Introduction
The COVID-19 pandemic, declared by the World Health
Organization (WHO) on March 11, 2020, highly affected elderly
fragile persons. The recently published data from the Global Burden
of Disease Study 2019 forecasted the global prevalence of dementia
in 2050 to 152.8 million globally, evolving from 57.4 million cases
in 2019(1). Age-related cognitive
impairment, such as the decline in decision-making or in executive
cognitive function, occur with the physiologic process of normal
aging, but the number of people with age-associated
neurodegenerative disorders is also increasing rapidly and there
are age-related conditions that accelerate the rate of neuronal
dysfunction, neuronal loss, and cognitive decline (2-4).
It was initially considered that COVID-19 clinical
presentation primarily affects the respiratory system causing an
interstitial pneumonia, but soon the medical body recognized it as
a systemic disease. The elderly are at a higher risk of developing
severe forms of COVID-19 due to factors associated with aging
(including the higher prevalence of medical comorbidities)
(5,6). SARS-CoV2 attaches to healthy cell
membranes primarily through the angiotensin-converting enzyme 2
(ACE-2) receptor present within many organs. There is widespread
viral organ damage identified at the level of the lungs, brain,
heart, kidney, liver, skin, endocrine system and gut (6-12),
decreasing the bioavailability of ACE-2 receptors within the
renin-angiotensin-aldosterone system. In individuals with
pre-existing ACE-2 deficiencies (such as in hypertension, diabetes
mellitus, and those of older age) the decrease in ACE-2
bioavailability as the infection spreads augments the risk of more
severe symptoms (12,13).
There are multiple reports identifying psychiatric
symptoms associated with COVID-19, such as: ‘brain fog’, insomnia,
depressed mood, anxiety, behavioral or affective disorders,
post-traumatic stress disorder, memory loss and cognitive
impairment in a large proportion of patients (14,15).
There are many hypotheses to explain the potential mechanisms
underlying these symptoms and why there is such a variability of
clinical phenotypes of the same disease. Most researchers tend to
attribute them as multifactorial, considering direct viral
neurotrophic effect of SARS-CoV-2, brain hypoxia, systemic
inflammation, consequences of intensive care unit interventions,
the use of mechanical ventilation and sedative drugs, secondary
effects of medications used to treat COVID-19-all connected with
dysfunction of peripheral organs triggering a cascade of neuronal
and cerebral dysfunctions (3,5).
One strong theory of the severe evolution within the
elder population relates to ‘inflammaging’, characterized as a
‘low-grade, controlled, asymptomatic, chronic, systemic,
inflammatory state that characterizes the aging process’ (16). Alterations in biological functions
of our body determine our biological age-distinct parameter against
chronological age-which strongly impact our ability to react and to
cope with the disease and also influence the effectiveness and
safety of specific therapies, including the ones used for
SARS-CoV-2 infection (3,17).
Our analysis focused on the prevalence and
manifestations of psychiatric disorders in relation to the COVID-19
acute phase and further reviewed the results in elder vs. younger
patients, comparing the two study subgroups split by the 60 year
age cut-off. The added value of this study to the wealth of
research data on COVID-19 is to provide detail on the psychiatric
dimension of the acute COVID-19 setting and to compare side by side
two age groups, with different morbidity/disease profiles, in order
to understand whether different age-associated long-term effects
may be expected.
Patients and methods
A study lot of 89 patients diagnosed with COVID-19
and admitted during a 12-month period (09/2020-09/2021) to the
‘Elisabeta Doamna’ Psychiatric Hospital was analyzed with regards
to psychiatric diagnosis, specific manifestations and the moment of
their onset. All patients were in the acute infectious phase
testing positive for SARS-CoV2 ARN (RT-PCR laboratory tests).
Diagnosis used the standard common International
Classification of Diseases, 10th edition (ICD-10) coding (18). The admittance to psychiatric
hospital was determined by the severity of the symptomatology
and/or the potential self-harming risk. The psychiatric clinical
manifestations were prevailing; only mild to moderate respiratory
COVID-19 forms were included in the study. Cognitive assessment was
based on Mini Mental State Evaluation (MMSE) (19), Clock-Drawing Test (20) and Montreal Cognitive Assessment
(MoCA) (21).
In the study lot, we comparatively assessed two
subgroups of patients, split by the 60 year age cut-off. The
relative risk (RR) of psychiatric symptomatology and the prevalence
of comorbidities were measured within the two subgroups using
MedCalc software (version 20.110; MedCalc Software Ltd.). A
descriptive statistical analysis was applied for this retrospective
study.
Results
Demographics
The demographic analysis of the population in the
study lot exhibited a median age of 51.3 years (with an age
interval of 19 to 91 years), the split between age groups being
66.3/33.7% for patients in the <60 years of age group and
respectively 60-91 years of age. A balanced sex ratio was
considered and most of the patients lived in an urban area. Median
hospitalization duration was 21.7 days (shortest 2 days, longest
111 days) (Table I).
 | Table IDemographics and hospital stay
analysis: Total lot and subgroup split in the study lot. |
Table I
Demographics and hospital stay
analysis: Total lot and subgroup split in the study lot.
| Items | Total study
set | Patients <60
years of age | Patients ≥60 years
of age |
|---|
| Study lot, no. of
patients (n) | 89 (%) | 59 (%) | 30 (%) |
| Area of
residence | | | |
|
Urban area,
n (%) | 52 (58.4) | 32 (54.2) | 20 (66.7) |
|
Rural area,
n (%) | 37 (41.6) | 27 (45.8) | 10 (33.3) |
| Sex | | | |
|
Female, n
(%) | 41 (46.1) | 24 (40.7) | 17 (56.7) |
|
Male, n
(%) | 48 (53.9) | 35 (59.3) | 13 (43.3) |
| Median period of
hospitalization (days) | 21.7 | 21 | 22.1 |
Moment of psychiatric diagnosis in
relation to COVID-19
Out of the 89 patients included in the analysis, 27%
were registered and previously treated for a psychiatric condition
before the onset of the SARS-CoV-2 infection; in the context of
COVID-19, their disease was aggravated or presented new symptoms
which required hospitalization. The majority of the hospital
admissions were related to newly occurring psychiatric
manifestations within the COVID-19 acute phase (73%), with
different prevalence in the two study subgroups (first psychiatric
symptoms during COVID-19 acute phase occurred in 63.3% of the
patients ≥60 years of age and in 78% of the patients <60 years
of age).
Psychiatric disorders during the acute
phase of COVID-19
The most prevalent type of diagnosis was of a
depressive disorder type, 24.7% in the general lot of all ages,
whereas the situation was different when analyzed by age subgroup.
For younger patients <60 years, the highest prevalence (30.5%)
was for paranoid/schizophrenic type of diagnosis (ICD-10 codes:
F21, Schizotypal personality disorder; F20.0, Paranoid
schizophrenia; F25, Schizoaffective disorder). For the older
patient subgroup (≥60 years) the most frequent diagnosis was for
depressive disorder, but occurring in a significantly higher
percentage vs. the study set (33.3%); the elderly patients received
ICD-10 diagnoses such as F33, Recurrent depressive disorder;
F32.2/F32.3, Severe depressive episode with/without psychotic
symptoms (Table II).
 | Table IITypes of psychiatric ICD-10 diagnoses
in the study lot. |
Table II
Types of psychiatric ICD-10 diagnoses
in the study lot.
| Diagnostic
class | Total lot (%) | Patients <60
years of age (%) | Patients ≥60 years
of age (%) |
|---|
| Psychotic disorder,
n (%) | 9 (10.1) | 8 (13.6) | 1 (3.3) |
| Alcohol dependence
and related, n (%) | 7 (7.9) | 6 (10.2) | 1 (3.3) |
| Dementia, n
(%) | 7 (7.9) | 0 (0) | 7 (23.3) |
| Depressive
disorder, n (%) | 22 (24.7) | 12 (20.3) | 10 (33.3) |
|
Behavioral/personality disorder, n
(%) | 16 (18.0) | 11 (18.6) | 5 (16.7) |
|
Schizophrenic/paranoid disorder, n
(%) | 18 (20.2) | 18 (30.5) | 0 (0) |
| Affective disorder,
n (%) | 10 (11.2) | 4 (6.8) | 6 (20.0) |
The RR of depression was 1.64 for the elderly
COVID-19 patients when compared with younger subjects; for this
older age group there was a significantly lower prevalence of
psychotic disorders, all types of alcohol dependence disorders and
paranoid/schizophrenic disorders. Affective disorders were found to
be more frequent in older COVID-19 patients when compared with the
younger subgroup (RR, 2.95).
Psychiatric manifestations during the
acute phase of COVID-19
The most frequent symptom which occurred during
COVID-19 in our study group was anxiety (in 78.7% of the patients
of all ages, with similar prevalence in the two study subgroups).
Clinical manifestations such as psychomotor agitation,
hallucinations, sleep disorders or delirium were presented by a
third of the patients in the general study set, but the prevalence
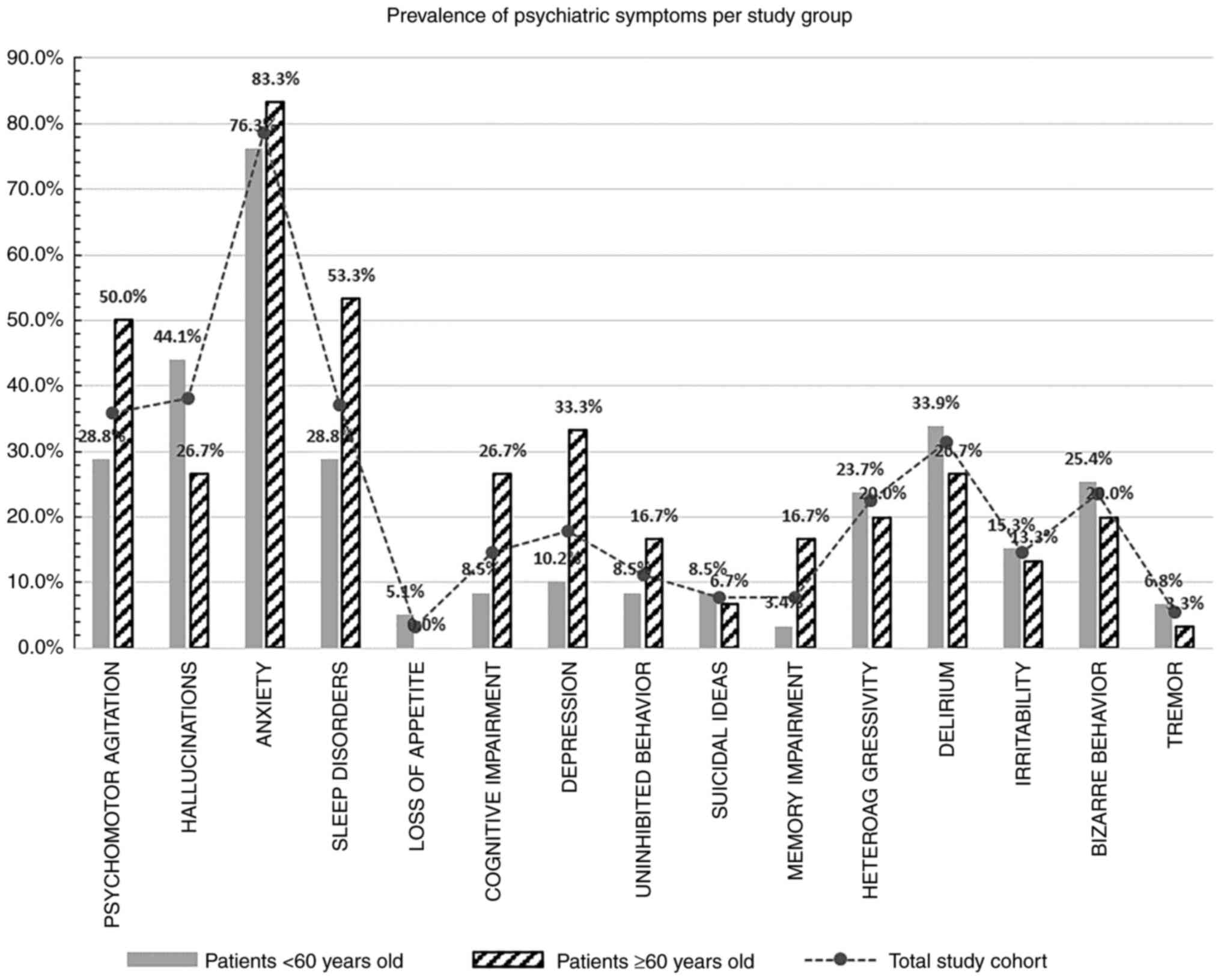
was different when analyzed by age split. Significantly more
patients ≥60 years showed cognitive impairment and subjective
memory deficits, as well as depression, sleep disorders and
psychomotor agitation as compared with the younger patient group
(Fig. 1).
Comorbidities as associated diagnosis
for the psychiatric manifestations during the acute phase of
COVID-19
A total of 68.5% of all study patients had at least
1 comorbidity: 80% in the older patient group as compared to 62.7%
in the younger group. The most prevalent were metabolic disorders
(29.2% of all patients had dyslipidemia or diabetes mellitus) in
the total study lot, significantly different when measured in each
age group; most patients ≥60 years of age had a cardiovascular
disorder-hypertension, angina pectoris, cardiac rhythm disorders or
arrhythmias, while in the <60 year subgroup the most frequent
comorbidity was metabolic (30.5% of younger patients) (Table III).
 | Table IIITypes of comorbidities in the study
lot. |
Table III
Types of comorbidities in the study
lot.
| Type of associated
diagnosis | Total study lot
(%) | Patients <60
years of age (%) | Patients ≥60 years
of age (%) |
|---|
| Metabolic
comorbidities, n (%) | 26 (29.2) | 18 (30.5) | 8 (26.7) |
| Cardiovascular
comorbidities, n (%) | 24 (27.0) | 8 (13.6) | 16 (53.3) |
| Neurologic
comorbidities, n (%) | 1 (1.1) | 1 (1.7) | 0 |
| Respiratory
comorbidities, n (%) | 16 (18.0) | 9 (15.3) | 7 (23.3) |
There was no correlation identified between the type
of comorbidity and the moment or the type of psychiatric
manifestations in relation to COVID-19, within any of the study
groups.
Discussion
It was demonstrated that SARS-CoV-2 is an
opportunistic pathogen of the nervous system, with ACE-2 expression
in the brain being a determinant for the viral tropism and
psychiatric manifestations associated with COVID-19, generated
either through direct neurotoxicity or immune-reactivity of the
host (22). Available data show
that COVID-19 is associated with cerebral oxidative stress,
neuroinflammation, deterioration within the white matter, brain
vessel endothelium and blood-brain barrier, cerebral hypoperfusion
and demyelination (23,24).
In addition, research focusing on the immediate
impact of COVID-19 on ageing has highlighted a consistently
accelerated biological age gap of 5.22 years and a significant
shortening of their telomeres (a known marker of ageing) in
COVID-19 survivors as compared with COVID-19 naïve individuals
(25). On medium to long term this
may trigger earlier and/or accelerated onset of age-related
neurodegenerative disorders which, together with direct cerebral
consequences of SARS-CoV-2 infection, may affect the evolution of
dementia prevalence and manifestations in the future (26,27).
Neuropsychological assessment during acute COVID-19
is difficult, especially in medium and severe cases (22). Our study focused on a group of 89
COVID-19 patients that required hospitalization because of the
severity of their psychiatric symptoms and not related to the
respiratory syndrome or other manifestations of the disease. We
analyzed comparatively two sub-groups of patients based on an age
cut-off (<60 years and ≥60 years of age) in order to assess
whether geriatric patients, with a demonstrated higher risk of
morbidity and mortality due to COVID-19, have psychiatric
manifestations similar to younger patients suffering from a
comparable clinical form of the infectious disease: mild to
moderate.
Related to the frequency of a new psychiatric
diagnosis during the acute COVID-19 phase, in our study lot there
was no significant difference between the two age groups. Regarding
available data, there is no consistency between studies. A
literature review regarding correlations between psychiatric
disorders and COVID-19 showed that the infectious disease may act
as a trigger for a new onset of psychiatric dysfunctions and may
also aggravate the severity of previously diagnosed mental
disorders (7,28,29).
This may have multiple causes and studies published previously to
the pandemic period demonstrate that self-isolation and stress are
determining a special form of inflammatory syndrome (30). Reports from Italy analyzing the
prevalence of psychiatric disorders in the database of COVID-19
deaths (where the median age at death was 80 years with an interval
of 72-86 years in the analyzed group) presented a frequency of 6.5%
of the patients with a psychiatric diagnosis prior to the infection
(31,32).
Immuno-senescence is a risk factor for more severe
manifestations of COVID-19 and is adding to the neuropsychiatric
damage that is directly induced by the viral infection (5).
From early pandemic phases, reports on psychiatric
symptomatology associated with COVID-19 have identified delirium,
agitation, depression, anxiety, insomnia or memory/cognitive
impairment (3,28,29).
The prevalence of these is not yet established, nor is the longer
term consequence of these types of complications; recent data
revealed a high incidence of 33.6% for neurologic and psychiatric
morbidity in the 6 months following COVID-19(33). In addition, it was demonstrated
that neurologic and psychiatric manifestations during COVID-19
acute phase could act as predictors of disease severity and
mortality (34).
In a meta-analysis published early in 2020, the
authors found that 63% of patients were affected by psychiatric
symptoms during acute SARS and MERS infections (including anxiety,
insomnia, confusion, depression, psychoses) (24). The prevalence of mental health
issues ranged from 20-36% in the general public and healthcare
workers during acute COVID-19(29), and a study from Wuhan reported a
high prevalence of anxiety (38.5%) and depression (35.9%) (35).
These findings are much lower in prevalence than our
reported data, but the major difference is that the present study
withdraws information regarding psychiatric issues requiring
hospitalization of acute COVID-19 patients. There is much detailed
research concerning post-acute neuropsychiatric sequelae of the
SARS-CoV-2 infection, with estimates up to 18.1% for the incidence
of first and recurrent psychiatric illness in the 90 days post
COVID-19 diagnosis (36,37).
We found significant differences between studies
coming from heterogeneous patient populations and study designs,
and scarce data regarding the variation in older vs. younger
COVID-19 patients manifesting psychiatric symptomatology in the
acute phase. Generally anxiety and depression have a high
incidence, also delirium is frequently mentioned as a symptom
during acute SARS-CoV-2 infection (30,32).
The underlying pathophysiological mechanisms of the
psychiatric spectrum of COVID-19 include direct viral damage of the
neuronal network, hypoxic injury and immune-mediated damage. The
elevation of biomarkers of inflammation strongly correlates with
neuro-cognitive impairment, as dysregulation in cytokine function
(a known phenomenon of this infectious disease) is associated with
psychiatric disorders (38,39).
Depression, presented by a third of the older patients in our set,
is linked with increased levels of cytokines such as interleukin
(IL)-6, tumor necrosis factor (TNF)-α, and chemokine (C-C motif)
ligand 2 (CCL2), which are also increased in COVID-19 patients and
are correlated with the severity of the disease (8,40-42).
In addition, other major psychiatric disorders including
schizophrenia, bipolar disorders, suicide or sleeping disorder have
been significantly associated with alterations of certain
immunomediators (43).
Corroborating these data with the research on immuno-senescence and
the immunologic substrate of the age-related neurocognitive
dysfunction, we can hypothesize that this may be the link
explaining higher incidence of some psychiatric COVID-19
manifestations in older vs. younger patients (psycho-motor
agitation, sleep disorders, depression, cognitive impairment)
(Fig. 1).
A high percentage of the psychiatric hospitalized
patients in our lot had at least one other diagnosis associated
with mental dysfunction and SARS-CoV-2 infection. The only
significant difference between our two study subgroups regarding
comorbidities is the prevalence of cardiovascular disorders, with a
relative risk (RR) of 3.93 for the senior group vs. patients <60
years of age. It is considered that there is a complex and
bidirectional relationship between cardiovascular disease and
mental illnesses such as depression, anxiety, schizophrenia or
bipolar disorder. Our study did not provide enough statistical
power to determine whether there is any correlation linking
psychiatric manifestations of COVID-19 with the difference in the
comorbidity prevalence within the two age subgroups.
After more than 2.5 years of living with the
pandemic, there are sufficient data to demonstrate that older
individuals are more prone to aggravated infections of SARS-CoV-2,
with more severe manifestations of pulmonary disease, heart
failure, hepatic and kidney dysfunction, neurocognitive damage; all
triggered by the direct viral invasion and cytokine storm with
microvascular coagulation among the main pathogenic mechanisms
(6,30). These initial alterations continue
in the majority of affected senior individuals beyond the acute
phase, and we still have to evaluate and develop strategies for
long term psychological and neuropsychiatric sequelae of the
pandemic. For example, it has been demonstrated that the
Ca2+ dysregulation in Alzheimer's disease is also a
mechanism for SARS-CoV-2 infection pathogenicity and it was
hypothesized that it may facilitate the viral lifecycle at the
brain level, together with the increased ACE-2 expression and other
common neuro-inflammatory substrates (6,43,44).
As data exist suggesting that COVID-19 may promote
the initiation and progression of dementia (including Alzheimer's
disease), contributing significantly to cognitive impairment
especially in the elderly, it is probable that the pandemic will
accelerate the already high trends of increasing dementia
prevalence (1,14,37).
In a recent report from Wuhan, China, at one year after being
discharged, senior COVID-19 survivors were associated with an
increased risk of longitudinal cognitive decline when compared with
uninfected control individuals; severe COVID-19 was associated with
a higher risk of early-onset [odds ratio (OR)=4.87], late-onset
(OR=7.58) or progressive (OR=19.00) cognitive decline (45).
Beyond direct disease consequences, COVID pandemic
restrictions also had a significant impact on the general
population, especially the elderly, due to social isolation, fear
of being infected and for the uncertain future, limited physical
activity and exposure to sunlight, complications of anxiety and
depression (2,29,44).
Therefore, healthcare systems need to adjust their dementia risk
reduction strategies in order to adjust for the post-pandemic
evolution in population's mental health and growing needs (15,46).
COVID-19 consequences on mental health are
significant and this is demonstrated by numerous studies, most of
them analyzing the clinical entity defined as Long COVID or
Post-acute sequelae of COVID (PASC) or Post-acute COVID-19 Syndrome
(PACS). Probably in the next years we may be able to further
explore long term implications for both COVID-19 survivors and
patients having an impact due to the pandemic reshaping of their
lives (22,47).
The limitations of the present study relate to the
small sample size which, in the context of scarce data in the
literature regarding differences between older vs. younger patients
with psychiatric manifestations of SARS-CoV-2 infection, need
extended data from broader cohorts to support our hypothesis and
complete the long-term projections and prognostic variables for
COVID-19 survivors. Another perspective is looking into comparative
data regarding the moment of psychiatric illness onset in order to
observe differences of manifestations in patients with previous vs.
new psychiatric disorder; the present study did not have a large
enough sample lot to provide relevant conclusions, thus, a broader
patient group may be considered for future research.
Relevant data identified a wide range of psychiatric
symptoms exhibited by COVID-19 patients during their acute disease,
with a severity which may decrease during the recovery period. In
addition to the direct results of the infectious disease, it is
also important to recognize the stress, fear of death, and anxiety
concerning the consequences of COVID-19 on their own health and
their family members (48).
In conclusion, an altered mental status is a
frequent manifestation of COVID-19. Our study assessed the
prevalence of the most often occurring manifestation and compared
two study subgroups of younger vs. older patients (60 year old age
cut-off). Common psychiatric disorders triggered by SARS-CoV-2
infection include depression, schizophrenic or paranoid disorders
and behavioral illness, but there is a different symptomatic
profile in older when compared with younger patients.
Anxiety is the most prevalent symptom during
COVID-19 acute phase (presented by 78.7% of all patients in our
research) and both elder and young adults are affected in similar
ratios. Our data showed that apart from anxiety, patients ≥60 years
of age suffer mostly from cognitive and memory impairment,
depression, sleep disorder and psychomotor agitation, while younger
patients present more frequently delirium, bizarre behavior,
hallucinations or hetero-aggressivity.
Age-related neuropsychiatric substrate could explain
some of these differences between the two study subgroups.
Furthermore, it is important to gather additional data on the
correlations between inflammation, cytokine involvement and
psychiatric disorders; a deeper understanding of the pathological
mechanisms could help develop new clinical algorithms and even new
therapeutic targets for a more custom approach.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization of the study was accomplished by
FS and AR, methodology design was the responsibility of FS and VDO.
Validation of the data was the responsibility of AR, CS, EPD and
GO. Formal analysis was conducted by FS; data curation and
statistical analysis was conducted by FS, AN, EPD and ALT. GO, CS
and AR substantially contributed to the interpretation of the
results; writing-original draft preparation was conducted by FS;
writing-review and editing were conducted by AR and AN. Data
representation was the responsibility of FS; supervision was
conducted by AR; project administration was the responsibility of
FS and GO; FS, CS and GO contributed equally to this research. FS
and VDO had full access to the database; EPD and GO confirm the
authenticity of all the raw data. All authors have discussed the
results, and read and approved the final manuscript.
Ethics approval and consent to
participate
The retrospective study publication was approved by
the Medical Ethics Committee of ‘Elisabeta Doamna’ Psychiatric
Hospital of Galati, Romania as comprehensive doctoral research (no.
4/11.03.2019) and according to the Helsinki Declaration (49). Due to the retrospective nature of
this study individual patient consent for use of their data was not
necessary.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2019 Dementia Forecasting
Collaborators. Estimation of the global prevalence of dementia in
2019 and forecasted prevalence in 2050: An analysis for the global
burden of disease study 2019. Lancet Public Health. 7:e105–e125.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pyne JD and Brickman AM: The impact of the
COVID-19 pandemic on dementia risk: Potential pathways to cognitive
decline. Neurodegener Dis. 21:1–23. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Han Y, Yuan K, Wang Z, Liu WJ, Lu ZA, Liu
L, Shi L, Yan W, Yuan JL, Li JL, et al: Neuropsychiatric
manifestations of COVID-19, potential neurotropic mechanisms, and
therapeutic interventions. Transl Psychiatry.
11(499)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Steardo L Jr, Steardo L and Verkhratsky A:
Psychiatric face of COVID-19. Transl Psychiatry.
10(261)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grolli RE, Mingoti MED, Bertollo AG,
Luzardo AR, Quevedo J, Réus GZ and Ignácio ZM: Impact of COVID-19
in the mental health in elderly: Psychological and biological
updates. Molecular Neurobiology. 58:1905–1916. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang S, Yang Z, Li ZN, Chen ZL, Yue SJ,
Fu RJ, Xu DO, Zhang S and Tang YP: Are older people really more
susceptible to SARS-CoV-2? Aging Dis. 2022.
|
|
7
|
da Rosa Mesquita R, Francelino Silva
Junior LC, Santos Santana FM, Farias de Oliveira T, Campos
Alcântara R, Monteiro Arnozo G, Rodrigues da Silva Filho E, Galdino
Dos Santos AG, Oliveira da Cunha EJ, Salgueiro de Aquino SH and
Freire de Souza CD: Clinical manifestations of COVID-19 in the
general population: Systematic review. Wien Klin Wochenschr.
133:377–382. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Davis HE, Assaf GS, McCorkell L, Wei H,
Low RJ, Re'em Y, Redfield S, Austin JP and Akrami A: Characterizing
long COVID in an international cohort: 7 Months of symptoms and
their impact. EClinicalMedicine. 38(101019)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tatu AL, Nadasdy T and Bujoreanu FC:
Familial clustering of COVID-19 skin manifestations. Dermatol Ther.
33(e14181)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tatu AL, Nadasdy T and Nwabudike LC:
Observations about sexual and other routes of SARS-CoV-2 (COVID-19)
transmission and its prevention. Clin Exp Dermatol. 45:761–762.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Spudich S and Nath A: Nervous system
consequences of COVID-19. Science. 375:267–269. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thye AY, Law JW, Tan LT, Pusparajah P, Ser
HL, Thurairajasingam S, Letchumanan V and Lee LH: Psychological
symptoms in COVID-19 patients: Insights into pathophysiology and
risk factors of long COVID-19. Biology (Basel).
11(61)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Frontera JA, Boutajangout A, Masurkar AV,
Betensky RA, Ge Y, Vedvyas A, Debure L, Moreira A, Lewis A, Huang
J, et al: Comparison of serum neurodegenerative biomarkers among
hospitalized COVID-19 patients versus non-COVID subjects with
normal cognition, mild cognitive impairment, or Alzheimer's
dementia. Alzheimer's Dement. 18:899–910. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Iodice F, Cassano V and Rossini PM: Direct
and indirect neurological, cognitive, and behavioral effects of
COVID-19 on the healthy elderly, mild-cognitive-impairment, and
Alzheimer's disease populations. Neurol Sci. 42:455–465.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Meier IB, Vieira Ligo Teixeira C, Tarnanas
I, Mirza F and Rajendran L: Neurological and mental health
consequences of COVID-19: Potential implications for well-being and
labour force. Brain Commun. 3(fcab012)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Giunta S: Is inflammaging an
auto[innate]immunity subclinical syndrome? Immun Ageing.
3(12)2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Candore G, Colonna-Romano G, Balistreri
CR, Di Carlo D, Grimaldi MP, Listì F, Nuzzo D, Vasto S, Lio D and
Caruso C: Biology of longevity: Role of the innate immune system.
Rejuvenation Res. 9:143–148. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
World Health Organization (WHO): The
ICD-10 classification of mental and behavioural disorders:
Diagnostic criteria for research. WHO, Geneva, 1993. https://www.who.int/standards/classifications/classification-of-diseases.
Accessed May 25, 2022.
|
|
19
|
Tombaugh TN, McDowell I, Kristjansson B
and Hubley AM: Mini-mental state examination (MMSE) and the
modified MMSE (3MS): A psychometric comparison and normative data.
Psychol Assess. 8:48–59. 1996.
|
|
20
|
Shulman KI: Clock-drawing: Is it the ideal
cognitive screening test? Int J Geriatr Psychiatry. 15:548–561.
2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nasreddine ZS, Phillips NA, Bédirian V,
Charbonneau S, Whitehead V, Collin I, Cummings JL and Chertkow H:
The montreal cognitive assessment, MoCA: A brief screening tool for
mild cognitive impairment. J Am Geriatr Soc. 53:695–699.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ciaccio M, Lo Sasso B, Scazzone C, Gambino
CM, Ciaccio AM, Bivona G, Piccoli T, Giglio RV and Agnello L:
COVID-19 and Alzheimer's disease. Brain Sci. 11(305)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Frontera JA, Sabadia S, Lalchan R, Fang T,
Flusty B, Millar-Vernetti P, Snyder T, Berger S, Yang D, Granger A,
et al: A prospective study of neurologic disorders in hospitalized
patients with COVID-19 in New York City. Neurology. 96:e575–e586.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Virhammar J, Nääs A, Fällmar D, Cunningham
JL, Klang A, Ashton NJ, Jackmann S, Westman G, Frithiof R, Blennow
K, et al: Biomarkers for central nervous system injury in
cerebrospinal fluid are elevated in COVID-19 and associated with
neurological symptoms and disease severity. Eur J Neurol.
28:3324–3331. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mongelli A, Barbi V, Gottardi Zamperla M,
Atlante S, Forleo L, Nesta M, Massetti M, Pontecorvi A, Nanni S,
Farsetti A, et al: Evidence for biological age acceleration and
telomere shortening in COVID-19 survivors. Int J Mol Sci.
22(6151)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mukaetova-Ladinska EB and Kronenberg G:
Psychological and neuropsychiatric implications of COVID-19. Eur
Arch Psychiatry Clin Neurosci. 271:235–248. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livingston G, Huntley J, Sommerlad A, Ames
D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J,
Cooper C, et al: Dementia prevention, intervention, and care: 2020
Report of the lancet commission. Lancet. 396:413–446.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rogers JP, Chesney E, Oliver D, Pollak TA,
McGuire P, Fusar-Poli P, Zandi MS, Lewis G and David AS:
Psychiatric and neuropsychiatric presentations associated with
severe coronavirus infections: A systematic review and
meta-analysis with comparison to the COVID-19 pandemic. Lancet
Psychiatry. 7:611–627. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
de Sousa GM, Tavares VDO, de Meiroz Grilo
MLP, Coelho MLG, de Lima-Araújo GL, Schuch FB and Galvão-Coelho NL:
Mental health in COVID-19 pandemic: A meta-review of prevalence
meta-analyses. Front Psychol. 12(703838)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He Y, Yu R and Ren J: The correlation
between psychiatric disorders and COVID-19: A narrative review.
Psychiatr Danub. 33:76–85. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lega I, Nisticò L, Palmieri L, Caroppo E,
Lo Noce C, Donfrancesco C, Vanacore N, Scattoni ML, Picardi A,
Gigantesco A, et al: Psychiatric disorders among hospitalized
patients deceased with COVID-19 in Italy. EClinicalMedicine.
35(100854)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hawkins M, Sockalingam S, Bonato S,
Rajaratnam T, Ravindran M, Gosse P and Sheehan KA: A rapid review
of the pathoetiology, presentation, and management of delirium in
adults with COVID-19. J Psychosom Res. 141(110350)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Taquet M, Geddes JR, Husain M, Luciano S
and Harrison PJ: 6-Month neurological and psychiatric outcomes in
236 379 survivors of COVID-19: A retrospective cohort study using
electronic health records. Lancet Psychiatry. 8:416–427.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nakamura ZM, Nash RP, Laughon SL and
Rosenstein DL: Neuropsychiatric complications of COVID-19. Curr
Psychiatry Rep. 23(25)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nie XD, Wang Q, Wang MN, Zhao S, Liu L,
Zhu YL and Chen H: Anxiety and depression and its correlates in
patients with coronavirus disease 2019 in Wuhan. Int J Psychiatry
Clin Pract. 25:109–114. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nalbandian A, Sehgal K, Gupta A, Madhavan
MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat
TS, et al: Post-acute COVID-19 syndrome. Nat Med. 27:601–615.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Taquet M, Luciano S, Geddes JR and
Harrison PJ: Bidirectional associations between COVID-19 and
psychiatric disorder: Retrospective cohort studies of 62 354
COVID-19 cases in the USA. Lancet Psychiatry. 8:130–140.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jones KA and Thomsen C: The role of the
innate immune system in psychiatric disorders. Mol Cell Neurosci.
53:52–62. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Miller AH and Raison CL: The role of
inflammation in depression: From evolutionary imperative to modern
treatment target. Nat Rev Immunol. 16:22–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Coperchini F, Chiovato L, Croce L, Magri F
and Rotondi M: The cytokine storm in COVID-19: An overview of the
involvement of the chemokine/chemokine-receptor system. Cytokine
Growth Factor Rev. 53:25–32. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Enache D, Pariante CM and Mondelli V:
Markers of central inflammation in major depressive disorder: A
systematic review and meta-analysis of studies examining
cerebrospinal fluid, positron emission tomography and post-mortem
brain tissue. Brain Behav Immun. 81:24–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Eyre HA, Air T, Pradhan A, Johnston J,
Lavretsky H, Stuart MJ and Baune BT: A meta-analysis of chemokines
in major depression. Prog Neuropsychopharmacol Biol Psychiatry.
68:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yuan N, Chen Y, Xia Y, Dai J and Liu C:
Inflammation-related biomarkers in major psychiatric disorders: A
cross-disorder assessment of reproducibility and specificity in 43
meta-analyses. Transl Psychiatry. 9(233)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xia X, Wang Y and Zheng J: COVID-19 and
Alzheimer's disease: How one crisis worsens the other. Transl
Neurodegener. 10(15)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu YH, Chen Y, Wang QH, Wang LR, Jiang L,
Yang Y, Chen X, Li Y, Cen Y, Xu C, et al: One-year trajectory of
cognitive changes in older survivors of COVID-19 in Wuhan, China: A
longitudinal cohort study. JAMA Neurol. 8:509–517. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Urazan JC, Padilla NE, Borda MG, Pinilla
J, Fernandez MAG Sr and Fan MV: Latin American older people and
neuropsychiatric symptoms: A mini-systematic review of effects of
COVID-19 pandemic. Alzheimers Dement. 17 (Suppl
12)(e058652)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hampshire A, Trender W, Chamberlain SR,
Jolly AE, Grant JE, Patrick F, Mazibuko N, Williams SC, Barnby JM,
Hellyer P and Mehta MA: Cognitive deficits in people who have
recovered from COVID-19. EClinicalMedicine.
39(101044)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xie Q, Liu XB, Xu YM and Zhong BL:
Understanding the psychiatric symptoms of COVID-19: A meta-analysis
of studies assessing psychiatric symptoms in Chinese patients with
and survivors of COVID-19 and SARS by using the symptom
checklist-90-revised. Transl Psychiatry. 11(290)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
World Medical Association (WMA): WMA
Declaration of Helsinki-Ethical Principles for Medical Research
Involving Human Subjects. 7th revision. WMA, Ferney-Voltaire, 2013.
https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
Accessed February 20, 2022.
|