Hepatitis C virus (HCV) infection is a global health
problem that human beings have struggled with for ~30 years.
Individuals infected with HCV develop chronic hepatitis, and a
proportion might develop cirrhosis and liver carcinoma. The World
Health Organization has set a goal to eliminate the public health
burden of HCV by 2030(1). A
platform in the use of medicines to treat chronic hepatitis C was
constructed in Australia for the collection and management of
treatment, virological outcome and other relevant clinical data of
patients with HCV to better inform the limitations of HCV infection
(2). Although the development of
direct-acting antiviral agents (DAAs) for chronic HCV has resulted
in a 95% cure rate for patients infected with HCV genotypes
(3), there are still millions of
new infections and tens of millions of re-infections worldwide
every year. In such cases, an effective prophylactic vaccine is
necessary to control HCV infection. Significant progress has been
made in the development of vaccines against HCV in the past 10
years despite no commercial vaccine having appeared so far. The
characteristics and properties of different forms of vaccines and
the advantages and disadvantages of various vaccine expression
systems are summarized in the present study, to provide new
insights into the research and development of HCV preventive
vaccines.
In the present review, a literature search was
performed using the PubMed, Elsevier Science Direct and China
National Knowledge Infrastructure databases with ‘Hepatitis C
virus’ and ‘vaccine’ as the primary key words. On this basis,
‘immunogenicity’, ‘immune response’, ‘production’, ‘expression’ and
‘rational design’ were used as key words for the secondary search.
Studies related to therapeutic vaccines were excluded, unless they
described the immune protection on HCV genotypes different from the
infected one. Studies not written in English or Chinese were
excluded.
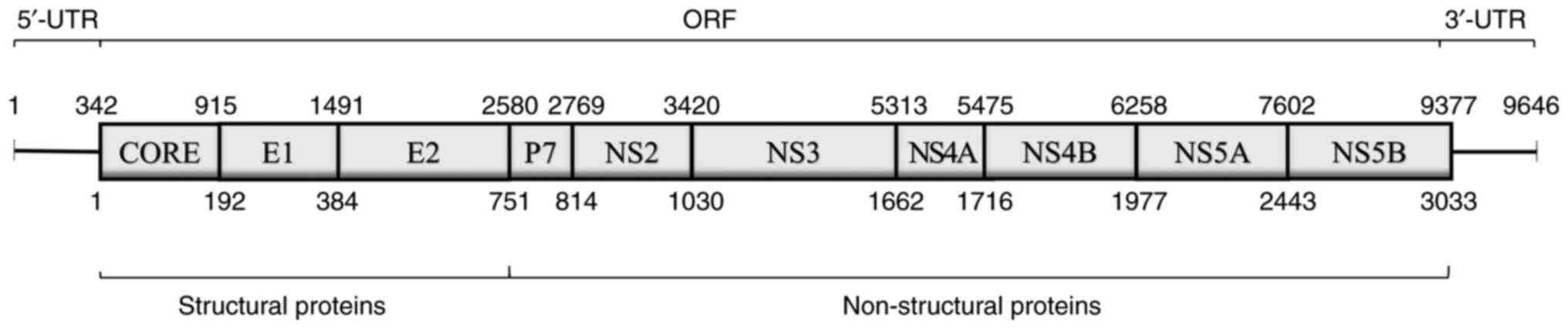
HCV is a positive-sense, single-stranded RNA virus
that belongs to the Flaviviridae family. The HCV genome contains
two untranslated regions and an open reading frame encoding
structural proteins [core, envelope protein 1 (E1) and envelope
protein 2 (E2)] and non-structural proteins (p7, NS2, NS3, NS4A,
NS4B, NS5A and NS5B) (Fig. 1)
(4). The complete or partial
genome has been used for vaccine preparation. Compared with the
attenuated vaccine or inactivated vaccine prepared using live
viruses, the preparation of vaccine using genetic engineering
technology has further improved its safety and immunogenicity
(5). Genetically engineered
vaccines are widely used to prevent infection by various viruses
(6). Different forms of
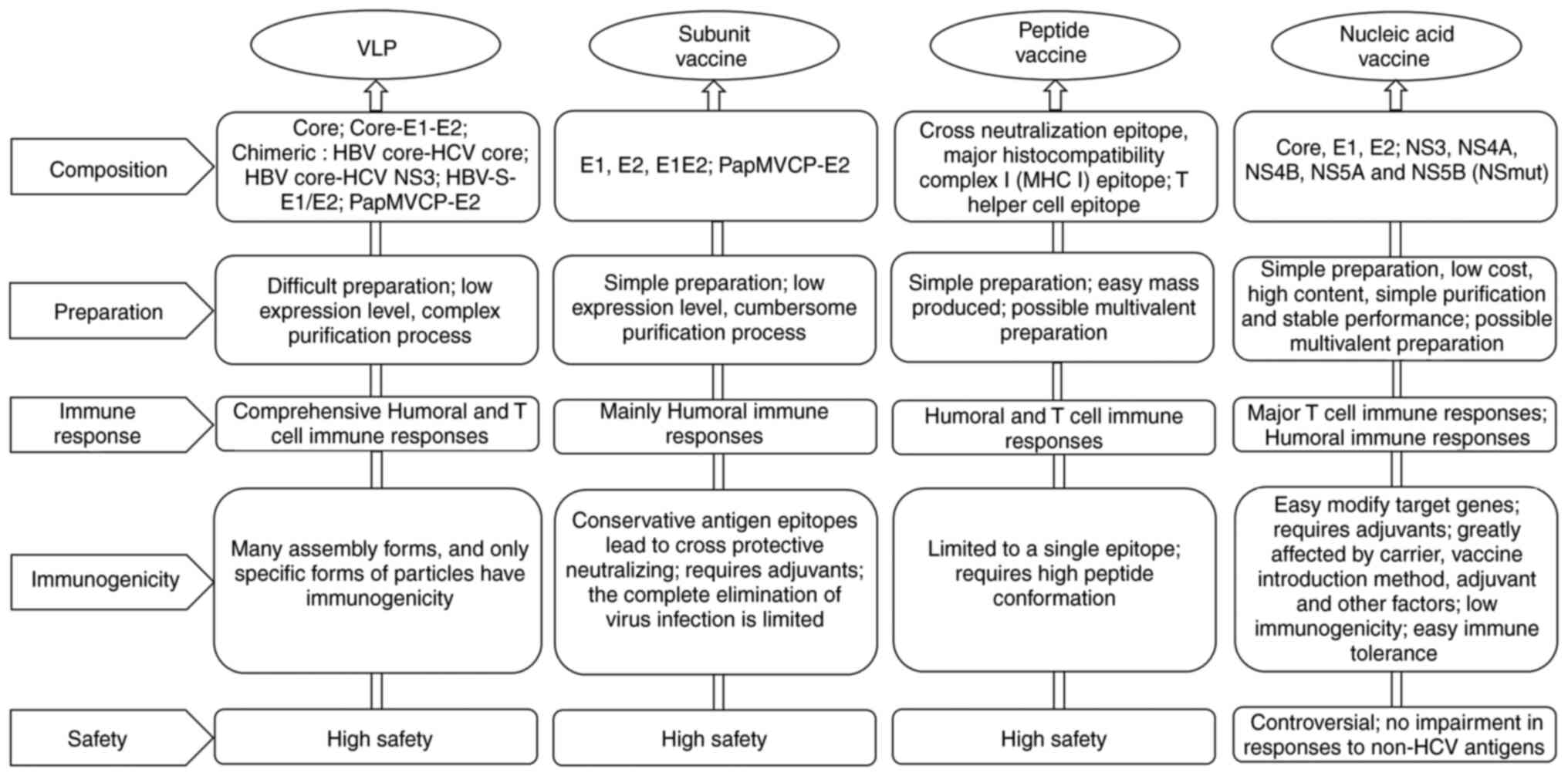
genetically engineered vaccines have been developed, and their
properties are summarized in Fig.
2.
The structural proteins of HCV can self-assemble
into viral particles without infection in vitro. VLP
vaccines can not only induce T-cell (CD4+ and
CD8+) immune responses, but also stimulate the formation
of protective neutralizing antibodies (7,8).
Research has found that different assembly forms lead to different
particle sizes (diameter ranging from 30-80 nm), in which only
particles of a specific size (55-60 nm in diameter) are infectious
and can cause specific antibody responses (9). VLPs with the same gene sequence can
be assembled into different structures in different expression
systems. For example, VLP vaccines containing the same structural
protein sequences have distinct immunogenicity due to the different
glycosylation mechanisms in insect and mammalian cells (10,11).
This suggests that the composition of the sugar chain could affect
the conformation of the whole virus particle. In addition, some
chimeric VLPs have been widely used. To date, the hepatitis B virus
(HBV) core protein (12), the
small (S) envelope protein of HBV (13), and papaya mosaic virus coat protein
(PapMVCP) (14) have been fused
with partial or total sequences of HCV envelope protein to prepare
VLP vaccines. However, the immunogenicity of chimeric VLPs is lower
than that of vaccines prepared using HCV self-proteins.
VLPs have been proven to induce protective immune
responses against viruses without adjuvants, and some studies have
shown that the use of adjuvants can significantly enhance the
immunogenicity of VLP vaccines (15-17).
Use of an anionic self-adjuvanting lipopeptide containing the
Toll-like receptor 2 agonist Pam2Cys (E8Pam2Cys) enhanced the
immunogenicity of VLPs composed of HCV structural proteins (core,
E1 and E2) (15). The improvement
in VLP and E2-specific antibody responses in VLP + E8Pam2Cys
vaccinated mice required up to three doses of non-adjuvanted VLPs
to match the antibody titers obtained with a single dose of VLPs
formulated with this lipopeptide. Further research found that
co-formulation of this lipopeptide with VLPs could improve
dendritic cell uptake and maturation, and could also induce better
VLP-specific interferon (IFN)-γ-mediated responses.
The recombinant subunit vaccine is mainly
concentrated in the envelope proteins E1 and E2 of the HCV, as the
epitopes on the envelope proteins are important for virus invasion
and neutralizing antibody identification (18,19).
Recombinant subunit vaccines based on envelope proteins can inhibit
viral infections by stimulating protective human neutralizing
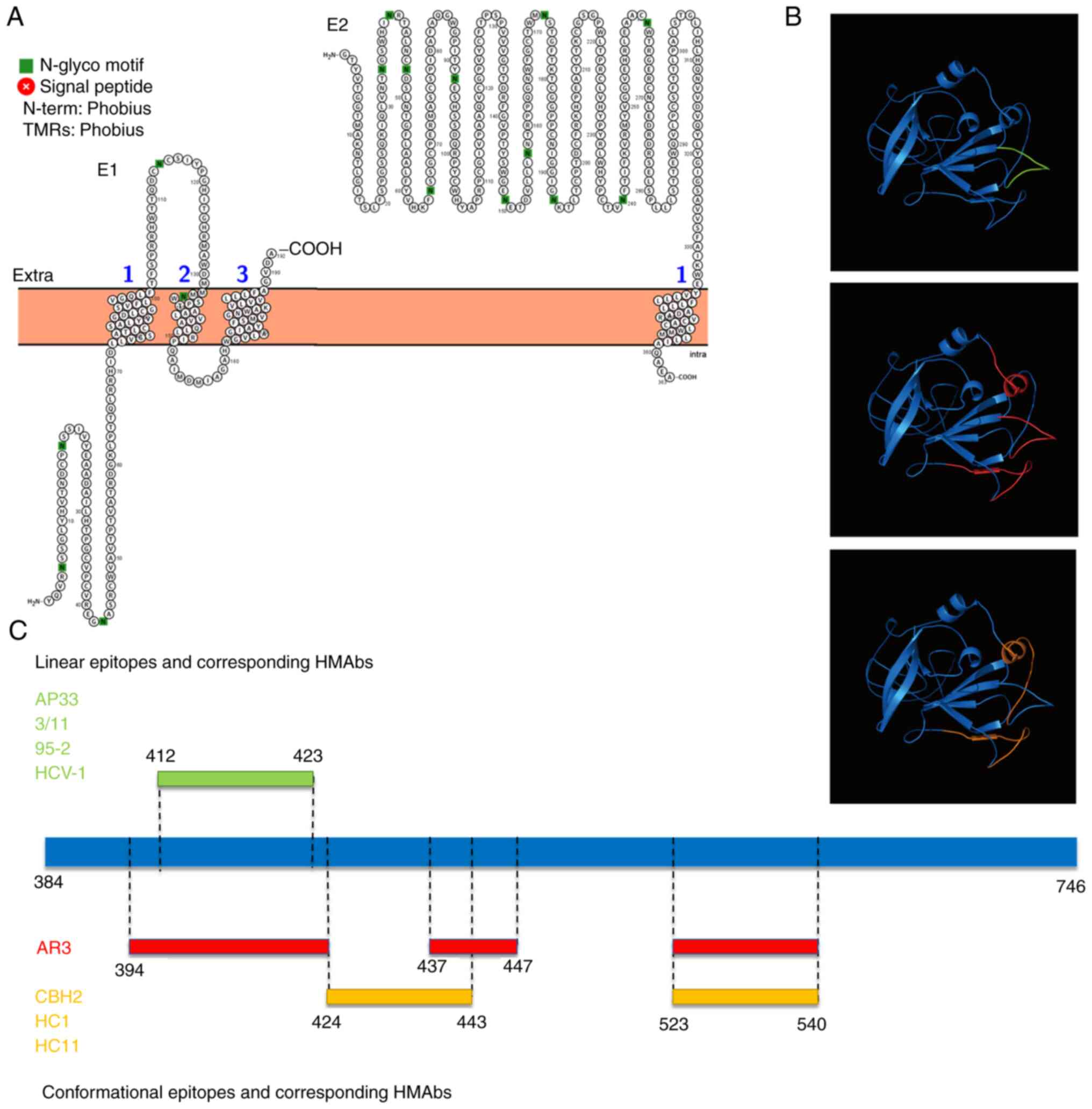
antibodies (HMAbs). Topological analysis showed that E1 is a
multi-pass transmembrane protein (crosses the membrane three times)
with most domains inside, and E2 is a single-pass transmembrane
protein with most domains outside (Fig. 3A). Antigenic epitopes are
concentrated in the E2 ectodomain due to the existence of binding
sites for cellular factors such as CD81, scavenger receptor class B
type I and claudin-1 (20-22).
Antigen epitopes on the surface of the envelope protein seriously
affect a broad spectrum of vaccines. HMAbs generated by
conservative antigen epitopes (divided into linear and
conformational epitopes) have a wide range of neutralization
characteristics and can cross-neutralize HCVs of different
genotypes. HMAbs (AP33, 3/11, 95-2 and HCV-1) corresponding to the
412-423 amino acid (aa) linear epitope show extensive neutralizing
activity against HCV pseudoparticles (HCVpp) of genotype 1-6
(Fig. 3B and C) (23);
HMAbs (CBH2, HC11 and HC1) or antigen region 3 (AR3) corresponding
to the conformational epitopes of two discontinuous sequences,
424-443/523-540 aa, or three discontinuous sequences,
394-424/437-447/523-540 aa, can also widely neutralize HCVpp of
genotypes 1-6 (Fig. 3B and
C) (24). Further experiements on
virus-escaping mutants showed that no virus escape body was found
in antibody HC1 at different experimental concentrations of
0.05-100 µg/ml (25). This
indicated that the influence of the conformational epitope on the
broad spectrum of the vaccine was greater than that of the linear
epitope. The discovery that epitopes determine the broad spectrum
of neutralizing antibodies will help in artificially designing
specific vaccine structures to stimulate broadly neutralizing
antibodies (26,27). Notably, not all epitopes could
induce the production of protective neutralizing antibodies. The
interfering antibody induced by the E2 region 434-446 aa does not
have neutralizing activity; however, its binding to E2 can mask the
binding of other neutralizing antibodies to the adjacent antigen
epitope region 412-426 aa, thereby reducing the effective
neutralizing activity corresponding to this epitope (28,29).
Thus, elicitation of antibodies with interfering capacity should be
avoided when producing an effective cross-neutralizing vaccine.
The epitopes of E2 are mostly concentrated in the
ectodomain; therefore, the removal of the C-terminal transmembrane
domain (TMD) has no significant effect on its immunogenicity
(30). In addition, the truncated
soluble E2, after removing hypervariable (HVR)1, HVR2 and
intergenotypic variable region, can still be folded correctly, but
the immunogenicity is reduced (31-33).
Changing the glycosylation modification mode of the envelope
protein also causes changes in its antigenicity and immunogenicity.
A comparison of the two glycosylation modification modes in insect
and mammalian cells showed that the sugar chain was essential for
the immunogenicity of the E2 vaccine, but the complex sugar chain
was not conducive to immunogenicity (34). Further research showed that insect
E2 induced stronger neutralizing antibody responses against the
homologous isolate used in the vaccine, but the two proteins
elicited comparable neutralization titers against heterologous
isolates (35). Adjuvants are
required to exert the immunogenicity of recombinant subunit
vaccines (36,37). Co-immunization with recombinant E2
vaccine and the saponin adjuvant QuilA, prepared by mammalian
cells, can produce anti-E2 antibody titer much higher than that
found using Freund's, monophosphoryl lipid A, cytosine
phosphorothioate guanine oligodeoxynucleotide (CpG ODN) or
α-galactosylceramide derivatives, and the effect of two adjuvant
combinations (QuilA and CpG ODN) is better than that of a single
adjuvant (36).
Peptide vaccines with immunogenicity can be screened
by constructing a phage expression peptide library or directly
synthesized using cross-neutralization epitopes, specific major
histocompatibility complex class I epitopes and T helper cell
epitopes. Such vaccines contain only limited epitopes and cannot
stimulate a wide range of immune responses. In patients, after
standard antiviral treatment, peptide vaccines can induce
HCV-specific T-cell responses to enhance the sustained virological
response and reduce relapse rates (38). However, the immunogenicity of
vaccines can be enhanced by modification. For example, in one
study, the affinity and immunogenicity of peptide vaccines were
improved after the leucine at position 8 of the cytotoxic T
lymphocyte epitope of HCV core antigen (132-140 aa) was mutated to
alanine (39). A nano-polypeptide
vaccine based on an NS3 polypeptide mixture can stimulate stronger
CD4+ T-cell responses and induce stronger
CD8+ T-cell immune responses than the NS3 polypeptide
vaccine (40). In addition, the
latest research showed that an overlapping peptide nanoparticle
vaccine prepared based on the p7 protein successfully stimulated
CD4+ and CD8+ T-cell responses (41). This was the first study to
demonstrate the immunogenicity of p7 as a vaccine target and
provides a new idea for the preparation of peptide vaccines.
Different application schedules and injection routes may also
influence the immunogenicity of HCV peptide vaccines. In a phase I
clinical trial for the dose and injection route of the HCV peptide
vaccine IC41, when increasing the frequency of vaccinations from 4
or 6 to 8 or 16 times per cycle (16 weeks), and decreasing the time
window from 4 weeks to 1 or 2 weeks, the T-cell response rates, in
particular the rates measuring CD8+ T-cell function,
were enhanced up to 2-fold compared with previous studies using the
same formulation of the IC41 vaccine (42-44).
DNA vaccines are prepared based on the coding
sequence of HCV structural proteins or non-structural proteins such
as E1, E2, NS3, NS4A, NS4B, NS5A or inactivated NS5B (NSmut) gene.
Viruses (adenovirus or vaccinia virus) and eukaryotic cytoplasmic
particles are usually used as vaccine vectors. DNA vaccines made of
structural protein-coding sequences usually stimulate humoral
immune responses and produce protective neutralizing antibodies
(45), whereas vaccines derived
from non-structural protein sequences mainly sustain cellular
immune responses. A prime-boost vaccine with chimpanzee-derived
adenovirus-3 NSmut and modified vaccinia Ankara NSmut could
successfully induce CD4+ and CD8+ T-cell
responses to all candidate HCV antigen epitopes, but its phase I
clinical trial results showed that the candidate vaccine did not
provide better protection against chronic HCV infection than the
placebo (clinicaltrials.gov identifier:
NCT01436357) (46). It was found
that the relative frequency of CD4+, CD25+
and Foxp3+ regulatory T cells (Tregs) was increased in
the blood and liver in patients with chronic persistent HCV
infection even after successful DAA treatment (47,48).
Tregs were demonstrated to attenuate vaccine-induced protective
CD4+ and CD8+ T-cell immune responses
(49). Therefore, a novel HCV DNA
vaccine, GLS-6150, consisting of the DNA plasmid encoding adjuvant
IFNL3 with DNA plasmids encoding the HCV non-structural proteins
(NS3/NS4A, NS4B and NS5A genes), reduced the frequency of Tregs and
increased HCV-specific T-cell responses in a phase I clinical trial
(NCT02027116) (50). Another study
reported that the immunogenicity of a DNA vaccine mixture (vaccine
cocktail) composed of conserved sequences of coding proteins of
multiple HCV genotypes was much greater than that of DNA vaccines
from a single genotype (51). The
breadth and intensity of the T-cell response have been improved,
although clinical experiments have not yet been performed.
Experiments on safety showed that the injection of DNA vaccines
does not impair the ability of the body to respond to non-HCV
antigens (52).
In the early stages of vaccine rational design
research, neutralizing antibodies associated with specific virus
clearance can be found by analyzing the antibody spectrum of the
virus, and then specific immunogens can be rationally designed to
facilitate the production of such antibodies. For example,
reasonable design of a specific epitope in E2 can produce HC33.1
antibody with strong antiviral effect (53). A recent study successfully
constructed an AP33 epitope structure simulant using an
anti-idiotypic method. In a mouse model, an antibody with the same
recognition site and residue as AP33 was induced as an immunogen,
and the antibody had the ability to resist HCV infection (54). This research opens up a new method
for the rational design of vaccines. Limited by technology, the
rational design thus far is restricted to linear epitopes and the
analog design of conformational epitopes cannot be realized.
The rational design of conformational epitope
vaccines must be based on an in-depth study of protein structure
and immune recognition information. Envelope glycoprotein E2 has a
highly conserved neutralization surface that is not covered by the
N-linked glycans and has three main overlapping neutralization
sites: Antigen site 412, 434 and AR3. Although it is composed of
highly conserved amino acid residues, its conformation is flexible
(21). It is difficult to obtain
ideal immune effects for peptides designed according to epitope
sequences, possibly since they do not have the correct
conformation. Analysis of the preferred conformation of the
neutralization site provides a basis for the design of a
conformational vaccine.
To date, candidate vaccine proteins composed of
partial or complete protein sequences of HCV have been successfully
produced in a variety of expression systems, such as mammalian and
insect cells, Pichia pastoris, Escherichia coli
expression systems, plant leaf expression systems, and even the
parasitic host Leishmania tarentolae. The hosts and
expression vectors used in different expression systems are
summarized in Table I.
A number of in-depth studies have focused on the
preparation of HCV vaccines using eukaryotic expression system, as
the recombinant vaccine protein is closest to the natural state of
the vaccine and has post-translational protein modifications such
as glycosylation. Mammalian cell-derived recombinant envelope
proteins have been reported to have higher immunogenicity and human
cell binding ability than those produced in yeast or insect cells
(55,56). Different vaccine candidates have
been successfully produced in mammalian cells, including Chinese
hamster ovary (CHO) (57), COS-7
(57,58), Huh-7 (59,60)
and human embryonic kidney 293T (13,61)
cells. A comparison of envelope protein expression in COS-7 and CHO
cells showed two different expression patterns: The former prepared
full-length protein with transient expression form, while the
latter produced truncated target protein in a stable manner
(58). This suggests that
transient expression is more suitable for maintaining protein
integrity than transient expression. The Pichia pastoris
expression system has the advantages of low cost and simple
operation for recombinant protein preparation. The structural
proteins of the core, E1 and E2, have been prepared as subunits or
VLP vaccines in Pichia pastoris under methanol induction,
and the target proteins have antigenicity. However, size-exclusion
chromatography and SDS-PAGE experiments have suggested that E2 is
mainly produced in a dimer or polymer form (62). The fragment from 612 to 620 aa has
been reported to be a dimerization sequence (63). The tendency to aggregate is
probably an intrinsic property of HCV glycoproteins, which leads to
low protein synthesis when using a non-viral vector (64). In fact, the maximum yield of HCV
glycoproteins prepared using yeast cells was 35 mg/l. The yield of
vaccine protein prepared by different hosts ranged from 1-10 mg,
and the highest yield was 100 mg/l from Drosophila S2 cells
with an expression cycle of up to 9 days. In recent years, some new
expression systems have been attempted for simpler genetic
manipulation, higher production levels and lower-cost production.
The HVR1/cholera toxin B subunit chimeric protein was expressed in
Nicotiana benthamiana plants with a production of 6-80 µg/g
of leaf tissue (65). Core-E1-E2
VLPs were successfully generated by the Leishmania
expression system (66). These
vaccines will not be suitable for use in clinics until their safety
and efficacy is confirmed.
Owing to the lack of protein modification by
prokaryotic cells, the immunogenicity of the vaccine in a
prokaryotic expression system is relatively lower than that
developed using eukaryotic cells. Most HCV vaccine proteins
prepared using the E. coli expression system are in the form
of inclusion bodies, and a few truncated envelope proteins can be
released into the periplasmic space of host cells using signal
peptides. The expression of the target protein accounts for 40-50%
of the total bacterial protein (58). It has been found that the expressed
core protein can also be assembled into particles in vitro
(with a diameter of 60 nm) (67).
In addition, due to the influence of bacterial and toxin proteins,
the purification cost is relatively high.
The vaccine protein, especially the envelope
glycoprotein, is mainly expressed intracellularly and is insoluble
through the addition of the signal peptide sequence during
recombinant expression (71). It
is generally believed that the strong hydrophobicity of the
C-terminus of the E2 protein is the main reason for this (75,76).
Therefore, several strategies have been developed to enhance
protein secretion: i) Fusion preparation with proteins having
strong secretory ability: The wild-type HBV S subviral particles
used in current HBV vaccines can be efficiently secreted into the
cell supernatant and are easily purified. Replacing the N-terminal
TMD of the HBV S protein with the TMD of HCV E1 or E2, the chimeric
HBV-HCV envelope proteins (E1-S or/and E2-S) can be effectively
secreted, co-expressed and assembled into VLPs [S+E1-S, S+E2-S and
S+(E1-S+E2-S)] with the wild-type HBV S protein (13). Unlike HCV VLPs, the chimeric
HBV-HCV VLPs could only induce a humoral immune response but not a
T-cell immune response. ii) Optimization of the structure of the
target protein: Following removal of the partial domain of the
C-terminal to prepare a truncated envelope protein, the truncated
E2 protein (384-521 or 605-680 aa) could be effectively secreted by
Pichia pastoris cells, while intact E2 (384-746 aa) was
mainly located in the insoluble part of ruptured cells (73). This is in concordance with another
study that found that C-terminal truncated enveloped proteins could
be efficiently secreted to the culture medium by mammalian cells
(77). Further research
demonstrated that the C-terminus of E2 that began with aa 718
contained an endoplasmic reticulum retention signal (75,76),
and topological analysis also showed that aa 718 was the beginning
of the TMD (Fig. 3A). iii)
Optimization of the signal peptide structure: When the α-factor
signal peptide of the Pichia pastoris expression system was
changed to the leader sequence of sucrose invertase 2,
extracellular expression of E2 could be realized (62,72).
However, the underlying mechanism remains unknown. In addition,
using the 374-383 aa of envelope protein E1 as a signal sequence,
the E2 protein could be expressed and secreted by mammalian cells
(78).
Due to a number of HCV genotypes, the vaccine that
is usually effective for one genotype is ineffective for other
genotypes. Therefore, broadening the spectrum of vaccines has
become a research challenge. Based on the published literature,
there are the following strategies to broaden the spectrum of
vaccines: i) Selection of antigen epitopes with strong
conservation. The appearance of broadly neutralizing antibodies
(bNAbs) is important for the body to remove HCV infection (79). Analyzing the distribution
characteristics of antigen epitopes in the tertiary structure of
HCV E2 protein, it was found that the antigen epitopes near the
receptor-binding amino acids were conserved and could easily
produce extensive neutralizing antibodies. HMAbs represented by
AP33, 3/11, 95-2 and HCV-1 bind to the 412-423 aa linear epitope
(23), which can show extensive
neutralizing activity against HCVpp of genotype 1-6. CBH2, HC11,
HC1 and other monoclonal antibodies bind to the conformational
epitope composed of 424-443 aa and 524-540 aa sequences, which can
widely neutralize the HCV 1-6 genotype (24). The molecular mechanism of the exact
cell entry process for HCV remains undefined, however, and only
limited antigen epitopes have been identified to this day. ii) The
antigen sequences of multiple genotypes are combined to prepare a
multi-antigen vaccine cocktail: A multi-antigen cocktail regimen
created by comparing a DNA vaccine cocktail encoding genotype
(Gt)1b and Gt3a NS3, NS4 and NS5B proteins elicited significantly
higher T-cell responses to Gt1b and Gt3a NS5B proteins than
single-genotype NS3/4/5B DNA vaccine (51). Obviously, the multi-antigen vaccine
cocktail method is not suitable for VLPs and subunit vaccines, for
which the correct conformation of the protein is necessary. iii)
Rational designing of a conservative structure suitable for
multiple genotypes: Structure-based vaccine designs have been
successfully used in influenza virus, human immunodeficiency virus
and other variable viruses for the purpose of optimizing the
presentation of key conserved epitopes, masking sites using
N-glycans or stabilizing the conformations of the envelope
glycoproteins (80,81). Similarly, the E2 antigen, in which
a mutation H449P was designed to stabilize the conformation of a
conservative immunogen domain D in the internal fluidity
neutralization surface, successfully induced bNAbs with
cross-neutralizing activities against HCVpp of the 1b, 2a, and 4a
genotypes (27). Analyzing the
sequence or structure of cross-neutralizing antibodies, the
corresponding epitope structure simulant was designed using
anti-idiotypic technology. The immunogenic effectiveness of AP33
linear epitope mimics has been confirmed in mouse models (54) and the design of structural epitope
mimics is the development direction of this strategy.
An effective vaccine for HCV is, essentially, an
antigen that elicits immune responses to key conserved epitopes. In
the present review, the composition, immunogenicity, advantages and
disadvantages of all different types of vaccines were summarized
and compared, and it was found that the source of antigen
determines the immunogenicity of vaccine. Structural proteins are
involved in the invasion of the virus into host cells and are used
in preference to stimulate the humoral immune response of the host.
Non-structural proteins are mainly involved in virus replication
and cause specific T-cell immune responses. HCV can be divided into
a number of genotypes due to its genetic variability. The conserved
key epitopes form flexible conformation. Vaccines using the
wild-type and full-length antigen cannot stimulate an ideal immune
effect. Future research will still focus on improving the
immunogenicity and broadening the spectrum of vaccines. Analysis
and optimization of the epitope structure corresponding to some
extremely effective neutralizing antibodies has significant
guidance value for the correct conformational expression of
vaccines. Therefore, the rational design, with structure as the
purpose and function as the starting point, is the most effective
way to obtain broad-spectrum vaccines. In addition, the vaccine
cocktail model can stimulate stronger and broader spectrum T-cell
responses and has higher immunogenicity and a broader spectrum than
vaccines from a single source.
Compared with other mature preparation technologies
of viral vaccines, improving the preparation level of an HCV
vaccine is also an important research direction in the future. By
summarizing the preparation methods and levels using different
expression hosts, it is indicated that deleting adverse protein
domains, sampling the sugar chains, or constructing multivalent
vaccines with proteins from other viruses, can effectively
neutralize the insoluble characteristics of HCV self-proteins.
Moreover, with the in-depth study of rational design, a new soluble
protein structure may be obtained.
In conclusion, all these successful advances
indicate that the high-level preparation of new vaccines with high
immunogenicity and a broad spectrum is possible. Rational design
will become the main focus in the future. At the same time, the
present review indicates an urgent need for in-depth research on
the structure and function of HCV proteins. A new vaccine will be
expected to completely eliminate new cases of HCV infection.
Not applicable.
Funding: This present study was supported by the Shandong Key
Research and Development Fund (grant no. 2018GSF121011), the Jinan
Clinical Medical Science and Technology Innovation Fund (grant no.
201805066) and the High-Level Project Cultivation Fund of Jinan
Central Hospital (grant no. 202105006).
Not applicable.
QZ conceived the idea of this study and was a major
contributor in writing the manuscript. KH, XHZ and MX assisted in
writing the manuscript. XPZ and HL reviewed the literature. All the
authors have read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Duncan JD, Urbanowicz RA, Tarr AW and Ball
JK: Hepatitis C Virus vaccine: Challenges and prospects. Vaccines
(Basel). 8(90)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ramsay J, Marsh J, Pedrana A, Andric N,
Norman R, Cheng W, Webb S, Zeps N, Bellgard M, Graves T, et al: A
platform in the use of medicines to treat chronic hepatitis C
(PLATINUM C): Protocol for a prospective treatment registry of
real-world outcomes for hepatitis C. BMC Infect Dis.
20(802)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Falade-Nwulia O, Suarez-Cuervo C, Nelson
DR, Fried MW, Segal JB and Sulkowski MS: Oral direct-acting agent
therapy for hepatitis C virus infection: A systematic review. Ann
Intern Med. 166:637–648. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Li D, Huang Z and Zhong J: Hepatitis C
virus vaccine development: Old challenges and new opportunities.
Natl Sci Rev. 2:285–295. 2015.
|
|
5
|
Forns X, Bukh J and Purcell RH: The
challenge of developing a vaccine against hepatitis C virus. J
Hepatol. 37:684–695. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Buonaguro L, Tagliamonte M, Tornesello ML
and Buonaguro FM: Developments in virus-like particle-based
vaccines for infectious diseases and cancer. Expert Rev Vaccines.
10:1569–1583. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Murata K, Lechmann M, Qiao M, Gunji T,
Alter HJ and Liang TJ: Immunization with hepatitis C virus-like
particles protects mice from recombinant hepatitis C virus vaccinia
infection. Proc Natl Acad Sci USA. 100:6753–6758. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Christiansen D, Earnest-Silveira L,
Grubor-Bauk B, Wijesundara DK, Boo I, Ramsland PA, Vincan E,
Drummer HE, Gowans EJ and Torresi J: Pre-clinical evaluation of a
quadrivalent HCV VLP vaccine in pigs following microneedle
delivery. Sci Rep. 9(9251)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gastaminza P, Dryden KA, Boyd B, Wood MR,
Law M, Yeager M and Chisari FV: Ultrastructural and biophysical
characterization of hepatitis C virus particles produced in cell
culture. J Virol. 84:10999–11009. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Matsuura Y, Harada S, Suzuki R, Watanabe
Y, Inoue Y, Saito I and Miyamura T: Expression of processed
envelope protein of hepatitis C virus in mammalian and insect
cells. J Virol. 66:1425–1431. 1992.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baumert TF, Ito S, Wong DT and Liang TJ:
Hepatitis C virus structural proteins assemble into virus like
particles in insect cells. J Virol. 72:3827–3836. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mihailova M, Boos M, Petrovskis I, Ose V,
Skrastina D, Fiedler M, Sominskaya I, Ross S, Pumpens P, Roggendorf
M and Viazov S: Recombinant virus-like particles as a carrier of B-
and T-cell epitopes of hepatitis C virus (HCV). Vaccine.
24:4369–4377. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Beaumont E, Patient R, Hourioux C,
Dimier-Poisson I and Roingeard P: Chimeric hepatitis B
virus/hepatitis C virus envelope proteins elicit broadly
neutralizing antibodies and constitute a potential bivalent
prophylactic vaccine. Hepatology. 57:1303–1313. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Denis J, Majeau N, Acosta-Ramirez E,
Savard C, Bedard MC, Simard S, Lecours K, Bolduc M, Pare C, Willems
B, et al: Immunogenicity of papaya mosaic virus-like particles
fused to a hepatitis C virus epitope: Evidence for the critical
function of multimerization. Virology. 363:59–68. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chua BY, Johnson D, Tan A,
Earnest-Silveira L, Sekiya T, Chin R, Torresi J and Jackson DC:
Hepatitis C VLPs delivered to dendritic cells by a TLR2 targeting
lipopeptide results in enhanced antibody and cell-mediated
responses. PLoS One. 7(e47492)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qiao M, Murata K, Davis AR, Jeong SH and
Liang TJ: Hepatitis C virus-like particles combined with novel
adjuvant systems enhance virus-specific immune responses.
Hepatology. 37:52–59. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Acosta-Rivero N, Poutou J,
Alvarez-Lajonchere L, Guerra I, Aguilera Y, Musacchio A, Rodríguez
A, Aguilar JC, Falcon V, Alvarez-Obregon JC, et al: Recombinant in
vitro assembled hepatitis C virus core particles induce strong
specific immunity enhanced by formulation with an oil-based
adjuvant. Biol Res. 42:41–56. 2009.PubMed/NCBI
|
|
18
|
Logan M, Law J, Wong JAJ, Hockman D, Landi
A, Chen C, Crawford K, Kundu J, Baldwin L, Johnson J, et al: Native
folding of a recombinant gpE1/gpE2 heterodimer vaccine antigen from
a precursor protein fused with Fc IgG. J Virol. 91:e01552–16.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Krapchev VB, Rychlowska M, Chmielewska A,
Zimmer K, Patel AH and Bienkowska-Szewczyk K: Recombinant
flag-tagged E1E2 glycoproteins from three hepatitis C virus
genotypes are biologically functional and elicit cross-reactive
neutralizing antibodies in mice. Virology. 519:33–41.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Owsianka AM, Timms JM, Tarr AW, Brown RJ,
Hickling TP, Szwejk A, Bienkowska-Szewczyk K, Thomson BJ, Patel AH
and Ball JK: Identification of conserved residues in the E2
envelope glycoprotein of the hepatitis C virus that are critical
for CD81 binding. J Virol. 80:8695–8704. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tzarum N, Wilson IA and Law M: The
neutralizing face of hepatitis C Virus E2 envelope glycoprotein.
Front Immunol. 9(1315)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kumar A, Hossain RA, Yost SA, Bu W, Wang
Y, Dearborn AD, Grakoui A, Cohen JI and Marcotrigiano J: Structural
insights into hepatitis C virus receptor binding and entry. Nature.
598:521–525. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Desombere I, Fafi-Kremer S, Van Houtte F,
Pessaux P, Farhoudi A, Heydmann L, Verhoye L, Cole S, Mckeating JA,
Leroux-Roels G, et al: Monoclonal anti-envelope antibody AP33
protects humanized mice against a patient-derived hepatitis C virus
challenge. Hepatology. 63:1120–1134. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Keck ZY and Foung SK: Neutralizing
antibody response to hepatitis C virus. Viruses. 3:2127–2145.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Keck ZY, Saha A, Xia J, Wang Y, Lau P,
Krey T, Rey FA and Foung SK: Mapping a region of HCV E2 that is
responsible for escape from neutralizing antibodies and a core
CD81-binding region that does not tolerate neutralization escape
mutations. J Virol. 85:10451–10463. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guest JD and Pierce BG: Structure-based
and rational design of a hepatitis C Virus vaccine. Viruses.
13(837)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pierce BG, Keck ZY, Wang R, Lau P,
Garagusi K, Elkholy K, Toth EA, Urbanowicz RA, Guest JD, Agnihotri
P, et al: Structure-based design of hepatitis C Virus E2
glycoprotein improves serum binding and cross-neutralization. J
Virol. 94:e00704–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kachko A, Frey SE, Sirota L, Ray R, Wells
F, Zubkova I, Zhang P and Major ME: Antibodies to an interfering
epitope in hepatitis C virus E2 can mask vaccine-induced
neutralizing activity. Hepatology. 62:1670–1682. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang P, Zhong L, Struble EB, Watanabe H,
Kachko A, Mihalik K, Virata ML, Alter HJ, Feinstone S and Major M:
Depletion of interfering antibodies in chronic hepatitis C patients
and vaccinated chimpanzees reveals broad cross-genotype
neutralizing activity. Proc Natl Acad Sci USA. 106:7537–7541.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ruwona TB, Giang E, Nieusma T and Law M:
Fine mapping of murine antibody responses to immunization with a
novel soluble form of hepatitis C virus envelope glycoprotein
complex. J Virol. 88:10459–10471. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vietheer PT, Boo I, Gu J, McCaffrey K,
Edwards S, Owczarek C, Hardy MP, Fabri L, Center RJ, Poumbourios P
and Drummer HE: The core domain of hepatitis C virus glycoprotein
E2 generates potent cross-neutralizing antibodies in guinea pigs.
Hepatology. 65:1117–1131. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
McCaffrey K, Boo I, Owczarek CM, Hardy MP,
Perugini MA, Fabri L, Scotney P, Poumbourios P and Drummer HE: An
optimized hepatitis C virus E2 glycoprotein core adopts a
functional homodimer that effciently blocks virus entry. J Virol.
91:e01668–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
McCaffrey K, Boo I, Poumbourios P and
Drummer HE: Expression and characterization of a minimal hepatitis
C virus glycoprotein E2 core domain that retains CD81 binding. J
Virol. 81:9584–9590. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li D, von Schaewen M, Wang X, Tao W, Zhang
Y, Li L, Heller B, Hrebikova G, Deng Q, Ploss A, et al: Altered
glycosylation patterns increase immunogenicity of a subunit
hepatitis C virus vaccine, inducing neutralizing antibodies which
confer protection in mice. J Virol. 90:10486–10498. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Urbanowicz RA, Wang R, Schiel JE, Keck ZY,
Kerzic MC, Lau P, Rangarajan S, Garagusi KJ, Tan L, Guest JD, et
al: Antigenicity and immunogenicity of differentially glycosylated
hepatitis C virus E2 envelope proteins expressed in mammalian and
insect Cells. J Virol. 93:e01403–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Naarding MA, Falkowska E, Xiao H and
Dragic T: Hepatitis C virus soluble E2 in combination with QuilA
and CpG ODN induces neutralizing antibodies in mice. Vaccine.
29:2910–2917. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Frey SE, Houghton M, Coates S, Abrignani
S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie AM, Rinella P, et
al: Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with
MF59 administered to healthy adults. Vaccine. 28:6367–6373.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wedemeyer H, Schuller E, Schlaphoff V,
Stauber RE, Wiegand J, Schiefke I, Firbas C, Jilma B, Thursz M,
Zeuzem S, et al: Therapeutic vaccine IC41 as late add-on to
standard treatment in patients with chronic hepatitis C. Vaccine.
27:5142–5151. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sarobe P, Pendieton CD, Akatsuka T, Lau D,
Engelhard VH, Feinstone SM and Berzofsky JA: Enhanced in vitro
potency and in vivo immunogenicity of a CTL epitope from hepatitis
C virus core protein following amino acid replacement at secondary
HLA-A2.1 binding positions. J Clin Invest. 102:1239–1248.
1998.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Filskov J, Mikkelsen M, Hansen PR,
Christensen JP, Thomsen AR, Andersen P, Bukh J and Agger EM:
Broadening CD4+ and CD8+ T cell responses
against hepatitis C virus by vaccination with NS3 overlapping
peptide panels in cross-priming liposomes. J Virol. 91:e00130–17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Filskov J, Andersen P, Agger EM and Bukh
J: HCV p7 as a novel vaccine-target inducing multifunctional
CD4+ and CD8+ T-cells targeting liver cells
expressing the viral antigen. Sci Rep. 9(14085)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Firbas C, Boehm T, Buerger V, Schuller E,
Sabarth N, Jilma B and Klade CS: Immunogenicity and safety of
different injection routes and schedules of IC41, a Hepatitis C
virus (HCV) peptide vaccine. Vaccine. 28:2397–2407. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Firbas C, Jilma B, Tauber E, Buerger V,
Jelovcan S, Lingnau K, Buschle M, Frisch J and Klade CS:
Immunogenicity and safety of a novel therapeutic hepatitis C virus
(HCV) peptide vaccine: A randomized, placebo controlled trial for
dose optimization in 128 healthy subjects. Vaccine. 24:4343–4353.
2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Klade CS, Wedemeyer H, Berg T, Hinrichsen
H, Cholewinska G, Zeuzem S, Blum H, Buschle M, Jelovcan S, Buerger
V, et al: Therapeutic vaccination of chronic hepatitis C
nonresponder patients with the peptide vaccine IC41.
Gastroenterology. 134:1385–1395. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yin D, Qi ZT, Yan S, Ding H, Hua X, Tong
YM, Wang YZ, Liu Y, Xu FH and Zhao P: Study on the induction of
mouse neutralization antibodies to HCVpp by HCV E2 DNA vaccines.
Chin J Exp Clin Infect Dis (Electronic Version). 2:24–32. 2008.(In
Chinese).
|
|
46
|
National Institute of Allergy and
Infectious Diseases (NIH): Trial Evaluating Experimental Hepatitis
C Vaccine Concludes. NIH, Bethesda, MD, 2019. https://www.niaid.nih.gov/news-events/trial-evaluating-experimental-hepatitis-c-vaccine-concludes.
Accessed July 8, 2021.
|
|
47
|
Shin EC, Sung PS and Park SH: Immune
responses and immunopathology in acute and chronic viral hepatitis.
Nat Rev Immunol. 16:509–523. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lu L, Barbi J and Pan F: The regulation of
immune tolerance by FOXP3. Nat Rev Immunol. 17:703–717.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Langhans B, Nischalke HD, Krämer B, Hausen
A, Dold L, van Heteren P, Hüneburg R, Nattermann J, Strassburg CP
and Spengler U: Increased peripheral CD4+ regulatory T
cells persist after successful direct-acting antiviral treatment of
chronic hepatitis C. J Hepatol. 66:888–896. 2017.PubMed/NCBI View Article : Google Scholar : (In Albanian,
English).
|
|
50
|
Han JW, Sung PS, Hong SH, Lee H, Koh JY,
Lee H, White S, Maslow JN, Weiner DB, Park SH, et al:
IFNL3-adjuvanted HCV DNA vaccine reduces regulatory T-cell
frequency and increases virus-specific T-cell responses. J Hepatol.
73:72–83. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wijesundara DK, Gummow J, Li Y, Yu W, Quah
BJ, Ranasinghe C, Torresi J, Gowans EJ and Grubor-Bauk B: Induction
of genotype cross-reactive, hepatitis C virus-specific,
cell-mediated immunity in DNA-vaccinated mice. J Virol.
92:e02133–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Castellanos M, Cinza Z, Dorta Z, Veliz G,
Vega H, Lorenzo I, Ojeda S, Dueñas-Carrera S, Alvarez-Lajonchere L,
Martínez G, et al: Immunization with a DNA vaccine candidate in
chronic hepatitis C patients is safe, well tolerated and does not
impair immune response induction after anti-hepatitis B
vaccination. J Gene Med. 12:107–116. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pierce BG, Boucher EN, Piepenbrink KH,
Ejemel M, Rapp CA, Thomas WD Jr, Sundberg EJ, Weng Z and Wang Y:
Structure-based design of hepatitis C virus vaccines that elicit
neutralizing antibody responses to a conserved epitope. J Virol.
91:e01032–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cowton VM, Owsianka AM, Fadda V,
Ortega-Prieto AM, Cole SJ, Potter JA, Skelton JK, Jeffrey N, Di
Lorenzo C, Dorner M, et al: Development of a structural epitope
mimic: An idiotypic approach to HCV vaccine design. NPJ Vaccines.
6(7)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Choo QL, Kuo G, Ralston R, Weiner A, Chien
D, Van Nest G, Han K, Berger K, Thudium K, Kuo C, et al:
Vaccination of chimpanzees against infection by the hepatitis C
virus. Proc Natl Acad Sci USA. 91:1294–1298. 1994.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Rosa D, Campagnoli S, Moretto C, Guenzi E,
Cousens L, Chin M, Dong C, Weiner AJ, Lau JY, Choo QL, et al: A
quantitative test to estimate neutralizing antibodies to the
hepatitis C virus: Cytofluorimetric assessment of envelope
glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA.
93:1759–1763. 1996.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shi X, Cao F, Du Y, Hou L, Ji Y and Wang
H: Protein expression and antigenicity detection of Hepatitis C
virus envelope protein E2. Medical Journal of Chinese People's
Liberation Army. 27:17–19. 2002.
|
|
58
|
Dai Z, Li G, Xu Q, Yao L, Han X, Yang L
and Chen W: Expression and Identification of Hepatitis C Virus
Envelop Protein E2. J Sun Yat-Sen Univ (Med Sci). 27:83–89.
2006.(In Chinese).
|
|
59
|
Earnest-Silveira L, Chua B, Chin R,
Christiansen D, Johnson D, Herrmann S, Ralph SA, Vercauteren K,
Mesalam A, Meuleman P, et al: Characterization of a hepatitis C
virus-like particle vaccine produced in a human hepatocyte-derived
cell line. J Gen Virol. 97:1865–1876. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Earnest-Silveira L, Christiansen D,
Herrmann S, Ralph SA, Das V, Gowans EJ and Torres J: Large scale
production of a mammalian cell derived quadrivalent hepatitis C
virus like particle vaccine. J Virol Methods. 236:87–92.
2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Shu F, Lei YF, Lin F, Wang X, Li B, Zhang
LJ, Dong K, Zhang HZ and Wei SH: Construction of eukaryotic
expression vector for chimeric gene of hepatitis C virus
neutralizing epitopes with hepatitis B virus S antigen and its
expression in 293T cells. Chinese Journal of Biologicals.
26:795–799. 2013.
|
|
62
|
Martínez-Donato G, Capdesuñer Y,
Acosta-Rivero N, Rodríguez A, Morales-Grillo J, Martínez E,
González M, Alvarez-Obregon JC and Dueñas-Carrera S: Multimeric HCV
E2 protein obtained from Pichia pastoris cells induces a strong
immune response in mice. Mol Biotechnol. 35:225–235.
2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yagnik AT, Lahm A, Meola A, Roccasecca RM,
Ercole BB, Nicosia A and Tramontano A: A model for the hepatitis C
virus envelope glycoprotein E2. Proteins. 40:355–366.
2000.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Duvet S, Cocquerel L, Pillez A, Cacan R,
Verbert A, Moradpour D, Eychoeski C and Dubuisson J: Hepatitis C
virus glycoprotein complex localization in the endoplasmic
reticulum involves a determinant for retention and not retrieval. J
Biol Chem. 273:32088–32095. 1998.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Nemchinov LG, Liang TJ, Rifaat MM, Mazyad
HM, Hadidi A and Keith JM: Development of a plant-derived subunit
vaccine candidate against hepatitis C virus. Arch Virol.
145:2557–2573. 2000.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bolhassani A, Davoudi N, Motevalli F and
Agi E: Comparison of HCV Core and CoreE1E2 virus-like particles
generated by stably transfected leishmania tarentolae for the
Stimulation of Th1 immune responses in mice. Curr Drug Deliv.
14:1040–1049. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kunkel M, Lorinczi M, Rijnbrand R, Lemon
SM and Watowich SJ: Self-assembly of nucleocapsid-like particles
from recombinant hepatitis C virus core protein. J Virol.
75:2119–2129. 2001.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Tarr AW, Backx M, Hamed MR, Urbanowicz RA,
McClure CP, Brown RJP and Ball JK: Immunization with a synthetic
consensus hepatitis C virus E2 glycoprotein ectodomain elicits
virus-neutralizing antibodies. Antivir Res. 160:25–37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Cao L, Yu B, Kong D, Cong Q, Yu T, Chen Z,
Hu Z, Chang H, Zhong J, Baker D and He Y: Functional expression and
characterization of the envelope glycoprotein E1E2 heterodimer of
hepatitis C virus. PLoS Pathog. 15(e1007759)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Fazlalipour M, Keyvani H, Monavari SH and
Mollaie HR: Expression, purification and immunogenic description of
a hepatitis C virus recombinant CoreE1E2 protein expressed by yeast
Pichia pastoris. Jundishapur J Microbiol. 8(e17157)2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Pechelyulko A, Andreeva-Kovalevskaya Z,
Dmitriev D, Lavrov V, Massino Y, Nagel A, Segal O, Sokolova OS,
Solonin A, Tarakanova Y and Dmitriev A: A simple method to purify
recombinant HCV core protein expressed in Pichia pastoris for
obtaining virus-like particles and producing monoclonal antibodies.
Protein Expr Purif. 183(105864)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Cai W, Su L, Liao Q, Ye L, Wu Y, Wu Z and
She Y: Expression, purification and immunogenic characterization of
hepatitis C virus recombinant E1E2 protein expressed by Pichia
pastoris yeast. Antivir Res. 88:80–85. 2010.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Martinez-Donato G, Acosta-Rivero N,
Morales-Grillo J, Musacchio A, Vina A, Alvarez C, Figueroa N,
Guerra I, Garcia J, Varas L, et al: Expression and processing of
hepatitis C virus structural proteins in Pichia pastoris yeast.
Biochem Biophys Res Commun. 342:625–631. 2006.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Yao M, Fang H, Yin W, Lei Y, Yang J, Sun
M, Tian J and Lv X: Prokaryotic expression and purification of
truncated Hepatitis C Virus envelope glycoprotein E2. Science
Technology and Engineering,. 9:2296–2303. 2009.
|
|
75
|
Cocquerel L, Meunier JC, Pillez A,
Wychowski C and Dubuisson J: A retention signal necessary and
sufficient for endoplasmic reticulum localization maps to the
transmembrane domain of hepatitis C virus glycoprotein E2. J Virol.
72:2183–2191. 1998.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cocquerel L, Wychowski C, Minner F, Penin
F and Dubuisson J: Charged residues in the transmembrane domains of
hepatitis C virus glycoproteins play a major role in the
processing, subcellular localization, and assembly of these
envelope proteins. J Virol. 74:3623–3633. 2000.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Matsuura Y, Suzuki T, Suzuki R, Sato M,
Aizaki H, Saito I and Miyamura T: Processing of E1 and E2
glycoproteins of hepatitis C virus expressed in mammalian and
insect cells. Virology. 205:141–150. 1994.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Mizushima H, Hijikata M, Asabe S, Hirota
M, Kimura K and Shimotohno K: Two hepatitis C virus glycoprotein E2
products with different C termini. J Virol. 68:6215–6222.
1994.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Morin TJ, Broering TJ, Leav BA, Blair BM,
Rowley KJ, Boucher EN, Wang Y, Cheslock PS, Knauber M, Olsen DB, et
al: Human monoclonal antibody HCV1 effectively prevents and treats
HCV infection in chimpanzees. PLoS Pathog.
8(e1002895)2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Impagliazzo A, Milder F, Kuipers H, Wagner
MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P,
Verspuij J, et al: A stable trimeric influenza hemagglutinin stem
as a broadly protective immunogen. Science. 349:1301–1306.
2015.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kulp DW, Steichen JM, Pauthner M, Hu X,
Schiffner T, Liguori A, Cottrell CA, Havenar-Daughton C, Ozorowski
G, Georgeson E, et al: Structure-based design of native-like HIV-1
envelope trimers to silence non-neutralizing epitopes and eliminate
CD4 binding. Nat Commun. 8(1655)2017.PubMed/NCBI View Article : Google Scholar
|