1. Introduction
The interleukin (IL)-1 family of cytokines plays a
major role in regulating the expression of genes related to
inflammation in autoimmune diseases (AIDs) (1,2).
IL-37 is also known as IL-1 family member 7 (IL-1F7), IL-1H4, and
IL-1RP1. It is a novel anti-inflammatory cytokine with
immunomodulatory effects. Specifically, it reduces the production
of anti-inflammatory cytokines and thereby inhibits the
inflammatory and immune responses by reducing the production of
anti-inflammatory cytokines. IL-37 functions in three ways, i.e.,
by reducing the synthesis of pro-inflammatory cytokines, by
lowering the expression of transcriptional cytokines, and by
inhibiting the activation of kinase signaling (3,4). The
aim of the present review was to summarize the immunomodulatory
roles of IL-37, as well as relevant clinical studies based on the
protective mechanisms of IL-37 in AIDs in order to develop
therapeutic strategies for treatment of AIDs.
2. Biological characteristics of IL-37
Structure
IL-37, commonly known as IL-1F7, is a member of the
IL-1 family of cytokines identified ten years ago (5). The IL-37 gene located at
2q12-q14.1 on chromosome 2, is typically composed of seven exons
(6). There are five basic subtypes
of IL-37, including IL-37a, IL-37b, IL-37c, IL-37d, and IL-37e.
Exons 3, 4, 5, and 6 encode IL-37a. IL-37b is encoded by exons 1,
2, 4, 5, and 6, while IL-37c is encoded by exons 1, 2, 5, and 6.
Exons 1, 4, and 6 encode IL-37d. IL-37e is encoded by exons 1, 5,
and 6(7). It has been suggested
that structural alterations induced in response to
caspase-1-mediated cleavage are responsible for the production of a
variety of IL-37 subtypes (8-10).
The action of IL-37 is mediated by a β-barrel structural unit in
its secondary structure. The 12-β-strand-containing proteins may be
formed by amino acid sequences encoded by exons 4, 5, and 6 of
IL-37a, IL-37b, and IL-37d (9,11).
The 12-hypothetical β-strand structural units that constitute the
primary secondary β-trefoil structure of IL-37 are responsible for
the function of the protein. Other members of the IL-1 family have
a similar barrel structure, which is intimately connected to the
binding of the IL-1 receptor (12). This construct has been shown to be
intimately involved in IL-1 receptor binding. Despite possessing
the same β-trefoil secondary structure, differential regulation of
signaling downstream of the receptor dictates differences in the
activity of other cytokine members of the IL-1 family (10,13).
However, further studies are required to reveal the detailed
structural basis of this phenomenon.
An additional structural feature of IL-37 is its
existence as a dimer (homodimer). It has been shown that this
structure is found mostly in the IL-37b subtype. A symmetrical
head-to-head IL-37b homodimer interface is created by the β3-β4
loops and β-trefoil sheet (β2-β3-β11) of each subunit. This dimer
is a negative regulator of IL-37 activity, and the formation of
such dimers weakens the anti-inflammatory effect of extracellular
IL-37(14). It is possible that
the binding of the homodimer to the IL-1 receptor is due to the
formation of the 12-β-trefoil structure (15).
Distribution, expression, and
release
IL-37 is widely expressed in multiple human tissues
and organs, including the skin, heart, kidney, gut, lymph node,
thymus, bone marrow, lung, testis, placenta, and uterus (16). However, the expression of distinct
subtypes differs according to the specific tissues and organs
involved. Under physiological conditions, IL-37a is mostly found in
the lymph nodes, thymus, bone marrow, placenta, colon, lung,
testicles, and brain, whereas IL-37b is mainly found in the
peripheral blood, lymph nodes, placenta, colon, lung, testicles,
and kidney. IL-37c is mostly expressed in the lymph nodes,
placenta, colon, lung, testis, and heart, whereas IL-37d is
predominantly expressed in the testis, bone marrow, blood system,
umbilical cord tissue, and adipose tissue mesenchymal stem cells.
The testicles and bone marrow are the primary sites of IL-37e
expression. Cells from the aforementioned tissues may express IL-37
in a number of ways; for instance, monocytes, macrophages, B cells,
plasma cells, endothelial cells, and skin keratinocytes are all
capable of producing IL-37 (17,18).
IL-37 is expressed at low levels under physiological
conditions, but can be upregulated in response to inflammatory
stimuli and pro-cytokines. For example, IL-37 is mainly produced by
macrophages in response to Toll-like receptor (TLR) activation
(19), and lipopolysaccharide
(LPS) can induce the expression of IL-37 in RAW264.7 mouse
macrophage cells (20). Triptolide
has been found to facilitate the expression of IL-37 in THP-1 cells
through activation of the p38 and extracellular regulated protein
kinase (ERK)1/2 pathways (21).
In different cells, such as peripheral blood
mononuclear cells (PBMCs), RAW-IL-37 cells, dendritic cells (DCs),
epithelial cells, endothelial cells, and T cells, IL-37 can be
upregulated by various pro-inflammatory cytokines, such as tumor
necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-1β,
transforming growth factor-β1 (TGF-β1; low concentrations), IL-4,
and IL-6(22). IL-12, IL-32, and
granulocyte-macrophage colony-stimulating factor (GM-CSF) are known
to limit IL-37 production (5).
In vivo evidence has shown that IL-37 can block the activity
of Th1/Th2/Th17 cells via PBMCs, M1 macrophages, and DCs (23,24),
while activating the function of Tregs (25,26).
However, specific signaling pathways remain poorly understood.
3. Biological functions of IL-37
IL-37 primarily reduces innate and acquired immune
responses through intracellular and extracellular inhibition by
reducing the secretion of pro-inflammatory chemokines (11,27).
IL-37 is a transcription factor that can be used to regulate gene
expression in cells. Caspase-1 cooperates with the signal
transduction protein Smad3 to regulate its transcription (17).
The IL-37a mRNA splicing site is positioned
at the N-terminus of the amino acid sequence encoded by exon 3,
which is located at the end of the exon. IL-37d also encodes
the 12-β-strand-containing protein structure as it comprises exons
1, 4, 5, and 6, while IL-37b encodes a transcript variant
containing exons 1 and 2, and includes an N-terminal pro-domain
that comprises a potential caspase-1 cleavage site (28). Caspase-1 is primarily responsible
for IL-37 splicing. Following translation, IL-37 exists in
the form of an immature precursor peptide, which is subsequently
cleaved by caspase-1 between amino acid residues D20 to E21 encoded
by exon 1 of IL-37. This cleavage leads to the formation of
active IL-37. Only mature IL-37 can perform biological tasks both
extracellularly and intracellularly (8).
IL-37 binds to Smad3, dimers of which eventually
enter the nucleus. Complexes formed in the cytoplasm by mature
IL-37 and phosphorylated activated Smad3 translocate into the
nucleus, where they are involved in regulating transcriptional
activity (8,29). In response to the interaction
between IL-37 and Smad3, the production of protein tyrosine
phosphatases (PTPNs), which can prevent the activation of tyrosine
phosphorylation-dependent signaling pathways, may be increased.
PTPNs have been shown to inhibit a number of inflammation- and
immune-related pathways, including ERK, mitogen-activated protein
kinase (MAPK), c-Jun N-terminal kinase (JNK),
phosphatidylinositol-3-kinase (PI3K), nuclear factor-κB (NF-κB),
and signal transduction and activator of transcription
(STAT)3(17). IL-37/Smad3
complexes can compete with Smad2/3/4 complexes to reduce the
phosphorylation of Smad2 and Smad4, allowing them to perform
additional biological functions in the nucleus. However, the
specific regulation remains to be elucidated (12).
The primary function of IL-37, an anti-inflammatory
cytokine, is the secretion of proteins to the exterior of cells,
which act as ligands for a functional receptor structure on the
target cell membrane. The β-barrel structure of IL-37b binds to the
α chain of the IL-18 receptor (IL-18R) and reduces the production
of inflammatory mediators (30).
Similar to IL-18, IL-37 is capable of non-competitively binding to
the receptors IL-18Ra and IL-18BP to create trimeric complexes,
which may then activate downstream transduction signals, such as
the NF-κB, the mammalian target protein of rapamycin (mTOR), MAPK,
ERK, AMP-dependent protein kinase (AMPK), and STAT3/6 signaling
pathways (11,31,32).
IL-37 also promotes the activation of M2 macrophages and
tolerogenic DCs by downregulating MHC Class II, CD40, and
CD86(33). These aforementioned
studies indicated that IL-37 may regulate immunosuppressive
responses by downregulating the expression of DC costimulatory
molecules.
Formation of triplex complexes is critical for the
anti-inflammatory effects of IL-37 and can coordinate innate immune
responses by inducing the activation of myeloid differentiation
factor 88 (MyD88) (34). IL-37 is
not expressed in mice. Transgenic human IL-37 (hIL-37tg) and
wild-type (WT) mice are widely used as animal models to investigate
IL-37 pathology (10).
IL-37 forms a complex with IL-18Rα and IL-1R8
(previously TIR8 or SIGIRR), which can markedly reduce the
anti-inflammatory activity of IL-37, indicating that the formation
of the IL-37 receptor complex is required for IL-37 to fulfill its
biological activities (35,36).
Furthermore, IL-37 negatively regulates inflammatory responses in
the innate immune system by downregulating IL-1 receptor-associated
kinase 1 (IRAK), phosphatase and tensin homolog (PTEN), TNF
receptor-associated factor 6 (TRAF6), NOD-like receptor family
pyrin domain containing 3 (NLRP3), mTOR, and thymic stromal
lymphopoietin (TSLP), and decreasing the levels of reactive oxygen
species (ROS) (37,38).
Currently, there is no evidence linking IL-37 to
autophagy. However, the potential role of IL-37 in autophagy is
currently unknown, although certain studies have revealed a
potential link between them. IL-37 enhances autophagy and induces
metabolic reprogramming by reducing mTOR expression and increasing
the AMPK levels, resulting in changes in the cellular redox state,
or by increasing oxidative phosphorylation (39,40).
IL-37 may be involved in the progression of lung fibrosis by
inhibiting TGF-β1 signaling and enhancing autophagy (41). IL-37/IL-1R8 exerts a
pseudo-starvation effect on mTOR (36). The molecular mechanisms of
IL-37-mediated autophagy also require investigation, and may
provide new insights into the development of IL-37-mediated
immunotherapy (Fig. 1).
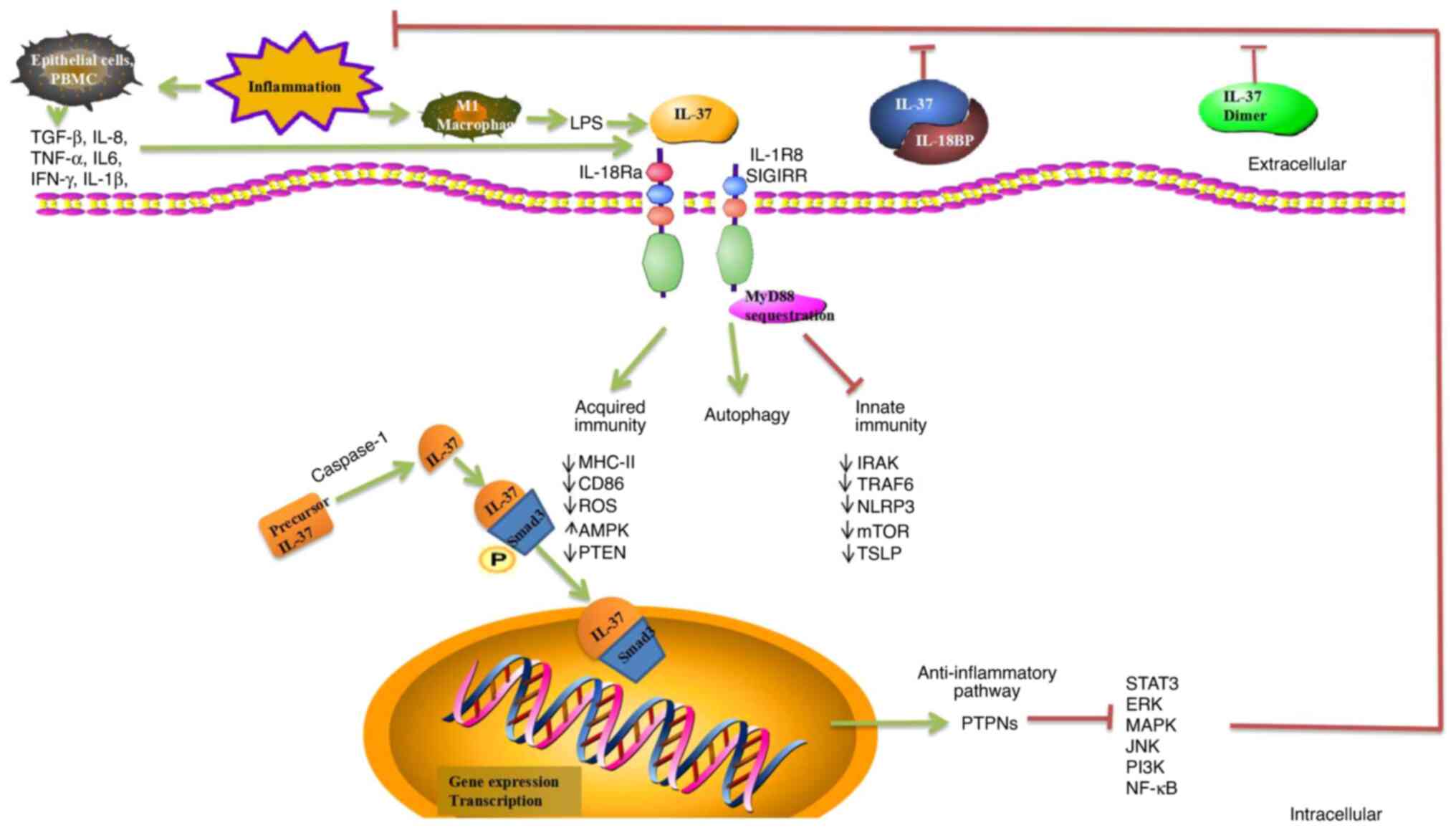 | Figure 1Role of IL-37 regulation of immunity.
IL-37 has significant anti-inflammatory, anticancer,
immuno-suppressive, and metabolic regulatory effects. IL-37 binds
to IL-18Ra or IL-18BP, which can enhance inhibition of IL-18 and
reduce inflammation. The homodimer of IL-37 is a negative regulator
of extracellular anti-inflammatory activity. IL-37 is translocated
to the nucleus after being processed by caspase-1, and precursor
IL-37 is processed into mature IL-37. Complexes formed by mature
IL-37 and phosphorylated activated Smad3 in the cytoplasm undergo
nuclear translocation into the nucleus, where they play a role in
regulating transcriptional activity. PTPNs are activated and
numerous related inflammatory and immune pathways are inhibited,
including ERK, MAPK, JNK, PI3K, NF-κB, and STAT3. IL-37 binds to
its receptor IL-18Rα, recruiting the co-receptor IL-1R8 to form the
IL-37/IL-18Rα/IL-1R8 complex at the plasma membrane induced by
inhibiting MyD88-dependent signaling. IL-37 negatively regulates
inflammatory responses in the innate and acquired immune system by
downregulating IRAK, PTEN, ROS, TRAF6, NLRP3, mTOR, TSLP, MHC-II,
and CD86. IL-37 acts as an anti-inflammatory cytokine by decreasing
the production of pro-inflammatory cytokines and chemokines. IL-37
enhances autophagy and induces metabolic reprogramming by reducing
the expression of mTOR and increasing the AMPK levels. However,
molecular mechanisms of IL-37-mediated autophagy remain unknown.
IL-18Ra, α-subunit of IL-18 receptor; IL-18BP, IL-18 binding
protein; PTPNs, protein tyrosine phosphatases; ERK, extracellular
signal-regulated kinase; MAPK, mitogen-activated protein kinase;
JNK, c-Jun N-terminal kinase; PI3K, phosphatidylinositol-3-kinase;
NF-κB, nuclear factor-κB; STAT, signal transduction and activator
of transcription; MyD88, myeloid differentiation factor 88; IRAK,
interleukin-1 receptor-associated kinase 1; PTEN, phosphatase and
tensin homolog; ROS, reactive oxygen species; TRAF6, TNF
receptor-associated factor 6; NLRP3, NOD-like receptor family pyrin
domain containing 3; mTOR, mammalian target of rapamycin; TSLP,
thymic stromal lymphopoietin; AMPK, AMP-dependent protein
kinase. |
4. IL-37 and AIDs
Accumulating evidence shows that the expression of
IL-37 is closely related to various AIDs such as rheumatoid
arthritis (RA), systemic lupus erythematosus (SLE), Sjögren's
syndrome (pSS), ankylosing spondylitis/spondyloarthritis (AS/SpA),
vasculitis, gout, and osteoarthritis (OA) (Table I).
 | Table IIL-37 in autoimmune disease. |
Table I
IL-37 in autoimmune disease.
| Disease | Role of IL-37 | (Refs.) |
|---|
| RA | Associated with
proinflammatory factors, TLR4 | (44,45,47,49-51) |
| | Associated with
disease activity | (44-46,49,51-53) |
| | Associated with
activated T cell function | (46) |
| | Associated with
bone loss | (49) |
| | Inhibits RAFLS
proliferation and migration; induces RAFLS apoptosis by inhibiting
the STAT3 pathway | (54) |
| AS/SPA | Associated with
bone density | (63) |
| | Associated with
disease activity | (63,64) |
| GOUT | Associated with
proinflammatory factors | (67,70) |
| | Associated with
disease activity | (67,69) |
| | Associated with
tophi forming, kidney deterioration | (67) |
| | rhIL-37 suppressed
MSU-induced innate immune responses by enhancing expression of
Smad3 and IL-1R8 to trigger multiple intracellular switches to
inhibit NLRP3 and the activation of SOCS3 | (68) |
| | Reduces the
transcription of pyrophosphate-related proteins and release of
inflammatory cytokines by enhancing phagocytosis of MSU, protects
mitochondrial function, and mediates metabolic reprogramming in
THP-1 cells treated by MSU, which depended on the mediation of
GSK-3 β | (70) |
| OA | Associated with
VAS | (75,76) |
| | Affects M1/M2-like
macrophage polarization | (75) |
| | Associated with
proinflammatory factors | (76,78) |
| | rhIL-37 may
regulate the key downstream target MMP-3 | (78) |
| SLE | Associated with
disease activity (SLEDAI) | (80,82,83) |
| | Associated with
kidney damage and skin lesion. | (80,83) |
| | Associated with
Asian race | (81) |
| | Associated with
proinflammatory factors | (82) |
| | Negatively
correlated with C3/C4, and antibodies | (83,84) |
| | Associated with
C3 | (84) |
| PSS | Associated with RF,
antibodies, proinflammatory factors | (87) |
| BD | Negatively
correlated with inflammatory response | (89-91) |
| | rhIL-37 may reduce
the levels of TSLP in vitro | (92) |
| ITP | Positive correlate
with the platelet count | (95) |
| | Positive correlate
with proinflammatory factors | (96,97) |
| MS | Act as a part of a
feed-back loop to control underlying inflammation | (99) |
| | Positive correlate
with disease activity | (99,100) |
| | Regulate autophagy,
apoptosis | (101) |
IL-37 and inflammatory joint
disease
Inflammatory arthritis (IA) is a prevalent joint
inflammatory illness, in addition to RA, OA and spondyloarthritis
(42).
RA
RA is a chronic inflammatory disease that can cause
irreversible joint damage and physical disability. It can involve
the skin, eyes, lungs, heart, and blood vessels (43).
As previously reported, the amount of IL-37 in
normal human plasma and bodily fluids is exceedingly low, but is
markedly increased in the synovial fluid, serum, and PBMCs of
patients with RA (24,44-55).
Increased IL-37 levels in the serum are positively associated with
inflammatory markers [erythrocyte sedimentation rate (ESR) and
C-reactive protein (CRP)], rheumatoid factor (RF), anti-cyclic
citrullinated peptide antibody (anti-CCP), disease activity
score-28 (DAS28), bone loss, and pro-inflammatory cytokine
expression (IL-6, IL-18, IL-4, IFN-γ, IL-17A).
The proportion of CD3+ CD26+ T
cells is associated with disease activity, implying that IL-37
levels are positively correlated with activated T lymphocytes
(46). It has been shown that
IL-37 affects the activity and phenotype of DCs and suppresses the
inflammatory responses mediated by Th17 and IL-17 in RA, while
failing to inhibit Th-17 cell differentiation (24). In a study involving 70 patients
with juvenile idiopathic arthritis (JIA), serum/synovial IL-37
levels and IL-37 mRNA expression in PBMCs were positively
associated with disease activity and angiogenesis indicators
[including vascular endothelial growth factor (VEGF) and VEGF
receptors] (56).
A previous study indicated that angiogenesis is a
crucial mechanism for the proliferation of synovial tissue and the
formation of invasive pannus in the early onset of RA (57), and is associated with the
regulation of VEGF and angiogenesis inhibitors. The inflammatory
response of the RA synovial tissue is triggered by stimuli. Local
macrophages and fibroblasts also respond to produce
pro-inflammatory cytokines, which can modulate the expression of
adhesion molecules, matrix metalloproteinases (MMPs), chemokines,
TLRs, and growth factors that are important at different stages of
angiogenesis (58). VEGF is a
cytokine that acts on the vascular endothelium of the synovium,
promotes angiogenesis, and binds to cognate receptors on
endothelial cells (ECs), thereby activating these cells to produce
greater levels of proteolytic enzymes. VEGF expression in the
synovial tissue is regulated by angiogenesis (59). Redox signaling is closely
associated with angiogenesis and can alter the angiogenic response
of synovial cells. Downregulation of hypoxia-inducible factor-1α
(HIF-1α) significantly reduces angiogenesis in VEGF-induced
rheumatoid arthritis fibroblast-like synoviocytes (RAFLS) in
macrophages of the synovial lining (60). Multiple studies have shown that
blocking angiogenic pathways may reduce inflammatory cell
infiltration and damage to joints (61,62).
In animal models of RA, prophylactic treatment with anti-VEGF
antibodies delays the onset, joint swelling, and vascularization in
collagen-induced arthritis (CIA) (63). Inhibition of angiogenesis has
emerged as a new option for the treatment of RA in recent years,
and numerous drugs targeting RA angiogenesis have been developed
(64).
In an in vitro study, recombinant IL-37
(rhIL-37) was used to stimulate PBMCs in RA patients. It was
discovered that rhIL-37 considerably decreased the levels of TNF-α,
IL-17, and IL-6 in RA patients (48).
The role of IL-37 single nucleotide polymorphisms
(SNPs) in RA is debatable. A Chinese RA population study revealed
that IL-37 rs3811047 is positively associated with disease
activity, indicating that the prognosis of RA patients with various
IL-37 genotypes varies (65). Two
other studies involving a Han RA population revealed that no
genotypes were associated with RA susceptibility (66,67).
In future, it is important to expand sample sizes and ethnic
diversity to identify distinct IL-37 phenotypes in patients with
RA.
Collectively, IL-37 may be presented as a novel
biomarker for predicting and monitoring disease
severity/therapeutic targets in RA by reducing inflammation.
AS/SPA
AS/SPA is a chronic inflammatory illness that
affects the sacroiliac joints, spine bony processes, paraspinal
soft tissues, and peripheral joints, with extra-articular symptoms
occurring in certain cases (68).
The level of IL-37 in the serum and PBMCs of
patients with AS was revealed to be higher than that in healthy
controls (HCs), and was was associated with ESR, CRP, Bath
Ankylosing Spondylitis Disease Activity Index (BASDAI), Ankylosing
Spondylitis Disease Activity Score (ASDAS), and bone density. It
was found that IL-37 can inhibit the expression of pro-inflammatory
cytokines (TNF-α, IL-6, IL-17, and IL-23) in PBMCs of patients with
AS, indicating a potential anti-inflammatory role of IL-37 in AS
(69,70). RhIL-37 can significantly reduce
LPS-stimulated PBMC proliferation and IL-6, IL-17, IL-23, and TNF-α
production (70).
The A/G frequency of IL-37 rs3811047 in AS
patients is significantly different from that observed in the
general population, and there is a link between this and alcohol
consumption (71). As it is
related to the susceptibility to AS in the Han population,
IL-37 A/G rs3811047 could be regarded as an independent risk
factor.
In conclusion, the aforementioned studies suggested
that IL-37 plays significant roles in the development of AS/SPA.
IL-37 may be used as a predictive biomarker for AS, as well as to
assess the degree of inflammation and bone loss.
Gout
Gout is caused by the precipitation of monosodium
urate (MSU) crystals within joints and soft tissues that can
progress to acute or chronic arthritis (72). Consequently, a negative feedback
mechanism for MSU-induced inflammation may be present. IL-37 has
been identified as a potential anti-inflammatory agent in response
to MSU. However, the association between IL-37 and clinical markers
and pro-inflammatory mediators in individuals with gout is not
fully understood.
Several studies have shown that IL-37 expression is
increased in PBMCs of patients with gout (73-75),
and is positively correlated with ESR, CRP, tophi formation, and
platelet counts.
Different doses of MSU have been shown to elicit
dose-dependent overexpression of IL-37 protein and mRNA in PMBCs
in vitro (75).
The mRNA level of pro-IL-37 in PBMCs from
patients with acute gout (AG) was significantly higher than that in
non-AG (NAG) PBMCs, indicating that IL-37 may act as a suppressor
of MSU-induced inflammation. Additionally, this study demonstrated
that rhIL-37 inhibited MSU-induced innate immune responses by
increasing the expression of Smad3 and IL-1R8, both in vitro
and in vivo. IL-37 controls MSU-induced inflammation, in
part through a MERTK-dependent signaling pathway (74). IL-37 inhibits gout inflammation and
exerts its effects in vitro by altering macrophage function
(76).
Therefore, rhIL-37 has both preventive and
therapeutic effects on gout, and the suppressive effect of
IL-37-mediated inflammation may partially depend on the activation
of MERTK (74,77). Recombinant human PDZ domain 1
protein (PDZK1) is a cytoskeletal protein expressed in renal
tubular epithelial cells that interacts with a variety of uric acid
transporters to control uric acid. A previous study revealed that
the transcription of PDZK1 expression stimulated with
various concentrations of IL-37 may regulate uric acid metabolism
through the NF-κB signaling pathway in HK-2 cells (78).
Using a molecular inversion probe sequencing
technique, four unusual IL-37 variations were detected in 675
individuals with gout. It is possible that carriers of p.(N182S)
(rs752113534) are at a higher risk of developing gout and undergo
early onset of disease (79).
These studies revealed that IL-37 may be a
potentially valuable treatment option for patients with chronic
gout, especially those with tophi and kidney damage.
OA
OA is one of the most frequent degenerative joint
disorders that affects people globally (80). A link between the expression and
function of IL-37 and OA remains unclear. IL-37 levels are elevated
in the blood, synovial fluid, synovial cells from lesions, and
chondrocytes of patients with OA. In addition, in patients with OA,
IL-37 levels are favorably linked with ESR, CRP, visual analog
scale (VAS) pain score, as well as other variables (81,82).
Luo et al determined that IL-37a and IL-37b receptors are
overexpressed in chondrocytes from patients with temporomandibular
joint (TMJ) OA. IL-1R8 is required for IL-37b to exert
anti-inflammatory effects on the TMJ. The therapeutic potential of
IL-37b in the treatment of TMJ inflammation was also elucidated,
suggesting that targeting the IL-37 pathway may provide a novel
therapeutic strategy for treating inflammation in OA patients.
RhIL-37b has also been shown to decrease the expression of
inflammatory cytokines in the TMJ, which is associated with reduced
inflammation and subchondral bone loss (81).
Ding et al revealed that IL-37 was
significantly upregulated in erosive osteoarthritis (EIOA)
[compared to primary generalized osteoarthritis (PGOA) and HCs],
and that the release of pro-inflammatory cytokines in synovial
cells treated with IL-37 was significantly inhibited in
vitro (82).
Another study, using immunohistochemical assays
revealed that the IL-37 protein was dysregulated in human OA
chondrocytes, which could inhibit osteoclast differentiation
directly (83). Overexpression of
IL-37 in a model of adenovirus construct (ad-IL-37)-induced OA
resulted in reduced levels of IL-1, IL-6, IL-8, and MMP3(84). MMP3 may be a critical downstream
target of rhIL-37 when interfering with cartilage breakdown.
These studies indicated that IL-37 may prevent
cartilage deterioration in individuals with OA. The presence of
IL-37 may be a useful marker to distinguish EIOA from PGOA. It is
anticipated that rhIL-37 will serve as a new therapeutic option for
individuals with specific types of OA.
IL-37 and SLE
SLE is an autoimmune disease involving the
activation of autoreactive B cells and the dysregulation of
numerous other types of immune cells, including CD4+ T cells, DCs,
macrophages, and neutrophils. SLE is highly heterogeneous in its
various presentations and is characterized by multiple organ damage
(85). The level of IL-37 in the
serum, plasma, and PBMCs of SLE patients is elevated, and has been
shown to be positively correlated with SLE disease activity index
(SLEDAI) scores (especially renal disease activity), the degree of
kidney and skin damage, and the levels of pro-inflammatory
cytokines (IL-6, IL-18, and IFN-γ) (86-90).
The opposite was observed in other studies, which found that the
amount of IL-37 was negatively correlated with the level of
complement proteins (89) and the
levels of anti-Sm and anti-RNP antibodies (90). This may be attributed to the
difference in sample sizes and cohorts.
Treatment with prednisone (1 mg/kg/day for 14 days)
substantially decreased plasma IL-37 expression in SLE patients
(88). RhIL-37 may play an
essential role in SLE pathogenesis by modulating pro-inflammatory
pathways in PBSCs from patients with SLE in vitro (89). Therefore, the level of IL-37 in
Asian patients with SLE may serve as a marker of disease activity.
Three IL-37 SNP variants (rs2723186, rs2723176, and rs4364030) may
also be associated with SLE susceptibility (91).
IL-37 may play an important role in the inhibition
of SLE pathogenesis. Thus, it is expected to serve as a diagnostic
and prognostic tool. Further studies are required to identify the
mechanisms underlying IL-37 regulation during the mediation of
immune reactions in SLE.
IL-37 and pSS and Behçet's disease
(BD)
pSS is an autoimmune disease characterized by focal
lymphocytic infiltration of exocrine glands such as the salivary
and lacrimal glands. Inflammatory response immune aberrations
underlying pSS are mediated by B and mast cells (MCs) (92). Liuqing et al revealed that
patients with pSS have increased IL-37 expression compared to HCs;
IL-37 expression was positively correlated with disease activity
and RF, IL-4, and IL-12 levels (93). Treatment with IL-37 may reduce
glandular inflammation and decrease systemic inflammatory
responses.
BD is a multisystem disorder characterized by
primary vasculitis of unknown etiology. Vasculitis and thrombotic
events are the most common causes of death (94). Currently, only a few studies have
examined the association between IL-37 levels and BD. According to
a previous study, the level of IL-37 in the cerebral fluid of
patients with neuro-BD (NBD) was increased and positively linked
with the level of TGF-β, indicating that IL-37 may be a significant
NBD biomarker (95).
Ye et al revealed that the amount of IL-37 in
the PBMCs of patients with active BD was considerably lower than
that in patients from the HC group. When DCs were stimulated with
rIL-37, the production of IL-6, IL-1, TNF-α, and ROS was reduced.
These stimulated DCs prevented the activation of ERK1/2, JNK, and
P38 MAPK (96). Treatment of
patients with corticosteroids is associated with increased IL-37
expression (97).
TSLP is upregulated during the acute phase of BD,
and is associated with skin lesions. It has been shown that rhIL-37
may reduce the levels of TSLP in vitro (98). Patients with BD tend to develop
ophthalmia. An IL-37 SNP (rs3811047) and IL-18RAP SNP (rs2058660)
have been shown to be associated with susceptibility to BD onset
(1,063 cases) instead of Vogt-Koyanagi-Hrada (VKH) uveitis (419
cases) in a case-control study involving the Chinese Han population
(99).
IL-37 alters immune dysregulation in pSS and BD. It
can be considered as a new biomarker with potential therapeutic
application.
IL-37 and immune thrombocytopenia
(ITP)
ITP is an autoimmune disorder characterized by
isolated thrombocytopenia (platelet count
<100x109/l), in the absence of other causes and
disorders that may be associated with thrombocytopenia (100).
Thus, IL-37 may be involved in ITP pathogenesis.
Current studies suggest that IL-37 levels are elevated in the PBMCs
or serum of patients with ITP (101-103).
Serum IL-37 levels and IL-18Rα+/CD4+ T-cell
ratios are negatively correlated with PLT counts (102). IL-37 expression is significantly
higher in patients with active ITP, and can regulate the expression
of several cytokines to exert anti-inflammatory effects (103). In terms of the possible
mechanisms involving IL-37 in the pathogenesis of ITP, it is
speculated that IL-37 may promote Th2 cell function by influencing
cytokine expression and inhibiting the immune response effects of
Th1 and Th17, thereby alleviating the inflammatory responses. This
may account for elevated IL-37 expression in patients with active
ITP. Thus, IL-37 may represent an important factor for ITP
diagnosis and treatment. Furthermore, rhIL-37 may exert therapeutic
effects with respect to refractory ITP.
IL-37 and multiple sclerosis (MS)
MS is one of the most prevalent neurological
dysfunctions and is an autoimmune disease that affects the central
nervous system (CNS), often leading to severe physical or cognitive
loss and neurological problems in patients (104).
Studies have shown that IL-37 is aberrantly
expressed in MS patients (105,106). There is a significant correlation
between serum IL-37 levels and MS disease severity, and IL-37 may
be involved in a feedback loop that controls the underlying
inflammation in MS pathogenesis (105).
The serum levels of IL-37 and sVEGFR2 and the
circulatory number of VEGFR2-expressing cells were higher in
patients with MS than in HCs (106). Serum levels of IL-37 and sVEGFR2
may represent important prognostic biomarkers for MS. IL-37 plays a
key role in the regulation of oxidative stress, autophagy, and
apoptosis markers in periodontal ligament cells from patients after
hypoxic preconditioning (107).
Thus, IL-37 may serve as a nucleator in a panel of new biomarkers
associated with MS.
5. Outlook
AIDs refer to the set of ailments that arise when
the immune system of the body launches an assault on self-tissues.
This involves the production of aberrant antibodies. IL-37, a
member of the IL-1 family, can decrease both congenital
inflammation and acquired immunological responses. Although the
mechanism underlying IL-37 function is not entirely understood, it
is known to be associated with the development of AIDs. IL-37 plays
a crucial role in protecting tissues from damage in AIDs by
suppressing excessive inflammatory responses. A transgenic IL-37tg
mouse model showed that IL-37 exerts considerable anti-inflammatory
effects (108). Therefore,
further elucidation of the mechanism by which IL-37 is involved in
other AIDs is critical for the therapeutic use of this cytokine.
Currently, clinical studies involving IL-18 inhibitors [glycogen
synthase kinase 1070806(109),
ABT-325(110), and rIL-18 binding
protein (111)] are underway, and
a clinical study involving an IL-33 inhibitor (CNTO-7160) has
commenced (112). IL-37 is
considered to be an AID biomarker, a predictive factor, and a
possible AID treatment.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported in part by
research grants from the Sanming Project of Medicine in Shenzhen
(grant no. SZSM201602087), the Shenzhen Science and Technology
Project (grant no. JCYJ20180302145033769), and the Research on
Public Welfare Project in Futian District, Shenzhen (grant no. FTWS
2021062).
Availability of data and materials
Not applicable.
Authors' contributions
HZ, KZ and ZY designed the study and wrote the
manuscript. HZ and KZ performed literature review. KZ and ZY
conceived the review and edited the manuscript. All authors have
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iwasaki A and Medzhitov R: Control of
adaptive immunity by the innate immune system. Nat Immunol.
16:343–353. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Dinarello CA, Nold-Petry C, Nold M, Fujita
M, Li S, Kim S and Bufler P: Suppression of innate inflammation and
immunity by interleukin-37. Eur J Immunol. 46:1067–1081.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Conti P, Lessiani G, Kritas SK, Ronconi G,
Caraffa A and Theoharides TC: Mast cells emerge as mediators of
atherosclerosis: Special emphasis on IL-37 inhibition. Tissue Cell.
49:393–400. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang X, Xu K, Chen S, Li Y and Li M: Role
of interleukin-37 in inflammatory and autoimmune diseases. Iran J
Immunol. 15:165–174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nold MF, Nold-Petry CA, Zepp JA, Palmer
BE, Bufler P and Dinarello CA: IL-37 is a fundamental inhibitor of
innate immunity. Nat Immunol. 11:1014–1022. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Sims JE and Smith DE: The IL-1 family:
Regulators of immunity. Nat Rev Immunol. 10:89–102. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Pan G, Risser P, Mao W, Baldwin DT, Zhong
AW, Filvaroff E, Yansura D, Lewis L, Eigenbrot C, Henzel WJ and
Vandlen R: IL-1H, an interleukin 1-related protein that binds IL-18
receptor/IL-1Rrp. Cytokine. 13:1–7. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sharma S, Kulk N, Nold MF, Gräf R, Kim SH,
Reinhardt D, Dinarello CA and Bufler P: The IL-1 family member 7b
translocates to the nucleus and down-regulates proinflammatory
cytokines. J Immunol. 180:5477–5482. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Quirk S and Agrawal DK: Immunobiology of
IL-37: Mechanism of action and clinical perspectives. Expert Rev
Clin Immunol. 10:1703–1709. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Allaire JM, Poon A, Crowley SM, Han X,
Sharafian Z, Moore N, Stahl M, Bressler B, Lavoie PM, Jacobson K,
et al: Interleukin-37 regulates innate immune signaling in human
and mouse colonic organoids. Sci Rep. 11(8206)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cavalli G and Dinarello CA: Suppression of
inflammation and acquired immunity by IL-37. Immunol Rev.
281:179–190. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao M, Li Y, Guo C, Wang L, Chu H, Zhu F,
Li Y, Wang X, Wang Q, Zhao W, et al: IL-37 isoform D downregulates
pro-inflammatory cytokines expression in a Smad3-dependent manner.
Cell Death Dis. 9(582)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dinarello CA: Introduction to the
interleukin-1 family of cytokines and receptors: Drivers of innate
inflammation and acquired immunity. Immunol Rev. 281:5–7.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mei Y and Liu H: IL-37: An
anti-inflammatory cytokine with antitumor functions. Cancer Rep
(Hoboken). 2(e1151)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ellisdon AM, Nold-Petry CA, D'Andrea L,
Cho SX, Lao JC, Rudloff I, Ngo D, Lo CY, Soares da Costa TP,
Perugini MA, et al: Homodimerization attenuates the
anti-inflammatory activity of interleukin-37. Sci Immunol.
2(eaaj1548)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Smithrithee R, Niyonsaba F, Kiatsurayanon
C, Ushio H, Ikeda S, Okumura K and Ogawa H: Human β-defensin-3
increases the expression of interleukin-37 through CCR6 in human
keratinocytes. J Dermatol Sci. 77:46–53. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bai J, Li Y, Li M, Tan S and Wu D: IL-37
as a potential biotherapeutics of inflammatory diseases. Curr Drug
Targets. 21:855–863. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pan Y, Wen X, Hao D, Wang Y, Wang L, He G
and Jiang X: The role of IL-37 in skin and connective tissue
diseases. Biomed Pharmacother. 122(109705)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Theoharides TC, Tsilioni I and Conti P:
Mast cells may regulate the anti-inflammatory activity of IL-37.
Int J Mol Sci. 20(3701)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Conti P, Caraffa A, Mastrangelo F,
Tettamanti L, Ronconi G, Frydas I, Kritas SK and Theoharides TC:
Critical role of inflammatory mast cell in fibrosis: Potential
therapeutic effect of IL-37. Cell Prolif. 51(e12475)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
He L, Liang Z, Zhao F, Peng L and Chen Z:
Modulation of IL-37 expression by triptolide and triptonide in
THP-1 cells. Cell Mol Immunol. 12:515–518. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tete S, Tripodi D, Rosati M, Conti F,
Maccauro G, Saggini A, Cianchetti E, Caraffa A, Antinolfi P,
Toniato E, et al: IL-37 (IL-1F7) the newest anti-inflammatory
cytokine which suppresses immune responses and inflammation. Int J
Immunopathol Pharmacol. 25:31–38. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang J, Shen Y, Li C, Liu C, Wang ZH, Li
YS, Ke X and Hu GH: IL-37 attenuates allergic process via
STAT6/STAT3 pathways in murine allergic rhinitis. Int
Immunopharmacol. 69:27–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ye L, Jiang B, Deng J, Du J, Xiong W, Guan
Y, Wen Z, Huang K and Huang Z: IL-37 alleviates rheumatoid
arthritis by suppressing IL-17 and IL-17-triggering cytokine
production and limiting Th17 cell proliferation. J Immunol.
194:5110–5119. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li S, Neff CP, Barber K, Hong J, Luo Y,
Azam T, Palmer BE, Fujita M, Garlanda C, Mantovani A, et al:
Extracellular forms of IL-37 inhibit innate inflammation in vitro
and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc
Natl Acad Sci USA. 112:2497–2502. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
An B, Liu X, Li G and Yuan H:
Interleukin-37 ameliorates coxsackievirus B3-induced viral
myocarditis by modulating the Th17/regulatory T cell immune
response. J Cardiovasc Pharmacol. 69:305–313. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu WD, Zhao Y and Liu Y: Insights into
IL-37, the role in autoimmune diseases. Autoimmun Rev.
14:1170–1175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gu J, Gao X, Pan X, Peng X, Li Y and Li M:
High-level expression and one-step purification of a soluble
recombinant human interleukin-37b in Escherichia coli. Protein Expr
Purif. 108:18–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li W, Ding F, Zhai Y, Tao W, Bi J, Fan H,
Yin N and Wang Z: IL-37 is protective in allergic contact
dermatitis through mast cell inhibition. Int Immunopharmacol.
83(106476)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Robuffo I, Toniato E, Tettamanti L,
Mastrangelo F, Ronconi G, Frydas I, Caraffa Al, Kritas SK and Conti
P: Mast cell in innate immunity mediated by proinflammatory and
antiinflammatory IL-1 family members. J Biol Regul Homeost Agents.
31:837–842. 2017.PubMed/NCBI
|
|
31
|
Wu W, Wang W, Wang Y, Li W, Yu G, Li Z,
Fang C, Shen Y, Sun Z, Han L, et al: IL-37b suppresses T cell
priming by modulating dendritic cell maturation and cytokine
production via dampening ERK/NF-κB/S6K signalings. Acta Biochim
Biophys Sin (Shanghai). 47:597–603. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Conti P, Caraffa A, Ronconi G, Kritas SK,
Mastrangelo F, Tettamanti L, Frydas I and Theoharides TC: Mast
cells participate in allograft rejection: Can IL-37 play an
inhibitory role? Inflamm Res. 67:747–755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Luo Y, Cai X, Liu S, Wang S, Nold-Petry
CA, Nold MF, Bufler P, Norris D, Dinarello CA and Fujita M:
Suppression of antigen-specific adaptive immunity by IL-37 via
induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA.
111:15178–15183. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhan Q, Zeng Q, Song R, Zhai Y, Xu D,
Fullerton DA, Dinarello CA and Meng X: IL-37 suppresses
MyD88-mediated inflammatory responses in human aortic valve
interstitial cells. Mol Med. 23:83–91. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Luo C, Shu Y, Luo J, Liu D, Huang DS, Han
Y, Chen C, Li YC, Zou JM, Qin J, et al: Intracellular IL-37b
interacts with Smad3 to suppress multiple signaling pathways and
the metastatic phenotype of tumor cells. Oncogene. 36:2889–2899.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nold-Petry CA, Lo CY, Rudloff I, Elgass
KD, Li S, Gantier MP, Lotz-Havla AS, Gersting SW, Cho SX, Lao JC,
et al: IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to
carry out its multifaceted anti-inflammatory program upon innate
signal transduction. Nat Immunol. 16:354–365. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Luo P, Peng S, Yan Y, Ji P and Xu J: IL-37
inhibits M1-like macrophage activation to ameliorate
temporomandibular joint inflammation through the NLRP3 pathway.
Rheumatology (Oxford). 59:3070–3080. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kim SK, Choe JY and Park KY: Activation of
CpG-ODN-induced TLR9 signaling inhibited by interleukin-37 in U937
human macrophages. Yonsei Med J. 62:1023–1031. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li T, Zhu D, Mou T, Guo Z, Pu J and Wu Z:
Interleukin-37 induces apoptosis and autophagy of SMMC-7721 cells
by inhibiting phosphorylation of mTOR. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 33:440–445. 2017.PubMed/NCBI(In Chinese).
|
|
40
|
Hou T, Sun X, Zhu J, Hon KL, Jiang P, Chu
IM, Tsang MS, Lam CW, Zeng H and Wong CK: IL-37 ameliorating
allergic inflammation in atopic dermatitis through regulating
microbiota and AMPK-mTOR signaling pathway-modulated autophagy
mechanism. Front Immunol. 11(752)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kim MS, Baek AR, Lee JH, Jang AS, Kim DJ,
Chin SS and Park SW: IL-37 attenuates lung fibrosis by inducing
autophagy and regulating TGF-β1 production in mice. J Immunol.
203:2265–2275. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rausch Osthoff AK, Niedermann K, Braun J,
Adams J, Brodin N, Dagfinrud H, Duruoz T, Esbensen BA, Günther KP,
Hurkmans E, et al: 2018 EULAR recommendations for physical activity
in people with inflammatory arthritis and osteoarthritis. Ann Rheum
Dis. 77:1251–1260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ngian GS: Rheumatoid arthritis. Aust Fam
Physician. 39:626–628. 2010.PubMed/NCBI
|
|
44
|
Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan
YX and Jiang YF: Plasma levels of IL-37 and correlation with TNF-α,
IL-17A, and disease activity during DMARD treatment of rheumatoid
arthritis. PLoS One. 9(e95346)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xia T, Zheng XF, Qian BH, Fang H, Wang JJ,
Zhang LL, Pang YF, Zhang J, Wei XQ, Xia ZF and Zhao DB: Plasma
interleukin-37 is elevated in patients with rheumatoid arthritis:
Its correlation with disease activity and Th1/Th2/Th17-related
cytokines. Dis Markers. 2015(795043)2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ragab D, Mobasher S and Shabaan E:
Elevated levels of IL-37 correlate with T cell activation status in
rheumatoid arthritis patients. Cytokine. 113:305–310.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Song L, Wang Y, Sui Y, Sun J, Li D, Li G,
Liu J, Li T and Shu Q: High interleukin-37 (IL-37) expression and
increased mucin-domain containing-3 (TIM-3) on peripheral T cells
in patients with rheumatoid arthritis. Med Sci Monit. 24:5660–5667.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xia L, Shen H and Lu J: Elevated serum and
synovial fluid levels of interleukin-37 in patients with rheumatoid
arthritis: Attenuated the production of inflammatory cytokines.
Cytokine. 76:553–557. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yang L, Zhang J, Tao J, Tao J and Lu T:
Elevated serum levels of Interleukin-37 are associated with
inflammatory cytokines and disease activity in rheumatoid
arthritis. APMIS. 123:1025–1031. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wan LL, Wang XD and Ci ZC: Expression of
TLR4 in peripheral blood of patients with 274 rheumatoid arthritis
and its correlation with IL-37 level. World J Complex Med. 2:23–25.
2016.
|
|
51
|
Chen X, Tian J, Zhang J and Su J:
Expression and clinical significance of serum IL-37 and soluble
PD-1 in patients with rheumatoid arthritis. Chin J Immunol.
33:422–425. 2017.
|
|
52
|
Akram N, Jamal A, Ullah S, Waqar AB and
Iqbal K: Expression level of serum interleukin-37 in rheumatoid
arthritis patients and its correlation with disease activity score.
Adv Life Sci. 5:159–165. 2018.
|
|
53
|
Ke Q, Huang Z, Yu H, et al: Expression and
significance of interleukin-37 in PBMCs from rheumatoid arthritis
patients. Int J Lab Med. 41:754–757. 2020.(In Chinese).
|
|
54
|
Liu Y and Gao W: Interleukin-37 inhibits
proliferation, migration and induces apoptosis of rheumatoid
arthritis fibroblast-like synoviocytes (RAFLS) by inhibiting STAT3.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 36:236–241. 2020.PubMed/NCBI(In Chinese).
|
|
55
|
Zhu J, Xie C, Qiu H and Shi L: Correlation
between level of interleukin-37 and rheumatoid arthritis
progression. Int J Gen Med. 14:1905–1910. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
El-Barbary AM, Hussein MS, Almedany SH,
Rageh EM, Alsalawy AM, Aboelhawa MA, Elkholy RM, Shafik NM and
Elharoun AS: Role of interleukin 37 as a novel proangiogenic factor
in juvenile idiopathic arthritis. J Clin Rheumatol. 25:85–90.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Elshabrawy HA, Chen Z, Volin MV, Ravella
S, Virupannavar S and Shahrara S: The pathogenic role of
angiogenesis in rheumatoid arthritis. Angiogenesis. 18:433–448.
2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
MacDonald IJ, Liu SC, Su CM, Wang YH, Tsai
CH and Tang CH: Implications of angiogenesis involvement in
arthritis. Int J Mol Sci. 19(2012)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sabi EM, Singh A, Althafar ZM, Behl T,
Sehgal A, Singh S, Sharma N, Bhatia S, Al-Harrasi A, Alqahtani HM
and Bungau S: Elucidating the role of hypoxia-inducible factor in
rheumatoid arthritis. Inflammopharmacology. 30:737–748.
2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hu F, Mu R, Zhu J, Shi L, Li Y, Liu X,
Shao W, Li G, Li M, Su Y, et al: Hypoxia and hypoxia-inducible
factor-1α provoke toll-like receptor signalling-induced
inflammation in rheumatoid arthritis. Ann Rheum Dis. 73:928–936.
2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang Y, Wu H and Deng R: Angiogenesis as a
potential treatment strategy for rheumatoid arthritis. Eur J
Pharmacol. 910(174500)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ba X, Huang Y, Shen P, Huang Y, Wang H,
Han L, Lin WJ, Yan HJ, Xu LJ, Qin K, et al: WTD attenuating
rheumatoid arthritis via suppressing angiogenesis and modulating
the PI3K/AKT/mTOR/HIF-1α pathway. Front Pharmacol.
12(696802)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhu J, Su C, Chen Y, Hao X and Jiang J:
Electroacupuncture on ST36 and GB39 acupoints inhibits synovial
angiogenesis via downregulating HIF-1α/VEGF expression in a rat
model of adjuvant arthritis. Evid Based Complement Alternat Med.
2019(5741931)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Feng X and Chen Y: Drug delivery targets
and systems for targeted treatment of rheumatoid arthritis. J Drug
Target. 26:845–857. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Pei B, Xu S, Liu T, Pan F, Xu J and Ding
C: Associations of the IL-1F7 gene polymorphisms with rheumatoid
arthritis in Chinese Han population. Int J Immunogenet. 40:199–203.
2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang XY, Zuo Y, Li C, Tu X, Xu HJ, Guo
JP, Li ZG and Mu R: IL1F7 gene polymorphism is not associated with
rheumatoid arthritis susceptibility in the Northern Chinese Han
population: A case-control study. Chin Med J (Engl). 131:171–179.
2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Shi LP, He Y and Liu ZD: Correlation
between single nucleotide polymorphism of rs3811047 in IL-1 F7 gene
and rheumatoid arthritis susceptibility among Han population in
central plains of China. Asian Pac J Trop Med. 6:73–75.
2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ward MM, Deodhar A, Gensler LS, Dubreuil
M, Yu D, Khan MA, Haroon N, Borenstein D, Wang R, Biehl A, et al:
2019 Update of the American college of rheumatology/spondylitis
association of America/spondyloarthritis research and treatment
network recommendations for the treatment of ankylosing spondylitis
and nonradiographic axial spondyloarthritis. Arthritis Care Res
(Hoboken). 71:1285–1299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Fawzy RM, Ganeb SS, Said EA and Fouad NA:
Serum level of interleukin-37 and expression of its mRNA in
ankylosing spondylitis patients: Possible role in osteoporosis.
Egypt J Immunol. 23:19–29. 2016.PubMed/NCBI
|
|
70
|
Chen B, Huang K, Ye L, Li Y, Zhang J,
Zhang J, Fan X, Liu X, Li L, Sun J, et al: Interleukin-37 is
increased in ankylosing spondylitis patients and associated with
disease activity. J Transl Med. 13(36)2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ge R, Pan F, Liao F, Xia G, Mei Y, Shen B,
Zhang T, Gao J, Zhang L, Duan Z, et al: Analysis on the interaction
between IL-1F7 gene and environmental factors on patients with
ankylosing spondylitis: A case-only study. Mol Biol Rep.
38:2281–2284. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Dalbeth N, Merriman TR and Stamp LK: Gout.
Lancet. 388:2039–2052. 2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ding L, Li H, Sun B, Wang T, Meng S, Huang
Q, Hong X and Liu D: Elevated interleukin-37 associated with tophus
and pro-inflammatory mediators in Chinese gout patients. Cytokine.
141(155468)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Liu L, Xue Y, Zhu Y, Xuan D, Yang X, Liang
M, Wang J, Zhu X, Zhang J and Zou H: Interleukin 37 limits
monosodium urate crystal-induced innate immune responses in human
and murine models of gout. Arthritis Res Ther.
18(268)2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zeng M, Dang W, Chen B, Qing Y, Xie W,
Zhao M and Zhou J: IL-37 inhibits the production of
pro-inflammatory cytokines in MSU crystal-induced inflammatory
response. Clin Rheumatol. 35:2251–2258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhao L, Zhao T, Yang X, Cao L, Xu R, Liu
J, Lin C, Yu Y, Xuan D, Zhu X, et al: IL-37 blocks gouty
inflammation by shaping macrophages into a non-inflammatory
phagocytic phenotype. Rheumatology (Oxford).
(keac009)2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
77
|
Onuora S: IL-37 linked to gout
pathogenesis and treatment. Nat Rev Rheumatol.
16(250)2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Wan W, Shi Y, Ji L, Li X, Xu X and Zhao D:
Interleukin-37 contributes to the pathogenesis of gout by affecting
PDZ domain-containing 1 protein through the nuclear factor-kappa B
pathway. J Int Med Res. 48(300060520948717)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Klück V, van Deuren RC, Cavalli G, Shaukat
A, Arts P, Cleophas MC, Crișan TO, Tausche AK, Riches P, Dalbeth N,
et al: Rare genetic variants in interleukin-37 link this
anti-inflammatory cytokine to the pathogenesis and treatment of
gout. Ann Rheum Dis. 79:536–544. 2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Mandl LA: Osteoarthritis year in review
2018: Clinical. Osteoarthritis Cartilage. 27:359–364.
2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Luo P, Feng C, Jiang C, Ren X, Gou L, Ji P
and Xu J: IL-37b alleviates inflammation in the temporomandibular
joint cartilage via IL-1R8 pathway. Cell Prolif.
52(e12692)2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ding L, Hong X, Sun B, Huang Q, Wang X,
Liu X, Li L, Huang Z and Liu D: IL-37 is associated with
osteoarthritis disease activity and suppresses proinflammatory
cytokines production in synovial cells. Sci Rep.
7(11601)2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
van Geffen EW, van Caam APM, Schreurs W,
van de Loo FA, van Lent PLEM, Koenders MI, Thudium CS, Bay-Jensen
AC, Blaney Davidson EN and van der Kraan PM: IL-37 diminishes
proteoglycan loss in human OA cartilage: Donor-specific link
between IL-37 and MMP-3. Osteoarthritis Cartilage. 27:148–157.
2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
van Geffen EW, van Caam AP, van Beuningen
HM, Vitters EL, Schreurs W, van de Loo FA, van Lent PL, Koenders
MI, Blaney Davidson EN and van der Kraan PM: IL37 dampens the
IL1β-induced catabolic status of human OA chondrocytes.
Rheumatology (Oxford). 56:351–361. 2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kiriakidou M and Ching CL: Systemic lupus
erythematosus. Ann Intern Med. 172:ITC81–ITC96. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Tawfik MG, Nasef SI, Omar HH and Ghaly MS:
Serum interleukin-37: A new player in lupus nephritis? Int J Rheum
Dis. 20:996–1001. 2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Godsell J, Rudloff I, Kandane-Rathnayake
R, Hoi A, Nold MF, Morand EF and Harris J: Clinical associations of
IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep.
6(34604)2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Song L, Qiu F, Fan Y, Ding F, Liu H, Shu
Q, Liu W and Li X: Glucocorticoid regulates interleukin-37 in
systemic lupus erythematosus. J Clin Immunol. 33:111–117.
2013.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, Yu
T, Chen B, Zhang J, Ding L, et al: IL-37 inhibits the production of
inflammatory cytokines in peripheral blood mononuclear cells of
patients with systemic lupus erythematosus: Its correlate with
disease activity. J Transl Med. 12(69)2014.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Wu GC, Li HM, Wang JB, Leng RX, Wang DG
and Ye DQ: Elevated plasma interleukin-37 levels in systemic lupus
erythematosus patients. Lupus. 25:1377–1380. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wu Q, Zhou J, Yuan ZC, Lan YY, Xu WD and
Huang AF: Association between IL-37 and systemic lupus
erythematosus risk. Immunol Invest: Jan 17, 2021 (Epub ahead of
print).
|
|
92
|
Bowman SJ: Primary Sjögren's syndrome.
Lupus. 27 (1 Suppl):S32–S35. 2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Liuqing W, Liping X, Hui S and Jing L:
Elevated IL-37, IL-18 and IL-18BP serum concentrations in patients
with primary Sjögren's syndrome. J Investig Med. 65:717–721.
2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Hatemi G, Christensen R, Bang D, Bodaghi
B, Celik AF, Fortune F, Gaudric J, Gul A, Kötter I, Leccese P, et
al: 2018 Update of the EULAR recommendations for the management of
Behçet's syndrome. Ann Rheum Dis. 77:808–818. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Ben Dhifallah I, Borhani-Haghighi A,
Hamzaoui A and Hamzaoui K: Decreased level of IL-37 correlates
negatively with inflammatory cytokines in cerebrospinal fluid of
patients with neuro-Behcet's disease. Iran J Immunol. 16:299–310.
2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Ye Z, Wang C, Kijlstra A, Zhou X and Yang
P: A possible role for interleukin 37 in the pathogenesis of
Behcet's disease. Curr Mol Med. 14:535–542. 2014.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Bouali E, Kaabachi W, Hamzaoui A and
Hamzaoui K: Interleukin-37 expression is decreased in Behçet's
disease and is associated with inflammation. Immunol Lett.
167:87–94. 2015.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Kacem O, Kaabachi W, Dhifallah IB,
Hamzaoui A and Hamzaoui K: Elevated expression of TSLP and IL-33 in
Behçet's disease skin lesions: IL-37 alleviate inflammatory effect
of TSLP. Clin Immunol. 192:14–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Tan H, Deng B, Yu H, Yang Y, Ding L, Zhang
Q, Qin J, Kijlstra A, Chen R and Yang P: Genetic analysis of innate
immunity in Behcet's disease identifies an association with IL-37
and IL-18RAP. Sci Rep. 6(35802)2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Provan D, Arnold DM, Bussel JB, Chong BH,
Cooper N, Gernsheimer T, Ghanima W, Godeau B, González-López TJ,
Grainger J, et al: Updated international consensus report on the
investigation and management of primary immune thrombocytopenia.
Blood Adv. 3:3780–3817. 2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Zhan Y, Cheng L, Wu B, Ji L, Chen P, Li F,
Cao J, Ke Y, Yuan L, Min Z, et al: Interleukin (IL)-1 family
cytokines could differentiate primary immune thrombocytopenia from
systemic lupus erythematosus-associated thrombocytopenia. Ann
Transl Med. 9(222)2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Chen Z, Qu W, Wang HQ, Xing LM, Wu YH, Liu
ZY, Zhang Y, Liu H, Dong XF, Tao JL and Shao ZH: Relationship of
peripheral blood IL-37 expression with T lymphocytes subsets and NK
cells in patients with primary immune thrombocytopenia. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 27:1201–1207. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
103
|
Liu L, Feng K, Wang ML, Shu XH, Zhou KS,
Zhou H, Liu XJ and Song YP: Expression of IL-37 in peripheral blood
of adults with primary immune thrombocytopenia. Zhonghua Xue Ye Xue
Za Zhi. 38:628–631. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
104
|
Olek MJ: Multiple sclerosis. Ann Intern
Med. 174:ITC81–ITC96. 2021.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Farrokhi M, Rezaei A, Amani-Beni A,
Etemadifar M, Kouchaki E and Zahedi A: Increased serum level of
IL-37 in patients with multiple sclerosis and neuromyelitis optica.
Acta Neurol Belg. 115:609–614. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Kouchaki E, Tamtaji OR, Dadgostar E,
Karami M, Nikoueinejad H and Akbari H: Correlation of serum levels
of IL-33, IL-37, soluble form of vascular endothelial growth factor
receptor 2 (VEGFR2), and circulatory frequency of VEGFR2-expressing
cells with multiple sclerosis severity. Iran J Allergy Asthma
Immunol. 16:329–337. 2017.PubMed/NCBI
|
|
107
|
Giacoppo S, Thangavelu SR, Diomede F,
Bramanti P, Conti P, Trubiani O and Mazzon E: Anti-inflammatory
effects of hypoxia-preconditioned human periodontal ligament cell
secretome in an experimental model of multiple sclerosis: A key
role of IL-37. FASEB J. 31:5592–5608. 2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Schauer AE, Klassert TE, von Lachner C,
Riebold D, Schneeweiß A, Stock M, Müller MM, Hammerschmidt S,
Bufler P, Seifert U, et al: IL-37 causes excessive inflammation and
tissue damage in murine pneumococcal pneumonia. J Innate Immun.
9:403–418. 2017.PubMed/NCBI View Article : Google Scholar
|
|
109
|
McKie EA, Reid JL, Mistry PC, DeWall SL,
Abberley L, Ambery PD and Gil-Extremera B: A study to investigate
the efficacy and safety of an anti-interleukin-18 monoclonal
antibody in the treatment of type 2 diabetes mellitus. PLoS One.
11(e0150018)2016.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Argiriadi MA, Xiang T, Wu C, Ghayur T and
Borhani DW: Unusual water-mediated antigenic recognition of the
proinflammatory cytokine interleukin-18. J Biol Chem.
284:24478–24489. 2009.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Gabay C, Fautrel B, Rech J, Spertini F,
Feist E, Kötter I, Hachulla E, Morel J, Schaeverbeke T, Hamidou MA,
et al: Open-label, multicentre, dose-escalating phase II clinical
trial on the safety and efficacy of tadekinig alfa (IL-18BP) in
adult-onset Still's disease. Ann Rheum Dis. 77:840–847.
2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Nnane I, Frederick B, Yao Z, Raible D, Shu
C, Badorrek P, van den Boer M, Branigan P, Duffy K, Baribaud F, et
al: The first-in-human study of CNTO 7160, an anti-interleukin-33
receptor monoclonal antibody, in healthy subjects and patients with
asthma or atopic dermatitis. Br J Clin Pharmacol. 86:2507–2518.
2020.PubMed/NCBI View Article : Google Scholar
|















