Introduction
Angiosarcoma is a rare, fatal, malignant vascular
tumor type. It may occur in any organ and is characterized by
aggressive clinical features, as angiosarcoma mainly originates
from endothelial cells. Angiosarcoma accounts for ~2% of all
sarcoma cases (1). The most common
primary sites of angiosarcoma include the skin and subcutaneous
tissues of the head and neck (2).
Of note, common metastasis sites of angiosarcoma include the lungs,
liver, bones and lymph nodes (3,4).
Pulmonary angiosarcoma is usually secondary tumors, whereas primary
pulmonary angiosarcoma (PPA) is rare and only ~30 cases have been
reported (5). Due to the lack of
specific clinical features and CT manifestations of PPA, it is
difficult to diagnose, particularly in patients with a history of
cancer.
The present study reports on a rare case of
pulmonary nodules in a middle-aged female patient with a history of
thyroid cancer. The patient presented with symptoms such as
persistent cough, expectoration and bloody sputum. Due to the
non-specificity of clinical features, according to the thyroid
cancer history, the case was initially considered to be pulmonary
metastasis of papillary thyroid carcinoma. The diagnosis was
confirmed through lung biopsy. Immunohistochemical staining was
positive for CD31, CD34, E26 transformation-specific-related gene
and vimentin, and no obvious tumor sign was observed in any other
sites, indicating that the case was a primary pulmonary epithelioid
angiosarcoma (PEA). The clinical features, diagnosis, differential
diagnosis and treatment of this disease are outlined below.
Case report
A 54-year-old female patient was admitted to the
Department of Pulmonary and Critical Care Medicine of Taihe
Hospital (Shiyan, China). The patient presented with cough and
expectoration with blood-stained sputum for >5 months and
aggravation for one day in May 2021. The patient underwent a series
of chest CT scans at our hospital (Fig. 1). A CT scan performed in July 2019
was normal (Fig. 1A and B). The patient had been admitted to the
hospital in February 2021 after reporting bloody sputum. The
patient underwent symptomatic and supportive treatment, which
improved the symptoms, and the patient was discharged. Chest CT
performed on admission in February 2021 revealed a small nodular
shadow (Fig. 1C and D). Hemoptysis recurred and the patient
was re-admitted to the Department of Pulmonary and Critical Care
Medicine in May 2021. The patient reported a medical history of
hypertension and coronary heart disease. In addition, the patient
had a history of papillary thyroid carcinoma diagnosed in 2003 and
had undergone treatment through surgery, postoperative chemotherapy
and iodine 131 therapy. Furthermore, the patient had a history of
postoperative recurrence, which was treated through residual
thyroid lobectomy. Thyroid tissue immunohistochemistry results
indicated the presence of cytokeratin 19 (CK19), human bone marrow
endothelial cell marker-1 (HBME-1) and galectin. Reexamination of
the patient through thyroid ultrasound indicated no significant
abnormality in February 2021. The patient was then treated with
Euthyrox and had no family history of tumor. The body temperature,
heart rate, respiration and blood pressure of the patient were
normal at the time of admission in May 2021. The thyroid gland was
not enlarged. Serum tumor biomarker analysis indicated that the
level of carbohydrate antigen 125 was 70.5 U/ml, whereas other
laboratory parameters had no obvious abnormality. The patient had
heart palpitations, chest tightness and other types of discomfort
after admission. Cardiac color ultrasound indicated large amounts
of pericardial effusion. Pericardiocentesis was performed on the
day after admission in May 2021. An ultrasound-guided
16G-percutaneous transhepatic cholangiography needle was used to
penetrate the pericardial effusion and 350 ml of bloody fluid was
withdrawn. Pericardial effusion cytology and microscopic
examination indicated a moderate level of mesothelial cells, a
small number of lymphocytes and no obvious atypia of cells with no
clear malignant cells. Basic pericardial effusion routine analysis
indicated a dark red color and the absence of clots, and the
Rivalta test was positive. The total blood cell count was
3.317x106/l, the nucleated cell count was
2,000x106/l, the monocyte percentage was 30% and the
multinucleated cell percentage was 70%. Biochemical analysis of
pericardial effusion indicated that the total protein content was
54.07 g/l, glucose level was 5.72 mmol/l, total cholesterol level
was 3.16 mmol/l, the lactate dehydrogenase content was 372.3 U/l,
adenosine deaminase levels were 9.3 U/l, the amylase content was
25.3 U/l and the quantitative level of high-sensitivity C-reactive
protein was 1.66 mg/l; these results indicate exudate.
Reexamination of the patient through chest CT on admission in May
2021 indicated multiple nodular and small patchy high-density
shadows of different sizes with clear boundaries. Certain lesions
displayed ground glass opacities (GGO) around them. Patchy
high-density shadows were observed on the left lower lobe with
blurred boundary and low levels of pleural effusion were observed
on the left side (Fig. 1E and
F). Thoracentesis was performed
and 600 ml of blood-stained pleural fluid was drawn. Pleural fluid
cytology indicated a small number mesothelial cells and a moderate
number of acute and chronic inflammatory cells under the
microscope. The cells exhibited no obvious atypia and had no clear
signs of malignancy on microscopic examination. CT-guided lung
biopsy was performed on the sixth day after admission in May 2021,
when the patient's condition was stable. Rapid on-site evaluation
of lung tissue revealed granulomatous inflammation and a small
number of lymphocytes were observed under the microscope without
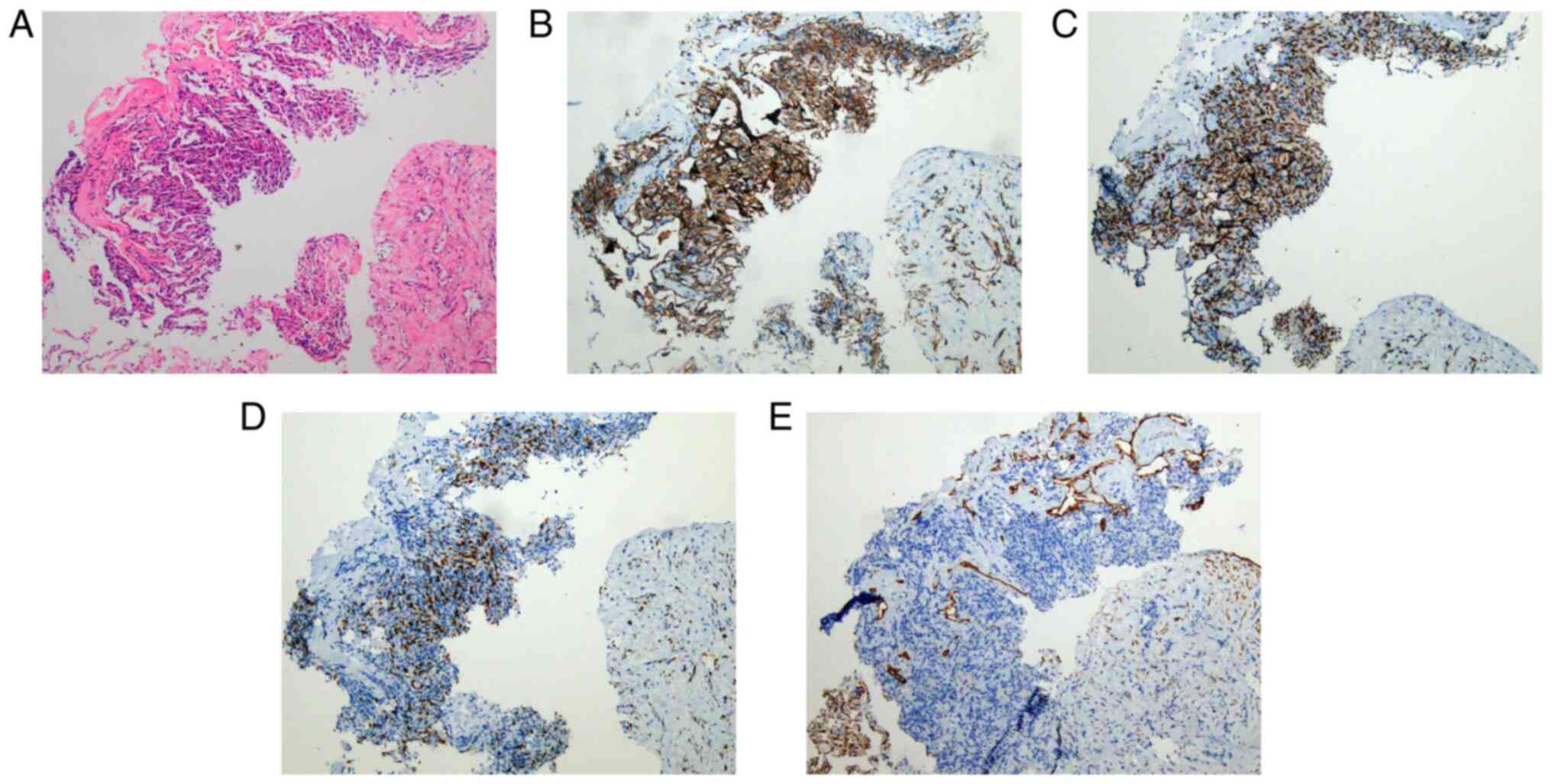
clear necrosis. Lung puncture tissue pathology revealed a
soft-tissue neoplasm with a sheet-like arrangement or storiform
pattern of spindle-like and epithelioid cells with prominent
nucleoli, with a certain amount of multinucleation. Furthermore,
interalveolar spindle cell proliferation was observed with a high
Ki67 index, an increased tendency to mesenchymal characteristics of
the tumor was present and it was not possible to exclude
angiosarcoma (Fig. 2A). Tissue
Mycobacterium tuberculosis PCR analysis was negative.
Special staining was performed and periodic acid Schiff reaction
was negative, silver staining was negative and acid-fast staining
was negative. Immunohistochemical results indicated the presence of
CD31 (Fig. 2B), ERG (Fig. 2C) and Ki67 (+30%) (Fig. 2D), and negative results for CD56,
CK19, galectin 3, HBME1, thyroid transcription factor (TTF1),
thyroglobulin antibody and napsin A; furthermore, positive staining
for vimentin and absence of progesterone receptor, epithelial
membrane antigen and CK pan were observed (Fig. 2E). At the Department of Pathology
(Tongji Hospital, Tongji Medical College of Huazhong University of
Science and Technology, Wuhan, China), lung tissue pathology
analysis was performed, indicating vascular tumor, possibly
angiosarcoma. Immunohistochemical analysis was positive for ERG,
CD34 and friend leukemia virus integration 1 (FLI1), and negative
for calretinin, Wilm's tumor 1 and transcription factor E3.
According to the histopathology and immunohistochemical results,
and the fact that no obvious tumor sign was observed in any other
sites, the patient was finally diagnosed with primary pulmonary
epithelioid angiosarcoma. Chest CT was conducted in the 10th, 13th
and 14th week after admission in May 2021 and the CT scan indicated
enlarged lung nodules, pleural nodules and pleural effusion as
compared with the previous CT scan, indicating that the patient
exhibited rapid progression of the tumor (Fig. 1G-L). The patient died 3 months
after the diagnosis.
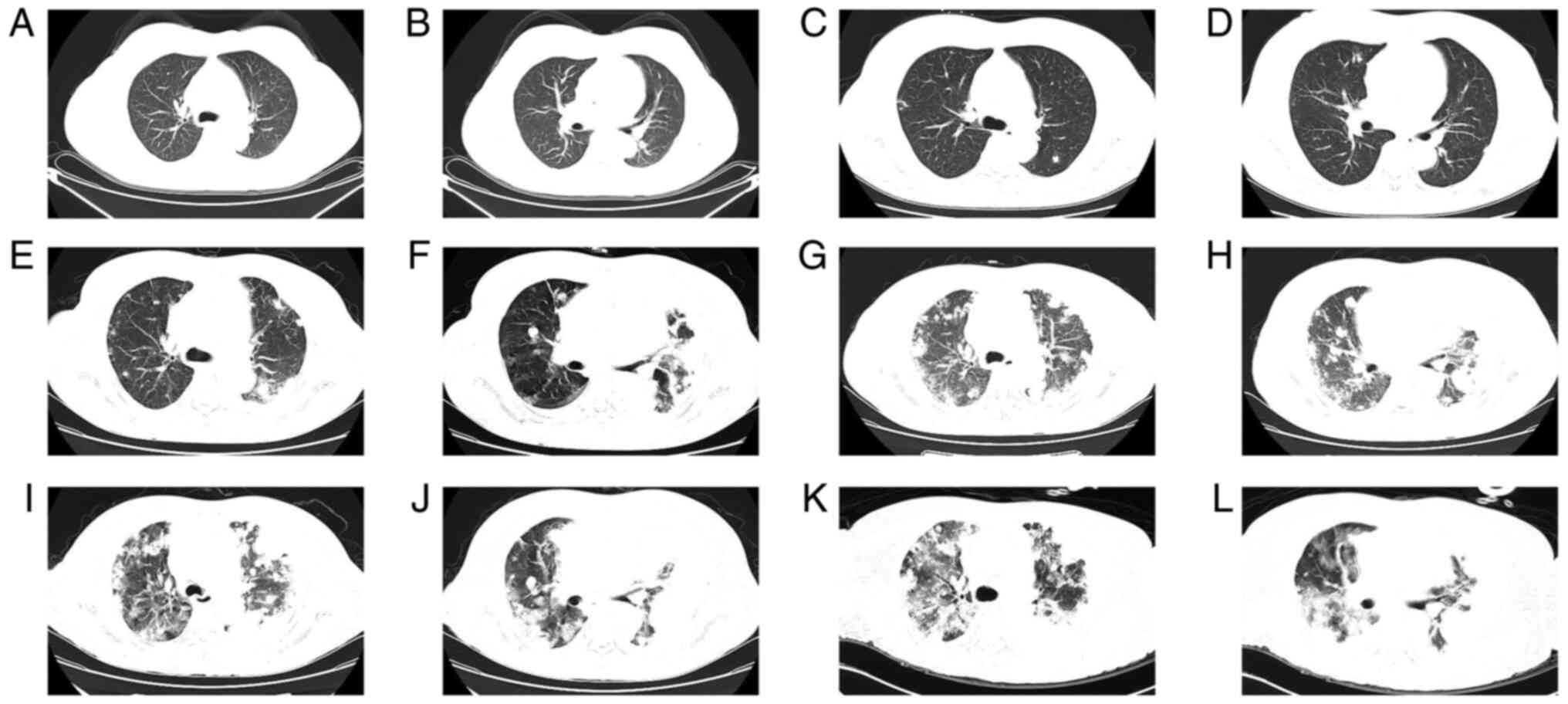 | Figure 1Chest CT scanning slice images of the
patient at different time-points. Normal lungs as displayed on CT
performed in July 2019, at (A) the carina level and (B)
intermediate bronchus level. Several nodules were observed in both
lungs as indicated by CT scan performed in February 2021, at (C)
the carina level and (D) intermediate bronchus level. Multiple
nodules in both lungs as revealed by CT scan in May 2021, at (E)
the carina level and (F) intermediate bronchus level. Enlarged
nodule with ground glass shadow as displayed by CT scan conducted
in July 2021, at (G) the carina level and (H) intermediate bronchus
level. Nodules were fused, as indicated by CT scan in August 2021,
and significantly larger compared with the previous time-points, at
(I) the carina level and (J) intermediate bronchus level. Enlarged
lung nodules, pleural nodules and pleural effusion as revealed by
CT scan performed in August 2021, 4 days after the previous scan,
at (K) the carina level and (L) intermediate bronchus level. CT,
computed tomography. |
Discussion
Angiosarcoma is a subtype of soft tissue sarcoma.
Angiosarcoma is an aggressive malignant endothelial cell tumor of
vascular or lymphatic origin (3).
Angiosarcoma most commonly occurs in the skin of the head and neck,
particularly on the scalp, in elderly individuals, accounting for
approximately half of all of these tumors. Other common sites
include the breast, thyroid, heart, liver, kidney, spleen,
pulmonary vessels and limbs (6,7).
Pulmonary angiosarcomas are invariably (>90%) metastatic tumors
from primary malignancies of the skin, bone, liver, breast or heart
(8). Primary pulmonary
angiosarcoma (PPA) is a rare tumor arising from arterial or venous
pulmonary vessels of various sizes (9), and only ~30 cases of PPA have been
reported to date (5,7). PPAs are more common in males and the
majority of patients are aged 40 years or above (7,10).
The pathogenesis of the PPA has remained to be fully
elucidated. Angiosarcoma originates from blood vessels or lymphatic
vessels; thus, abnormal activation of vascular endothelial growth
factor (VEGF) and its receptor VEGFR may result in angiosarcoma
(6). According to previous
reports, the risk factors for PPA are radiotherapy, thorium
dioxide, poly-vinyl chloride, radon, thorotrast, copper mining
dust, mastectomy, chronic empyema and tuberculosis (3,8,10-12).
The patient of the present study received iodine 131 therapy. The
mechanism of immunosuppression in angiosarcoma has not been fully
elucidated and immunodeficiency may be correlated with the
pathogenesis of angiosarcoma (13). However, epidemiological studies
should be performed to confirm this.
PPA is associated with symptoms such as cough,
hemoptysis, chest pain, shortness of breath, pneumothorax,
spontaneous hemothorax, cyanosis, syncope or rarely massive
pulmonary hemorrhage and weight loss (6-8,10,11).
In addition, most patients present with cough and hemoptysis as the
initial symptoms (6). However, up
to 20% of PPA cases are asymptomatic and diagnosis mainly occurs
incidentally during autopsy (3,6,11).
PPA does not have any specific clinical manifestations and thus, it
is easily misdiagnosed as similar diseases, such as lung cancer,
metastatic malignancies, malignant pleural mesothelioma, malignant
endobronchial lesions, pulmonary embolism, interstitial pneumonia
associated with autoimmunological disease or infectious pneumonia
(e.g. invasive pulmonary mycoses, tuberculosis) (6-9).
The patient of the current study was admitted to the hospital after
presenting with cough and hemoptysis as the initial symptoms.
The main CT features include a solitary or multiple
lung nodules, interstitial infiltration, diffuse consolidation,
GGO, pulmonary nodules surrounding GGO, diffuse pleural thickening,
pleural effusion, chest wall invasion, ipsilateral thorax volume
loss, hemothorax, pneumothorax and bilateral cystic lung disease
(5,9,14-21).
An endobronchial presentation of a PPA is extraordinarily rare
(8,17,22).
In the present case, the chest CT revealed multiple nodules and
small patches of increased density in both lungs. Furthermore,
halo-like ground glass shadows were observed around certain
lesions. The patient reported a previous history of thyroid cancer
and chest CT was performed after admission. Multiple pulmonary
nodules accompanied by rapid growth of pericardial effusion
occurred in a short period of time, indicating that the potential
cause was multiple metastases of thyroid cancer and other tumors.
However, the pulmonary nodules were not typical in shape and no
significant abnormalities were observed for thyroglobulin and
thyroid function. Lung tissue immunohistochemistry results
indicated that TTF1, CK19, HBME-1 and galectin were negative,
implying that it was not a case of thyroid cancer metastasis.
Immunohistochemistry also revealed the presence of vascular
endothelium-related markers and thus, the possibility of primary
pulmonary angiosarcoma was considered.
18F-fluorodeoxyglucose (18F-FDG) positron
emission tomography (PET)/CT is a valuable auxiliary tool to stage
or restage and monitor tumor response and recurrence (7). A previous study suggested that
high-grade angiosarcomas had significantly higher maximum
standardized uptake value for the primary tumor (pSUVmax) and
primary tumor-to-blood ratio (TBR) according to
18F-FDG-PET/CT (23).
Furthermore, a higher pSUVmax, metabolic tumor volume, whole-body
total lesion glycolysis (TLG), primary TBR and whole-body TLG ratio
were significantly associated with unfavorable overall survival in
angiosarcomas (23).
Due to the lack of characteristic clinical
manifestations of PPA, histopathological and immunohistochemical
results are required. More importantly, due to certain patients
exhibiting multiple nodules in the lungs, it is similar to
metastatic angiosarcoma of the lung; a diagnosis of primary disease
requires a complete clinical and radiological examination of the
body in order to ensure that there are no primary lesions outside
of the chest (20).
The histological features of PPA may range from
well-differentiated tumors with variable endothelial atypia to
high-grade spindle cell malignancies (12). In well-differentiated areas,
abnormal endothelial cells form functioning vascular sinusoidal
channels that are continuous with normal vascular channels. In
patients with progressively more aggressive disease, the
architecture becomes more chaotic, with less clearly defined
vascular spaces. In poorly differentiated areas, the malignant
endothelial cells form continuous monolayers, usually with an
epithelioid morphology that defines the epithelioid angiosarcoma
(12,17). PEA is a rare type of angiosarcoma,
with a structure characterized by single or multifocal tumors
composed of sheets of atypical epithelioid cells. The main
pathological manifestations of PEA include diffuse patchy
distributions of spindle cells, abundant cytoplasm of cancer cells,
prominent capillary-like vasoformative elements, hemorrhage pools,
papillary growth, prominent nucleoli, marked atypical nuclei and
necrosis (7,24).
Angiosarcoma is characterized by high expression
levels of vascular endothelial markers, including factor
VIII-related antigens, CD31, CD34, FLI-1 and ERG (6-8,17,24).
Factor VIII-related antigen has the highest specificity; however,
it has the lowest sensitivity (17). CD31 may be detected in ~90% of
angiosarcoma cases, which is relatively specific and highly
sensitive, particularly in poorly differentiated cases (6,7,17).
Angiosarcoma express cytokeratin in ~30% of all cases (17). As with all types of angiosarcoma,
the epithelioid variant is strongly vimentin-positive. Factor
VIII-related antigen, CD34 and CD31 are specific markers for tumors
derived from the endothelium (12). Among these markers, CD31 and ERG
are considered to be the most reliable markers of vascular
differentiation (7,25). In the present case,
immunohistochemical staining was positive for CD31 and ERG and also
positive for CD34, FLI-1 and vimentin (data not shown). Abdominal
CT and brain CT did not indicate any obvious tumor signs and
echocardiography did not reveal any obvious structural
abnormalities (data not shown). The patient was diagnosed with
primary epithelioid pulmonary angiosarcoma based on her
histopathological and immunohistochemical results.
Angiosarcoma is a rare disease, and thus, there is
currently no standard treatment plan, particularly not for PPA.
Patients with pulmonary angiosarcoma have been treated with
surgical resection (5,11,17,22),
adjuvant radiotherapy (8,20,22),
chemotherapy (20,26), immunotherapy (27,28)
and combined therapy (10,16,20).
It has been proposed that surgery was the most effective treatment,
which should be considered as early as possible for resectable
tumors (5,17). Yang et al (7) reported that one patient was still
alive 59 months postoperatively, despite the development of
postoperative adjacent pleural invasion. The most common
chemotherapy regimens may be gemcitabine plus docetaxel (20,26).
Wilson et al (26)
presented a case of PPA with complete radiographic response to
gemcitabine and docetaxel, with a sustained complete response for
over 12 months and markedly prolonged survival of 39 months. The
other effective chemotherapy regimens include
doxorubicin/ifosfamide/mesna (10), adriamycin/ifosfamide (29),
cyclophosphamide/gemcitabine/docetaxel (30) and docetaxel/cisplatin (16). Previous studies reported that
immunotherapy is effective against angiosarcoma (31,32).
Radiotherapy and immunotherapy with recombinant interleukin-2
(rIL-2) was also reported to be effective (33). However, the potential efficacy of
rIL-2 alone cannot be assessed (34). Immunotherapy [anti-programmed cell
death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1)] has
exhibited efficacy against certain sarcoma subtypes, including
angiosarcoma (35,36). Xu et al (28) reported that a patient with PPA
benefited from anti-PD-L1 treatment (pembrolizumab 200 mg) with
high PD-L1 expression (70%); 9 days after the first pembrolizumab
infusion, a CT scan demonstrated a confirmed size reduction of
certain lesions compared with original lesions. A previous study
indicated that a case of stage IV PPA had an excellent response to
immunotherapy (pembrolizumab 2 mg/kg every 21 days) after
progression on first-line chemotherapy (paclitaxel) and had stable
disease after 1 year of immunotherapy treatment, which the patient
was able to tolerate well (27).
It has been indicated that patients with low expression of aldehyde
dehydrogenase (ALDH) in primary pulmonary angiosarcoma have a
favorable prognosis, whereas patients with high expression of ALDH
have poor prognosis (20). These
findings provide a basis for ALDH targeted therapy, which may be an
effective treatment strategy for this malignant tumor type. The
patient in the present study was in a poor condition and was not
able to tolerate surgery, further radiotherapy and chemotherapy.
The patient only received symptomatic and supportive treatment.
While no treatment has been clinically proven to be effective,
anti-PD-1 or anti-PD-L1 in combination with chemotherapy may be a
promising strategy for advanced-stage PPA. The prognosis of PEA is
particularly poor and the survival time ranged from less than one
month to >59 months after clinical presentation (5,7,17,22).
In conclusion, the rate of early diagnosis of PPA is
low owing to nonspecific respiratory manifestations and atypical
laboratory test results. Diagnosis of PPA mainly relies on
pathological analyses. Surgical resection is the first-line
treatment strategy for patients in the early stage. However, the
disease is frequently diagnosed at a late stage and beyond the
indications for surgery. For advanced-stage patients, chemotherapy
may be effective with the combined regimen of gemcitabine and
docetaxel. Anti-PD-1 or anti-PD-L1 in combination with chemotherapy
may be a promising strategy for advanced-stage PPA. As the disease
may develop and progress rapidly and aggressively, timely diagnosis
and early treatment are important to effectively prolong the
survival time of patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed in the present study
are included in this published article.
Authors' contributions
LY and YS obtained and analyzed the patient's
information and wrote the manuscript. MW and XQ obtained and
analyzed the patient's information and reviewed the discussion part
of the clinical manifestations and imaging features. LY analyzed
pathological figures and reviewed the discussion part of the
pathological analysis. QW and XQ designed the study and reviewed
the manuscript. All authors read and approved the final manuscript.
LY and XQ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Taihe Hospital (Shiyan, China).
Patient consent for publication
Written informed consent for publication of the
clinical details and clinical images was obtained from the
patient's guardian.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Naka N, Ohsawa M, Tomita Y, Kanno H,
Uchida A and Aozasa K: Angiosarcoma in Japan. A review of 99 cases.
Cancer. 75:989–996. 1995.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Penel N, Marréaud S, Robin YM and
Hohenberger P: Angiosarcoma: State of the art and perspectives.
Crit Rev Oncol Hematol. 80:257–263. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Young RJ, Brown NJ, Reed MW, Hughes D and
Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang H, Shi J, Liu H, Chen Y, Wang Y, Wang
W and Zhang L: Clinical and diagnostic features of angiosarcoma
with pulmonary metastases: A retrospective observational study.
Medicine (Baltimore). 96(e8033)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shimabukuro I, Yatera K, Noguchi S,
Kawanami Y, Iwanami T, Nishida C, Yamasaki K, Kawanami T, Ishimoto
H, So T, et al: Primary pulmonary angiosarcoma presenting with
hemoptysis and ground-glass opacity: A case report and literature
review. Tohoku J Exp Med. 237:273–278. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang P, Xu L and Yang Y: A rare cause of
pulmonary nodules diagnosed as angiosarcoma was misdiagnosed as
Vasculitis and Wegener's granuloma in an elderly man: A case
report. Front Oncol. 11(629597)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang X, Jiang J, Dong X, Liang J and Guan
Y: Correlations between computed tomography and positron emission
tomography/computed tomography findings and pathology in 6 cases of
pulmonary epithelioid angiosarcoma. Medicine (Baltimore).
97(e12107)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Krishnamurthy A, Nayak D, Ramshankar V and
Majhi U: Fluorine-18 fluorodeoxyglucose positron emission
tomography/computed tomography in the detection of primary
pulmonary angiosarcomas. Indian J Nucl Med. 30:142–144.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Piechuta A, Przybylowski T, Szolkowska M
and Krenke R: Hemoptysis in a patient with multifocal primary
pulmonary angiosarcoma. Pneumonol Alergol Pol. 84:283–289.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sayan M, Valiyev E, Akarsu I, Esendagli G,
Korpeoglu T and Celik A: Primary pulmonary angiosarcoma presenting
as Pancoast tumor. Asian Cardiovasc Thorac Ann. 29:434–437.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang Y, Huang X, Peng C, Wang Y, Wu Q, Wu
Z, Shao H and Wang W: Primary pulmonary epithelioid angiosarcoma: A
case report and literature review. J Cancer Res Ther. 14 (Suppl
1):S533–S535. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ng FH, Yu SM, Wai OK and Chan JC: Primary
epithelioid angiosarcoma of lung: Radiologic and clinicopathologic
correlation. J Clin Imaging Sci. 7(33)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Goedert JJ, Coté TR, Virgo P, Scoppa SM,
Kingma DW, Gail MH, Jaffe ES and Biggar RJ: Spectrum of
AIDS-associated malignant disorders. Lancet. 351:1833–1839.
1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma J, Zhang W, Huang Y, Wang L, Wang J,
Yuan L and Zhang J: Radiologic findings of primary pulmonary
angiosarcoma: A case report. Medicine (Baltimore).
97(e11105)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ren Y, Zhu M, Liu Y, Diao X and Zhang Y:
Primary pulmonary angiosarcoma: Three case reports and literature
review. Thorac Cancer. 7:607–613. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen YB, Guo LC, Yang L, Feng W, Zhang XQ,
Ling CH, Ji C and Huang JA: Angiosarcoma of the lung: 2 cases
report and literature reviewed. Lung Cancer. 70:352–356.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Grafino M, Alves P, de Almeida MM, Garrido
P, Hasmucrai D, Teixeira E and Sotto-Mayor R: Angiosarcoma of the
lung. J Bras Pneumol. 42:68–70. 2016.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
18
|
Atasoy C, Fitoz S, Yiğit H, Atasoy P,
Erden I and Akyar S: Radiographic, CT, and MRI findings in primary
pulmonary angiosarcoma. Clin Imaging. 25:337–340. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang CF, Chen TW, Tseng GC and Chiang IP:
Primary pulmonary epithelioid angiosarcoma presenting as a solitary
pulmonary nodule on image. Pathol Int. 62:424–428. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Aramini B, Masciale V, Bianchi D,
Manfredini B, Banchelli F, D'Amico R, Bertolini F, Dominici M,
Morandi U and Maiorana A: ALDH expression in angiosarcoma of the
lung: A potential marker of aggressiveness? Front Med (Lausanne).
7(544158)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Faiek S, Tariq H, Upparapalli D, Bansal A
and Sompalli S: Primary pulmonary epithelioid angiosarcoma: A rare
case presentation of bilateral pneumothoraces. Cureus.
11(e6514)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kakegawa S, Kawashima O, Ibe T, Ujita M,
Iwashina M, Nakano T and Shimizu K: A case of primary angiosarcoma
of the lung presenting as a hemorrhagic bronchial tumor. Ann Thorac
Cardiovasc Surg. 18:347–351. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kato A, Nakamoto Y, Ishimori T, Saga T and
Togashi K: Prognostic value of quantitative parameters of
18F-FDG PET/CT for patients with angiosarcoma. AJR Am J
Roentgenol. 214:649–657. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Modrzewska K, Radzikowska E, Szołkowska M,
Oniszh K, Szczęsna M and Roszkowski-Śliż K: Diffuse pulmonary
haemorrhage accompanied by haemothorax as a rare presentation of
primary lung angiosarcoma. Kardiochir Torakochirurgia Pol.
12:367–371. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Anderson T, Zhang L, Hameed M, Rusch V,
Travis WD and Antonescu CR: Thoracic epithelioid malignant vascular
tumors: a clinicopathologic study of 52 cases with emphasis on
pathologic grading and molecular studies of WWTR1-CAMTA1 fusions.
Am J Surg Pathol. 39:132–139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wilson R, Glaros S, Brown RK, Michael C
and Reisman D: Complete radiographic response of primary pulmonary
angiosarcomas following gemcitabine and taxotere. Lung Cancer.
61:131–136. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nguyen QT, Pham AT, Nguyen TT, Nguyen TTT
and Van Le K: Role of immunotherapy in pulmonary angiosarcoma: A
case report. Case Rep Oncol. 14:797–801. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu F, Zheng J, Fu M and Zhou H:
Antiprogrammed cell death protein 1 immunotherapy for angiosarcoma
with high programmed death-ligand 1 expression: A case report.
Immunotherapy. 12:771–776. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Carillo GA, Carretero MA, Vazquez JE,
Fontan EG, Ramos MB, Ventura JA, Rodriguez AP, Salmon AS and
Tejedor JL: Epithelioid angiosarcoma of the lung with pleural
metastases: A rare cause of haemoptysis clinicopathological
conference. Heart Lung Circ. 19:624–628. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shirey L, Coombs D, Talwar A and Mickus T:
Pulmonary epithelioid angiosarcoma responsive to chemotherapy: A
case report. Radiol Case Rep. 13:479–484. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sindhu S, Gimber LH, Cranmer L, McBride A
and Kraft AS: Angiosarcoma treated successfully with anti-PD-1
therapy-a case report. J Immunother Cancer. 5(58)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Florou V, Rosenberg AE, Wieder E,
Komanduri KV, Kolonias D, Uduman M, Castle JC, Buell JS, Trent JC
and Wilky BA: Correction to: Angiosarcoma patients treated with
immune checkpoint inhibitors: A case series of seven patients from
a single institution. J Immunother Cancer. 7(285)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kojima K, Okamoto I, Ushijima S, Yoshinaga
T, Kitaoka M, Suga M and Sasaki Y: Successful treatment of primary
pulmonary angiosarcoma. Chest. 124:2397–2400. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Duck L, Baurain JF and Machiels JP:
Treatment of a primary pulmonary angiosarcoma. Chest. 126:317–318.
2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Florou V and Wilky BA: Current management
of angiosarcoma: Recent advances and lessons from the past. Curr
Treat Options Oncol. 22(61)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dajsakdipon T, Siripoon T, Ngamphaiboon N,
Ativitavas T and Dejthevaporn T: Immunotherapy and biomarkers in
sarcoma. Curr Treat Options Oncol. 23:415–438. 2022.PubMed/NCBI View Article : Google Scholar
|