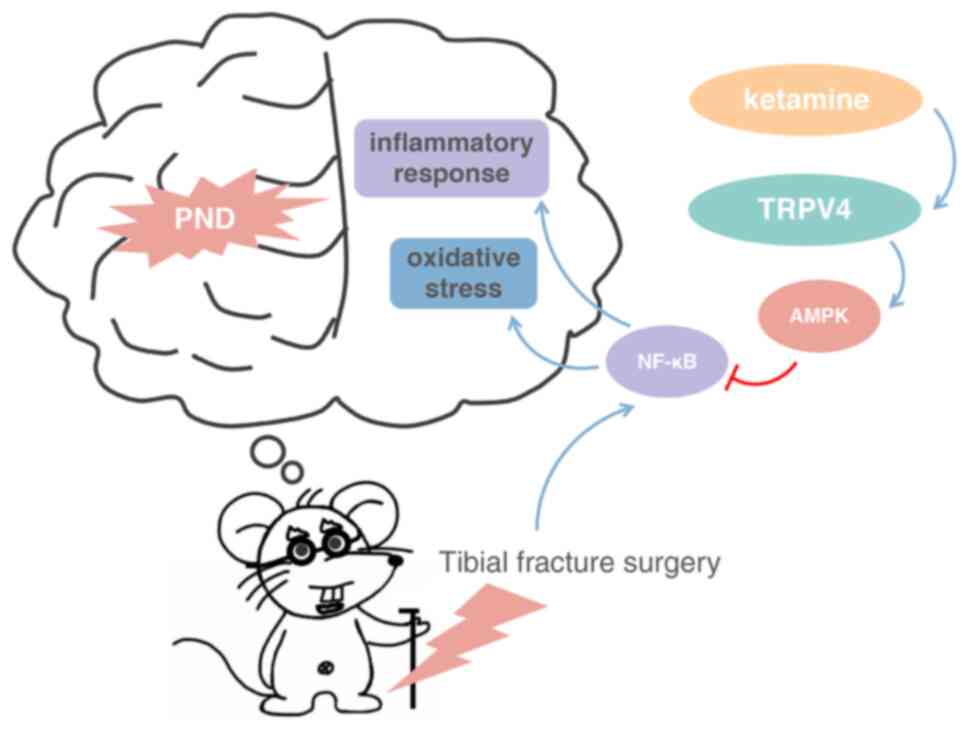
Introduction
Postoperative cognitive dysfunction (POCD) is a
complication of anesthesia and surgery prevalent in the elderly,
which is characterized by memory loss and cognitive deficiencies
(1,2). A study recommended that the clinical
terminology for cognitive dysfunction temporally relative to
surgery and/or anesthesia should be modified from POCD to
perioperative neurocognitive disorder (PND) (2). PNDs are usually associated with a
decrease in the quality of life in elderly patients (3,4). As
with other neurodegenerative diseases, including Alzheimer's, the
precise mechanisms and therapies of PND should be further
investigated.
In clinical practice, ketamine has been used to
induce short-term anesthesia and analgesia via inhibiting
N-methyl-D aspartic acid (NMDA) receptors (5). Notably, the use of ketamine in other
domains, including managing acute and chronic pain, and as an
antidepressant for mental disorders, has caused widespread concern
(6,7). Ketamine exerts neuroprotective
effects by decreasing the systemic production of inflammatory
factors induced by surgery (8) and
attenuates cognitive dysfunction in patients undergoing cardiac
surgery with a concomitant anti-inflammatory effect (9). Until now, the mechanisms underlying
the efficiency of ketamine in treating cognitive dysfunction have
not been extensively studied.
The transient receptor potential vanilloid 4 (TRPV4)
channel, which belongs to the mammalian transient receptor
potential superfamily of cation channels, has been reported to be
widely distributed in neurons and glial cells (10,11).
As a cell surface-expressed, non-selective cation channel, TRPV4
can be activated by chemical, mechanical and osmotic stimuli via
regulation of calcium ion influx (12). Notably, the inhibition of TRPV4
channel opening reportedly contributes to neuronal death and
inflammatory response in a model of intracerebral hemorrhage
(13), while activation of TRPV4
channel opening can participate in the regulation of neuronal
excitability and behavior in mammals (14). Most importantly, it has been
reported that silencing TRPV4 can block excessive ketamine-induced
neurotoxic effects (15). Based on
these findings, the present study explored whether the opening of
TRPV4 channels mediated ketamine-induced neuroprotection against
PND.
The present study established a tibial fracture
model and then investigated whether ketamine administration
ameliorated PND in aged mice after surgical treatment. In addition,
the role of TRPV4 channels in mediating the neuroprotective effects
of ketamine was determined.
Materials and methods
Experimental animals
A total of 120, 20-month-old adult male C57BL/6 mice
(weight, 35-40 g) were purchased from Changsheng Biotechnology Co.,
Ltd. As per a previous study, 20-month-old mice were considered as
aged mice (16). All mice were
allowed free access to food and water, and kept in a 12-h
light/dark cycle facility at 25±1˚C (humidity, 50-70%). All animal
procedures conformed to the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (17). The present study was approved by
the Animal Review Board of The Second Affiliated Hospital of
Jiaxing University (JXEY-2021JX122) (Jiaxing, China). In addition,
all experiments complied with the Animal Research: Reporting In
Vivo Experiments (ARRIVE) guidelines (18).
Group assignment
Male C57/BL mice were divided into one of the
following five groups according to a computer-based randomization
method (n=24/group): i) Vehicle; ii) PND; iii) PND + ketamine
(Ket); iv) PND + Ket + HC-067047 (HC); v) PND + HC. Mice in the PND
groups were subjected to tibial fracture surgery as described in
previous studies (19,20). Ketamine administration (0.5 mg/kg;
Gutian Fuxing Pharmaceutical Co., Ltd.) was performed once a day
for 3 days (the first injection from 2 h after surgical treatment)
as indicated by previous studies (20,21)
and our preliminary experiment. 2 µl HC-067047 (1 µmol, cat. no.
HY-100208; MedChemExpress), a selective antagonist of TRPV4, was
dissolved in 10% dimethyl sulfoxide (DMSO; cat. no. D8418; Beyotime
Institute of Biotechnology) and 90% corn oil (cat. no. HY-Y1888;
MedChemExpress) and injected into the left lateral ventricle at 30
min before ketamine administration. The co-ordinates of the left
lateral ventricle located using a stereotaxic instrument were as
follows: 0.3 mm posterior to the bregma, 1.0 mm lateral to the
midline and 2.5 mm below the skull. Vehicle (2 µl) for HC-067047
was administered with an equivalent volume of DMSO plus corn oil as
a control to all groups not treated with HC-067047. If mice lost
their appetite for 5 days or stopped drinking for 3 days, lost
>20% of their body weight, and were unable to eat and drink
before the scheduled experimental endpoint, they were sacrificed.
After mice were anesthetized with 7-8% sevoflurane for 5-7 min,
reflexes disappeared. When the monitor indicated cardiac arrest,
respiratory arrest and pupil dilation, the mice were euthanized by
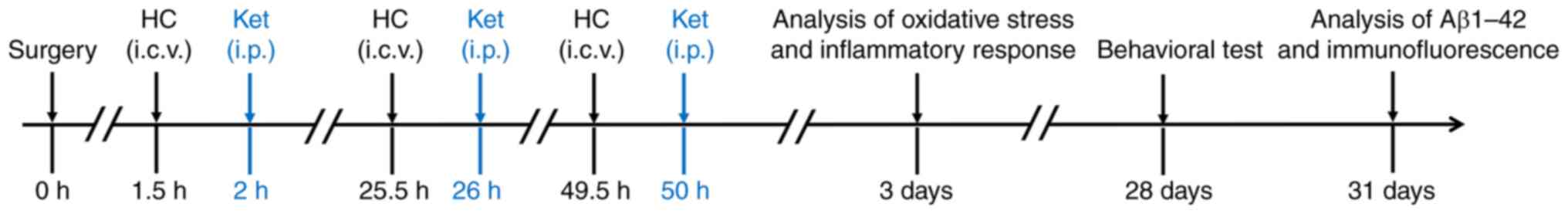
cervical dislocation. A total of 60 mice (n=12/group) were sampled
3 days after surgery to assess oxidative stress (n=6) and
inflammatory response (n=6) in the five groups. A total of 60 mice
(n=12/group) were assigned to behavioral tests 28 days after
surgery and sacrificed at day 31 for Aβ1-42 analysis (n=6) and
immunofluorescence (n=6) staining. The timeline of the experimental
procedure is shown in Fig. 1.
Tibial fracture surgery
Tibial fracture surgery was used to stimulate PND in
mice, as described by previous studies (22,23).
Mice were subjected to 7-8% sevoflurane in self-made inhalation
anesthesia boxes as anesthesia induction. Mice were then
anesthetized with 3-4% sevoflurane with a face mask for anesthesia
maintenance and kept warm at 37-38˚C with a warming pad; a heart
rate between 100 and 120 bpm was maintained. Tibial fracture
surgery was performed as follows: i) The left hind paw was shaved
and disinfected; ii) a 0.38-mm pin was inserted in the
intramedullary canal after making a median incision on the left
hind paw; iii) the periosteum was stripped with a periosteal
elevator; iv) the osteotomy was performed with scissors; v) the
median incision was sutured and disinfected; vi) the incision was
blocked by 1.0% ropivacaine (0.1 ml) for postoperative analgesia.
Under anesthesia, a skin incision, suture and incision block on the
left hind paw were performed in mice of the vehicle group.
Novel object recognition
The cognitive ability of mice was assessed via a
novel object recognition test (24). At days 28 and 29, after the tibial
fracture surgery, mice were placed in a 60x60x40-cm box with black
walls for 5 min/day. On day 30 after surgery, mice were exposed to
two identical cubic objects placed in the right and left corners of
the box until they had explored for a total of 5 min. During the
test phase, the right cubic object was replaced by a novel
spherical object; subsequently, the mice were allowed to probe the
two objects for 5 min. A video analysis system provided by Shanghai
Jiliang Software Science & Technology Co., Ltd. was used to
analyze the trajectory of mice. Cognitive ability was assessed
using recognition index (RI), which was calculated as follows:
RI=novel object exploration time/(novel object exploration time +
familiar object exploration time).
Fear conditioning
On day 28 after surgery, mice were assigned to a
fear conditioning test. The mice were kept in a dark chamber wiped
with 70% alcohol and subjected to three tone-foot shock pairings
(tone: 2,000 Hz; 80 dB; 60 sec; foot shock: 1 mA; 2 sec). After the
final foot shock, the animals were kept in the chamber for 60 sec
and then returned to the home cage. The next day, the mice were
exposed to the same chamber without tone-foot shock pairings for 3
min. After 2 h, the mice were placed in a novel chamber with a
different context (transparent walls and light) and smell (wiped
with 1% acetic acid) from the first test chamber. The freezing
behaviors with and without the tone stimulus were recorded for 3
min using a video analysis system (XR-XZ301; Shanghai XinRuan
Information Technology Co., Ltd.). The freezing behaviors,
including 3 min in the dark chamber (context-related) and 3 min in
the transparent chamber (tone-related), were used to assess the
rodent's memory.
Amyloid β1-42, IL-1β and IL-6
ELISA
The mice were deeply anesthetized with 8%
sevoflurane and perfused with heparin saline via the aorta. The
hippocampal tissues for ELISA, reverse transcription-quantitative
(RT-q) PCR and western blot analysis were isolated and homogenized.
Total protein was extracted using cell lysis buffer (cat. no.
P0013, Beyotime Institute of Biotechnology) and quantified by BCA
assay. Based on the manufacturer's instructions, the levels of
Aβ1-42 (cat. no. CS-ELISA2397; Chuntest Biotechnology Co., Ltd.),
IL-1β (cat. no. PI301; Beyotime Institute of Biotechnology) and
IL-6 (cat. no. EK0411; Wuhan Boster Biological Technology, Ltd.)
were analyzed using sandwich ELISA kits.
Immunofluorescence
Mice were anesthetized with 8% sevoflurane, and were
then perfused with heparin saline and 4% paraformaldehyde via the
aorta. Following fixing with 4% paraformaldehyde at room
temperature for 24 h, the cerebral tissue containing the
hippocampus was dehydrated with 50-90% ethanol at room temperature,
then the cerebral tissue was permeabilized in 50% xylene-ethanol at
room temperature for 30 min then in 50% xylene-paraffin at 60˚C for
15 min and embedded in paraffin. Subsequently, 4-µm
paraffin-embedded sections were dewaxed and rehydrated with
alcohol, incubated with 0.1% Triton X-100 for 30 min at room
temperature (cat. no. T8200; Beijing Solarbio Science &
Technology Co., Ltd.) and sealed with 5% standard bovine serum
(cat. no. A8010; Beijing Solarbio Science & Technology Co.,
Ltd.) for 1 h at room temperature. After rinsing with PBS, a
polyclonal goat anti-ionized calcium binding adaptor molecule 1
(Iba1) primary antibody (cat. no. ab5076; 1:400; Abcam) was used to
incubate the sections at 4˚C overnight. The secondary antibody
(Cy3-conjugated donkey anti-goat IgG; cat. no. A0502; 1:1,000;
Beyotime Institute of Biotechnology) was then used to incubate the
sections for 1 h after washing three times with PBS.
4',6-diamidino-2-phenylindole (DAPI; 10 µg/ml, cat. no. C1002;
Beyotime Institute of Biotechnology) was used to label nuclei.
Fluorescence images were captured by a fluorescence microscope
(DP70; Olympus Corporation), while the intensity and number were
analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Inc.).
Measurement of total superoxide
dismutase (SOD), malondialdehyde (MDA) and lipid peroxidation (LPO)
content
At 3 days following surgery, the total SOD activity,
and MDA and lipid LPO levels in the hippocampus were assessed using
a SOD assay kit (WST-8 method; cat. no. S0103; Beyotime Institute
of Biotechnology), an MDA assay kit (cat. no. S0131; Beyotime
Institute of Biotechnology) and an LPO kit (cat. no. JLC13854,
Gelatins; Jiang Lanchun), respectively, according to the
manufacturers' instructions.
Reverse transcription-quantitative
(RT-q) PCR
At 3 days following surgery, total RNA was extracted
from hippocampal tissues with Beyozol (cat. no. R0011; Beyotime
Institute of Biotechnology) and then reverse transcribed into cDNA
using the BeyoRT First Strand cDNA Synthesis kit (cat. no. D7166;
Beyotime Institute of Biotechnology) according to the
manufacturers' protocols. The RT-qPCR system (Step One; Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to assess the
reaction mixtures, which contained: 1 µl cDNA, 0.2 µl forward
primer, 0.2 µl reverse primer, 5 µl BeyoFast SYBR Green qPCR Mix
(cat. no. D7260; Beyotime Institute of Biotechnology) and 3.6 µl
nuclease-free water. The PCR amplification conditions were as
follows: 95˚C for 10 min and 45 cycles of 95˚C for 10 sec and 60˚C
for 1 min. The mRNA expression levels were normalized to β-actin
and calculated with the 2-ΔΔCq method (25). The sequence of primers used for
qPCR were: TNF-α, forward (F) 5'-GACCCTCACACTCAGATCATCTTCT-3' and
reverse (R) 5'-CCTCCACTTGGTGGTTTGCT-3'; IFN-β, F
5'-GCCCTCTCCATCGACTACAAG-3' and R 5'-AAGACATTCTGGAGCATCACTTG-3';
β-actin, F 5'-TTTGCAGCTCCTTCGTTGC-3' and R
5'-TCGTCATCCATGGCGAACT-3'.
Western blotting
At 3 days following surgery, total protein was
extracted using cell lysis buffer (cat. no. P0013, Beyotime
Institute of Biotechnology) and quantified by BCA assay. Protein
samples (30 µg) from hippocampal tissues were mixed with loading
buffer (cat. no. P0015; Beyotime Institute of Biotechnology),
boiled, separated by SDS-PAGE (12% gel) and transferred to a PVDF
membrane. Skimmed milk (5%) was used to block the PVDF membrane for
2 h at 25˚C. After washing three times with TBS-0.05% Tween (5
min/wash), the PVDF membrane was incubated with the following
primary antibodies: Monoclonal rabbit anti-phosphorylated
(p)-adenosine monophosphate-activated protein kinase (AMPK)
(Ser496; cat. no. AF2677; 1:500), monoclonal total AMPK (cat. no.
AF1627; 1:500), polyclonal p-NF-κB p65 (cat. no. AF5875; 1:500) and
polyclonal NF-κB p65 (cat. no. AF0246; 1:1,000) (all from Beyotime
Institute of Biotechnology) at 4˚C overnight. The next day, a
secondary antibody (HRP-labeled Goat Anti-Rabbit IgG; cat. no.
A0208, 1:2,000; Beyotime Institute of Biotechnology) was used to
incubate the PVDF membrane at 25˚C for 1 h. Following incubation
with BeyoECL Plus (cat. no. P0018; Beyotime Institute of
Biotechnology) for 5 min at 25˚C, protein bands were analyzed using
Image Lab software (version 6.0; Bio-Rad Laboratories, Inc.). GAPDH
(1:1,000; cat. no. K106389P; Beijing Solarbio Science &
Technology Co., Ltd.) was used as an internal reference.
Statistical analysis
Unless stated otherwise, all data were expressed as
mean ± SD and analyzed using GraphPad Prism 5 (GraphPad Software,
Inc.). One-way analysis of variance followed by Bonferroni's post
hoc test was used to compare the differences among groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Ketamine administration alleviates
tibial fracture surgery-induced cognitive dysfunction
A total of 120 mice were involved in this current
study and no mice were excluded or sacrificed prior to the
endpoint. Mice exposed to tibial fracture surgery exhibited less
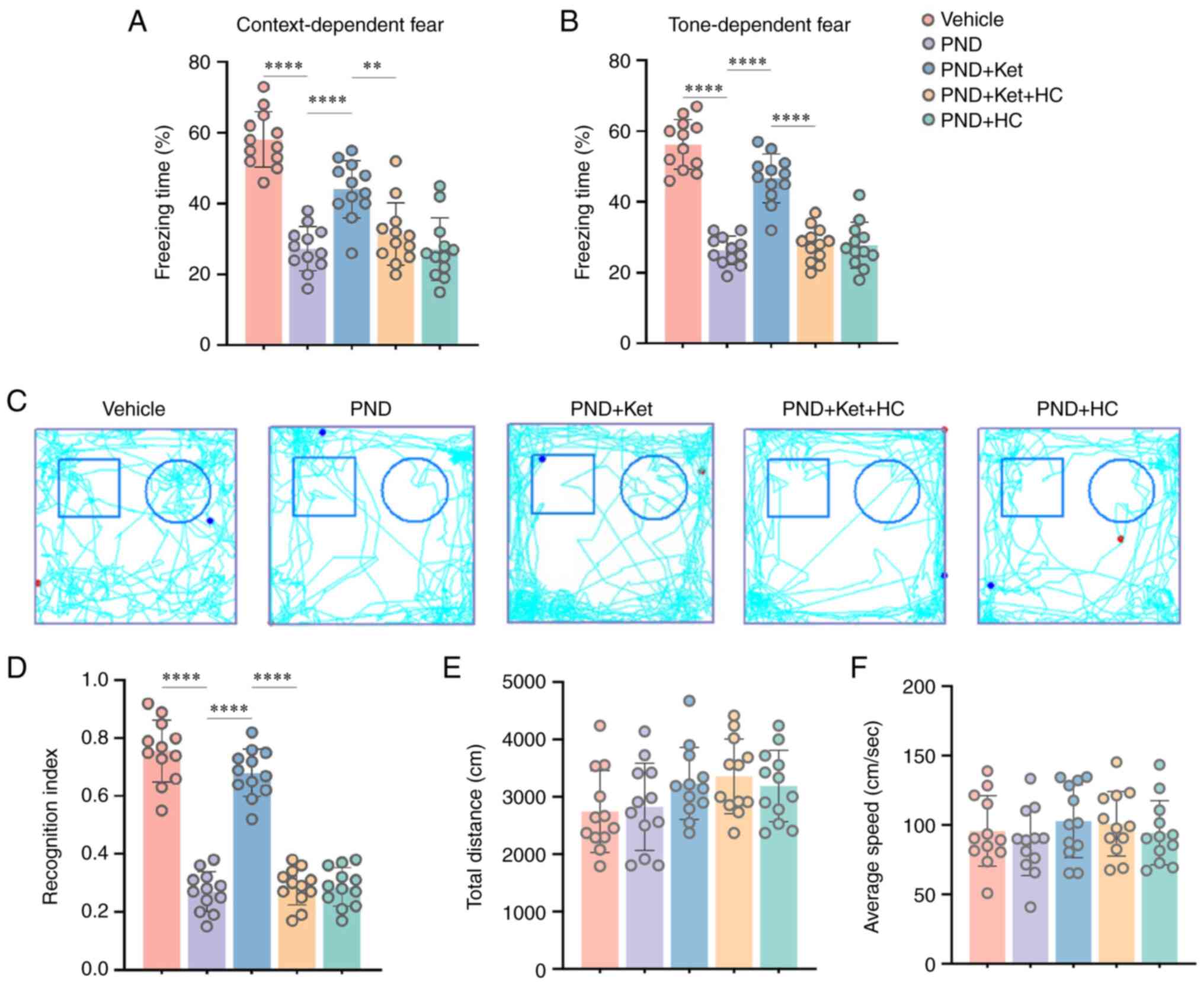
context-related (P<0.0001; Fig.
2A) and tone-related (P<0.0001; Fig. 2B) freezing behaviors in terms of
the total time spent motionless than vehicle-treated mice. However,
these context-related (Fig. 2A)
and tone-related (Fig. 2B)
freezing behaviors were significantly elevated after ketamine
administration (P<0.0001); however, HC-067047, an antagonist of
TRPV4 channels, could reverse these changes (P=0.0027 for
context-related; P<0.0001 for tone-related; Fig. 2A and B). There was no difference in the
context-related and tone-related freezing behaviors in aged mice
among the PND, PND + Ket + HC and PND + HC groups (Fig. 2A and B).
The novel object recognition test was performed
before the fear conditioning test since the emotional effect of
tone-foot shocks could influence the results of the former test
(26). It is acknowledged that
mice exhibit an innate tendency to explore new objects (16). Accordingly, the present study
evaluated the cognition and memory of mice models of tibial
fracture via the novel object recognition test. During the test
phase, the mice in the PND group showed a significantly decreased
RI following tibial fracture surgery compared with mice in the
vehicle group (P<0.0001; Fig.
2C and D). However, ketamine
administration significantly elevated the RI in the PND + Ket group
compared with in the PND group (P<0.0001; Fig. 2C and D); this change could be reversed by
HC-067047 to a certain extent (P<0.0001; Fig. 2C and D). No difference in RI was found in aged
mice among the PND, PND + Ket + HC and PND + HC groups (Fig. 2C and D). In addition, no significant difference
in the total distance and the average speed was found among the
above five groups (Fig. 2E and
F), which indicates no significant
motor deficits occurred at 29 days after tibial fracture
surgery.
Ketamine administration mitigates
neuronal degeneration and microglial activation after surgery
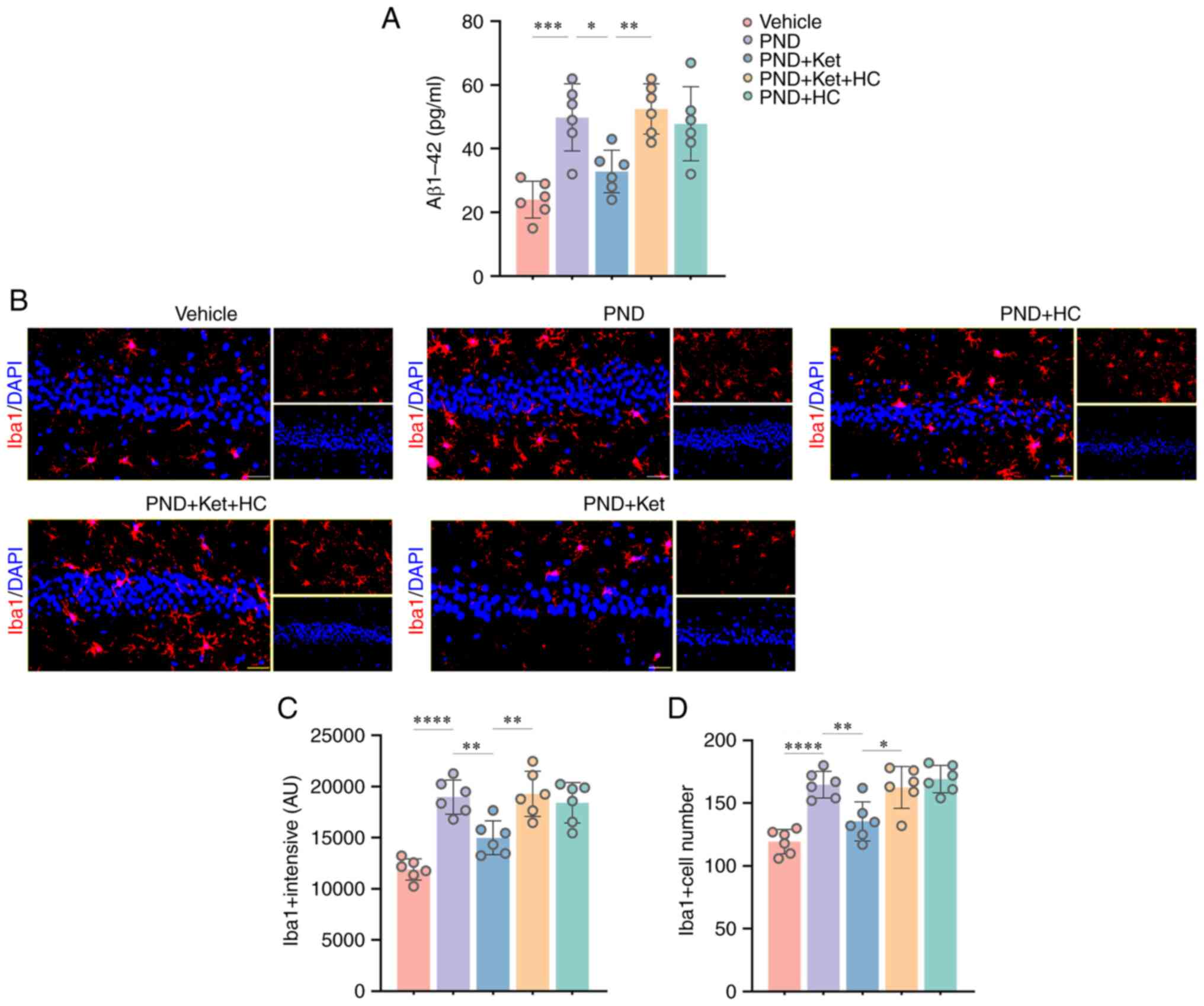
The transmembrane glycoprotein amyloid precursor
protein can be cleaved into Aβ peptides by enzymes β-secretase and
γ-secretase (27). Notably, Aβ
plaque formation has been associated with memory and cognitive
dysfunction (28). In the present
study, the results of an ELISA showed that Aβ1-42 levels were
significantly increased in mice that underwent surgery compared
with mice in the vehicle group (P=0.0003; Fig. 3A). Studies have shown that
microglial activation participates in the accumulation of Aβ plaque
and neuronal degeneration (29).
In the present study, the immunofluorescence results showed
increased microglial intensity (P<0.0001; Fig. 3B and C) and number (P<0.0001; Fig. 3B and D), measured by the number of
Iba1-positive microglia in the hippocampus of mice that underwent
surgery compared with the vehicle group. However, ketamine
administration in the PND + Ket group significantly attenuated
Aβ1-42 levels (P=0.0201; Fig. 3A)
and microglial activation intensity (P=0.0050; Fig. 3B and C) and number (P=0.0055; Fig. 3B and D), compared with mice in the PND group;
these changes could be reversed by HC-067047 in the PND + Ket + HC
group to a certain extent (for Aβ1-42, P=0.0057; for intensity,
P=0.0023; for number, P=0.0111) (Fig.
3B-D). In addition, there was no significant difference in
Aβ1-42 levels, microglial intensity and number in mice among the
PND, PND + Ket + HC and PND + HC groups.
Ketamine administration mitigates
oxidative stress and the inflammatory response in the early stage
after surgery
In the present study, the LPO and MDA contents were
determined as biomarkers of oxidative stress, whereas the total SOD
activity was assessed as an indicator of antioxidant status, as
described in the literature (30,31).
As shown in Fig. 4, compared with
in vehicle-treated mice, a significant reduction was observed in
the total SOD activity (P<0.0001; Fig. 4A), whereas the MDA (P<0.0001;
Fig. 4B) and LPO contents
(P<0.0001; Fig. 4C) were
increased 3 days after surgery. In addition, IL-1β and IL-6 have
been used to reflect neuronal inflammation in previous studies
(32,33). IL-1β (P<0.0001; Fig. 4D) and IL-6 levels (P<0.0001;
Fig. 4E) in the hippocampal tissue
were significantly upregulated 3 days following surgery. However,
compared with mice in the PND group, ketamine administration
significantly restored total SOD activity (P<0.0001; Fig. 4A), and reduced the MDA
(P<0.0001; Fig. 4B) and LPO
contents (P=0.0001; Fig. 4C), and
IL-1β (P=0.0118; Fig. 4D) and IL-6
levels (P=0.0013; Fig. 4E) in mice
in the PND + Ket group. Notably, HC-067047 partially reversed these
improvements, including total SOD (P<0.0001; Fig. 4A), MDA (P<0.0001; Fig. 4B), LPO (P=0.0005; Fig. 4C), IL-1β (P=0.0184; Fig. 4D) and IL-6 levels (P=0.0007;
Fig. 4E) in the PND + Ket + HC
group compared with in the PND + Ket group. In addition, there was
no significant difference in the aforementioned indexes in mice
among the PND, PND + Ket + HC and PND + HC groups.
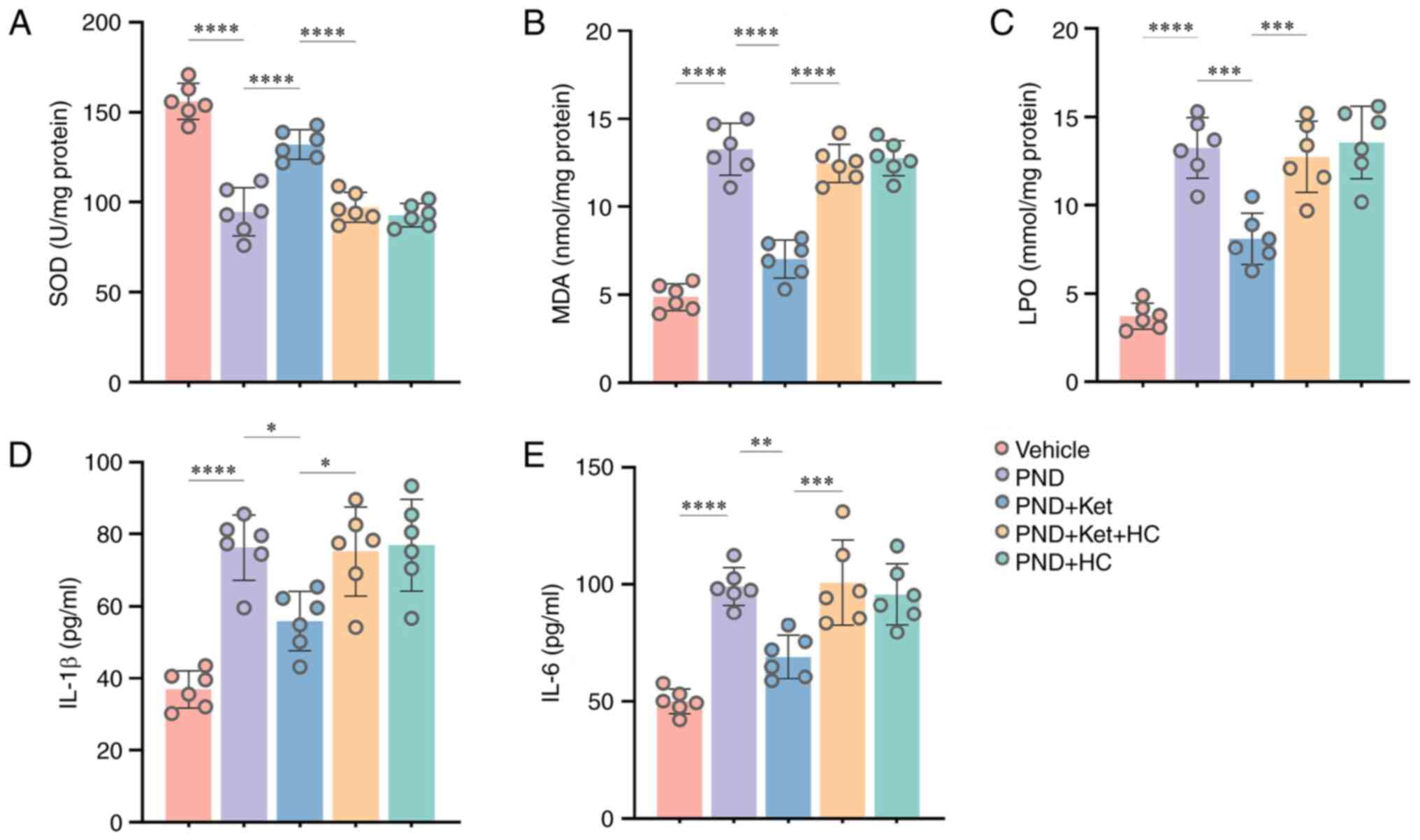 | Figure 4Ketamine administration attenuates
tibial fracture surgery-induced oxidative stress and inflammatory
response. Changes in the total (A) SOD, (B) MDA and (C) LPO content
in the hippocampal tissue 3 days post-PND. Changes in the (D) IL-1β
and (E) IL-6 levels in the hippocampal tissue 3 days post-PND. Data
are presented as the mean ± SD (n=6/group). *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. SOD, superoxide dismutase; MDA,
malondialdehyde; LPO, lipid peroxidation; PND, perioperative
neurocognitive disorder; Ket, ketamine; HC, HC-067047. |
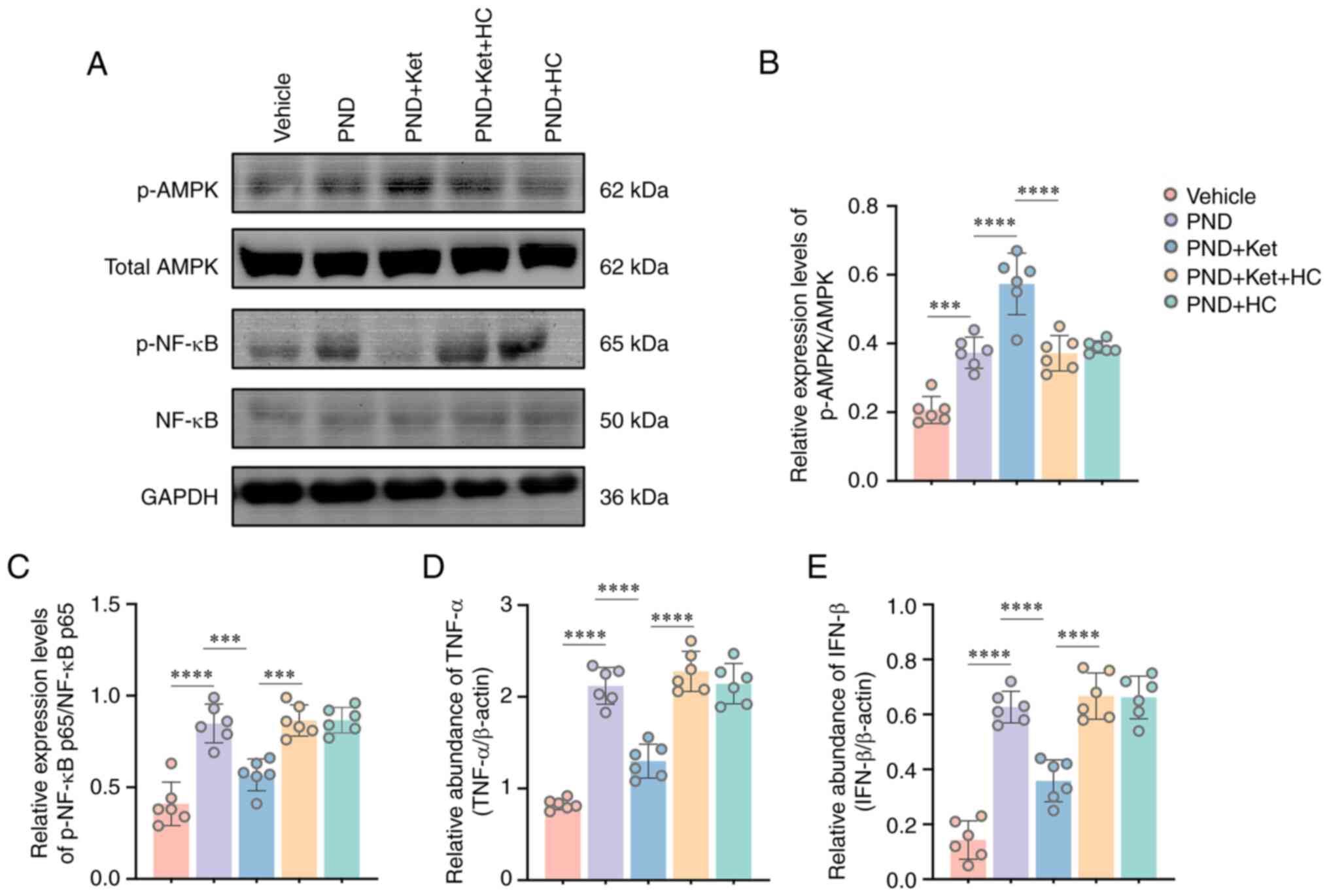
Protective effects of ketamine are
mediated by the AMPK/NF-κB signaling pathway following surgery
The protective effects induced by TRPV4 channel
opening have been associated with phosphorylation of AMPK and
inhibition of the NF-κB signaling pathway (34,35).
In the present study, the effect of surgery on individual proteins
in the AMPK/NF-κB signaling pathway was determined using western
blotting with pathway-specific antibodies. It was found that tibial
fracture surgery led to a significant increase in p-AMPK/total AMPK
ratio (P<0.0001; Fig. 5A and
B) and p-NF-κB p65 levels
(P<0.0001; Fig. 5A and C). Notably, ketamine administration
significantly enhanced AMPK phosphorylation (P<0.0001; Fig. 5A and B) and attenuated p-NF-κB p65 expression
in the PND + Ket group compared with in the PND group (P=0.0002;
Fig. 5A and C). Conversely, HC-067047 treatment
induced a significant decrease in p-AMPK/total AMPK ratio
(P<0.0001; Fig. 5A and B), but a significant increase in p-NF-κB
p65 was detected in mice in the PND + Ket + HC group compared with
in the PND + Ket group (P=0.0001; Fig.
5A and C). The expression
levels of the downstream inflammatory mediators of NF-κB, TNF-α and
IFN-β, were further explored by RT-Qpcr (36,37).
It was shown that tibial fracture surgery led to marked elevations
in TNF-α (P<0.0001; Fig. 5D)
and IFN-β mRNA expression (P<0.0001; Fig. 5E), whereas ketamine administration
significantly attenuated TNF-α (P<0.0001; Fig. 5D) and IFN-β (P<0.0001; Fig. 5E) mRNA expression levels in the PND
+ Ket group compared with in the PND group. By contrast,
significant increases in TNF-α (P<0.0001; Fig. 5D) and IFN-β (P<0.0001; Fig. 5E) mRNA expression levels were found
in mice in the PND + Ket + HC group compared with in the PND + Ket
group. In addition, there was no significant difference in p-AMPK,
p-NF-κB p65, TNF-α and IFN-β expression levels in mice among the
PND, PND + Ket + HC and PND + HC groups (Fig. 5B-E).
Discussion
The present study demonstrated that ketamine
administration alleviated tibial fracture surgery-induced cognitive
dysfunction by triggering TRPV4 channel opening, which
significantly increased the time spent exploring a novel object,
and context- and tone-related freezing behaviors, whereas Aβ1-42
levels and microglial activation were attenuated. Furthermore,
TRPV4 activation induced by ketamine administration decreased MDA
and LPO contents, as well as IL-1β and IL-6 levels, whereas total
SOD activity was increased. In addition, it was revealed that
p-AMPK levels were upregulated, whereas p-NF-κB p65 protein, and
TNF-α and IFN-β mRNA expression levels were downregulated at an
early stage following tibial fracture surgery. To a certain extent,
these changes could be reversed by HC-067047, an antagonist of
TRPV4 channel opening. Collectively, the present study documented
the pivotal role of the TPRV4/AMPK/NF-κB signaling pathway in
regulating oxidative stress and inflammatory response in PND
(Fig. 6).
The present study explored the mechanisms of PNDs in
a tibial fracture model established in aged mice. In the population
of older adult humans, total joint arthroplasty of the hip and knee
has become a common major surgery due to fracture or degeneration
(38). It has been demonstrated
that aged patients may still experience short- or long-term PND
after total joint arthroplasty (39-41).
A number of studies have demonstrated that tibial fracture surgery
in rodents can induce the physiopathologic process of PND,
subsequently inducing significant long-term effects on cognitive
dysfunction (19,42). Notably, the data of the present
study revealed that tibial fracture surgery triggered a significant
decline in cognition documented by decreased context- and
tone-related freezing behaviors, and attenuated the time spent
exploring a novel object, which is consistent with previous studies
(24,43). Once the brain is prone to disease,
systemic inflammation resulting from endotoxemia, including
lipopolysaccharide (LPS), can lead to an elevation in the
microglial activation, subsequently contributing to neuronal death
(44). There is overwhelming
evidence that clinically relevant tibial fracture surgery in rodent
models can cause memory dysfunction through induction of systemic
cytokine release and disruption of the blood-brain barrier (BBB),
which allows macrophages to migrate into the central nervous system
and shifts microglial morphology (45,46).
In addition, systemic inflammation induced by tibial fracture
surgery has been suggested to be a vital initiator of
neuroinflammation and delirium-like behavior (47). Notably, several studies have
suggested that PND following surgery may be associated with
neuronal degeneration and microglial activation (48,49).
In the present study, tibial fracture surgery significantly
increased Aβ1-42 levels and exacerbated microglial activation in
the hippocampus. These results suggested that cognitive dysfunction
was successfully triggered in the PND model, and neuronal
degeneration and microglial activation in the hippocampus were
related to PND.
Oxidative stress and inflammatory response have been
demonstrated to participate in the pathophysiology of PNDs. Several
studies have reported that following surgery, oxidative stress and
inflammatory response can lead to neuronal degeneration and
microglial activation (16,19,50).
Furthermore, it has been reported that proinflammatory cytokines,
such as IL-1β and IL-6, can lead to neuronal damage during ischemia
or infection (51-53).
Proteins, lipids and nucleic acids can be modified by free radicals
and lipid peroxidation following activation of the central nervous
system and the immune system under inflammatory conditions
(19). Given the high metabolic
rate and peroxidation induced by fatty acids and the low
antioxidant capacity, neurons are more susceptible to the
destructive effects of oxidative stress (54). In this regard, BBB dysfunction
following surgery reportedly facilitates entry of inflammatory
factors and oxidative species in the central nervous system,
resulting in microglial activation (55). In addition, lipid peroxidation
products, along with proinflammatory factors released from
activated microglia, form a toxic environment for neurons (56). Furthermore, the present study
demonstrated that oxidative stress (LPO and MDA) and inflammatory
factors (IL-1β and IL-6) were significantly increased at an early
stage following tibial fracture surgery. Recently, it was reported
that the occurrence of an inflammatory response in neurons
contributes to neuronal degeneration and cognitive dysfunction
following surgical exposure (57).
Besides neurons, glial cells, including astrocytes and microglia,
generally exhibit a quiescent phenotype under normal physiological
conditions and can be activated after peripheral surgery and
secrete appreciable levels of pro-inflammatory cytokines within the
brain (58). An increasing body of
evidence has suggested that postoperative astrocytic and microglial
activation participate in oxidative stress at the early stage of
PND (47,59,60).
In addition, mast cells, vital sentinel cells for host defense
against selected pathogens, are typically found in the choroid
plexus, meninges and the brain side of the BBB (61). Notably, there is a definite
communication between neurons, glia and mast cells in the process
of PND (62). It is suggested that
a marked attenuation in BBB permeability can be induced through
pharmacological inhibition of mast cells, which contributes to
neuroinflammation under PND (63).
Although which cell types in the brain exhibit changes in oxidative
stress and inflammatory response should be further explored, these
findings suggested that oxidative stress and inflammatory response
may participate in cognitive dysfunction after tibial fracture
surgery.
Ketamine has been reported to act as a
neuroprotective agent that can inhibit the inflammatory response,
oxidative stress and cellular dysfunction (64-66).
It was suggested that ketamine could attenuate LPS-induced injury
in BV2 cells via inhibiting NMDA receptors, and reducing
Ca2+ levels and NF-κB phosphorylation (67). Under hypoxic conditions, ketamine
can attenuate inflammatory pathways, mirrored by decreased IL-6 and
IL-1β levels (68). In addition,
ketamine may suppress oxidative stress via activation of Nrf2 and
its downstream proteins in a rodent model of traumatic brain
injury, thus exhibiting neuroprotective effects (64). The results of the present study
indicated that both AMPK phosphorylation and NF-κB phosphorylation
were increased after surgery. The mechanism may be associated with
NF-κB phosphorylation can lead to AMPK phosphorylation (69). It has been suggested that ketamine
can exert a rapid antidepressive effect via the phosphorylation of
AMPK and its downstream proteins, including brain-derived
neurotrophic factor (70). In
addition, studies have shown that AMPK phosphorylation exerts an
excellent analgesic effect by inhibiting the NF-κB signaling
pathway (71) and ketamine
administration suppresses endotoxin-induced NF-κB phosphorylation
both in vivo and in vitro (72). The results of the present study
indicated that ketamine administration significantly increased AMPK
phosphorylation, but decreased p-NF-κB p65, as well as the
expression levels of the downstream mediators TNF-α and IFN-β.
These results revealed that the ketamine-induced improvement in
cognitive dysfunction may be mediated by suppressing the AMPK/NF-κB
signaling pathway.
TRPV4 channels have been reported to be chemical,
mechanical stimuli and osmotic sensors, and changes in the internal
environment can induce TRPV4 activation (73,74).
TRPV4 can sense mechanical stimulation, eicosanoid metabolites and
cell swelling, and then can adjust its opening state (75). Studies have demonstrated that
internal environment changes, such as oxidative stress and
inflammation, can activate TRPV4 channels opening (12,76,77).
The present study showed that treatment with an antagonist of TRPV4
channels reversed the inhibition of oxidative stress and
inflammatory response in mice administered with ketamine after
tibial fracture surgery. In addition, the antagonist significantly
attenuated ketamine-induced inhibition of AMPK/NF-κB signaling
following tibial fracture surgery. Moreover, the antagonist of
TRPV4 channel opening abolished the improvement in cognitive
dysfunction. The current data only showed the pivotal role of the
TPRV4/AMPK/NF-κB signaling pathway in regulating oxidative stress
and inflammatory response in PND through HC-067047, an antagonist
of TRPV4. However, there is a limitation in that inhibitors
specific to AMPK pathways, such as compound C and MRT199665, should
be further investigated to determine underlying AMPK signal
mechanisms of ketamine (78,79).
The present study indicated inhibition of TRPV4 channel opening may
be associated with the neuroprotective effects of ketamine under
PND conditions.
In conclusion, ketamine administration improved
cognitive dysfunction in mice that underwent tibial fracture
surgery, and the mechanism may involve inhibition of oxidative
stress and inflammatory response mediated by suppression of the
TRPV4/AMPK/NF-κB signaling pathway. Notwithstanding that the
present study provided compelling evidence that ketamine has huge
prospects for clinical application in PND treatment, further
studies are required to explore the underlying mechanisms.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Public Welfare
Research Plan of Jiaxing (grant no. 2021AD30098).
Availability of data and materials
All data generated and analyzed during this study
are included in this published article.
Authors' contributions
QL was responsible for methodology, funding
acquisition, data analysis and writing the original draft. YQT was
responsible for methodology, formal analysis and software. XWW was
responsible for design of methodology and formal analysis. DQP was
responsible for design of methodology and analysis of
immunofluorescence staining. YX was responsible for methodology and
formal analysis. DNZ was responsible for conceptualization,
supervision, writing, review and editing. QL and DNZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was carried out in compliance with
the ARRIVE guidelines. Experiments were performed according to
institutional and national guidelines, and were approved by the
Animal Ethics Committee of The Second Affiliated Hospital of
Jiaxing University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Evered LA and Silbert BS: Postoperative
cognitive dysfunction and noncardiac surgery. Anesth Analg.
127:496–505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Evered L, Silbert B, Knopman DS, Scott DA,
DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG,
et al: Recommendations for the nomenclature of cognitive change
associated with anaesthesia and surgery-2018. Br J Anaesth.
121:1005–1012. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Urits I, Orhurhu V, Jones M, Hoyt D, Seats
A and Viswanath O: Current perspectives on postoperative cognitive
dysfunction in the ageing population. Turk J Anaesthesiol Reanim.
47:439–447. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rundshagen I: Postoperative cognitive
dysfunction. Dtsch Arztebl Int. 111:119–125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haas DA and Harper DG: Ketamine: A review
of its pharmacologic properties and use in ambulatory anesthesia.
Anesth Prog. 39:61–68. 1992.PubMed/NCBI
|
|
6
|
Jonkman K, van de Donk T and Dahan A:
Ketamine for cancer pain: What is the evidence? Curr Opin Support
Palliat Care. 11:88–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Corriger A and Pickering G: Ketamine and
depression: A narrative review. Drug Des Devel Ther. 13:3051–3067.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ibrahim TH, Abdelrahman HS, Alharbi MA,
Zabani IA, Ismail MF and Kary H: Effect of ketamine on pro- and
anti-inflammatory cytokine response in paediatric cardiac surgery:
A prospective randomised controlled study. Indian J Anaesth.
61:549–555. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hudetz JA, Iqbal Z, Gandhi SD, Patterson
KM, Byrne AJ, Hudetz AG, Pagel PS and Warltier DC: Ketamine
attenuates post-operative cognitive dysfunction after cardiac
surgery. Acta Anaesthesiol Scand. 53:864–872. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Z, Zhou L, An D, Xu W, Wu C, Sha S,
Li Y, Zhu Y, Chen A, Du Y, et al: TRPV4-induced inflammatory
response is involved in neuronal death in pilocarpine model of
temporal lobe epilepsy in mice. Cell Death Dis.
10(386)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Woolums BM, McCray BA, Sung H, Tabuchi M,
Sullivan JM, Ruppell KT, Yang Y, Mamah C, Aisenberg WH,
Saavedra-Rivera PC, et al: TRPV4 disrupts mitochondrial transport
and causes axonal degeneration via a CaMKII-dependent elevation of
intracellular Ca(2). Nat Commun. 11(2679)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vriens J, Watanabe H, Janssens A,
Droogmans G, Voets T and Nilius B: Cell swelling, heat, and
chemical agonists use distinct pathways for the activation of the
cation channel TRPV4. Proc Natl Acad Sci USA. 101:396–401.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Asao Y, Tobori S, Kakae M, Nagayasu K,
Shibasaki K, Shirakawa H and Kaneko S: Transient receptor potential
vanilloid 4 agonist GSK1016790A improves neurological outcomes
after intracerebral hemorrhage in mice. Biochem Biophys Res Commun.
529:590–595. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shibasaki K, Sugio S, Takao K, Yamanaka A,
Miyakawa T, Tominaga M and Ishizaki Y: TRPV4 activation at the
physiological temperature is a critical determinant of neuronal
excitability and behavior. Pflugers Arch. 467:2495–2507.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang C, Si M and Zhou J: Silencing TRPV4
partially reverses the neurotoxic effects caused by excess
ketamine. J Toxicol Sci. 46:69–81. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Z, Liu T, Yin C, Li Y, Gao F, Yu L
and Wang Q: Electroacupuncture pretreatment ameliorates anesthesia
and surgery-induced cognitive dysfunction via activation of an
α7-nAChR signal in aged rats. Neuropsychiatr Dis Treat.
17:2599–2611. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stephenson W: Deficiencies in the national
institute of health's guidelines for the care and protection of
laboratory animals. J Med Philos. 18:375–388. 1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG: NC3Rs Reporting Guidelines Working Group: NC3Rs
reporting guidelines working group. Animal research: Reporting in
vivo experiments: The ARRIVE guidelines. J Gene Med. 12:561–563.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Netto MB, de Oliveira Junior AN, Goldim M,
Mathias K, Fileti ME, da Rosa N, Laurentino AO, de Farias BX, Costa
AB, Rezin GT, et al: Oxidative stress and mitochondrial dysfunction
contributes to postoperative cognitive dysfunction in elderly rats.
Brain Behav Immun. 73:661–669. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Feng X, Valdearcos M, Uchida Y, Lutrin D,
Maze M and Koliwad SK: Microglia mediate postoperative hippocampal
inflammation and cognitive decline in mice. JCI Insight.
2(e91229)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Amiri S, Haj-Mirzaian A, Amini-khoei H,
Momeny M, Shirzadian A, Rahimi-Balaei M, Zarrinrad G,
Ghazi-Khansari M, Azizi R, Dehpour AR and Mehr SE: NMDA receptor
antagonists attenuate the proconvulsant effect of juvenile social
isolation in male mice. Brain Res Bull. 121:158–168.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Berrino L, Oliva P, Massimo F, Aurilio C,
Maione S, Grella A and Rossi F: Antinociceptive effect in mice of
intraperitoneal N-methyl-D-aspartate receptor antagonists in the
formalin test. Eur J Pain. 7:131–137. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jiang XL, Gu XY, Zhou XX, Chen XM, Zhang
X, Yang YT, Qin Y, Shen L, Yu WF and Su DS: Intestinal
dysbacteriosis mediates the reference memory deficit induced by
anaesthesia/surgery in aged mice. Brain Behav Immun. 80:605–615.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Meng B, Li X, Lu B, Liu R, Yuan H, Zhai X,
Qin J, Chen Z, Zheng J and Chen J: The investigation of
hippocampus-dependent cognitive decline induced by
anesthesia/surgery in mice through integrated behavioral Z-scoring.
Front Behav Neurosci. 13(282)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Faria RS, Gutierres LF, Sobrinho FC, do
Vale Miranda I, Reis JD, Dias EV, Sartori CR and Moreira DAR:
Effects of the swimming exercise on the consolidation and
persistence of auditory and contextual fear memory. Neurosci Lett.
628:147–152. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun L, Zhou R, Yang G and Shi Y: Analysis
of 138 pathogenic mutations in presenilin-1 on the in vitro
production of Aβ42 and Aβ40 peptides by γ-secretase. Proc Natl Acad
Sci USA. 114:E476–E485. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tamano H and Takeda A: Is interaction of
amyloid β-peptides with metals involved in cognitive activity?
Metallomics. 7:1205–1212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Socodato R, Portugal CC, Canedo T,
Rodrigues A, Almeida TO, Henriques JF, Vaz SH, Magalhães J, Silva
CM, Baptista FI, et al: Microglia dysfunction caused by the loss of
rhoa disrupts neuronal physiology and leads to neurodegeneration.
Cell Rep. 31(107796)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koskenkorva-Frank TS, Weiss G, Koppenol WH
and Burckhardt S: The complex interplay of iron metabolism,
reactive oxygen species, and reactive nitrogen species: Insights
into the potential of various iron therapies to induce oxidative
and nitrosative stress. Free Radic Biol Med. 65:1174–1194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
El Tabaa MM, Sokkar SS, Ramadan ES, Abd El
Salam IZ and Zaid A: Neuroprotective role of Ginkgo biloba against
cognitive deficits associated with bisphenol a exposure: An animal
model study. Neurochem Int. 108:199–212. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang J, Yu C, Zhang X, Chen H, Dong J, Lu
W, Song Z and Zhou W: Porphyromonas gingivalis lipopolysaccharide
induces cognitive dysfunction, mediated by neuronal inflammation
via activation of the TLR4 signaling pathway in C57BL/6 mice. J
Neuroinflammation. 15(37)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Y, Zhang Y, Zheng X, Fang T, Yang X,
Luo X, Guo A, Newell KA, Huang XF and Yu Y: Galantamine improves
cognition, hippocampal inflammation, and synaptic plasticity
impairments induced by lipopolysaccharide in mice. J
Neuroinflammation. 15(112)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tian Y, Qi M, Wang Z, Wu C, Sun Z, Li Y,
Sha S, Du Y and Chen L and Chen L: Activation of transient receptor
potential vanilloid 4 impairs the dendritic arborization of newborn
neurons in the hippocampal dentate gyrus through the AMPK and Akt
signaling pathways. Front Mol Neurosci. 10(190)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hattori K, Takahashi N, Terabe K, Ohashi
Y, Kishimoto K, Yokota Y, Suzuki M, Kojima T and Imagama S:
Activation of transient receptor potential vanilloid 4 protects
articular cartilage against inflammatory responses via
CaMKK/AMPK/NF-κB signaling pathway. Sci Rep.
11(15508)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Feng J, Xu Y, Lin P, Peng X, Wang Y and
Zhang Z: Identification of IκBα in Japanese eel Anguilla japonica
that impairs the IKKα-dependent activation of NF-κB, AP1, and type
I IFN signaling pathways. Dev Comp Immunol.
122(104044)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang B, Wang PP, Hu KL, Li LN, Yu X, Lu Y
and Chang HS: Antidepressant-like effect and mechanism of action of
honokiol on the mouse lipopolysaccharide (LPS) depression model.
Molecules. 24(2035)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Harris WH and Sledge CB: Total hip and
total knee replacement (1). N Engl J Med. 323:725–731.
1990.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ancelin ML, de Roquefeuil G, Scali J,
Bonnel F, Adam JF, Cheminal JC, Cristol JP, Dupuy AM, Carrière I
and Ritchie K: Long-term post-operative cognitive decline in the
elderly: the effects of anesthesia type, apolipoprotein E genotype,
and clinical antecedents. J Alzheimers Dis. 22 (Suppl 3):S105–S113.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Evered L, Scott DA, Silbert B and Maruff
P: Postoperative cognitive dysfunction is independent of type of
surgery and anesthetic. Anesth Analg. 112:1179–1185.
2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Williams-Russo P, Sharrock NE, Mattis S,
Liguori GA, Mancuso C, Peterson MG, Hollenberg J, Ranawat C,
Salvati E and Sculco T: Randomized trial of hypotensive epidural
anesthesia in older adults. Anesthesiology. 91:926–935.
1999.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li PJ, Guo YQ, Ding PY, Liu RB, Deng F,
Feng XX and Yan WJ: Neuroprotective effects of a Smoothened
receptor agonist against postoperative cognitive dysfunction by
promoting autophagy in the dentate gyrus of aged rats. Neurol Res.
41:867–874. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang X, Jiang X, Huang L, Tian W, Chen X,
Gu X, Yu W, Tian J and Su D: Central cholinergic system mediates
working memory deficit induced by anesthesia/surgery in adult mice.
Brain Behav. 8(e00957)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cibelli M, Fidalgo AR, Terrando N, Ma D,
Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS
and Maze M: Role of interleukin-1beta in postoperative cognitive
dysfunction. Ann Neurol. 68:360–368. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang P, Velagapudi R, Kong C, Rodriguiz
RM, Wetsel WC, Yang T, Berger M, Gelbard HA, Colton CA and Terrando
N: Neurovascular and immune mechanisms that regulate postoperative
delirium superimposed on dementia. Alzheimers Dement. 16:734–749.
2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Subramaniyan S and Terrando N:
Neuroinflammation and perioperative neurocognitive disorders.
Anesth Analg. 128:781–788. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu Q, Sun YM, Huang H, Chen C, Wan J, Ma
LH, Sun YY, Miao HH and Wu YQ: Sirtuin 3 protects against
anesthesia/surgery-induced cognitive decline in aged mice by
suppressing hippocampal neuroinflammation. J Neuroinflammation.
18(41)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Guéniot L, Lepere V, De Medeiros GF,
Danckaert A, Flamant P, Dudal ML, Langeron O, Goossens PL, Chrétien
F and Jouvion G: Muscle injury induces postoperative cognitive
dysfunction. Sci Rep. 10(2768)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xu J, Dong H, Qian Q, Zhang X, Wang Y, Jin
W and Qian Y: Astrocyte-derived CCL2 participates in
surgery-induced cognitive dysfunction and neuroinflammation via
evoking microglia activation. Behav Brain Res. 332:145–153.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lai IK, Valdearcos M, Morioka K, Saxena S,
Feng X, Li R, Uchida Y, Lijun A, Li W, Pan J, et al: Blocking Kv1.3
potassium channels prevents postoperative neuroinflammation and
cognitive decline without impairing wound healing in mice. Br J
Anaesth. 125:298–307. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lambertsen KL, Finsen B and Clausen BH:
Post-stroke inflammation-target or tool for therapy? Acta
Neuropathol. 137:693–714. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Diaz-Cañestro C, Reiner MF, Bonetti NR,
Liberale L, Merlini M, Wüst P, Amstalden H, Briand-Schumacher S,
Semerano A and Giacalone G: AP-1 (Activated Protein-1)
transcription factor JunD regulates ischemia/reperfusion brain
damage via IL-1β (Interleukin-1β). Stroke. 50:469–477.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Patterson SL: Immune dysregulation and
cognitive vulnerability in the aging brain: Interactions of
microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology.
96:11–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang X and Michaelis EK: Selective
neuronal vulnerability to oxidative stress in the brain. Front
Aging Neurosci. 2(12)2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd
S, Lewis M, Marriott A, Moore EM, Morris G, Page RS and Gray L:
Post-operative cognitive dysfunction: An exploration of the
inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev.
84:116–133. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang X, Dong H, Li N, Zhang S, Sun J,
Zhang S and Qian Y: Activated brain mast cells contribute to
postoperative cognitive dysfunction by evoking microglia activation
and neuronal apoptosis. J Neuroinflammation. 13(127)2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yuan N, Wang X, Zhang Y, Kong L, Yuan L
and Ge Y: Intervention of NF-Κb signaling pathway and preventing
post-operative cognitive dysfunction as well as neuronal apoptosis.
Iran J Public Health. 51:124–132. 2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Konsman JP, Parnet P and Dantzer R:
Cytokine-induced sickness behaviour: Mechanisms and implications.
Trends Neurosci. 25:154–159. 2002.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Peng S, Li P, Liu P, Yan H, Wang J, Lu W,
Liu C and Zhou Y: Cistanches alleviates sevoflurane-induced
cognitive dysfunction by regulating PPAR-γ-dependent antioxidant
and anti-inflammatory in rats. J Cell Mol Med. 24:1345–1359.
2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Liu PR, Cao F, Zhang Y and Peng S:
Electroacupuncture reduces astrocyte number and oxidative stress in
aged rats with surgery-induced cognitive dysfunction. J Int Med
Res. 47:3860–3873. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Nelissen S, Lemmens E, Geurts N, Kramer P,
Maurer M, Hendriks J and Hendrix S: The role of mast cells in
neuroinflammation. Acta Neuropathol. 125:637–650. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Li N, Zhang X, Dong H, Hu Y and Qian Y:
Bidirectional relationship of mast cells-neurovascular unit
communication in neuroinflammation and its involvement in POCD.
Behav Brain Res. 322:60–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang S, Dong H, Zhang X, Li N, Sun J and
Qian Y: Cerebral mast cells contribute to postoperative cognitive
dysfunction by promoting blood brain barrier disruption. Behav
Brain Res. 298:158–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Liang J, Wu S, Xie W and He H: Ketamine
ameliorates oxidative stress-induced apoptosis in experimental
traumatic brain injury via the Nrf2 pathway. Drug Des Devel Ther.
12:845–853. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zanos P, Moaddel R, Morris PJ, Riggs LM,
Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ,
Zarate CA Jr and Gould TD: Ketamine and ketamine metabolite
pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev.
70:621–660. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Yang Y, Song Y, Zhang X, Zhao W, Ma T, Liu
Y, Ma P, Zhao Y and Zhang H: Ketamine relieves depression-like
behaviors induced by chronic postsurgical pain in rats through
anti-inflammatory, anti-oxidant effects and regulating BDNF
expression. Psychopharmacology (Berl). 237:1657–1669.
2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Lu Y, Ding X, Wu X and Huang S: Ketamine
inhibits LPS-mediated BV2 microglial inflammation via NMDA receptor
blockage. Fundam Clin Pharmacol. 34:229–237. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Li X, Wang J, Song X, Wu H, Guo P, Jin Z,
Wang C, Tang C, Wang Y and Zhang Z: Ketamine ameliorates
ischemia-reperfusion injury after liver autotransplantation by
suppressing activation of Kupffer cells in rats. Can J Physiol
Pharmacol. 96:886–892. 2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wang C, Gao Y, Zhang Z, Chi Q, Liu Y, Yang
L and Xu K: Safflower yellow alleviates osteoarthritis and prevents
inflammation by inhibiting PGE2 release and regulating
NF-κB/SIRT1/AMPK signaling pathways. Phytomedicine.
78(153305)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Xu SX, Zhou ZQ, Li XM, Ji MH, Zhang GF and
Yang JJ: The activation of adenosine monophosphate-activated
protein kinase in rat hippocampus contributes to the rapid
antidepressant effect of ketamine. Behav Brain Res. 253:305–309.
2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Xiang HC, Lin LX, Hu XF, Zhu H, Li HP,
Zhang RY, Hu L, Liu WT, Zhao YL, Shu Y, et al: AMPK activation
attenuates inflammatory pain through inhibiting NF-κB activation
and IL-1β expression. J Neuroinflammation. 16(34)2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Sakai T, Ichiyama T, Whitten CW, Giesecke
AH and Lipton JM: Ketamine suppresses endotoxin-induced NF-kappaB
expression. Can J Anaesth. 47:1019–1024. 2000.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Liedtke W and Kim C: Functionality of the
TRPV subfamily of TRP ion channels: Add mechano-TRP and osmo-TRP to
the lexicon! Cell Mol Life Sci. 62:2985–3001. 2005.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Suzuki T, Notomi T, Miyajima D, Mizoguchi
F, Hayata T, Nakamoto T, Hanyu R, Kamolratanakul P, Mizuno A,
Suzuki M, et al: Osteoblastic differentiation enhances expression
of TRPV4 that is required for calcium oscillation induced by
mechanical force. Bone. 54:172–178. 2013.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Liu L, Guo M, Lv X, Wang Z, Yang J, Li Y,
Yu F, Wen X, Feng L and Zhou T: Role of transient receptor
potential vanilloid 4 in vascular function. Front Mol Biosci.
8(677661)2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Dutta B, Arya RK, Goswami R, Alharbi MO,
Sharma S and Rahaman SO: Role of macrophage TRPV4 in inflammation.
Lab Invest. 100:178–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
De Logu F, Trevisan G, Marone IM, Coppi E,
Dalenogare DP, Titiz M, Marini M, Landini L, de Araujo DSM, Puma
SL, et al: Oxidative stress mediates thalidomide-induced pain by
targeting peripheral TRPA1 and central TRPV4. BMC Biol.
18(197)2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Liu X, Chhipa RR, Nakano I and Dasgupta B:
The AMPK inhibitor compound C is a potent AMPK-independent
antiglioma agent. Mol Cancer Ther. 13:596–605. 2014.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ross FA, Hawley SA, Auciello FR, Gowans
GJ, Atrih A, Lamont DJ and Hardie DG: Mechanisms of paradoxical
activation of AMPK by the kinase inhibitors SU6656 and sorafenib.
Cell Chem Biol. 24:813–824. 2017.PubMed/NCBI View Article : Google Scholar
|