Introduction
Necrosis of the femoral head (NFH) is an orthopedic
disease that results in a decrease in blood supply to the femoral
head under the joint action of numerous factors. This leads to the
apoptosis or death of cartilage cells and a decrease in the number
of osteocytes, which reduces the mechanical strength of the femoral
head (1,2). Finally, the femoral head collapses,
resulting in functional disorders of the hip joint (3). Although a number of studies have
investigated the etiology, pathology and pathogenesis of NFH, the
exact pathogenesis and related risk genes remain unclear (4-7).
Therefore, it is important to identify regulatory genes and targets
to improve the understanding of the molecular pathological
mechanism of this disorder.
Osteoprotegerin (OPG) belongs to the tumor necrosis
factor receptor superfamily, members of which are implicated in
multiple functions, including blocking osteoclast maturation,
controlling vascular calcification, and promoting tumor growth and
metastasis (8). A large number of
studies have suggested that the competitive binding between OPG and
its ligand, OPGL, is the most important regulatory mechanism in the
process of osteoclast differentiation, proliferation, apoptosis and
physiological function (9-11).
There is increasing evidence that OPG not only protects bone but
also inhibits apoptotic factors and possibly vasoprotective and
cytoprotective factors (4,12-15).
The expression of OPG has been detected in xenogeneic
antigen-extracted cancellous bone/lentiviral basic fibroblast
growth factor/mesenchymal stem cell transplantation for the repair
of rabbit femoral head defect necrosis (16). Chondrocytes have been demonstrated
to be an ideal material for studying the biological role of OPG in
NFH (2). Small molecules can
diffuse from the bone marrow through the subchondral bone and deep
layers of cartilage to the synovial fluid (17). OPG expressed by chondrocytes is
capable of binding to certain receptors to prevent the resorption
of subchondral bone and protect cartilage from degradation
(2). However, the expression of
OPG during NFH and its potential anti-apoptotic effect on human
chondrocytes are unclear. The present study explored the expression
levels of OPG in a large number of tissue samples from patients
with NFH and investigated the potential anti-apoptotic effect of
OPG in human chondrocytes.
Materials and methods
Case information
The present study was approved by the Ethics
Committee of Dezhou People's Hospital (approval no.
EC-20210120-1006; Dezhou, China) and was conducted in compliance
with the Declaration of Helsinki for medical research involving
human subjects. A total of 50 femoral head tissue samples were
collected from hospitalized patients in Dezhou People's Hospital
(Dezhou, China) who were admitted between October 2012 and October
2015 and diagnosed with steroid- or alcohol-induced NFH by clinical
diagnosis and histopathological examination (Table SI). The inclusion criteria were as
follows: i) Volunteered to participate in the study and agreed to
take femoral head tissues; ii) met the diagnostic criteria of NFH;
iii) aged ≥18 years old; iv) subjects were conscious and could
communicate correctly; and v) no tumor or other serious physical
diseases. Exclusion criteria: i) Aged <18 years old; and ii)
suffering from metabolic disease and neuropsychiatric diseases. The
age and sex distribution of patients in each group are presented in
Table SI. A total of 30 patients
without joint or bone disease who underwent treatment for femoral
neck fractures at Dezhou People's Hospital (Dezhou, China)
volunteered to donate femoral head tissues for the control group
between March 2013 and December 2018. All samples were obtained
from a tissue bank at the hospital.
Hematoxylin-eosin staining
Hematoxylin-eosin (HE) staining is a technical
method widely used in pathology research. The femoral head tissues
obtained during the operation were fixed in 4% formalin for 48 h at
room temperature and then embedded in paraffin. The specific steps
of HE staining were as follows: ⅰ) Dewaxing: Dried 4-µm-thick
slices were immediately placed in xylene for dewaxing for 5-10 min
at room temperature, then placed into gradient alcohol (100, 90,
80, 70 and 0%) for 2 min for each step, and then moved into water
for ~2 min; ⅱ) staining: Slices were successively transferred into
hematoxylin for 8-15 min at room temperature, followed by 1-2 min
in water at room temperature. They were transferred into
differentiation solution (1 ml hydrochloric acid with 99 ml 70%
alcohol) and differentiation was allowed to occur for 1-30 sec at
room temperature. The slices were moved into water and washed for
30-60 min at room temperature to make the tissues appear dark blue
in a light microscope. The slices were transferred into eosin
solution and soaked for 2-5 min at room temperature. The eosin
floating solution was removed with water and the excess dye on the
glass slide was wiped away with gauze; ⅲ) dehydration: Samples were
sliced 4-µm thick and placed in 80% alcohol (1-2 min), 90% alcohol
(2-4 min) and 100% ethanol (4-8 min); ⅳ) permeabilization: The
slices were transferred into xylene for permeabilization for 3-5
min. Then, the xylene was replaced and permeabilized the
aforementioned slices again for 5-10 min; and ⅴ) Sealing: First,
the slices were removed from the xylene, the xylene around the
tissue slice was quickly wiped off, and then a drop of neutral gum
was dropped on the tissue slice. A clean cover glass was taken,
carefully aligned, added to the sealing agent and flattened slowly,
and the sample was dried at room temperature.
Cell culture and lentiviral infection
of chondrocytes
Small pieces of cartilage tissue were taken from the
joint surface during surgery, washed with PBS three times, cut into
1-mm3 pieces and transferred to a small conical bottle.
To digest the tissue samples, these were incubated with 0.5 ml 2%
type II collagenase and digested at 37˚C for 45 min. Free
chondrocytes in the enzyme solution were filtered through a
120-mesh nylon filter using a pipette (18). The obtained filtrate was
transferred to a sterile centrifuge tube and centrifuged at 1,200 x
g for 8 min at room temperature. The resulting supernatant was
discarded and, to terminate the digestion reaction, 5 ml DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) was added and the samples were
mixed. The chondrocyte suspension was then diluted to
3x105 cells/ml and inoculated into a culture dish
containing DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and supplementary
antibiotics (50 U/ml penicillin and 50 µg/ml streptomycin) at 37˚C
with 5% CO2 in a humidified atmosphere (18).
Lentiviral transduction was performed in 293T cells
(Shanghai Institute of Biochemistry and Cell Biology) by transient
co-transfection of a three-plasmid expression system involving an
OPG plasmid (pLVX-TRE3G-OPG) or a control plasmid (pLVX-TRE3G)
(Shanghai Zeye, Inc.) and the procedure was performed according to
the previous reports (19,20). The primary chondrocytes for
lentiviral infection were obtained from individuals without joint
or bone disease. Primary chondrocytes were cultured in a Petri dish
to 80-90% confluence and then trypsinized into a cell suspension
(21). The number of cells was
adjusted to 1x105 cells per well using culture medium,
and the cells were inoculated into 24-well plates and cultured at
37˚C with 5% CO2 for 24 h. After the cells adhered to
the wall of the well, they were ready for transfection. Lentivirus
vectors were added to each well at a MOI of 25, 50, 100 or 200.
After 72 h of culture, fluorescent protein expression was observed
in the cells under an inverted fluorescence microscope, and the
virus infection efficiency was calculated. Cells with a
transfection efficiency of 80% (MOI=100) were selected for
follow-up experiments (data not shown).
RT-qPCR assay
Total RNA was extracted from the primary
chondrocytes and cartilage tissues using TRIzol® reagent
(Thermo Fisher Scientific, Inc.) and then reverse transcribed into
cDNA using a PrimeScript™ RT reagents kit (Takara Bio, Inc.)
according to the manufacturer's instructions. qPCR reactions were
performed using a KAPA SYBR® FAST qPCR Kit (Kapa
Biosystems; Roche Diagnostics) and a CFX96 real-time PCR system
(Bio-Rad Laboratories, Inc.). The OPG-specific primers used were:
5'-CATTCTTCAGGTTTGCTGTTCCT-3' (forward) and
5'-TTGCCGTTTTATCCTCTCTACACTC-3' (reverse). GAPDH was used as an
internal reference gene for the RT-qPCR experiments, and the
primers used were: 5'-CAATGACCCCTTCATTGACC-3' (forward) and
5'-TTGATTTTGGAGGGATCTCG-3' (reverse). The RT-qPCR conditions were
as follows: Pre-denaturation at 95˚C for 10 min, then at 95˚C for
10 sec and at 60˚C for 60 sec, repeating for 40 cycles (22,23).
OPG expression was normalized to GAPDH and was presented as a
relative expression ratio (2-ΔΔCq;
ΔCq=CqOPG-CqGAPDH) (24).
MTT assay of cell viability
After infection with lentivirus for 72 h, 200 µl
cell suspension was transferred into 96-well plates at a density of
4,000-5,000 cells per well. Preliminary experiments revealed that
tert-butyl hydroperoxide (tBHP) exerted a significant
biological effect on cell viability at a minimum concentration of
100 µM and at a minimum time of 1 day (Figs. S1 and S2). Under these conditions, the
interference of other factors on the experimental results can be
eliminated to the greatest extent. After 12 h of cell culture, the
cells were treated with a final concentration of 100 µM tBHP for 24
h. The cells were then treated with 10 µl 5 mg/ml MTT at 37˚C for
an additional 4 h. After removing the culture medium, dimethyl
sulfoxide (150 µl) was added to dissolve the formazan crystals. The
optical density values of the samples were determined using a
microplate spectrophotometer (BioTek Instruments, Inc.) at 490 nm
wavelength.
Flow cytometric analysis of
apoptosis
Human chondrocytes infected with virus particles
containing the OPG plasmid or control plasmid were treated with 100
µM tBHP or vehicle for 24 h at 37˚C. Human chondrocytes were
harvested with ethylenediaminetetraacetic acid-free trypsin, washed
with PBS and then incubated in 100 µl binding buffer containing 20
µg PI and 5 µl Annexin V-FITC (R&D Systems, Inc.) at 4˚C in the
dark for 15 min. Binding buffer (900 µl) was then added and the
fluorescence of Annexin V-FITC and PI was detected using a Gallios
Flow Cytometer (Beckman Coulter, Inc.). Data were analyzed using
FlowJo software v7.6.1 (FlowJo LLC). Cells positive for Annexin
V-FITC and negative for PI were considered to be early apoptotic,
and cells positive for both Annexin V-FITC and PI were considered
to be late apoptotic, whereas those positive for PI and negative
for Annexin V-FITC were considered to be necrotic.
TUNEL assay
A terminal deoxynucleotidyl transferase (TdT)
-mediated dUTP nick-end labeling (TUNEL) kit (Beyotime Institute of
Biotechnology) was used to detect DNA fragmentation resulting from
apoptosis according to the manufacturer's instructions. Briefly,
terminal deoxynucleotidyl transferase was used to incorporate
residues of digoxigenin nucleotide into the 3' OH ends of DNA
fragments. The DNA breaking points (nicks) expose the 3' OH-end of
DNA, which were labelled, thus allowing the identification of
apoptotic cells. Human chondrocytes infected with virus particles
were seeded on coverslips and incubated in culture medium
containing tBHP (100 µM) for 24 h. After incubation in PBS with
0.2% Triton X-100 for 5 min, the cells were fixed with 4%
paraformaldehyde at room temperature for 20 min. The cells were
then treated with a mixture of terminal deoxynucleotidyl
transferase enzyme, terminal deoxynucleotidyl transferase reaction
buffer and fluorescent labeling buffer (1:24:25) for 60 min at
37˚C. DAPI staining (100 ng/ml, 25˚C, 20 min) was used to count the
total number of cells, and apoptotic cells were identified as those
with green nuclei. Apoptotic cells were quantitated by counting the
number of TUNEL-positive cells in four random microscopic fields of
a DM2500 fluorescence microscope (Leica Microsystems GmbH).
Flow cytometric detection of
intracellular reactive oxygen species (ROS)
To measure ROS production, human chondrocytes
infected with virus particles containing the OPG plasmid or control
virus particles were cultured in the presence or absence of 100 µM
tBHP. After treatment, the cells were incubated with 10 µM
2'-7'-dichlorofluorescin diacetate for 30 min at 37˚C and
intracellular ROS levels were determined using a Gallios Flow
Cytometer with Kaluza analysis software v2.1.1 (Beckman Coulter,
Inc.).
Western blotting
Membrane protein levels were determined by western
blotting as described previously (15). Briefly, cells or tissue fragments
were lysed with modified RIPA buffer (Beyotime Institute of
Biotechnology) for 30 min at 4˚C, and the lysates were then
centrifuged at 12,000 x g for 30 min at 4˚C. After transferring the
supernatant to a fresh ice-cold tube, the protein concentration was
determined using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Equal concentrations of proteins were
mixed with SDS sample buffer, denatured at 95˚C for 5 min and a
total of 40 µg protein was loaded per lane and resolved on 8%
SDS-polyacrylamide gel. The separated proteins were then
transferred onto nitrocellulose membranes, which were blocked with
5% non-fat dried milk in TBS with 0.1% Tween-20 (TBST) at room
temperature for 1 h. After blocking, the blots were incubated
overnight at 4˚C with the primary antibodies (dilution, 1:1,000).
The membranes were then washed with TBST and incubated with
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
ProteinTech Group, Inc.) for 1 h at room temperature. The membranes
were washed again with TBST and then processed using an enhanced
chemiluminescence detection system (Beyotime Institute of
Biotechnology). Relative band intensities were measured using
Gel-Pro Analyzer image analysis software v4.5 (Media Cybernetics,
Inc.). The antibody details are presented in Table SII.
Statistical analysis
Preliminary experiments revealed no significant
difference in results between a blank control and the negative
control (pLVX-TRE3G) (Figs. S3
and S4). All experiments were
performed in triplicate. Data are presented as the mean ± standard
error of the mean, and statistical differences among groups were
further evaluated by one-way ANOVA followed by Bonferroni's post
hoc test for multiple groups. An unpaired Student's t-test was used
to compare two groups. The categorical data were analyzed using
chi-squared test (χ2 test). A receiver operating
characteristic (ROC) curve was constructed by calculating the
sensitivity and specificity of OPG expression in a logistic
regression model at different cut-off points to differentiate
patients with NFH from controls (25-27).
The area under the curve (AUC) can be statistically interpreted as
the probability of correctly distinguishing patients with NFH from
controls (25-27).
All statistical analyses were calculated using SPSS software 17.0
(SPSS Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of OPG in patients
with NFH and their association with NFH
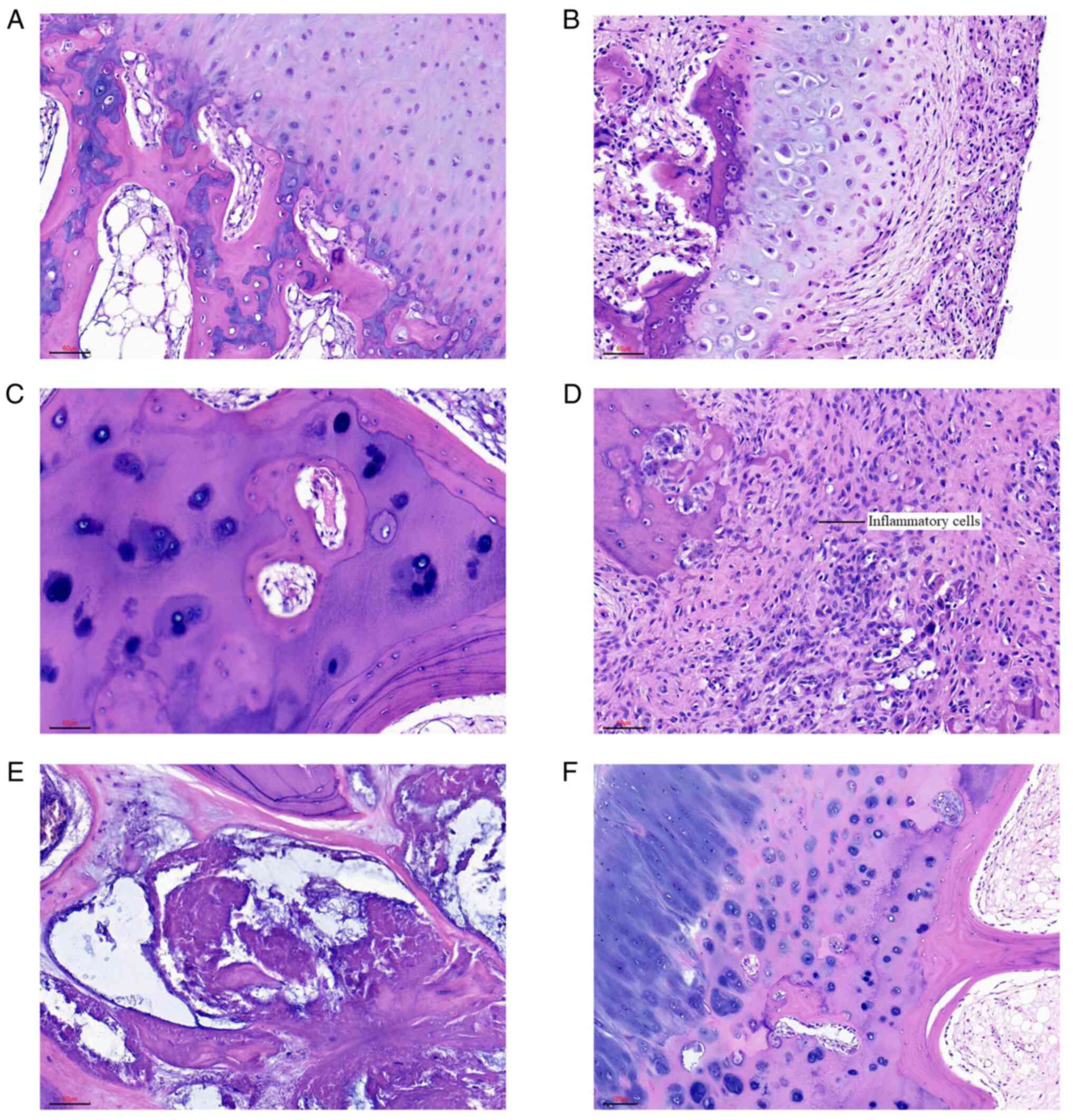
Fig. 1 shows the
results of hematoxylin and eosin staining of normal bone, dead
bone, infiltrating inflammatory cells and femoral head tissues in
patients with steroid-induced NFH and alcoholic NFH. The normal
bone color was uniform and smooth (Fig. 1A), whereas the dead bone and
steroid-induced femoral head necrotic lesions had blood cells with
inflammatory cell infiltration.
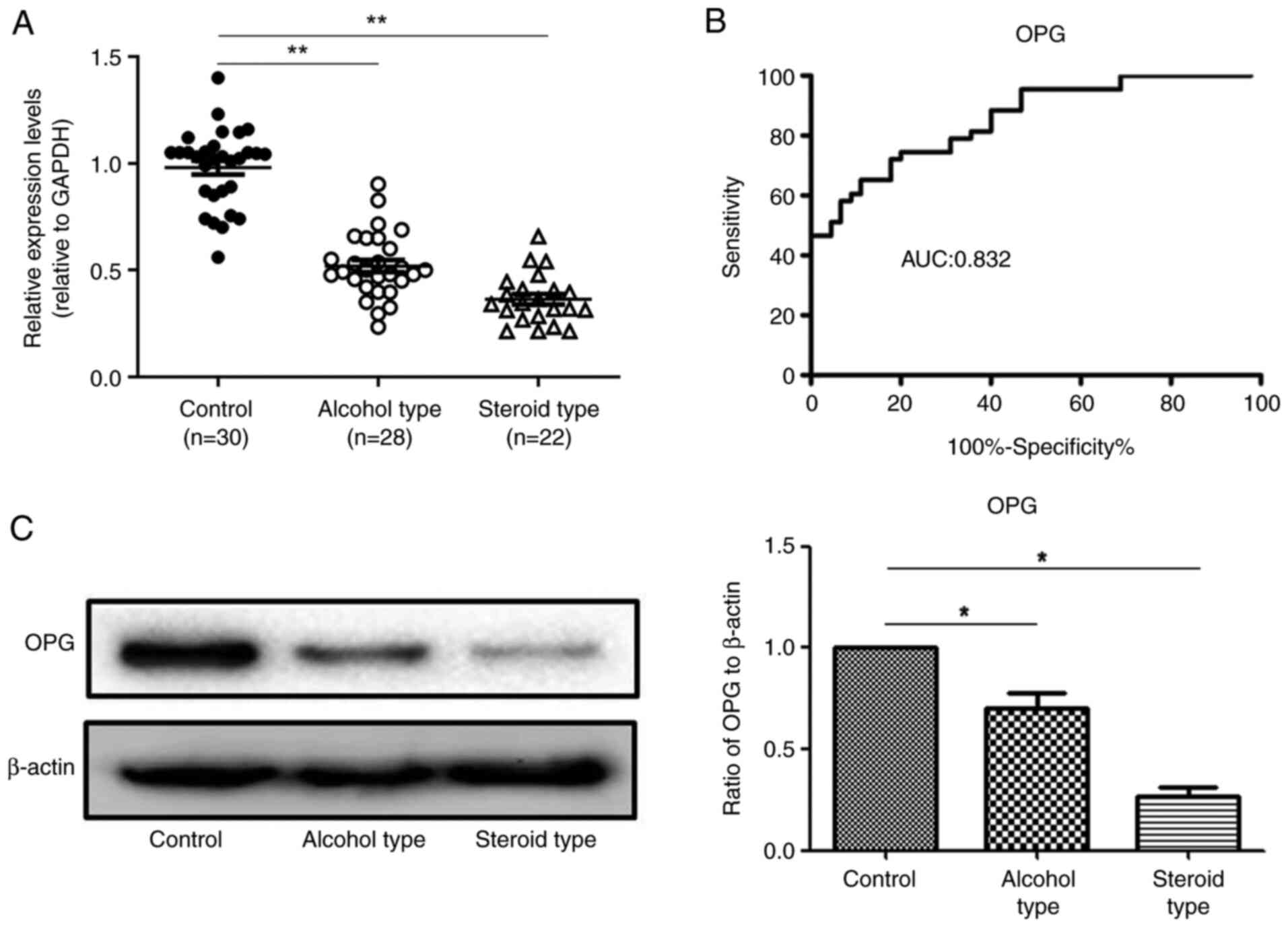
RT-qPCR was used to examine OPG gene
expression in specimens from patients with NFH and those with a
normal femoral head. The OPG levels were lower in patients with
steroid-(0.31±0.02) or alcohol-induced (0.49±0.06) NFH than in
patients with normal femoral heads (1.01±0.12; P<0.05; Fig. 2A). A sensitivity correlation
analysis of the ROC curve was performed on OPG mRNA
expression levels in 50 femoral head samples with necrosis and 30
normal femoral head samples. The AUC for OPG expression was
0.832 (95% confidence interval, 0.723-0.881). The cut-off value was
0.452 with sensitivity and specificity values of 85 and 72%,
respectively (Fig. 2B). OPG
protein expression was also significantly decreased in both
alcoholic NFH specimens and steroid-induced NFH specimens compared
with normal femoral head specimens (Fig. 2C).
Effect of OPG overexpression on
tBHP-induced apoptosis of human chondrocytes
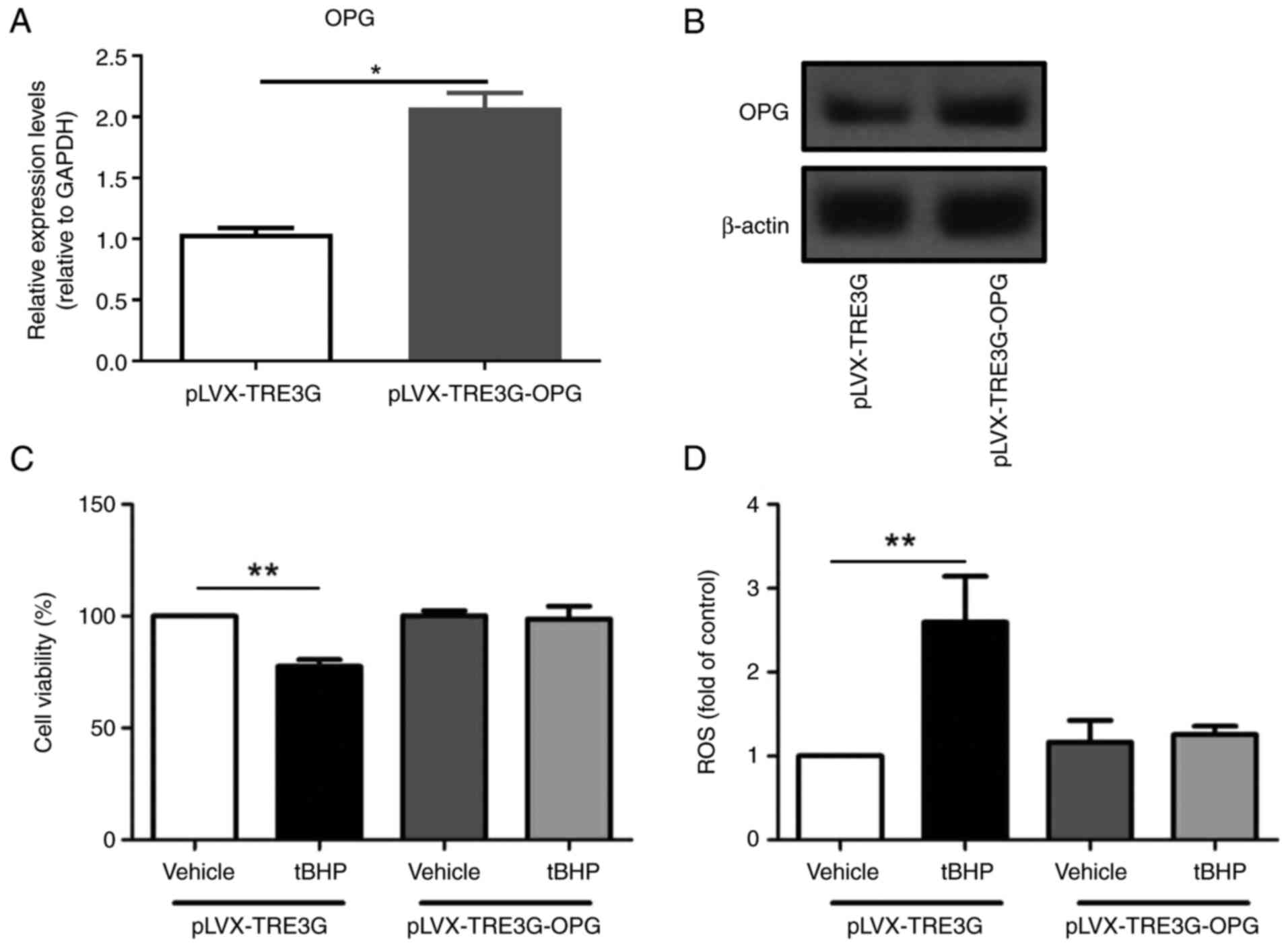
To determine whether OPG has a protective effect on
chondrocytes, an MTT assay was performed to examine the viability
of tBHP-treated human chondrocytes transfected with OPG or control
plasmids. There was a significantly increased mRNA level of OPG in
the cells transfected with the OPG plasmid compared with cells
transfected with the control plasmid (Fig. 3A). The cells transfected with the
OPG plasmid showed higher OPG protein level compared with that in
cells transfected with the control plasmid (Fig. 3B). As shown in Fig. 3C, the viability of human
chondrocytes transfected with the control plasmid (pLVX-TRE3G) was
significantly reduced by ~20% after treatment with 100 µM tBHP,
whereas there was little change in the viability of OPG-transfected
chondrocytes (pLVX-TRE3G-OPG). This result indicated that
overexpression of OPG in human chondrocytes protects them from
tBHP-induced cell death.
Overexpression of OPG suppresses
tBHP-induced ROS production in human chondrocytes
ROS are important regulators of cell death and
mitochondrial-related apoptosis (28). ROS production in human chondrocytes
was detected using flow cytometry. As shown in Fig. 3D, the ROS levels significantly
increased after tBHP treatment of human chondrocytes transfected
with the pLVX-TRE3G (P<0.01). The representative flow cytometry
plots of ROS are shown in Figs.
S4 (blank control and negative control: pLVX-TRE3G) and S5
(pLVX-TRE3G, pLVX-TRE3G + tBHP, pLVX-TRE3G-OPG and pLVX-TRE3G-OPG +
tBHP). However, no difference in ROS production was observed
between cells overexpressing OPG and tBHP-treated OPG
overexpression cells (Fig.
3D).
Overexpression of OPG reduces
tBHP-induced human chondrocyte apoptosis
OPG has been demonstrated to have a protective
effect against apoptosis (28);
however, to the best of our knowledge, there are no reports on the
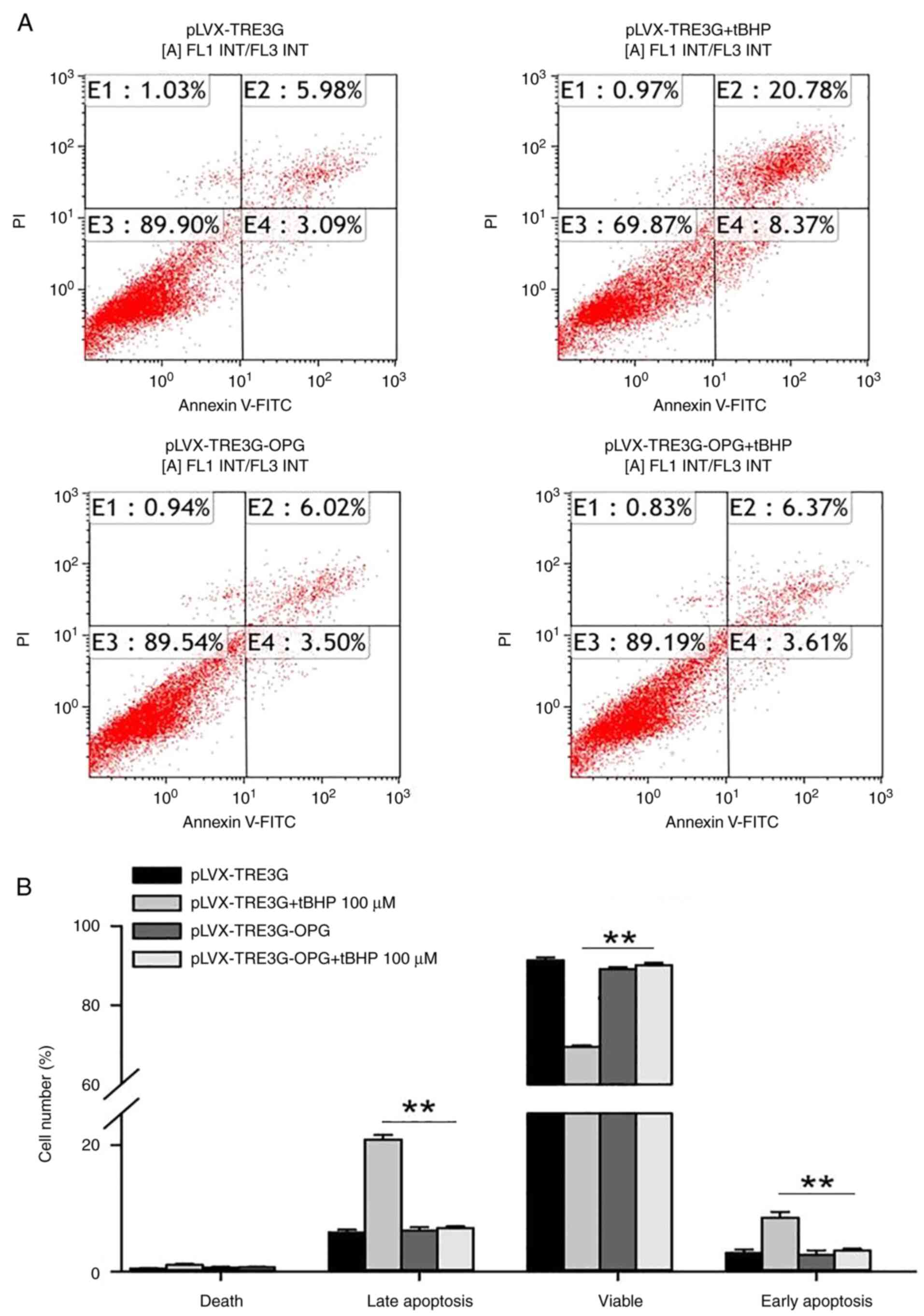
anti-apoptotic effect of OPG in human chondrocytes. In the human
chondrocytes with pLVX-TRE3G transfection, tBHP treatment increased
the percentage of late apoptotic cells from 5.98±0.36 to
20.78±0.72% and the percentage of early apoptotic cells from
3.09±0.42 to 8.37±0.74% compared with that of the negative control
group (pLVX-TRE3G) (Fig. 4A).
However, in human chondrocytes transfected with OPG, no significant
increase in the number of apoptotic cells was observed after
treatment with 100 µM tBHP. Thus, overexpression of OPG
significantly inhibited the tBHP-induced apoptosis of human
chondrocytes (Fig. 4B,
P<0.01).
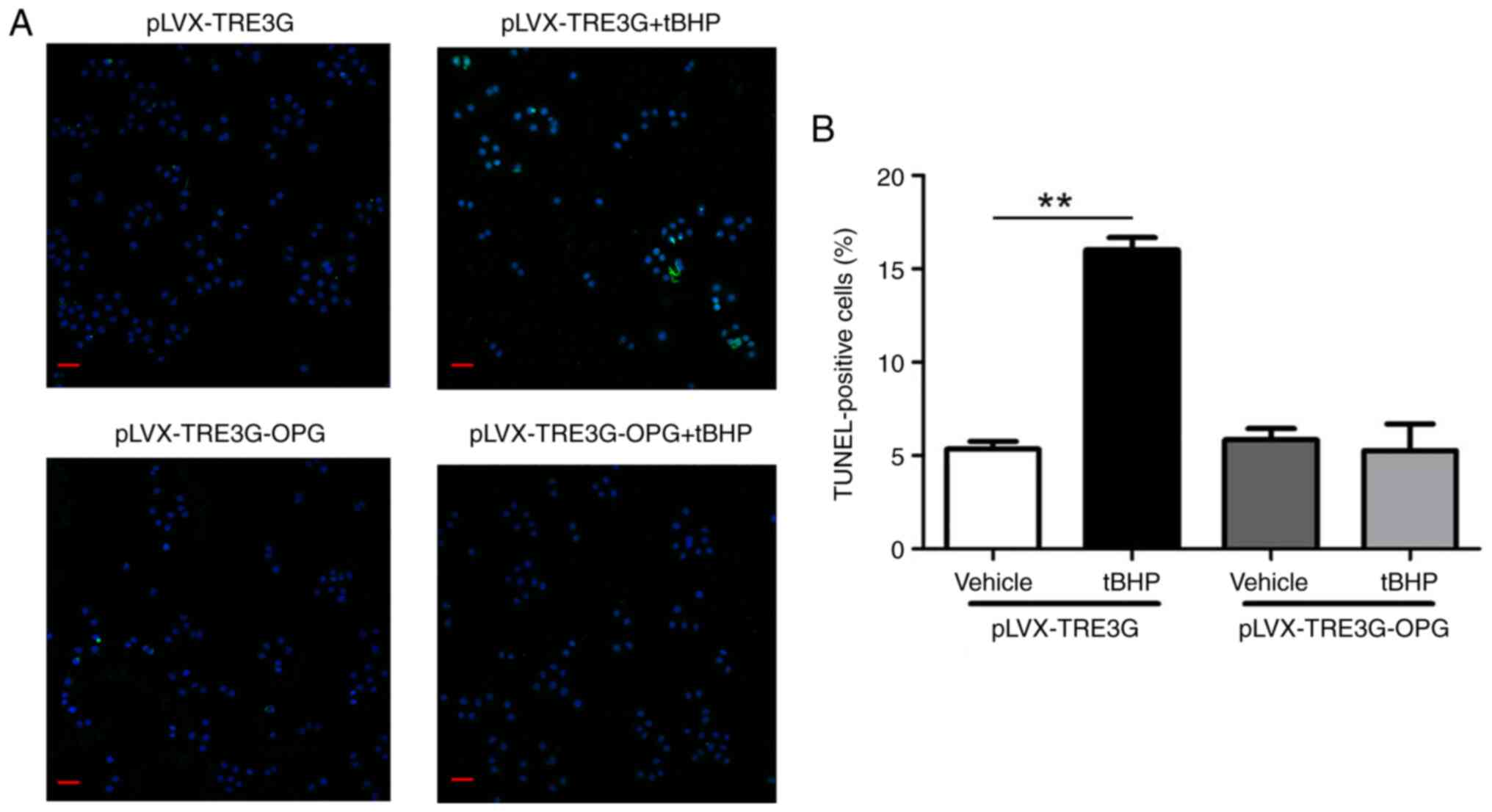
A TUNEL assay was also employed to confirm the
tBHP-induced apoptosis of human chondrocytes. As shown in Fig. 5, only ~5% of vehicle-treated human
chondrocytes transfected with the pLVX-TRE3G control plasmid were
TUNEL-positive, whereas 16% were TUNEL-positive after tBHP
treatment. However, only 6% of chondrocytes transfected with
OPG were TUNEL-positive after tBHP treatment (Fig. 5A and B).
Molecular mechanism of tBHP-induced
apoptosis in OPG-overexpressing human chondrocytes
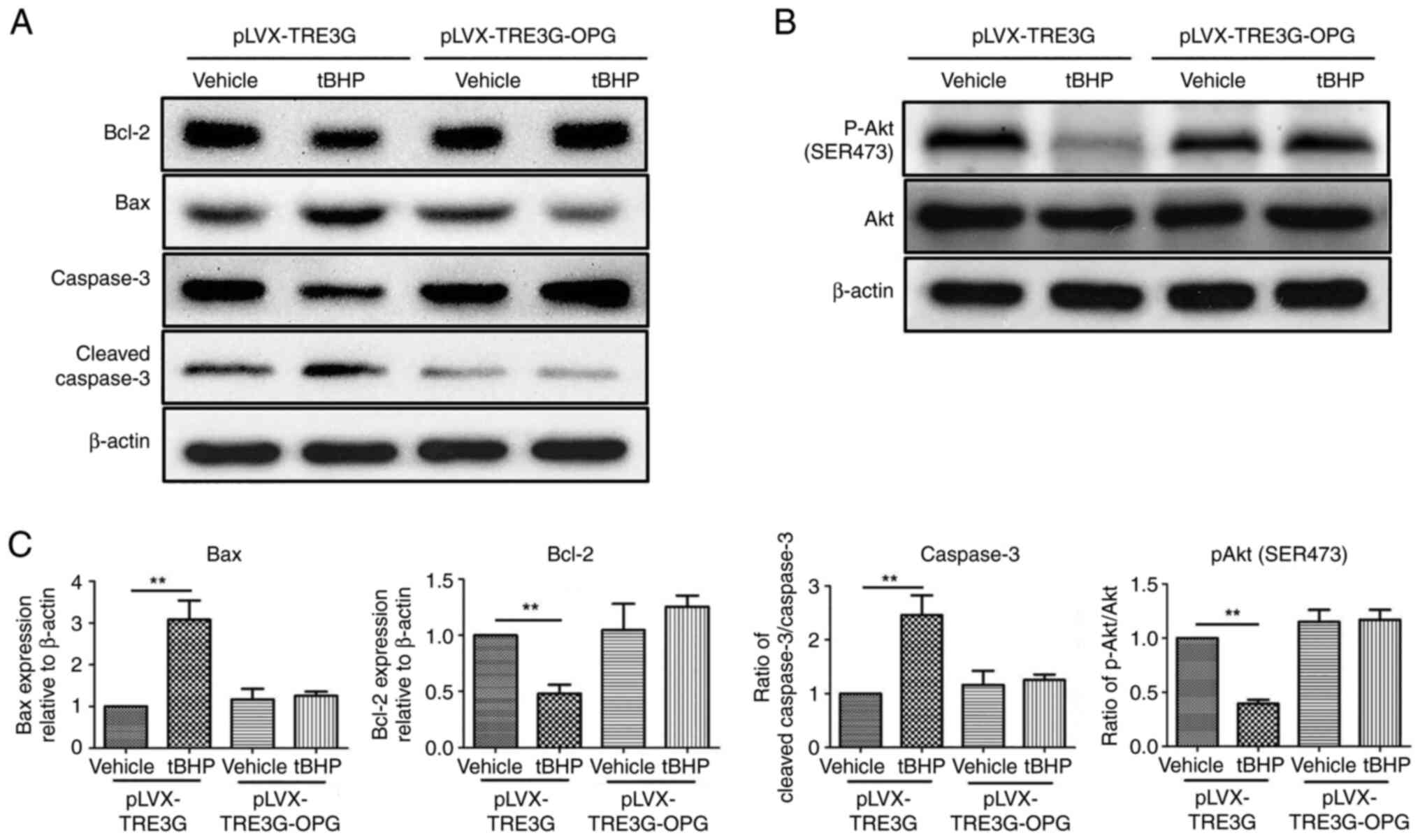
Western blotting was used to explore the expression
levels of tBHP-induced apoptosis-related proteins in human
chondrocytes overexpressing OPG. As shown in Fig. 6, tBHP increased the levels of the
pro-apoptotic proteins Bax (310%) and cleaved caspase-3 (250%), and
decreased the levels of the anti-apoptotic proteins Bcl-2 (0.45)
and phosphorylated (p-) Akt (0.41), in the vector control group. In
human chondrocytes overexpressing OPG, tBHP treatment had no effect
on the levels of Bax, cleaved caspase-3, Bcl-2 and p-Akt. This
result indicated that overexpression of OPG in human
chondrocytes inhibited Bax expression and caspase-3 cleavage and
promoted Bcl-2 expression and Akt phosphorylation.
Discussion
OPG, also known as osteoclastogenesis inhibitory
factor, is a cytokine receptor of the TNF receptor superfamily
encoded by the TNF receptor superfamily member 11B gene (2). In the present study, OPG gene
expression in the femoral head tissue of patients with
steroid-induced NFH and alcoholic NFH was determined by RT-qPCR.
The results demonstrated that OPG expression was markedly decreased
in patients with steroid-induced and alcoholic NFH compared with
normal controls without NFH. Apoptosis can be divided into early
apoptosis, characterized by changes in cell membrane structure and
phosphatidylserine eversion, early-to-middle apoptosis,
characterized by an increase in cytoplasmic density, disappearance
of mitochondrial membrane potential, changes in permeability and a
release of cytochrome C into the cytoplasm, middle-to-late
apoptosis, characterized by apoptosis-related signal transduction,
and late apoptosis, characterized by DNA degradation into 180-200
bp fragments (29,30). Recent studies have suggested that
the downregulation of OPG expression in patients with NFH may
interfere with the differentiation of osteoclasts and their
secretory function in the transversing axis of bone remodeling and
the inhibition of bone resorption, thus weakening their protective
effect on cartilage and causing osteosclerosis and orthopedic
diseases, such as osteoporosis (31-36).
OPG is synthesized by osteoblasts and stromal cells
and it regulates osteoclast differentiation and activity (37). It can directly affect the
differentiation and efficacy of osteoclasts and regulate bone
remodeling (35,36,38).
The secretory system adjacent to the horizontal axis is involved in
bone resorption (39). OPG has a
regulatory effect on bone and it protects cartilage (40). It can also participate in the
development of a series of orthopedic diseases, such as
osteoporosis, osteosclerosis and bone tumors, in combination with
receptor activator of NF-κB ligand (RANKL), and it has been
demonstrated to serve an important role in bone diseases (41). OPG was first discovered as a novel
secreted tumor necrosis factor receptor-related protein that served
a role in the regulation of bone density, and it was later found to
be a decoy receptor for RANKL (28,42).
OPG also binds to TNF-related apoptosis-inducing ligand (TRAIL) and
inhibits the TRAIL-induced apoptosis of specific cells, including
tumor cells (43). Other OPG
ligands include syndecan-1, glycosaminoglycans, von Willebrand
factor and the factor VIII-von Willebrand factor complex (44). OPG-knockout (OPG-KO) mice show
decreased chondrocyte proliferation and increased chondrocyte
apoptosis (1,40). The isolated chondrocytes from
OPG-KO mice also show impaired survival and increased chondrogenic
differentiation (1,40). Numerous studies have reported that
OPG has anti-apoptotic effects (3,45).
In addition to severe osteoporosis and multiple fractures, OPG-KO
mice also have calcification in the middle layers of the aorta and
renal arteries (8). In the bone
marrow mesenchymal stem cells, OPG has been found to inhibit
TRAIL-induced apoptosis (46).
OPGα or β3 can prevent the apoptosis of rat aortic endothelial
cells and human capillary endothelial cells, and the anti-apoptotic
effect of OPG is further achieved by activating NF-κB to increase
OPG expression (42). In the
osteoclasts, OPG regulates apoptosis via Bcl-2/Bax and caspase-3 in
the classic Fas/Fas ligand apoptosis pathway (47). A previous study has demonstrated
that OPG inhibits the apoptosis of human mammary epithelial cells,
in part because it blocks the binding of TRAIL to death receptors,
and the apoptosis of endothelial cells, in which high expression
levels of OPG and vascular endothelial growth factor are more
viable (48).
In the absence of OPG, mice show thinned articular
cartilage and extensive remodeling of the subchondral bone in the
femoral head (2). The articular
cartilage also shows decreased levels of aggrecan, collagen
(Col)-II and Col-X, but increased levels of Col-I and matrix
metalloproteinase-13 in the femoral head of OPG-KO mice compared
with wild-type mice (2).
Furthermore, OPG-KO mice have increased chondrocyte apoptosis and
decreased chondrocyte proliferation in the femoral head compared
with wild-type mice (2).
Additionally, the present study demonstrated that overexpression of
OPG in human chondrocytes by lentivirus-mediated transfection
inhibited the decrease in cell viability induced by tBHP. The
present study revealed that increasing OPG expression could inhibit
the production of ROS caused by tBHP. Since ROS is a key stimulator
related to apoptosis (28), this
strongly suggested that OPG might be involved in the process of
apoptosis. Further investigation revealed that this effect of OPG
occurred by inhibiting late and early apoptosis. Furthermore, the
present study investigated apoptosis-related signaling pathways and
revealed that overexpression of OPG increased Bcl-2 expression and
Akt phosphorylation levels and decreased the levels of Bax and
cleaved caspase-3. The present results are also consistent with
those reported by other groups (8,17,49,50).
Cheng et al (51) found
that graphene oxide nanoparticles may be used to protect cartilage
by modifying the RANKL/OPG axis. This line of evidence suggests
that OPG is essential for maintaining the physiological function of
chondrocytes in the femoral head.
In summary, the present study revealed that the
expression levels of OPG were lower in femoral heads from patients
with NFH than those from control patients. Overexpression of OPG
decreased tBHP-induced cell death, cellular ROS production and late
and early apoptosis by inhibiting Bax and cleaved caspase-3 and
promoting Bcl2 expression and Akt phosphorylation in human
chondrocytes. The present study provides novel strategies for the
clinical treatment and basic research of NFH.
Supplementary Material
Effect of tBHP on cell viability in
chondrocytes is concentration-dependent. ***P<0.01
vs. control. OPG, osteoprotegerin; tBHP, tert-butyl
hydroperoxide.
Effect of tBHP on cell viability in
chondrocytes is time-dependent. ***P<0.01 vs.
control. OPG, osteoprotegerin; tBHP, tert-butyl
hydroperoxide.
No difference between the blank
control and negative control (pLVX-TRE3G) groups was observed in
terms of the apoptosis ratio (early plus late apoptosis). NS, not
significant.
No difference between blank control
and negative control (pLVX-TRE3G) groups was observed in terms of
(A) chondrocyte viability and (B) ROS production. The
representative flow cytometry plots of ROS in (C) blank control and
(D) pLVX-TRE3G. NS, not significant; ROS, reactive oxygen
species.
Representative flow cytometry plots
for ROS detection. (A) Negative control (pLVX-TRE3G), (B)
pLVX-TRE3G + tBHP, (C) pLVX-TRE3G-OPG and (D) pLVX-TRE3G OPG +
tBHP. OPG, osteoprotegerin; ROS, reactive oxygen species; tBHP,
tert-butyl hydroperoxide.
Clinical characteristics of
subjectswith NFHand controls.
Information of primary and secondary
antibodies used for western blotting.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Medical and Health
Science and Technology Development Program of Shandong Province
(grant no. 2019ws015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and ML conceived and designed the study. QR, WZ,
PL, JZ and ZL performed the study experiments. YZ and ML wrote the
paper. All authors have read and approved the final manuscript. QR,
YZ and ML confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All procedures in the present study were approved by
the Medical Ethics Committee of Dezhou People's Hospital (Dezhou,
China) and written informed consent was obtained from all
subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen D, Liu Y, Liu Z and Wang P: OPG is
required for the postnatal maintenance of condylar cartilage.
Calcif Tissue Int. 104:461–474. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu Y, Ge J, Chen D, Weng Y, Du H, Sun Y
and Zhang Q: Osteoprotegerin deficiency leads to deformation of the
articular cartilage in femoral head. J Mol Histol. 47:475–483.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bouredji Z, Hamoudi D, Marcadet L, Argaw A
and Frenette J: Testing the efficacy of a human full-length OPG-Fc
analog in a severe model of cardiotoxin-induced skeletal muscle
injury and repair. Mol Ther Methods Clin Dev. 21:559–573.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Manolagas SC, O'Brien CA and Almeida M:
The role of estrogen and androgen receptors in bone health and
disease. Nat Rev Endocrinol. 9:699–712. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Al-Omari AA, Aleshawi AJ, Marei OA, Younes
HMB, Alawneh KZ, ALQuran E and Mohaidat ZM: Avascular necrosis of
the femoral head after single steroid intra-articular injection.
Eur J Orthop Surg Traumatol. 30:193–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang T, Azeddine B, Mah W, Harvey EJ,
Rosenblatt D and Séguin C: Osteonecrosis of the femoral head:
Genetic basis. Int Orthop. 43:519–530. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee SW, Lim KH, Lee KJ, Heo YR and Lee JH:
No association between telomere length and osteonecrosis of the
femoral head. BMC Musculoskelet Disord. 22(176)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Y, Guo Y, Wang Q, Ouyang Y, Cao Y, Jin
T and Wang J: Osteoprotegerin polymorphisms are associated with
alcohol-induced osteonecrosis of femoral head in Chinese Han
population from Henan province. J Genet. 95:983–989.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hardy R, Juarez M, Naylor A, Tu J, Rabbitt
EH, Filer A, Stewart PM, Buckley CD, Raza K and Cooper MS: Synovial
DKK1 expression is regulated by local glucocorticoid metabolism in
inflammatory arthritis. Arthritis Res Ther. 14(R226)2012.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Holt V, Caplan AI and Haynesworth SE:
Identification of a subpopulation of marrow MSC-derived medullary
adipocytes that express osteoclast-regulating molecules: Marrow
adipocytes express osteoclast mediators. PLoS One.
9(e108920)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Walsh MC and Choi Y: Biology of the
RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol.
5(511)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kuhn MC, Willenberg HS, Schott M,
Papewalis C, Stumpf U, Flohé S, Scherbaum WA and Schinner S:
Adipocyte-secreted factors increase osteoblast proliferation and
the OPG/RANKL ratio to influence osteoclast formation. Mol Cell
Endocrinol. 349:180–188. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Berendsen AD, Jia S, Lotinun S,
Baron R, Ferrara N and Olsen B: Intracellular VEGF regulates the
balance between osteoblast and adipocyte differentiation. J Clin
Invest. 122:3101–3113. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Milanova V, Ivanovska N and Dimitrova P:
TLR2 elicits IL-17-mediated RANKL expression, IL-17, and OPG
production in neutrophils from arthritic mice. Mediators Inflamm.
2014(643406)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng J, Zhuo H, Wang L, Zheng W, Chen X,
Hou J, Zhao J and Cai J: Identification of the combinatorial effect
of miRNA family regulatory network in different growth patterns of
GC. Mol Ther Oncolytics. 17:531–546. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Peng W, Zhang J, Zhang F, Zhao Y and Dong
W: Expression of osteoprotegerin and receptor activator for the
nuclear factor-κB ligand in XACB/LV-bFGF/MSCs transplantation for
repair of rabbit femoral head defect necrosis. J Cell Biochem: Oct
18, 2018 (Epub ahead of print).
|
|
17
|
Kovács B, Vajda E and Nagy EE: Regulatory
effects and interactions of the Wnt and OPG-RANKL-RANK signaling at
the bone-cartilage interface in osteoarthritis. Int J Mol Sci.
20(4653)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou Y, Chen X, Qu N, Zhang B and Xia C:
Chondroprotection of PPARα activation by WY14643 via autophagy
involving Akt and ERK in LPS-treated mouse chondrocytes and
osteoarthritis model. J Cell Mol Med. 23:2782–2793. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang M, Thomas D, Li MX, Feng W, Chan SM,
Majeti R and Mitchell BS: Role of cysteine 288 in nucleophosmin
cytoplasmic mutations: Sensitization to toxicity induced by arsenic
trioxide and bortezomib. Leukemia. 27:1970–1980. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yenerall P, Kollipara RK, Avila K, Peyton
M, Eide CA, Bottomly D, McWeeney SK, Liu Y, Westover KD, Druker BJ,
et al: Lentiviral-driven discovery of cancer drug resistance
mutations. Cancer Res. 81:4685–4695. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cheng L, Zeng G, Liu Z, Zhang B, Cui X,
Zhao H, Zheng X, Song G, Kang J and Xia C: Protein kinase B and
extracellular signal-regulated kinase contribute to the
chondroprotective effect of morroniside on osteoarthritis
chondrocytes. J Cell Mol Med. 19:1877–1886. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang P, Qiu Z, Jiang Y, Dong L, Yang W, Gu
C, Li G and Zhu Y: Silencing of cZNF292 circular RNA suppresses
human glioma tube formation via the Wnt/β-catenin signaling
pathway. Oncotarget. 7(63449)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhong Z, Lv M and Chen J: Screening
differential circular RNA expression profiles reveals the
regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in
bladder carcinoma. Sci Rep. 6(30919)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao S, Cheng J, Li G, Sun T, Xu Y, Wang Y,
Du X, Xu G and Duan S: Catechol-O-methyltransferase gene promoter
methylation as a peripheral biomarker in male schizophrenia. Eur
Psychiatry. 44:39–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tian H, Li G, Xu G, Liu J, Wan X, Zhang J,
Xie S, Cheng J and Gao S: Inflammatory cytokines derived from
peripheral blood contribute to the modified electroconvulsive
therapy-induced cognitive deficits in major depressive disorder.
Eur Arch Psychiatry Clin Neurosci. 271:475–485. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng J, Zhuo H, Xu M, Wang L, Xu H, Peng
J, Hou J, Lin L and Cai J: Regulatory network of circRNA-miRNA-mRNA
contributes to the histological classification and disease
progression in gastric cancer. J Transl Med. 16:1–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shafiey SI, Mohamed WR and Abo-Saif AA:
Paroxetine and rivastigmine mitigates adjuvant-induced rheumatoid
arthritis in rats: Impact on oxidative stress, apoptosis and
RANKL/OPG signals. Life Sci. 212:109–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Voss AK and Strasser A: The essentials of
developmental apoptosis. F1000Res 9: F1000 Faculty Rev-148,
2020.
|
|
31
|
Tanaka H, Mine T, Ogasa H, Taguchi T and
Liang CT: Expression of RANKL/OPG during bone remodeling in vivo.
Biochem Biophys Res Commun. 411:690–694. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Upton AR, Holding CA, Dharmapatni AA and
Haynes DR: The expression of RANKL and OPG in the various grades of
osteoarthritic cartilage. Rheumatol Int. 32:535–540.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nishida D, Arai A, Zhao L, Yang M,
Nakamichi Y, Horibe K, Hosoya A, Kobayashi Y, Udagawa N and
Mizoguchi T: RANKL/OPG ratio regulates odontoclastogenesis in
damaged dental pulp. Sci Rep. 11(4575)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Guo Y, Xu C, Wu X, Zhang W, Sun Y and
Shrestha A: Leptin regulates OPG and RANKL expression in gingival
fibroblasts and tissues of chronic periodontitis patients. Int J
Med Sci. 18:2431–2437. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Song HM, Wei YC, Li N, Wu B, Xie N, Zhang
KM, Wang SZ and Wang HM: Effects of Wenyangbushen formula on the
expression of VEGF, OPG, RANK and RANKL in rabbits with
steroid-induced femoral head avascular necrosis. Mol Med Rep.
12:8155–8161. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen K, Liu Y, He J, Pavlos N, Wang C,
Kenny J, Yuan J, Zhang Q, Xu J and He W: Steroid-induced
osteonecrosis of the femoral head reveals enhanced reactive oxygen
species and hyperactive osteoclasts. Int J Biol Sci. 16:1888–1900.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hardaway AL, Herroon MK, Rajagurubandara E
and Podgorski I: Bone marrow fat: Linking adipocyte-induced
inflammation with skeletal metastases. Cancer Metastasis Rev.
33:527–543. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sakamoto K, Osaki M, Hozumi A, Goto H,
Fukushima T, Baba H and Shindo H: Simvastatin suppresses
dexamethasone-induced secretion of plasminogen activator
inhibitor-1 in human bone marrow adipocytes. BMC Musculoskelet
Disord. 12(82)2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shimizu S, Asou Y, Itoh S, Chung UI,
Kawaguchi H, Shinomiya K and Muneta T: Prevention of cartilage
destruction with intraarticular osteoclastogenesis inhibitory
factor/osteoprotegerin in a murine model of osteoarthritis.
Arthritis Rheum. 56:3358–3365. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Busillo JM and Cidlowski JA: The five Rs
of glucocorticoid action during inflammation: Ready, reinforce,
repress, resolve, and restore. Trends Endocrinol Metab. 24:109–119.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Soliman S and Ahmed M: The effect of
orthognathic surgery on osteoprotegerin as immunological caliper of
bone healing. Open Access Maced J Med Sci. 4:705–708.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Reid PE, Brown NJ and Holen I: Breast
cancer cells stimulate osteoprotegerin (OPG) production by
endothelial cells through direct cell contact. Mol Cancer.
8(49)2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Baud'huin M, Duplomb L, Teletchea S,
Lamoureux F, Ruiz-Velasco C, Maillasson M, Redini F, Heymann MF and
Heymann D: Osteoprotegerin: Multiple partners for multiple
functions. Cytokine Growth Factor Rev. 24:401–409. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bayer CM, Beckmann MW and Fasching PA:
Updates on the role of receptor activator of nuclear factor
κB/receptor activator of nuclear factor kappaB
ligand/osteoprotegerin pathway in breast cancer risk and treatment.
Curr Opin Obstet Gynecol. 29:4–11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang L, Liu M, Zhou X, Liu Y, Jing B,
Wang X, Zhang Q and Sun Y: Role of osteoprotegerin (OPG) in bone
marrow adipogenesis. Cell Physiol Biochem. 40:681–692.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu W, Xu C, Zhao H, Xia P, Song R, Gu J,
Liu X, Bian J, Yuan Y and Liu Z: Osteoprotegerin induces apoptosis
of osteoclasts and osteoclast precursor cells via the Fas/Fas
ligand pathway. PLoS One. 10(e0142519)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim CS, Bae EH, Ma SK, Han SH, Choi KH,
Lee J, Chae DW, Oh KH, Ahn C and Kim SW: Representatives of the
KNOW-CKD Investigator Group. Association of serum osteoprotegerin
levels with bone loss in chronic kidney disease: Insights from the
KNOW-CKD study. PLoS One. 11(e0166792)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Van Poznak C, Cross SS, Saggese M, Hudis
C, Panageas KS, Norton L, Coleman RE and Holen I: Expression of
osteoprotegerin (OPG), TNF related apoptosis inducing ligand
(TRAIL), and receptor activator of nuclear factor kappaB ligand
(RANKL) in human breast tumours. J Clin Pathol. 59:56–63.
2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ren H, Ren H, Li X, Yu D, Mu S, Chen Z and
Fu Q: Effects of intermedin on proliferation, apoptosis and the
expression of OPG/RANKL/M-CSF in the MC3T3-E1 osteoblast cell line.
Mol Med Rep. 12:6711–6717. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cheng Z, Landish B, Chi Z, Nannan C,
Jingyu D, Sen L and Xiangjin L: 3D printing hydrogel with graphene
oxide is functional in cartilage protection by influencing the
signal pathway of Rank/Rankl/OPG. Mater Sci Eng C Mater Biol Appl.
82:244–252. 2018.PubMed/NCBI View Article : Google Scholar
|