Introduction
The incidence of drug-induced Parkinsonism (DIP)
increases with older age, and the most common cause is
antipsychotic medication (1). In
patients with schizophrenia who show exacerbation or progression of
Parkinsonism under treatment with antipsychotics, rare
neurodegenerative disorders may have to be considered in the
differential diagnosis of Parkinsonism (2).
Progressive supranuclear palsy (PSP) is a rare
progressive neurodegenerative disease characterized by vertical
supranuclear gaze palsy, pseudobulbar palsy, dysarthria, early
postural instability, and axial dystonia (3). An important advance was made in 2016
with the development of the new International Parkinson's and
Movement Disorder Society (MDS) Criteria for the Diagnosis of PSP
(4). In brief, essential features
of PSP are required for all patients based on MDS criteria, where
mandatory eligibility criteria indicate the presence of sporadic,
adult-onset and, gradually progressive disease-related symptoms.
Conversely, mandatory exclusion criteria indicate other
neurological disease such as Alzheimer's disease, dementia with
Lewy bodies, and severe leukoencephalopathy should be ruled out in
clinical and neuroimaging manifestation for any patients.
Furthermore, four core functional domains [i.e., ocular motor
dysfunction (O), postural instability (P), akinesia (A) and
cognitive dysfunction (C)] are proposed as diagnostic clinical
symptom of PSP. Each of functional domains is stratified into three
stages, which could contribute to diagnose different clinical
subtypes of PSP in clinical practice. For instance, the combination
of falls with vertical ocular motor dysfunction (O) and early onset
postural instability (P) is generally referred to as progressive
supranuclear palsy-Richardson's syndrome (PSP-RS) (4). The prevalence of PSP-RS is ~5-7 cases
per 100,000 individuals, the average age at onset in the mid-60s,
and the disease duration is ~6 years (5-7).
The pathological specificity of probable PSP is
95-100%, and the clinical distinction between genuine DIP and PSP
is likely identifiable (4).
However, in patients with newly developed DIP, the diagnosis of PSP
may be overlooked. This is because symptoms such as different
resting tremors [e.g., Parkinson's disease (PD)] on the left and
right sides, which are less likely to occur in DIP, are more
pronounced in PSP.
Here, we report a case involving a 64-year-old male
inpatient with schizophrenia who had received long-term treatment
with antipsychotic medication and likely developed PSP-RS.
Case report
The patient had no family history of psychiatric or
neurodegenerative diseases. In 1980, at 27 years of age, he first
presented with hallucination/delusions and was diagnosed with
schizophrenia on the basis of the DSM-3 criteria in our hospital.
In July 2002, at 49 years of age, he was admitted to the hospital
for the third time because of frequent insomnia, hallucinations, a
tendency to become excited, and increasing incidences of violence.
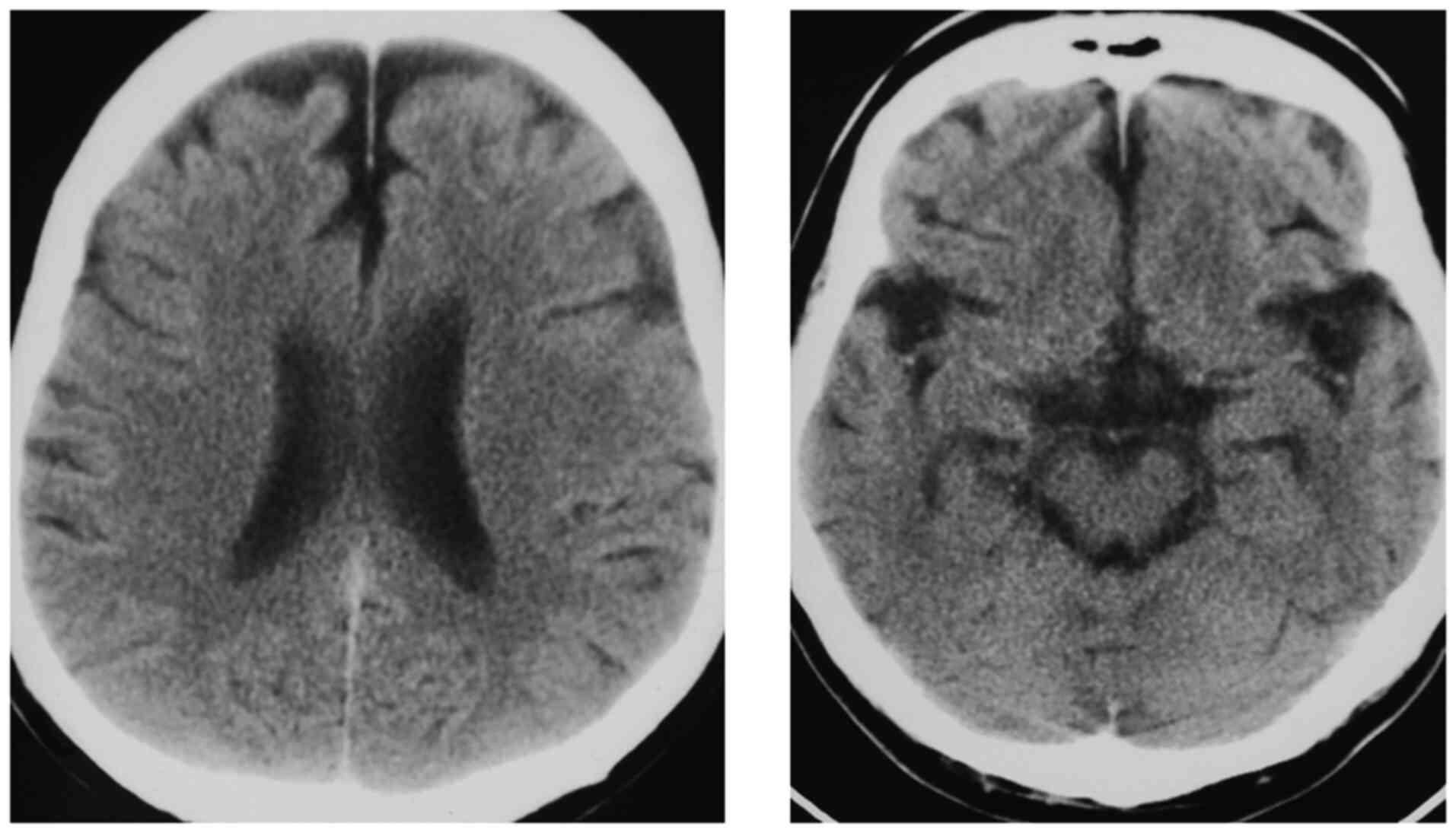
Head computed tomography at the time of admission showed no atrophy
of the midbrain tectum or frontal lobe (Fig. 1).
Since 2002, he received typical antipsychotic
medications, such as levomepromazine (125 mg) and haloperidol (4.5
mg), for ~8 years. However, he could not be discharged home due to
severe asociality, avolition, and the fact that he usually stayed
in bed throughout the day. Since 2010, his motility worsened under
management with atypical antipsychotic drugs [quetiapine (600 mg)
and olanzapine (20 mg)], although he was never prescribed
anticholinergic drugs.
In 2015, at 62 years of age, he began experiencing
short-term memory impairment and bradykinesia. In April 2016, he
showed increased dysphagia, upper-limb muscle rigidity, and
extrapyramidal symptoms (EPS) and began experiencing vision loss
and photophobia. However, his psychological symptoms included
chronic depression and irritability with bouts of euphoric mood,
which had never been observed before. DIP was suspected because he
began to cough while eating. Therefore, biperiden was prescribed at
a dosage of 2 mg/day starting in July 2016. However, his speech was
difficult to comprehend because of the sialorrhea. Moreover, his
inactivity, muscular rigidity, and slouching gait gradually
worsened, following which he began to use a walking aid. In
December 2016, he experienced repeated falls due to suspected DIP,
and risperidone (from 9 mg) and olanzapine (from 20 mg) were
gradually tapered off over 12 months. Although antipsychotic
medications were completely discontinued, he showed persistent
Parkinsonism symptoms with an apathetic mood, and he became mostly
wheelchair-dependent in his daily activities, during which he had
fallen at least five times. In December 2017, at 64 years of age,
he was referred to the neurology department, where he presented
with severe supranuclear vertical gaze palsy, retrocollis, mild
limb muscle rigidity with little laterality, and total akinesia.
Although standing was difficult due to severe retropulsion, no
rapid eye movement sleep behavior disorder, autonomic symptoms such
as orthostatic hypotension, illusion, or signs of upper and lower
motor neuron disorders were noted. Based on the MDS-PSP criteria,
this patient had presented severe supranuclear vertical gaze palsy
(O1) and repeated falls with severe retropulsion within 3 years
(P1) without gait freezing and speech disorder, which was most
likely to meet a key requirement for diagnosis of probable PSP-RS.
The bedside assessment of cognitive abilities using the Mini-Mental
State Examination score was 16/30 points, indicating moderate
cognitive decline. Further, while blood examination showed normal
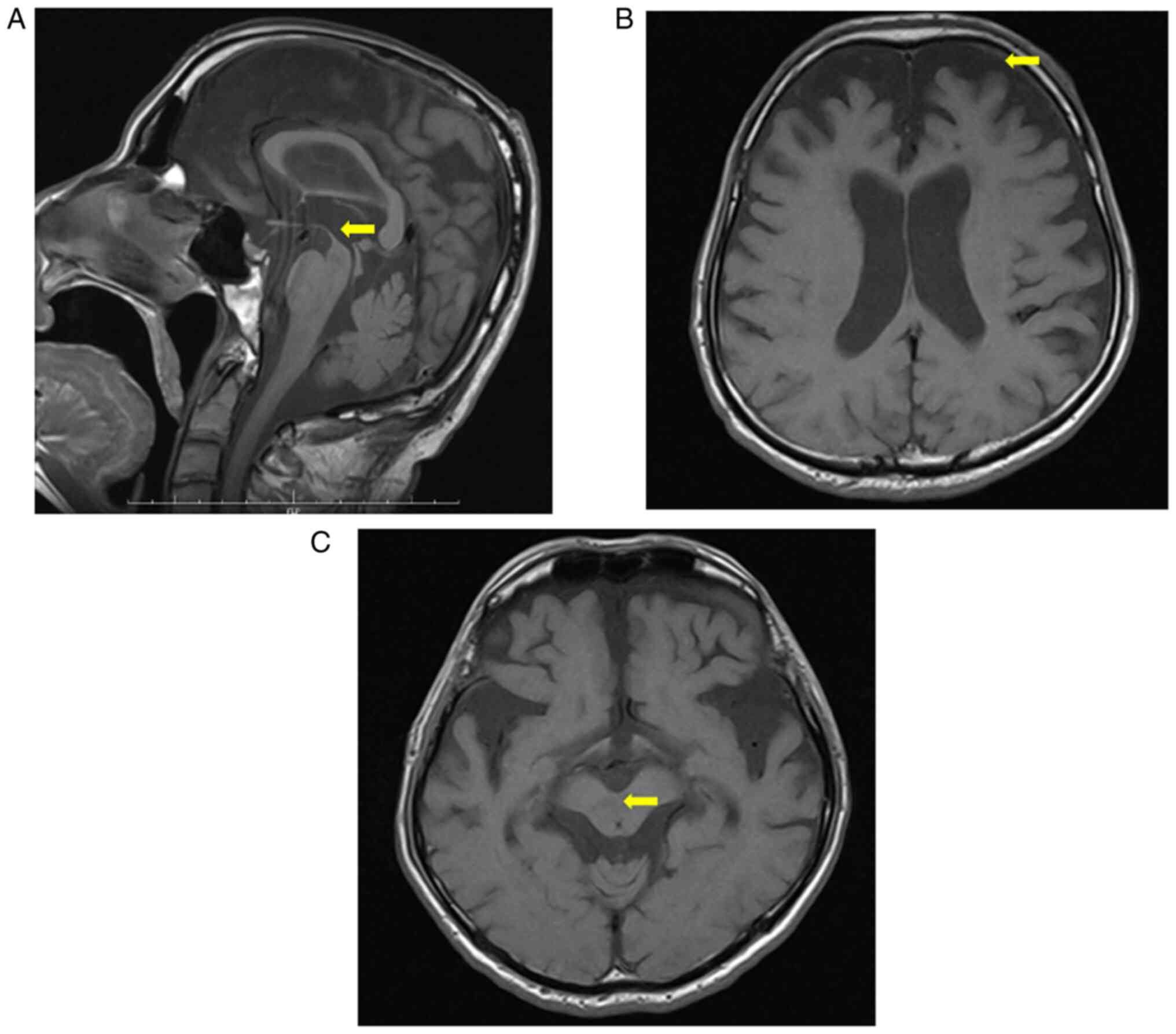
results, brain MRI showed features of PSP, such as atrophy of the
mesencephalic tegmentum (hummingbird sign) and atrophy of the
frontal lobe (Fig. 2A-C).
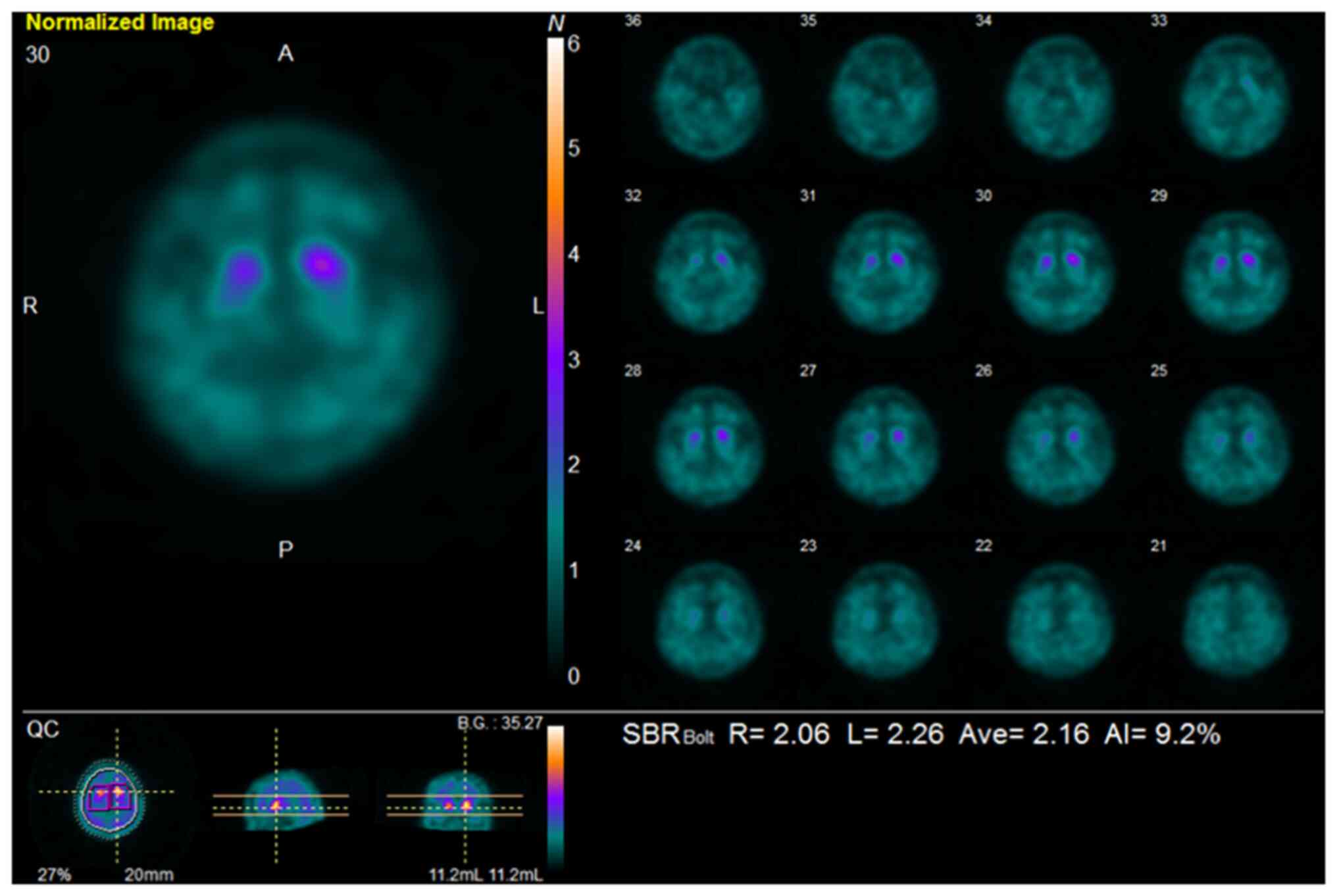
123I-ioflupane SPECT revealed decreased striatal uptake
(Fig. 3). On the basis of these
findings, the patient was finally diagnosed with probable PSP-RS by
a neurologist (4).
In March 2018, a swallowing test indicated the need
for an alternative mode of nutrition because of the aspiration of
saliva. In May 2018, because of repeated aspiration pneumonia and
hemophagocytic syndrome, the patient was transferred to another
medical hospital, following which he died soon from worsening
multi-organ failure.
Discussion
PSP is a rare neurodegenerative disorder. To our
knowledge, this is the first report of an older male patient with
chronic schizophrenia and probable PSP-RS.
In addition to the psychiatric symptoms of chronic
schizophrenia, our patient presented with EPS as an exacerbation of
PSP. Komatsu et al recently reported a case of multiple
system atrophy (MSA) in a 60-year-old female patient with chronic
schizophrenia who developed possible MSA with predominant
Parkinsonism (2). Her Parkinsonism
was reported to be unremedied, although antipsychotic medication
was switched from typical to atypical antipsychotics and tapered
off. In our case, a 64-year-old male patient developed PSP-RS with
chronic schizophrenia when his antipsychotics were discontinued for
several months. However, his Parkinsonism was exacerbated and he
was mostly wheelchair-dependent in his daily activities.
Parkinsonism that appears in patients taking antipsychotics for
chronic schizophrenia can be generally considered a drug-induced
syndrome. However, the combination of MRI and
123I-ioflupane SPECT plays a critical role in the
differential diagnosis of DIP in these cases, suggesting that the
comorbidity of schizophrenia and other degenerative disorders such
as PSP and MSA should be taken into consideration in clinical
practice.
In Boxer's previous review, the most frequently
reported symptoms of PSP-RS at onset were unexplained falls,
unsteady gait, bradykinesia, subtle personality changes (apathy,
disinhibition), cognitive slowing (bradyphenia), executive
dysfunction (difficulty planning or multitasking), slow, ataxic,
spastic, and hypophonic speech, dysphagia, and impaired ocular
movement (i.e., slowing of vertical saccades, difficulty reading,
or apraxia of eyelid opening) (8,9). In
the MDS criteria, a diagnosis of probable PSP-RS requires O1
(ocular motor dysfunction, vertical supranuclear gaze palsy) and P1
(postural instability, repeated unprovoked falls within three
years) (4). In addition to the
diagnostic criteria (supranuclear ophthalmoplegia and repeated
falls) for MDS, our patient also showed unsteady gait,
bradykinesia, slowness, ataxia, dystonic stiffness of the neck and
upper body, personality changes, cognitive decline, dysarthria, and
dysphagia (pseudobulbar palsy), and he was diagnosed as having
probable PSP-RS.
During the course of the illness, patients with PSP
typically exhibit psychotic symptoms. Behavioral abnormalities were
common in Gerstenecker's cohort of patients with PSP, with more
than half experiencing apathy, depression, and sleeping problems,
and approximately one-third displaying agitation, irritability,
disinhibition, and eating problems (10). Neuropsychiatric symptoms such as
hallucinations and delusions, however, were only observed in 5-10%
of patients (10). The patient in
the present report showed cognitive impairments, apathy,
depression, sleeping problems, irritability, and hallucinations as
psychiatric symptoms of PSP during 2 years after onset, however, he
had positive symptoms (i.e., hallucination/delusion, insomnia,
agitation) and negative symptoms (i.e., asociality, avolition) of
schizophrenia for 35 years.
In addition to clinical features, brain MRI may help
distinguish PSP from other Parkinsonian syndromes. Atrophy of the
midbrain and superior cerebellar peduncles is a useful marker in
differentiating PSP-RS from other Parkinsonian syndromes (11). Conventional structural MRI is more
specific but less sensitive than a clinical diagnosis of PSP, and
the hummingbird and morning glory flower signs each showed 100%
specificity for PSP (11). The
presence of frontal atrophy and hypometabolism are also prominent
features of PSP-RS and they may improve diagnosis when considered
together with midbrain atrophy (12). The current case showed MRI
characteristics of PSP-RS: the hummingbird sign and frontal
atrophy.
DIP should be considered in the differential
diagnosis because it is one of the few reversible causes of the
disorder (13). The management of
DIP involves identifying and discontinuing the contributing
medications, which usually resolves the symptoms, although the
symptoms may linger for a few months or up to a year or two in some
cases (14). Hayes' review
suggests that dopamine active transporter imaging using
123I-ioflupane SPECT may be useful in diagnosing DIP, as
it is normal in those cases (15,16).
The present case showed significantly reduced bilateral striatal
reuptake on 123I-ioflupane SPECT, and was not diagnosed
as DIP.
Traditionally, schizophrenia is considered to be a
result of dopaminergic hyperactivity, whereas dopaminergic
deficiency underlies the pathology of PD (17). This conflicting pathophysiology
makes coexisting schizophrenia and PD seemingly impossible.
However, they have been shown to coexist in clinical practice, and
comorbidity of idiopathic PD and schizophrenia represent a rare
scenario that is often difficult to manage (17,18).
These findings support the hypothesis that the nigrostriatal and
mesolimbic dopaminergic pathways largely function independently, or
time- and site-dependent variations in the severity of dysfunction
within a common cortical-striatal-thalamocortical neuronal network
results in the development of idiopathic PD in patients with
schizophrenia as they age (19).
Moreover, there is renewed interest in the motor aspects of
schizophrenia's neurodevelopmental disorders, including spontaneous
Parkinsonism, which appears to be independent of antipsychotic
treatment (20). By examining a
small number of cases of schizophrenia combined with degenerative
disorders such as PD, PSP, and MSA, it may be possible to approach
the neurodevelopmental disorder hypothesis for the pathophysiology
of schizophrenia.
This case report had some limitations to make a
diagnosis of PSP. First, a definite diagnosis of PSP could not be
confirmed because of a lack of qualified pathologists capable of
performing an autopsy in a terminal hospital. Second, other brain
imaging modalities (e.g., 123I-meta-iodobenzylguanidine
scintigraphy) could not be sufficiently investigated at other time
points. Third, genetic analyses that can help to support the
clinical diagnosis of PSP describing in context dependent exclusion
criteria were not examined in this case (4).
In conclusion, this is the first known report to
describe probable PSP-RS in an older patient with chronic
schizophrenia. The comorbidity of PSP-RS in patients with
schizophrenia is extremely rare, but PSP should be considered when
such patients present with worsening symptoms of Parkinsonism,
especially when accompanied by supranuclear ophthalmoplegia,
pseudobulbar palsy, dysarthria, and dystonic stiffness of the neck
and upper body. In this case, the combination of
123I-ioflupane SPECT, brain MRI, and the patient's
clinical features, helped us to discriminate PSP-RS from other
Parkinsonian syndromes. Future research examining subjects with
schizophrenia comorbid with neurodegenerative disorders such as PD,
PSP, and MSA may provide a greater insight into understanding the
shared common mechanisms that may contribute to overlapping
pathophysiological and clinical features between the disorders,
leading to an avenue for the novel drug discovery and
development.
Acknowledgements
We thank Professor Satoshi Ukai at Wakayama Medical
University (Wakayama, Japan) for his encouragement in writing this
manuscript. We would also like to thank English editor Mr Benjamin
Phillis from the Clinical Study Support Center at Wakayama Medical
University (Wakayama, Japan) for proofreading and editing this
document.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AK, TT, MY, YS and HI contributed to the patient
treatment. JK performed neurological examinations and treatment.
AK, TT, MY, YS, HI and JK collected data and wrote the first draft
of the manuscript. AK, TT, ST and SK supervised the project, were
critically involved in its design, and assisted in editing the
final manuscript. All authors contributed toward drafting the paper
and agree to be accountable for all aspects of the work. AK and SK
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Medical Ethics
Commission for Clinical Studies in the Wakayama Medical University
(approval no. 3428 on 21/02/2022).
Patient consent for publication
Patient consent for publication could not be
obtained because of his death; however, consent in written form was
provided by his family on 18/05/2018.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Savica R, Grossardt BR, Bower JH, Ahlskog
JE, Mielke MM and Rocca WA: Incidence and time trends of
drug-induced parkinsonism: A 30-year population-based study. Mov
Disord. 32:227–234. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Komatsu H, Kato M, Kinpara T, Ono T and
Kakuto Y: Possible multiple system atrophy with predominant
parkinsonism in a patient with chronic schizophrenia: A case
report. BMC Psychiatry. 18(141)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Steele JC, Richardson JC and Olszewski J:
Progressive supranuclear palsy. A heterogeneous degeneration
involving the brain stem, basal ganglia and cerebellum with
vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia.
Arch Neurol. 10:333–359. 1964.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Höglinger GU, Respondek G, Stamelou M,
Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C,
Whitwell JL, et al: Clinical diagnosis of progressive supranuclear
palsy: The movement disorder society criteria. Mov Disord.
32:853–864. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Coyle-Gilchrist IT, Dick KM, Patterson K,
Vázquez Rodríquez P, Wehmann E, Wilcox A, Lansdall CJ, Dawson KE,
Wiggins J, Mead S, et al: Prevalence, characteristics, and survival
of frontotemporal lobar degeneration syndromes. Neurology.
86:1736–1743. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nath U, Ben-Shlomo Y, Thomson RG, Morris
HR, Wood NW, Lees AJ and Burn DJ: The prevalence of progressive
supranuclear palsy (Steele-Richardson-Olszewski syndrome) in the
UK. Brain. 124:1438–1449. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Respondek G, Kurz C, Arzberger T, Compta
Y, Englund E, Ferguson LW, Gelpi E, Giese A, Irwin DJ, Meissner WG,
et al: Which ante mortem clinical features predict progressive
supranuclear palsy pathology. Mov Disord. 32:995–1005.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boxer AL, Yu JT, Golbe LI, Litvan I, Lang
AE and Höglinger GU: Advances in progressive supranuclear palsy:
New diagnostic criteria, biomarkers, and therapeutic approaches.
Lancet Neurol. 16:552–563. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Williams DR and Lees AJ: Progressive
supranuclear palsy: Clinicopathological concepts and diagnostic
challenges. Lancet Neurol. 8:270–279. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gerstenecker A, Duff K, Mast B and Litvan
I: ENGENE-PSP Study Group. Behavioral abnormalities in progressive
supranuclear palsy. Psychiatry Res. 210:1205–1210. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Massey LA, Micallef C, Paviour DC,
O'Sullivan SS, Ling H, Williams DR, Kallis C, Holton JL, Revesz T,
Burn DJ, et al: Conventional magnetic resonance imaging in confirmed
progressive supranuclear palsy and multiple system atrophy. Mov
Disord. 27:1754–1762. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Whitwell JL, Höglinger GU, Antonini A,
Bordelon Y, Boxer AL, Colosimo C, van Eimeren T, Golbe LI, Kassubek
J, Kurz C, et al: Radiological biomarkers for diagnosis in PSP:
Where are we and where do we need to be. Mov Disord. 32:955–971.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
DeMaagd G and Philip A: Parkinson's
disease and its management: Part 1: Disease entity, risk factors,
pathophysiology, clinical presentation, and diagnosis. P T.
40:504–532. 2015.PubMed/NCBI
|
|
14
|
Lee PE, Sykora K, Gill SS, Mamdani M,
Marras C, Anderson G, Shulman KI, Stukel T, Normand SL and Rochon
PA: Antipsychotic medications and drug-induced movement disorders
other than parkinsonism: A population-based cohort study in older
adults. J Am Geriatr Soc. 53:1374–1379. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hayes MT: Parkinson's disease and
parkinsonism. Am J Med. 132:802–807. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tolosa E, Coelho M and Gallardo M: DAT
imaging in drug-induced and psychogenic parkinsonism. Mov Disord.
18 (Suppl 7):S28–S33. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Oh J, Shen G and Nan G: Comorbid
schizophrenia and Parkinson's disease: A case series and brief
review. Neurol Asia. 22:139–142. 2017.
|
|
18
|
Grover S, Sahoo S and Goyal MK:
Schizophrenia with comorbid idiopathic Parkinson's disease: A
difficult clinical management scenario. Indian J Psychol Med.
39:823–827. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Winter C, Juckel G, Plotkin M, Niehaus L
and Kupsch A: Paranoid schizophrenia and idiopathic Parkinson's
disease do coexist: A challenge for clinicians. Psychiatry Clin
Neurosci. 60(639)2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Waddington JL: Psychosis in Parkinson's
disease and parkinsonism in antipsychotic-naive schizophrenia
spectrum psychosis: Clinical, nosological and pathobiological
challenges. Acta Pharmacol Sin. 41:464–470. 2020.PubMed/NCBI View Article : Google Scholar
|