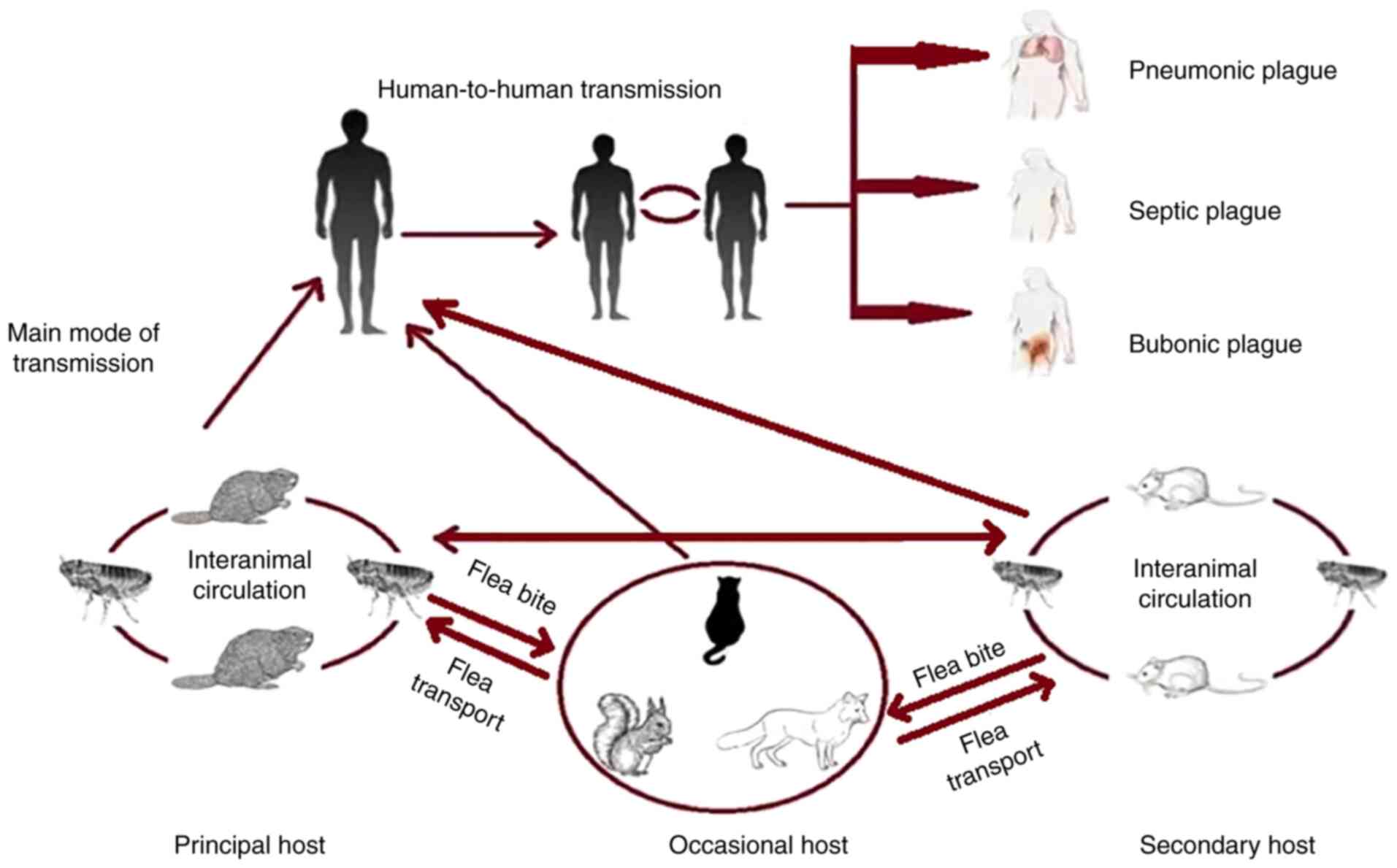
Plague is a zoonotic infection disease having a high
mortality rate without treatment. It may present three distinct
clinical forms: bubonic, septicemic and pneumonic (1). Yersinia pestis (Y.
pestis), a member of the genus Yersinia which belongs to
the Enterobacteriaceae family, is the etiological agent of
plague (2). Y. pestis is a
highly pathogenic gram-negative coccobacillus, which are
nonmsotile, non-spore-forming, oxidase-negative, catalase-positive
and lactose-negative, exhibiting bipolar staining with Giemsa,
Wright's and Wayson stains (3). It
grows at temperatures ranging from 4-40˚C and the optimal
temperature for growth is 28-30˚C (4). At present, four biotypes of Y.
pestis are recognized, including Antiqua,
Orientalis, Mediaevalis and Microtus, on the
basis of their ability to ferment glycerol and form nitrite from
nitrate (5,6). Among them, three classic biotypes
(Antiqua, Orientalis and Mediaevalis) of Y.
pestis demonstrate no difference in their pathology in animals
or humans (7). By contrast,
Microtus is nonpathogenic for humans (8). Y. pestis has a complex
infectious cycle, which starts within an insect vector (fleas)
followed by transmission to a mammalian host (rodents and humans)
(9) (Fig. 1).
Early diagnosis and treatment can effectively reduce
the mortality of bubonic plague and septicemic plague (13,14).
Polymerase chain reaction (PCR)-based methods have enabled the
rapid identification of cultured or uncultured bacteria (15). Previous reviews describing
microbiological and molecular aspect, molecular typing and
molecular diagnostic techniques of Y. pestis, are available
(16-20).
The present review focused on the applications of PCR-based methods
for detection of Y. pestis and attempt to compile and update
technical aspects of PCR strategies in diagnosis of Y.
pestis infection.
At present, there are various laboratory tests for
diagnosis of plague, such as bacterial culture, staining
techniques, serological evidence, phage tests, DNA hybridization
and PCR analysis (21). Isolation
and identification of pathogen in the laboratory is gold standard
for plague diagnosis (22).
Clinical specimens for analysis can include blood, bubo aspirates,
sputum, or cerebrospinal fluid. Y. pestis can be cultivated
on culture media, such as brain heart infusion broth, MacConkey
agar and sheep blood agar. Isolation of Y. pestis should be
performed under biosafety level 3 conditions. However,
bacteriological evidence is time consuming due to the low growth
rate of Y. pestis. Serological tests are often used to
diagnosis plague, including the agar-gel precipitin inhibition, the
complement fixation, passive hemagglutination (PHA) test (23), immunochromatography test (24), enzyme-linked immunosorbent assay
(ELISA) (25), dot
enzyme-immunosorbent assay (DOT-ELISA) (26) and the dissociation-enhanced
lanthanide fluorescent immunoassays (DELFIA) (27). Serological tests seem to be more
effective but are expensive and labor intensive. Moreover, it can
be unspecific due to serological cross-reactivity with other
enteropathogenic bacteria (24).
DNA hybridization using Y. pestis-specific DNA probe may be
used for plague diagnosis (28).
The minimum detection limits of this method are ~105
bacteria, which limits its clinical application. PCR is well suited
molecular biology tool for diagnosis of pathogens. At present,
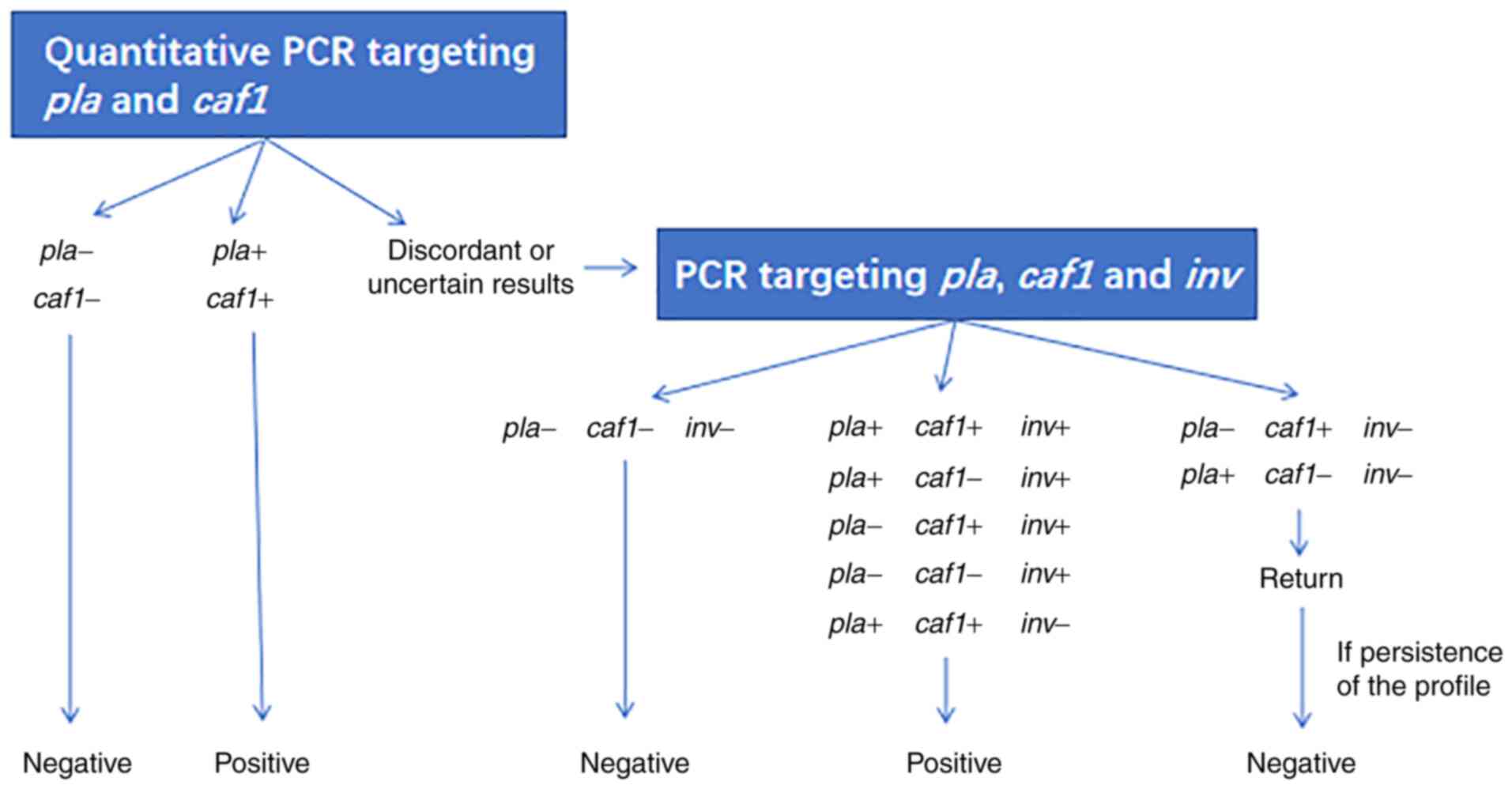
confirmation of plague is performed using reverse transcription PCR
targeting a plasminogen activator gene (pla) and 60-Md
plasmid-located gene (caf1) and in the case of discordant or
uncertain results, a PCR targeting pla, caf1 and an
invasin protein gene (inv) is performed (Fig. 2).
Standard PCR is replacing the more traditional
microbiological assays in the detection of Y. pestis. This
approach requires development of highly specific oligonucleotide
primers unique to Y. pestis. Primer pairs include the
primers for sequences of caf1, pla, inv, a
Y. pestis-specific region of a yopM gene, 23S
ribosomal DNA interspace region and insertion sequence (56-60).
Table I gives the different
primers for standard PCR.
By using specific probe for the amplicon detection,
standard PCR is considered sensitive and specific. However, it
cannot be monitored in real time and requires the performance of
any postreaction processing, such as the electrophoresis gel.
Moreover, standard PCR method is relatively poor in detecting the
low numbers of pathogens in the biopsy sample. So far, there have
been numerous modifications of the PCR technology for increasing
the sensitivity of detection.
Compared to conventional PCR, reverse transcription
PCR has several advantages, including speed, simplicity,
reproducibility, quantitative capability and low risk of
contamination (67-70).
Reverse transcription PCR for the rapid detection and
differentiation of Y. pestis has been developed, targeting
caf1, Ymt, pla, hemin storage genes
(hmsH, hmsF and hmsR) and irp2
iron-regulating gene (71,72). Table
II gives the different primers and probes for the reverse
transcription PCR.
Reverse transcription PCR is proposed as a timely,
cost-effective and accurate diagnostic assay (73,74).
The reliability of this method was evaluated in 1,050 clinical
specimens and high values of specificity were obtained (75). An autonomous pathogen detection
system was developed by coupling reverse transcription TaqMan
assay, which generate extremely low false positive rate (76). Woubit et al (77) also identify the genomic targets of
Y. pestis to design the primers. Primer sets are used to
specifically detect pathogen with reverse transcription PCR assays
and this assay is found to be sensitive. A 5' nuclease PCR assay
for detection of the Y. pestis has been developed with a
detection threshold of 1.6 pg of total cell DNA (78). Tomaso et al (79) established a reverse transcription
PCR assay for the specific detection of Y. pestis. The lower
limit of detection is ~0.1 genome equivalent. Skottman et al
(80) report the development of
reverse transcription PCR assays for detection of Y. pestis
with a sensitivity of 1 fg of total DNA in the PCR tube. In
addition, some researchers develop and validate reverse
transcription PCR for the differentiation and quantification of
Y. pestis. Comer et al (81) report reverse transcription PCR
assays to determine absolute bacterial numbers in flea vector and
mammalian host tissues. A quadruplex reverse transcription PCR
assay proved to be successful in differentiating Y. pestis
from Y. pseudotuberculosis (82). Chase et al (83) also designed reverse transcription
PCR assays to discriminate Y. pestis DNA from all other
Yersinia species tested and from the closely related Y.
pseudotuberculosis. Moreover, reverse transcription PCR assays
have been developed for simultaneous detection of various
organisms. Liu et al (75)
developed a reverse transcription PCR-based TaqMan array card that
can simultaneously detect 26 organisms, including Y. pestis.
Notably, reverse transcription PCR allows the detection of only
live Y. pestis using amplification of plague diagnostic
bacteriophages (84). It is
therefore a useful method for the differentiation among inactive
and active states of Y. pestis.
Some researchers develop reverse transcription PCR
for the specific detection and quantification of Y. pestis
from various samples, such as complex food, synthetic building
debris and leachate and spleen samples of animals (85-89).
Hennebique et al (90) also
report the development of a reverse transcription PCR assay for the
detection of Y. pestis in various types of samples and
demonstrate good performances.
Some researchers have compared reverse transcription
PCR assay performance across various platforms. Christensen et
al (91) detect Y.
pestis by reverse transcription PCR on the R.A.P.I.D., the
LightCycler and the Smart Cycler platforms. They find that the
tested assays have comparable sensitivity and specificity on these
rapid cycling instruments. Matero et al (92) also compare this assay performance
between the Applied Biosystems 7300/7500 and the RAZOR instruments
for detection of Y. pestis. Although no notable differences
between two platforms were observed in analytical sensitivity or
specificity, the duration of thermocycling with the RAZOR
instrument was significantly shorter (40 min vs. 100 min with ABI
7300/7500). Mölsä et al (93) compare the performance of a novel
portable reverse transcription PCR thermocycler PikoReal to ABI
7300 for the detection of Y. pestis. The PikoReal system may
be a more efficient alternative to detect biothreat agents under
field conditions.
Multiplex PCR is a type of PCR technique which
amplifies more than one target DNA in one reaction system at one
time. Elsholz et al (94)
designed a multiplex PCR method for the parallel detection of a
panel of the pathogens, including B. anthracis, Y.
pestis, F. tularensis and ortho pox viruses
(genus). Stenkova et al (95) show that the multiplex PCR provides
an improved method for detection of the Yersinia genus with
identification of pathogenic species (Y. pestis, Y.
pseudotuberculosis, Y. enterocolitica). Stevenson et
al (96) further detect
flea-associated microorganisms, such as Bartonella strains
and Y. pestis, in prairie dogs and their fleas using
multiplex PCR. Additionally, the multiplex PCR can be used to
detect and identify Y. pestis using multiplex primers,
including caf1, yopM, pla and inv genes
(97). Woron et al also
reported the 4-target multiplex reverse transcription PCR assay for
Y. pestis (98).
Multiplex PCR can be a powerful tool for the
simultaneous quantification of more than one pathogen in a single
reaction by combination of primers and probes. The advantages of
this method include ease of sample collection, improvement in
amplification efficiency and reduction of laboratory time. This
technique is more suitable for screening of pathogenic
bacteria.
The nested and semi-nested PCR assays have
advantages of high sensitivity and easy applicability for the
detection of Y. pestis in various samples. Trebesius et
al (108) present the
semi-nested PCR approach based on 16S and 23S rDNAs with respect to
diagnosis of plague. A single-tube nested-PCR technique targeting
the caf1 gene was evaluated for plague diagnosis, which
showed more sensitive than conventional PCR (109). Glukhov et al (110) develop a nested PCR method to
distinguish the culture of Y. pestis from cultures of other
microorganism, demonstrating a higher sensitivity and specificity
than standard PCR.
The sensitivity limit of PCR depends on the method
used for preparing the sample (124) and the presence of PCR inhibitors
that are often found in biological samples (125). A previous study showed that some
components in the tissues can inhibit PCR (126). Leal et al (127) found that the spleen suspension of
animals experimentally infected with Y. pestis can be used
as PCR amplification template without DNA extraction. The
sensitivity and specificity were enhanced by amplification after
the second-round PCR. Afanas'ev et al (128) treated the samples of
plague-infected fleas with an affine sorbent prior to PCR analysis.
They found that the use of magnoimmunosorbent prevents the
inhibitory effect of flea tissues and makes it possible to have a
specific concentration of plague microbial DNA. The high-quality
DNA before PCR gene amplification is essential for the diagnostic
of pathogenic bacteria. Coyne et al (129) evaluate the Schleicher and Schuell
IsoCode Stix DNA isolation device and the Qiagen QIAamp DNA Mini
kit for isolating Y. pestis DNA from serum and whole-blood
samples. They find that the two methods achieve comparable
detection limits. Dauphin et al (130) evaluate five commercially
available DNA extraction kits. TaqMan reverse transcription PCR
analysis revealed that the MasterPure kit was best extraction
method for Y. pestis suspensions and spiked environmental
samples. Gilbert et al (131) show that various methods of tooth
manipulation can influence the PCR-based detection of Y.
pestis DNA in human teeth from European excavations of putative
plague victims. They use a novel contamination-minimizing embedding
technique to reduce the levels of environmental bacterial DNA
presented in DNA extracts. Hong-Geller et al (132) evaluate the sample recovery
efficiencies of two collection methods (swabs and wipes) for Y.
pestis from nonporous surfaces. They found that collection
efficiency was surface-dependent, indicating the importance of
surface interactions in pathogen detection.
The developed approach based on PCR is applicable
for identifying and confirming Y. pestis (133,134). This system also allows for
effective differentiation of Yersinia strains of various
subspecies. In addition, the PCR assay is able to determine
bacterial susceptibility to antibiotics and prominent virulence
markers of Y. pestis. Compared with traditional techniques,
PCR-based is simple, rapid, highly sensitive and specific and it
has proven useful in application as a diagnostic strategy for
routine plague surveillance of epidemics. However, the PCR
inhibitors may be present in samples. The suboptimal field
conditions, sample recovery efficiency and DNA extraction quality
directly influence the sensitivity and specificity of most
PCR-based methods. Therefore, future studies should focus on the
standardization of sample processing.
Not applicable.
Funding: The present study was supported by Key Scientific and
Technology Project of Inner Mongolia Autonomous Region (grant no.
2021ZD0006).
Not applicable.
YZ contributed to the acquisition, analysis and
systematization of data and manuscript writing. ZW and WW
contributed to the acquisition and analysis of data. HY and MJ
contributed to the systematization of data and critical revision.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zietz BP and Dunkelberg H: The history of
the plague and the research on the causative agent Yersinia
pestis. Int J Hyg Environ Health. 207:165–178. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Drancourt M and Raoult D: Molecular
history of plague. Clin Microbiol Infect. 22:911–915.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Devignat R: Varieties of Pasteurella
pestis; new hypothesis. Bull World Health Organ. 4:247–263.
1951.PubMed/NCBI(In Undetermined Language).
|
|
4
|
Brubaker RR: Factors promoting acute and
chronic diseases caused by yersiniae. Clin Microbiol Rev.
4:309–324. 1991.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dai E, Tong Z, Wang X, Li M, Cui B, Dai R,
Zhou D, Pei D, Song Y, Zhang J, et al: Identification of different
regions among strains of Yersinia pestis by suppression
subtractive hybridization. Res Microbiol. 156:785–789.
2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Drancourt M: Plague in the genomic area.
Clin Microbiol Infect. 18:224–230. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brubaker RR: The genus Yersinia:
Biochemistry and genetics of virulence. Curr Top Microbiol Immunol.
57:111–158. 1972.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou D, Tong Z, Song Y, Han Y, Pei D, Pang
X, Zhai J, Li M, Cui B, Qi Z, et al: Genetics of metabolic
variations between Yersinia pestis biovars and the proposal
of a new biovar, microtus. J Bacteriol. 186:5147–5152.
2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Perry RD and Fetherston JD: Yersinia
pestis-etiologic agent of plague. Clin Microbiol Rev. 10:35–66.
1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mordechai L, Eisenberg M, Newfield TP,
Izdebski A, Kay JE and Poinar H: The justinianic plague: An
inconsequential pandemic? Proc Natl Acad Sci USA. 116:25546–25554.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Susat J, Bonczarowska JH,
Pētersone-Gordina E, Immel A, Nebel A, Gerhards G and Krause-Kyora
B: Yersinia pestis strains from Latvia show depletion of the
pla virulence gene at the end of the second plague pandemic. Sci
Rep. 10(14628)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bramanti B, Dean KR, Walløe L and
Chr*Stenseth N: The third plague pandemic in Europe. Proc Biol Sci.
286(20182429)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nikiforov VV, Gao H, Zhou L and Anisimov
A: Plague: Clinics, diagnosis and treatment. Adv Exp Med Biol.
918:293–312. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jullien S, Dissanayake HA and Chaplin M:
Rapid diagnostic tests for plague. Cochrane Database Syst Rev.
6(CD013459)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hinnebusch J and Schwan TG: New method for
plague surveillance using polymerase chain reaction to detect
Yersinia pestis in fleas. J Clin Microbiol. 31:1511–1514.
1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wake A: Pathogenicity of Yersinia
pestis: Microbiological and molecular aspect. Nihon Saikingaku
Zasshi. 50:651–669. 1995.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
17
|
Platonov ME, Evseeva VV, Dentovskaya SV
and Anisimov AP: Molecular typing of Yersinia pestis. Mol
Gen Mikrobiol Virusol. 3–12. 2013.PubMed/NCBI(In Russian).
|
|
18
|
Wolkowicz T: The utility and perspectives
of NGS-based methods in BSL-3 and BSL-4 laboratory-sequencing and
analysis strategies. Brief Funct Genomics. 17:471–476.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Neubauer H, Sprague LD, Scholz H and
Hensel A: Diagnosis o Yersinia enterocolitica infections: A
review on classical identification techniques and new molecular
biological methods. Berl Munch Tierarztl Wochenschr. 114:1–7.
2001.PubMed/NCBI(In German).
|
|
20
|
Jones SW, Dobson ME, Francesconi SC,
Schoske R and Crawford R: DNA assays for detection, identification
and individualization of select agent microorganisms. Croat Med J.
46:522–529. 2005.PubMed/NCBI
|
|
21
|
Gaweł J, Bartoszcze M and Osiak B:
Yersinia pestis pathogenesis and diagnostics. Przegl
Epidemiol. 60:315–321. 2006.PubMed/NCBI(In Polish).
|
|
22
|
Yang R: Plague: Recognition, treatment and
prevention. J Clin Microbiol. 56:e01519–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Marshall JD Jr, Mangiafico JA and
Cavanaugh DC: Comparison of the reliability and sensitivity of
three serological procedures in detecting antibody to Yersinia
pestis (Pasteurella pestis). Appl Microbiol. 24:202–204.
1972.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kopylov PKh, Platonov ME, Ablamunits VG,
Kombarova TI, Ivanov SA, Kadnikova LA, Somov AN, Dentovskaya SV,
Uversky VN and Anisimov AP: Yersinia pestis caf1 protein:
Effect of sequence polymorphism on intrinsic disorder propensity,
serological cross-reactivity and cross-protectivity of isoforms.
PLoS One. 11(e0162308)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shepherd AJ, Leman PA, Hummitzsch DE and
Swanepoel R: A comparison of serological techniques for plague
surveillance. Trans R Soc Trop Med Hyg. 78:771–773. 1984.PubMed/NCBI View Article : Google Scholar
|
|
26
|
de*Almeida AM and Ferreira LC: Evaluation
of three serological tests for the detection of human plague in
northeast Brazil. Mem Inst Oswaldo Cruz. 87:87–92. 1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Smith DR, Rossi CA, Kijek TM, Henchal EA
and Ludwig GV: Comparison of dissociation-enhanced lanthanide
fluorescent immunoassays to enzyme-linked immunosorbent assays for
detection of staphylococcal enterotoxin B, Yersinia
pestis-specific F1 antigen and Venezuelan equine encephalitis
virus. Clin Diagn Lab Immunol. 8:1070–1075. 2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McDonough KA, Schwan TG, Thomas RE and
Falkow S: Identification of a Yersinia pestis-specific DNA
probe with potential for use in plague surveillance. J Clin
Microbiol. 26:2515–2519. 1988.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bulat SA, Mikhaĭlo NV and Koroliuk AM: The
gene identification of bacterial species and serovariants by the
polymerase chain reaction with universal oligonucleotides: The
reidentification of earlier isolated strains of Yersinia
pseudotuberculosis. Zh Mikrobiol Epidemiol Immunobiol. 2–7.
1991.PubMed/NCBI(In Russian).
|
|
30
|
Eroshenko GA, Odinokov GN, Kukleva LM,
Pavlova AI, Krasnov IaM, Shavina NIu, Guseva NP, Vinogradova NA and
Kutyrev VV: Standard algorithm of molecular typing of Yersinia
pestis strains. Zh Mikrobiol Epidemiol Immunobiol. 25–35.
2012.PubMed/NCBI(In Russian).
|
|
31
|
Tong ZZ, Zhou DS, Song YJ, Zhang L, Pei D,
Han YP, Pang X, Li M, Cui BZ, Wang J, et al: Genetic variations in
the pgm locus among natural isolates of Yersinia pestis. J
Gen Appl Microbiol. 51:11–19. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kim W, Song MO, Song W, Kim KJ, Chung SI,
Choi CS and Park YH: Comparison of 16S rDNA analysis and rep-PCR
genomic fingerprinting for molecular identification of Yersinia
pseudotuberculosis. Antonie Van Leeuwenhoek. 83:125–133.
2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Y, Dai E, Cui Y, Li M, Zhang Y, Wu M,
Zhou D, Guo Z, Dai X, Cui B, et al: Different region analysis for
genotyping Yersinia pestis isolates from China. PLoS One.
3(e2166)2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kingston JJ, Tuteja U, Kapil M, Murali HS
and Batra HV: Genotyping of Indian Yersinia pestis strains
by MLVA and repetitive DNA sequence based PCRs. Antonie Van
Leeuwenhoek. 96:303–312. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Motin VL, Georgescu AM, Elliott JM, Hu P,
Worsham PL, Ott LL, Slezak TR, Sokhansanj BA, Regala WM, Brubaker
RR and Garcia E: Genetic variability of Yersinia pestis
isolates as predicted by PCR-based IS100 genotyping and analysis of
structural genes encoding glycerol-3-phosphate dehydrogenase
(glpD). J Bacteriol. 184:1019–1027. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bogdanovich T, Carniel E, Fukushima H and
Skurnik M: Use of O-antigen gene cluster-specific PCRs for the
identification and O-genotyping of Yersinia
pseudotuberculosis and Yersinia pestis. J Clin
Microbiol. 41:5103–5112. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Savostina EP, Popov IuA, Kashmanova TN and
Iashechkin IuI: Analysis of genomic polymorphism of typical and
atypical strains of the plague pathogen using polymerase chain
reaction with universal primers. Mol Gen Mikrobiol Virusol. 22–26.
2004.PubMed/NCBI(In Russian).
|
|
38
|
Nikiforov KA, Oglodin EG, Kukleva LM,
Eroshenko GA, Germanchuk VG, Devdariani ZL and Kutyrev VV:
Subspecies differentiation of Yersinia pestis strains by PCR
with hybridization-fluorescent detection. Zh Mikrobiol Epidemiol
Immunobiol. 22–27. 2017.PubMed/NCBI(In English, Russian).
|
|
39
|
Matero P, Pasanen T, Laukkanen R, Tissari
P, Tarkka E, Vaara M and Skurnik M: Real-time multiplex PCR assay
for detection of Yersinia pestis and Yersinia
pseudotuberculosis. APMIS. 117:34–44. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bai Y, Motin V, Enscore RE, Osikowicz L,
Rosales Rizzo M, Hojgaard A, Kosoy M and Eisen RJ: Pentaplex
real-time PCR for differential detection of Yersinia pestis
and Y. pseudotuberculosis and application for testing fleas
collected during plague epizootics. Microbiologyopen.
9(e1105)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Franklin HA, Stapp P and Cohen A:
Polymerase chain reaction (PCR) identification of rodent blood
meals confirms host sharing by flea vectors of plague. J Vector
Ecol. 35:363–371. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Engelthaler DM, Hinnebusch BJ, Rittner CM
and Gage KL: Quantitative competitive PCR as a technique for
exploring flea-Yersina pestis dynamics. Am J Trop Med Hyg.
62:552–560. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hinnebusch BJ, Gage KL and Schwan TG:
Estimation of vector infectivity rates for plague by means of a
standard curve-based competitive polymerase chain reaction method
to quantify Yersinia pestis in fleas. Am J Trop Med Hyg.
58:562–569. 1998.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Dai R, He J, Zha X, Wang Y, Zhang X, Gao
H, Yang X, Li J, Xin Y, Wang Y, et al: A novel mechanism of
streptomycin resistance in Yersinia pestis: Mutation in the
rpsL gene. PLoS Negl Trop Dis. 15(e0009324)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Steinberger-Levy I, Shifman O, Zvi A,
Ariel N, Beth-Din A, Israeli O, Gur D, Aftalion M, Maoz S and Ber
R: A rapid molecular test for determining Yersinia pestis
susceptibility to ciprofloxacin by the quantification of
differentially expressed marker genes. Front Microbiol.
7(763)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lindler LE, Fan W and Jahan N: Detection
of ciprofloxacin-resistant Yersinia pestis by fluorogenic
PCR using the LightCycler. J Clin Microbiol. 39:3649–3655.
2001.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shifman O, Steinberger-Levy I,
Aloni-Grinstein R, Gur D, Aftalion M, Ron I, Mamroud E, Ber R and
Rotem S: A rapid antimicrobial susceptibility test for determining
Yersinia pestis susceptibility to Doxycycline by RT-PCR
quantification of RNA markers. Front Microbiol.
10(754)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ehlers J, Krüger A, Rakotondranary SJ,
Ratovonamana RY, Poppert S, Ganzhorn JU and Tappe D: Molecular
detection of Rickettsia spp., Borrelia spp.,
Bartonella spp. and Yersinia pestis in ectoparasites
of endemic and domestic animals in southwest Madagascar. Acta Trop.
205(105339)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Leal NC and Almeida AM: Diagnosis of
plague and identification of virulence markers in Yersinia
pestis by multiplex-PCR. Rev Inst Med Trop Sao Paulo.
41:339–342. 1999.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Griffin KA, Martin DJ, Rosen LE, Sirochman
MA, Walsh DP, Wolfe LL and Miller MW: Detection of Yersinia
pestis DNA in prairie dog-associated fleas by polymerase chain
reaction assay of purified DNA. J Wildl Dis. 46:636–643.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Neubauer H, Meyer H, Prior J, Aleksic S,
Hensel A and Splettstösser W: A combination of different polymerase
chain reaction (PCR) assays for the presumptive identification of
Yersinia pestis. J Vet Med B Infect Dis Vet Public Health.
47:573–580. 2000.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mize EL and Britten HB: Detections of
Yersinia pestis east of the known distribution of active
plague in the United States. Vector Borne Zoonotic Dis. 16:88–95.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Safari Foroshani N, Karami A and Pourali
F: Simultaneous and rapid detection of Salmonella typhi,
Bacillus anthracis, and Yersinia pestis by using
multiplex polymerase chain reaction (PCR). Iran Red Crescent Med J.
15(e9208)2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Engelthaler DM, Gage KL, Montenieri JA,
Chu M and Carter LG: PCR detection of Yersinia pestis in
fleas: Comparison with mouse inoculation. J Clin Microbiol.
37:1980–1984. 1999.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Nyirenda SS, Hang'ombe BM, Mulenga E and
Kilonzo BS: Serological and PCR investigation of Yersinia
pestis in potential reservoir hosts from a plague outbreak
focus in Zambia. BMC Res Notes. 10(345)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Rahalison L, Vololonirina E, Ratsitorahina
M and Chanteau S: Diagnosis of bubonic plague by PCR in Madagascar
under field conditions. J Clin Microbiol. 38:260–263.
2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Nyirenda SS, Hang Ombe BM, Simulundu E,
Mulenga E, Moonga L, Machang U RS, Misinzo G and Kilonzo BS:
Molecular epidemiological investigations of plague in Eastern
Province of Zambia. BMC Microbiol. 18(2)2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Radnedge L, Gamez-Chin S, McCready PM,
Worsham PL and Andersen GL: Identification of nucleotide sequences
for the specific and rapid detection of Yersinia pestis.
Appl Environ Microbiol. 67:3759–3762. 2001.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tsukano H, Itoh K, Suzuki S and Watanabe
H: Detection and identification of Yersinia pestis by
polymerase chain reaction (PCR) using multiplex primers. Microbiol
Immunol. 40:773–775. 1996.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang Z, Wu L, Liang Y, Wang S, He J, Yu D
and Li W: Identification of Yersinia pestis of Xilingele
plateau ecotype isolated from China using insertion sequences as
target. Ann Clin Lab Sci. 49:656–660. 2019.PubMed/NCBI
|
|
61
|
Ziwa MH, Matee MI, Kilonzo BS and
Hang'ombe BM: Evidence of Yersinia pestis DNA in rodents in
plague outbreak foci in Mbulu and Karatu Districts, northern
Tanzania. Tanzan J Health Res. 15:152–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zasada AA, Formińska K and Zacharczuk K:
Fast identification of Yersinia pestis, Bacillus
anthracis and Francisella tularensis based on
conventional PCR. Pol J Microbiol. 62:453–455. 2013.PubMed/NCBI
|
|
63
|
Singh R, Pal V, Kumar M, Tripathi NK and
Goel AK: Development of a PCR-lateral flow assay for rapid
detection of Yersinia pestis, the causative agent of plague.
Acta Trop. 220(105958)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Arnold T, Hensel A, Hagen R, Aleksic S,
Neubauer H and Scholz HC: A highly specific one-step PCR-assay for
the rapid discrimination of enteropathogenic Yersinia
enterocolitica from pathogenic Yersinia
pseudotuberculosis and Yersinia pestis. Syst Appl
Microbiol. 24:285–289. 2001.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Trukhachev AL, Ivanova VS, Arsen'eva TE,
Lebedeva SA and Goncharenko EV: Search for primers on the basis of
Yersinia pestis chromosomal DNA for effective PCR
identification of typical and atypical plague pathogen strains.
Klin Lab Diagn. 49–52. 2008.PubMed/NCBI(In Russian).
|
|
66
|
Zhou D, Han Y, Dai E, Pei D, Song Y, Zhai
J, Du Z, Wang J, Guo Z and Yang R: Identification of signature
genes for rapid and specific characterization of Yersinia
pestis. Microbiol Immunol. 48:263–269. 2004.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Fenollar F and Raoult D: Molecular genetic
methods for the diagnosis of fastidious microorganisms. APMIS.
112:785–807. 2004.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Anderson B, Rashid MH, Carter C,
Pasternack G, Rajanna C, Revazishvili T, Dean T, Senecal A and
Sulakvelidze A: Enumeration of bacteriophage particles: Comparative
analysis of the traditional plaque assay and real-time QPCR- and
nanosight-based assays. Bacteriophage. 1:86–93. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Tomaso H, Jacob D, Eickhoff M, Scholz HC,
Al Dahouk S, Kattar MM, Reischl U, Plicka H, Olsen JS, Nikkari S,
et al: Preliminary validation of real-time PCR assays for the
identification of Yersinia pestis. Clin Chem Lab Med.
46:1239–1244. 2008.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Rachwal PA, Rose HL, Cox V, Lukaszewski
RA, Murch AL and Weller SA: The potential of TaqMan array cards for
detection of multiple biological agents by real-time PCR. PLoS One.
7(e35971)2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Gaddy CE, Cuevas PF, Hartman LJ, Howe GB,
Worsham PL and Minogue TD: Development of real-time PCR assays for
specific detection of hmsH, hmsF, hmsR, and irp2 located within the
102-kb pgm locus of Yersinia pestis. Mol Cell Probes.
28:288–295. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Riehm JM, Rahalison L, Scholz HC, Thoma B,
Pfeffer M, Razanakoto LM, Al Dahouk S, Neubauer H and Tomaso H:
Detection of Yersinia pestis using real-time PCR in patients
with suspected bubonic plague. Mol Cell Probes. 25:8–12.
2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Yang S, Rothman RE, Hardick J, Kuroki M,
Hardick A, Doshi V, Ramachandran P and Gaydos CA: Rapid polymerase
chain reaction-based screening assay for bacterial biothreat
agents. Acad Emerg Med. 15:388–392. 2008.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Amoako KK, Goji N, Macmillan T, Said KB,
Druhan S, Tanaka E and Thomas EG: Development of multitarget
real-time PCR for the rapid, specific, and sensitive detection of
Yersinia pestis in milk and ground beef. J Food Prot.
73:18–25. 2010.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Liu J, Ochieng C, Wiersma S, Ströher U,
Towner JS, Whitmer S, Nichol ST, Moore CC, Kersh GJ, Kato C, et al:
Development of a TaqMan array card for acute-febrile-illness
outbreak investigation and surveillance of emerging pathogens,
including Ebola virus. J Clin Microbiol. 54:49–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hindson BJ, McBride MT, Makarewicz AJ,
Henderer BD, Setlur US, Smith SM, Gutierrez DM, Metz TR, Nasarabadi
SL, Venkateswaran KS, et al: Autonomous detection of aerosolized
biological agents by multiplexed immunoassay with polymerase chain
reaction confirmation. Anal Chem. 77:284–289. 2005.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Woubit A, Yehualaeshet T, Habtemariam T
and Samuel T: Novel genomic tools for specific and real-time
detection of biothreat and frequently encountered foodborne
pathogens. J Food Prot. 75:660–670. 2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Higgins JA, Ezzell J, Hinnebusch BJ,
Shipley M, Henchal EA and Ibrahim MS: 5' nuclease PCR assay to
detect Yersinia pestis. J Clin Microbiol. 36:2284–2288.
1998.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Tomaso H, Reisinger EC, Al Dahouk S,
Frangoulidis D, Rakin A, Landt O and Neubauer H: Rapid detection of
Yersinia pestis with multiplex real-time PCR assays using
fluorescent hybridisation probes. FEMS Immunol Med Microbiol.
38:117–126. 2003.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Skottman T, Piiparinen H, Hyytiäinen H,
Myllys V, Skurnik M and Nikkari S: Simultaneous real-time PCR
detection of Bacillus anthracis, Francisella
tularensis and Yersinia pestis. Eur J Clin Microbiol
Infect Dis. 26:207–211. 2007.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Comer JE, Lorange EA and Hinnebusch BJ:
Examining the vector-host-pathogen interface with quantitative
molecular tools. Methods Mol Biol. 431:123–131. 2008.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Stewart A, Satterfield B, Cohen M, O'Neill
K and Robison R: A quadruplex real-time PCR assay for the detection
of Yersinia pestis and its plasmids. J Med Microbiol.
57:324–331. 2008.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Chase CJ, Ulrich MP, Wasieloski LP Jr,
Kondig JP, Garrison J, Lindler LE and Kulesh DA: Real-time PCR
assays targeting a unique chromosomal sequence of Yersinia
pestis. Clin Chem. 51:1778–1785. 2005.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Sergueev KV, He Y, Borschel RH, Nikolich
MP and Filippov AA: Rapid and sensitive detection of Yersinia
pestis using amplification of plague diagnostic bacteriophages
monitored by real-time PCR. PLoS One. 5(e11337)2010.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Satterfield BC, Kulesh DA, Norwood DA,
Wasieloski LP Jr, Caplan MR and West JA: Tentacle probes:
Differentiation of difficult single-nucleotide polymorphisms and
deletions by presence or absence of a signal in real-time PCR. Clin
Chem. 53:2042–2050. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Sting R, Eisenberg T and Hrubenja M: Rapid
and reasonable molecular identification of bacteria and fungi in
microbiological diagnostics using rapid real-time PCR and sanger
sequencing. J Microbiol Methods. 159:148–156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Saikaly PE, Barlaz MA and de*Los*Reyes
FL*III: Development of quantitative real-time PCR assays for
detection and quantification of surrogate biological warfare agents
in building debris and leachate. Appl Environ Microbiol.
73:6557–6565. 2007.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Thomas MC, Janzen TW, Huscyzynsky G,
Mathews A and Amoako KK: Development of a novel multiplexed qPCR
and pyrosequencing method for the detection of human pathogenic
yersiniae. Int J Food Microbiol. 257:247–253. 2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Mostafavi E, Ghasemi A, Rohani M,
Molaeipoor L, Esmaeili S, Mohammadi Z, Mahmoudi A, Aliabadian M and
Johansson A: Molecular survey of tularemia and plague in small
mammals from Iran. Front Cell Infect Microbiol.
8(215)2018.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Hennebique A, Gas F, Batina H, De Araujo
C, Bizet K and Maurin M: Evaluation of the biotoxis qPCR detection
kit for Francisella tularensis detection in clinical and
environmental samples. J Clin Microbiol. 59:e01434–20.
2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Christensen DR, Hartman LJ, Loveless BM,
Frye MS, Shipley MA, Bridge DL, Richards MJ, Kaplan RS, Garrison J,
Baldwin CD, et al: Detection of biological threat agents by
real-time PCR: Comparison of assay performance on the R.A.P.I.D.,
the LightCycler, and the smart cycler platforms. Clin Chem.
52:141–145. 2006.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Matero P, Hemmilä H, Tomaso H, Piiparinen
H, Rantakokko-Jalava K, Nuotio L and Nikkari S: Rapid field
detection assays for Bacillus anthracis, Brucella
spp., Francisella tularensis and Yersinia pestis.
Clin Microbiol Infect. 17:34–43. 2011.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Mölsä M, Hemmilä H, Katz A, Niemimaa J,
Forbes KM, Huitu O, Stuart P, Henttonen H and Nikkari S: Monitoring
biothreat agents (Francisella tularensis, Bacillus
anthracis and Yersinia pestis) with a portable real-time
PCR instrument. J Microbiol Methods. 115:89–93. 2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Elsholz B, Nitsche A, Achenbach J,
Ellerbrok H, Blohm L, Albers J, Pauli G, Hintsche R and Wörl R:
Electrical microarrays for highly sensitive detection of multiplex
PCR products from biological agents. Biosens Bioelectron.
24:1737–1743. 2009.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Stenkova AM, Isaeva MP and Rasskazov VA:
Development of a multiplex PCR for detection of the Yersinia genus
with identification of pathogenic species (Y. pestis, Y.
pseudotuberculosis, Y. enterocolitica). Mol Gen
Mikrobiol Virusol. 18–23. 2008.PubMed/NCBI(In Russian).
|
|
96
|
Stevenson HL, Bai Y, Kosoy MY, Montenieri
JA, Lowell JL, Chu MC and Gage KL: Detection of novel
Bartonella strains and Yersinia pestis in prairie
dogs and their fleas (Siphonaptera: Ceratophyllidae and Pulicidae)
using multiplex polymerase chain reaction. J Med Entomol.
40:329–337. 2003.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Demeure CE, Dussurget O, Mas Fiol G, Le
Guern AS, Savin C and Pizarro-Cerdá J: Yersinia pestis and
plague: An updated view on evolution, virulence determinants,
immune subversion, vaccination, and diagnostics. Genes Immun.
20:357–370. 2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Woron AM, Nazarian EJ, Egan C, McDonough
KA, Cirino NM, Limberger RJ and Musser KA: Development and
evaluation of a 4-target multiplex real-time polymerase chain
reaction assay for the detection and characterization of
Yersinia pestis. Diagn Microbiol Infect Dis. 56:261–268.
2006.PubMed/NCBI View Article : Google Scholar
|
|
99
|
He J, Kraft AJ, Fan J, Van Dyke M, Wang L,
Bose ME, Khanna M, Metallo JA and Henrickson KJ: Simultaneous
detection of CDC category ‘A’ DNA and RNA bioterrorism agents by
use of multiplex PCR & RT-PCR enzyme hybridization assays.
Viruses. 1:441–459. 2009.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Vanlalhmuaka Thavachelvam K, Tuteja U,
Sarika K, Nagendra S and Kumar S: Reverse line blot macroarray for
simultaneous detection and characterization of four biological
warfare agents. Indian J Microbiol. 53:41–47. 2013.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Batra SA, Krupanidhi S and Tuteja U: A
sensitive & specific multiplex PCR assay for simultaneous
detection of Bacillus anthracis, Yersinia pestis,
Burkholderia pseudomallei & Brucella species.
Indian J Med Res. 138:111–116. 2013.PubMed/NCBI
|
|
102
|
Regan JF, Makarewicz AJ, Hindson BJ, Metz
TR, Gutierrez DM, Corzett TH, Hadley DR, Mahnke RC, Henderer BD,
Breneman JW IV, et al: Environmental monitoring for biological
threat agents using the autonomous pathogen detection system with
multiplexed polymerase chain reaction. Anal Chem. 80:7422–7429.
2008.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Wilson WJ, Erler AM, Nasarabadi SL,
Skowronski EW and Imbro PM: A multiplexed PCR-coupled liquid bead
array for the simultaneous detection of four biothreat agents. Mol
Cell Probes. 19:137–144. 2005.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Tran TN, Signoli M, Fozzati L, Aboudharam
G, Raoult D and Drancourt M: High throughput, multiplexed pathogen
detection authenticates plague waves in medieval Venice, Italy.
PLoS One. 6(e16735)2011.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Melo AC, Almeida AM and Leal NC:
Retrospective study of a plague outbreak by multiplex-PCR. Lett
Appl Microbiol. 37:361–364. 2003.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Deshpande A, Gans J, Graves SW, Green L,
Taylor L, Kim HB, Kunde YA, Leonard PM, Li PE, Mark J, et al: A
rapid multiplex assay for nucleic acid-based diagnostics. J
Microbiol Methods. 80:155–163. 2010.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Weller SA, Cox V, Essex-Lopresti A,
Hartley MG, Parsons TM, Rachwal PA, Stapleton HL and Lukaszewski
RA: Evaluation of two multiplex real-time PCR screening
capabilities for the detection of Bacillus anthracis,
Francisella tularensis and Yersinia pestis in blood
samples generated from murine infection models. J Med Microbiol.
61:1546–1555. 2012.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Trebesius K, Harmsen D, Rakin A, Schmelz J
and Heesemann J: Development of rRNA-targeted PCR and in situ
hybridization with fluorescently labelled oligonucleotides for
detection of Yersinia species. J Clin Microbiol.
36:2557–2564. 1998.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Souza G, Abath F, Leal N, Farias A and
Almeida A: Development and evaluation of a single tube nested PCR
based approach (STNPCR) for the diagnosis of plague. Adv Exp Med
Biol. 603:351–359. 2007.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Glukhov AI, Gordeev SA, Al'tshuler ML,
Zykova IE and Severin SE: Use of nested PCR in detection of the
plague pathogen. Klin Lab Diagn. 48–50. 2003.PubMed/NCBI(In Russian).
|
|
111
|
Belgrader P, Benett W, Hadley D, Long G,
Mariella R Jr, Milanovich F, Nasarabadi S, Nelson W, Richards J and
Stratton P: Rapid pathogen detection using a microchip PCR array
instrument. Clin Chem. 44:2191–2194. 1998.PubMed/NCBI
|
|
112
|
Pingle MR, Granger K, Feinberg P, Shatsky
R, Sterling B, Rundell M, Spitzer E, Larone D, Golightly L and
Barany F: Multiplexed identification of blood-borne bacterial
pathogens by use of a novel 16S rRNA gene PCR-ligase detection
reaction-capillary electrophoresis assay. J Clin Microbiol.
45:1927–1935. 2007.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Jacob D, Sauer U, Housley R, Washington C,
Sannes-Lowery K, Ecker DJ, Sampath R and Grunow R: Rapid and
high-throughput detection of highly pathogenic bacteria by Ibis
PLEX-ID technology. PLoS One. 7(e39928)2012.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Song J, Li PE, Gans J, Vuyisich M,
Deshpande A, Wolinsky M and White PS: Simultaneous pathogen
detection and antibiotic resistance characterization using
SNP-based multiplexed oligonucleotide ligation-PCR (MOL-PCR). Adv
Exp Med Biol. 680:455–464. 2010.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Souza RA, Frazão MR, Almeida AM and Falcão
JP: Rapid and efficient differentiation of Yersinia species
using high-resolution melting analysis. J Microbiol Methods.
115:6–12. 2015.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Jeng K, Hardick J, Rothman R, Yang S, Won
H, Peterson S, Hsieh YH, Masek BJ, Carroll KC and Gaydos CA:
Reverse transcription-PCR-electrospray ionization mass spectrometry
for rapid detection of biothreat and common respiratory pathogens.
J Clin Microbiol. 51:3300–3307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Jelinkova P, Hrdy J, Markova J, Dresler J,
Pajer P, Pavlis O, Branich P, Borilova G, Reichelova M, Babak V, et
al: Development and inter-laboratory validation of diagnostics
panel for detection of biothreat bacteria based on MOL-PCR assay.
Microorganisms. 9(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Woubit A, Yehualaeshet T, Roberts S,
Graham M, Kim M and Samuel T: Customizable PCR-microplate array for
differential identification of multiple pathogens. J Food Prot.
76:1948–1957. 2013.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Malou N, Tran TN, Nappez C, Signoli M, Le
Forestier C, Castex D, Drancourt M and Raoult D: Immuno-PCR-a new
tool for paleomicrobiology: The plague paradigm. PLoS One.
7(e31744)2012.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Mayboroda O, Gonzalez Benito A, Sabaté del
Rio J, Svobodova M, Julich S, Tomaso H, O'Sullivan CK and Katakis
I: Isothermal solid-phase amplification system for detection of
Yersinia pestis. Anal Bioanal Chem. 408:671–676.
2016.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Kane SR, Shah SR and Alfaro TM:
Development of a rapid viability polymerase chain reaction method
for detection of Yersinia pestis. J Microbiol Methods.
162:21–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Iqbal SS, Chambers JP, Goode MT, Valdes JJ
and Brubaker RR: Detection of Yersinia pestis by pesticin
fluorogenic probe-coupled PCR. Mol Cell Probes. 14:109–114.
2000.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Raoult D, Aboudharam G, Crubézy E, Larrouy
G, Ludes B and Drancourt M: Molecular identification by ‘suicide
PCR’ of Yersinia pestis as the agent of medieval black
death. Proc Natl Acad Sci USA. 97:12800–12803. 2000.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Norkina OV, Kulichenko AN, Gintsburg AL,
Tuchkov IV, Popov YuA, Aksenov MU and Drosdov IG: Development of a
diagnostic test for Yersinia pestis by the polymerase chain
reaction. J Appl Bacteriol. 76:240–245. 1994.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Loïez C, Herwegh S, Wallet F, Armand S,
Guinet F and Courcol RJ: Detection of Yersinia pestis in
sputum by real-time PCR. J Clin Microbiol. 41:4873–4875.
2003.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Feng N, Zhou Y, Fan Y, Bi Y, Yang R, Zhou
Y and Wang X: Yersinia pestis detection by loop-mediated
isothermal amplification combined with magnetic bead capture of
DNA. Braz J Microbiol. 49:128–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Leal NC, Abath FG, Alves LC and de*Almeida
AM: A simple PCR-based procedure for plague diagnosis. Rev Inst Med
Trop Sao Paulo. 38:371–373. 1996.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Afanas'ev EN, Briukhanov AF, Briukhanova
GD, Tiumentseva IS, Chzhichzhou S, Zharinova NV, Efremenko VI and
Zharnikova IV: Detection of plague microbe in the fleas by
polymerase chain reaction by using magnetic immunosorbents. Med
Parazitol (Mosk). 33–36. 2004.PubMed/NCBI(In Russian).
|
|
129
|
Coyne SR, Craw PD, Norwood DA and Ulrich
MP: Comparative analysis of the schleicher and schuell IsoCode stix
DNA isolation device and the qiagen qiaamp DNA mini kit. J Clin
Microbiol. 42:4859–4862. 2004.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Dauphin LA, Stephens KW, Eufinger SC and
Bowen MD: Comparison of five commercial DNA extraction kits for the
recovery of Yersinia pestis DNA from bacterial suspensions
and spiked environmental samples. J Appl Microbiol. 108:163–172.
2010.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Gilbert MTP, Cuccui J, White W, Lynnerup
N, Titball RW, Cooper A and Prentice MB: Absence of Yersinia
pestis-specific DNA in human teeth from five European
excavations of putative plague victims. Microbiology (Reading).
150:341–354. 2004.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Hong-Geller E, Valdez YE, Shou Y, Yoshida
TM, Marrone BL and Dunbar JM: Evaluation of Bacillus
anthracis and Yersinia pestis sample collection from
nonporous surfaces by quantitative real-time PCR. Lett Appl
Microbiol. 50:431–437. 2010.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Ramasindrazana B, Parany MN, Rasoamalala
F, Rasoanoro M, Rahajandraibe S, Vogler AJ, Sahl JW,
Andrianaivoarimanana V, Rajerison M and Wagner DM: Local-scale
diversity of Yersinia pestis: A case study from
Ambohitromby, Ankazobe District, Madagascar. Zoonoses Public
Health. 69:61–70. 2022.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Essbauer S, Baumann K, Schlegel M, Faulde
MK, Lewitzki J, Sauer SC, Frangoulidis D, Riehm JM, Dobler G,
Teifke JP, et al: Small mammals as reservoir for zoonotic agents in
Afghanistan. Mil Med. 187:e189–e196. 2022.PubMed/NCBI View Article : Google Scholar
|