1. Introduction
Dyslipidemia is a lipid metabolism disorder in which
the levels of total cholesterol (TC), triglyceride (TG) and
low-density lipoprotein cholesterol (LDL-C) in the blood are
abnormally elevated and/or those of high-density lipoprotein
cholesterol (HDL-C) in the blood are abnormally reduced (1). According to the World Health
Organization, the total number of deaths associated with
cardiovascular diseases worldwide reached 17.9 million in
2019(2). In particular,
dyslipidemia is considered to be a major risk factor of
cardiovascular diseases, including atherosclerosis, coronary artery
disease and myocardial infarction, all of which pose a serious
threat to human health (3).
Therefore, development of novel treatment options for dyslipidemia
is required.
Following advances in molecular biology, previous
studies have demonstrated that multiple signaling pathways,
including the peroxisome proliferator-activated receptor (PPAR),
adenosine monophosphate-activated protein kinase (AMPK), farnesoid
X receptor (FXR), forkhead box O (FOXO), adipocytokine and cyclic
adenosine monophosphate (cAMP) signaling pathways, are closely
associated with the occurrence and development of dyslipidemia
(4-9).
Specifically, certain drugs can target specific aspects of lipid
metabolism and serve a role in lowering the levels of lipids by
activating or inhibiting these signaling pathways (4-9).
Therefore, it is key to study the aforementioned signaling pathways
for the development of novel treatment options and for the
prevention of dyslipidemia. The majority of previous studies on
signaling pathways altered by natural substances that have been
proposed to treat dyslipidemia are experimental (4-11).
However, at present, review articles on natural substances
(components or metabolites of animals, plants, marine organisms and
microorganisms as well as endogenous active components of
organisms) that can be used to treat dyslipidemia remain lacking.
Therefore, the present review summarizes the mechanistic
information of these six signaling pathways following treatment
with the natural substances that have been reported to effectively
control dyslipidemia. In addition, it provides an overview on the
available experimental data to provide a basis for the treatment of
dyslipidemia.
2. PPAR signaling pathway in
dyslipidemia
The PPAR signaling pathway is one of the most widely
studied dyslipidemia-associated signaling pathways. PPARs are
ligand-activated transcription factors that consist of the
following three subtypes: PPARα, PPARβ and PPARγ (12). Following endogenous ligand (e.g.,
lipids such as polyunsaturated fatty acid and arachidonate)
binding, PPARs form heterodimers with retinoic X receptors and
become activated to transcribe target genes associated with lipid
metabolism (Fig. 1; generated
using Adobe Illustrator CC version 23.0; Adobe Systems, Inc.)
(13).
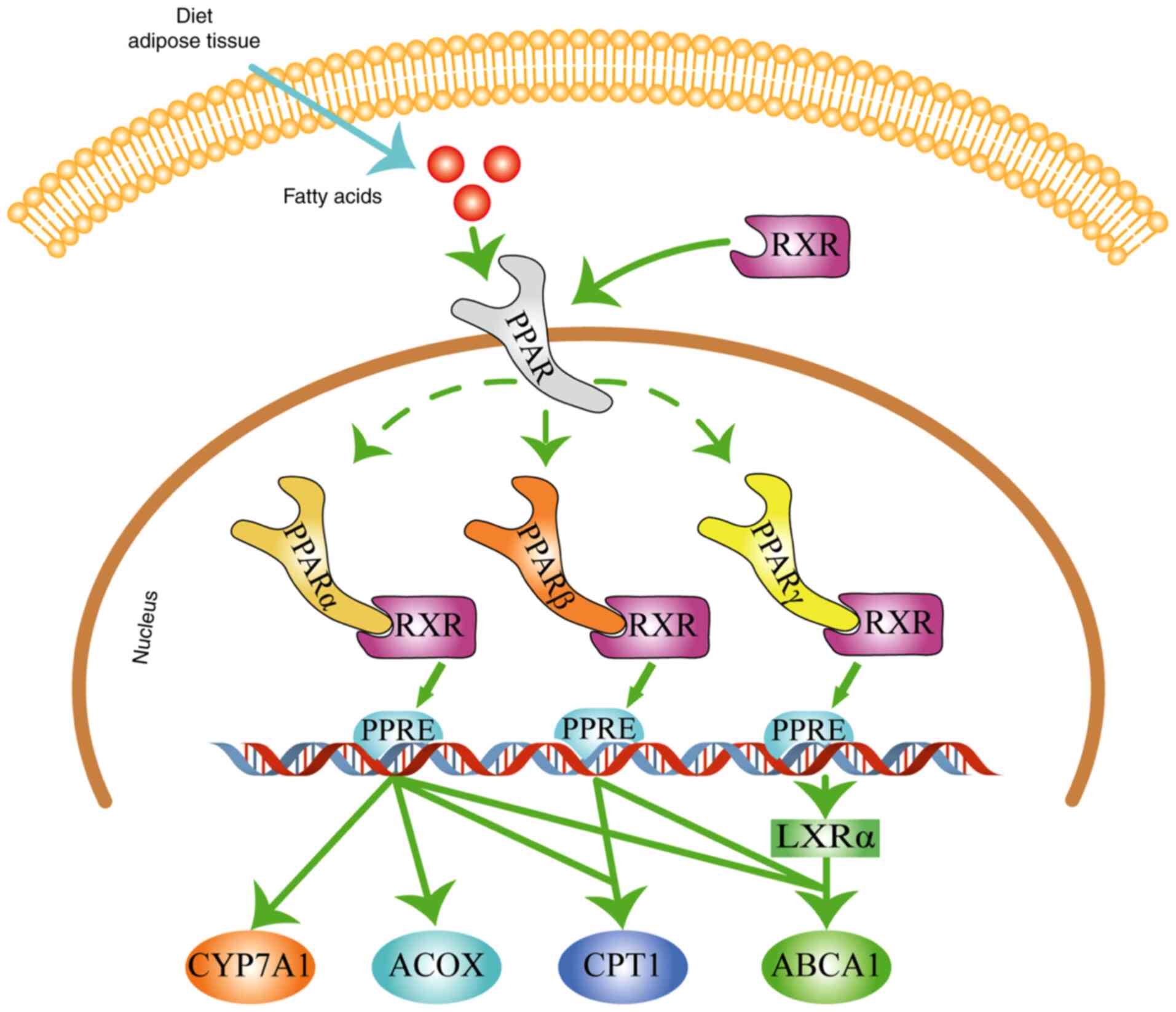 | Figure 1PPAR signaling pathway in
dyslipidemia. PPAR regulates lipid metabolism by promoting the
expression or activity of CYP7A1, ACOX, CPT1 and ABCA1. The blue
arrow represents fatty acids obtained from dietary intake or the
breakdown of adipose tissue. The green arrows represent stimulatory
modifications. Dashed lines indicate various types of PPAR. The
figure was generated using Adobe Illustrator CC version 23.0 (Adobe
Systems, Inc.). RXR, retinoid X receptor; PPAR, peroxisome
proliferator-activated receptor; LXRα, liver X receptor α; CYP7A1,
cholesterol 7 α-hydroxylase; ACOX, acyl-CoA oxidase; CPT1,
carnitine palmitoyl transferase 1; ABCA1, ATP-binding cassette
transporter A1; PPRE, peroxisome proliferator response element. |
PPARα signaling pathway in
dyslipidemia
PPARα can upregulate the expression of cholesterol 7
alpha-hydroxylase (CYP7A1), acyl-CoA oxidase (ACOX), carnitine
palmitoyl transferase 1 (CPT1) and adenosine triphosphate
(ATP)-binding cassette transporter A1 (ABCA1) (14-16).
This in turn increases HDL synthesis, cholesterol conversion and
fatty acid oxidation (14-16).
CYP7A1 is a rate-limiting enzyme of bile acid synthesis that
promotes the transformation of cholesterol to bile acids, thereby
reducing TC levels in the blood (17). By contrast, ACOX and CPT1 are
rate-limiting enzymes in the fatty acid oxidation pathway that
reduces free fatty acid (FFA) and TG levels in the blood (18-20).
ABCA1 is a rate-limiting factor in the process of HDL assembly that
promotes the production and secretion of HDL to enhance the levels
of HDL-C in the blood (21).
To investigate the hypolipidemic mechanisms mediated
by Danhe granules (Danhe is a mixture prepared based on a
prescription in clinical settings consisting of Salvia
miltiorrhiza Bunge, Reynoutria japonica Houtt,
Crataegus pinnatifida Bunge, Citrus x aurantium L.,
Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf and
Nelumbo nucifera Gaertn), Chen et al (22) administered 3.30 g/kg Danhe to
hyperlipidemic hamsters by oral gavage for 8 weeks. The results of
this study demonstrated that Danhe administration significantly
increased the expression levels of liver PPARα, CYP7A1 and ABCA1,
decreased serum TC levels by 32.41% and enhanced serum HDL-C levels
by 40.96% in hyperlipidemic hamsters (22). These results suggest that Danhe
exerted hypolipidemic effects by activating the PPARα signaling
pathway. To evaluate the effects of K-877 (a novel selective PPARα
agonist) on lipid metabolism, LDL receptor-knockout mice were fed
with a high-fat diet (HFD) supplemented with 0.001% K-877 for 1
week. K-877 administration was found to increase the expression of
PPARα, ACOX, CPT1 and ABCA1 in the liver and increased the plasma
levels of HDL-C in the plasma whilst decreasing the levels of TG in
the plasma. Therefore, K-877 improved lipid metabolism by
activating the PPARα signaling pathway (23).
PPARβ signaling pathway in
dyslipidemia
Following activation, PPARβ upregulates the
expression of ABCA1 and CPT1 to increase HDL synthesis and fatty
acid oxidation (24,25). To unravel the cholesterol-lowering
mechanism of ombuin-3-O-β-d-glucopyranoside (ombuine), HepG2 cells
were supplemented with oleic acid. Treatment with 10 µmol/l ombuine
was found to significantly increase the expression of PPARβ and
ABCA1 whilst reducing intracellular cholesterol concentrations in
HepG2 cells. This suggests that ombuine exerts a
cholesterol-lowering role by activating the PPARβ signaling pathway
(24). In another previous study,
Jiang et al previously (26) investigated the effects of
Li-Gan-Shi-Liu-Ba-Wei-San (LGSLBWS; a type of Mongolian medicine
comprising eight types of Chinese herbal medicine, namely,
pomegranate, cinnamon, cardamom, piper longum, safflower, amomum
tsao-ko, dried ginger and nutmeg) on lipid metabolism in rats with
non-alcoholic fatty liver disease (NAFLD). Following the
administration of LGSLBWS (1.5 g/kg/day) for 4 weeks, the
expression of PPARβ and the levels of fatty acid oxidation were
markedly increased, whilst TG and FFA levels in the serum were
decreased. Although CPT1 levels were not detected, the serum FFA
and TG levels were decreased, suggesting that LGSLBWS may increase
fatty acid oxidation through the PPARβ signaling pathway.
PPARγ signaling pathway in
dyslipidemia
Following activation, PPARγ upregulates the
transcription of liver X receptor α (LXRα) and ABCA1 to increase
HDL synthesis (27). Li et
al (28) applied a rat model
of hyperlipidemia to explore the effects of Agaricus blazei
Murill acidic polysaccharide (WABM-A) on the regulation of blood
lipid levels. WABM-A (640 mg/kg/day) treatment for 8 weeks
significantly upregulated the liver expression levels of PPARγ,
LXRα and ABCA1, reduced the serum levels of TC and increased the
serum levels of HDL-C in rats with hyperlipidemia (28). These results suggest that the
lipid-lowering mechanisms underlying WABM-A may be associated with
activation of the PPARγ signaling pathway.
3. AMPK signaling pathway in
dyslipidemia
The AMPK signaling pathway is one of the most
important homeostatic signaling pathways in the body and has been
implicated in various metabolic diseases, including dyslipidemia
and diabetes (29). Specifically,
AMPK is a serine/threonine protein kinas that is widely expressed
in the liver, heart and other metabolic organs, including skeletal
muscle, kidney and adipose tissue (30). AMPK is typically activated by two
methods. When the AMP/ATP ratio is increased, which indicates that
the intracellular energy levels are low, liver kinase B1
translocates from the nucleus into the cytosol to activate AMPK by
phosphorylation at Thr172(31).
Alternatively, AMPK can be activated by adiponectin (APN), which
binds to its receptor to activate phospholipase C to convert
phosphatidylinositol 4,5-bisphosphate (PIP2) into
inositol triphosphate (IP3) (32). IP3 then binds with the
IP3 receptor on the endoplasmic reticulum membrane to
promote Ca2+ release (32). Ca2+ then activates
calmodulin-dependent protein kinase kinase, which in turn activates
AMPK also by phosphorylation at the Thr172 residue (33). AMPK regulates lipid metabolism
mainly through three mechanistic pathways (Fig. 2; generated using Adobe Illustrator
CC version 23.0; Adobe Systems, Inc.). In the first mode of
activation, AMPK can downregulate the expression of lipogenic
enzymes acetyl CoA carboxylase (ACC) and fatty acid synthase (FAS)
by inhibiting the expression of sterol regulatory binding
protein-1c (SREBP-1c) (34). Since
ACC and FAS are key enzymes in the fatty acid anabolic pathway,
lowering their expression levels would reduce fatty acid synthesis
(34). In addition, fatty acids
are the main component within the molecular structure of TG
(20). Therefore, reducing the
levels of fatty acids would in turn reduce the levels of TG in the
blood (20). In the second mode of
mechanism, AMPK serves to inactivate ACC by phosphorylation,
reducing the synthesis of the CPT1 inhibitor malonyl-CoA (35). This increases the activity of CPT1,
which facilitates fatty acid oxidation to reduce fatty acid and TG
content in the blood (36). In the
third pathway, AMPK can inactivate 3-hydroxy-3-methylglutaryl CoA
reductase (HMGCR) by phosphorylation, which is the rate-limiting
enzyme in the cholesterol synthesis pathway (37). Inactivation of HMGCR reduces the
production of cholesterol, which in turn leads to lower blood TC
levels. Therefore, activation of the AMPK signaling pathway can
inhibit fatty acid synthesis, promote fatty acid oxidation and
suppress cholesterol synthesis, which finally serves to reduce
blood TG and TC levels.
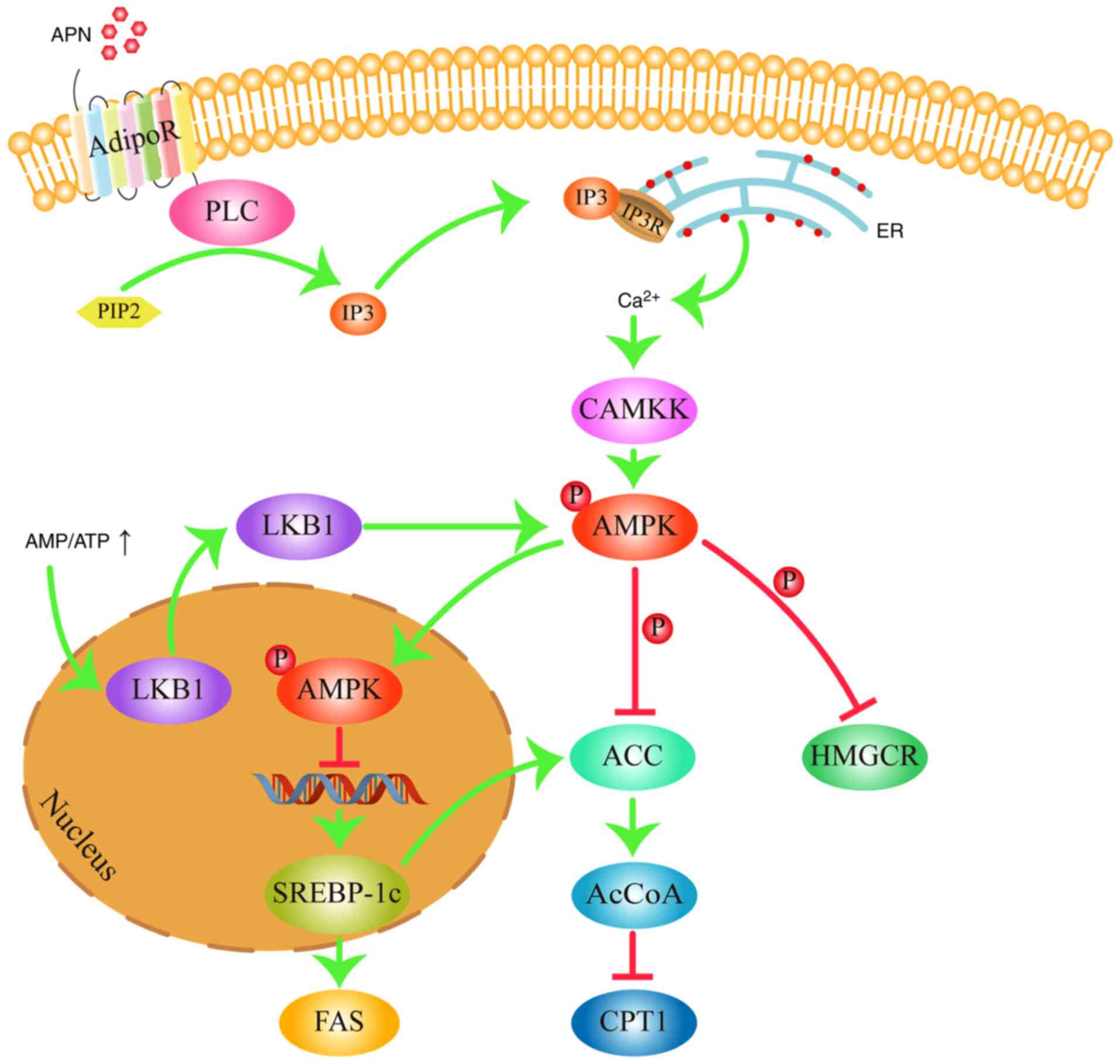 | Figure 2AMPK signaling pathway in
dyslipidemia. AMPK regulates lipid metabolism by inhibiting the
expression of FAS, decreasing the activity of HMGCR and ACC, and
increasing the activity of CPT1. Arrows represent stimulatory
modifications. T-shaped arrows represent inhibitory modifications.
The picture was generated using Adobe Illustrator CC version 23.0
(Adobe Systems, Inc.). APN, adiponectin; AdipoR, adiponectin
receptor; PLC, phospholipase C; PIP2,
phosphatidylinositol 4,5-bisphosphate; IP3, inositol
triphosphate; IP3R, inositol triphosphate receptor; ER,
endoplasmic reticulum; CAMKK, calmodulin-dependent protein kinase
kinase; AMP, adenosine monophosphate; ATP, adenosine triphosphate;
AMPK, adenosine monophosphate-activated protein kinase; LKB1, liver
kinase B1; SREBP-1c, sterol regulatory binding protein-1c; ACC,
acetyl-CoA carboxylase; AcCoA, malonyl-CoA; FAS, fatty acid
synthase; HMGCR, 3-hydroxy-3-methylglutaryl coA reductase. |
To investigate the effects of the Herba
houttuyniae aqueous extract (HAE) on hyperlipidemia, Cao et
al (38) randomly divided male
C57BL/6J mice into three groups: Control, model and HAE. The mice
in the HAE group were treated with HAE from day 1 to 9. On day 10,
all mice except for those in the control group were
intraperitoneally injected with 0.5 g/kg poloxamer 407 to induce
acute hyperlipidemia, whereas mice in the control group were
injected with saline. The results of the study demonstrated that
treatment with 200 mg/kg/day HAE significantly decreased the
expression levels of SREBP-1c, ACC and FAS in the liver whilst
reducing the serum TG content (38). This suggests that HAE exerts
protective effects against hyperlipidemia by upregulating the AMPK
signaling pathway. In another study, to explore the pharmacological
activity of arctigenin (a phenylpropanoid dibenzyl butyrolactone
lignan isolated from Arctium lappa Linné using a
crystallization method) on lipid metabolism, Song et al
(39) established a hyperlipidemic
rat model by feeding them on an HFD for 8 weeks. Following 4 weeks
of arctigenin administration (100 mg/kg/day), the serum levels of
TG were significantly reduced compared with those in the model
group (39). By contrast, the
phosphorylation levels of AMPK and ACC, in addition to the
expression of CPT1, were significantly increased compared with
those in the model group (39).
The results of this study suggest that arctigenin may exert a
therapeutic effect on lipid metabolism through activation of the
AMPK signaling pathway. In addition, Lee et al (40) previously investigated the
cholesterolemic effects of unripe R. coreanus (5-uRCK) and
ellagic acid on a high cholesterol diet-induced
hypercholesterolemia rat model. The results demonstrated that
5-uRCK at 150 mg/kg and ellagic acid at 4 mg/kg significantly
increased AMPK and HMGCR phosphorylation whilst markedly reducing
the serum levels of TC and LDL-C (40). These results suggest that 5-uRCK
and ellagic acid can inhibit the activity of HMGCR through
activation of the AMPK signaling pathway, which lowered cholesterol
levels.
4. FXR signaling pathway in
dyslipidemia
The FXR signaling pathway is the principal signaling
pathway in cholesterol conversion and serves a key role in
maintaining lipid homeostasis. It can regulate cholesterol
metabolism through two main mechanism (Fig. 3; generated using Adobe Illustrator
CC version 23.0; Adobe Systems, Inc.). The first mechanism involves
the FXR-small heterodimer partner (SHP) pathway in the liver, which
is activated by bile acids (41).
FXR activation induces the expression of SHP by binding to the FXR
response element (FXRE) within the promoter region of the SHP gene
(42). Subsequently, SHP binds to
and inactivates liver receptor homolog-1 (LRH-1), which is a
transcription factor required for the transcription of the CYP7A1
gene (43). Therefore, LRH-1
inactivation in response to activated FXR results in the inhibition
of CYP7A1 transcription (43).
CYP7A1 is a rate-limiting enzyme of bile acid synthesis that
promotes the transformation of cholesterol into bile acids
(17). Reductions in CYP7A1
transcription reduce the conversion of cholesterol and increases
the TC content in the blood. The second mechanism is through the
FXR/fibroblast growth factor 15 (FGF15) pathway in the small
intestine, following activation by bile acids (44). FXR promotes the expression and
secretion of FGF15 by combining with the FXRE in the promoter
region of the FGF15 gene (45).
Subsequently, FGF15 enters the liver through the portal circulation
and binds to FGF receptor 4 (FGFR4) on the surface of hepatocytes
(45). JNK is then activated
following the complex formation between FGF15 and FGFR4(45). Activated JNK then inhibits the
expression of CYP7A1 to increase the TC content in the blood
(45). Therefore, activation of
the FXR/SHP pathway in the liver and the FXR/FGF15 pathway in the
small intestine combine to reduce the expression of CYP7A1, which
suppresses the conversion of cholesterol to increase the content of
TC in the blood.
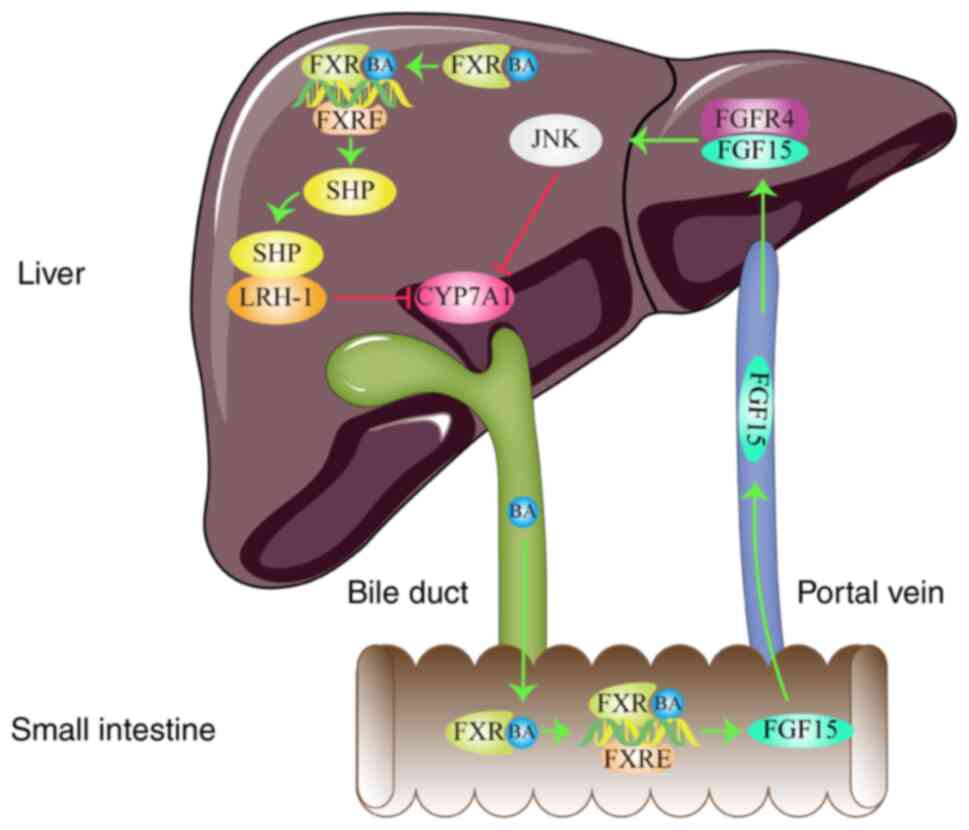 | Figure 3FXR signaling pathway in
dyslipidemia. FXR regulates lipid metabolism by inhibiting CYP7A1
expression. Arrows represent stimulatory modifications. T-shaped
arrows represent inhibitory modifications. The picture was
generated using Adobe Illustrator CC version 23.0 (Adobe Systems,
Inc.). BA, bile acids; FXR, farnesoid X receptor; SHP, small
heterodimer partner; LRH-1, liver receptor homolog-1; JNK, c-Jun
N-terminal kinase; FGFR4, fibroblast growth factor receptor 4;
FGF15, fibroblast growth factor 15; CYP7A1, cholesterol 7
alpha-hydroxylase; FXRE, farnesoid X response element. |
To clarify the mechanism underlying the
cholesterolemic activity of the bitter gourd fruit (BGF), Matsui
et al (46) measured the
changes in the expression of cholesterol-regulating proteins and
serum cholesterol levels in rats fed on a high-cholesterol diet
following BGF intake. BGF administration not only increased hepatic
LRH-1 and CYP7A1 expression but also significantly decreased
hepatic SHP expression, serum TC and LDL-C levels (P<0.05).
These data suggest that BGF exerted hypocholesterolemic activity by
facilitating the conversion of cholesterol to bile acids, which was
induced by CYP7A1 upregulation and concomitant downregulation of
the FXR signaling pathway in the liver. Huang et al
(47) also previously established
a mouse model with high cholesterol induced by HFD to investigate
the underlying cholesterol handling mechanism after theabrownin
treatment. The results of this study demonstrated that treatment
with 225 mg/kg/day theabrownin for 8 weeks significantly reduced
ileal FXR and FGF15 expression whilst increasing the expression
levels of hepatic CYP7A1(47). In
addition, theabrownin decreased serum TC levels in mice with high
cholesterol (47). These results
suggest that the cholesterol-lowering effect of theabrownin
depended on the inhibition of the intestinal FXR signaling pathway
in the ileum.
5. FOXO signaling pathway in
dyslipidemia
The FOXO signaling pathway is important in
maintaining the balance of blood lipids in the body and regulates
the processes of lipoprotein (chylomicron and very low-density
lipoprotein) synthesis and hydrolysis (48,49).
FOXO1 is a key member of the FOXO family, the activity of which is
negatively regulated by insulin (INS) (50). After the INS receptor is activated,
it activates the INS receptor substrate (IRS) to activate PI3K,
which in turn converts PIP2 into phosphatidylinositol
3,4,5-triphosphate (PIP3) (51). Subsequently, PIP3 binds
to phosphoinositide-dependent kinase 1 (PDK1), which contains a PH
domain in the cell, to promote the phosphorylation and activation
of AKT (52). Activated AKT
phosphorylates FOXO1 in the nucleus to induce export into the
cytoplasm (53,54). In the nucleus, FOXO1 promotes the
expression of its target genes, such as microsomal triglyceride
transfer protein (MTP) and apolipoprotein C-III (ApoC-III)
(Fig. 4; generated using Adobe
Illustrator CC version 23.0; Adobe Systems, Inc.) (53,54).
MTP is an essential factor in the assembly and secretion of very
low-density lipoprotein (VLDL) by taking part in their
rate-limiting steps (55). VLDL is
converted to LDL under the influence of lipoprotein lipase (LPL)
(56). MTP can indirectly increase
LDL production, contributing to increased blood LDL-C levels. By
contrast, ApoC-III is an inhibitor of LPL that reduces the
hydrolysis of TG in chylomicrons and VLDL in the blood to increase
blood TG levels (48,49). However, following FOXO1
phosphorylation by AKT, it is exported from the nucleus into the
cytoplasm, resulting in the reduced expression of MTP and ApoC-III
(48,49). This then reduces the content of
LDL-C and TG in the blood. Therefore, activation of the FOXO
signaling pathway may inhibit VLDL synthesis, decrease TG
hydrolysis in the blood chylomicron and VLDL whilst reducing LDL-C
and TG content in the blood.
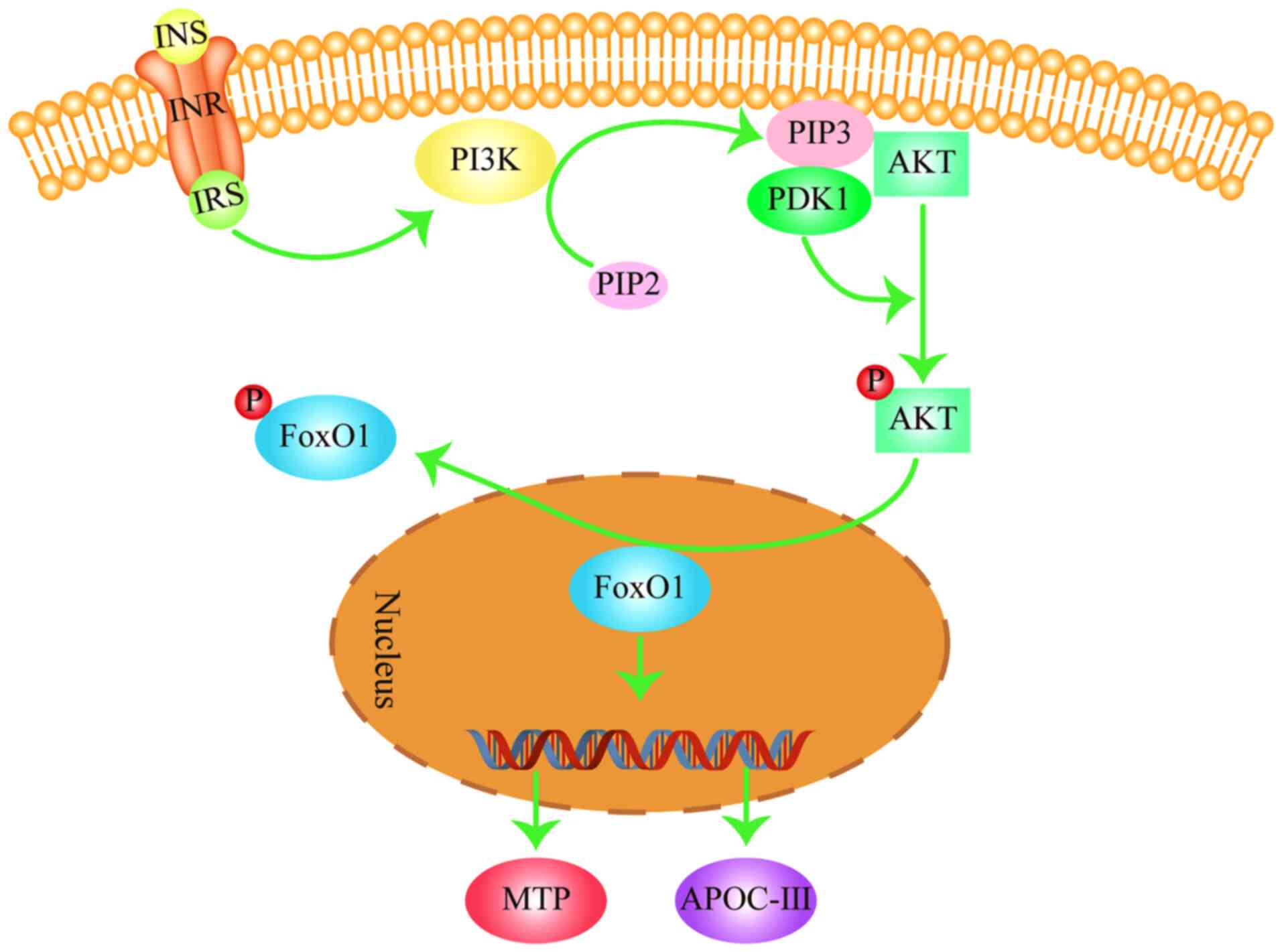 | Figure 4FOXO signaling pathway in
dyslipidemia. FOXO1 regulates lipid metabolism by promoting the
expression of MTP and APOC-III. Arrows represent stimulatory
modifications. The picture was generated using Adobe Illustrator CC
version 23.0 (Adobe Systems, Inc.). INS, insulin; INR, insulin
receptor; IRS, insulin receptor substrate; PI3K,
phosphoinositide 3-kinase; PIP3, phosphatidylinositol
3,4,5-triphosphate; PDK1, phosphoinositide-dependent kinase 1;
FOXO1, forkhead box subgroup O1; MTP, microsomal triglyceride
transfer protein; APOC-III, apolipoprotein C-III. |
To determine the mechanism underlying the beneficial
effects of docosahexaenoic acid (DHA) on lipid homeostasis, 30
weaned crossbred pigs were randomly divided into groups and fed
with a standard diet supplemented with either 2% beef tallow, 2%
soybean oil or 2% DHA oil for 30 days (54). The results of the study
demonstrated that feeding with the 2% DHA-supplemented diets
decreased the expression levels of FOXO1, ApoC-III and MTP in the
liver, which decreased TG and TC levels in the plasma (54). These findings suggest that the
reduced FOXO1 expression may contribute to the beneficial effects
of DHA on lipid homeostasis (54).
A type 2 diabetes mellitus rat model induced by HFD and
streptozotocin was previously used by Xu et al (57) to investigate the effects of dioscin
(a natural steroidal saponin isolated from the rhizome of
Dioscorea nipponica Makino, Dioscorea opposita Thunb
and Dioscorea zingiberensis Wright (Dioscoreaceae) by
chromatography) on glycolipid metabolism. Administration of 60
mg/kg/day dioscin was found to significantly elevate the expression
of PI3K, AKT and FOXO1 phosphorylation, whilst serum TC, TG and
LDL-C levels were markedly reduced (57). Although the expression levels of
MTP and ApoC-III were not measured, the serum TC, TG and LDL-C
levels in this rat model were decreased (57). Therefore, these regulatory effects
of dioscin on lipid metabolism may have been due to the regulation
of the FOXO signaling pathway. However, the specific mechanism
remains to be verified.
6. Adipocytokine signaling pathway in
dyslipidemia
Adipocytokines, such as APN, leptin (LEP) and
resistin. are biologically active polypeptides that are produced
and secreted by adipocytes (58).
They serve key and complex roles in the maintenance of energy
homeostasis, glucose and lipid metabolism (58). In particular, APN and LEP are
closely associated with lipid metabolism in the body (59). APN regulates lipid metabolism by
activating the AMPK signaling pathway as previously described
(32,33). LEP serves a key role in the process
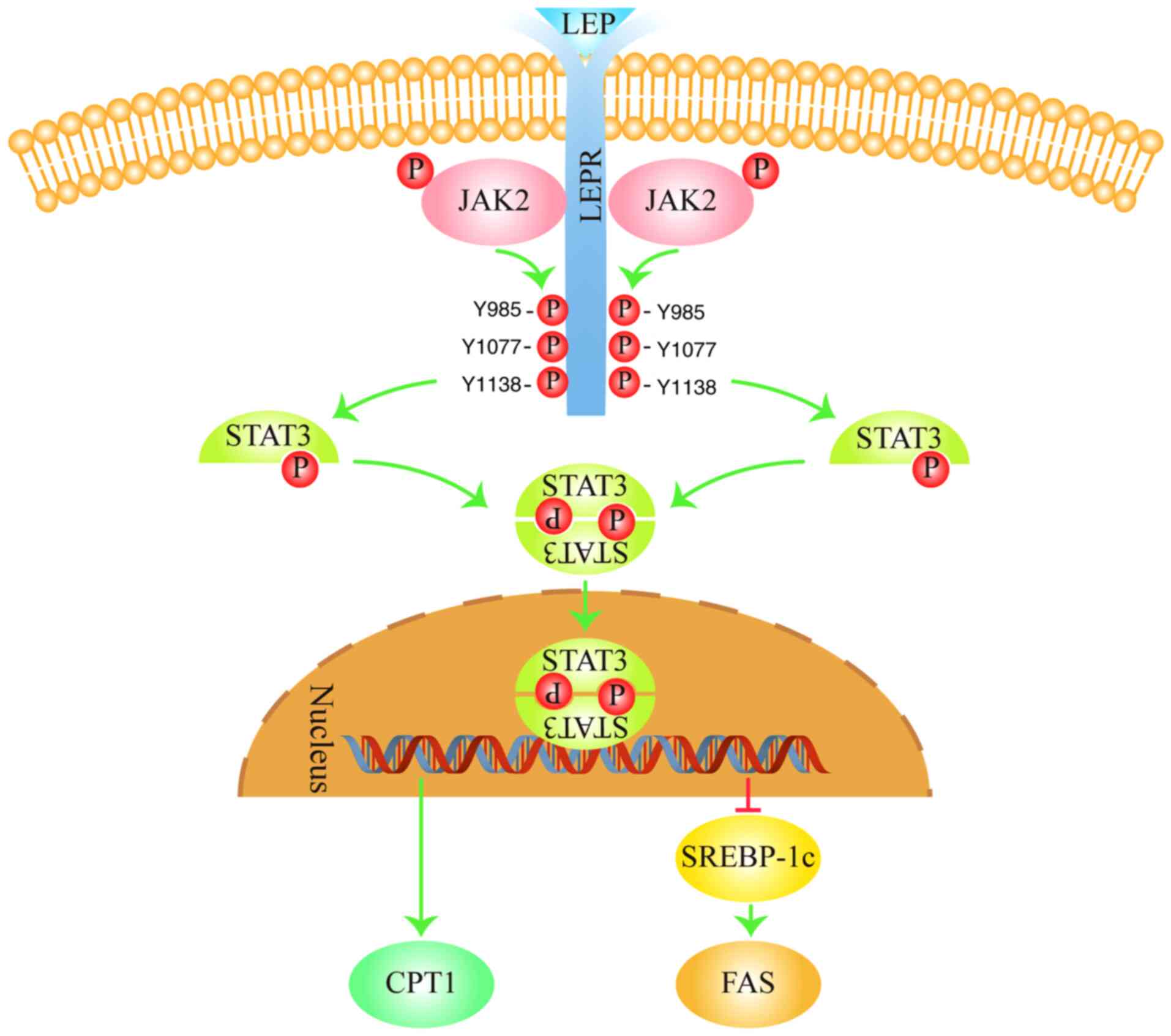
of lipid metabolism through a continuous process (Fig. 5; generated using Adobe Illustrator
CC version 23.0; Adobe Systems, Inc.). LEP can bind to its receptor
(LEPR) to activate Janus kinase 2 (JAK2), which in turn
phosphorylates three tyrosine residues on LEPR (Y985, Y1077 and
Y1138) (60). Y1138 then recruits
and activates the STAT3 by phosphorylation (60). Subsequently, activated STAT3 binds
to another STAT3 to form a homodimer and is transported into the
nucleus, where they bind to DNA (61). This STAT3 homodimer complex
promotes the expression of CPT1(62), the rate-limiting enzyme in fatty
acid oxidation. This promotes fatty acid oxidation into
CO2 and H2O, which in turn reduces FFA and TG
levels in the blood (19). In
addition, this STAT3 homodimer can also inhibit the expression of
SREBP-1c and its target gene FAS (63). FAS is a key enzyme in the fatty
acid synthesis pathway (34).
Reduced FAS expression leads to the reduction of fatty acid
synthesis, which decreases TG levels in the blood (34). Therefore, activation of the
adipocytokine signaling pathway can promote fatty acid oxidation
whilst inhibiting fatty acid synthesis to reduce blood TG
levels.
To investigate the effects of Dendrobium mixture (a
mixture prepared based on a prescription in clinical settings,
comprising dendrobium, astragalus, schisandra, pueraria, salvia,
rehmannia and earthworms) on glucose and lipid metabolism in
diabetic rats, Lin et al (64) established a diabetic rat model
induced by high-glucose, HFD and the intraperitoneal injection of
streptozotocin. Dendrobium mixture treatment for 15 weeks was
demonstrated to significantly increase the protein expression of
LEPR, CPT1 and STAT3 phosphorylation in the liver, increased the
mRNA expression of LEPR and CPT1 and decreased the plasma TG
content (64). These results
indicate that the dendrobium mixture may regulate lipid metabolism
in diabetic rats by activating the adipokine signaling pathway. In
another study, to explore the mechanism underlying the effects of
carbenoxolone in the regulation of lipid metabolism, Chen et
al (65) fed mice on an HFD
for 8 weeks and administered carbenoxolone (15 mg/kg) every day by
oral gavage for 12 weeks. Treatment with carbenoxolone was observed
to increase the activation of JAK2 and STAT3 whilst decreasing the
expression of SREBP-1c and FAS, which in turn decreased the
concentrations of TG in the serum (65). These results suggest that
carbenoxolone may regulate lipid metabolism by improving the
activity of the adipocytokine signaling pathway.
7. cAMP signaling pathway in
dyslipidemia
The cAMP signaling pathway is one of the most
extensively studied signaling pathways and regulates lipid
metabolism by regulating lipolysis in the adipose tissue (66). cAMP is a second messenger that can
transmit vital information inside the cells by activating
downstream effector molecules (67). Intracellular cAMP concentrations
are modulated by adenylyl cyclases (AC) and phosphodiesterases
(PDEs) (68). Growth hormones and
other ligands, including adrenaline and glucagon, first interact
with G-protein coupled receptors on the cell membrane to activate
the G-proteins. This G-protein then binds to guanosine triphosphate
(GTP) before binding to AC to activate it, which then catalyzes the
biosynthesis of cAMP from ATP (69). PDEs hydrolyzes cAMP into AMP to
switch off the signal (70). Under
physiological conditions, cAMP synthesis by AC and degradation by
PDEs is maintained in a balance. Excessive caloric intake leads to
the decreased expression of PDEs, increasing cAMP production in
adipose tissues (71). After cAMP
binds to the regulatory subunits of protein kinase A (PKA), the
catalytic subunits of PKA then dissociate and activate
hormone-sensitive lipase (HSL) through phosphorylation at the
Ser563, Ser659 and Ser660 residues (Fig. 6; generated using Adobe Illustrator
CC version 23.0; Adobe Systems, Inc.). HSL is a critical
rate-limiting enzyme of adipose degradation and is responsible for
the breakdown of TG and cholesteryl esters into glycerin, fatty
acid and cholesterol in the adipose tissue, which are then released
into the blood (72). Therefore,
inhibition of the cAMP signaling pathway can decreases the
phosphorylation of HSL to reduce the content of blood TC and
TG.
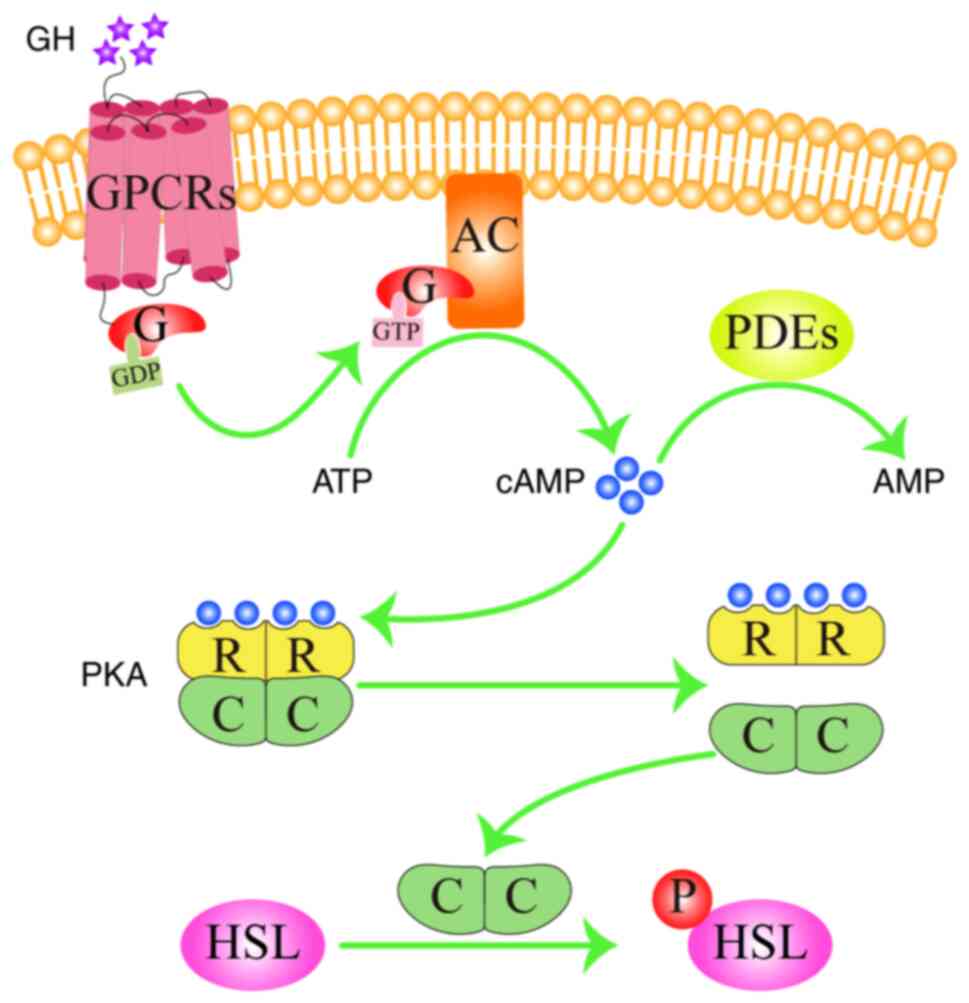 | Figure 6cAMP signaling pathway in
dyslipidemia. cAMP binds to the regulatory subunit of PKA,
phosphorylates and activates HSL, thereby regulating lipid
metabolism. Arrows represent stimulatory modifications. The picture
was generated using Adobe Illustrator CC version 23.0 (Adobe
Systems, Inc.). GH, growth hormone; GPCRs, G protein-coupled
receptors; AC, adenylyl cyclase; cAMP, cyclic adenosine
monophosphate; ATP, adenosine triphosphate; GTP, guanosine
triphosphate; GDP, guanosine diphosphate; PDEs, phosphodiesterases;
PKA, protein kinase A; HSL, hormone sensitive lipase; P, phosphate
group; G, G-protein; R, regulatory subunit; C, catalytic
subunit. |
To investigate the effects of ginsenoside Rg5 (the
ginsenosides isolated from steamed ginseng by chromatography and
crystallization) on the regulation of lipolysis, mice were fed on
an HFD for 10 days along with the simultaneous administration of
ginsenoside Rg5 (50 mg/kg) by oral gavage every day. Compared with
those in the HFD mice, ginsenoside Rg5 treatment markedly increased
the expression of PDE3B whilst cAMP accumulation and PKA 62 KDa
substrate phosphorylation were reduced in the adipose tissue
(73). In line with reduced cAMP
accumulation and PKA substrate phosphorylation, oral gavage
administration of ginsenoside Rg5 also decreased the
phosphorylation of HSL in the adipose tissue, which reduced the
levels of FFA, glycerol and TG in the blood (73). These findings suggest that
ginsenoside Rg5 can inhibit lipolysis by inhibiting the cAMP
signaling pathway (73). In
addition, in another study, to investigate the effects of
astragaloside IV (a saponin and main active component isolated from
the medicinal plant Astragalus membranaceus by
chromatography) on adipose lipolysis, mice were fed on an HFD for 2
weeks combined with the simultaneous oral gavage administration of
astragaloside IV (50 or 100 mg/kg) daily (74). Compared with those in the HFD mice,
astragaloside IV treatment significantly increased PDE3B expression
and prevented cAMP accumulation (74). Consistent with the reduced cAMP
accumulation, oral gavage administration of astragaloside IV also
suppressed PKA substrate and HSL phosphorylation. Downstream, the
levels of serum FFA and glycerol were also reduced, whilst the
content of TC and TG in the serum were not affected (74). This may have been due to the short
administration time of 2 weeks (74). These results suggest that
astragaloside IV can inhibit lipolysis by downregulating the cAMP
signaling pathway (74).
8. Conclusions
It should be noted that each of the six signaling
pathways aforementioned are not mutually exclusive and can
coordinate together to maintain lipid homeostasis (75-77).
As such, certain drugs can simultaneously activate or inhibit
multiple signaling pathways to exert lipid-lowering effects. Gong
et al (78) previously used
a NAFLD mouse model to study the effects of cordycepin on lipid
metabolism and the primary molecular mechanisms. Compared with
those in the model group, treatment with 50 mg/kg cordycepin
markedly increased the protein levels of p-AMPK, CPT-1 and PPARα
whilst decreasing the expression of SREBP1-c and ACC in the liver
of NAFLD model mice (78). By
contrast, cordycepin treatment reduced the levels of TC, TG and
LDL-C in the serum of these same NAFLD model mice (78). These results suggest that
cordycepin can exert a lipid-lowering role by activating two
signaling pathways. Specifically, cordycepin may activate the PPARα
signaling pathway to upregulate the expression of PPARα and CPT1
whilst at the same time activating the AMPK signaling pathway to
downregulate the expression of SREBP-1c and ACC. Furthermore, Cao
et al (79) previously
investigated the cholesterol-lowering effects and mechanism of
coptis alkaloids (50, 100 and 200 mg/kg) on high lipid diet-induced
hyperlipidemic rats. Compared with those in the model group, the
mRNA expression levels of PPARα and CYP7A1 were increased in a
dose-dependent manner, which were accompanied by the decreased
levels of FXR mRNA expression, in the livers of coptis
alkaloid-treated rats (79). In
addition, the levels of serum TC and LDL-C were reduced in a
dose-dependent manner following treatment with coptis alkaloids
(79). These results suggest that
the cholesterol-lowering effects of coptis alkaloid extract may be
attributed to the promotion of cholesterol conversion into bile
acids, mediated by the increased expression of CYP7A1(79). This may be associated with the
regulation of two signaling pathways, including the positive
regulation of the PPARα signaling pathway and the negative
modulation of the FXR signaling pathway.
In conclusion, the six dyslipidemia-related
signaling pathways, including the PPAR, AMPK, FXR, FOXO,
adipocytokine and cAMP signaling pathways, have all been
demonstrated to exhibit altered activity in dyslipidemia. The
results found by the aforementioned experiments demonstrated that
different drugs can mediate effects on dyslipidemia by targeting
the six signaling pathways. However, further investigations into
alternative dyslipidemia-associated signaling pathways are
required. In particular, future studies should focus on the
application of multiomics technologies, including transcriptomics,
proteomics and metabolomics, to systematically study the
dyslipidemia-associated signaling pathways. This may uncover novel
signaling pathways to provide novel therapeutic targets for the
treatment of dyslipidemia.
Acknowledgements
Not applicable.
Funding
Funding: The present review was supported by the National
Natural Science Foundation of China (grant no. 81973557), Natural
Science Fund of Tianjin City (grant no. 20JCZDJC00010), Open
Research fund of State Key Laboratory of Drug Delivery and
Pharmacokinetics, Tianjin Institute of Pharmaceutical Research
(grant no. 010162001) and Graduate Research Innovation Project of
Tianjin University of Traditional Chinese Medicine (grant no.
YJSKC-20201013).
Availability of data and materials
Not applicable.
Authors' contributions
NC analyzed the literature data and modified the
manuscript. XL and WZ wrote the manuscript. QW and YL designed the
figures using Adobe Illustrator CC version 23.0 (Adobe Systems,
Inc.). FZ and XX revised the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chu SM, Shih WT, Yang YH, Chen PC and Chu
YH: Use of traditional Chinese medicine in patients with
hyperlipidemia: A population-based study in Taiwan. J
Ethnopharmacol. 168:129–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
World Health Organization. World health
statistics 2021: Monitoring health for the SDGs, sustainable
development goals. Geneva: World Health Organization. Licence: CC
BY-NC-SA 3.0 IGO, 2021.
|
|
3
|
Navar-Boggan AM, Peterson ED, D'Agostino
RB Sr, Neely B, Sniderman AD and Pencina MJ: Hyperlipidemia in
early adulthood increases long-term risk of coronary heart disease.
Circulation. 131:451–458. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tang H, Zeng Q, Tang T, Wei Y and Pu P:
Kaempferide improves glycolipid metabolism disorder by activating
PPARγ in high-fat-diet-fed mice. Life Sci.
270(119133)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cheon SY, Chung KS, Lee KJ, Choi HY, Ham
IH, Jung DH, Cha YY and An HJ: HVC1 ameliorates hyperlipidemia and
inflammation in LDLR-/- mice. BMC Complement Altern Med.
17(222)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Deng YF, Huang XL, Su M, Yu PX, Zhang Z,
Liu QH, Wang GP and Liu MY: Hypolipidemic effect of SIPI-7623, a
derivative of an extract from oriental wormwood, through farnesoid
X receptor antagonism. Chin J Nat Med. 16:572–579. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu M, Hao S, Liu T, Yang L, Zheng P,
Zhang L and Ji G: Lingguizhugan decoction improves non-alcoholic
fatty liver disease by altering insulin resistance and lipid
metabolism related genes: A whole trancriptome study by RNA-Seq.
Oncotarget. 8:82621–82631. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen HL, Tsai TC, Tsai YC, Liao JW, Yen CC
and Chen CM: Kefir peptides prevent high-fructose corn
syrup-induced non-alcoholic fatty liver disease in a murine model
by modulation of inflammation and the JAK2 signaling pathway. Nutr
Diabetes. 6(e237)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu CP, Chau PC, Chang CT, An LM, Yeh JL,
Chen IJ and Wu BN: KMUP-1, a GPCR modulator, attenuates
triglyceride accumulation involved MAPKs/Akt/PPARγ and PKA/PKG/HSL
signaling in 3T3-L1 preadipocytes. Molecules.
23(2433)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin LY, Huang BC, Chen KC and Peng RY:
Integrated anti-hyperlipidemic bioactivity of whole Citrus
grandis [L.] osbeck fruits-multi-action mechanism evidenced using
animal and cell models. Food Funct. 11:2978–2996. 2020.
|
|
11
|
Deng N, He Z, Guo R, Zheng B, Li T and Liu
RH: Highland barley whole grain (Hordeum vulgare L.) ameliorates
hyperlipidemia by modulating cecal microbiota, miRNAs, and AMPK
pathways in leptin receptor-deficient db/db mice. J Agric Food
Chem. 68:11735–11746. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manickam R and Wahli W: Roles of
peroxisome proliferator-activated receptor β/δ in skeletal muscle
physiology. Biochimie. 136:42–48. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Capitão A, Lopes-Marques M, Páscoa I,
Ruivo R, Mendiratta N, Fonseca E, Castro LFC and Santos MM: The
echinodermata PPAR: Functional characterization and exploitation by
the model lipid homeostasis regulator tributyltin. Environ Pollut.
263(114467)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Du H, Li C, Wang Z, He Y, Wang Y, Zhou H,
Wan H and Yang J: Effects of Danhong injection on dyslipidemia and
cholesterol metabolism in high-fat diets fed rats. J
Ethnopharmacol. 274(114058)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rong Q, Han B, Li Y, Yin H, Li J and Hou
Y: Berberine reduces lipid accumulation by promoting fatty acid
oxidation in renal tubular epithelial cells of the diabetic kidney.
Front Pharmacol. 12(729384)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Song J, Qiu H, Du P, Mou F, Nie Z, Zheng Y
and Wang M: Polyphenols extracted from Shanxi-aged vinegar exert
hypolipidemic effects on OA-induced HepG2 cells via the
PPARα-LXRα-ABCA1 pathway. J Food Biochem. 46(e14029)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu K, Tan F, Mu J, Yi R, Zhou XR and Zhao
X: Anti-obesity effects of lactobacillus fermentum CQPC05 isolated
from sichuan pickle in high-fat diet-induced obese mice through
PPAR-α signaling pathway. Microorganisms. 7(194)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen XF, Tian MX, Sun RQ, Zhang ML, Zhou
LS, Jin L, Chen LL, Zhou WJ, Duan KL, Chen YJ, et al: SIRT5
inhibits peroxisomal ACOX1 to prevent oxidative damage and is
downregulated in liver cancer. EMBO Rep. 19(e45124)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schlaepfer IR and Joshi M: CPT1A-mediated
fat oxidation, mechanisms, and therapeutic potential.
Endocrinology. 161(bqz046)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yan S, Wang X, Yang C, Wang J, Wang Y, Wu
B, Qiao L, Zhao J, Mohammad P, Zheng X, et al: Insights into walnut
lipid metabolism from metabolome and transcriptome analysis. Front
Genet. 12(715731)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hafiane A and Genest J: ATP binding
cassette A1 (ABCA1) mediates microparticle formation during
high-density lipoprotein (HDL) biogenesis. Atherosclerosis.
257:90–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen K, Ma Z, Yan X, Liu J, Xu W, Li Y,
Dai Y, Zhang Y and Xiao H: Investigation of the lipid-lowering
mechanisms and active ingredients of danhe granule on
hyperlipidemia based on systems pharmacology. Front Pharmacol.
11(528)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takei K, Nakagawa Y, Wang Y, Han SI, Satoh
A, Sekiya M, Matsuzaka T and Shimano H: Effects of K-877, a novel
selective PPARα modulator, on small intestine contribute to the
amelioration of hyperlipidemia in low-density lipoprotein receptor
knockout mice. J Pharmacol Sci. 133:214–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Malek MA, Hoang MH, Jia Y, Lee JH, Jun HJ,
Lee DH, Lee HJ, Lee C, Lee MK, Hwang BY and Lee SJ:
Ombuin-3-O-β-D-glucopyranoside from Gynostemma pentaphyllum is a
dual agonistic ligand of peroxisome proliferator-activated
receptors α and δ/β. Biochem Biophys Res Commun. 430:1322–1328.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Toral M, Romero M, Jiménez R, Mahmoud AM,
Barroso E, Gómez-Guzmán M, Sánchez M, Cogolludo Á, García-Redondo
AB, Briones AM, et al: Carnitine palmitoyltransferase-1
up-regulation by PPAR-β/δ prevents lipid-induced endothelial
dysfunction. Clin Sci (Lond). 129:823–837. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jiang Y, Chen L, Wang H, Narisi B and Chen
B: Li-Gan-Shi-Liu-Ba-Wei-San improves non-alcoholic fatty liver
disease through enhancing lipid oxidation and alleviating oxidation
stress. J Ethnopharmacol. 176:499–507. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun L, He X, Zhang T, Han Y and Tao G:
Knockdown of mesenchymal stem cell-derived exosomal LOC100129516
suppresses the symptoms of atherosclerosis via upregulation of the
PPARγ/LXRα/ABCA1 signaling pathway. Int J Mol Med.
48(208)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Y, Sheng Y, Lu X, Guo X, Xu G, Han X,
An L and Du P: Isolation and purification of acidic polysaccharides
from Agaricus blazei Murill and evaluation of their
lipid-lowering mechanism. Int J Biol Macromol. 157:276–287.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang D, Tian M, Qi Y, Chen G, Xu L, Zou X,
Wang K, Dong H and Lu F: Jinlida granule inhibits palmitic acid
induced-intracellular lipid accumulation and enhances autophagy in
NIT-1 pancreatic β cells through AMPK activation. J Ethnopharmacol.
161:99–107. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jiang S, Li T, Yang Z, Yi W, Di S, Sun Y,
Wang D and Yang Y: AMPK orchestrates an elaborate cascade
protecting tissue from fibrosis and aging. Ageing Res Rev.
38:18–27. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang T, Yamamoto N and Ashida H:
Chalcones suppress fatty acid-induced lipid accumulation through a
LKB1/AMPK signaling pathway in HepG2 cells. Food Funct.
5:1134–1141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang S, Li C, Sun P, Shi J, Wu X, Liu C,
Peng Z, Han H, Xu S, Yang Y, et al: PCV2 triggers PK-15 Cell
apoptosis through the PLC-IP3R-Ca2+ signaling pathway. Front
Microbiol. 12(674907)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fujiwara Y, Kawaguchi Y, Fujimoto T,
Kanayama N, Magari M and Tokumitsu H: Differential AMP-activated
protein kinase (AMPK) recognition mechanism of
Ca2+/calmodulin-dependent protein kinase kinase
isoforms. J Biol Chem. 291:13802–13808. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shin MR, Shin SH and Roh SS: Diospyros
kaki and Citrus unshiu mixture improves disorders of lipid
metabolism in nonalcoholic fatty liver disease. Can J Gastroenterol
Hepatol. 2020(8812634)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chang JJ, Hsu MJ, Huang HP, Chung DJ,
Chang YC and Wang CJ: Mulberry anthocyanins inhibit oleic acid
induced lipid accumulation by reduction of lipogenesis and
promotion of hepatic lipid clearance. J Agric Food Chem.
61:6069–6076. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kang J, Park J, Kim HL, Jung Y, Youn DH,
Lim S, Song G, Park H, Jin JS, Kwak HJ and Um JY:
Secoisolariciresinol diglucoside inhibits adipogenesis through the
AMPK pathway. Eur J Pharmacol. 820:235–244. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang X, Song Y, Feng M, Zhou X, Lu Y, Gao
L, Yu C, Jiang X and Zhao J: Thyroid-stimulating hormone decreases
HMG-CoA reductase phosphorylation via AMP-activated protein kinase
in the liver. J Lipid Res. 56:963–971. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cao K, Lv W, Liu X, Fan Y, Wang K, Feng Z
and Liu J, Zang W, Xing L and Liu J: Herba houttuyniae
extract benefits hyperlipidemic mice via activation of the
AMPK/PGC-1α/Nrf2 cascade. Nutrients. 12(164)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Song Y, Li X, Liu Y, Hu Y and Yang R:
Arctigenin improves lipid metabolism by regulating AMP-activated
protein kinase and downstream signaling pathways. J Cell Biochem.
120:13275–13288. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee KH, Jeong ES, Jang G, Na JR, Park S,
Kang WS, Kim E, Choi H, Kim JS and Kim S: Unripe rubus coreanus
miquel extract containing ellagic acid regulates AMPK, SREBP-2,
HMGCR, and INSIG-1 signaling and cholesterol metabolism in vitro
and in vivo. Nutrients. 12(610)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gallo-Ebert C, Francisco J, Liu HY, Draper
R, Modi K, Hayward MD, Jones BK, Buiakova O, McDonough V and
Nickels JT Jr: Mice lacking ARV1 have reduced signs of metabolic
syndrome and non-alcoholic fatty liver disease. J Biol Chem.
293:5956–5974. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang C, Wang J, Hu W, Wang C, Lu X, Tong
L, Wu F and Zhang W: Identification of functional farnesoid X
receptors in brain neurons. FEBS Lett. 590:3233–3242.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang T, Zhao M, Lu D, Wang S, Yu F, Guo
L, Wen S and Wu B: REV-ERBα regulates CYP7A1 Through repression of
liver receptor homolog-1. Drug Metab Dispos. 46:248–258.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huang M, Kong B, Zhang M, Rizzolo D,
Armstrong LE, Schumacher JD, Chow MD, Lee YH, Joseph LB, Stofan M,
et al: Enhanced alcoholic liver disease in mice with
intestine-specific farnesoid X receptor deficiency. Lab Invest.
100:1158–1168. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jung D, York JP, Wang L, Yang C, Zhang A,
Francis HL, Webb P, McKeehan WL, Alpini G, Lesage GD, et al:
FXR-induced secretion of FGF15/19 inhibits CYP27 expression in
cholangiocytes through p38 kinase pathway. Pflugers Arch.
466:1011–1019. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Matsui S, Yamane T, Takita T, Oishi Y and
Kobayashi-Hattori K: The hypocholesterolemic activity of Momordica
charantia fruit is mediated by the altered cholesterol- and bile
acid-regulating gene expression in rat liver. Nutr Res. 33:580–585.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Huang F, Zheng X, Ma X, Jiang R, Zhou W,
Zhou S, Zhang Y, Lei S, Wang S, Kuang J, et al: Theabrownin from
Pu-erh tea attenuates hypercholesterolemia via modulation of gut
microbiota and bile acid metabolism. Nat Commun.
10(4971)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Altomonte J, Cong L, Harbaran S, Richter
A, Xu J, Meseck M and Dong HH: Foxo1 mediates insulin action on
apoC-III and triglyceride metabolism. J Clin Invest. 114:1493–1503.
2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kamagate A, Qu S, Perdomo G, Su D, Kim DH,
Slusher S, Meseck M and Dong HH: FoxO1 mediates insulin-dependent
regulation of hepatic VLDL production in mice. J Clin Invest.
118:2347–2364. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Link W: Introduction to FOXO biology.
Methods Mol Biol. 1890:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bao S, Wu YL, Wang X, Han S, Cho S, Ao W
and Nan JX: Agriophyllum oligosaccharides ameliorate hepatic injury
in type 2 diabetic db/db mice targeting
INS-R/IRS-2/PI3K/AKT/PPAR-γ/Glut4 signal pathway. J Ethnopharmacol.
257(112863)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhu M, Qin YC, Gao CQ, Yan HC, Li XG and
Wang XQ: Extracellular glutamate-induced mTORC1 activation via the
IR/IRS/PI3K/Akt pathway enhances the expansion of porcine
intestinal stem cells. J Agric Food Chem. 67:9510–9521.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang W, Hou C, Du L, Zhang X, Yang M,
Chen L and Li J: Protective action of pomegranate peel polyphenols
in type 2 diabetic rats via the translocation of Nrf2 and FoxO1
regulated by the PI3K/Akt pathway. Food Funct. 12:11408–11419.
2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chen YJ, Chen CC, Li TK, Wang PH, Liu LR,
Chang FY, Wang YC, Yu YH, Lin SP, Mersmann HJ and Ding ST:
Docosahexaenoic acid suppresses the expression of FoxO and its
target genes. J Nutr Biochem. 23:1609–1616. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hooper AJ, Burnett JR and Watts GF:
Contemporary aspects of the biology and therapeutic regulation of
the microsomal triglyceride transfer protein. Circ Res.
116:193–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Upton GV: Lipids, cardiovascular disease,
and oral contraceptives: A practical perspective. Fertil Steril.
53:1–12. 1990.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Xu LN, Yin LH, Jin Y, Qi Y, Han X, Xu YW,
Liu KX, Zhao YY and Peng JY: Effect and possible mechanisms of
dioscin on ameliorating metabolic glycolipid metabolic disorder in
type-2-diabetes. Phytomedicine. 67(153139)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Maximus PS, Al Achkar Z, Hamid PF, Hasnain
SS and Peralta CA: Adipocytokines: Are they the theory of
everything? Cytokine. 133(155144)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pyrzak B, Ruminska M, Popko K and Demkow
U: Adiponectin as a biomarker of the metabolic syndrome in children
and adolescents. Eur J Med Res. 15 (Suppl 2):S147–S151.
2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Münzberg H and Morrison CD: Structure,
production and signaling of leptin. Metabolism. 64:13–23.
2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Yadav A, Kataria MA, Saini V and Yadav A:
Role of leptin and adiponectin in insulin resistance. Clin Chim
Acta. 417:80–84. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Richard AJ and Stephens JM: The role of
JAK-STAT signaling in adipose tissue function. Biochim Biophys
Acta. 1842:431–439. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang T, Fahrmann JF, Lee H, Li YJ,
Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, et al:
JAK/STAT3-regulated fatty acid β-oxidation is critical for breast
cancer stem cell self-renewal and chemoresistance. Cell Metab.
27:136–150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Lin X, Shi H, Cui Y, Wang X, Zhang J, Yu W
and Wei M: Dendrobium mixture regulates hepatic gluconeogenesis in
diabetic rats via the phosphoinositide-3-kinase/protein kinase B
signaling pathway. Exp Ther Med. 16:204–212. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chen Y, Lu W, Jin Z, Yu J and Shi B:
Carbenoxolone ameliorates hepatic lipid metabolism and inflammation
in obese mice induced by high fat diet via regulating the
JAK2/STAT3 signaling pathway. Int Immunopharmacol.
74(105498)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Olmos-Ortiz A, Olivares-Huerta A,
García-Quiroz J, Zariñán T, Chavira R, Zaga-Clavellina V, Avila E,
Halhali A, Durand M, Larrea F and Díaz L: Placentas associated with
female neonates from pregnancies complicated by urinary tract
infections have higher cAMP content and cytokines expression than
males. Am J Reprod Immunol. 86(e13434)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gold MG, Gonen T and Scott JD: Local cAMP
signaling in disease at a glance. J Cell Sci. 126:4537–4543.
2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hanna R, Nour-Eldine W, Saliba Y,
Dagher-Hamalian C, Hachem P, Abou-Khalil P, Mika D, Varin A, El
Hayek MS, Pereira L, et al: Cardiac phosphodiesterases are
differentially increased in diabetic cardiomyopathy. Life Sci.
283(119857)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Frezza E, Martin J and Lavery R: A
molecular dynamics study of adenylyl cyclase: The impact of ATP and
G-protein binding. PLoS One. 13(e0196207)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Leukes V, Walzl G and du Plessis N:
Myeloid-derived suppressor cells as target of phosphodiesterase-5
inhibitors in host-directed therapeutics for tuberculosis. Front
Immunol. 11(451)2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
El Awdan SA, Abdel Rahman RF, Ibrahim HM,
Hegazy RR, El Marasy SA, Badawi M and Arbid MS: Regression of
fibrosis by cilostazol in a rat model of thioacetamide-induced
liver fibrosis: Up regulation of hepatic cAMP, and modulation of
inflammatory, oxidative stress and apoptotic biomarkers. PLoS One.
14(e0216301)2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ge M, Guo R, Lou HX and Zhang W: Extract
of paecilomyces hepiali mycelia induces lipolysis through
PKA-mediated phosphorylation of hormone-sensitive lipase and
ERK-mediated downregulation of perilipin in 3T3-L1 adipocytes. BMC
Complement Altern Med. 18(326)2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Xiao N, Yang LL, Yang YL, Liu LW, Li J,
Liu B, Liu K, Qi LW and Li P: Ginsenoside Rg5 inhibits
succinate-associated lipolysis in adipose tissue and prevents
muscle insulin resistance. Front Pharmacol. 8(43)2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Du Q, Zhang S, Li A, Mohammad IS, Liu B
and Li Y: Astragaloside IV inhibits adipose lipolysis and reduces
hepatic glucose production via Akt dependent PDE3B expression in
HFD-Fed mice. Front Physiol. 9(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wu L, Wang Y, Chi G, Shen B, Tian Y, Li Z,
Han L, Zhang Q and Feng H: Morin reduces inflammatory responses and
alleviates lipid accumulation in hepatocytes. J Cell Physiol.
234:19785–19798. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Xu HY, Yu L, Chen JH, Yang LN, Lin C, Shi
XQ and Qin H: Sesamol alleviates obesity-related hepatic steatosis
via activating hepatic PKA pathway. Nutrients.
12(329)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhao Y, Peng L, Yang LC, Xu XD, Li WJ, Luo
XM and Jin X: Wedelolactone regulates lipid metabolism and improves
hepatic steatosis partly by AMPK activation and up-regulation of
expression of PPARα/LPL and LDLR. PLoS One.
10(e0132720)2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Gong X, Li T, Wan R and Sha L: Cordycepin
attenuates high-fat diet-induced non-alcoholic fatty liver disease
via down-regulation of lipid metabolism and inflammatory responses.
Int Immunopharmacol. 91(107173)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Cao Y, Bei W, Hu Y, Cao L, Huang L, Wang
L, Luo D, Chen Y, Yao X, He W, et al: Hypocholesterolemia of
rhizoma coptidis alkaloids is related to the bile acid by
up-regulated CYP7A1 in hyperlipidemic rats. Phytomedicine.
19:686–692. 2012.PubMed/NCBI View Article : Google Scholar
|