Introduction
Gas explosions accidents in coal mining threaten
public safety and social economy. The mortality associated with gas
accidents reached 54.5% of the total coal industry-associated
mortalities from 2010 to 2019 in China (1). Gas explosions are able to cause
complex injuries with severe inhalation injury, which puts patients
at a high risk of developing systemic infection and respiratory
failure (2); however, the
mechanism of injury remains unknown.
As a cavity and gas-bearing organ, the lung is
affected by in gas explosion-induced blast injury. Thus it can be
severely torn and fragmented by gas explosion-induced shockwaves
(3). Recently, a study indicated
that type I and type II alveolar epithelial cells are the targets
in BLI induced by gas explosion, and that the characteristics of
the injury are nuclear contraction and mitochondrial structural
abnormalities (4). A previous
study on canines demonstrated that levels of serum malondialdehyde
increased with a blast, suggesting that blast wave injury resulted
in oxidative stress in the organism (5). Mitochondria are important organelles
involved in adenosine triphosphate (ATP) synthesis that produce ATP
and numerous other biosynthetic intermediates. Our previous study
revealed that energy is consumed rapidly and that galactose
metabolism and the tricarboxylic acid (TCA) cycle are downregulated
in rats exposed to gas explosion (6). This abnormality in energy metabolism
is probably closely associated with mitochondrial function. The
mitochondrial quality control system plays an important role, not
only in maintaining the biological production of energy, but also
in controlling oxidant formation by the electron transport chain
(ETC) (7). Reactive oxygen species
(ROS) produced by the ETC can oxidize lipid molecules and induce
DNA damage, especially in mitochondrial DNA (mtDNA) (8). Owing to the lack of a protective
histone shield around mtDNA and limited DNA repair mechanisms,
mtDNA is more sensitive to oxidative damage compared with nuclear
DNA (9,10). This suggests mitochondrial mass,
function and stabilization are intimately involved in energy
metabolism and oxidative stress (9,10).
AMP-activated protein kinase (AMPK), a major energy
sensor at both cellular and whole-body levels (11), maintains energy homeostasis in
eukaryotic cells. A previous study observed a correlation between
nicotinamide adenine dinucleotide phosphate oxidase (NOX2)
expression and energy metabolism (12). The NOX2 heterodimer is composed of
p22phox and gp91phox, and contributes to the production of ROS,
especially superoxide anions, which are very harmful to cells
(13). ROS generated by NOX2 act
as the initiator of the pathophysiology of acute lung injury
(14). Recent study have proposed
that AMPK and NOX2 are mutually regulated (15). Furthermore, AMPK increases
mitochondrial biogenesis by activating peroxisome
proliferator-activated receptor-γ coactivator-1α (PGC1α) (16,17).
Therefore, AMPK may be a potential target in the treatment of gas
explosion-induced blast lung injury.
Metformin, an activator of AMPK, is a key regulator
of energy metabolism and balance, and it improves mitochondrial
respiration, restores the mitochondrial life cycle by activating
the protein kinase AMPK and inhibits oxidative stress through the
upregulation of PGC1α and superoxide dismutase 1 in acute lung
injury (18,19). The aforementioned studies confirmed
that metformin can regulate energy metabolism and oxidative stress
via the activation of AMPK.
The present study aimed to explore the role of the
AMPK/NOX2 pathway in gas blast-induced lung injury by designing
experiments that simulated the effects of a gas explosion on a real
roadway with a rat model, which provided evidence to demonstrate
the pulmonary-protective effects of metformin and its specific
molecular mechanism with respect to targeting and alleviating gas
explosion-induced blast lung injury.
Materials and methods
Chemicals and reagents
Metformin (MET) was purchased from Bristol-Myers
Squibb Pharmaceuticals Ltd. The total antioxidant capacity (T-AOC)
detection kit (cat. no. #A015-3-1) was purchased from the Nanjing
Jiancheng Bioengineering Institute. The micro isocitrate
dehydrogenase mitochondrial (ICDHm) assay kit (cat. no. #BC2165),
micro α-ketoglutarate dehydrogenase (α-KGDH) assay kit (cat. no.
#BC0715), phosphofructokinase (PFK) activity assay kit (#BC0535),
ATP content assay kit (cat. no. #BC0305) were purchased from
Beijing Solarbio Science & Technology Co., Ltd.
TRIzol® was purchased from Ambion (Thermo Fisher
Scientific, Inc.). The Pierce™ BCA protein assay kit and
SuperScript III reverse transcriptase were purchased from Thermo
Fisher Scientific, Inc. SDS-PAGE sample loading buffer was
purchased from Beyotime Institute of Biotechnology. β-actin (cat.
no. #AF7018), AMPK (cat. no. #AF6423) and p-AMPK antibodies (cat.
no. #AF3423) were purchased from Affinity Biosciences, Ltd.; NOX2
antibody (cat. no. #A19701) and PGC-1α (cat. no. #A19674) antibody
were purchased from ABclonal Biotech Co., Ltd.; and catalase (CAT;
cat. no. #12980) antibody was purchased from Cell Signaling
Technology, Inc. SYBR® GreenER qPCR SuperMix Universal
was purchased from Invitrogen (Thermo Fisher Scientific, Inc.).
Experimental animals and grouping
In total, 90 six-month-old male specific
pathogen-free (SPF) Sprague-Dawley rats with an average weight of
200±20 g were purchased from Speifu (Beijing) Biotechnology Co.,
Ltd. and the animal license number was SCXK 2019-0010. The animals
were housed in a SPF animal room and maintained under a controlled
environment (24±1˚C temperature, relative humidity of 40-50% and 12
h light/dark cycle) for 1 week with adaptive feeding to ensure rats
adaptation to the new environment and free diet before commencement
of the experiments. The rats were randomly divided into three
groups (n=30/group) as follows: Control group, gas explosion injury
(BLI) group and gas explosion injury + metformin (BLI + MET) group.
A total of 10 rats, randomly selected from each group, were
euthanized on days 1, 3 and 7 after treatment. Rats were dissected
and the lungs of rats were isolated and sectioned. The animal
experiments were performed in accordance with Guide for the Care
and Use of Laboratory Animals (20), and were approved by the Medical
Ethics Committee of Xinxiang Medical University (approval no.
XYLL-2019001; Jan 13, 2019).
BLI model and intervention
Preliminary experiments confirmed that the explosion
flame range was 160 m (data not shown); thus, rats were placed 240
m away from the center of the explosion to ensure that they were
only affected by shock waves. The explosion chamber, with a total
volume of 100 m3 contained air with 9.3% methane gas, and the
ignition energy was 20 J. Rats in the BLI and MET groups were
deeply anesthetized with 1% sodium pentobarbital (50 mg/kg) and
placed in a custom iron cage. After the explosion, rats of the BLI
+ MET group were administered metformin daily by intraperitoneal
injection (10 mg/kg). A total of 10 rats from each group were
randomly selected and euthanized on the 1st, 3rd and 7th day.
Before collecting the blood, rats were deeply anesthetized as
aforementioned. The rats were cervically dislocated after the blood
was collected and the organs were retained after death was
confirmed. Lung tissues were isolated and weighed. The right lower
lung tissue was fixed in 4% paraformaldehyde at 25˚C for 24 h, and
the remaining lung tissue was frozen in liquid nitrogen at -80˚C
for further tests.
Lung function detection in rats
Rats were placed in the test room for at least 30
min for habituation. Indicators of lung function, peak inspiratory
flow rate (PIFR) and minute ventilation (MV), were measured using a
whole-body plethysmography system (Buxco Electronics, Inc.).
Assessment of lung injury based on
hematoxylin and eosin (HE) staining
The lower lobe of right lung tissue was fixed with
4% formaldehyde at 25˚C for 24 h, dehydrated, embedded in paraffin,
cut into 4-µm sections and kept at 60˚C for 1 h. Tissue was
deparaffinized with xylene for twice 20 min and then rehydrated,
stained with hematoxylin at 25˚C for 5 min and eosin at 25˚C for 3
min before histopathological observation. Overall, five visual
fields were randomly selected for each section under a light
microscope and scored based on the following four aspects: Alveolar
wall thickness, congestion, hemorrhage and inflammatory cell
infiltration. A five-point scale from 0 to 4 was used to generate a
systematic scoring system for acute lung injury as follows: 0=No
damage; l=mild damage; 2=moderate damage; 3=severe damage; and
4=very severe damage (21).
Detection of energy metabolism-related
indicators using a biochemical assay kit
Lung tissues frozen at -80˚C were used for
biochemical analyses using the aforementioned ICDHm, α-KGDH, PFK
activity and ATP content assay kit according to the manufacturer's
instructions. Approximately 30 mg of lung tissue was added to 300
µl of homologous extraction solution for ice-bath grinding and then
centrifuged at 8,000-11,000 x g at 4˚C for 10-15 min. The
supernatant was collected and placed on ice for further testing.
Working reagents and the sample supernatant were added to each well
of a 96-well plate and incubated for several minutes at 37˚C
according to the manufacturer's instructions. Product formations
were detected using a standard microplate reader (PerkinElmer,
Inc.; EnSpire Multimode Plate Reader) at a specific absorbance
wavelength (340 and 505 nm).
Observation of mitochondrial
morphology via transmission electron microscopy (TEM)
Fresh rat lung tissue was obtained and cut into
cubes of ~1 mm3 to minimize mechanical damage, such as
pulling, contusion and extrusion. The small lung tissue cubes were
fixed in 4% glutaraldehyde in 0.1 M phosphate buffer overnight at
4˚C and then washed in ice-cold PBS three times. The fixed samples
were post-fixed in 1% osmium tetroxide in 0.1 M PBS overnight at
25±3˚C and rinsed in PBS as previously described. Next the samples
were dehydrated, infiltrated, embedded in resin and polymerized
overnight in an oven at 60˚C. The embedded tissue was cut into
ultrathin sections of 60-80 nm using an ultrathin slicer (Leica
UC7; Leica Microsystems GmbH). After staining with 2% uranium
acetate saturated alcohol solution and 2% lead citrate solution
(25˚C for 15 min each), the ultrastructures of cells were observed
under a transmission electron microscope (HT7700; Hitachi, Ltd.)
and the captured images were analyzed by Image J 1 (National
Institutes of Health).
Detection of the total serum
antioxidant capacity
Blood was obtained from the abdominal aorta before
the animals were euthanized. The collected blood samples were
clotted at 25±3˚C for 30 min and then centrifuged at 4,000 x g at
4˚C for 15 min. The serum was removed and stored in the tube at
-80˚C. Freshly prepared FeSO4-7H2O standard
solutions were used; the remaining steps in the experiment were
performed according to the instructions of the T-AOC detection
kit.
Reverse-transcription-quantitative
(RT-qPCR)
Total RNA from lung tissues was extracted using
TRIzol®. The extracted RNA was reverse-transcribed into
cDNA using SuperScript III reverse transcriptase according to the
manufacturer's instructions. All mRNA levels were detected using
SYBR® GreenER™ qPCR SuperMix Universal. The cycling
conditions were as follows: 95˚C Pre-denaturation for 1 min, 40
cycles of 95˚C for 15 sec, 60˚C for 15 sec and 72˚C for 30 sec.
Finally, 65˚C for 1 min, 95˚C for 20 sec and 37˚C for 1 min.
Fold-changes in mRNA expression levels were calculated using
2-ΔΔCq values (22).
The primer sequences of the targeted genes used in this study were
as follows: Receptor of advanced glycation end products
(RAGE), forward, 5'-CAATGGTTCACTCCTCCTT-3', and reverse
5'-TCTGGTAGACTCGGACTC-3'; AMPK, forward,
5'-AATTCGCAGGGAGATTCAGA-3', and reverse,
5'-ACAGCTCTCCTCCAGAAACG-3'; PGC1-α, forward
5'-GCACTGACAGATGGAGACGTGA-3', and reverse,
5'-TCATTGTAGCTGAGCTGAGTGTTGG-3'; Transcription factor A of
mitochondrial (TFAM), forward
5'-TGAAGCTTGTAAATCAGGCTTGGA-3', and reverse,
5'-GAGATCACTTCGCCCAACTTCAG-3'; Gp91phox (GP91), forward
5'-CTGCCAGTGTGTCGGAATCT-3', and reverse,
5'-TGTGAATGGCCGTGTGAAGT-3'; CAT, forward,
5'-AAGCTGGTTAATGCGAATGG-3', and reverse,
5'-AAGTTTTTGATGCCCTGGTC-3'; GADPH forward,
5'-TCAACGGCACAGTCAAGG-3', and reverse 5'-TCAACGGCACAGTCAAGG-3'.
Western blotting
Rat lung tissues were rapidly homogenized using an
ultrasonic tissue disruptor (SCIENTZ-48; Ningbo Xinzhi
Biotechnology Co., Ltd.) with cold RIPA extraction buffer (Beyotime
Institute of Biotechnology) to ensure complete homogenization. Lung
tissue remained on ice for 30 min, and then it was centrifuged at
15,000 x g at 4˚C for 15 min to obtain the supernatant. Protein
concentration was measured using the Pierce™ BCA protein assay kit
and denatured with SDS-PAGE sample loading buffer at 100˚C for 5
min. 30 ng denatured protein samples were separated using 10%
sodium dodecyl sulfate polyacrylamide gel electrophoresis (30 min
at 90 V, 90 min at 120 V), and electro-transferred to a 0.45 µm
polyvinylidene difluoride membrane for 70 min at 300 mA. The
membranes were incubated in blocking solution (5% skim milk) at
25±3˚C for 90 min, and subsequently incubated with the antibodies
targeting the following proteins at 4˚C overnight: β-actin
(1:3,000), AMPK (1:2,000), p-AMPK (1:2,500), NOX2 (1:2,000), PGC-1α
(1:2,000) and CAT (1:2,000). The next day, the membrane was washed
three times with 5% tris-buffered saline with tween for 30 min (10
min each) and incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:3,000, cat. no. #S0001, Affinity Biosciences,
Ltd.) for 2 h at 25±3˚C. Finally, the membranes were washed three
times for 10 min each, and chemiluminescence detection was
performed using the Amersham Image Quant800 (Cytiva) system. The
images were analyzed by Image J 1 (National Institutes of
Health).
Statistical analysis
All data were analyzed using SPSS 20.0 (IBM Corp.).
Continuous variables of normally distributed were expressed as the
mean ± SD, and significant differences between groups were
determined using one-way ANOVA followed by Tukey's post hoc test.
Ordinal variables were expressed as the median with interquartile
range and were analyzed using Kruskal-Wallis test and Dunn's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
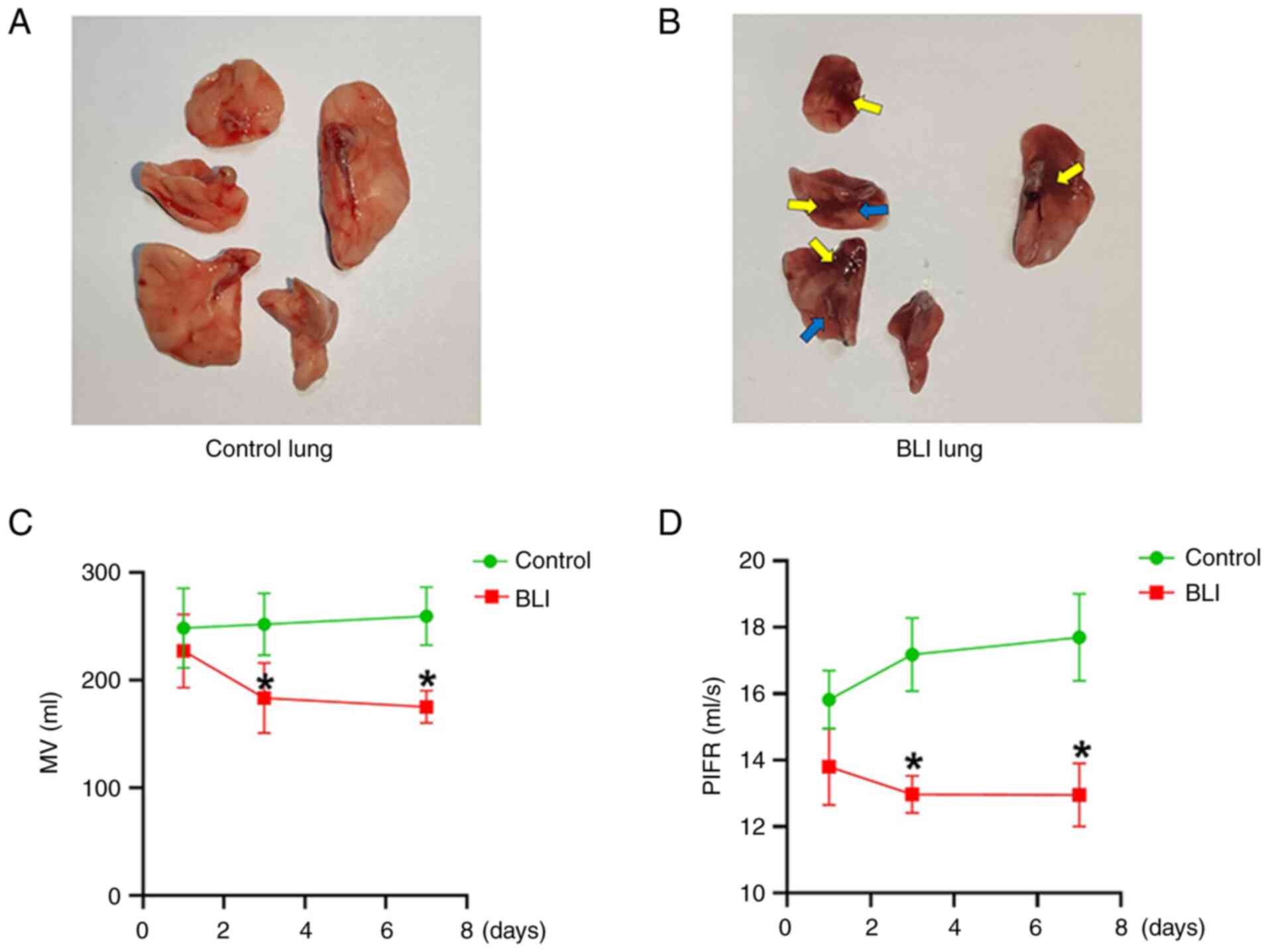
Gas explosions cause lung tissue
damage and pulmonary dysfunction
In the present study, lung injuries were clearly
observed in rats exposed to gas explosions. Compared with that in
the control group, gas explosion exerted marked effects on the
lungs of rats in the BLI group, characterized by evident lung
bleeding points (yellow arrows) and lung alveolar collapse (the
blue arrows) (Fig. 1A and B). Furthermore, gas explosion reduced
lung function as follows: MV and PIFR of rats in the BLI group were
significantly lower compared with those in the control group
(Fig. 1C and D). Compared with those in the control
group, the differences in lung function of rats in the BLI group at
all time points were statistically significant, and this trend
became more pronounced over time. These results indicate the BLI
rat model was established successfully.
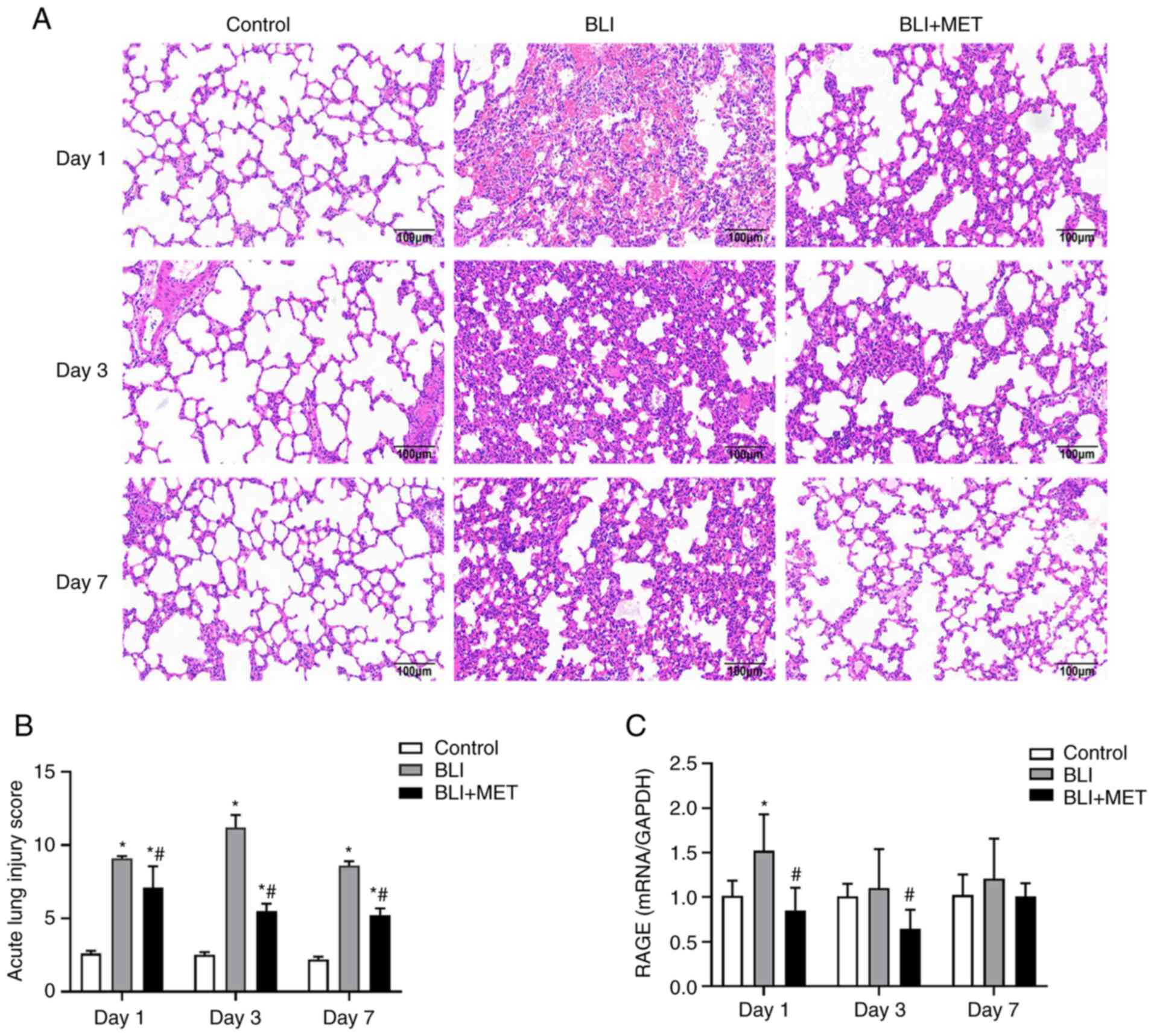
MET attenuates pathological and endothelial factor
damage to lungs caused by gas explosion. The lungs of the rats in
the control group had intact alveolar structures, thin, clear
alveolar walls and no edema (Fig.
2). Compared with those in the normal control group, alveolar
structure disorder, alveolar wall collapse, bleeding, and numerous
inflammatory cells were observed in the BLI group (Fig. 2). Histological assessment revealed
that the degree of inflammation was most severe on the third day
after the gas explosion. To determine the effect of MET on the
established BLI rat model, MET (10 mg/kg) was injected
intraperitoneally into the rats. As expected, the lungs of the MET
group had a significantly reduced amount of inflammatory cells and
thinner alveolar walls compared with those of the BLI group. The
pathological scores of acute lung injury are presented in Fig. 2B. In addition, the expression of an
endothelial factor, RAGE, was detected, which was associated with
acute lung injury in the control and BLI group (23) using RT-qPCR. The results (Fig. 2C) revealed that activation of the
RAGE damage pathway by the gas explosion was rescued by MET on day
1. The expression of RAGE in the BLI group was not significantly
increased at days 3 and 7 compared with the control; however,
treatment with MET did markedly decrease the expression of RAGE in
the BLI + MET group compared with the BLI group, and this decrease
was significant on day 3.
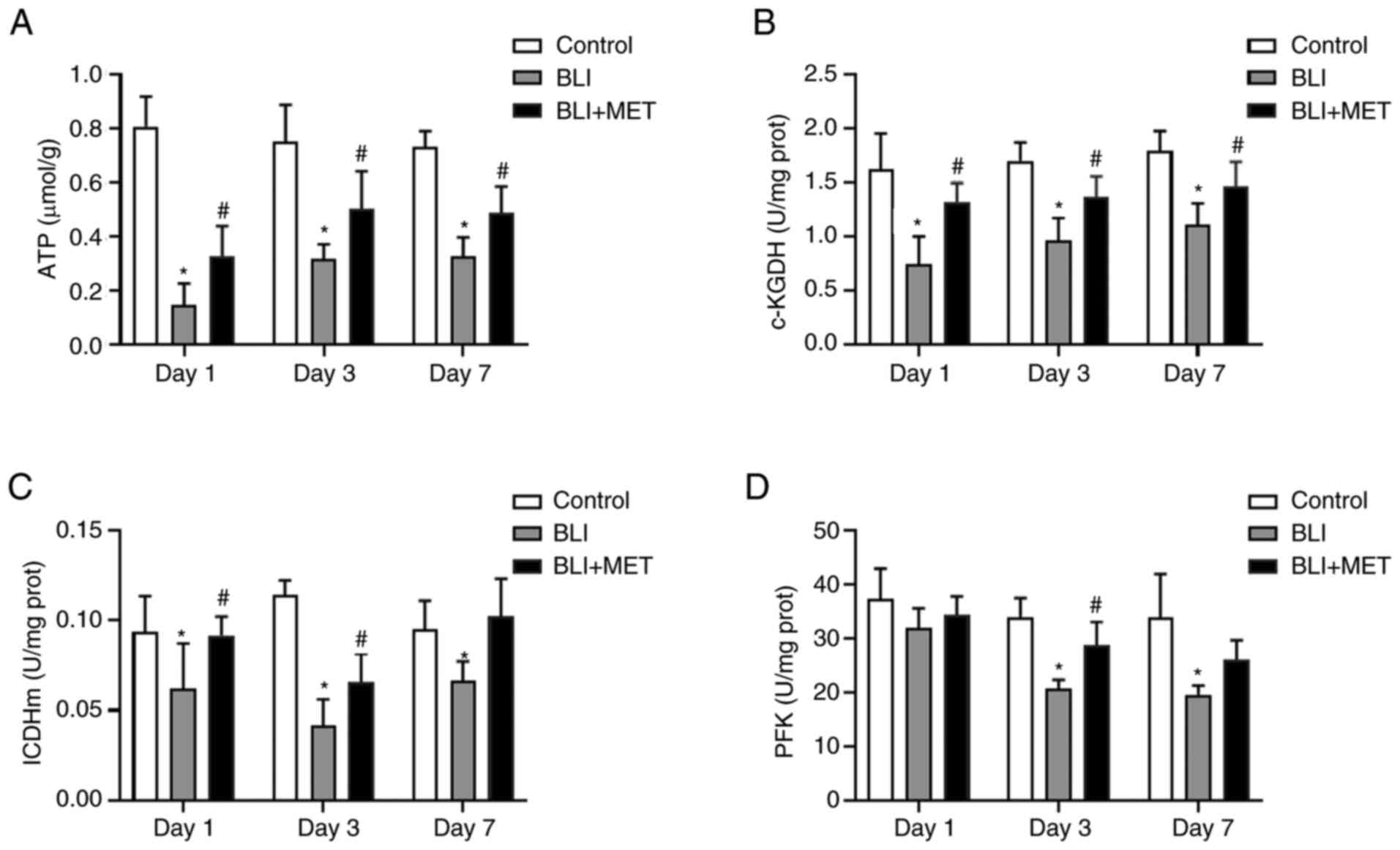
MET regulates the energy metabolic
disturbances caused by gas explosions
Pulmonary ATP levels were significantly lower in the
BLI group compared with in the control group, and MET treatment
significantly increased pulmonary ATP levels in gas
explosion-induced rats lung injury (Fig. 3A). Therefore, the activities of
several metabolic enzymes in the TCA cycle and glycolysis were
investigated to confirm that MET regulated energy metabolism.
Similarly, the enzymatic activities of rate-limiting
enzymes α-KGDH and ICDHm in the TCA cycle (24,25)
were significantly lower in the BLI group compared with the control
group (Fig. 3B and C), and MET treatment markedly improved
these enzymatic activities in rats exposed to a gas explosion at
each time point. The same effect was observed in terms of the
activity of PFK, which is the rate-limiting enzyme in glycolysis.
However, the changes between the MET and the BLI group varied
significantly only on days 3 and 7 (Fig. 3D). Overall, these data indicated
that MET ameliorated the deregulated energy metabolism attributed
to gas explosion-induced injury.
MET activates the AMPK/PGC1
mitochondrial protection pathway
The aforementioned results showed that MET
alleviated pathological damage and regulated energy metabolism in
the blast-injured lung of rats. Therefore, the present study
detected the expression of AMPK and PGC-1α using western blotting
and RT-qPCR to confirm the effect of MET on the mitochondrial
protective pathway (Fig. 4). The
ratio of p-AMPK/t-AMPK levels were significantly lower in the BLI
group compared with the control group on the 3rd day, and PGC-1α
levels were significantly lower in the BLI group compared with the
control group at each time point (Fig.
4A-D); whereas the ratio of p-AMPK/t-AMPK levels and PGC-1α
levels were significantly higher in the MET compared with the BLI
group on day 3 and 7 (Fig. 4C and
D). Similarly, results of RT-qPCR
revealed that the mRNA expression levels of AMPK,
PGC-1α and TFAM in the rats exposed to gas were lower
compared with those in the control group, while MET treatment
significantly increased the expression of these mRNA markers in gas
blast-injured rats compared with BLI group (Fig. 4E-G).
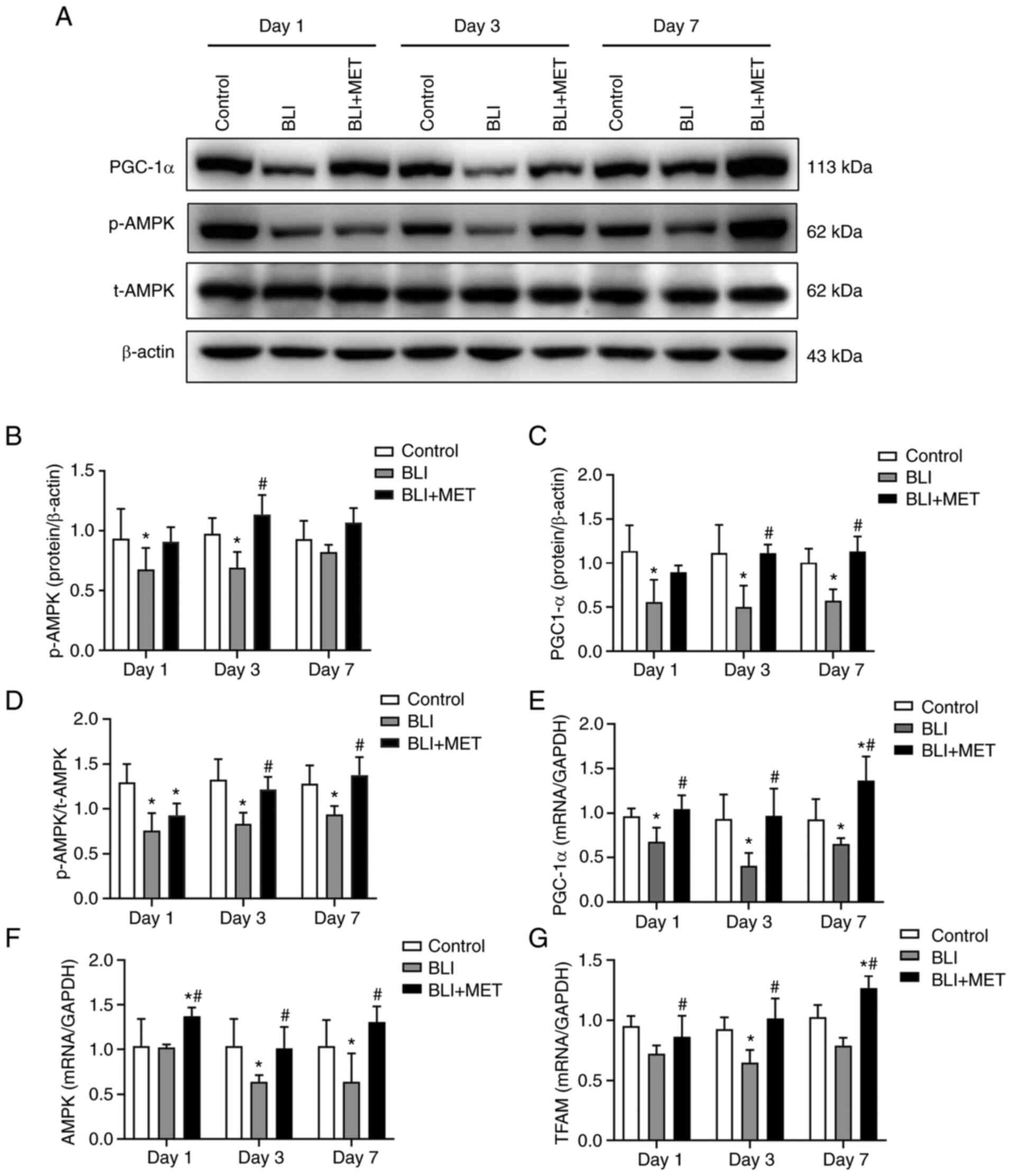 | Figure 4MET activates the AMPK/PGC1α
mitochondrial protection pathway. (A) Expression of p-AMPK, t-AMPK
and PGC-1α. Protein levels of (B) p-AMPK, (C) PGC-1α and (D)
p-AMPK/t-AMPK were quantified using Image J Software. n=8. RT-qPCR
analysis of the relative quantitative expression levels of (E)
PGC-1α and (F) AMPK and (G) TFAM in lung
tissues of rats, and the relative quantitative expression of each
gene was normalized to that of GAPDH. n=6.
*P<0.05 compared with the control group;
#P<0.05 compared with the BLI group. RT-qPCR,
reverse-transcription-quantitative; BLI, blast lung injury; MET,
metformin; p-, phosphorylated; t-, total; AMPK, AMP-activated
protein kinase; PGC-1α, peroxisome proliferator-activated
receptor-γ coactivator-1α; TFAM, transcription factor A of
mitochondrial. |
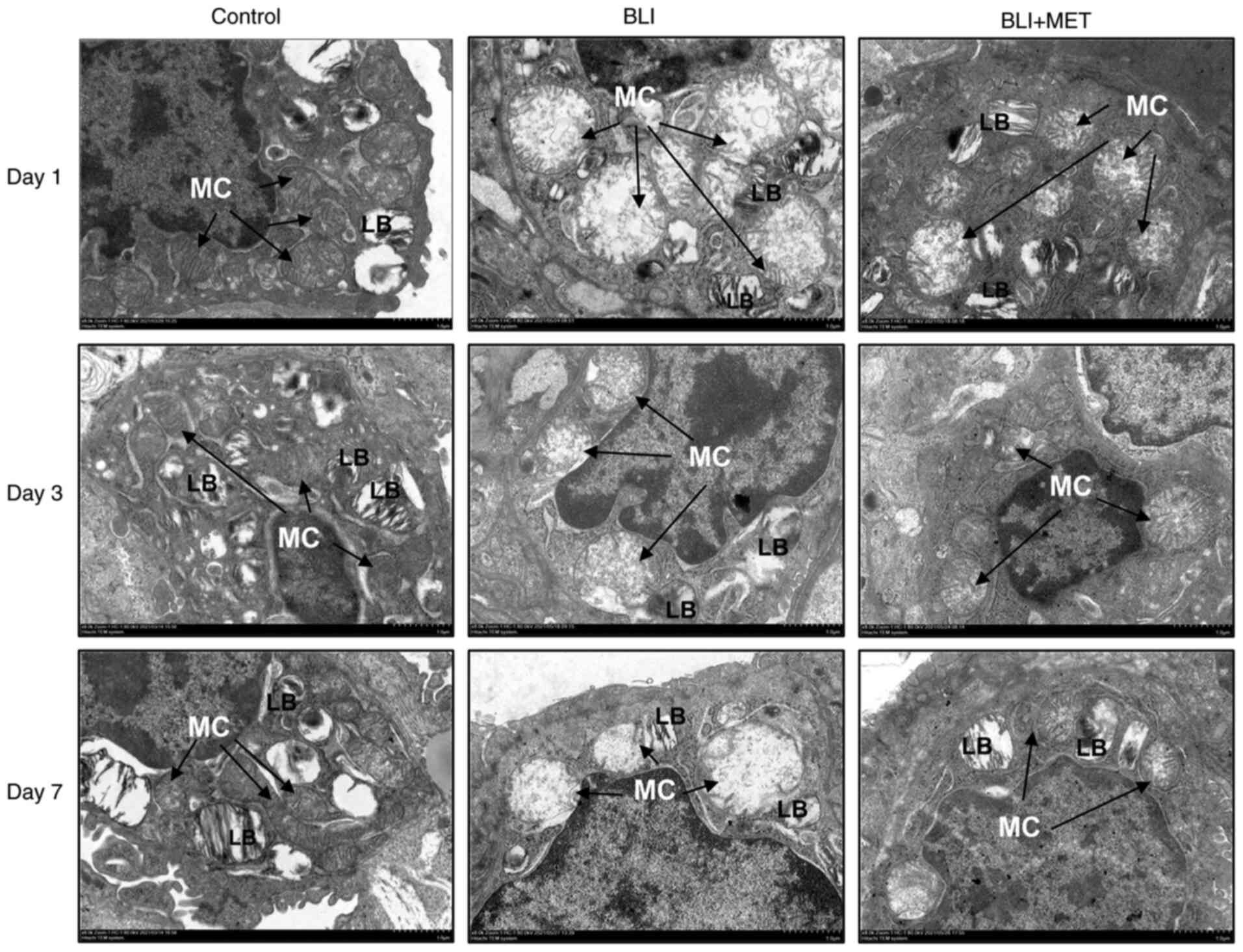
MET maintains mitochondrial
morphological stability
Ultrastructural changes in the lung tissue were
observed using TEM (Fig. 5). The
results indicated that the mitochondria of rats in the control
group had the same normal shape, size and arrangement, and that
mitochondrial cristae were arranged in layers. However, the rats
with gas explosion-related swelling demonstrated a decrease or
disappearance of the mitochondria crista and vacuolization.
Moreover, loss of the lamellar body structure of alveolar type II
cells was observed. As expected, the number of mitochondrial
cristae in rats treated with MET increased, and the structures of
mitochondria and lamellar bodies were more stable compared with
those in the rats exposed to a gas explosion.
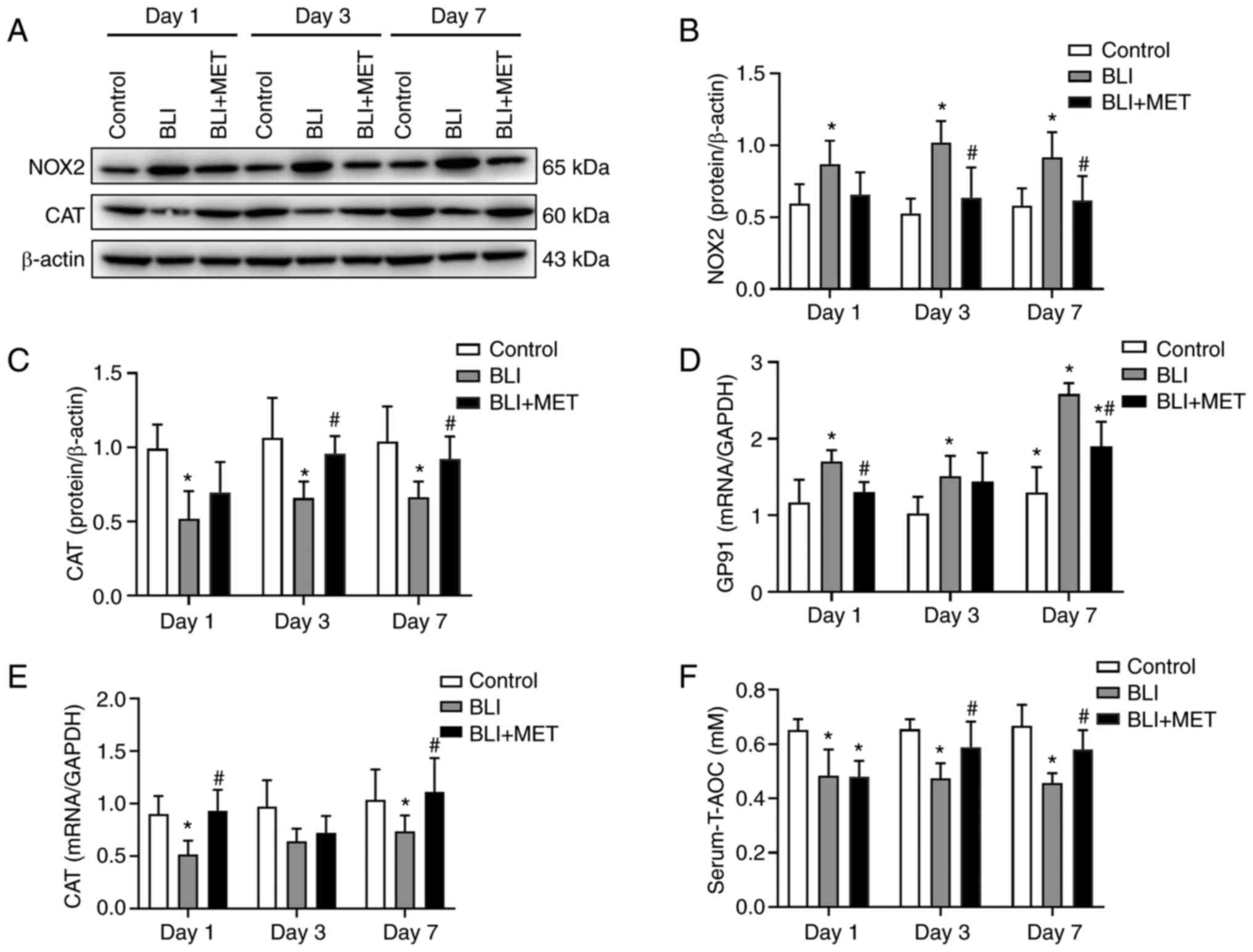
MET downregulates oxidative stress
caused by NOX2 due to gas explosions
Western blotting revealed that NOX2 levels in lung
tissues were significantly increased in the BLI group compared with
those in the control group, and MET treatment decreased NOX2
levels, the main source of ROS in acute lung injury (Fig. 6A and B). In addition, CAT levels were
significantly decreased in the lung tissues of gas
explosion-exposed rats compared with the control group; however,
MET supplementation increased the antioxidant capacity of the lung
(Fig. 6A and C). Similarly, RT-qPCR revealed that the
levels of GP91, which is the main subunit of the superoxide
anion-generating NADPH oxidase complex, and CAT were
consistent with the western blotting results (Fig. 6D and E). Finally, the serum T-AOC of rats
exposed to gas explosions was significantly lower compared with
that in the rats of the control group, whereas MET treatment
increased this capacity in gas explosion-exposed rats (Fig. 6F).
Discussion
The shock wave pressure caused by a gas explosion
increases following multiple reflections, resulting in extensive
damage and serious harm due to the complex structure of the mine.
The high-temperature flame from a gas explosion can cause serious
damage to the upper and lower respiratory tracts and alveolae
(26). Previous studies have
indicated that shock waves from a gas explosion contribute damage
to the cavity viscera, such as the lungs and brain (27,28).
The mechanism of gas explosion-induced lung injury is unclear, and
our previous metabolomic study identified nine different
metabolites that differ significantly between the control rats and
explosion-induced acute lung injury rats (6). The identified biomarkers reflect
well-known adverse health effects of a gas explosion, such as
inflammation and impairment of glucose homeostasis (29). In addition, NOX2 is an important
source of ROS in acute lung injury, and NOX2-induced ROS causes
oxidative damage to macromolecules, such as proteins, DNA and
lipids (13). AMPK, an important
energy sensor for the maintenance of cellular energy homeostasis,
can be activated by MET (11).
Recent studies have indicated that in addition to the treatment of
type 2 diabetes, MET helps alleviate the clinical symptoms of
cancer, aging (30),
cardiovascular disease (31) and
kidney disease (32). Therefore,
the present study explored the effect of MET on energy metabolism
during gas explosion-exposed pulmonary injury in rats. The results
indicated that MET could attenuate gas explosion-induced lung
injury via AMPK-mediated energy metabolism and the NOX2-related
oxidase pathway.
The present study used an actual roadway to imitate
a real gas explosion situation in a tunnel. There were bleeding
points in the lungs and a large number of free red blood cells were
observed under the microscope; this indicated that shock waves
caused by a gas explosion could induce pulmonary hemorrhage and
decreased MV and PIFR, indicating that a gas explosion in a real
roadway environment could lead to a decrease in respiratory
function and lung tissue damage in a rat model. The peak of
inflammatory cell influx was related to the impairment of lung
function. Pulmonary edema induced by inflammation often shows an
enhanced respiration pause (33),
and the reduction of MV and PIFR indicates the occurrence of airway
stenosis. These results prove the applicability of the present gas
explosion blast lung injury-induced rat model.
To investigate whether MET could mitigate gas
explosion-induced blast injury, 10 mg/kg of MET was administered to
rats in the present study. The results revealed that MET alleviated
the pathological injury induced by exposure to a gas explosion. To
demonstrate that MET could positively regulate the energy
metabolism associated with injured alveolar cells in gas explosion,
the changes in enzyme activity were measured. Under normal
circumstances, >80% of ATP required by the human body is
provided by oxidative phosphorylation (34). However, when oxidative
phosphorylation is inhibited, glycolysis can rapidly produce ATP to
provide energy to the human body by converting glucose into lactic
acid (34). Jin et al
(35) revealed that ATP production
and metabolic reorganization from the TCA cycle to glycolysis are
reduced in the lungs of rats exposed to different dosages of
seasonal particulate matter less than 2.5 µm in diameter. By
investigating the activities of the rate-limiting enzymes in the
TCA cycle, specifically α-KGDH and ICDHm, the present study
revealed that ATP content was decreased, and the TCA cycle was
inhibited after the gas explosion. Similarly, a previous study
demonstrated that the rapid consumption of energy by rats with gas
explosion-induced injury downregulates galactose metabolism and the
TCA cycle, both of which are consistent with the Warburg effect
(6). In addition, the present
study revealed that gas explosions not only inhibited oxidative
phosphorylation but also affected glycolysis. PFK is the source
rate-limiting enzyme of glycolysis, and its enzymatic activity was
inhibited by gas explosion injuries. Therefore, ATP production is
directly inhibited, but MET can improve metabolic abnormalities to
some extent.
In the present study, MET promoted the
phosphorylation of AMPKα and increased the expression of PGC1α. In
addition, AMPK, PGC1α and TFAM mRNA levels
were increased in the BLI + MET group. The transcription of mtDNA
requires the participation of nucleus-encoded proteins, such as
additional factors necessary for TFAM (36). In turn, factors are activated
directly or indirectly by the PGC-1 family (37). PGC-1α increases the number of
mitochondria to enhance oxidative phosphorylation in lung
epithelial cells (38). Similarly,
the AMPK/PGC1α pathway is activated to regulate mitochondrial
homeostasis and then attenuate gas explosion-induced BLI (17).
The results of the present study are consistent with
the results aforementioned. Ultrastructural changes in lung tissue
demonstrated the improvement in the structural stability of the
mitochondria after MET treatment. In previous studies, considerable
damage to the right lower lobe, mitochondrial abnormalities and the
loss of a lamellar body structure have been observed after acute
primary blast injury by an electron microscope. Moreover, the
lesions noted in the lung may be progressive in the first 24 h
after injury (39). However, the
present study revealed that the most severe lung injury was
observed after 3 days using TEM. In summary, MET activated the
AMPK/PGC1α mitochondrial protective pathway and maintained the
mitochondrial structural stability.
Finally, the present study demonstrated that MET
downregulated oxidative stress caused by NOX2, which was activated
in gas explosion-induced BLI. Oxidative stress is known to induce
mitochondrial functional protein destruction or mtDNA damage
(9). A previous study indicated
that the activation of NOX2 increases cardiac superoxide
production, causing mitochondrial dysfunction and decreased
contractility (40). The present
results confirmed that NOX2 mediated oxidative stress in lung
injury caused by a gas explosion. By contrast, AMPK agonists can
improve mitochondrial function, increase energy levels and thereby
reduce oxidative stress to improve overall cell viability and
tissue health (41). In a study by
Schuhmacher et al (42),
alveolar type II intervention in AMPKα1-knockout mice resulted in a
significant upregulation of NOX2 mRNA and protein in the vascular
endothelium of mice, while AMPK downregulates NOX2 expression,
reduces oxidative stress, decreases mitochondrial inner membrane
ROS production and protects mitochondrial structures from being
compromised (42). Thus, the
inhibition of NOX2 and promotion of antioxidation may be another
mechanism by which MET protects mitochondria from gas
explosion-induced acute lung injury.
On a temporal level, the damage to the lung function
due to gas explosions is continuously aggravated. The results from
the present experiment indicated that the situation was the most
severe on day 3 after the injury. This is consistent with the
pre-experimental results of our previous studies. However, between
days 3 and 7 after the gas explosion, there was a weak tendency of
abnormal molecular indicators to return to their normal levels, but
this self-recovery was particularly limited. The present results
provided evidence that if MET was administered as early as possible
after a gas explosion-induced injury, it could reduce the
persistent aggravation of intrapulmonary injury caused by a gas
explosion. In summary, MET mitigated gas explosion-induced blast
lung injuries through AMPK-mediated energy metabolism and
NOX2-related oxidase pathway in rats.
In the present study, only one dose intervention
group was used. Future experiments should use multiple dose groups
of metformin to determine the best dose of treatment.
In conclusion, gas explosions can cause the energy
metabolism abnormalities associated with acute lung injury and lead
to the onset of oxidative stress in the lungs. MET activates AMPK
and downregulates NOX2 expression. This maintains the mitochondrial
structure, enhances intrapulmonary antioxidant capacity, promotes
intrapulmonary ATP production and finally alleviates the extent of
BLI induced by a gas explosion.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by the Key Projects of Henan
Union Fund of NSFC (grant no. U1904209) and the Innovation Research
Program for Postgraduates of Xinxiang Medical University (grant no.
YJSCX202005Z).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ, WJR and SQY conceived the study and designed
experiments. CJD and SH performed animal experiments. MZ, YZS and
XWD performed the main experiments. SQY and WJR confirm the
authenticity of all the raw data. NL, YG and LZ were responsible
for data acquisition and analysis. WY and JC analyzed and
interpreted data. MZ and SQY wrote and revised the manuscript. WY,
JC, WJR and SQY were accountable for all aspects of the work in
ensuring that questions are appropriately investigated and
resolved. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal study protocol was approved by the
Medical Ethics Committee of Xinxiang Medical University (approval
no. XYLL-2019001; Jan 13, 2019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li J, Qin Y, Wang Z and Xin Y: How to
analyse the injury based on 24Model: A case study of coal mine gas
explosion injury. Inj Prev. 27:542–553. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li N, Geng C, Hou S, Fan H and Gong Y:
Damage-associated molecular patterns and their signaling pathways
in primary blast lung injury: New research progress and future
directions. Int J Mol Sci. 21(6303)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wolf SJ, Bebarta VS, Bonnett CJ, Pons PT
and Cantrill SV: Blast injuries. Lancet. 374:405–415.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Smith JE and Garner J: Pathophysiology of
primary blast injury. J R Army Med Corps. 165:57–62.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Z, Li H, Liang Z, Li C, Yang Z, Li
Y, Cao L, She Y, Wang W, Liu C and Chen L: Vaporized
perfluorocarbon inhalation attenuates primary blast lung injury in
canines by inhibiting mitogen-activated protein kinase/nuclear
factor-κB activation and inducing nuclear factor, erythroid 2 like
2 pathway. Toxicol Lett. 319:49–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dong X, Wu W, Yao S, Cao J, He L, Ren H
and Ren W: Evaluation of gas explosion injury based on analysis of
rat serum profile by ultra-performance liquid chromatography/mass
spectrometry-based metabonomics techniques. Biomed Res Int.
2020(8645869)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Quijano C, Trujillo M, Castro L and
Trostchansky A: Interplay between oxidant species and energy
metabolism. Redox Biol. 8:28–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kellner M, Noonepalle S, Lu Q, Srivastava
A, Zemskov E and Black SM: ROS signaling in the pathogenesis of
acute lung injury (ALI) and acute respiratory distress syndrome
(ARDS). Adv Exp Med Biol. 967:105–137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu X and Chen Z: The pathophysiological
role of mitochondrial oxidative stress in lung diseases. J Transl
Med. 15(207)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fathi H, Ebrahimzadeh MA, Ziar A and
Mohammadi H: Oxidative damage induced by retching; antiemetic and
neuroprotective role of Sambucus ebulus L. Cell Biol Toxicol.
31:231–239. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hardie DG: AMP-activated protein kinase:
Maintaining energy homeostasis at the cellular and whole-body
levels. Annu Rev Nutr. 34:31–55. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Magnani ND, Marchini T, Vanasco V, Tasat
DR, Alvarez S and Evelson P: Reactive oxygen species produced by
NADPH oxidase and mitochondrial dysfunction in lung after an acute
exposure to residual oil fly ashes. Toxicol Appl Pharmacol.
270:31–38. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aviello G and Knaus UG: NADPH oxidases and
ROS signaling in the gastrointestinal tract. Mucosal Immunol.
11:1011–1023. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nadeem A, Al-Harbi NO, Ahmad SF, Ibrahim
KE, Siddiqui N and Al-Harbi MM: Glucose-6-phosphate dehydrogenase
inhibition attenuates acute lung injury through reduction in NADPH
oxidase-derived reactive oxygen species. Clin Exp Immunol.
191:279–287. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rodríguez C, Contreras C, Sáenz-Medina J,
Muñoz M, Corbacho C, Carballido J, García-Sacristán A, Hernandez M,
López M, Rivera L and Prieto D: Activation of the AMP-related
kinase (AMPK) induces renal vasodilatation and downregulates
Nox-derived reactive oxygen species (ROS) generation. Redox Biol.
34(101575)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nunnari J and Suomalainen A: Mitochondria:
In sickness and in health. Cell. 148:1145–1159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chang M, Xu G, Xiong C, Yang X, Yan S, Tao
Y, Li H, Li Y, Yao S and Zhao Y: Alpha-lipoic acid attenuates
silica-induced pulmonary fibrosis by improving mitochondrial
function via AMPK/PGC1α pathway activation in C57BL/6J mice.
Toxicol Lett. 350:121–132. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Y, An H, Liu T, Qin C, Sesaki H, Guo
S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, et al:
Metformin improves mitochondrial respiratory activity through
activation of AMPK. Cell Rep. 29:1511–1523.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang G, Song Y, Feng W, Liu L, Zhu Y, Xie
X, Pan Y, Ke R, Li S, Li F, et al: Activation of AMPK attenuates
LPS-induced acute lung injury by upregulation of PGC1α and SOD1.
Exp Ther Med. 12:1551–1555. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Research Council (US) Institute
for Laboratory Animal Research. Guide for the care and use of
laboratory animals, 8th edition. National Academies
Press,Washington, DC, 2011.
|
|
21
|
Mikawa K, Nishina K, Takao Y and Obara H:
ONO-1714, a nitric oxide synthase inhibitor, attenuates
endotoxin-induced acute lung injury in rabbits. Anesth Analg.
97:1751–1755. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Creagh-Brown BC, Quinlan GJ, Evans TW and
Burke-Gaffney A: The RAGE axis in systemic inflammation, acute lung
injury and myocardial dysfunction: An important therapeutic target?
Intensive Care Med. 36:1644–1656. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tretter L and Adam-Vizi V:
Alpha-ketoglutarate dehydrogenase: A target and generator of
oxidative stress. Philos Trans R Soc Lond B Biol Sci.
360:2335–2345. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Legendre F, MacLean A, Appanna VP and
Appanna VD: Biochemical pathways to α-ketoglutarate, a
multi-faceted metabolite. World J Microbiol Biotechnol.
36(123)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang JN, Li HB, Dong XW, Wu WD, Ren WJ and
Yao SQ: Role of pyroptosis pathway related molecules in acute lung
injury induced by gas explosion in rats. Zhonghua Lao Dong Wei
Sheng Zhi Ye Bing Za Zhi. 40:97–102. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
27
|
Svetlov SI, Larner SF, Kirk DR, Atkinson
J, Hayes RL and Wang KK: Biomarkers of blast-induced neurotrauma:
Profiling molecular and cellular mechanisms of blast brain injury.
J Neurotrauma. 26:913–921. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mathews ZR and Koyfman A: Blast injuries.
J Emerg Med. 49:573–587. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dong X, Yao S, Wu W, Cao J, Sun L, Li H,
Ren H and Ren W: Gas explosion-induced acute blast lung injury
assessment and biomarker identification by a LC-MS-based serum
metabolomics analysis. Hum Exp Toxicol. 40:608–621. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Podhorecka M, Ibanez B and Dmoszyńska A:
Metformin-its potential anti-cancer and anti-aging effects. Postepy
Hig Med Dosw (Online). 71:170–175. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Luo F, Das A, Chen J, Wu P, Li X and Fang
Z: Metformin in patients with and without diabetes: A paradigm
shift in cardiovascular disease management. Cardiovasc Diabetol.
18(54)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ren H, Shao Y, Wu C, Ma X, Lv C and Wang
Q: Metformin alleviates oxidative stress and enhances autophagy in
diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell
Endocrinol. 500(110628)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Håkansson HF, Smailagic A, Brunmark C,
Miller-Larsson A and Lal H: Altered lung function relates to
inflammation in an acute LPS mouse model. Pulm Pharmacol Ther.
25:399–406. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Papa S, Martino PL, Capitanio G, Gaballo
A, De Rasmo D, Signorile A and Petruzzella V: The oxidative
phosphorylation system in mammalian mitochondria. Adv Exp Med Biol.
942:3–37. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jin X, Su H, Ding G, Sun Z and Li Z:
Exposure to ambient fine particles causes abnormal energy
metabolism and ATP decrease in lung tissues. Chemosphere.
224:29–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao M, Wang Y, Li L, Liu S, Wang C, Yuan
Y, Yang G, Chen Y, Cheng J, Lu Y and Liu J: Mitochondrial ROS
promote mitochondrial dysfunction and inflammation in ischemic
acute kidney injury by disrupting TFAM-mediated mtDNA maintenance.
Theranostics. 11:1845–1863. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Scarpulla RC: Transcriptional paradigms in
mammalian mitochondrial biogenesis and function. Physiol Rev.
88:611–638. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schoors S, Bruning U, Missiaen R, Queiroz
KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia
J, et al: Fatty acid carbon is essential for dNTP synthesis in
endothelial cells. Nature. 520:192–197. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Brown RF, Cooper GJ and Maynard RL: The
ultrastructure of rat lung following acute primary blast injury.
Int J Exp Pathol. 74:151–162. 1993.PubMed/NCBI
|
|
40
|
Joseph LC, Kokkinaki D, Valenti MC, Kim
GJ, Barca E, Tomar D, Hoffman NE, Subramanyam P, Colecraft HM,
Hirano M, et al: Inhibition of NADPH oxidase 2 (NOX2) prevents
sepsis-induced cardiomyopathy by improving calcium handling and
mitochondrial function. JCI Insight. 2(e94248)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Moore T, Yanes RE, Calton MA, Vollrath D,
Enns GM and Cowan TM: AMP-independent activator of AMPK for
treatment of mitochondrial disorders. PLoS One.
15(e0240517)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Schuhmacher S, Foretz M, Knorr M, Jansen
T, Hortmann M, Wenzel P, Oelze M, Kleschyov AL, Daiber A, Keaney JF
Jr, et al: α1AMP-activated protein kinase preserves endothelial
function during chronic angiotensin II treatment by limiting Nox2
upregulation. Arterioscler Thromb Vasc Biol. 31:560–566.
2011.PubMed/NCBI View Article : Google Scholar
|