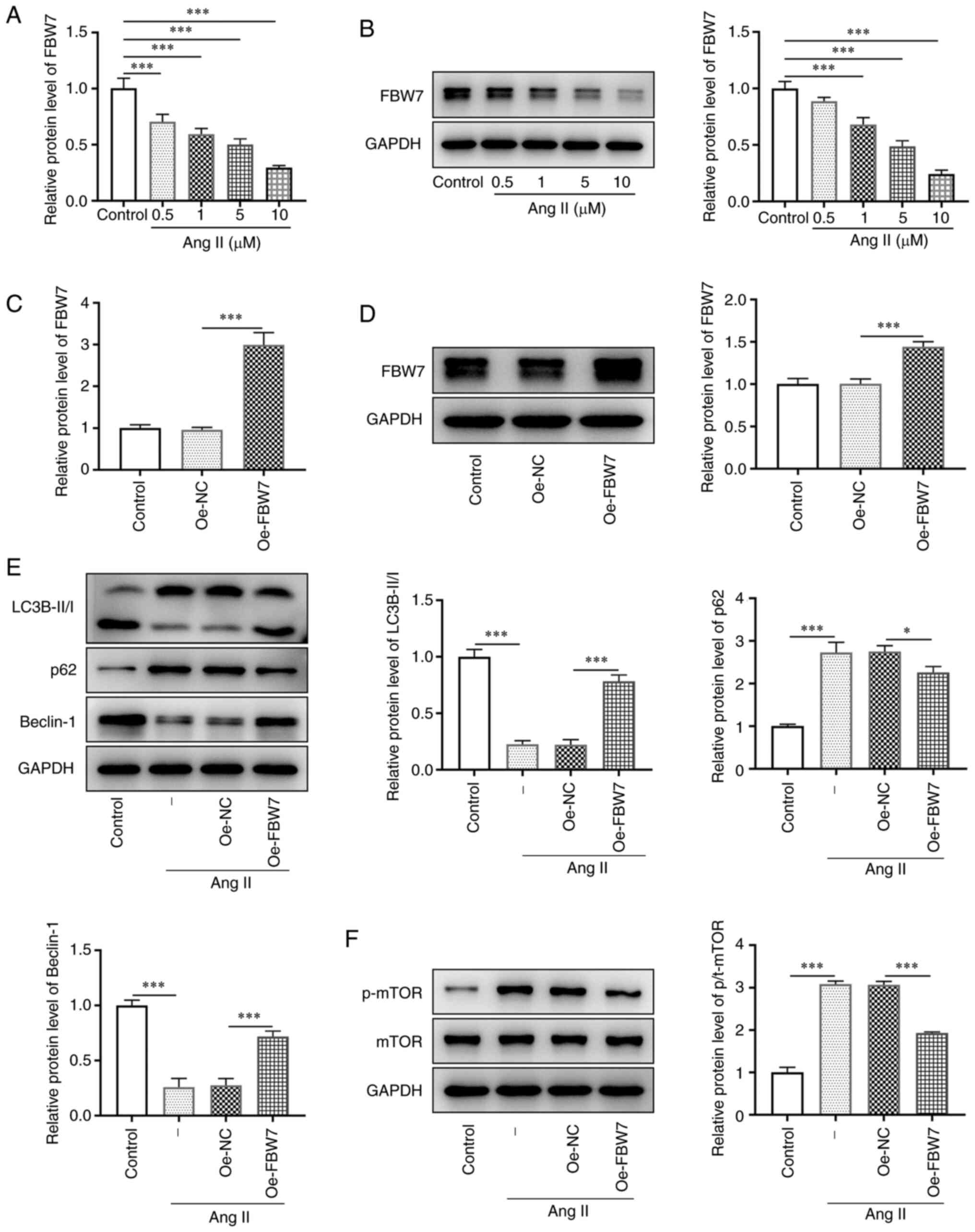 | Figure 1FBW7 expression is downregulated in
Ang II-induced H9C2 cells and its upregulation activates
mTOR-mediated cell autophagy. (A) mRNA expression of FBW7 in the
control and Ang II (0.5, 1, 5 and 10 µM) groups. (B) Protein
expression level of FBW7 in the control and Ang II (0.5, 1, 5 and
10 µM) groups. Overexpression efficiency of FBW7 in the control,
Oe-NC and Oe-FBW7 groups was measured using (C) RT-qPCR and (D)
western blotting. (E) Protein expression levels of LC3B-II/I, P62
and Beclin-1 related to autophagy in the control, Ang II, Ang
II+Oe-NC and Ang II+Oe-FBW7 groups. (F) Protein levels of p-mTOR
and mTOR related to mTOR signaling pathway in the control, Ang II,
Ang II+Oe-NC and Ang II+Oe-FBW7 groups. *P<0.05,
***P<0.001. FBW7, F-box and WD repeat-containing
protein 7; Ang, angiotensin; Oe, overexpression; NC, negative
control; LC3B, microtubule-associated proteins 1A/1B light chain
3B; p-, phosphorylated; -, Ang II treatment without
transfection. |
FBW7 inhibits Ang II-induced apoptosis
in H9C2 cells via the activation of autophagy
To determine whether FBW7 affects Ang II-induced
H9C2 cell viability via induction of autophagy, the autophagy
inhibitor 3-MA was added to the cells. The viability of H9C2 cells
was decreased by ~45% in the Ang II group compared with that of the
control group. It was also increased by ~30% in the Ang II+Oe-FBW7
group compared with that of the negative control group. The
addition of 3-MA significantly decreased the cell viability of H9C2
cells compared with the Ang II+Oe-FBW7 group (Fig. 2A). This result indicated that FBW7
overexpression could largely prevent Ang II-induced loss of cell
viability, whereas 3-MA reversed partly the effect of FBW7 on H9C2
cells. The apoptotic rate in the Ang II group was estimated to be
~25%, which was markedly higher than that in the control group
(Fig. 2B and C). FBW7 overexpression reduced this
percentage to ~10% (vs. Oe-NC), whereas 3-MA increased it further
to ~20% compared with the Oe-FBW7 group. In addition, the
expression level of the anti-apoptotic protein Bcl-2 in H9C2 cells
was significantly decreased, whereas the levels of Bax and cleaved
caspase 3 were increased in the Ang II group compared with those in
the control group (Fig. 2D). The
expression level of Bcl-2 was significantly elevated, and the
levels of Bax and cleaved caspase 3 were decreased in Ang
II-treated H9C2 cells transfected with Oe-FBW7 compared with those
in the negative control group, whereas the Bcl-2 level was reduced
and the levels of Bax and cleaved caspase 3 were elevated in the
Ang II+Oe-FBW7+3-MA group compared with in the Ang II+Oe-FBW7 group
(Fig. 2D). This evidence suggested
that FBW7 could inhibit Ang II-induced apoptosis in H9C2 cells via
the autophagic pathway.
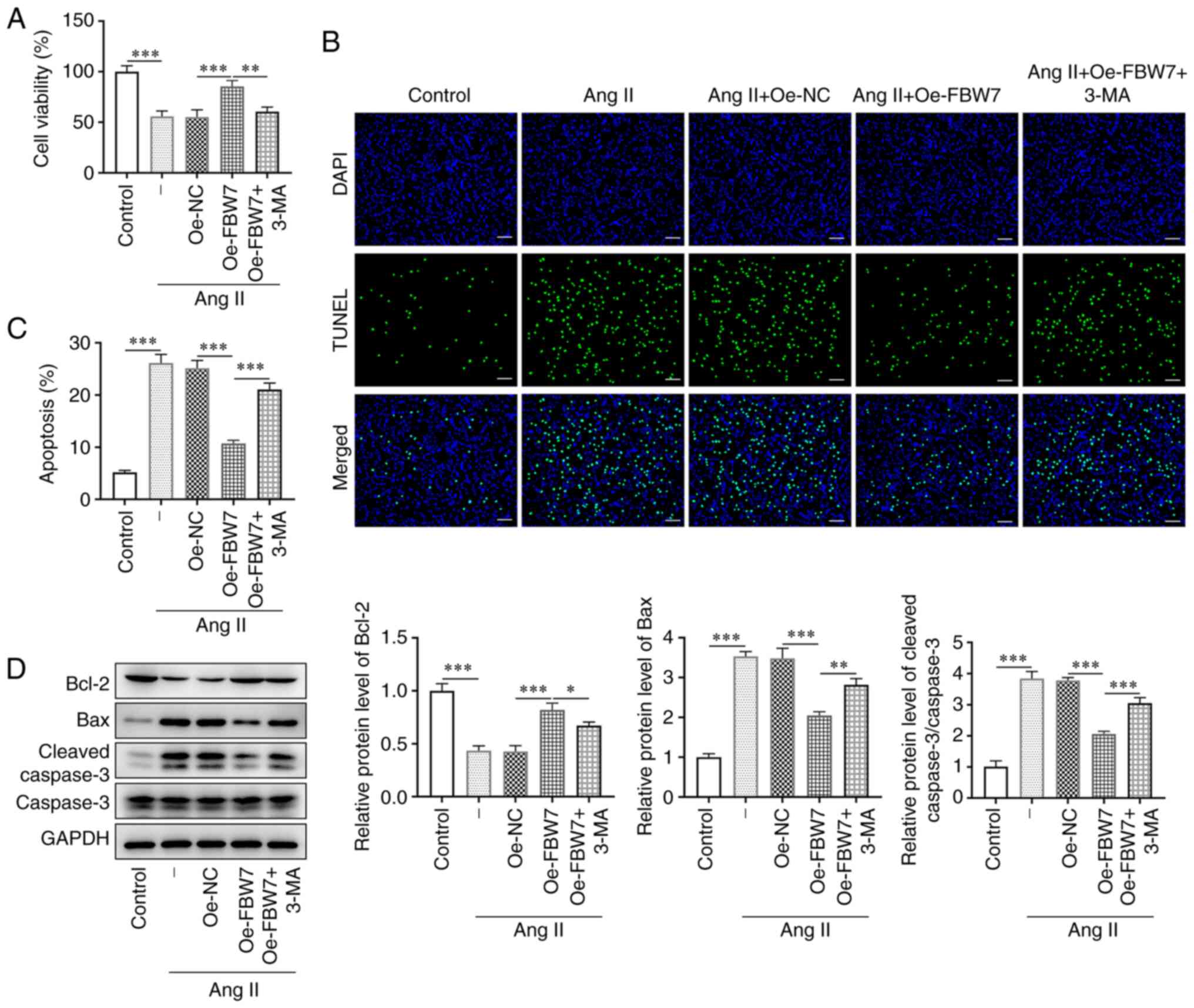 | Figure 2FBW7 inhibits Ang II-induced
apoptosis in H9C2 cells via autophagy. (A) Cell viability of H9C2
cells in the control, Ang II, Ang II+Oe-NC, Ang II+Oe-FBW7 and Ang
II+Oe-FBW7+3-MA groups. (B and C) Apoptosis of H9C2 cells in the
control, Ang II, Ang II+Oe-NC, Ang II+Oe-FBW7 and Ang
II+Oe-FBW7+3-MA groups. Scale bar, 50 µm. (D) Protein levels of
Bcl-2, Bax and cleaved caspase 3 associated with apoptosis in the
control, Ang II, Ang II+Oe-NC, Ang II+Oe-FBW7 and Ang
II+Oe-FBW7+3-MA groups. *P<0.05,
**P<0.01, ***P<0.001. FBW7, F-box and
WD repeat-containing protein 7; Ang, angiotensin; Oe,
overexpression; NC, negative control; 3-MA, 3-methyladenine; -, Ang
II treatment without transfection. |
FBW7 inhibits Ang II-induced
hypertrophy and fibrosis in H9C2 cells via induction of
autophagy
The size of the cytoskeleton was observed using
confocal microscopy to assess whether the Ang II-induced
cardiomyocyte hypertrophy model was successfully established. Ang
II effectively induced H9C2 cytoskeletal expansion as determined by
an increase in cell length, whereas FBW7 overexpression
successfully prevented the Ang II-stimulated irease in the
cytoskeletal size (Fig. 3A). The
length of Ang II-induced H9C2 cells transfected with Oe-FBW7 was
increased again following treatment with 3-MA. In addition, the
protein levels of the hypertrophic markers β-MHC, BNP and ANF were
also significantly increased in response to Ang II treatment
compared with the control group, whereas they were significantly
decreased following FBW7 oncverexpression compared with the Ang
II+Oe-NC group. 3-MA caused a significant increase in the levels of
these hypertrophic markers compared with the Ang II+Oe-FBW7 group
(Fig. 3B). Concomitantly, the
cells underwent fibrotic changes. The expression levels of the
fibrosis-related proteins α-SMA, fibronectin, vimentin, collagen I
and III were significantly increased in Ang II-induced H9C2 cells
compared with the control group, whereas they were significantly
decreased following FBW7 overexpression compared with the Ang
II+Oe-NC group (Fig. 3C). However,
the expression levels of these proteins were markedly increased
again following 3-MA treatment. Collectively, these results
demonstrated that FBW7 overexpression could inhibit Ang II-induced
hypertrophy and fibrosis in H9C2 cells via the autophagic
pathway.
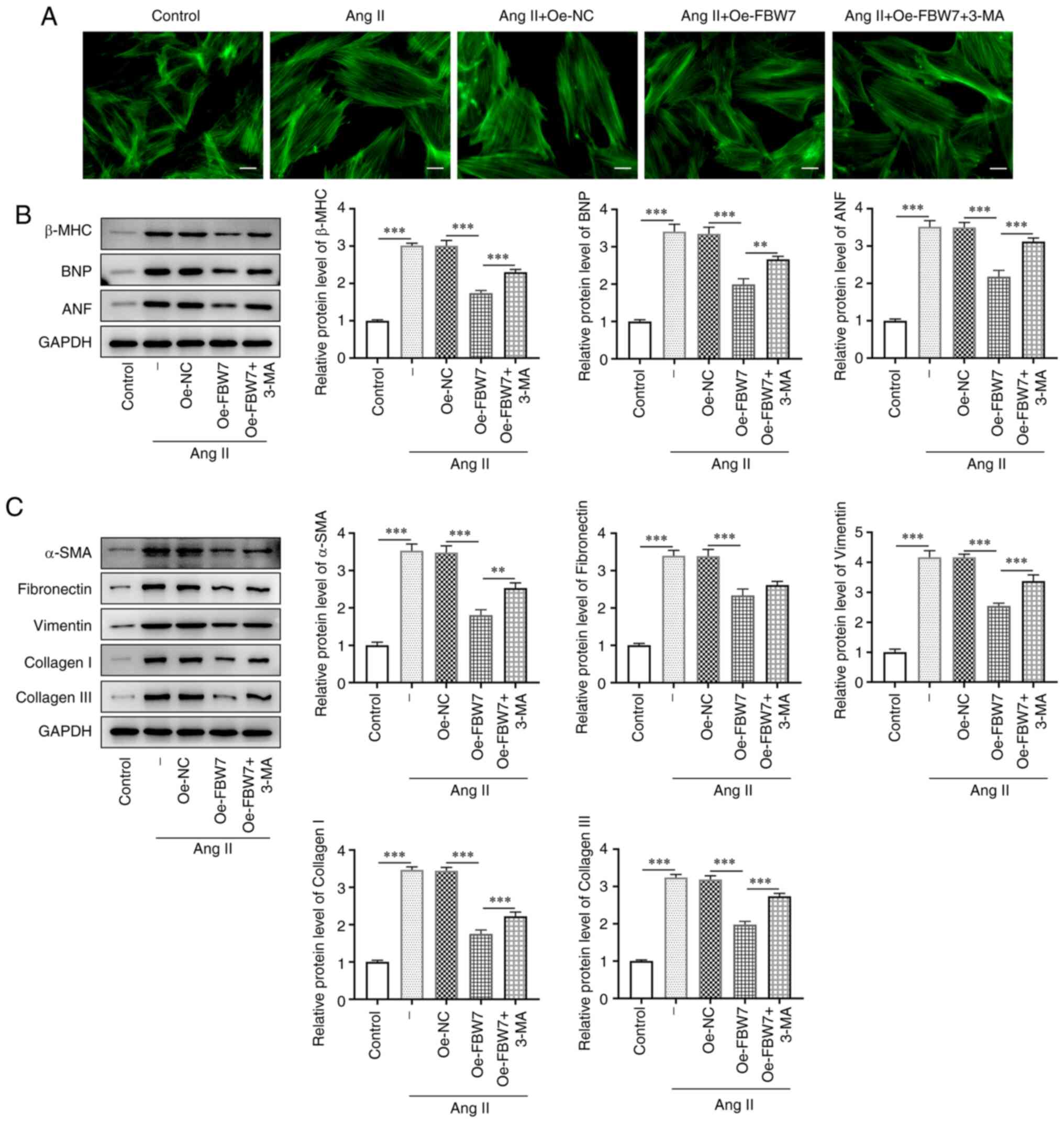 | Figure 3FBW7 inhibits Ang II-induced
hypertrophy and fibrosis in H9C2 cells through autophagy. (A) Cell
length of H9C2 cells in the control, Ang II, Ang II+Oe-NC, Ang
II+Oe-FBW7 and Ang II+Oe-FBW7+3-MA groups. Scale bar, 25 µm. (B)
Protein levels of hypertrophic markers β-MHC, BNP and ANF in H9C2
cells in the control, Ang II, Ang II+Oe-NC, Ang II+Oe-FBW7 and Ang
II+Oe-FBW7+3-MA groups. (C) Protein levels of α-SMA, Fibronectin,
Vimentin Collagen I and Collagen III associated fibrosis in H9C2
cells in the control, Ang II, Ang II+Oe-NC, Ang II+Oe-FBW7 and Ang
II+Oe-FBW7+3-MA groups. **P<0.01,
***P<0.001. FBW7, F-box and WD repeat-containing
protein 7; Ang, angiotensin; Oe, overexpression; NC, negative
control; 3-MA, 3-methyladenine; β-MHC, β-myosin heavy chain; BNP,
brain natriuretic peptide; ANF, atrial natriuretic factor; atrial
natriuretic factor; SMA, smooth muscle actin; -, Ang II treatment
without transfection. |
FBW7 suppresses Ang II-induced
inflammation and oxidative stress in H9C2 cells via induction of
autophagy
In order to determine whether FBW7 affects
inflammation and oxidative stress in Ang II-induced H9C2 cells, the
expression levels of the inflammatory factors and the levels of
oxidative stress were assessed. The levels of the pro-inflammatory
cytokines TNF-α and IL-1β were significantly increased in the Ang
II group compared with the control, whereas they significantly
declined in the Ang II+Oe-FBW7 group compared with Oe-NC (Fig. 4A and B). By contrast, the expression levels of
TNF-α and IL-1β were increased in the Ang II+Oe-FBW7+3-MA group
compared with the Ang II+Oe-FBW7 group (Fig. 4A and B). Moreover, Ang II induced a significant
increase in the protein levels of iNOS and COX-2 in H9C2 cells
compared with the control group. This increase was significantly
reduced by FBW7 overexpression. 3-MA partially abrogated the
inhibitory effect of FBW7 on the protein levels of iNOS and COX-2
compared with the Ang II+Oe-FBW7 group (Fig. 4C). Furthermore, the activity of SOD
was significantly reduced in the Ang II group compared with that of
the control group, whereas it was increased in the Ang II+Oe-FBW7
group compared with that of the Oe-NC group. The activity of SOD
declined in the Ang II+Oe-FBW7+3-MA group compared with the Ang
II+Oe-FBW7 group, while the levels of MDA exhibited the opposite
trend to that of MDA (Fig. 4D and
E). Overall, these results
illustrated that FBW7 could suppress Ang II-induced inflammation
and oxidative stress in H9C2 cells via the autophagic pathway.
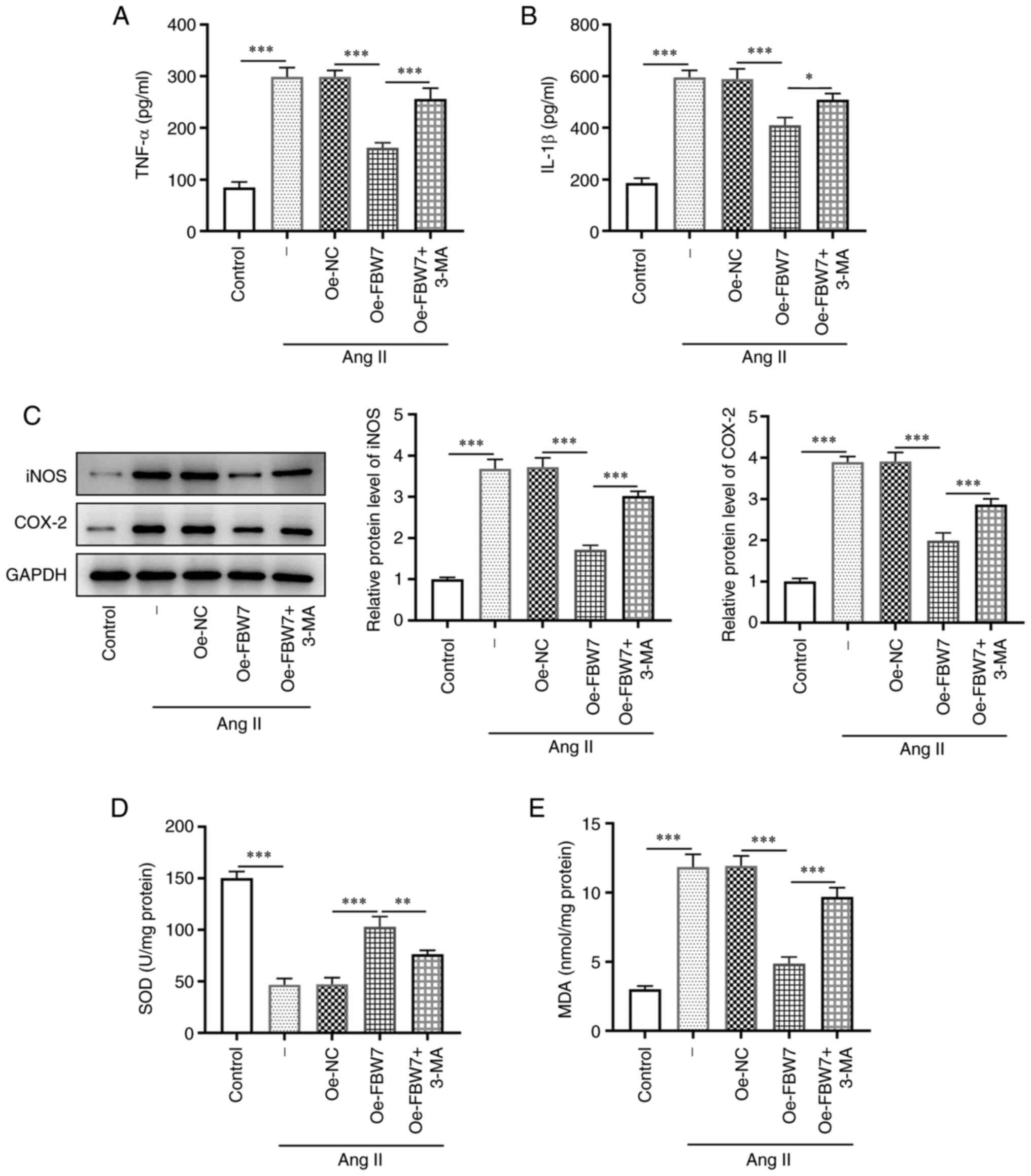 | Figure 4FBW7 suppresses Ang II-induced
inflammation and oxidative stress in H9C2 cells via autophagy.
Levels of inflammatory cytokines (A) TNF-α and (B) IL-1β in H9C2
cells in the control, Ang II, Ang II+Oe-NC, Ang II+Oe-FBW7 and Ang
II+Oe-FBW7+3-MA groups. (C) Protein levels of iNOS and COX-2
associated with inflammation in H9C2 cells in the control, Ang II,
Ang II+Oe-NC, Ang II+Oe-FBW7 and Ang II+Oe-FBW7+3-MA groups. Levels
of (D) SOD and (E) MDA associated with oxidative stress in H9C2
cells in the control, Ang II, Ang II+Oe-NC, Ang II+Oe-FBW7 and Ang
II+Oe-FBW7+3-MA groups. *P<0.05,
**P<0.01, ***P<0.001. FBW7, F-box and
WD repeat-containing protein 7; IL, interleukin; Ang, angiotensin;
Oe, overexpression; NC, negative control; 3-MA, 3-methyladenine;
iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2;
SOD, superoxide dismutase; MDA, malondialdehyde; -, Ang II
treatment without transfection. |
Discussion
Myocardial hypertrophy is an adaptive response to
hemodynamic stress and serves as compensation to improve cardiac
performance and reduce ventricular wall tension and oxygen
consumption (22). However,
pathological myocardial hypertrophy is more likely to lead to a
variety of cardiovascular diseases, such as myocardial infarction
and sudden death (23). As
aforementioned, FBW7 has been reported to exert an important
regulatory role in cardiac development (10,11).
Therefore, to explore the regulatory role of FBW7 in cardiac
hypertrophy, the present study established a myocardial hypertrophy
model using Ang II-treated cells and determined the protective role
of FBW7 in Ang II-induced H9C2 cell injury.
The F-box protein FBW7, also termed FBXW7, is a
component of the SCF-type E3 ubiquitin ligase (24). As previously described, FBW7 plays
a key role in cardiomyocyte development. Its dysregulated
expression is considered to be a pivotal step in malignant
transformation (16). The present
experiments indicated that FBW7 expression was significantly
downregulated in Ang II-induced H9C2 cells in a
concentration-dependent manner. In addition, the induction of
autophagy is associated with clearance of pathogens and antigen
expression and has been indicated to be inhibited by the mTOR
complex 1 (mTORC1) (25). It has
been demonstrated that the induction of autophagy in cardiomyocytes
plays a protective role during hemodynamic stress (26). Moreover, the FBXW7-SHOC2-RPTOR axis
can regulate mTORC1-mediated autophagy (27). The present experiments revealed a
significant increase in the autophagy-related proteins LC3B-II and
Beclin-1, and a decrease in the levels of the proteins LC3B-I and
p62 in Ang II-induced H9C2 cells following overexpression of FBW7
in comparison with the Oe-NC group. Furthermore, a drop in the
protein levels of p-mTOR was noted in Ang II-induced H9C2 cells
transfected with Oe-FBW7 compared with that the levels in Ang
II-induced H9C2 cells transfected with Oe-NC, indicating that
upregulation of FBW7 could activate mTOR-mediated autophagy in Ang
II-treated H9C2 cells.
Apoptosis is a unique form of cell death that can
cause excessive cardiomyocyte death, leading to cardiac
dysfunction. Apoptosis is also involved in the transition from
adaptive cardiac hypertrophy to pathological cardiac hypertrophy
(28). The process of apoptotic
dysregulation is associated with a decrease in the expression
levels of the anti-apoptotic protein Bcl-2 and an increase in the
expression levels of the pro-apoptotic protein Bax as well as in
the levels of cleaved caspase 3(29). A previous study demonstrated that
FBW7 can regulate apoptosis by targeting induced myeloid leukemia
cell differentiation protein MCL1 for ubiquitylation and
destruction (30). The present
experiments demonstrated that Ang II induced a significantly lower
cell viability, reduced the rate of apoptosis and the expression
levels of the anti-apoptotic protein Bcl-2 in H9C2 cells. These
effects were inhibited by overexpression of FBW7. Subsequently, the
autophagy inhibitor 3-MA was used to pretreat H9C2 cells for 3 h.
3-MA significantly reversed the inhibitory effects of FBW7 on Ang
II-induced apoptosis in H9C2 cells. These results suggested that
the inhibitory effects of FBW7 on the Ang II-induced apoptosis in
H9C2 cells were achieved via the autophagic pathway.
Pathological cardiac hypertrophy is often
accompanied by myocardial dysfunction and fibrosis (31). β-MHC, BNP and ANF are markers of
cardiomyocyte hypertrophy. Previous studies have indicated that
FBXW7 negatively regulates physiological cardiac hypertrophy by
inhibiting the pro-hypertrophic hypoxia-inducible factor-1α-post
signaling pathway and promoting the inactivation of Akt (32). In the present study, cell length
and the levels of the hypertrophic marker proteins β-MHC, BNP and
ANF were increased by Ang II in H9C2 cells. The expression levels
of these markers were markedly decreased following FBW7
overexpression, whereas following the addition of 3-MA they were
elevated.
Furthermore, circular RNA circFBXW4 inhibits hepatic
fibrosis by targeting the miR-18b-3p/FBXW7 axis (33). Ang II induced increased expression
levels of the fibrosis-associated proteins α-SMA, fibronectin,
vimentin, collagen I and III in H9C2 cells, whereas these effects
were decreased by FBW7 overexpression and the latter effect was
partially reversed by the addition of 3-MA to the cells. This
evidence suggested that FBW7 could inhibit Ang II-induced
hypertrophy and fibrosis in H9C2 cells via the autophagic
pathway.
According to previous studies, a reduction in
oxidative stress levels is beneficial in mitigating the development
of cell hypertrophy (34,35). In addition, inflammation also plays
an important role in the pathogenesis of hypertrophy (36). This is due to the ability of the
inflammatory cytokine TNF-α to suppress cardiac contractility and
to provoke myocardial hypertrophy, cardiac fibrosis and
cardiomyocyte apoptosis (37-39).
Moreover, IL-1β, which is a downstream factor of caspase-1 plays an
important role in cardiac hypertrophy (40). A previous study demonstrated that
FBW7β contributes to the protection of cells from oxidative stress
(41). FBW7 overexpression can
increase autophagy by inhibiting mTOR signaling and ameliorating
inflammation (18). In the present
study, the expression levels of the inflammatory cytokines TNF-α
and IL-1β and of the inflammation-associated proteins iNOS and
COX-2 were significantly increased by Ang II in H9C2 cells, whereas
they were decreased following overexpression of FBW7 and partially
reversed by application of 3-MA.
Furthermore, the level of the antioxidant enzyme SOD
was decreased in Ang II-treated H9C2 cells, whereas it was
decreased following FBW7 overexpression. However, the level of SOD
was decreased again following the addition of 3-MA. These results
revealed that FBW7 overexpression could inhibit Ang II-induced
inflammation and oxidative stress in H9C2 cells via the autophagic
pathway.
Notably, Gao et al (42) revealed that FBXW7 promotes
pathological cardiac hypertrophy by targeting EZH2-SIX1 signaling,
which shows discrepant results with the present results. However,
another study used the same cell line as that in the current study
and demonstrated results that are consistent with the present paper
(11). In addition, several
studies have demonstrated that FBW7 is beneficial for
cardiovascular physiological activity and metabolism (16,32,43).
Thus, the roles of FBW7 in cardiac hypertrophy still need to be
explored. Furthermore, Ang II concentration used in the present
in vitro study was different compared with that of plasma
level of human, thus the result of the present study may not be
suitable for the phenomenon of in vivo. Future in
vivo experiments will be performed to confirm the role of FBW7
and the involvement of mTOR-mediated autophagic pathway in
myocardial hypertrophy in further study.
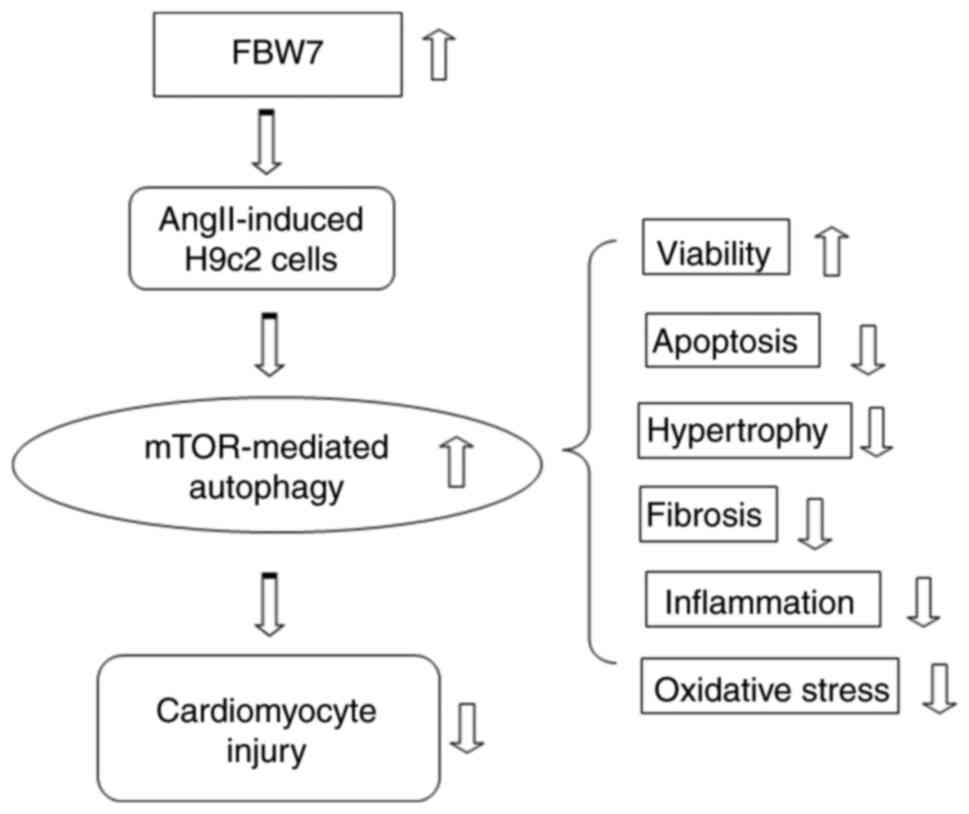
The studies presented so far provide evidence that
FBW7 inhibits Ang II-induced H9C2 apoptosis, hypertrophy and
fibrosis, inflammation and oxidative stress via the mTOR-mediated
autophagic pathway (Fig. 5). The
findings may provide a novel fundamental insight into how FBW7
ameliorates myocardial hypertrophy and enhance the potential
applicability of FBW7 in clinical practice.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QL and WZ designed the study, drafted and revised
the manuscript. CH, XW and JZ analyzed the data and searched the
literature. QL, CH and XW performed the experiments. All authors
read and approved the final manuscript. QL and CH confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lazzeroni D, Rimoldi O and Camici PG: From
left ventricular hypertrophy to dysfunction and failure. Circ J.
80:555–564. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shimizu I and Minamino T: Physiological
and pathological cardiac hypertrophy. J Mol Cell Cardiol.
97:245–262. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Frey N and Olson EN: Cardiac hypertrophy:
The good, the bad, and the ugly. Annu Rev Physiol. 65:45–79.
2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Luo M, Chen PP, Yang L, Wang P, Lu YL, Shi
FG, Gao Y, Xu SF, Gong QH, Xu RX and Deng J: Sodium ferulate
inhibits myocardial hypertrophy induced by abdominal coarctation in
rats: Involvement of cardiac PKC and MAPK signaling pathways.
Biomed Pharmacother. 112(108735)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li X, Lan Y, Wang Y, Nie M, Lu Y and Zhao
E: Telmisartan suppresses cardiac hypertrophy by inhibiting
cardiomyocyte apoptosis via the NFAT/ANP/BNP signaling pathway. Mol
Med Rep. 15:2574–2582. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rai V, Sharma P, Agrawal S and Agrawal DK:
Relevance of mouse models of cardiac fibrosis and hypertrophy in
cardiac research. Mol Cell Biochem. 424:123–145. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsutsui H, Kinugawa S and Matsushima S:
Oxidative stress and heart failure. Am J Physiol Heart Circ
Physiol. 301:H2181–H2190. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang C, Wang F, Zhang Y, Kang Y, Wang H,
Si M, Su L, Xin X, Xue F, Hao F, et al: Celecoxib prevents pressure
overload-induced cardiac hypertrophy and dysfunction by inhibiting
inflammation, apoptosis and oxidative stress. J Cell Mol Med.
20:116–127. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Uddin S, Bhat AA, Krishnankutty R, Mir F,
Kulinski M and Mohammad RM: Involvement of F-BOX proteins in
progression and development of human malignancies. Semin Cancer
Biol. 36:18–32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang B, Xu M, Li M, Wu F, Hu S, Chen X,
Zhao L, Huang Z, Lan F, Liu D and Wang Y: miR-25 promotes
cardiomyocyte proliferation by targeting FBXW7. Mol Ther Nucleic
Acids. 19:1299–1308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang L, Qin D, Shi H, Zhang Y, Li H and
Han Q: MiR-195-5p promotes cardiomyocyte hypertrophy by targeting
MFN2 and FBXW7. Biomed Res Int. 2019(1580982)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thompson BJ, Buonamici S, Sulis ML,
Palomero T, Vilimas T, Basso G, Ferrando A and Aifantis I: The
SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell
leukemia. J Exp Med. 204:1825–1835. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Welcker M and Clurman BE: FBW7 ubiquitin
ligase: A tumour suppressor at the crossroads of cell division,
growth and differentiation. Nat Rev Cancer. 8:83–93.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Tan Y, Sangfelt O and Spruck C: The
Fbxw7/hCdc4 tumor suppressor in human cancer. Cancer Lett.
271:1–12. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fu Q, Lu Z, Fu X, Ma S and Lu X: MicroRNA
27b promotes cardiac fibrosis by targeting the FBW7/Snail pathway.
Aging (Albany NY). 11:11865–11879. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tetzlaff MT, Yu W, Li M, Zhang P, Finegold
M, Mahon K, Harper JW, Schwartz RJ and Elledge SJ: Defective
cardiovascular development and elevated cyclin E and Notch proteins
in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci USA.
101:3338–3345. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Gao C, Fan F, Chen J, Long Y, Tang S,
Jiang C and Xu Y: FBW7 regulates the autophagy signal in mesangial
cells induced by high glucose. Biomed Res Int.
2019(6061594)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alebiosu CO, Odusan O and Jaiyesimi A:
Morbidity in relation to stage of diabetic nephropathy in type-2
diabetic patients. J Natl Med Assoc. 95:1042–1047. 2003.PubMed/NCBI
|
|
20
|
Diep QN, El Mabrouk M, Yue P and Schiffrin
EL: Effect of AT(1) receptor blockade on cardiac apoptosis in
angiotensin II-induced hypertension. Am J Physiol Heart Circ
Physiol. 282:H1635–H1641. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Berenji K, Drazner MH, Rothermel BA and
Hill JA: Does load-induced ventricular hypertrophy progress to
systolic heart failure? Am J Physiol Heart Circ Physiol.
289:H8–H16. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kavey RE: Left ventricular hypertrophy in
hypertensive children and adolescents: predictors and prevalence.
Curr Hypertens Rep. 15:453–457. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shimizu K, Nihira NT, Inuzuka H and Wei W:
Physiological functions of FBW7 in cancer and metabolism. Cell
Signal. 46:15–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Yamaguchi O: Autophagy in the heart. Circ
J. 83:697–704. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xie CM and Sun Y: The MTORC1-mediated
autophagy is regulated by the FBXW7-SHOC2-RPTOR axis. Autophagy.
15:1470–1472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Oldfield CJ, Duhamel TA and Dhalla NS:
Mechanisms for the transition from physiological to pathological
cardiac hypertrophy. Can J Physiol Pharmacol. 98:74–84.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kvansakul M, Caria S and Hinds MG: The
Bcl-2 family in host-virus interactions. Viruses.
9(290)2017.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Samak M, Fatullayev J, Sabashnikov A,
Zeriouh M, Schmack B, Farag M, Popov AF, Dohmen PM, Choi YH,
Wahlers T and Weymann A: Cardiac hypertrophy: An introduction to
molecular and cellular basis. Med Sci Monit Basic Res. 22:75–79.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang L, Li Y, Wang X, Mu X, Qin D, Huang
W, Alshahrani S, Nieman M, Peng J, Essandoh K, et al:
Overexpression of miR-223 tips the balance of pro- and
anti-hypertrophic signaling cascades toward physiologic cardiac
hypertrophy. J Biol Chem. 291:15700–15713. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen X, Li HD, Bu FT, Li XF, Chen Y, Zhu
S, Wang JN, Chen SY, Sun YY, Pan XY, et al: Circular RNA circFBXW4
suppresses hepatic fibrosis via targeting the miR-18b-3p/FBXW7
axis. Theranostics. 10:4851–4870. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sawyer DB, Siwik DA, Xiao L, Pimentel DR,
Singh K and Colucci WS: Role of oxidative stress in myocardial
hypertrophy and failure. J Mol Cell Cardiol. 34:379–388.
2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sugden PH and Clerk A: Oxidative stress
and growth-regulating intracellular signaling pathways in cardiac
myocytes. Antioxid Redox Signal. 8:2111–2124. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Guan XH, Hong X, Zhao N, Liu XH, Xiao YF,
Chen TT, Deng LB, Wang XL, Wang JB, Ji GJ, et al: CD38 promotes
angiotensin II-induced cardiac hypertrophy. J Cell Mol Med.
21:1492–1502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Grandel U, Fink L, Blum A, Heep M, Buerke
M, Kraemer HJ, Mayer K, Bohle RM, Seeger W, Grimminger F and
Sibelius U: Endotoxin-induced myocardial tumor necrosis
factor-alpha synthesis depresses contractility of isolated rat
hearts: Evidence for a role of sphingosine and
cyclooxygenase-2-derived thromboxane production. Circulation.
102:2758–2764. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yokoyama T, Nakano M, Bednarczyk JL,
McIntyre BW, Entman M and Mann DL: Tumor necrosis factor-alpha
provokes a hypertrophic growth response in adult cardiac myocytes.
Circulation. 95:1247–1252. 1997.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bradham WS, Bozkurt B, Gunasinghe H, Mann
D and Spinale FG: Tumor necrosis factor-alpha and myocardial
remodeling in progression of heart failure: a current perspective.
Cardiovasc Res. 53:822–830. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bai Y, Sun X, Chu Q, Li A, Qin Y, Li Y,
Yue E, Wang H, Li G, Zahra SM, et al: Caspase-1 regulate
AngII-induced cardiomyocyte hypertrophy via upregulation of IL-1β.
Biosci Rep. 38(BSR20171438)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Matsumoto A, Tateishi Y, Onoyama I, Okita
Y, Nakayama K and Nakayama KI: Fbxw7β resides in the endoplasmic
reticulum membrane and protects cells from oxidative stress. Cancer
Sci. 102:749–755. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gao W, Guo N, Zhao S, Chen Z, Zhang W, Yan
F, Liao H and Chi K: FBXW7 promotes pathological cardiac
hypertrophy by targeting EZH2-SIX1 signaling. Exp Cell Res.
393(112059)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shen Y, Chen X, Chi C, Wang H, Xue J, Su
D, Wang H, Li M, Liu B and Dong Q: Smooth muscle cell-specific
knockout of FBW7 exacerbates intracranial atherosclerotic stenosis.
Neurobiol Dis. 132(104584)2019.PubMed/NCBI View Article : Google Scholar
|