Introduction
Acute pancreatitis (AP) is a potentially lethal
disease accompanied by systemic injury, and ~20% of patients
develop moderate or severe acute pancreatitis (SAP) (1). According to global estimates, the
annual morbidity of AP is ~33.74 cases (95% confidence interval:
23.33-48.81) per 100,000 person-years (2). The mortality rate of SAP may reach
20-40% when accompanied by pancreatic or peripancreatic tissue
necrosis or organ failure (3-5).
The severity of AP is classified as mild, moderately severe, or
severe based on the presence of organ failure and local or systemic
complications according to the revised Atlanta classification
(6). Although numerous advances
have been made in the treatment of AP, there is still a lack of
specific and effective drug therapies because the pathophysiology
of the disease is poorly understood (7).
Sodium taurocholate (NaT), a natural bile salt, may
rapidly and directly impair the surrounding acinar cells in a few
minutes (8,9). NaT may also trigger pathological
acinar cell calcium transients, cell death and calcium-dependent
trypsinogen activation (10,11).
Retrograde pancreatic duct infusion of NaT is a common method of
inducing AP (10,12). Different extent of the pancreatic
injury can be induced by changing the concentration, perfusion rate
and volume of NaT (12,13). However, different researchers have
redesigned the dosage of NaT and timing for study to meet their own
research needs, resulting in difficulties in the application of
this model, as well as further efficacy evaluation. Based on
previous studies, several NaT concentrations between 3.5 and 6% (1
ml/kg) have been reported to induce SAP in rats (14-17).
However, differences in the modeling conditions of rat models
result in poor comparability between different SAP efficacy and
mechanism experiments.
Moreover, previous studies have strongly emphasized
local rather than systemic pancreatic injury, and systemic organ
dysfunction in this animal model remains controversial and unclear.
Organ failure is the most critical factor affecting mortality in
patients with SAP (18). Early
organ dysfunction is attributed to sterile injury and inflammation
caused by damage-associated molecular patterns and unsaturated
fatty acids (19). Patients with
persistent systemic inflammatory response syndrome (SIRS) are prone
to developing systemic organ dysfunction and later organ failure
due to pathogen-associated molecular patterns (20,21).
The lungs, liver, kidneys and heart are often involved in
AP-related multiple organ failure (3). Secondary infection with pancreatic or
peripancreatic necrosis is considered to be a result of bacterial
translocation of intestinal microorganisms (22). Disruption of the tight junction of
the intestinal mucosa is a frequent cause of AP-related SIRS and
sepsis. Zonula occludens-1 (ZO-1) and occludin are the most
extensively used tight junction structural markers. ZO-1 expression
is downregulated in AP and inversely correlated with the intensity
of inflammation (23).
In the present study, the damage to numerous organs
(pancreas, lung, ileum, liver and kidney) and systemic
inflammation, induced by retrograde injection of 3.5 or 5% NaT into
the rat pancreatic duct, were evaluated.
Materials and methods
Animals
A total of 192 male specific pathogen-free
Sprague-Dawley rats (weight, 300±20 g) were purchased from the
Experimental Animal Center of Guangzhou University of Chinese
Medicine (Guangzhou, China) and housed under standard laboratory
conditions (12/12 h light-dark cycle, 21±2˚C, humidity 50±10%) with
food and water provided ad libitum during the study. All
animal procedures were conducted in accordance with the Guidelines
for Animal Care of Guangzhou University of Chinese Medicine and
were approved (approval no. ZYD-2020-028) by the Animal Care and
Use Committee of Guangzhou University of Chinese Medicine
(Guangzhou, China). All efforts were made to minimize animal
suffering.
NaT-induced pancreatitis
Male Sprague-Dawley rats were divided into the
following four groups using the random weight method: i) Sham, ii)
normal saline (NS), iii) 3.5% NaT, and iv) 5% NaT. Sham group rats
underwent Sham operation and were treated similarly without
infusion (n=16), NS group rats were infused with NS solution and
underwent Sham operation (n=16), whereas SAP was induced in 3.5%
NaT group rats (n=80) (3, 6, 12, 24, 48 and 72 h) and 5% NaT group
rats (n=80) (3, 6, 12, 24, 48 and 72 h) according to a previously
reported method (13,15,16).
Rats were fasted for 12 h before the operation. Anesthesia was
induced using isoflurane (RWD Life Science) and an R550
Multi-output Anesthesia Machine (RWD Life Science) before the onset
of surgery. Briefly, a midline laparotomy was performed after
sterilization, and the duodenum was identified and gently rotated
to expose the posterior surface and pancreas. The proximal
biliopancreatic duct was then temporarily occluded with a
microclip. The cannula was passed through the duodenal wall to the
papilla and into the pancreatic duct. Next, 3.5 or 5% NaT
(Sigma-Aldrich; Merck KGaA) at 1 ml/kg was infused into the
pancreatic duct at a constant rate (6 ml/h) and pressure, using a
syringe pump (Leienly Company). After the infusion, the cannula and
microclips were removed from the biliopancreatic duct. Finally, the
peritoneum and skin were sutured using a 4-0 prolene suture. After
surgery, all the rats were administered NS (1 ml/kg) and placed on
a heated pad (37˚C) until they recovered. Animals in the Sham and
NS groups were euthanized at 72 h, whereas NaT-induced SAP animals
were euthanized at 3, 6, 12, 24, 48 and 72 h after overdosage of
isoflurane anesthesia (≥5% for >1 min after cessation of
breathing stops). Due to the difficulty of surgery and the high
mortality rate in this model, particularly in the 5% NaT group, no
rats survived in the 5% NaT-72 h group. The rest of the sample
distribution was as follows: Sham group (n=16), NS group (n=16),
3.5% NaT-3 h (n=15), 3.5% NaT-6 h (n=15), 3.5% NaT-12 h (n=13),
3.5% NaT-24 h (n=14), 3.5% NaT-48 h (n=5), 3.5% NaT-72 h (n=4), 5%
NaT-3 h (n=15), 5% NaT-6 h (n=13), 5% NaT-12 h (n=11), 5% NaT-24 h
(n=7), 5% NaT-48 h (n=3), and 5% NaT-72 h (n=none).
Blood was collected from the abdominal aorta, and
ascitic fluid was collected under isoflurane anesthesia before
euthanasia. All blood and ascitic fluid samples were centrifuged at
1,000 x g, 4˚C for 15 min, and maintained at -80˚C until use.
Tissue samples from the pancreas, lung, ileum, liver and kidneys
were harvested and fixed in 4% paraformaldehyde (Dalian Meilun
Biology Technology Co., Ltd.) at room temperature for over 24 h,
embedded in paraffin blocks, or stored at -80˚C until use.
Histopathological analysis
Tissue sections were stained with hematoxylin and
eosin (H&E) for histopathological evaluation. After dewaxed and
dehydration, tissue sections were stained with hematoxylin at room
temperature for 5 min, followed by differentiation and bluing, then
stained with eosin at room temperature for 1 min. For each group,
the pancreatic histopathological scores of three independent tissue
sections were blindly assessed by two pathologists according to the
scoring criteria developed by Schmidt et al (23) and simplified by Liu et al
(15). Briefly, the pancreatic
histopathological scoring criteria included four aspects: i)
interstitial edema: 0 points=none, 1 point=interlobular, 2
points=lobule involvement, and 3 points=isolated island-like acinar
cells; ii) leukocyte infiltration: 0 points=none, 1 point=<20%,
2 points=20-50%, and 3 points=>50%; iii) acinar cell necrosis: 0
points=none, 1 point=<5%, 2 points=5-20%, and 3 points=>20%;
(iv) hemorrhage: 0 points=none, 1 point=1-2, 2 points=3-5, and 3
points=>20%. Pulmonary histopathological changes were assessed
by areas of alveolar septal thickening, leukocyte infiltration,
alveolar capillaries dilation and congestion and hemorrhage.
Serum and ascitic fluid amylase
levels
The serum and ascitic fluid amylase levels were
determined using starch-iodine colorimetry and α-amylase assay kits
(Nanjing Jiancheng Bioengineering Institute). Phosphate-buffered
saline (PBS) was used to dilute the pancreatitis rat serum and
ascites fluid samples. First, 0.1 ml of serum and ascitic fluid
samples were added into 0.5 ml 4 g/ml substrate buffer and placed
in a water bath with a water temperature of 37˚C for 7.5 min. Then,
0.5 ml 0.1 mol/l iodine solution and 3 ml distilled water were
added. The absorbance was immediately detected at 660 nm on a UV
spectrophotometer (Shanghai Metash Instrument Co., Ltd.).
Serum and ascitic fluid lipase
levels
The serum and ascitic fluid lipase levels were
determined after dilution (1:1 and 1:10) with PBS using a routine
colorimetric method and a lipase assay kit (Nanjing Jiancheng
Bioengineering Institute). The substrate buffer was preheated at
37˚C and added to 50 µl fresh serum or ascitic fluid, and the
absorbance was detected at 420 nm in the first 30 sec as A1. After
10 min of reaction, the absorbance (A2) was read using a UV
spectrophotometer. The lipase activity was measured according to
the manufacturer's protocol.
Enzyme-linked immunosorbent assay
(ELISA)
The ileum tissues were lysed in normal saline
containing a protease inhibitor. Tissues were ground using an
automatic tissue lyser (Jingxin Industrial Development Co., Ltd.).
The lysate was collected and centrifuged at 1,000 x g and 4˚C for
15 min. The protein concentration was measured using a BCA Protein
Assay Kit (Beyotime Institute of Biotechnology). All samples were
stored at -80˚C until use. Enzymatic immunoassays for IL-1β, IL-6,
and TNF-α were performed using ELISA kits (cat. nos. CRE0006,
CRE0005 and CRE0003; Beijing 4A Biotech Co., Ltd.) according to the
manufacturer's protocol. The standard, serum, and ascitic fluid
samples were added into a 96-well plate (100 µl/well), which was
precoated with IL-1β, IL-6, and TNF-α, respectively. Then, they
were incubated at 37˚C for 2 h and washed five times with washing
buffer. The corresponding biotinylated antibodies (IL-1β 1:100,
IL-6 1:100 and TNF-α 1:100), enzyme-binding antibodies, and
tetramethylbenzidine solution were added. Finally, 100 µl stop
solution was added to stop the reaction. The absorbance was
detected at 450 nm using MULTISKAN GO (Thermo Fisher Scientific,
Inc.), the concentrations of each sample were calculated according
to the standard curve, and protein concentrations were normalized
to tissue cytokine levels.
Immunofluorescence
Ileum tissue was embedded in an Optimum Cutting
Temperature embedding agent, sliced into 10-µm thick sections, and
fixed with 4% paraformaldehyde at 4˚C for 30 min. Next, the tissue
sections were incubated with 100 µl of 5% donkey serum blocker
solution at room temperature for 2 h to block non-specific
antigens. A diluted antibody-containing 5% donkey serum PBST (0.05%
Tween-20) was prepared of ZO-1 (1:50; cat. no. sc-33725, Santa Cruz
Biotechnology, Inc.) or occludin (1:50; cat. no. sc-133256, Santa
Cruz Biotechnology, Inc.). Next, 50 µl of the diluted antibody
solution was added to each sample and incubated overnight at 4˚C.
After washing with PBST for 10 min thrice, the samples were
incubated with a PBST-diluted AF488-conjugated secondary antibody
(1:200; cat. no. 1010-30, Southern Biotech) for 2 h at room
temperature. Then, they were washed with PBST thrice for 10 min
each, and stained with Hoechst 33342 (1:1,000; cat. no. 561908, BD
Pharmingen) at room temperature for 30 min. An LSM 8000 laser
confocal microscope (Carl Zeiss AG) equipped with a camera was used
to observe the stained tissue samples and to obtain images.
Biochemical assay
Serum alanine transaminase (ALT), aspartate
transaminase (AST), creatinine (CRE), and urea levels were measured
using a Hitachi 7080 automatic biochemical analyzer (Hitachi,
Ltd.). Lung myeloperoxidase (MPO) levels were determined using MPO
ELISA kits (cat. no. CSB-E13689Rb, Cusabio Technology LLC) and
malondialdehyde (MDA) activity was determined using the Lipid
Peroxidation MDA Assay kit (cat. no. S0131S, Beyotime Institute of
Biotechnology) according to the manufacturer's protocol.
Statistical analysis
All data were analyzed using the SPSS software
(version 25.0; IBM Corp.) and are presented as the mean ± standard
error. After the homogeneity of variance test, one-way ANOVA
followed by the least significant difference (LSD) or Games-Howell
test was performed for multiple group comparisons. Data were
displayed using GraphPad Prism (version 7.0; GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
General condition (mortality, ascites
volume, and amylase and lipase activities in the serum and ascitic
fluid) of the rats with NaT-induced SAP
SAP rat serum and ascitic fluid were collected at
different time points (3, 6, 12, 24, 48 and 72 h) and the amylase
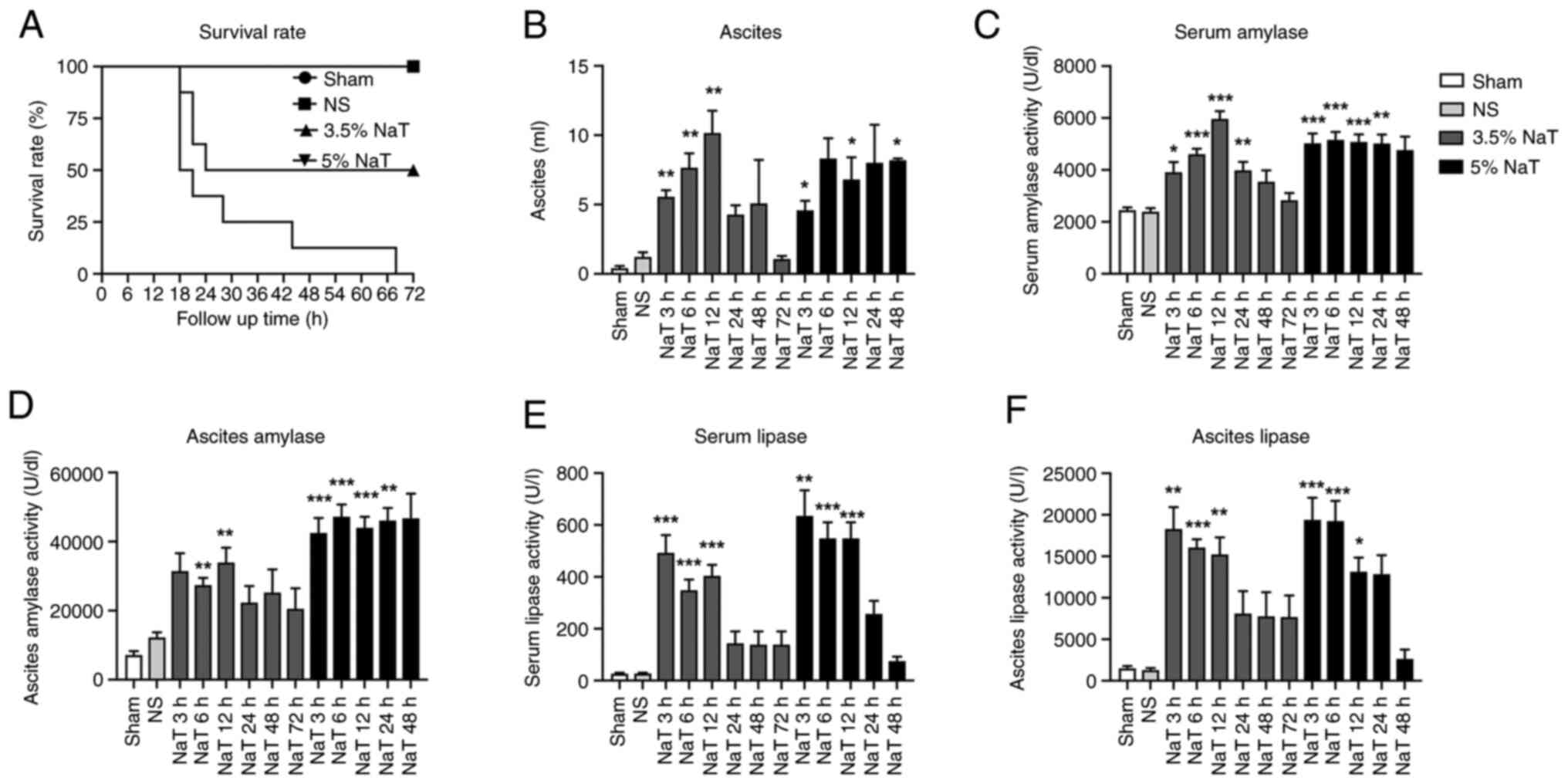
and lipase levels were detected. As revealed in Fig. 1A, the mortality rate in the 3.5%
NaT-72 h group was ~50%, and most animals succumbed within 24 h.
The mortality rate in the 5% NaT-72 h group was ~100%; therefore, a
sample from this group was unobtainable.
The ascites volume increased 3 h after surgery.
Significant increases in the ascites volume were observed in the
3.5% NaT (3, 6 and 12 h) and 5% NaT (3, 12 and 48 h) groups
compared with those in the NS group (Fig. 1B). Serum amylase levels
significantly increased after pancreatic duct perfusion with 3.5%
NaT (3, 6, 12 and 24 h) and 5% NaT (3, 6, 12 and 24 h) compared
with those in the NS group (Fig.
1C). The amylase levels in ascitic fluid also significantly
increased after perfusion with 3.5% NaT (6 and 12 h) and 5% NaT (3,
6, 12 and 24 h), as demonstrated in Fig. 1D. In the 5% NaT group, the serum
and ascitic fluid amylase levels exhibited a sustained increase and
were maintained at a high level without reduction. No statistically
significant difference in amylase levels was observed in the 5%
NaT-48 h group. Lipase activity in the 3.5% NaT and 5% NaT groups
significantly increased in the serum and ascitic fluid at 3, 6 and
12 h after the infusion and recovered within 24 h (Fig. 1E and F).
Histopathological changes in the
pancreas of NaT-induced SAP rats
Histopathological scores were assessed (Table I) according to the levels of
pancreatic interstitial edema, leukocyte infiltration, acinar cell
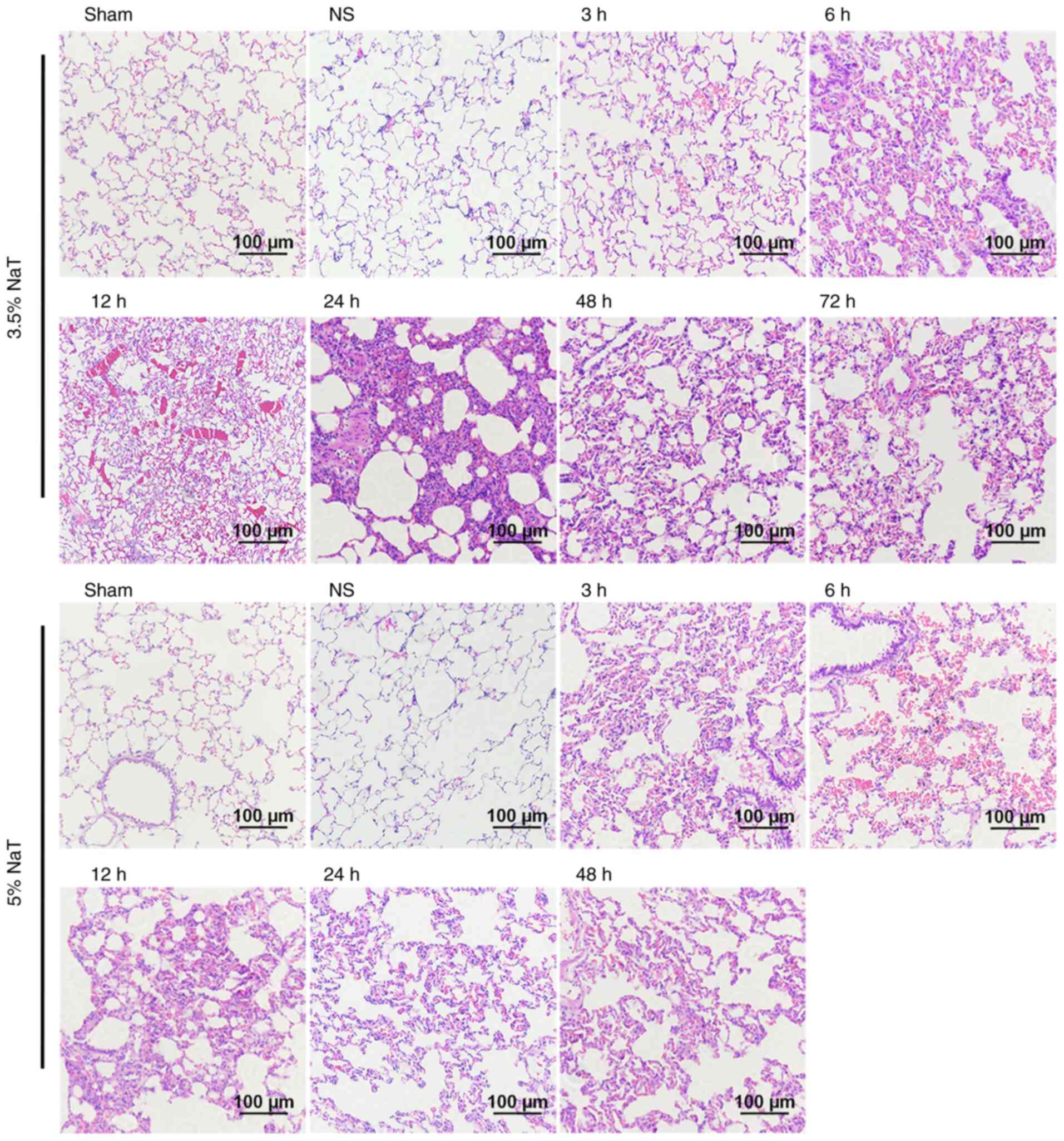
necrosis and hemorrhage using H&E staining. Representative
images (magnification, x200) of the H&E-stained pancreatic
sections are revealed in Fig. 2.
No remarkable difference was observed in the histopathological
images of the pancreas between the Sham and NS groups. In the 3.5
and 5% NaT groups, the pancreas was critically damaged in a time-
and dose-dependent manner, compared with the NS group. Furthermore,
the pancreas in the 5% NaT group exhibited earlier severe acinar
cell necrosis, interstitial edema, and hemorrhage compared with
that in the 3.5% NaT group (Fig. 2
and Table I). However, leukocyte
infiltration was mild in both groups (mostly lymphocytes,
plasmacytes, and eosinophils rather than neutrophils).
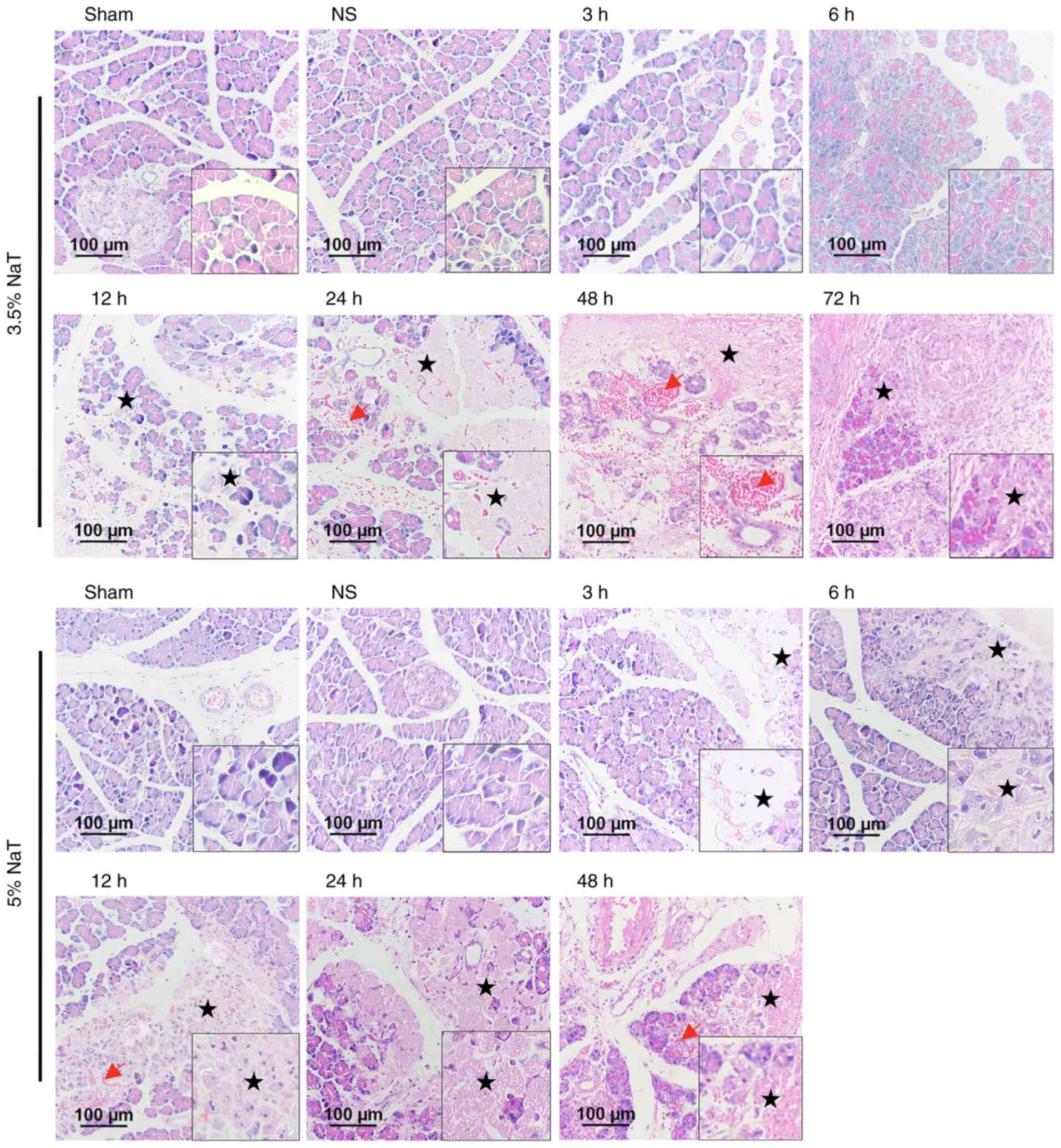 | Figure 2Histopathological changes of the
pancreas in NaT-induced severe acute pancreatitis rats.
Representative images of hematoxylin and eosin-stained pancreatic
sections (magnification, x200; scale bar: 100 µm). Normal
pancreatic histology in the Sham and NS groups. In the 3.5% NaT
groups, interstitial edema occurred at 3 h and extended at 6 h
after pancreatitis induction. Extensive acinar cell necrosis (star)
was observed at 12, 24, 48 and 72 h after surgery, with hemorrhage
(red arrow). In the 5% NaT groups, the typical pathological
features at 3 h were edema and small areas of acinar cell necrosis.
Hemorrhage was observed at 6 h, which was exacerbated at 12, 24,
and 48 h with severe necrosis. NaT, sodium taurocholate; NS, normal
saline. |
 | Table IPancreatitis histopathological
scores. |
Table I
Pancreatitis histopathological
scores.
| | Score |
|---|
| Groups (n=3) | Interstitial
edema | Leukocyte
infiltration | Acinar cell
necrosis | Hemorrhage | Total |
|---|
| Sham | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| NS | 0.33±0.33 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.33±0.33 |
| 3.5% NaT | | | | | |
|
3 h | 1.33±0.33 | 0.00±0.00 | 1.00±0.00 | 0.00±0.00 | 2.33±0.33 |
|
6 h |
2.33±0.67b | 0.00±0.00 | 0.67±0.33 | 0.00±0.00 | 3.00±0.58 |
|
12 h |
2.00±0.58b | 0.67±0.67 |
3.00±0.00b |
2.17±0.44b |
7.83±1.30b |
|
24 h |
2.00±0.00b | 0.67±0.33 |
2.00±0.58b |
1.83±0.44a |
6.50±1.04b |
|
48 h |
1.83±0.16a | 0.83±0.44 |
2.67±0.17b |
1.83±0.60a |
7.17±1.20b |
|
72 h |
2.00±0.00b |
1.67±0.33b |
3.00±0.00b |
3.00±0.00b |
9.67±0.33b |
| 5% NaT | | | | | |
|
3 h | 1.00±0.00 | 0.67±0.17 |
1.50±0.29a | 1.17±0.73 |
4.33±0.93b |
|
6 h | 1.33±0.33 | 1.00±0.00 |
2.67±0.33b | 1.00±0.58 |
6.00±0.00b |
|
12 h | 1.00±0.00 | 1.00±0.00 |
2.67±0.33b |
2.67±0.33b |
7.33±0.33b |
|
24 h | 1.33±0.33 |
1.17±0.17a |
3.00±0.00b |
2.67±0.33b |
8.17±0.44b |
|
48 h |
2.50±0.00b |
1.67±0.17b |
3.00±0.00b |
2.33±0.17b |
9.50±0.00b |
Proinflammatory cytokines in the serum
and ascitic fluid of NaT-induced SAP rats
The protein levels of IL-1β, IL-6, and TNF-α were
measured using ELISA to evaluate the inflammatory response in the
serum and ascitic fluid of rats with NaT-induced pancreatitis. The
levels of IL-1β in ascitic fluid were significantly increased in
the 5% NaT-12 h group compared with those in the NS group
(P<0.05; Fig. 3D). The levels
of IL-6 in ascitic fluid significantly increased in the 5% NaT
group at 3, 6 and 12 h, and were alleviated within 48 h (P<0.05
compared with the 12 h group; Fig.
3E). The levels of IL-6 significantly increased in serum at 5%
NaT-48 h, Whereas the level of IL-1β and TNF-α in the serum
scarcely increased after 3.5 or 5% NaT infusion (Fig. 3A-C). Again, the levels of TNF-α in
the serum and ascitic fluid were not elevated (Fig. 3C and F).
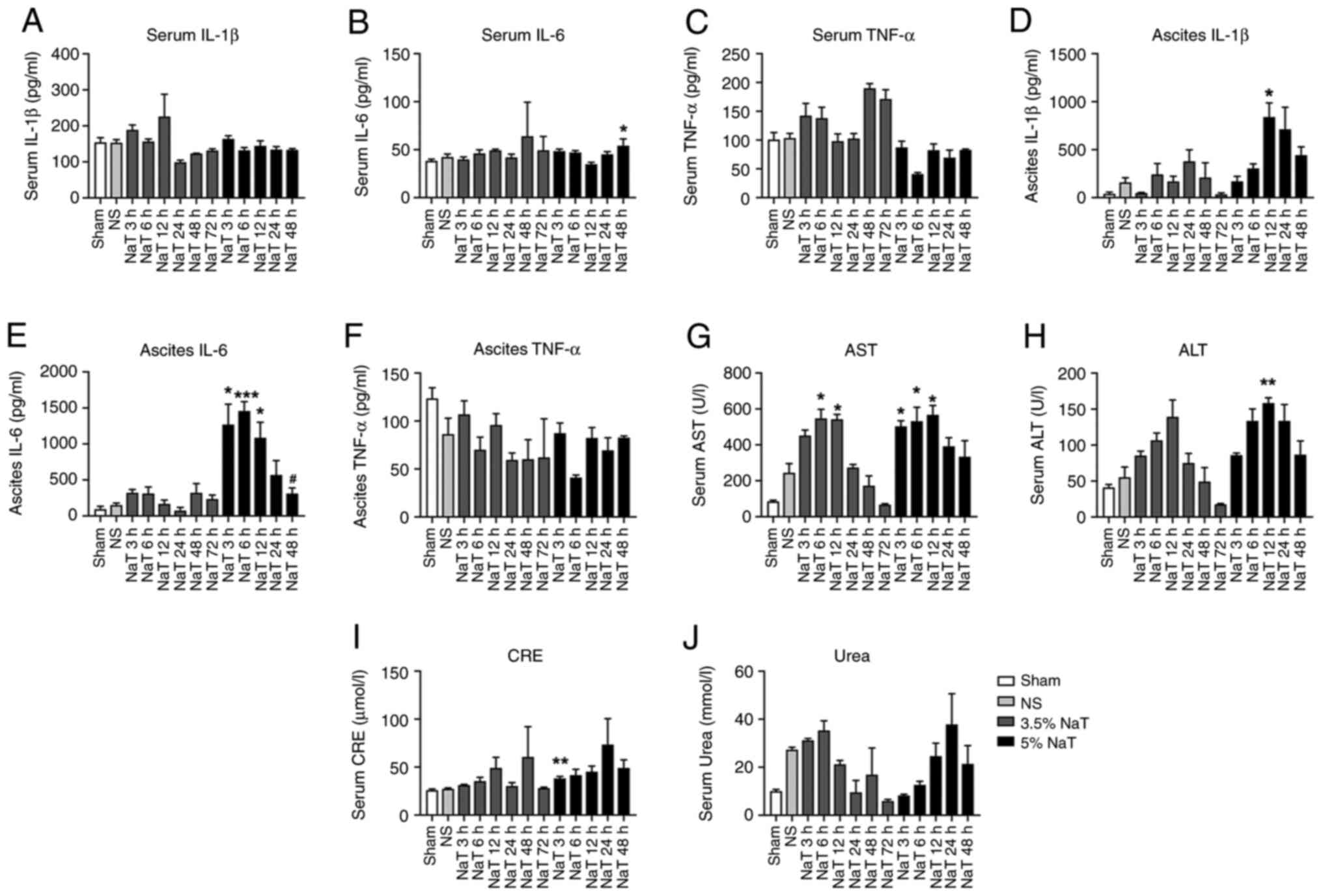 | Figure 3The levels of proinflammatory
cytokines, liver function and renal function in NaT-induced severe
acute pancreatitis rats. The serum levels of (A) IL-1β, (B) IL-6
and (C) TNF-α, respectively. The ascitic fluid levels of (D) IL-1β,
(E) IL-6 and (F) TNF-α, respectively. The serum levels of (G) AST,
(H) ALT, (I) CRE, and (J) Urea, respectively. Data are expressed as
the mean ± standard error of the mean. *P<0.05,
**P<0.01 and ***P<0.001 vs. the NS
group; #P<0.05 vs. the 5% NaT-12 h group, (n=3-16).
NaT, sodium taurocholate; ALT, alanine transaminase; AST, aspartate
aminotransferase; CRE, creatinine; NS, normal saline. |
Pancreatitis-associated liver and
kidney injuries in NaT-induced SAP rats
Compared to the NS group, the AST levels were
significantly elevated after infusion in the 3.5% (6 and 12 h) and
5% NaT groups (3, 6 and 12 h; P<0.05; Fig. 3G). The level of ALT in 5% NaT
groups (12 h) was significantly increased (P<0.01; Fig. 3H). In 3.5% NaT groups, ALT and AST
levels declined back to almost normal levels at 72 h, whereas in
the 5% NaT groups a nonsignificant downward trend was observed
after 24 h (Fig. 3G and H). Furthermore, the CRE levels increased
only in the 5% NaT-3 h group (P<0.01), compared with those in
the NS group (Fig. 3I), whereas no
statistically significant increase in the serum urea levels was
observed (Fig. 3J).
According to the liver histopathology results
(Fig. S1), only slight
hepatocellular edema and hepatic sinusoid dilation were observed at
12 h (1/3) and 24 h (1/3) in the 5% NaT group. In the kidneys, ~1/3
of the 5% NaT rats at each time point exhibited slight renal tubule
edema or renal vascular congestion, and only one rat among all
animals developed periglomerular interstitial hemorrhage and
ischemic tubular necrosis at 24 h. The most severe pathological
findings are shown in Fig. S1.
However, changes in the liver and kidneys were considered
insufficient for the diagnosis of pathological damage. In addition,
no apparent pathological changes in the kidneys and livers were
observed in the 3.5% NaT group, even after 72 h (data not
shown).
Pancreatitis-associated acute lung
injury in NaT-induced SAP rats
Lung sections were stained with H&E and images
were obtained (Fig. 4).
Histopathological results showed that the 3.5 and 5% NaT groups
showed alveolar septum thickening with partial alveolar collapse,
alveolar capillary dilation with congestion, or local hemorrhage.
Alveolar septum thickening was widely observed in all NaT groups
(Table II). Slight neutrophil
infiltration was observed in certain cases (Table II). MPO and MDA levels in lung
tissue were not significantly increased (Fig. S2).
 | Table IIHistopathological findings of lungs
tissue. |
Table II
Histopathological findings of lungs
tissue.
| | Number of
case/Total number of each group |
|---|
| | Alveolar septum
thickening area | |
|---|
| Groups | None | ≤5% | <20% | ≥20% | Leukocyte
infiltration | Alveolar
capillaries dilation and congestion | Hemorrhage |
|---|
| Sham | 5/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| NS | 2/5 | 2/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 3.5% NaT | | | | | | | |
|
3 h | 1/5 | 3/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 |
|
6 h | 0/5 | 0/5 | 2/5 | 3/5 | 1/5 | 0/5 | 1/5 |
|
12 h | 0/5 | 0/5 | 2/5 | 3/5 | 0/5 | 0/5 | 2/5 |
|
24 h | 0/5 | 0/5 | 2/5 | 3/5 | 1/5 | 0/5 | 0/5 |
|
48 h | 0/5 | 0/5 | 0/5 | 5/5 | 2/5 | 4/5 | 1/5 |
|
72 h | 0/4 | 0/4 | 1/4 | 3/4 | 0/4 | 2/4 | 0/4 |
| 5% NaT | | | | | | | |
|
3 h | 0/5 | 0/5 | 1/5 | 4/5 | 0/5 | 0/5 | 0/5 |
|
6 h | 0/5 | 1/5 | 2/5 | 2/5 | 3/5 | 0/5 | 1/5 |
|
12 h | 0/5 | 2/5 | 2/5 | 1/5 | 2/5 | 0/5 | 1/5 |
|
24 h | 0/5 | 3/5 | 0/5 | 2/5 | 1/5 | 0/5 | 1/5 |
|
48 h | 0/3 | 2/3 | 1/3 | 0/3 | 1/3 | 0/3 | 0/3 |
Pancreatitis-associated intestinal
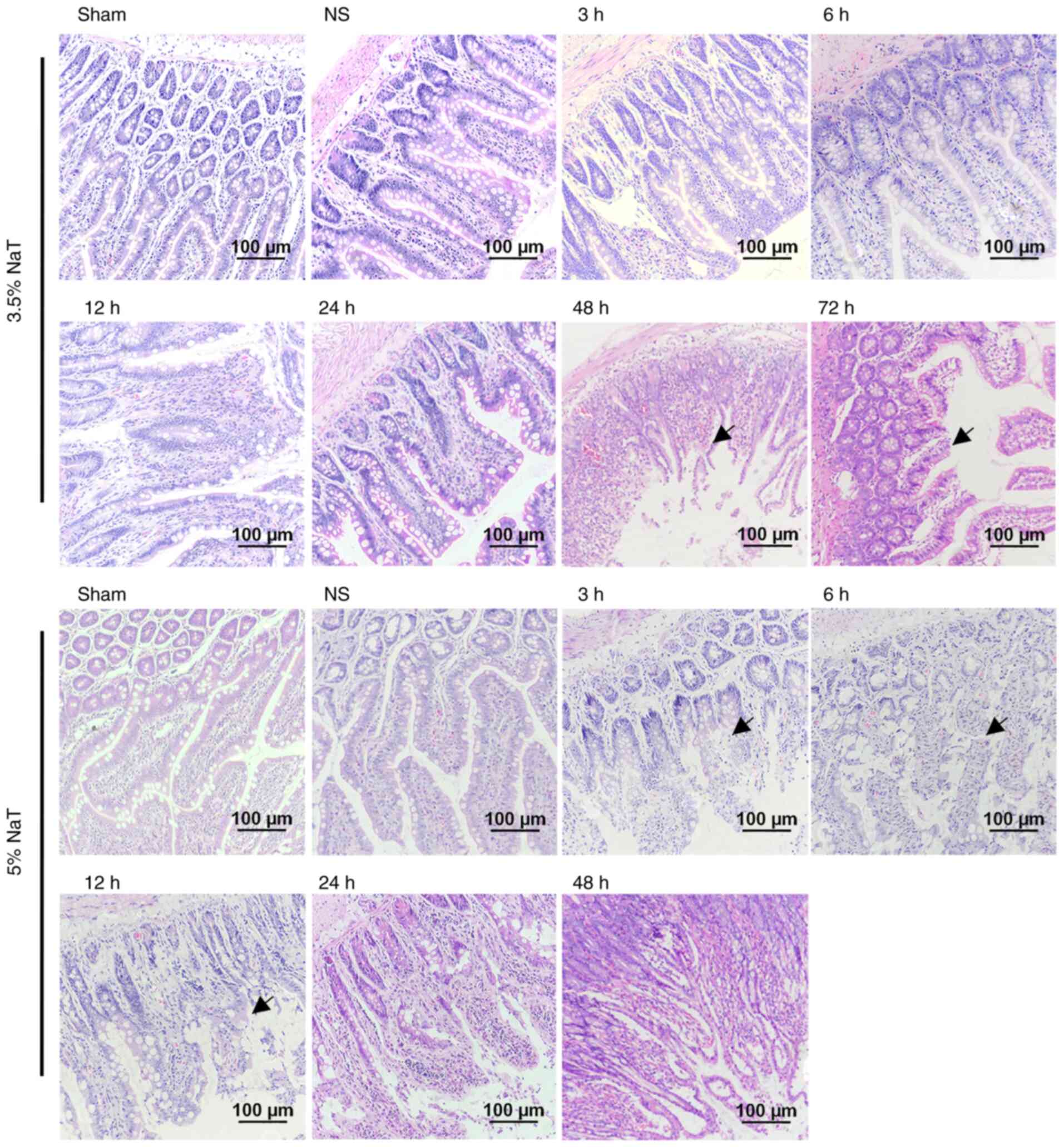
mucosal injury in NaT-induced SAP rats
Intestinal mucosal injury was observed in the ileum
in the 5% NaT groups using H&E staining (magnification, x200;
Fig. 5). In the 5% NaT group, the
intestinal mucosal epithelium exhibited abscission, necrosis and
hemorrhage at 3 and 6 h after infusion, suggesting that 5% NaT
induced mucosal injury in the early stage of SAP. No significant
pathological changes in the ileum were observed in the 3.5% NaT
group compared with those in the NS group until 48 h (1/3) and 72 h
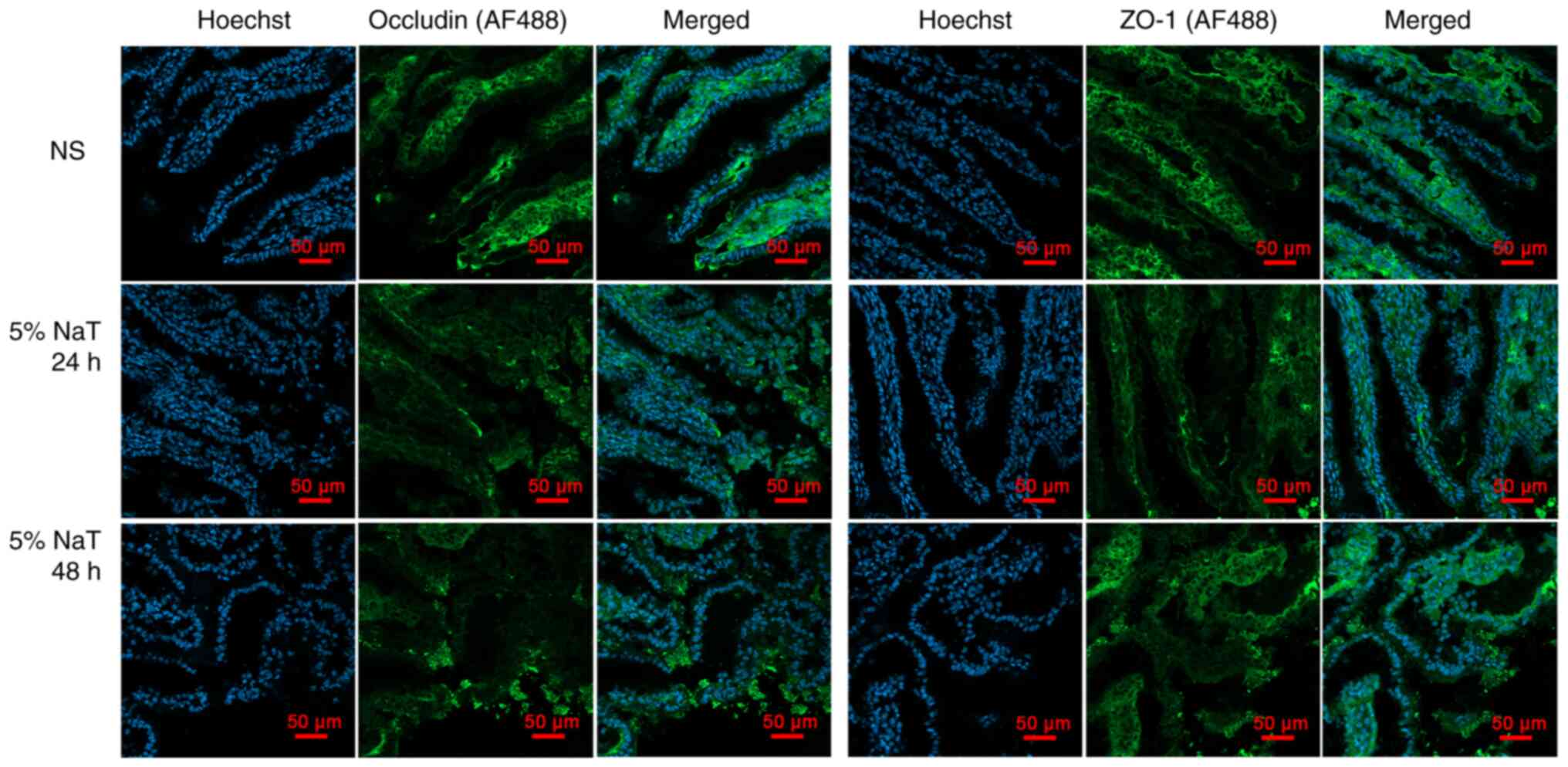
(1/3). Furthermore, immunofluorescence was used to detect
alterations in ileal tight junction morphology in the 5% NaT group
(24 and 48 h). As expected, disruption of ZO-1 and occludin tight
junction constituents was detected in the 5% NaT-48 h group
(Fig. 6).
The levels of IL-1β, IL-6 and TNF-α in ileum tissue
were detected using ELISA (Fig.
S3). The results showed that the IL-1β and TNF-α levels in the
5% NaT group were significantly increased in the ileum at 12 h
compared with those in the NS group (P<0.01). No statistical
difference in the IL-6 levels was observed in either the 3.5 NaT or
5% NaT groups.
Discussion
SAP is a devastating condition characterized by
local pancreatitis and systemic complications, commonly originating
from biliary (45%) and alcoholic (21%) AP (24). Overall, the mortality rate of
patients with SAP may reach 50%, whereas that of all forms of AP is
~2-5% (25). One of the most
critical risk factors for mortality in SAP is organ failure
(18). However, the mechanisms
underlying SAP development remain unclear. To perform a more
systematic assessment of organ dysfunction and systemic
inflammatory responses in this animal model, multiple organs
treated with different NaT concentrations and at different time
points were observed and studied.
In the present study, a SAP rat model with two
different concentrations of NaT was successfully established and
the potential systemic injury in this model was examined. The
present results revealed that the ascites volume, amylase and
lipase activity in the serum and ascitic fluid significantly
increased at both NaT concentrations after infusion, suggesting
that our model was successfully established. The mechanism of
ascites production is related to the leakage of pancreatic
secretions from damaged pancreatic ducts, which occurs when
pancreatic secretions accumulate in the peritoneum (26). The severity of ascites production
varies widely, often depending on the location and degree of ductal
injury and infection in the fluid (26).
In a previous study, the mortality of AP mice that
received 4 and 5% taurocholate solutions in the first 24 h was ~10
and 60%, respectively (27).
Considering the high mortality rate of this model, the effects of
3.5 and 5% NaT were compared. The histological changes in
pancreatic damage in NaT-induced pancreatitis became progressively
more severe in all SAP groups during the experiment. The 5% NaT
group exhibited earlier and more severe acinar cell necrosis,
interstitial edema and hemorrhage than the 3.5% NaT group. However,
only mild infiltration of lymphocytes, plasmacytes, and
eosinophils, rather than neutrophils, was observed, indicating that
pancreatic injury may be considered a hemorrhagic necrotizing
injury rather than an inflammatory lesion.
The development of SIRS is an important risk factor
for AP (28-30).
Therefore, the levels of proinflammatory cytokines, such as IL-1β,
Il-6 and TNF-α, were determined. Although it was attempted to
monitor the occurrence of SIRS in rats with SAP using cytokine
levels, the results were unsatisfactory. Only IL-6 showed a
substantial increase in the circulation after 48 h of 5% NaT
infusion in the present study, whereas cytokines, such as IL-1β and
TNF-α, did not. One possible explanation is that pancreatitis
begins with the premature activation of digestive enzymes in
pancreatic acinar cells, resulting in cell damage (11,31).
This process parallels the infiltration of inflammatory cells and
causes organ and pancreatic damage (32). H&E staining of the pancreas
revealed more necrosis and hemorrhage in the early stage, while
leukocyte infiltration appeared later in this animal model, at 72 h
in the 3.5% NaT group, and at 24 and 48 h in the 5% NaT group.
Furthermore, intestinal ischemia/reperfusion injuries sustained by
pancreatic inflammation may lead to mucosal barrier disruption and
bacterial translocation, which may be the first stage in the
pathogenesis of sepsis (33,34).
Tight junctions between intestinal epithelial cells play an
essential role in intestinal permeability. In the present study,
ZO-1 and occludin tight junction constituents were disrupted after
infusion of 5% NaT for 48 h. This may explain why early stage
elevation of serum inflammatory cytokines was minimal. It is
hypothesized that this model exhibited a systemic inflammatory
response.
Although it was not possible to assess the
occurrence of SIRS in terms of serum inflammatory cytokines, a
significant release of a large number of inflammatory factors
(IL-1β and IL-6) in ascites was observed in the 5% NaT group.
Pancreatic ascites results from the leakage of pancreatic
secretions into the peritoneum, mostly occurring after a pseudocyst
or walled-off necrosis (26). The
ascitic fluid contains a large number of lipases, amylase,
inflammatory cytokines and necrotic substances, which may cause
intra-abdominal hypertension and adhesion of the abdominal organs
(35). Symptoms of SAP can be
improved by decreasing the levels of cytokines and abdominal
paracentesis drainage has been reported to be beneficial to animals
and patients (36-38).
The current clinical diagnosis of SIRS is mainly
related to the evaluation of vital signs (body temperature, heart
rate and respiration) and leukocyte count, thus lacking specificity
(39). Matsumoto et al
(40) used HMGB1 as a marker of
SIRS in rats intraperitoneally injected with cerulein and
lipopolysaccharide. However, the serum levels of IL-1β, IL-6, and
TNF-α were not measured. Moreover, the activation of NLRP3 is
confirmed to regulate the development of SIRS and compensatory
anti-inflammatory response syndromes in mice with cerulein-induced
AP (37). More sensitive
indicators are required to determine the occurrence of SIRS in
SAP.
Pancreatic necrosis is associated with poor
prognosis, higher mortality and organ failure (41). Multiple organ injury in the SAP rat
model was also investigated in the present study. Acute lung
injury, acute kidney injury and cardiovascular dysfunction are the
most common pancreatitis-associated organ dysfunctions in patients
and autopsy findings (19,25). Pancreatitis-associated acute lung
injury (12,19), kidney injury (42-44),
and liver injury (45) have been
reported in experimental models. Clinical findings of respiratory
failure (acute respiratory distress syndrome) are correlated with
autopsy findings of pleural effusions, acute diffuse alveolar
damage and pulmonary congestion (46,47).
In the present study, alveolar septum thickening with partial
alveolar collapse was widely observed after NaT infusion,
representing a possible insufficiency of lung function. Dilation
and congestion of alveolar capillaries, which primarily appeared in
the 3.5% NaT group at 48 and 72 h, have also been observed in some
cases. Neutrophil infiltration was also observed in some cases in
our study, but the MPO and MDA levels did not increase in lung
tissue.
Shi et al (42) reported the features of kidney
pathologic injury, such as glomerular degeneration, blurred cell
boundaries, tubule epithelial cell swelling and necrosis,
interstitial hemorrhage, tubule lumen narrowing, formation of a
large number of tubular casts and inflammatory cell infiltration,
at 12 h after administration of 5% NaT. However, it was found that
even after 5% NaT infusion, most of the animals did not present
such pathological changes in the kidneys, whereas only one rat
showed periglomerular interstitial hemorrhage and ischemic tubular
necrosis in the kidneys after 24 h. Yet, numerous researchers have
used this model to investigate drugs related to liver and kidney
injury. Occasionally, slight hepatocellular edema and hepatic
sinusoid dilation in the liver were observed in the present study;
however, these changes may not be sufficient for further efficacy
studies. The present results are consistent with those previously
reported in a mouse model, in which NaT-induced AP did not show
severe kidney and liver pathological damage (27). One possible explanation is that
pancreatic necrosis caused by NaT mainly affects the respiratory
circulatory system in the early stage, causing a large number of
deaths. While the liver adjacent to the pancreas has a strong
compensatory capacity, only a transient increase in liver enzymes
occurs in the early stage.
Intestinal mucosal ischemia-reperfusion during AP
can compromise the integrity of the intestinal barrier and cause
intestinal bacterial translocation, resulting in local and systemic
infections (48). The
gastrointestinal complications of pancreatitis are important and
may result in high mortality rates. Bowel complications associated
with AP are potentially due to the pancreatic enzymes released to
the mesentery, which may result in inflammation, ischemia, necrosis
and obstruction of different segments of the intestine (49). Small intestinal mucosal barrier
injury may contribute to bacterial translocation and infected
pancreatic necrosis, resulting in the pathophysiology of systemic
inflammation (19). Intestinal
injury has been reported in the NaT-induced SAP model, but most
studies have focused on the results obtained at 12, 24 h, or even
24 days after infusion (50-53).
In the present study, it was identified that the ileum villi were
exfoliated and necrotic in the 5% NaT group, but these injuries
were virtually absent in the 3.5% NaT group, indicating that the
onset of injury to the intestinal mucosal barrier may occur at the
early stage of pancreatitis in this animal model using 5% NaT.
Moreover, local inflammation and intestinal barrier
tight junction proteins in ileum tissue were examined. The levels
of IL-1β and TNF-α were significantly elevated in the ileum in the
5% NaT-12 h group, and no further increase in the levels of IL-1β,
IL-6 and TNF-α was observed after 24 h. According to the
pathologist, intestinal mucosal injuries that occurred in the early
stage of this animal model are caused by acute ischemic injury
rather than bacteria, which is consistent with the finding that the
levels of inflammatory factors were not elevated in the early
stage. In addition, the expression of the tight junction proteins
ZO-1 and occludin was disrupted in the 5% NaT group at 48 h. This
result indicated that ischemic injury of the ileum progressed to
severe intestinal barrier injury in this model; therefore, there
was significant inflammatory infiltration in the intestinal tract
or pancreatic necrotic tissue in the 5% NaT group at 48 h.
A likely explanation is that the high mortality in
this model is a result of severe pancreatic damage and acute
respiratory dysfunction, leading to hemodynamic instability.
Unfortunately, indices such as heart rate, blood pressure, blood
oxygen saturation, arterial blood gas and other physiological
indicators were not included; basic experiments may be helpful in
monitoring the severity of AP in rats.
The present study may hopefully provide important
information to researchers who intend to carry out animal
experiments using NaT-induced SAP models. The present findings will
be useful for conducting experiments according to the 3R principles
of replacement, reduction, and refinement for the ethical use of
animals in research. However, it was also emphasized that there are
certain limitations in the application of this model to the study
of mechanisms and treatments for pancreatitis-associated acute
liver and kidney injuries. Therefore, it is considered that
multiple model comparisons are important for efficacy assessment
and further mechanistic exploration.
Supplementary Material
Pancreatitis-associated liver and
kidney injuries in NaT-induced severe acute pancreatitis rats.
Representative images of hematoxylin and eosin-stained (A) liver
and (B) kidney sections (magnification, x200; scale bar: 100 μm).
NaT, sodium taurocholate; NS, normal saline.
MPO and MDA activities in NaT-induced
severe acute pancreatitis rats. The levels of (A) MPO and (B) MDA
activity in lung tissues showed no significant increase. Data are
expressed as the mean ± standard error of the mean (n=3 5). MPO,
lung myeloperoxidase; MDA, malondialdehyde; NaT, sodium
taurocholate; NS, normal saline.
Proinflammatory cytokines in the ileum
of NaT-induced severe acute pancreatitis rats. The protein levels
of IL-1%#x03B2;, IL-6, and TNF-α in the ileum of rats in the (A)
3.5% NaT and (B) 5% NaT groups detected using ELISA. Data are
expressed as the mean ± standard error of the mean.
**P<0.01 vs. the NS group (n=3 12). NaT, sodium
taurocholate; NS, normal saline.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81703859) and the Health and
Family Planning Commission of Shenzhen Municipality (grant no.
SZBC2017017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XXH conducted the experiment and wrote the
manuscript. HYW designed the study and was involved in revisions.
JMY, BFL and YZL carried out the establishment of animal models,
including animal feeding and animal surgery. PDH and ZYZ collected
and analyzed the data. XXH and JMY confirm the authenticity of all
the raw data. QQM and SJG performed part of the experiments and
provided professional pathological advice. BH was involved in the
design of the study and revised the important intellectual content.
YFX contributed to the conception of this study, was involved in
the animal surgery and gave final approval of the version to be
published. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were conducted according to
the Guidelines for Animal Care of Guangzhou University of Chinese
Medicine and approved (approval no. ZYD-2020-028) by the Animal
Care and Use Committee of Guangzhou University of Chinese Medicine
(Guangzhou, China). All efforts were made to minimize the suffering
of animals as much as possible.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boxhoorn L, Voermans RP, Bouwense SA,
Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC and
Besselink MG: Acute pancreatitis. Lancet. 396:726–734.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor
JA, Yadav D and Petrov MS: Global incidence and mortality of
pancreatic diseases: A systematic review, meta-analysis, and
meta-regression of population-based cohort studies. Lancet
Gastroenterol Hepatol. 1:45–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schepers NJ, Bakker OJ, Besselink MG,
Ahmed Ali U, Bollen TL, Gooszen HG, van Santvoort HC and Bruno MJ:
Dutch Pancreatitis Study Group. Impact of characteristics of organ
failure and infected necrosis on mortality in necrotising
pancreatitis. Gut. 68:1044–1051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van Santvoort HC, Bakker OJ, Bollen TL,
Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor
H, Dejong CH, van Eijck CH, et al: A conservative and minimally
invasive approach to necrotizing pancreatitis improves outcome.
Gastroenterology. 141:1254–1263. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bang JY, Wilcox CM, Arnoletti JP and
Varadarajulu S: Superiority of endoscopic interventions over
minimally invasive surgery for infected necrotizing pancreatitis:
Meta-analysis of randomized trials. Dig Endosc. 32:298–308.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group. Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moggia E, Koti R, Belgaumkar AP, Fazio F,
Pereira SP, Davidson BR and Gurusamy KS: Pharmacological
interventions for acute pancreatitis. Cochrane Database Syst Rev.
4(CD011384)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aho HJ and Nevalainen TJ: Experimental
pancreatitis in the rat. Ultrastructure of sodium
taurocholate-induced pancreatic lesions. Scand J Gastroenterol.
15:417–424. 1980.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aho HJ, Koskensalo SM and Nevalainen TJ:
Experimental pancreatitis in the rat. Sodium taurocholate-induced
acute haemorrhagic pancreatitis. Scand J Gastroenterol. 15:411–416.
1980.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Laukkarinen JM, Van Acker GJ, Weiss ER,
Steer ML and Perides G: A mouse model of acute biliary pancreatitis
induced by retrograde pancreatic duct infusion of Na-taurocholate.
Gut. 56:1590–1598. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Saluja A, Dudeja V, Dawra R and Sah RP:
Early intra-acinar events in pathogenesis of pancreatitis.
Gastroenterology. 156:1979–1993. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Su KH, Cuthbertson C and Christophi C:
Review of experimental animal models of acute pancreatitis. HPB
(Oxford). 8:264–286. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Serra MB, Koike MK, Barbeiro DF, Machado
MCC and de Souza HP: Sodium taurocholate induced severe acute
pancreatitis in C57BL/6 Mice. J Vis Exp. 172(e61547)2021.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Yang X, Geng H, You L, Yuan L, Meng J, Ma
Y, Gu X and Lei M: Rhein protects against severe acute pancreatitis
in vitro and in vivo by regulating the JAK2/STAT3 pathway. Front
Pharmacol. 13(778221)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu ZH, Peng JS, Li CJ, Yang ZL, Xiang J,
Song H, Wu XB, Chen JR and Diao DC: A simple taurocholate-induced
model of severe acute pancreatitis in rats. World J Gastroenterol.
15:5732–5739. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guo P, Liu L, Yang X, Li M, Zhao Q and Wu
H: Irisin improves BBB dysfunction in SAP rats by inhibiting MMP-9
via the ERK/NF-κB signaling pathway. Cell Signal.
93(110300)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qi H, Lu Q, Yin C, Xiao H, Wen Y, Zhang S,
Cui Q and Yang W: Exogenous leptin protects rat models of sodium
taurocholate-induced severe acute pancreatitis through endocrinal
and immunological pathways. Mol Med Rep. 16:6306–6312.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo Q, Li A, Xia Q, Liu X, Tian B, Mai G,
Huang Z, Chen G, Tang W, Jin X, et al: The role of organ failure
and infection in necrotizing pancreatitis: A prospective study. Ann
Surg. 259:1201–1207. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Garg PK and Singh VP: Organ failure due to
systemic injury in acute pancreatitis. Gastroenterology.
156:2008–2023. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Johnson CD and Abu-Hilal M: Persistent
organ failure during the first week as a marker of fatal outcome in
acute pancreatitis. Gut. 53:1340–1344. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mofidi R, Duff MD, Wigmore SJ, Madhavan
KK, Garden OJ and Parks RW: Association between early systemic
inflammatory response, severity of multiorgan dysfunction and death
in acute pancreatitis. Br J Surg. 93:738–744. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Mowbray NG, Ben-Ismaeil B, Hammoda M,
Shingler G and Al-Sarireh B: The microbiology of infected
pancreatic necrosis. Hepatobiliary Pancreat Dis Int. 17:456–460.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Matta B, Gougol A, Gao X, Reddy N,
Talukdar R, Kochhar R, Goenka MK, Gulla A, Gonzalez JA, Singh VK,
et al: Worldwide variations in demographics, management, and
outcomes of acute pancreatitis. Clin Gastroenterol Hepatol.
18:1567–1575.e2. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gliem N, Ammer-Herrmenau C, Ellenrieder V
and Neesse A: Management of severe acute pancreatitis: An update.
Digestion. 102:503–507. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gapp J, Hoilat GJ and Chandra S:
Pancreatic ascites. In: StatPearls. Treasure Island (FL):
StatPearls Publishing, 2020.
|
|
27
|
Wittel UA, Wiech T, Chakraborty S, Boss B,
Lauch R, Batra SK and Hopt UT: Taurocholate-induced pancreatitis: A
model of severe necrotizing pancreatitis in mice. Pancreas.
36:e9–e21. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Malmstrøm ML, Hansen MB, Andersen AM,
Ersbøll AK, Nielsen OH, Jørgensen LN and Novovic S: Cytokines and
organ failure in acute pancreatitis: Inflammatory response in acute
pancreatitis. Pancreas. 41:271–277. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hirota M, Nozawa F, Okabe A, Shibata M,
Beppu T, Shimada S, Egami H, Yamaguchi Y, Ikei S, Okajima T, et al:
Relationship between plasma cytokine concentration and multiple
organ failure in patients with acute pancreatitis. Pancreas.
21:141–146. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Brivet FG, Emilie D and Galanaud P: Pro-
and anti-inflammatory cytokines during acute severe pancreatitis:
An early and sustained response, although unpredictable of death.
Parisian study group on acute pancreatitis. Crit Care Med.
27:749–755. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sendler M, Maertin S, John D, Persike M,
Weiss FU, Krüger B, Wartmann T, Wagh P, Halangk W, Schaschke N, et
al: Cathepsin B activity initiates apoptosis via digestive protease
activation in pancreatic acinar cells and experimental
pancreatitis. J Biol Chem. 291:14717–14731. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sendler M, Weiss FU, Golchert J, Homuth G,
van den Brandt C, Mahajan UM, Partecke LI, Döring P, Gukovsky I,
Gukovskaya AS, et al: Cathepsin B-mediated activation of
trypsinogen in endocytosing macrophages increases severity of
pancreatitis in mice. Gastroenterology. 154:704–718.e10.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang J, Yu WQ, Wei T, Zhang C, Wen L,
Chen Q, Chen W, Qiu JY, Zhang Y and Liang TB: Effects of
short-peptide-based enteral nutrition on the intestinal
microcirculation and mucosal barrier in mice with severe acute
pancreatitis. Mol Nutr Food Res. 64(e1901191)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
de Madaria E, Martínez J, Lozano B,
Sempere L, Benlloch S, Such J, Uceda F, Francés R and Pérez-Mateo
M: Detection and identification of bacterial DNA in serum from
patients with acute pancreatitis. Gut. 54:1293–1297.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
ShaykhoIslami A, Ghasemian M, Zardast M
and Farzad M: Effect of intra-abdominal administration of ascites
fluid on postoperative peritoneal adhesion in rat model: A
randomized controlled trail. Ann Med Surg (Lond).
71(102928)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Baroja-Mazo A, Martín-Sánchez F, Gomez AI,
Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe
J, Ruiz-Ortiz E, Antón J, et al: The NLRP3 inflammasome is released
as a particulate danger signal that amplifies the inflammatory
response. Nat Immunol. 15:738–748. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sendler M, van den Brandt C, Glaubitz J,
Wilden A, Golchert J, Weiss FU, Homuth G, De Freitas Chama LL,
Mishra N, Mahajan UM, et al: NLRP3 inflammasome regulates
development of systemic inflammatory response and compensatory
anti-inflammatory response syndromes in mice with acute
pancreatitis. Gastroenterology. 158:253–269.e14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu RH, Wen Y, Sun HY, Liu CY, Zhang YF,
Yang Y, Huang QL, Tang JJ, Huang CC and Tang LJ: Abdominal
paracentesis drainage ameliorates severe acute pancreatitis in rats
by regulating the polarization of peritoneal macrophages. World J
Gastroenterol. 24:5131–5143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kaukonen KM, Bailey M, Pilcher D, Cooper
DJ and Bellomo R: Systemic inflammatory response syndrome criteria
in defining severe sepsis. N Engl J Med. 372:1629–1638.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Matsumoto M, Kamei K, Chikugo T, Matsumoto
I, Kawaguchi K and Takeyama Y: Efficacy of recombinant
human-soluble thrombomodulin for severe acute pancreatitis in a rat
experimental model. Pancreas. 49:503–508. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rashid MU, Hussain I, Jehanzeb S, Ullah W,
Ali S, Jain AG, Khetpal N and Ahmad S: Pancreatic necrosis:
Complications and changing trend of treatment. World J Gastrointest
Surg. 11:198–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shi Q, Liao KS, Zhao KL, Wang WX, Zuo T,
Deng WH, Chen C, Yu J, Guo WY, He XB, et al: Hydrogen-rich saline
attenuates acute renal injury in sodium taurocholate-induced severe
acute pancreatitis by inhibiting ROS and NF-κB pathway. Mediators
Inflamm. 2015(685043)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhu S, Zhang C, Weng Q and Ye B: Curcumin
protects against acute renal injury by suppressing JAK2/STAT3
pathway in severe acute pancreatitis in rats. Exp Ther Med.
14:1669–1674. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang XH, Li ML, Wang B, Guo MX and Zhu
RM: Caspase-1 inhibition alleviates acute renal injury in rats with
severe acute pancreatitis. World J Gastroenterol. 20:10457–10463.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li YY, Li XL, Yang CX, Zhong H, Yao H and
Zhu L: Effects of tetrandrine and QYT on ICAM-1 and SOD gene
expression in pancreas and liver of rats with acute pancreatitis.
World J Gastroenterol. 9:155–159. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Stoppacher R: Sudden death due to acute
pancreatitis. Acad Forensic Pathol. 8:239–255. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang A, Zhang L and Vege SS: Autopsy study
of patients dying due to acute pancreatitis: 262. Am Coll
Gastroenterol. 109(S81)2014.
|
|
48
|
Li XY, He C, Zhu Y and Lu NH: Role of gut
microbiota on intestinal barrier function in acute pancreatitis.
World J Gastroenterol. 26:2187–2193. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bansal A, Gupta P, Singh H, Samanta J,
Mandavdhare H, Sharma V, Sinha SK, Dutta U and Kochhar R:
Gastrointestinal complications in acute and chronic pancreatitis.
JGH Open. 3:450–455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Piao X, Liu B, Sui X, Niu W, Zhang Q, Shi
X, Cai S and Fan Y: Picroside II improves severe acute
pancreatitis-induced intestinal barrier injury by inactivating
oxidative and inflammatory TLR4-dependent PI3K/AKT/NF-κB signaling
and improving gut microbiota. Oxid Med Cell Longev.
2020(3589497)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Su S, Liang T, Zhou X, He K, Li B and Xia
X: Qingyi decoction attenuates severe acute pancreatitis in rats
via inhibition of inflammation and protection of the intestinal
barrier. J Int Med Res. 47:2215–2227. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ye J, Dai H, Liu Y, Yu B, Yang J and Fei
A: Blockade of C3a/C3aR axis alleviates severe acute
pancreatitis-induced intestinal barrier injury. Am J Transl Res.
12:6290–6301. 2020.PubMed/NCBI
|
|
53
|
Ning JW, Zhang Y, Yu MS, Gu ML, Xu J,
Usman A and Ji F: Emodin alleviates intestinal mucosal injury in
rats with severe acute pancreatitis via the caspase-1 inhibition.
Hepatobiliary Pancreat Dis Int. 16:431–436. 2017.PubMed/NCBI View Article : Google Scholar
|