Introduction
Sepsis, characterized by life-threatening organ
dysfunction resulting from a maladaptive host response to
infection, is the leading cause of death in intensive care units
worldwide (1). As the kidney is
one of the most vulnerable organs to sepsis, acute kidney injury
(AKI) is generally observed to be the most commonly occurring and
serious complication of sepsis. As reported previously (2,3), the
incidence of septic AKI accounts for 45-70% of all cases of AKI,
and the majority of the supportive therapies for septic AKI
treatment that are currently available are largely ineffective,
leading to an unsatisfactory clinical outcome. An emerging body of
evidence has indicated that the occurrence of septic AKI portends
an increased mortality rate and longer hospital stays compared with
non-septic AKI (4,5). Therefore, there is an urgent need to
develop novel effective strategies for the treatment of septic
AKI.
Pinocembrin
[(2S)-5,7-dihydroxy-2-phenyl-2,3-dihydrochromen-4-one; PINO] is a
major bioactive flavonoid that is mainly isolated from honey,
propolis, wild marjoram and the roots of ginger, and it is
tolerated well without any obvious adverse reactions (6,7). At
present, a great deal of attention is being focused on the study of
PINO for its diverse pharmacological activities, including its
anti-inflammatory, antioxidative, antimicrobial and neuroprotective
properties, and its ability to exert protective effects in multiple
diseases (8). It is worth noting
that PINO has been approved by China Food and Drug Administration
as a novel drug for ischemic stroke, and it is currently in phase
II clinical trials (8-10).
In addition, as a potential anti-inflammatory drug, its ability to
control inflammation has been demonstrated in situations of lung
injury, liver injury and intestinal injury (11-13).
A previous study has uncovered a role for PINO in attenuating
gentamicin-induced nephrotoxicity in rats, suggesting a protective
effect for PINO against kidney injury (14). In addition, PINO has been proposed
as a new candidate drug for preventing the progression of septic
shock, as PINO has been demonstrated to improve host survival
against lipopolysaccharide (LPS)-induced lethal endotoxemia by
lowering the overproduction of pro-inflammatory cytokines (15). Furthermore, PINO has been reported
to alleviate septic cardiomyopathy, a complication of sepsis
(16), suggesting that PINO may
exert protective functions against both sepsis and its
complications.
To the best of the authors' knowledge, with respect
to septic AKI, the benefits of PINO have not yet been investigated.
Based on the findings above, the present study aimed to investigate
whether PINO may exert a protective role in septic AKI, and to
elucidate the potential underlying mechanisms in an LPS-induced
in vitro septic AKI model in HK-2 cells.
Materials and methods
Cell culture and treatment
Human renal tubular epithelial cells (HK-2 cells)
were obtained from American Type Culture Collection and kept in
culture in RPMI-1640 medium supplemented with 10%
Invitrogen® fetal bovine serum (Thermo Fisher
Scientific, Inc.) at 37˚C in an incubator in an atmosphere of 5%
CO2. To simulate sepsis-induced AKI, HK-2 cells were
treated with 1 µg/ml LPS (Sigma-Aldrich; Merck KGaA) at 37˚C for 24
h. To explore the role of PINO in LPS-induced HK-2 cells,
increasing concentrations of PINO (0, 50, 100 and 200 µg/ml; Merck
KGaA) were applied for the various treatments 1 h prior to LPS
induction (17). In addition,
tunicamycin (TM; MedChemExpress), an agonist of endoplasmic
reticulum stress (ERS), was introduced into HK-2 cells at a
concentration of 1 µg/ml at 37˚C at 2 h prior to 200 µg/ml PINO
treatment to explore the regulatory mechanism of PINO.
Cell viability assay
HK-2 cells were seeded into 96-well plates
(2x103 cells/well) and incubated at 37˚C in an
atmosphere of 5% CO2. After adherence of the cells, 20
µl 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide
(MTT; Merck KGaA) solution was added to each well, and the cells
were cultured at 37˚C for a further 4 h. Subsequently, the culture
medium was discarded, 100 µl dimethyl sulfoxide (Merck KGaA) was
added to each well, and the cell culture was allowed to continue
for a further 15 min at 37˚C to dissolve the crystalline
substances. Finally, the absorbance at 490 nm was measured using a
microplate reader.
Measurement of malondialdehyde (MDA)
and glutathione (GSH)
HK-2 cells were seeded into 96-well plates
(2x103 cells/well) and incubated at 37˚C in an
atmosphere of 5% CO2. After LPS induction for 24 h, with
or without pre-treatment of PINO as aforementioned, 100 µl
supernatant of each well was collected. Subsequently, the
concentrations of MDA and GSH in the supernatant were measured
using the corresponding commercial detection kits for MDA (cat. no.
A003-1 for MDA and cat. no. A006-2 for GSH; from Nanjing Jiancheng
Bioengineering Institute), strictly following the manufacturer's
instructions; the absorbances at 532 and 420 nm for MDA and GSH,
respectively, were measured using a microplate reader.
TUNEL analysis
Apoptosis was assessed using a TUNEL Apoptosis Assay
kit (Beyotime Institute of Biotechnology). In brief, after LPS
induction for 24 h, with or without pre-treatment of PINO, HK-2
cells were subjected to fixation with 4% paraformaldehyde for 30
min at room temperature, and 5 min permeabilization with 0.3%
Triton X-100 at room temperature. Subsequently, the TUNEL detection
solution mixture was added to the HK-2 cells at 37˚C for 1 h in the
dark, followed by an incubation with 4',6-diamidino-2-phenylinodole
(50 µg/ml) for 10 min at room temperature and mounting in an
anti-fade reagent (Beijing Solarbio Science & Technology Co.,
Ltd.). The TUNEL-positive cells from at least five random fields
were then observed under an inverted fluorescence microscope
(Olympus Corporation).
Western blotting
Total protein was extracted from HK-2 cells using
ice-cold radio-immunoprecipitation assay (RIPA) lysis buffer
(Beyotime Institute of Biotechnology). After the protein
concentration had been determined using the bicinchoninic acid
protein assay (Beyotime Institute of Biotechnology), the protein
samples were adjusted to the same amount (30 µg/lane) and subjected
to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis,
before being transferred onto polyvinylidene difluoride membranes.
After blocking with 5% skimmed milk for 1 h at 4˚C, the membranes
were probed with primary antibodies against IL-1β (cat. no. ab9722;
Abcam), IL-6 (cat. no. ab233706; Abcam), TNF-α (cat. no. ab215188;
Abcam), Bcl-2 (cat. no. ab32124; Abcam), Bax (cat. no. ab32503;
Abcam), cleaved caspase 9 (cat. no. ab2324; Abcam), caspase 9 (cat.
no. 10380-1-AP; ProteinTech Group, Inc.), activating transcription
factor 4 (ATF4; cat. no. ab184909; Abcam), C/EBP homologous protein
(CHOP; cat. no. ab11419; Abcam), phosphorylated eukaryotic
translation initiation factor 2 subunit 1 (p-eIF2α; cat. no.
ab32157; Abcam), eIF2α (cat. no. ab157478; Abcam) (all 1:1,000),
and GAPDH (1:2,500, cat. no. ab9485; Abcam) at 4˚C overnight. On
the following day, the membranes were washed three times with
TBS-0.1% Tween-20, and incubated with HRP-conjugated goat
anti-rabbit IgG (cat. no. ab6721) or goat anti-mouse IgG (cat. no.
ab6789) (both 1:2,000; Abcam) secondary antibodies at room
temperature for 2 h. The bands were visualized using an ECL Western
Blotting Detection kit (Beijing Solarbio Science & Technology
Co., Ltd.) and quantified using ImageJ software (version 1.48;
National Institutes of Health), with GAPDH as the internal
control.
Statistical analysis
GraphPad Prism 8 software (version 8.0; GraphPad
Software, Inc.) was used for the statistical analysis. The
experimental data are presented as the mean ± standard deviation
from at least three independent experiments. Comparisons among
groups were made using one-way analysis of variance (ANOVA),
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
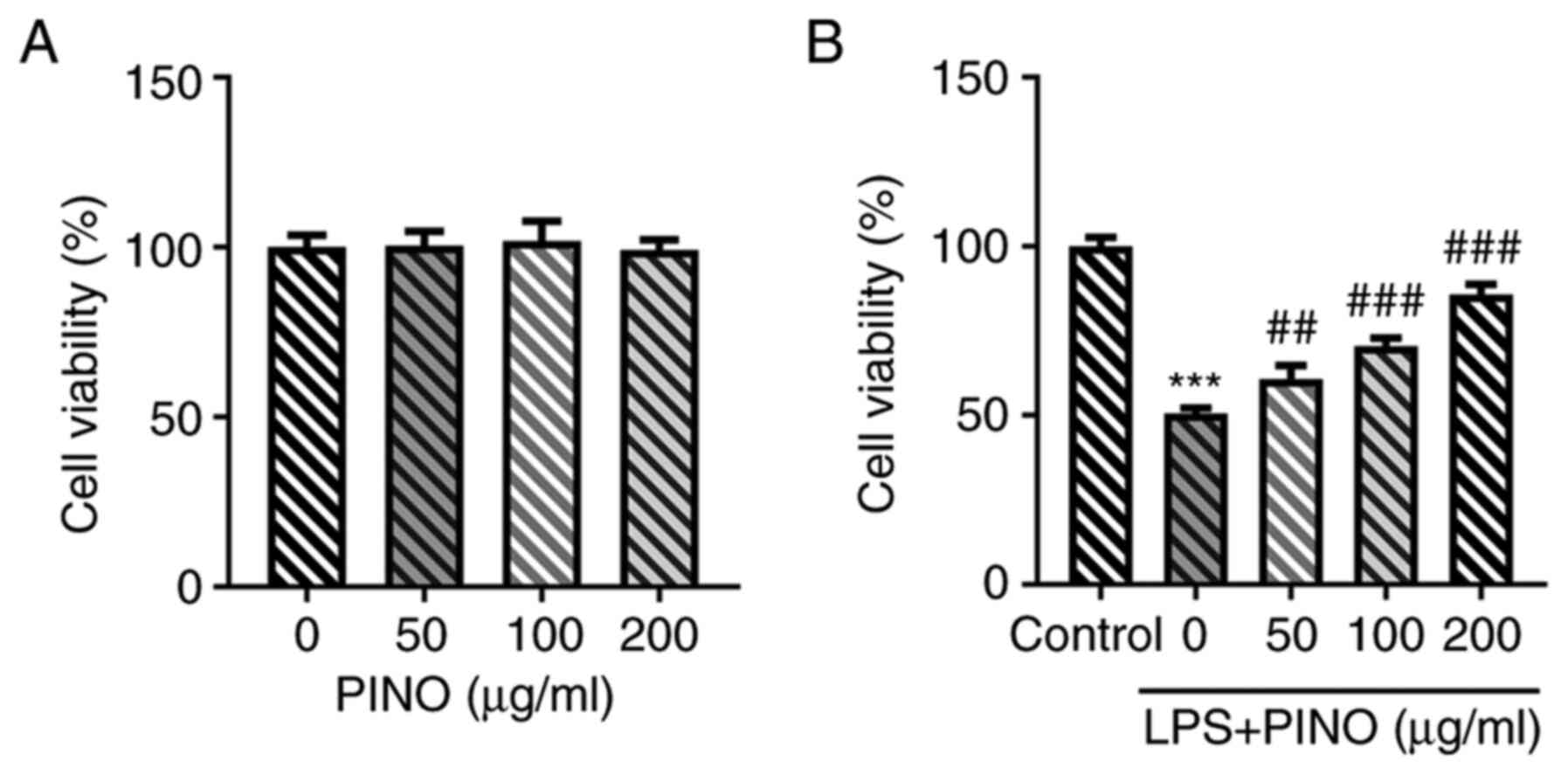
Effect of PINO on cell viability in
HK-2 cells with or without LPS induction
To assess whether PINO was toxic to HK-2 cells, the
effects of different concentrations of PINO (0, 50, 100 and 200
µg/ml) on cell viability were first examined. It was observed that
no significant differences in cell viability occurred when HK-2
cells were treated with PINO at concentrations of 0-200 µg/ml
(Fig. 1A). Subsequently, HK-2
cells were pretreated with various doses of PINO for 1 h prior to
LPS treatment for 24 h. These experiments revealed that treatment
with LPS led to a significant reduction in cell viability compared
with the control, whereas pretreatment with PINO led to significant
improvements in the viability of LPS-challenged HK-2 cells in a
concentration-dependent manner compared with the LPS group
(Fig. 1B).
Effects of PINO on inflammation,
oxidative stress and apoptosis in LPS-induced HK-2 cells
Subsequently, the biological activity of PINO in
sepsis-induced AKI in vitro was investigated. As expected,
LPS significantly promoted the secretion of pro-inflammatory
cytokines compared with the control, including interleukin (IL)-6,
IL-1β and tumor necrosis factor-α (TNF-α), in HK-2 cells, thereby
simulating the inflammatory environment surrounding kidney cells
exposed to sepsis. By contrast, PINO treatment exerted significant
inhibitory effects on the levels of IL-6, IL-1β and TNF-α in
LPS-induced HK-2 cells compared with the LPS group in a
concentration-dependent manner (Fig.
2A). The level of MDA, a marker of oxidative stress, was
significantly increased in LPS-induced HK-2 cells compared with the
control, although this enhancement was inhibited by PINO in a
concentration-dependent manner (Fig.
2B). Compared with the control, the concentration of GSH, one
of the major cellular antioxidants, was revealed to be
significantly decreased in HK-2 cells upon exposure to LPS,
although this decrease in the LPS group was attenuated upon PINO
treatment in a concentration-dependent manner (Fig. 2C). Furthermore, the results of the
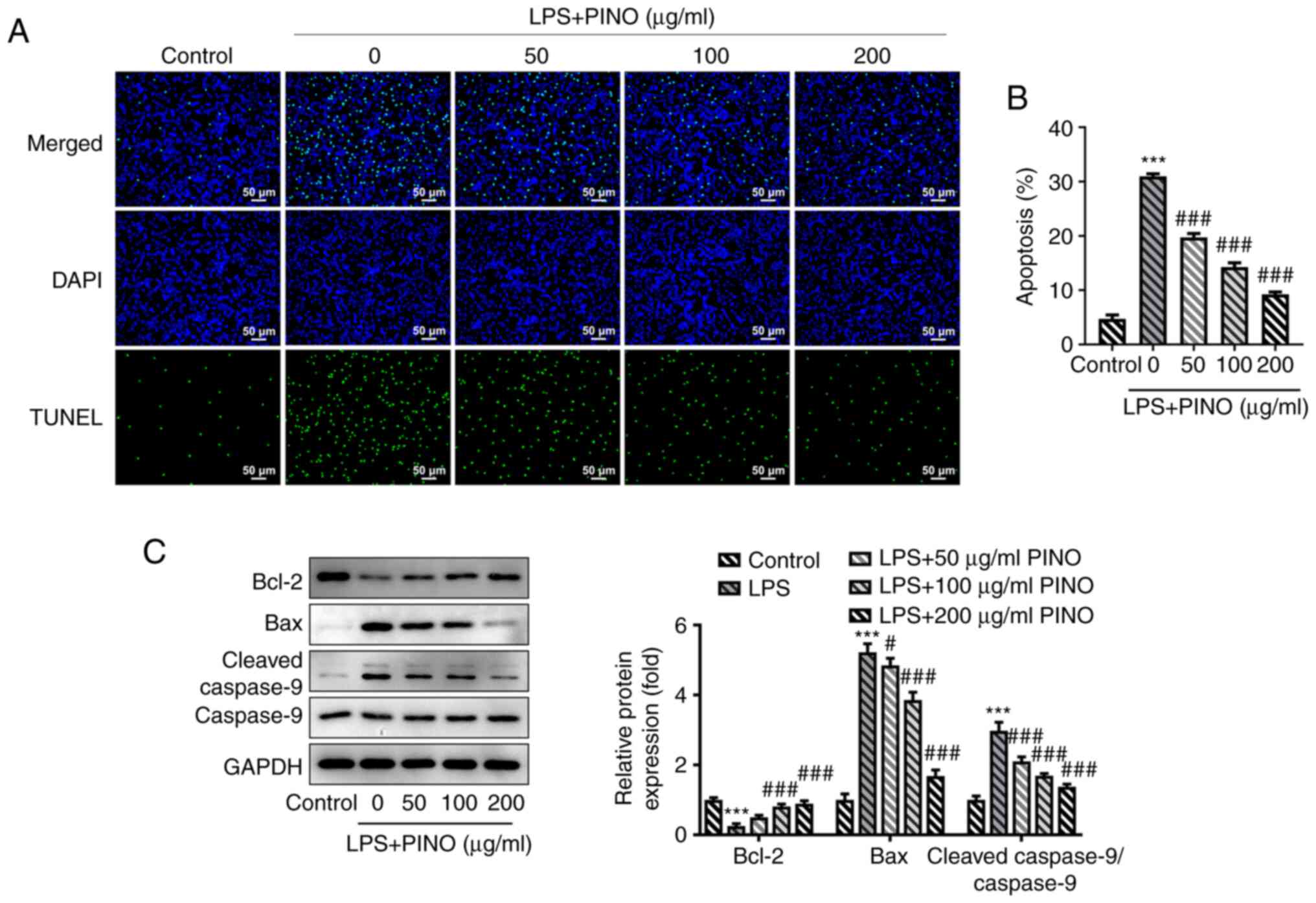
TUNEL assay demonstrated that there was a significant increase in
TUNEL-positive cells in LPS-challenged HK-2 cells compared with the
numbers of cells in the control group, whereas the numbers of
TUNEL-positive cells were gradually reduced following treatment
with increasing concentrations of PINO (Fig. 3A and B). In addition, a series of
apoptosis-associated proteins were detected using western blotting.
The expression level of Bcl-2 was significantly downregulated upon
LPS induction, whereas the protein expression levels of Bax and
cleaved-caspase 9 exhibited a significantly upregulated trend,
which suggested that LPS induced apoptosis of the HK-2 cells.
However, these changes upon LPS induction were subsequently
reversed by treatment with PINO in a concentration-dependent manner
(Fig. 3C). Collectively, these
findings suggested that PINO could exert anti-inflammatory,
anti-oxidative and anti-apoptosis effects on the LPS-induced HK-2
cells.
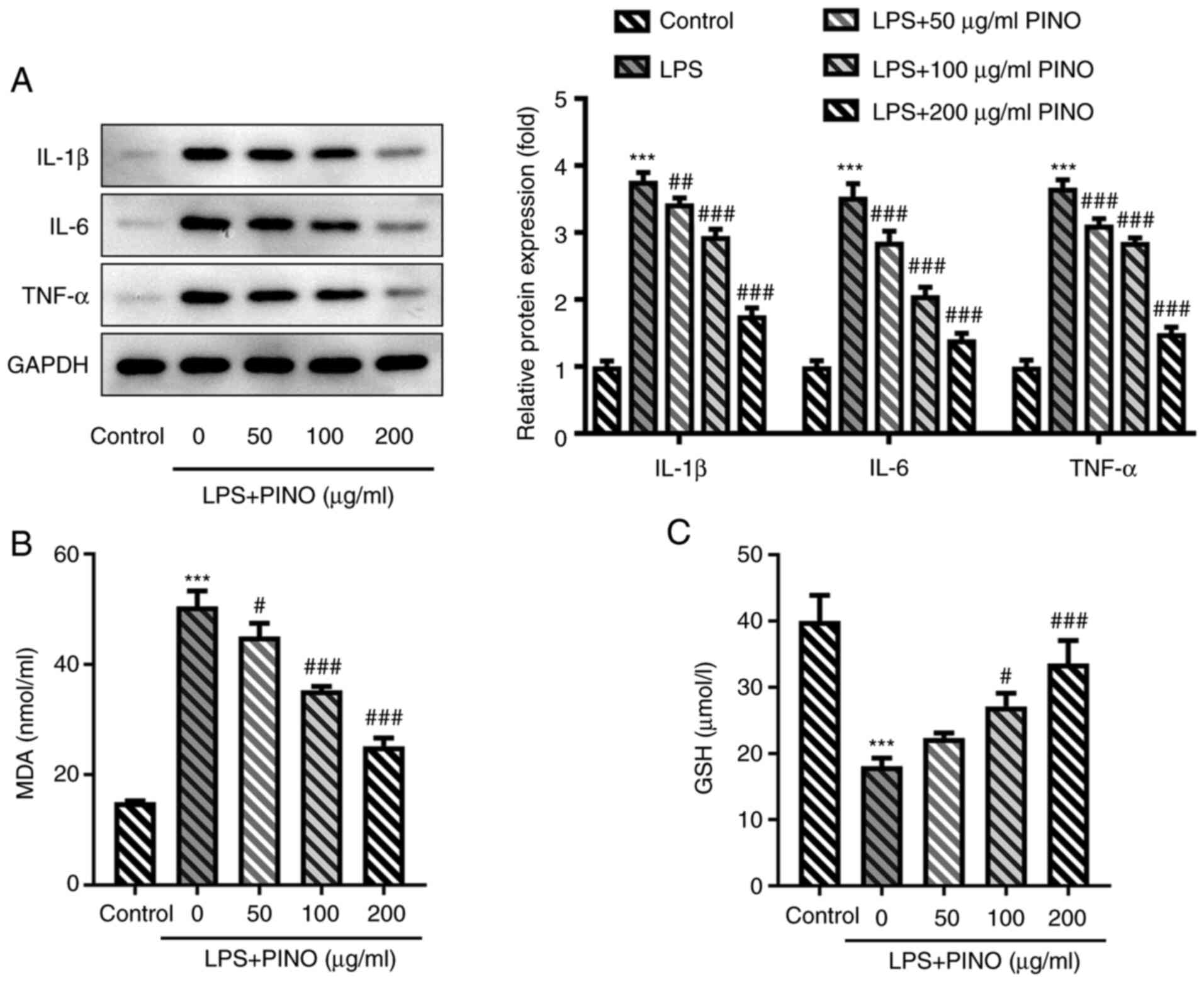 | Figure 2Effects of PINO on inflammation and
oxidative stress in LPS-induced HK-2 cells. HK-2 cells were
pre-treated with PINO (0, 50, 100 and 200 µg/ml) for 1 h, and then
treated with LPS for 24 h. (A) Protein expression levels of TNF-α,
IL-6 and IL-1β were measured using western blotting. The
concentration of (B) MDA and (C) GSH in the supernatant of culture
media was determined using the corresponding commercial kits.
***P<0.005 vs. control; #P<0.05,
##P<0.01, ###P<0.001 vs. the LPS group.
PINO, pinocembrin; LPS, lipopolysaccharide; IL, interleukin; TNF-α,
tumor necrosis factor-α; GSH, glutathione; MDA,
malondialdehyde. |
Effect of PINO on ERS in LPS-induced
HK-2 cells
To explore the potential regulatory mechanism of
PINO, its possible influence on ERS, which is involved in multiple
pathological processes and stress reactions, was explored. As
presented in Fig. 4, the levels of
ATF4, CHOP and p-eIF2α, which are considered important mediators
and markers of ERS, were significantly enhanced in LPS-induced HK-2
cells compared with the control, demonstrating the occurrence of
ERS in LPS-challenged HK-2 cells. Subsequently, the suppressive
effects of PINO on the protein expression levels of ATF4, CHOP and
p-eIF2α in HK-2 cells exposed to LPS implied that PINO could
ameliorate ERS in the LPS-challenged HK-2 cells.
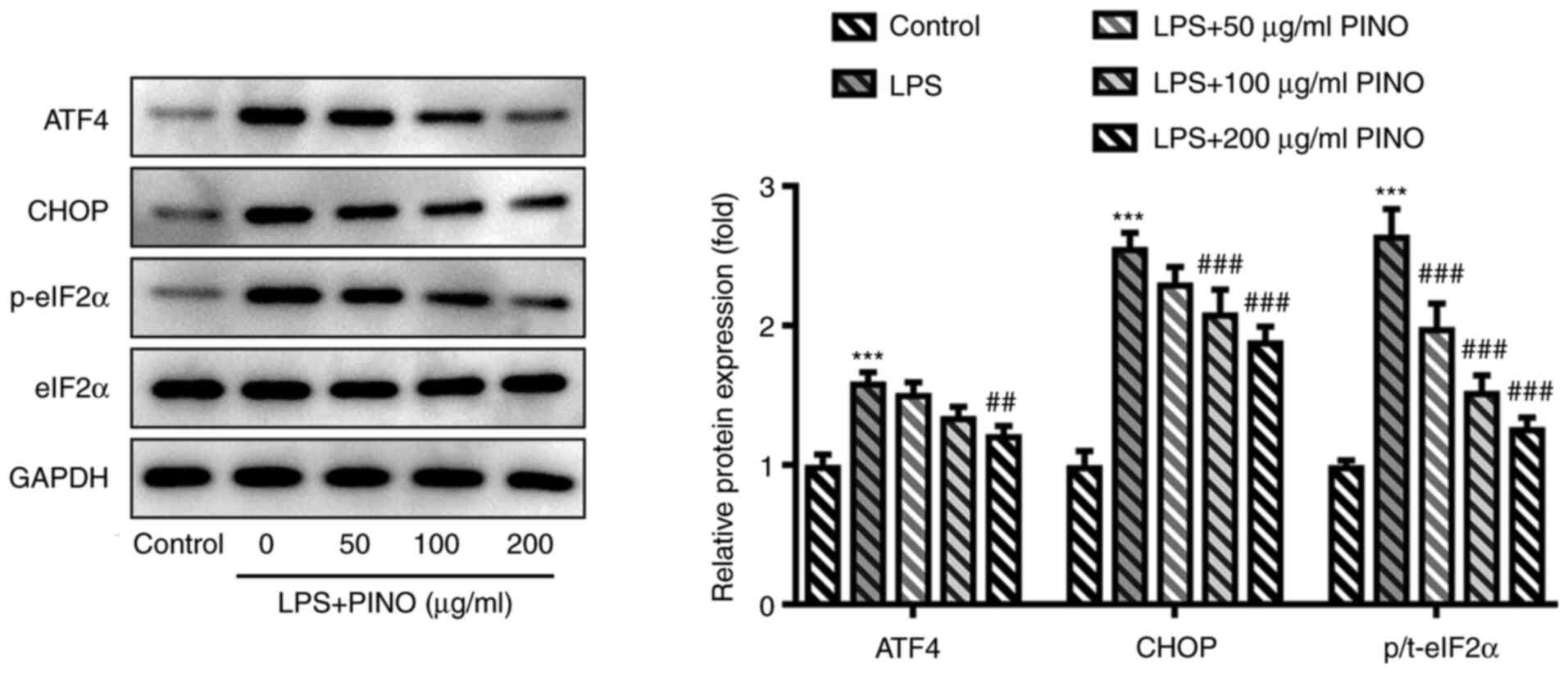 | Figure 4Effect of PINO on ERS in LPS-induced
HK-2 cells. HK-2 cells were pretreated with PINO (0, 50, 100 and
200 µg/ml) for 1 h, and then treated with LPS for 24 h. The
ERS-associated proteins were detected using western blotting.
***P<0.005 vs. control; ##P<0.01,
###P<0.001 vs. the LPS group. PINO, pinocembrin; LPS,
lipopolysaccharide; ERS, endoplasmic reticulum stress; ATF4,
activating transcription factor 4; CHOP, C/EBP homologous protein;
p-, phosphorylated; t-, total; eIF2α, eukaryotic translation
initiation factor 2 subunit 1. |
PINO exerts effects on inflammation,
oxidative stress and apoptosis in LPS-induced HK-2 cells via
regulating ERS
To establish whether ERS may have an important
influence on the biological activity of PINO in LPS-induced HK-2
cells, TM, an agonist of ERS, was introduced into HK-2 cells at a
concentration of 1 µg/ml at 2 h prior to PINO treatment (200
µg/ml). These experiments revealed that the expression levels of
TNF-α, IL-6 and IL-1β in the TM + LPS + PINO group were
significantly elevated in comparison with the LPS + PINO group
(Fig. 5A). Furthermore, the
reduced MDA level caused by PINO was significantly increased upon
additional treatment of TM in the LPS-induced HK-2 cells;
conversely, the upregulated level of GSH caused by PINO was
significantly reduced upon the additional treatment with TM in the
LPS-induced HK-2 cells (Fig. 5B
and C). Furthermore, treatment
with TM also led to a partial significant decrease in the
inhibitory effect of PINO on apoptosis in LPS-induced HK-2 cells,
as evidenced by increased numbers of TUNEL-positive cells,
significantly upregulated protein expression levels of Bax and
cleaved-caspase 9 and decreased protein expression of Bcl-2 in the
TM + LPS + PINO group compared with the LPS + PINO group (Fig. 5D-F). Overall, these findings
suggested that the activation of ERS could partly weaken the
protective role of PINO against cell injuries sustained through
exposure to LPS.
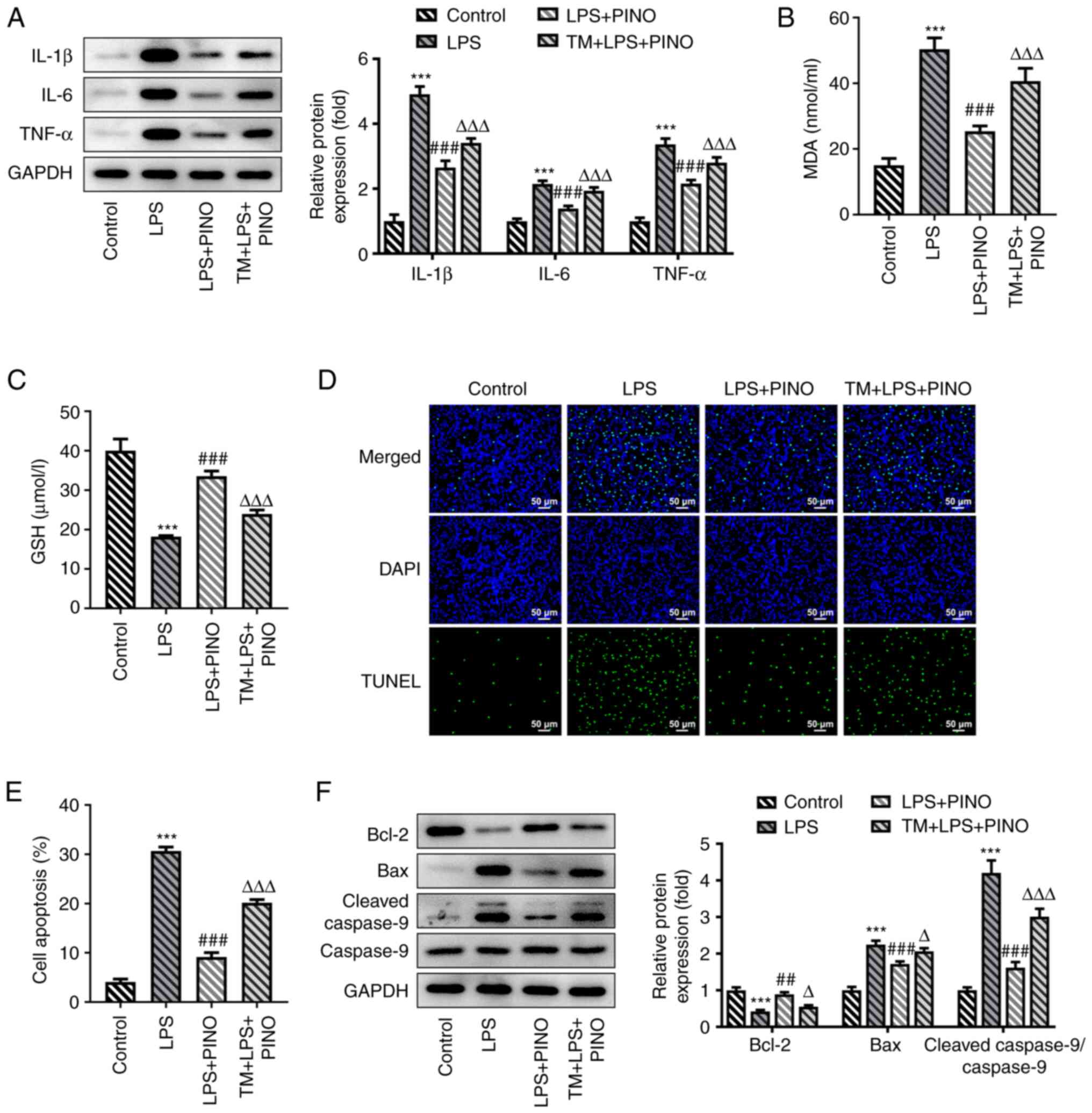 | Figure 5PINO exerts effects on inflammation,
oxidative stress and apoptosis in LPS-induced HK-2 cells by
regulating ERS. HK-2 cells were treated with TM (1 µg/ml) for 2 h
prior to PINO treatment and LPS stimulation. (A) Protein expression
levels of TNF-α, IL-6 and IL-1β were measured using western
blotting. The concentrations of (B) MDA and (C) GSH in the
supernatant of the culture media were determined using their
corresponding commercial kits. (D) TUNEL assay was performed to
assess apoptosis. (E) Quantification of the apoptosis rate. (F)
Apoptosis-associated proteins were detected using western blotting.
***P<0.005 vs. control; ##P<0.01,
###P<0.001 vs. the LPS group; ΔP<0.05,
ΔΔΔP<0.001 vs. the LPS + PINO group. PINO,
pinocembrin; LPS, lipopolysaccharide; IL, interleukin; TNF-α, tumor
necrosis factor-α; GSH, glutathione; MDA, malondialdehyde; ERS,
endoplasmic reticulum stress; TM, tunicamycin. |
Discussion
Sepsis is a systemic inflammatory response caused by
multiple infections, which results in damage to multiple organs,
ultimately leading to a high level of mortality worldwide (18). Sepsis has long been regarded as the
foremost precipitant of AKI, which is attributed to the high
susceptibility of the kidney to sepsis (2,3).
PINO, a bioactive flavonoid isolated from honey, propolis, wild
marjoram and the roots of ginger, possesses multiple biological
properties and is recognized as a promising natural small-molecule
drug in inflammation-associated diseases (8,11,15).
The present study was designed to determine the functional roles of
PINO in septic AKI, and to investigate the underlying mechanism.
The findings obtained have revealed that treatment with PINO
clearly alleviated LPS-induced inflammation, oxidative stress and
apoptosis in HK-2 cells. Furthermore, the aforementioned protective
effects of PINO were weakened upon activating ERS. Hence, it was
possible to surmise that PINO may act in a protective role in
LPS-challenged HK-2 cells through regulating ERS.
The endotoxin LPS, which serves as an important
component of Gram-negative bacteria, is involved in the
pathogenesis of septic AKI, and has been widely applied to
establish a septic AKI model (19). LPS stimulation usually results in a
severe inflammatory response, accompanied by the excessive
production of pro-inflammatory cytokines, including TNF-α and IL-6,
leading to subsequent apoptosis and renal injury (20,21).
The results of the present study also revealed increased expression
levels of TNF-α, IL-6 and IL-1β in LPS-challenged HK-2 cells.
Several previous studies have indicated that drug candidates with
anti-inflammatory properties may protect the kidney against
sepsis-triggered organ damage (20,22).
In the present study, decreased expression levels of these
cytokines were observed after PINO treatment, suggesting that PINO
acted in a protective role in septic AKI.
An imbalance of the oxidant-antioxidant system
leaning towards the dominance of oxidants during inflammation will
lead to oxidative stress. Accumulating evidence has revealed that
oxidative stress is as important as inflammation with respect to
the pathophysiology of sepsis, as numerous drug candidates exert
their protective effects on septic AKI via the suppression of
inflammation as well as oxidative stress, and these drugs include
etanercept, honokiol and glycyrrhizic acid (23-25).
In the present study, the results also illustrated that PINO could
significantly attenuate oxidative stress by reducing MDA production
and increasing the level of GSH in LPS-challenged HK-2 cells,
further affirming the protective role of PINO in septic AKI. In
addition, a previous study has revealed that aberrant inflammation
and oxidative stress in the kidney initiated apoptosis, which
subsequently led to kidney epithelial cell apoptosis and promoted
kidney cell viability loss (26).
Likewise, abundant apoptotic cells were observed in LPS-challenged
HK-2 cells in the present study, demonstrating that severe injury
of kidney cells was manifested upon LPS stimulation; however, the
aforementioned injuries were ameliorated by PINO owing to its
inhibitory action on apoptosis. Therefore, overall, the data in the
present study suggested that PINO exerted a protective role in
LPS-stimulated HK-2 cells by reducing inflammation, oxidative
stress and apoptosis.
ER serves an important role in maintaining protein
homeostasis, and is sensitive to various stimuli, including
infection and trauma, which further leads to the accumulation of
misfolded or unfolded proteins and ERS (27). During ERS, the unfolded protein
response (URP) is activated to maintain cellular homeostasis
(28); however, a prolonged URP
can lead to excessive ERS and cause CHOP-mediated apoptosis
(29). An increasing body of
evidence suggests that sepsis is associated with the activation of
ERS, which has been identified as a contributor to kidney injury
(30,31). Therefore, focusing on the
modulation of ERS may be a potential approach to elucidating the
underlying mechanism of the pathogenesis of sepsis-induced AKI. The
study performed by Jia et al (32) revealed that methane-rich saline
solution can exert its anti-inflammatory, antioxidative and
antiapoptotic properties in terms of ameliorating sepsis-induced
AKI by suppressing the ERS-associated GRP78/ATF4/CHOP/caspase-12
apoptotic signaling pathway. Wang et al (33) considered resveratrol as a promising
drug for protecting against sepsis-induced kidney injury, and
demonstrated that resveratrol inhibits the renal inflammatory
response by regulating the ERS-mediated NF-κB pathway. Notably,
PINO has been demonstrated to reduce the expression levels of CHOP
and caspase-12 in ischemia/reperfusion-induced brain injury,
suggesting that PINO may prevent brain injury by attenuating
ERS-induced apoptosis (34).
Therefore, to elucidate the mechanism contributing
to the protective effects of PINO on LPS-induced kidney injury, the
present study also evaluated the expression levels of
ERS-associated proteins in LPS-induced kidney injury. Likewise, the
results obtained revealed that ERS was triggered upon LPS
stimulation, and that this was then inhibited by PINO treatment,
whereas additional treatment with tunicamycin, an agonist of ERS,
served to weaken the protective function of PINO against
inflammation, oxidative stress and apoptosis in LPS-challenged HK-2
cells, suggesting that PINO may protect the kidney against
sepsis-triggered inflammation, oxidative stress and apoptosis
partly through inhibiting ERS.
In conclusion, the present study demonstrated that
PINO protected against LPS-induced septic AKI by reducing the
levels of inflammation, oxidative stress and apoptosis, at least in
part through regulating ERS. Consequently, PINO is a potential drug
candidate for the prevention and treatment of sepsis and its
complications, including AKI.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YF designed the study. YZ, CY and YF collected,
analyzed and interpreted the data. YZ and CY drafted the manuscript
and YF revised the manuscript. YF and YZ confirm the authenticity
of all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The Third International consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alobaidi R, Basu RK, Goldstein SL and
Bagshaw SM: Sepsis-associated acute kidney injury. Semin Nephrol.
35:2–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hoste EAJ, Kellum JA, Selby NM, Zarbock A,
Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J and Chawla LS:
Global epidemiology and outcomes of acute kidney injury. Nat Rev
Nephrol. 14:607–625. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cruz MG, Dantas JG, Levi TM, Rocha Mde S,
de Souza SP, Boa-Sorte N, de Moura CG and Cruz CM: Septic versus
non-septic acute kidney injury in critically ill patients:
Characteristics and clinical outcomes. Rev Bras Ter Intensiva.
26:384–391. 2014.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
5
|
Mehta RL, Bouchard J, Soroko SB, Ikizler
TA, Paganini EP, Chertow GM and Himmelfarb J: Program to Improve
Care in Acute Renal Disease (PICARD) Study Group. Sepsis as a cause
and consequence of acute kidney injury: Program to improve care in
acute renal disease. Intensive Care Med. 37:241–248.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cao G, Ying P, Yan B, Xue W, Li K, Shi A,
Sun T, Yan J and Hu X: Pharmacokinetics, safety, and tolerability
of single and multiple-doses of pinocembrin injection administered
intravenously in healthy subjects. J Ethnopharmacol. 168:31–36.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Danert FC, Zampini C, Ordonez R, Maldonado
L, Bedascarrasbure E and Isla MI: Nutritional and functional
properties of aqueous and hydroalcoholic extracts from Argentinean
propolis. Nat Prod Commun. 9:167–170. 2014.PubMed/NCBI
|
|
8
|
Shen X, Liu Y, Luo X and Yang Z: Advances
in biosynthesis, pharmacology, and pharmacokinetics of pinocembrin,
a promising natural small-molecule drug. Molecules.
24(2323)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Menezes da Silveira CCS, Luz DA, da Silva
CCS, Prediger RDS, Martins MD, Martins MAT, Fontes-Júnior EA and
Maia CSF: Propolis: A useful agent on psychiatric and neurological
disorders? A focus on CAPE and pinocembrin components. Med Res Rev.
41:1195–1215. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Parrella E, Gussago C, Porrini V, Benarese
M and Pizzi M: From preclinical stroke models to humans:
Polyphenols in the prevention and treatment of stroke. Nutrients.
13(85)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gan W, Li X, Cui Y, Xiao T, Liu R, Wang M,
Wei Y, Cui M, Ren S, Helian K, et al: Pinocembrin relieves
lipopolysaccharide and bleomycin induced lung inflammation via
inhibiting TLR4-NF-κB-NLRP3 inflammasome signaling pathway. Int
Immunopharmacol. 90(107230)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cao P, Chen Q, Shi C, Pei M, Wang L and
Gong Z: Pinocembrin ameliorates acute liver failure via activating
the Sirt1/PPARalpha pathway in vitro and in vivo. Eur J Pharmacol.
915(174610)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yue B, Ren J, Yu Z, Luo X, Ren Y, Zhang J,
Mani S, Wang Z and Dou W: Pinocembrin alleviates ulcerative colitis
in mice via regulating gut microbiota, suppressing TLR4/MD2/NF-κB
pathway and promoting intestinal barrier. Biosci Rep.
40(BSR20200986)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Promsan S, Jaikumkao K, Pongchaidecha A,
Chattipakorn N, Chatsudthipong V, Arjinajarn P, Pompimon W and
Lungkaphin A: Pinocembrin attenuates gentamicin-induced
nephrotoxicity in rats. Can J Physiol Pharmacol. 94:808–818.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Soromou LW, Jiang L, Wei M, Chen N, Huo M,
Chu X, Zhong W, Wu Q, Baldé A, Deng X and Feng H: Protection of
mice against lipopolysaccharide-induced endotoxic shock by
pinocembrin is correlated with regulation of cytokine secretion. J
Immunotoxicol. 11:56–61. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li C, Wan W, Ye T, Sun Y, Chen X, Liu X,
Shi S, Zhang Y, Qu C, Yang B and Zhang C: Pinocembrin alleviates
lipopolysaccharide-induced myocardial injury and cardiac
dysfunction in rats by inhibiting p38/JNK MAPK pathway. Life Sci.
277(119418)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Giri SS, Sen SS, Sukumaran V and Park SC:
Pinocembrin attenuates lipopolysaccharide-induced inflammatory
responses in Labeo rohita macrophages via the suppression of the
NF-κB signalling pathway. Fish Shellfish Immunol. 56:459–466.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dellepiane S, Marengo M and Cantaluppi V:
Detrimental cross-talk between sepsis and acute kidney injury: New
pathogenic mechanisms, early biomarkers and targeted therapies.
Crit Care. 20(61)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Doi K, Leelahavanichkul A, Yuen PS and
Star RA: Animal models of sepsis and sepsis-induced kidney injury.
J Clin Invest. 119:2868–2878. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Ren Q, Guo F, Tao S, Huang R, Ma L and Fu
P: Flavonoid fisetin alleviates kidney inflammation and apoptosis
via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways
in septic AKI mice. Biomed Pharmacother. 122(109772)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang G, Bao J, Shao X, Zhou W, Wu B, Ni Z
and Wang L: Inhibiting pannexin-1 alleviates sepsis-induced acute
kidney injury via decreasing NLRP3 inflammasome activation and cell
apoptosis. Life Sci. 254(117791)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gui Y, Yang Y, Xu D, Tao S and Li J:
Schisantherin A attenuates sepsis-induced acute kidney injury by
suppressing inflammation via regulating the NRF2 pathway. Life Sci.
258(118161)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aydin E, Yildirim Y, Aydin FY, Bahadir MV,
Kaplan I, Kadiroglu B, Ketani MA, Yılmaz Z, Kadiroğlu AK and Yılmaz
ME: Evaluation of the effect of intraperitoneal etanercept
administration on oxidative stress and inflammation indicators in
the kidney and blood of experimental sepsis-induced rats. Rev Soc
Bras Med Trop. 53(e20200016)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xia S, Lin H, Liu H, Lu Z, Wang H, Fan S
and Li N: Honokiol attenuates sepsis-associated acute kidney injury
via the inhibition of oxidative stress and inflammation.
Inflammation. 42:826–834. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao H, Liu Z, Shen H, Jin S and Zhang S:
Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney
injury via suppressing inflammation, apoptosis and oxidative
stress. Eur J Pharmacol. 781:92–99. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Peerapornratana S, Manrique-Caballero CL,
Gomez H and Kellum JA: Acute kidney injury from sepsis: Current
concepts, epidemiology, pathophysiology, prevention and treatment.
Kidney Int. 96:1083–1099. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou H, Wang K, Wang M and Zhao W, Zhang
C, Cai M, Qiu Y, Zhang T, Shao R and Zhao W: ER-phagy in the
occurrence and development of cancer. Biomedicines.
10(707)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen X, Wang Y, Xie X, Chen H, Zhu Q, Ge
Z, Wei H, Deng J, Xia Z and Lian Q: Heme oxygenase-1 reduces
sepsis-induced endoplasmic reticulum stress and acute lung injury.
Mediators Inflamm. 2018(9413876)2018.
|
|
29
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Khan MM, Yang WL and Wang P: Endoplasmic
reticulum stress in sepsis. Shock. 44:294–304. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thiessen SE, Van den Berghe G and
Vanhorebeek I: Mitochondrial and endoplasmic reticulum dysfunction
and related defense mechanisms in critical illness-induced multiple
organ failure. Biochim Biophys Acta Mol Basis Dis. 1863(10 Pt
B):2534–2545. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jia Y, Li Z, Feng Y, Cui R, Dong Y, Zhang
X, Xiang X, Qu K, Liu C and Zhang J: Methane-Rich saline
ameliorates sepsis-induced acute kidney injury through
anti-inflammation, antioxidative, and antiapoptosis effects by
regulating endoplasmic reticulum stress. Oxid Med Cell Longev.
2018(4756846)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang N, Mao L, Yang L, Zou J, Liu K, Liu
M, Zhang H, Xiao X and Wang K: Resveratrol protects against early
polymicrobial sepsis-induced acute kidney injury through inhibiting
endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget.
8:36449–36461. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF
and Du GH: Pinocembrin protects brain against ischemia/reperfusion
injury by attenuating endoplasmic reticulum stress induced
apoptosis. Neurosci Lett. 546:57–62. 2013.PubMed/NCBI View Article : Google Scholar
|