Introduction
Acute-on-chronic liver failure (ACLF) refers to
acute liver injury that occurs on the basis of underlying liver
disease, with jaundice and coagulation disorders as the main
manifestations, short-term complications of ascites and hepatic
encephalopathy, often combined with multiple organ failure, and
high short-term mortality rates (1-3).
HBV-ACLF is the most common type of ACLF in China. The
international time window for liver failure and ACLF progression
has been changed several times, from the initial 12 to 4 weeks, and
again back to 12 weeks (4). Data
from the large Asia-Pacific Association for the Study of Liver
(APASL)-ACLF Research Consortium database showed that ~70% of
patients surviving >90 days exhibited a reversal of ACLF
syndrome, showing sustained regression of the disease after 1 year.
Therefore, the 90-day survival rate of patients with ACLF not only
reflects their short-term prognosis, but also has great
implications for their long-term prognosis (1). The disease progresses rapidly in
patients with ACLF, and liver failure or extrahepatic organ failure
caused by acute events can occur rapidly, with a dangerous
prognosis (5). Therefore, the
early and correct diagnosis of ACLF and the determination of
indicators related to the poor prognosis of the disease are crucial
for the prognosis prediction and treatment of patients (6).
The reconstituted hemostasis pattern in the
disturbed internal environment of patients with ACLF is a subtle
balanced hemostasis characterized by the coexistence of
hypocoagulability and hypercoagulation, which may lead to the
occurrence of hemorrhagic or prothrombotic states (7-9).
In the progression of ACLF, subsequent organ failure may further
exacerbate the hemostatic imbalance caused by cirrhosis (10,11).
Secondary fibrinolysis due to intrahepatic hypercoagulability may
be related to elevated serum D-dimer levels in patients with ACLF
(12). D-dimer, a marker of
activation of the coagulation and fibrinolytic systems, and an
indirect marker of thrombotic activity (13), is elevated in critically ill
patients with cirrhosis (14). The
level of serum D-dimer of patients with liver cirrhosis gradually
increases with the aggravation of liver dysfunction (15,16).
Higher D-dimer levels can significantly predict in-hospital
mortality in patients with cirrhosis; therefore, D-dimer detection
can be used for prognostic stratification in cirrhosis (17,18).
The association of D-dimer with multiple organ injury confirms its
association with systemic inflammatory responses, thus, as well as
being a marker of fibrinolytic activation, D-dimer is also a sign
of severe systemic inflammation (12). A retrospective study by Qi et
al (12) showed that D-dimer
levels were related to the 28-day mortality rate of patients with
ACLF, and the risk of 28-day mortality in patients with ACLF
increased when the level of D-dimer reached 6.5 mg/l FEU. The
research conclusions need to be further confirmed.
Materials and methods
Study design
A single center retrospective study was conducted to
screen patients who were hospitalized in The Second Affiliated
Hospital of Chongqing Medical University (Chongqing, China) between
August 2017 and May 2021. The inclusion criteria included: i) met
the diagnostic criteria of APASL ACLF; ii) a diagnosis of ACLF
combined with HBV infection; and iii) age within 12-80 years old.
The exclusion criteria included: i) a diagnosis of ACLF combined
with deep vein thrombosis or portal vein thrombosis; ii) a
diagnosis of ACLF combined with liver cancer or other malignant
tumors; iii) a diagnosis of ACLF combined with severe chronic
extrahepatic diseases; iv) a diagnosis of ACLF combined with atrial
fibrillation, coronary heart disease or acute aortic dissection; v)
a diagnosis of ACLF combined with human immunodeficiency virus
infection; vi) major surgery or trauma within the last 6 months;
vii) received anticoagulation therapy within the past month; viii)
received immunosuppressive therapy; and ix) pregnant women. A total
of 201 patients with HBV-ACLF were included. All patients received
standardized medical treatment [according to the Diagnostic and
Treatment Guideline for Liver Failure 2018(19)] and artificial liver support system
treatment during hospitalization, and none of the patients received
liver transplantation.
Data collection
Patient data, including age, sex, comorbidities,
laboratory test results and prognostic scores, were collected via
electronic medical records, and 28 and 90-day survival rates were
collated using electronic medical record review and/or standardized
telephone interviews. Serum D-dimer level was detected as part of
the initial patient tests during the hospitalization period and it
was detected by the immunofluorescence method. This method is based
on immunofluorescence technology and adopts the double-antibody
sandwich method. The sample to be tested is mixed with the
detection buffer, and the immunolabeled detection antibody in the
buffer will bind to the D-dimer antigen. The intensity of the
fluorescent antibody signal is proportional to the concentration of
the captured D-dimer, and the concentration of the antigen in the
sample can be calculated after being analyzed by an immunoassay
analyzer. The type of the analyzer was Jet-iStar3000 produced by
Joinstar company, and the D-dimer detection kit produced by
Joinstar company was used. The analyzer and kit was used to test
the serum samples from 160 healthy individuals and the normal
reference range of D-dimer was confirmed to be <550 ng/ml.
According to whether the serum D-dimer level was increased (>550
ng/ml), the patients were divided into the elevated D-dimer group
and the normal D-dimer group.
Statistical analysis
Continuous variables with normal distribution were
expressed as the mean ± standard deviation, non-normally
distributed continuous variables were expressed as the median
(inter-quartile range) and nominal variables were expressed as n
(%). The influencing factors of 28 and 90-day prognosis were
determined by univariate and multivariate logistic regression
analysis, and the sensitivity and specificity of influencing
factors were determined by receiver operating characteristic (ROC)
curves. Among the influencing factors, the binary variable was
determined by the χ2 test, and its specificity and
sensitivity were calculated. Pearson's correlation analysis was
performed between the serum D-dimer levels and various baseline
data (the point biserial correlation coefficient of continuous
variables and dichotomous variables was consistent with the values
of Pearson's correlation coefficient). A Kaplan-Meier curve was
drawn, and Breslow's test was used to compare the difference in
survival between the two groups. All statistical analyses were
performed using SPSS v.26.0 (IBM Corp.) and all figures were drawn
with GraphPad Prism 9.0 (GraphPad Software, Inc.). P<0.05 was
used to indicate a statistically significant difference.
Results
Baseline data
Of the 201 patients, 18 were lost to follow-up.
Among the 183 patients, 162 were male and 21 were female, with a
mean age of 47.9 years. Overall, 71.6% of patients had underlying
cirrhosis, 53.0% had spontaneous peritonitis (SBP) and 61.7% had an
elevated serum D-dimer level at baseline (Table I).
 | Table IBaseline data of patients (n=183). |
Table I
Baseline data of patients (n=183).
| Variable | Value | Normal range |
|---|
| Age, years | 47.9±11.3 | |
| Male sex | 162 (88.5%) | |
| Cirrhosis | 131 (71.6%) | |
| SBP | 97 (53.0%) | |
| Red blood cell count
(x1012/l) | 4.1±0.7 | 4.3-5.8 |
| Hemoglobin, g/l | 131.9±20.8 | 130-175 |
| Leucocyte count
(x109/l) | 6.4 (4.9-8.6) | 3.5-9.5 |
| Neutrophil
percentage | 72.0 (66.8-80.7) | 45-75% |
| Platelet count
(x109/l) | 94 (68.0-125.0) | 100-300 |
| Alanine
aminotransferase, U/l | 578
(193.0-1,222.0) | 9-50 |
| Aspartate
aminotransferase, U/l | 385
(150.0-931.0) | 15-40 |
| Serum total
bilirubin, µmol/l | 298.3
(236.7-373.7) | 5.1-28 |
| Albumin, g/l | 31.1±4.1 | 40-55 |
| Serum creatinine,
µmol/l | 57.7 (49.5-71.0) | 57-97 |
| Serum sodium,
mmol/l | 134.9±4.0 | 137-147 |
| INR | 2.2 (1.9-2.9) | 0.7-1.3 |
| Fibrinogen, g/l | 1.5 (1.2-1.9) | 2-4 |
| MELD-Na score | 24.8 (21.0-28.8) | |
|
Log10HBV-DNA | 5.0 (3.8-6.7) | |
| Serum D-dimer,
ng/ml | 902
(345.2-1,634.6) | 0-550 |
| Elevated D-dimer
group | 113 (61.7%) | |
| 28-day mortality | 50 (27.3%) | |
| 90-day mortality | 89 (48.6%) | |
Logistic analysis and ROC curve
Multivariate logistic analysis was performed on the
patient's baseline data and the 28 and 90-day prognosis of the
patients, and the independent risk factors affecting the 28 and
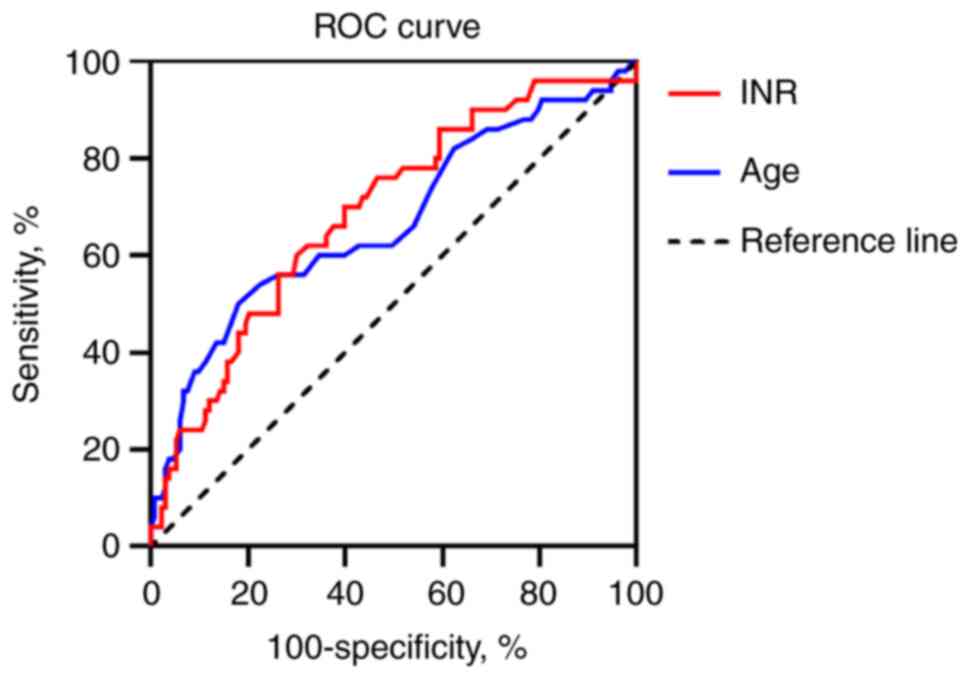
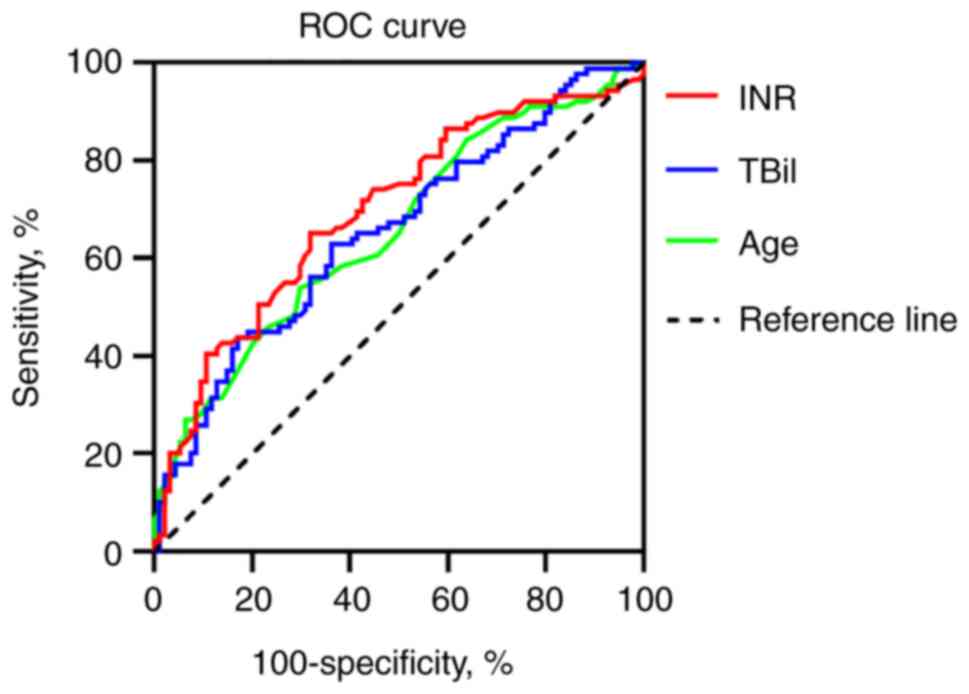
90-day survival rates were obtained. ROC curve was used to analyze
the predictive value of these factors on 28 and 90-day survival.
The critical value of each indicator was calculated using Jordan
index (20).
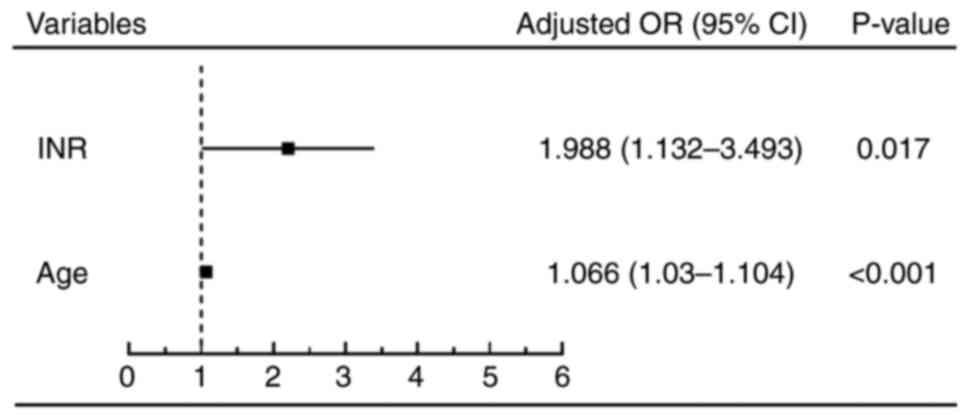
The results showed that international normalized
ratio (INR) >2.3 and age >53 years were independent risk
factors affecting 28-day survival (P<0.05) (Figs. 1 and 2; Table
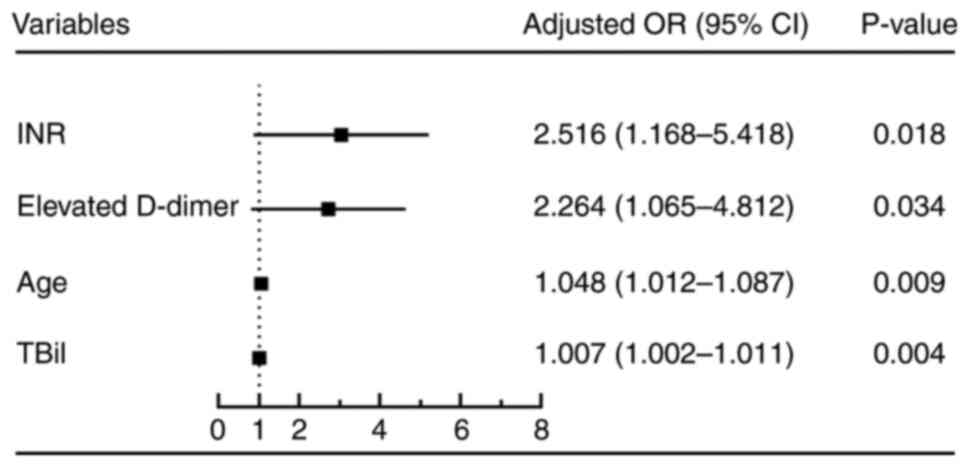
II). INR >2.3, serum total bilirubin (TBil) >358.2
µmol/l, age >49 years and elevated serum D-dimer (>550 ng/ml)
were independent risk factors affecting 90-day survival (P<0.05)
(Figs. 3 and 4; Tables
III and IV).
 | Table IIReceiver operating characteristic
curve analysis of 28-day prognostic factors. |
Table II
Receiver operating characteristic
curve analysis of 28-day prognostic factors.
| Factor | AUC | 95% Confidence
interval | P-value | Specificity, % | Sensitivity, % | Associated
criterion |
|---|
| INR | 0.688 | 0.616-0.754 | <0.0001 | 60.15 | 70.00 | >2.3 |
| Age | 0.668 | 0.595-0.736 | 0.0005 | 81.95 | 50.00 | >53 years |
 | Table IIIReceiver operating characteristic
curve analysis of 90-day prognostic factors. |
Table III
Receiver operating characteristic
curve analysis of 90-day prognostic factors.
| Factor | AUC | 95% Confidence
interval | P-value | Specificity, % | Sensitivity, % | Associated
criterion |
|---|
| INR | 0.696 | 0.624-0.762 | <0.0001 | 68.09 | 65.17 | >2.3 |
| TBil | 0.656 | 0.582-0.724 | 0.0001 | 82.98 | 43.82 | >358.2
µmol/l |
| Age | 0.655 | 0.581-0.724 | 0.0001 | 70.21 | 53.93 | >49 years |
 | Table IVχ2 test of elevated serum
D-dimer and 90-day survival. |
Table IV
χ2 test of elevated serum
D-dimer and 90-day survival.
| | 90-Day survival, n
(%) | |
|---|
| Patient group | Died | Survived | χ2 | P-value | Specificity, % | Sensitivity, % |
|---|
| Elevated
D-dimer | 66 (36.07) | 47 (25.68) | 11.295 | 0.001 | 50.00 | 74.16 |
| Normal D-dimer | 23 (12.57) | 47 (25.68) | | | | |
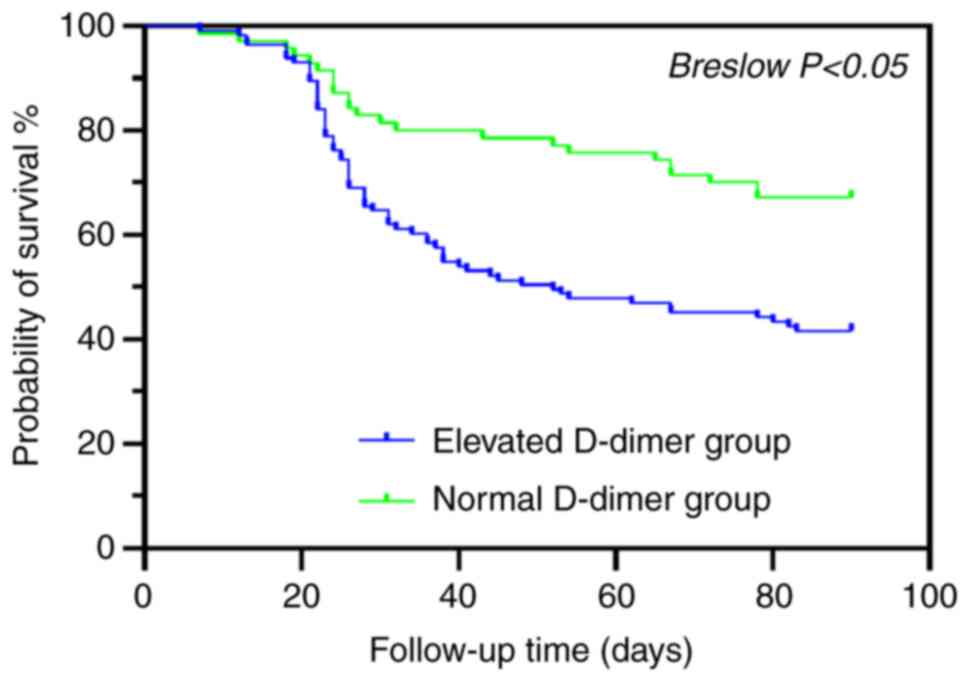
Survival analysis
After 90 days of follow-up, 18 of the 201 patients
were censored. A Kaplan-Meier curve was drawn, and the Breslow test
was used to compare the difference in survival between the elevated
D-dimer and normal D-dimer groups. The results showed that there
were significant differences in the 90-day survival rate and the
survival time between the two groups (P<0.05). The 90-day
survival rate and the survival time of patients in the elevated
D-dimer group were significantly lower than those in the normal
D-dimer group (Fig. 5).
Correlation analysis
Pearson's correlation analysis was performed between
the serum D-dimer levels and various baseline data (the point
biserial correlation coefficient of continuous variables and
dichotomous variables was consistent with the values of Pearson's
correlation coefficient). The results showed that serum D-dimer
level was negatively associated with male sex (r=-0.146, P=0.049),
red blood cell count (r=-0.173, P=0.019), serum sodium (r=-0.158,
P=0.033) and fibrinogen (r=-0.273, P<0.001), and positively
associated with age (r=0.155, P=0.037), combined SBP (r=0.149,
P=0.044), albumin (r=0.160, P=0.031), INR (r=0.149, P=0.044) and
MELD-Na score (r=0.174, P=0.018) (Table V).
 | Table VCorrelation analysis of serum D-dimer
with baseline data. |
Table V
Correlation analysis of serum D-dimer
with baseline data.
| Variable | Correlation
coefficient (r) | P-value |
|---|
| Age, years | 0.155 | 0.037 |
| Male sex | -0.146 | 0.049 |
| Cirrhosis | 0.030 | 0.688 |
| SBP | 0.149 | 0.044 |
| Red blood cell
count (x1012/l) | -0.173 | 0.019 |
| Hemoglobin,
g/l | -0.119 | 0.109 |
| Leucocyte count
(x109/l) | 0.050 | 0.501 |
| Neutrophil
percentage | 0.040 | 0.586 |
| Platelet count
(x109/l) | 0.047 | 0.527 |
| Alanine
aminotransferase, U/l | -0.126 | 0.090 |
| Aspartate
aminotransferase, U/l | -0.013 | 0.857 |
| Serum total
bilirubin, µmol/l | 0.064 | 0.389 |
| Albumin, g/l | 0.160 | 0.031 |
| Serum creatinine,
µmol/l | -0.081 | 0.278 |
| Serum sodium,
mmol/l | -0.158 | 0.033 |
| INR | 0.149 | 0.044 |
| Fibrinogen,
g/l | -0.273 | <0.001 |
| MELD-Na score | 0.174 | 0.018 |
|
Log10HBV-DNA | -0.040 | 0.587 |
Discussion
D-dimer is a sensitive marker of coagulation and
fibrinolysis, and studies have found that serum D-dimer is elevated
in liver failure and portal vein thrombosis (21). In the mouse model of acute liver
failure, both hepatic hypercoagulability and fibrin deposition are
present (13,14,22).
The results of the present study showed that the level of serum
D-dimer was significantly negatively associated with fibrinogen
(r=-0.273), which reflected the presence of hypercoagulable states
in the patients with ACLF. It has been found that SBP may play an
active role in the pathogenesis of accelerated plasma fibrinolysis
in patients with liver cirrhosis (23). The present study showed that plasma
D-dimer levels were significantly positively associated with
combined SBP (r=0.149). Thus, it can be inferred that secondary
fibrinolysis caused by intrahepatic hypercoagulability may be
associated with the elevation of serum D-dimer in patients with
ACLF, and the presence of SBP may play a certain role in promoting
this process. In addition, a study reports that a significant
increase in baseline serum von Willebrand factor (vWF) level in
patients with ACLF is associated with a marked reduction in
survival time, which may be due to vWF further promoting the
formation of a hypercoagulable state in patients with ALCF
(24). The significance of both
coagulation and fibrinolysis in ACLF progression was underscored in
these findings.
Infection and sepsis are common in patients with
ACLF, and their presence or development is associated with poor
outcomes (25,26). Sepsis is usually associated with
infections such as SBP, pulmonary infections and urinary tract
infections (27). A study by El
Gohary et al (23)
confirmed that the level of serum D-dimer in patients with SBP was
significantly higher than that in normal subjects and those without
SBP, and serum D-dimer was efficient in the early diagnosis of SBP
in patients with liver cirrhosis. The results of the present study
also showed that the serum D-dimer level was significantly
positively associated with SBP (r=0.149). Therefore, it can be
considered that the increase in serum D-dimer level can reflect
whether patients with ACLF have SBP complications to a certain
extent.
Microvascular thrombosis is associated with the
activation of the coagulation system and inflammation, which causes
multiple organ failure in patients with sepsis (28). It has also been found that acute
inflammatory responses in patients with ACLF may trigger
endothelial activation, resulting in markedly elevated vWF levels,
leading to progressive occlusion of small vessels and secondary
thrombotic microangiopathy, which is associated with multiorgan
failure and short-term mortality (29). It has also been found that
hemostatic changes in patients with ACLF partially overlap with
those of patients with sepsis-related disseminated intravascular
coagulation (DIC). Therefore, interventions that improve the
prognosis of sepsis-related DIC may also be beneficial for patients
with ACLF (30).
The study by El Gohary et al (23) found that the serum D-dimer
concentration increased with the deterioration of liver function,
and that the serum D-dimer level increased significantly with the
severity of liver disease. The clinical indicators used to evaluate
liver function mainly include TBil, INR and albumin. The results of
the present study showed that serum D-dimer level was significantly
positively associated with INR (r=0.149), which was an independent
risk factor affecting both the 28 and 90-day survival of the
patients. Serum D-dimer level was significantly positively
associated with albumin (r=0.160), but there was no significant
association between serum D-dimer level and TBil (P=0.389),
although TBil was an independent risk factor affecting the 90-day
survival of patients, which may be due to the limited sample
size.
The limitations of the present study are, first,
that all patients included were diagnosed with HBV-ACLF, so the
predictive value of serum D-dimer for short-term prognosis in
patients with ACLF due to other etiologies remains to be
determined. Second, ACLF progresses rapidly, but due to the
limitation of retrospective studies, the changes in serum D-dimer
levels in the patients with HBV-ACLF could not be dynamically
monitored. Whether the short-term increase of serum D-dimer levels
has predictive value for the short-term prognosis of HBV-ACLF still
needs further research to confirm.
In conclusion, elevated serum D-dimer (>550
ng/ml) was an independent risk factor affecting the 90-day survival
rate of the patients with HBV-ACLF (P<0.05). The 90-day survival
rate and the survival time of the patients in the elevated D-dimer
group were significantly lower compared with those in the normal
D-dimer group (P<0.05). Serum D-dimer is associated with the
short-term prognosis of patients with HBV-ACLF, and the detection
of serum D-dimer at admission can help predict the short-term
prognosis of these patients, especially the 90-day prognosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QC and ZM confirm the authenticity of all the raw
data. QC is responsible for data collection, data analysis and
writing the paper. ZM and QC designed and performed the study
together. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Chongqing Medical University,
Chongqing, China (approval no. 2022032).
Patient consent for publication
All patients in this study provided oral consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sarin SK, Choudhury A, Sharma MK, Maiwall
R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H,
et al: Correction to: Acute-on-chronic liver failure: Consensus
recommendations of the Asian Pacific association for the study of
the liver (APASL): An update. Hepatol Int. 13:826–828.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jalan R, Gines P, Olson JC, Mookerjee RP,
Moreau R, Garcia-Tsao G, Arroyo V and Kamath PS: Acute-on chronic
liver failure. J Hepatol. 57:1336–1348. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bajaj JS, O'Leary JG, Reddy KR, Wong F,
Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik
R, et al: Survival in infection-related acute-on-chronic liver
failure is defined by extrahepatic organ failures. Hepatology.
60:250–256. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bajaj JS, Moreau R, Kamath PS, Vargas HE,
Arroyo V, Reddy KR, Szabo G, Tandon P, Olson J, Karvellas C, et al:
Acute-on-chronic liver failure: Getting ready for prime time?
Hepatology. 68:1621–1632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jalan R, Pavesi M, Saliba F, Amorós A,
Fernandez J, Holland-Fischer P, Sawhney R, Mookerjee R, Caraceni P,
Moreau R, et al: The CLIF consortium acute decompensation score
(CLIF-C ADs) for prognosis of hospitalised cirrhotic patients
without acute-on-chronic liver failure. J Hepatol. 62:831–840.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wlodzimirow KA, Eslami S, Abu-Hanna A,
Nieuwoudt M and Chamuleau RA: A systematic review on prognostic
indicators of acute on chronic liver failure and their predictive
value for mortality. Liver Int. 33:40–52. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Saxena P, Bihari C, Rastogi A, Agarwal S,
Anand L and Sarin SK: Sonoclot signature analysis in patients with
liver disease and its correlation with conventional coagulation
studies. Adv Hematol. 2013(237351)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lisman T and Porte RJ: Rebalanced
hemostasis in patients with liver disease: Evidence and clinical
consequences. Blood. 116:878–885. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Blasi A, Calvo A, Prado V, Reverter E,
Reverter JC, Hernández-Tejero M, Aziz F, Amoros A, Cardenas A and
Fernández J: Coagulation failure in patients with acute-on-chronic
liver failure and decompensated cirrhosis: Beyond the international
normalized ratio. Hepatology. 68:2325–2337. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tripodi A, Primignani M, Chantarangkul V,
Dell'Era A, Clerici M, de Franchis R, Colombo M and Mannucci PM: An
imbalance of pro-vs anti-coagulation factors in plasma from
patients with cirrhosis. Gastroenterology. 137:2105–2111.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Caldwell SH, Hoffman M, Lisman T, Macik
BG, Northup PG, Reddy KR, Tripodi A and Sanyal AJ: Coagulation in
Liver Disease Group. Coagulation disorders and hemostasis in liver
disease: Pathophysiology and critical assessment of current
management. Hepatology. 44:1039–1046. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qi T, Zhu C, Lu G, Hao J, He Q, Chen Y,
Zhou F, Chen J and Hou J: Elevated D-dimer is associated with
increased 28-day mortality in acute-on-chronic liver failure in
China: A retrospective study. BMC Gastroenterol.
19(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weitz JI, Fredenburgh JC and Eikelboom JW:
A test in context: D-dimer. J Am Coll Cardiol. 70:2411–2420.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Drolz A, Horvatits T, Roedl K, Rutter K,
Staufer K, Kneidinger N, Holzinger U, Zauner C, Schellongowski P,
Heinz G, et al: Coagulation parameters and major bleeding in
critically ill patients with cirrhosis. Hepatology. 64:556–568.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gram J, Duscha H, Zurborn KH and Bruhn HD:
Increased levels of fibrinolysis reaction products (D-dimer) in
patients with decompensated alcoholic liver cirrhosis. Scand J
Gastroenterol. 26:1173–1178. 1991.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cioni G, Cristani A, Mussini C, Grandi S,
Pentore R, Zeneroli ML, Tizzanini W, Zagni G and Ventura E:
Incidence and clinical significance of elevated fibrin(ogen)
degradation product and/or D-dimer levels in liver cirrhosis
patients. Ital J Gastroenterol. 22:70–74. 1990.PubMed/NCBI
|
|
17
|
Li Y, Qi X, Li H, Dai J, Deng H, Li J,
Peng Y, Liu X, Sun X and Guo X: D-dimer level for predicting the
in-hospital mortality in liver cirrhosis: A retrospective study.
Exp Ther Med. 13:285–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou J, Mao W, Shen L and Huang H: Plasma
D-dimer as a novel biomarker for predicting poor outcomes in
HBV-related decompensated cirrhosis. Medicine (Baltimore).
98(e18527)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liver Failure and Artificial Liver Group,
Chinese Society of Infectious Diseases, Chinese Medical
Association; Severe Liver Disease and Artificial Liver Group,
Chinese Society of Hepatology, Chinese Medical Association.
Guideline for diagnosis and treatment of liver failure. Zhonghua
Gan Zang Bing Za Zhi. 27:18–26. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
20
|
Yin J and Tian L: Joint confidence region
estimation for area under ROC curve and Youden index. Stat Med.
33:985–1000. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Fimognari FL, De Santis A, Piccheri C,
Moscatelli R, Gigliotti F, Vestri A, Attili A and Violi F:
Evaluation of D-dimer and factor VIII in cirrhotic patients with
asymptomatic portal venous thrombosis. J Lab Clin Med. 146:238–243.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Violi F, Ferro D, Basili S, Quintarelli C,
Musca A, Cordova C and Balsano F: Hyperfibrinolysis resulting from
clotting activation in patients with different degrees of
cirrhosis. The CALC group. Coagulation abnormalities in liver
cirrhosis. Hepatology. 17:78–83. 1993.PubMed/NCBI
|
|
23
|
El Gohary AM, Elyamany AS, Mikhael NL,
Mahmoud MG and Tawfik MMR: Serum and ascitic D-dimer in cirrhotic
patients with spontaneous bacterial peritonitis. Clin Exp Hepatol.
7:134–140. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Eidelberg A, Kirubakaran R, Nair SC, Eapen
CE, Elias E and Goel A: Systematic review: Role of elevated plasma
von-Willebrand factor as predictor of mortality in patients with
chronic liver disease. Eur J Gastroenterol Hepatol. 31:1184–1191.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Piano S, Bartoletti M, Tonon M,
Baldassarre M, Chies G, Romano A, Viale P, Vettore E, Domenicali M,
Stanco M, et al: Assessment of Sepsis-3 criteria and quick SOFA in
patients with cirrhosis and bacterial infections. Gut.
67:1892–1899. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fernández J, Acevedo J, Wiest R, Gustot T,
Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, et
al: Bacterial and fungal infections in acute-on-chronic liver
failure: Prevalence, characteristics and impact on prognosis. Gut.
67:1870–1880. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Moreau R, Jalan R, Gines P, Pavesi M,
Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et
al: Acute-on-chronic liver failure is a distinct syndrome that
develops in patients with acute decompensation of cirrhosis.
Gastroenterology. 144:1426–1437.e1-e9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rittirsch D, Flierl MA and Ward PA:
Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 8:776–787.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Prasanna KS, Goel A, Amirtharaj GJ,
Ramachandran A, Balasubramanian KA, Mackie I, Zachariah U, Sajith
KG, Elias E and Eapen CE: Plasma von Willebrand factor levels
predict in-hospital survival in patients with acute-on-chronic
liver failure. Indian J Gastroenterol. 35:432–440. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lisman T, Arefaine B, Adelmeijer J,
Zamalloa A, Corcoran E, Smith JG, Bernal W and Patel VC: Global
hemostatic status in patients with acute-on-chronic liver failure
and septics without underlying liver disease. J Thromb Haemost.
19:85–95. 2021.PubMed/NCBI View Article : Google Scholar
|