Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common non-skin-related cancer worldwide. Each year,
600,000 patients are diagnosed with this cancer, and 50% succumb
(1). Salivary gland carcinoma is a
relatively rare malignant tumor, accounting for <5% of HNSCC
cases (2). Patients with
high-grade salivary carcinoma have a 5-year survival rate of ~40%,
whereas those with low-grade salivary carcinoma have a 5-year
survival rate of 85-90% (3,4). The
standard treatments for major and minor salivary gland tumors
include surgical excision, radiotherapy and chemotherapy.
Cisplatin, 5-fluorouracil and methotrexate are common drugs used to
treat squamous cell carcinoma of the salivary glands (5). However, doxorubicin hydrochloride
(Adriamycin), cisplatin, cyclophosphamide or cisplatin with
5-fluorouracil can be used for the primary treatment of patients
with recurrent, metastatic or unresectable salivary gland tumors
(6,7). Only a few effective treatment options
are available for patients with recurrent or unresectable tumors.
Despite the effective treatment features of cisplatin, various
studies have revealed only a 10-month survival period when this
drug is used alone or combined with other medicines. Chemotherapy
causes speech/swallowing defects, chronic pain, muscle atrophy and
other side effects (8). Therefore,
novel chemotherapy and treatment regimens are required for salivary
carcinoma (9).
Esculetin, also known as 6,7-dihydroxy coumarin, is
derived from coumarin and can be obtained from various plants, such
as Citrus limonia, Artemisia capillaries and
Euphorbia lathyris (10,11).
Pleiotropic biological activity is a well-known characteristic of
esculetin. Moreover, esculetin has various advantages, including
antioxidant and xanthine oxidase inhibitory activities, platelet
aggregation and anticancer behavior (12-16).
Esculetin also induces the apoptosis of oral squamous cell
carcinoma (OSCC), resulting in the suppression of cancer cell
proliferation (13,17). To date, no study has evaluated the
effect of esculetin on human salivary gland tumors. Thus, in the
present study, in vitro and in vivo experiments were
performed to assess the induction of apoptosis and the
antiproliferative effect of esculetin on the human submandibular
carcinoma A253 cell line.
Materials and methods
Materials
Esculetin with a purity >98% was obtained from
Tokyo Chemical Industry. DMSO, cisplatin and
3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) were
purchased from Sigma-Aldrich; Merck KGaA. The apoptosis detection
kit for TUNEL-FITC and the human apoptosis proteome profiler kit
were supplied by Promega Corporation and R&D Systems, Inc.,
respectively. All the required antibodies, including Bax (cat. no.
ab53154), Bcl-2 (cat. no. ab196495), poly (ADP-ribose) polymerase
(PARP; cat. no. ab32139), caspase-3 (cat. no. ab184786), caspase-9
(cat. no. ab184786), and β-actin (cat. no. ab8227), were purchased
from Abcam. The A253 cells were obtained from the American Type
Culture Collection.
Cell culture
A253 cells were cultured in modified RPMI 1640
medium (Welgene, Inc.) supplemented with 10% fetal bovine serum
(Welgene, Inc.), streptomycin (100 µg/ml), and penicillin (100
U/ml), and maintained in an incubator containing 5% CO2
at 37˚C.
Cell viability and apoptosis
assay
The MTT assay was used to estimate the effect of
esculetin on cell viability. The A253 cells were seeded in a
96-well cell culture plate at a density of 5x104 cells
per well. Thereafter, the cultured cells were treated with
different doses of esculetin (0, 50, 100 and 150 µM in 0.1% DMSO)
for 24 and 48 h. MTT (5 mg/ml; 10 µl) was then added to the cells,
which were further incubated at 37˚C for 4 h. After MTT removal,
the violet formazan crystals were dissolved in 1 ml DMSO. A
microplate reader (540 nm; Tecan Group, Ltd.) was used to obtain
the MTT assay results. The cells were cultured on autoclaved
coverslips to determine esculetin-treated apoptotic cells. TUNEL
staining was conducted to detect apoptotic cells, after 24 and 48 h
of pre-incubation with esculetin. Cells were then washed with PBS,
and fixed with 4% paraformaldehyde in PBS at room temperature for
20 min. Fixed cells were washed with PBS and treated with the rTdT
reaction mix at 37˚C for 1 h; reactions were terminated by
immersing in 2X SSC solution for 15 min at room temperature. Cells
were stained with 0.2 mg/ml of DAPI in PBS at room temperature for
15 mi and mounted onto glass slides with the mounting medium
(Vectashield, Vector Laboratories, Inc.) and analyzed under a
fluorescent microscope (Olympus Corporation). Images of at least
five random fluorescent fields were captured, and TUNEL-positive
cells were counted. Data were expressed as the percentage of
apoptotic cells per field.
Western blot analysis
A protein extraction RIPA lysis buffer (Elpis
Biotech) containing a Halt™ Protease Inhibitor Cocktail
(Thermo Fisher Scientific, Inc.) was used to lyse the treated
cells. Thereafter, Protein Assay Dye Reagent Concentrate (Bio-Rad
Laboratories, Inc.) was used to quantify the extracted protein.
Equal amounts of proteins (50 µg/lane) were separated by 12.5%
SDS-PAGE gel and transferred to a PVDF membrane (MilliporeSigma).
The membranes were then blocked with 5% skimmed milk in
Tris-buffered saline containing 0.05% Tween-20 (TBST) at room
temperature for 2 h. The primary antibodies used were anti-Bax
(1:500), anti-Bcl-2 (1:500), anti-PARP (1:500), anti-caspase-3
(1:500), anti-caspase-9 (1:500), and β-actin (1:1,000). The
membranes were incubated with primary antibodies diluted in TBST
overnight at 4˚C, washed three times with TBST. Finally, a western
blotting detection kit (ECL Western blotting substrate kit; Abcam)
was used to observe the protein bands after treatment with a
horseradish peroxidase-linked secondary antibody (1:1,000,
Advansta) at room temperature for 1 h.
Proteome profiling of human apoptosis
array
Different doses of esculetin (0, 50, 100, and 150
µM) were used to treat A253 cells for 48 h. A proteome profiler
human apoptosis antibody array kit (cat. no. ARY009, R&D
systems) was used to analyze the lysed cells according to the
manufacturer's instructions. ImageJ software version 1.52a
(National Institutes of Health) was used to desensitize the
obtained signals, where the average pixel density was expressed by
normalizing the pixel density to untreated samples.
Quantitative polymerase chain reaction
(qPCR)
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to extract total RNA from the collected
cells based on the manufacturer's instructions. Total RNA (1 µg)
was reverse transcribed in a final volume of 30 µl using the
ImProm-II reverse transcriptase system kit (Promega Corporation)
according to the manufacturer's instructions. cDNAs were used for
PCR. The primer sequences are summarized in Table I. The AniQ5 Continuous Fluorescence
Detector System (Bio-Rad Laboratories, Inc.) and a 2X
SYBR® Green PCR Master Mix (cat. no. RR420A, Takara Bio,
Inc.) were used to perform the qPCR at 95˚C for 30 sec, followed by
40 cycles of 95˚C for 3 sec and 60˚C for 30 sec and a single cycle
of 95˚C for 15 sec, 60˚C for 60 sec, and 95˚C for 15 sec to
generate dissociation curves. All PCR reactions were performed in
triplicate, and the specificity of the reaction was determined by
melting curve analysis. Comparative quantification of each target
gene was performed based on cycle threshold (Ct) normalized to
β-actin using the 2-ΔΔCq method (18).
 | Table IList of primer sequences used in this
study. |
Table I
List of primer sequences used in this
study.
| Gene | Forward primer | Reverse primer |
|---|
| Caspase-3 |
5'-AATGGACCTGTTGACCTGAAA-3' |
5'-ATAATAACCAGGTGCTGTGGAG-3' |
| Bcl-2 |
5'-GGAGGATTGTGGCCTTCTTT-3' |
5'-GAGACAGCCAGGAGAAATCAA-3' |
| Bax |
5'-TTGCTTCAGGGTTTCATCCA-3' |
5'-AGTTGAAGTTGCCGTCAGAA-3' |
| β-actin |
5'-TGACGATATCGCTGCGCTC-3' |
5'-CAGTTGGTGACAATGCCGTG-3' |
Tumor xenograft model
A total of 18 male BALB/c nude mice (age, 6 weeks
old; weight, 15-20 g) were obtained from Central Lab Animal Inc.
Mice were housed under a 12-h light/dark cycle at a temperature of
23±1˚C and 55±5% humidity and were provided with standard rodent
pellets and filtered water ad libitum. The protocols
approved by the Institutional Animal Care and Use Committee of the
Jeonbuk National University Hospital (Jeonju, South Korea; approval
no. JBNUH 2021-019) were used for the assessment. Briefly, A253
cells were suspended in RPMI-1640 culture medium containing 10%
FBS, and maintained in a humidified atmosphere containing 5%
CO2 at a controlled temperature of 37.6˚C. Before tumor
inoculation, all mice were anesthetized with ketamine (80 mg/kg)
and xylazine (10 mg/kg). To obtain a xenograft model, mouse
shoulders were inoculated with A253 cells at a total of
2x106 cells in 50 µl Dulbecco's modified Eagle's medium
(Welgene, Inc.) mixed with 50 µl Matrigel (Becton, Dickinson and
Company) for a total volume of 100 µl per injection site. After 7
days, mice were randomly divided into three groups, each containing
six mice. Esculetin and cisplatin were dissolved in 0.5%
carboxymethylcellulose. Thereafter, esculetin (100 mg/kg per day)
or cisplatin (7.5 mg/kg per day), as a positive control, was orally
administered for 18 days (19,20).
Mice in the negative control group were orally administered the
vehicle (0.5% carboxymethylcellulose). The in vivo antitumor
activity of esculetin was assessed by measuring tumor size at 3-day
intervals. Esculetin was administered for 18 days until the tumor
size in the negative control group reached 200 mm3.
Tumor volume was calculated using the following equation: π/6 x
(a)2 x (b), where ‘a’ and ‘b’, correspond to the
shortest and longest tumor diameters, respectively (21). No adverse reactions or
compound-related side effects were observed in the mice. Body
weight was measured and the mice were sacrificed with ketamine (500
mg/kg) and xylazine (50 mg/kg) to excise the tumor xenografts at
necropsy.
H&E and TUNEL staining
For evaluation of the tissue structures, the tumor
tissues were fixed in formaldehyde solution (4%) at room
temperature for 24 h, dehydrated using gradient ethanol, embedded
in paraffin, incised into smaller sections (4 µm) and then stained
with H&E. The TUNEL assay was also performed using the one-step
TUNEL kit, according to the defined guidelines. After paraffin
removal, the sections were incubated in 100 µl Proteinase K (20
µg/ml) for 10 min at room temperature, transferred into a TUNEL
reaction mixture with a volume of 50 µl, and placed in a dark
incubator for 1 h at 37˚C. A solution of Vectashield Mounting
Medium with 4',6-diamidino-2-phenylindole (Vector Laboratories,
Inc.) was used to stain the prepared sections. Subsequently, a
fluorescence microscope was used to capture images of the prepared
slices. Images of at ≥5 random fluorescent fields were captured. In
this evaluation, the number of TUNEL-positive apoptotic cells per
100 cells was determined using green fluorescence exerted by the
cells.
Statistical analysis
The results are presented as the mean ± standard
deviation. One-way analysis of variance was used to analyze the
data, followed by Tukey's multiple comparison test. The assessments
were conducted using GraphPad Prism 6.0 (GraphPad Prism Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of esculetin on the
proliferation and apoptosis of A253 cells
Several concentrations of esculetin were used to
treat A253 cells for 24 and 48 h to explore the therapeutic
potential of esculetin. Further, MTT assays were performed to
determine the viability of the cells. The proliferation of A253
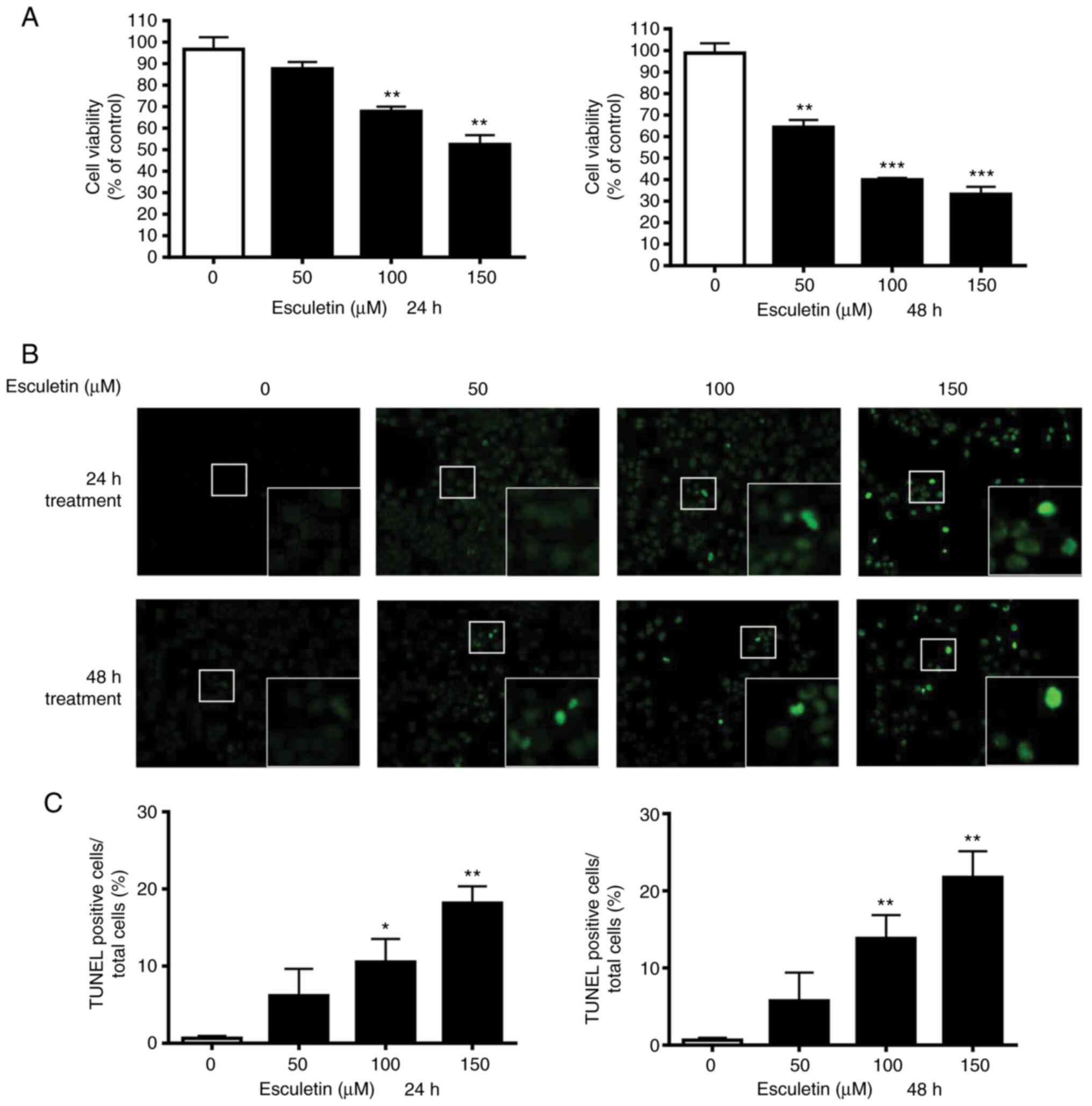
cells was inhibited by esculetin after both 24 and 47 h of
treatment in a concentration-dependent manner (Fig. 1A). The 50% maximal inhibitory
concentration (IC50) values of esculetin at 24 and 48 h
were 157.4±30.0 and 78.5±5.3 µM, respectively. TUNEL staining was
performed to determine the effect of esculetin on apoptosis. As
shown in Fig. 1B and C, the apoptotic cell ratio was enhanced
in the treated cancer cells compared with that in the control cells
in a concentration-dependent manner. Based on the observed trend,
apoptosis was the main cause of the decrease in cell viability of
cancer cells treated with esculetin.
Role of esculetin in apoptosis
induction via the regulation of apoptosis regulatory factors in
A253 cells
The role of esculetin in apoptosis induction through
the regulation of apoptosis regulatory proteins in tumor cells has
been widely reported (22-24).
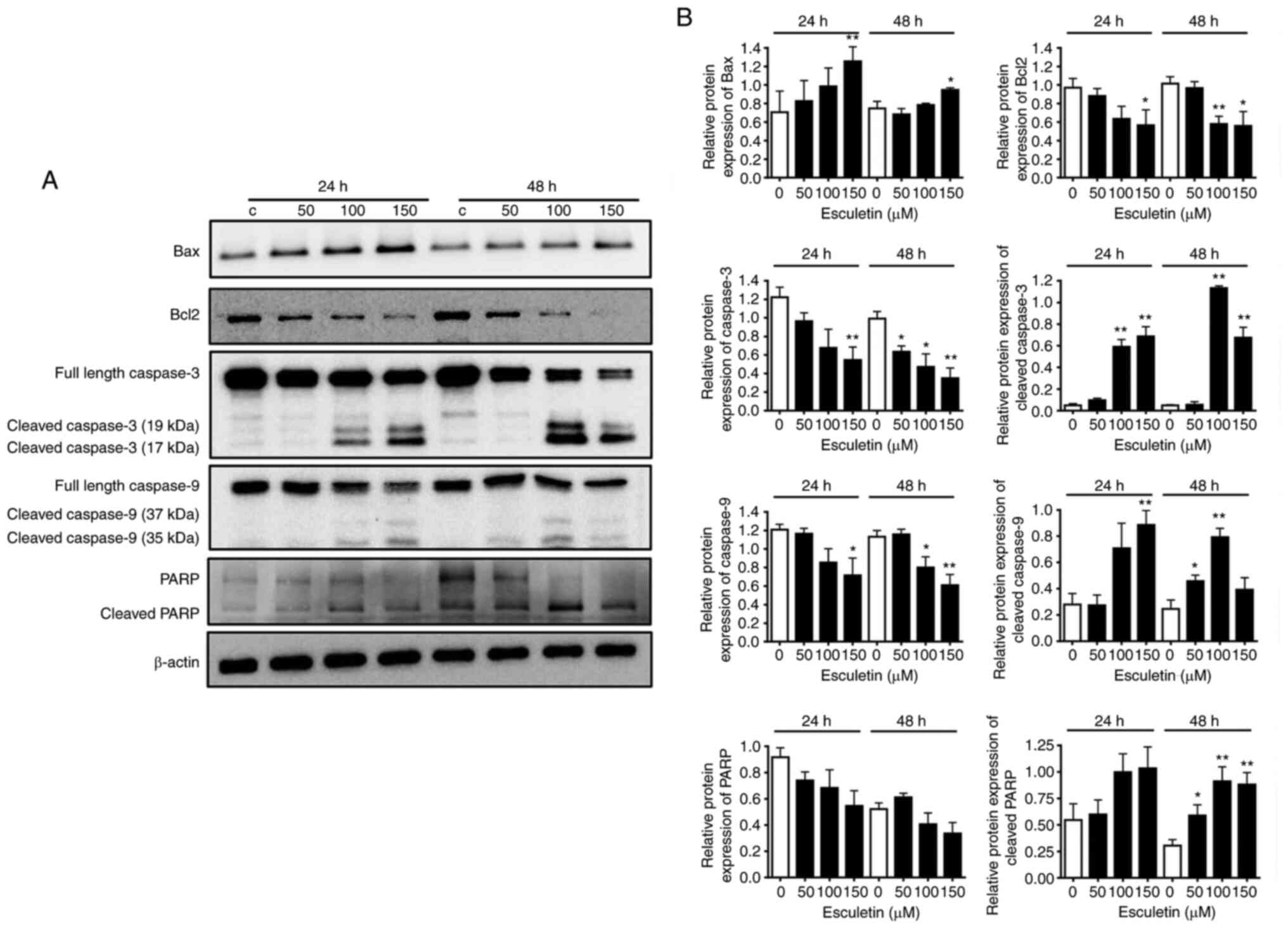
To determine the expression level of apoptosis-related proteins,
A253 cells were treated with various concentrations of esculetin
(0, 50, 100 and 150 µM) for 24 or 48 h. The levels of Bax, cleaved
caspase-3, cleaved caspase-9 and cleaved-PARP apoptosis-related
proteins increased by esculetin after both 24 and 47 h of
treatment. However, the level of the anti-apoptotic protein, Bcl-2,
decreased in an esculetin dose-dependent manner (Fig. 2). These results indicated that
esculetin induced apoptosis in the A253 cell line. To further
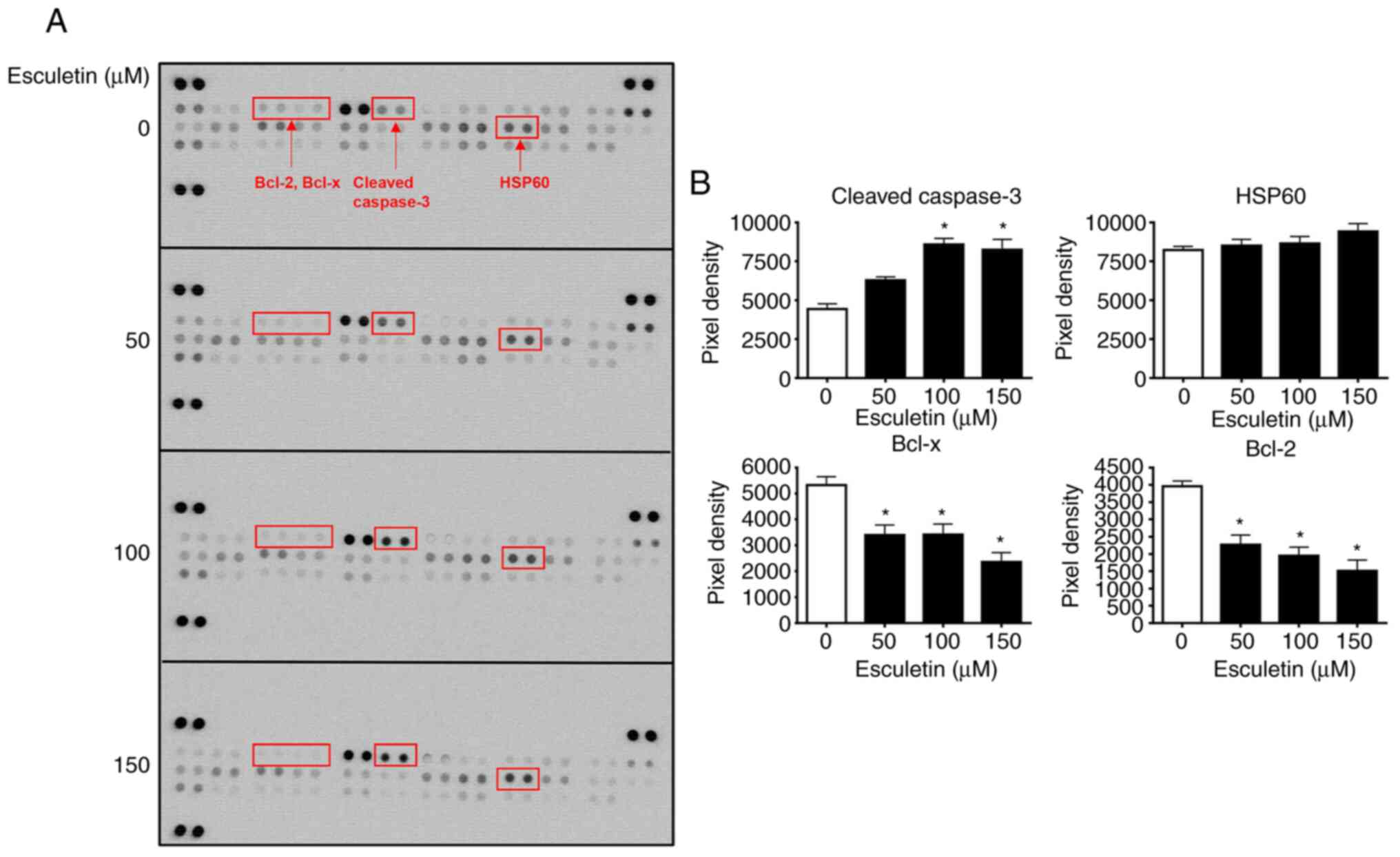
elucidate the effect of esculetin on the differential expression of
pro- and anti-apoptotic proteins, 56 proteins were identified using
a human apoptosis proteome profiler array kit (Fig. 3A). As predicted, cleaved caspase-3
was significantly upregulated in cells treated with esculetin
compared with untreated control cells. Further, HSP60 expression
was found to be raised, whereas the expression levels of the
anti-apoptotic proteins, including Bcl-2 and Bcl-x, was decreased
in esculetin-treated cancer cells (Fig. 3A and B). Therefore, esculetin may promote A253
cell death through pro-apoptotic protein induction and
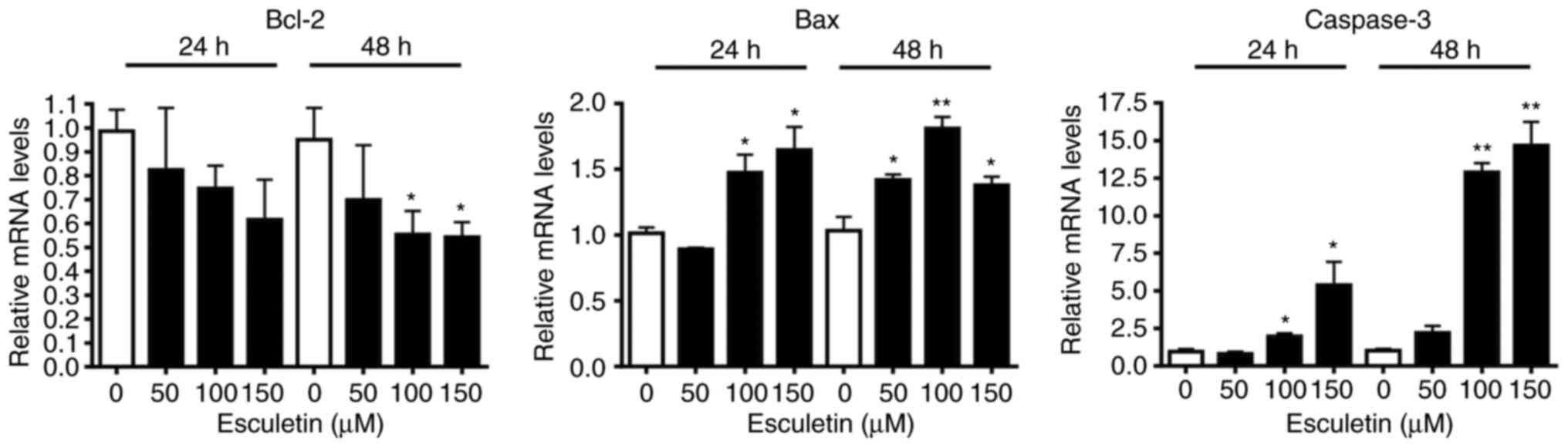
anti-apoptotic protein reduction. To confirm the aforementioned
results, the mRNA expression levels of pro- and anti-apoptotic
genes in esculetin-treated A253 cells were evaluated. Caspase-3 and
Bax mRNA expression levels were significantly increased, while
those of Bcl-2 were decreased in a dose-dependent manner (Fig. 4).
Effect of esculetin on the
proliferation and apoptosis of A253 cells
An in vivo examination was performed using a
xenograft nude mouse model of A253 cells to determine the role of
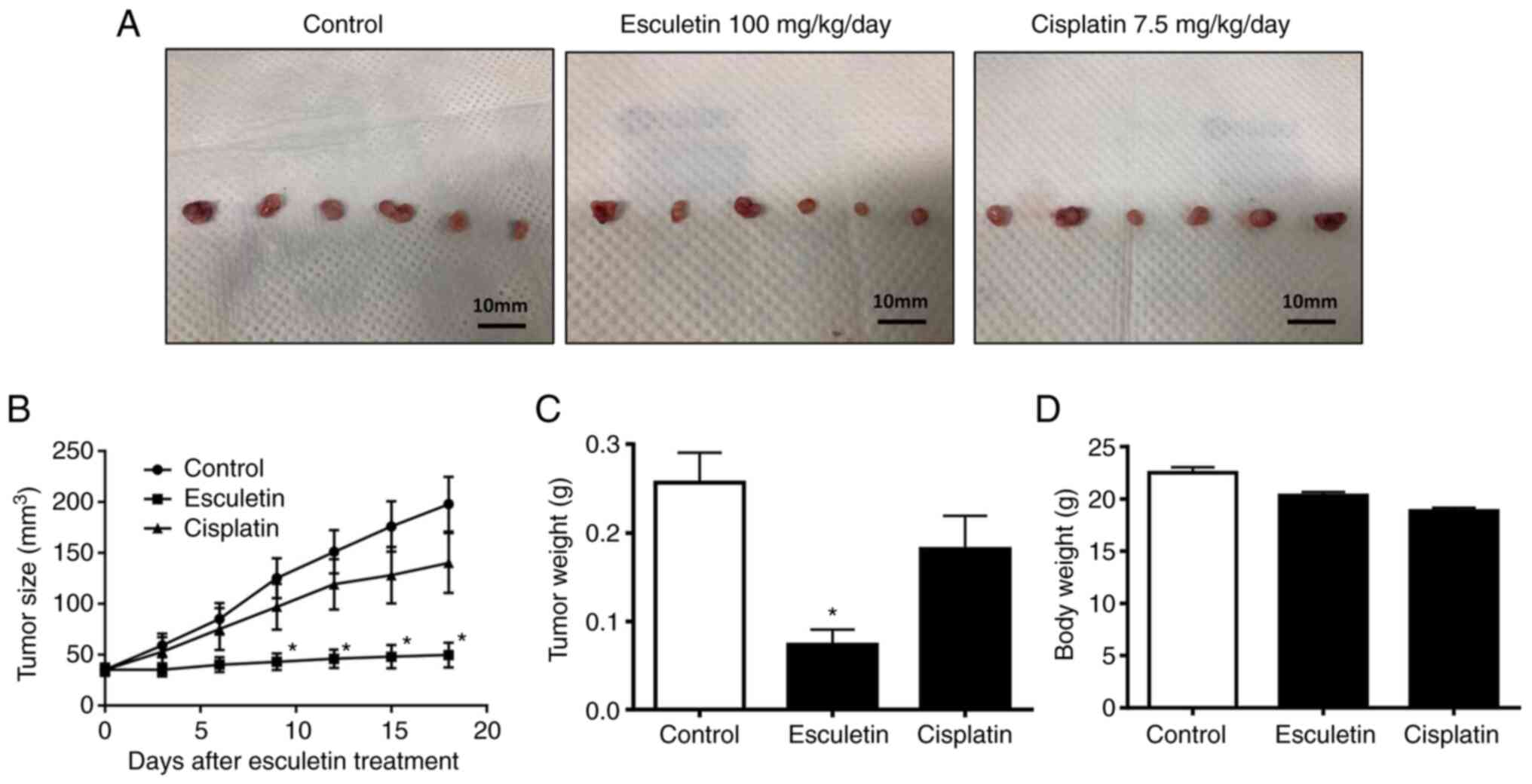
esculetin in the inhibition of tumor growth. The data revealed the
inhibitory effect of esculetin on the growth of tumors (Fig. 5). Esculetin was found to reduce
tumor weight and size. In fact, at necropsy, tumor sizes in the
vehicle, esculetin and cisplatin-treated groups were 197.9±66.0,
49.8±29.8, and 140±72.0 mm3, respectively. Thus,
esculetin suppressed xenograft tumor development in mice by 74%
relative to that of vehicle-treated mice. Nevertheless, the body
weight of xenograft nude mice was not significantly affected by
esculetin. Cisplatin did not significantly suppress A253 tumor
growth (P>0.05). Thus, esculetin has more potent antitumor
activity than cisplatin. These findings suggest that the growth of
human submandibular salivary gland tumors can be suppressed by the
application of esculetin.
Effect of esculetin on apoptosis
induction in the A253 cell xenograft model
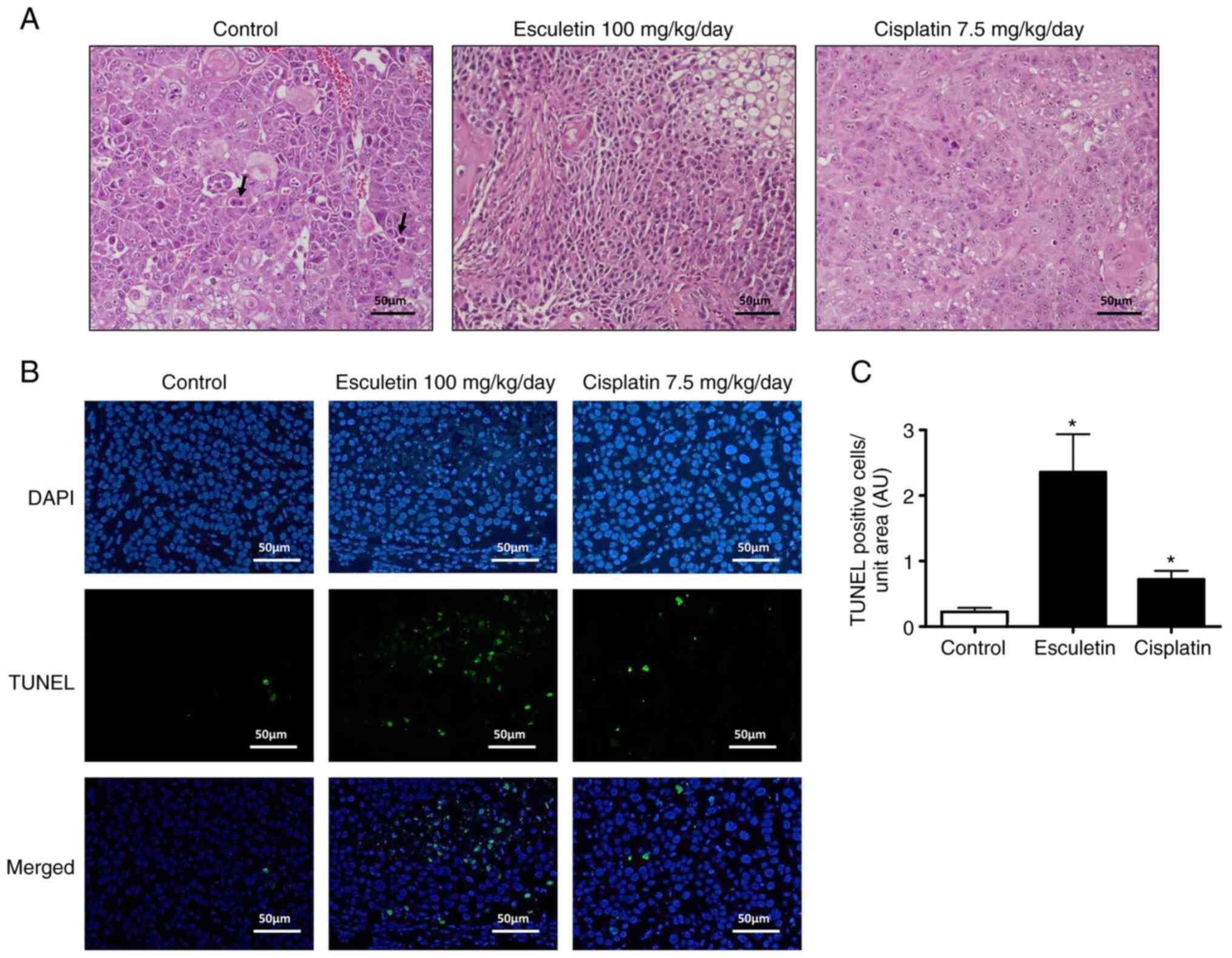
Based on H&E staining, the xenograft tumor
tissues of the control group showed vigorous growth, irregular
morphology, nuclear hypertrophy, numerous abnormal mitoses, overt
dysplasia, giant tumor cells and increased tumor angiogenesis
(Fig. 6A). However, the esculetin
group rarely showed necrotic lesions, with small nuclei, decreased
dysplasia and reduced tumor angiogenesis. Notably, these
tumor-related histopathological changes were not suppressed in the
cisplatin-treated group. To detect apoptotic cells in tumor
tissues, TUNEL analysis was performed, as shown in Fig. 6B and C. The treated group exhibited more
TUNEL-positive cells than the control group, which aligns with the
in vitro results. Hence, induction of apoptotic cells and
inhibition of cell proliferation could occur in an A253 cell
xenograft model using esculetin. Overall, both in vitro and
in vivo analyses suggested that esculetin induces apoptosis
in submandibular salivary gland tumors.
Discussion
The proliferation, migration and survival of
carcinoma cells can occur through several pathways, such as the
RAS/RAF/ERK, PI3K and JAK/STAT pathways (25). Some chemotherapy drugs, including
cisplatin, 5-fluorouracil, docetaxel, methotrexate, bleomycin and
cetuximab, are considered targeted agents. In addition, various
signals, such as those of VEGFR, EGFR, tyrosine protein kinase Met
and insulin-like growth factor 1 (IGF-1) receptor, have been
considered as therapeutic molecular targets (26). Cetuximab is the only EGFR-targeting
agent approved by the Food and Drug Administration in the United
States, but has insignificant effects due to intrinsic or acquired
resistance, despite ubiquitous EGFR expression in HNSCC tumors
(26).
In the present study, the antiproliferative and
pro-apoptotic activities of esculetin against an HNSCC tumor cell
line were investigated using in vitro and in vivo
examinations. Based on recent studies, esculetin exerts antitumor
activity in several cancer types. For example, esculetin (20-100
µM) inhibits the proliferation and expression of cyclin D1,
cyclin-dependent kinase 4 and matrix metalloproteinase-2, and
prevents the production of transforming and vascular endothelial
growth factors in osteosarcoma LM8 cells (27). Esculetin also induces gastric
cancer MGC-803 cell apoptosis by triggering the activation of the
mitochondrial apoptotic pathway; this is linked to the
mitochondrial membrane potential reduction, Bax/Bcl-2 ratio
increase, agitation of the activities of caspase-3 and caspase-9,
and enhancement of released cytochrome c from the
mitochondria (28). Esculetin
prevents cancer cell proliferation and induces the apoptosis of
gastric cancer cells, which is mediated by the mitochondrial
apoptosis pathway involving IGF-1/PI3K/Akt (28). Similarly, esculetin enhances the
activities of caspase-3 and caspase-9, and promotes Bax expression,
Bcl-2 expression reduction, mitochondrial membrane potential
collapse, cytochrome c release and IGF-1/PI3K/Akt
inactivation in SMMC-7721 cells (29). Lee et al (30) reported that the Wnt pathway could
be a versatile therapeutic and suppressive target for various human
cancer types. The β-catenin-T-cell complex factor, a key canonical
Wnt signaling mediator, has been implicated in the development of
human colon cancer. Esculetin, with its small molecular structure,
is considered an effective Wnt/β-catenin pathway inhibitor. In
another study, esculetin was reported to act as an effective lead
chemotherapy agent for human metastatic colorectal cancer
management; this can be linked to the ability of esculetin to
prevent E-cadherin-mediated Wnt signaling via Axin2 inhibitors
(31).
In several cancer cell lines, esculetin is known to
inhibit cell proliferation and induce apoptosis or cell cycle
arrest. Several studies have shown that esculetin inhibits lung
cancer cell growth by adversely affecting c-Myc, cyclin D1 and
NF-κB (32). Esculetin also causes
Akt phosphorylation suppression and enhances protein expression of
tumor suppressor phosphatase and tensin homologs by arresting the
G1 phase of the cell cycle (33). Esculetin markedly inhibits STAT3
phosphorylation, blocks translocation of STAT3 to the nucleus and
restricts the G1/S phase of the cell cycle in laryngeal
cancer (34). A Bax/Bcl-2 ratio
increase, caspase-3 and -9 activation, and mitochondria-mediated
apoptosis pathway induction have been observed in hepatocellular
carcinoma cells following the application of this therapy (35). Furthermore, 2-aminoethoxydiphenyl
borate-sensitive store-operated Ca2+ entry,
Ca2+ release from the endoplasmic reticulum and
activation of the mitochondrial apoptosis pathway result in
Ca2+ influx via esculetin, ultimately leading to cell
cycle arrest in ZR-75-1 human breast cancer (36).
Previously, esculetin was revealed to play an
anti-proliferative role in head and neck cancer (13). The induction of apoptosis was
caused by the inhibition of the specific protein 1 transcription
factor (Sp1) (22), as well as
modulation regulation of the p27, p21 and cyclin D1 target genes in
OSCC, HSC-4 and HN22, and in human malignant melanoma cell lines
(37). Sp1 is an essential
transcription factor for a number of genes required for the
regulation of multiple aspects of tumor cell survival, growth and
angiogenesis (38). Previous
studies have demonstrated that Sp1 is a drug target, and several
antineoplastic agents have been shown to inhibit Sp1 expression
(39). Sp1 is involved in the
regulation of apoptosis. In fact, the promoters of a number of
anti-apoptotic genes (bcl-2, bcl-x and survivin) and pro-apoptotic
genes (bax, trail, fas, fas-ligand, caspase-9 and caspase-3)
contain Sp1-binding sites (40).
The inhibition of Sp1 expression regulates these genes. Indeed,
inhibition of Sp1-DNA binding was shown to induce caspase
9-dependent apoptosis in bone marrow stromal cells (41). The inhibition of Sp1 by esculetin
may lead to an increase in levels of Bax, cleaved caspase-3,
cleaved-9 and cleaved PARP, and a decrease in Bcl-2 anti-apoptotic
protein levels in A253 cells. Although the present study did not
provide concrete scientific evidence of the effect of esculetin on
the tumorigenesis of A263 cells, the observed results were
consistent with its reported effects on various tumors.
The antitumor activity of esculetin was compared
with that of cisplatin, which is a well-known anticancer drug. As
previously reported, cisplatin is a positive control drug that
reduces tumor size (42). In the
present study, esculetin displayed more potent antitumor activity
than cisplatin. In fact, cisplatin did not suppress A253 tumor
growth (P>0.05). Similarly, in previous studies, the tumor
growth of Cal-27 human head and neck squamous cell carcinoma cells
did not show any growth delay when treated with cisplatin
(P>0.05), whereas that of FaDu human head and neck squamous cell
carcinoma cells showed a slight but significant growth delay when
treated with cisplatin (P<0.01) (43). These results suggest that the
antitumor activity of cisplatin varies depending on the head and
neck carcinoma cell line used. Notably, the clinical use of
cisplatin is often limited by undesirable side effects, such as
severe nephrotoxicity and hepatotoxicity (44). Although the precise mechanism for
this cisplatin-induced toxicity is not well understood, cisplatin
is preferentially taken up and accumulates in the human liver and
kidney cells, resulting in enhanced production of reactive oxygen
species and a decrease in levels of antioxidant enzymes (45,46).
In the in vitro system of the present study,
the effective dose of esculetin to induce apoptosis in A253 cells
was as low as 50 µM, whereas the maximum dose was 150 µM. In
several previous reports, esculetin (50 and 100 mg/kg/day)
dose-dependently inhibited xenograft tumor growth in laryngeal
cancer cells (33). Zhu et
al (32) reported that
esculetin (100 mg/kg/day) suppressed tumor growth in a mouse model
of lung cancer. Based on these reports, the oral dosage of
esculetin (100 mg/kg/day) in mice was determined. In the in
vivo investigation, 150 mg/kg/day of esculetin was identified
as the most influential dose. In the present study, there was no
difference in body weight between the esculetin-treated and
vehicle-treated mice. Injecting 700 mg/kg esculetin per day was
suggested to have no adverse effects on the body weight of the mice
(34). Esculetin had a median
lethal dose (LD50) of 1,450 mg/kg via intraperitoneal
injection and >2,000 mg/kg by oral gavage (47). Based on the guidelines of the
Organization for Economic Cooperation and Development, an
LD50 >2,000 mg/kg indicates the safety of the applied
compound (48). Collectively,
these results suggest that esculetin has relatively low toxicity in
mice, highlighting the safety of esculetin for medical
applications.
In the present study, the antiproliferative and
pro-apoptotic activities of esculetin against human submandibular
salivary gland tumor cells were evaluated. However, the
antiproliferative activity of cisplatin was not evaluated in
vitro, thereby serving as a study limitation. Previously, Lee
et al (49) reported that
the IC50 value of cisplatin on the proliferation of A253
cells was ~6.7 µM. In the present study, multiple oral doses of
esculetin were required to determine the effective dose range in
the animal experiment, which served as another limitation of the
study. Therefore, the detailed beneficial role of esculetin in
submandibular salivary gland tumor cells requires further
study.
Overall, the in vitro and in vivo
experiments of the present study confirmed the apoptosis and cell
proliferation inhibitory effects of esculetin on human
submandibular salivary gland tumors. These findings clearly
indicate that esculetin may be a promising treatment option for
HSNCC.
Supplementary Material
Original blot images for Fig. 2A.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a National Research
Foundation of Korea and funded by the Korean government (MEST;
grant no. NRF-2019R1A2C1008773).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SBP, WKJ, HRK, HYY and YHK performed the
experiments, collected data and wrote the manuscript. SBP and JK
analyzed the data and wrote the manuscript. SBP and JK confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of the Jeonbuk National
University Hospital, Jeonju, South Korea (approval no. JBNUH
2021-019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Laurie SA and Licitra L: Systemic therapy
in the palliative management of advanced salivary gland cancers. J
Clin Oncol. 24:2673–2678. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bell RB, Dierks EJ, Homer L and Potter BE:
Management and outcome of patients with malignant salivary gland
tumors. J Oral Maxillofac Surg. 63:917–928. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lima RA, Tavares MR, Dias FL, Kligerman J,
Nascimento MF, Barbosa MM, Cernea CR, Soares JR, Santos IC and
Salviano S: Clinical prognostic factors in malignant parotid gland
tumors. Otolaryngol Head Neck Surg. 133:702–708. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guzzo M, Locati LD, Prott FJ, Gatta G,
McGurk M and Licitra L: Major and minor salivary gland tumors. Crit
Rev Oncol Hematol. 74:134–148. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kaplan MJ, Johns ME and Cantrell RW:
Chemotherapy for salivary gland cancer. Otolaryngol Head Neck Surg.
95:165–170. 1986.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lagha A, Chraiet N, Ayadi M, Krimi S,
Allani B, Rifi H, Raies H and Mezlini A: Systemic therapy in the
management of metastatic or advanced salivary gland cancers. Head
Neck Oncol. 4(19)2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Larson DL, Lindberg RD, Lane E and
Goepfert H: Major complications of radiotherapy in cancer of the
oral cavity and oropharynx. A 10 year retrospective study. Am J
Surg. 146:531–536. 1983.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pendleton KP and Grandis JR:
Cisplatin-based chemotherapy options for recurrent and/or
metastatic squamous cell cancer of the head and neck. Clin Med
Insights Ther. 2013(10.4137/CMT.S10409)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chang WS, Lin CC, Chuang SC and Chiang HC:
Superoxide anion scavenging effect of coumarins. Am J Chin Med.
24:11–17. 1996.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Masamoto Y, Ando H, Murata Y, Shimoishi Y,
Tada M and Takahata K: Mushroom tyrosinase inhibitory activity of
esculetin isolated from seeds of Euphorbia lathyris L.
Biosci Biotechnol Biochem. 67:631–634. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Egan D, O'Kennedy R, Moran E, Cox D,
Prosser E and Thornes RD: The pharmacology, metabolism, analysis,
and applications of coumarin and coumarin-related compounds. Drug
Metab Rev. 22:503–529. 1990.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kok SH, Yeh CC, Chen ML and Kuo MY:
Esculetin enhances TRAIL-induced apoptosis through DR5 upregulation
in human oral cancer SAS cells. Oral Oncol. 45:1067–1072.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin WL, Wang CJ, Tsai YY, Liu CL, Hwang JM
and Tseng TH: Inhibitory effect of esculetin on oxidative damage
induced by t-butyl hydroperoxide in rat liver. Arch Toxicol.
74:467–472. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Okada Y, Miyauchi N, Suzuki K, Kobayashi
T, Tsutsui C, Mayuzumi K, Nishibe S and Okuyama T: Search for
naturally occurring substances to prevent the complications of
diabetes. II. Inhibitory effect of coumarin and flavonoid
derivatives on bovine lens aldose reductase and rabbit platelet
aggregation. Chem Pharm Bull (Tokyo). 43:1385–1387. 1995.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang CJ, Hsieh YJ, Chu CY, Lin YL and
Tseng TH: Inhibition of cell cycle progression in human leukemia
HL-60 cells by esculetin. Cancer Lett. 183:163–168. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Noguchi M, Kitagawa H, Miyazaki I and
Mizukami Y: Influence of esculetin on incidence, proliferation, and
cell kinetics of mammary carcinomas induced by
7,12-dimethylbenz[a]anthracene in rats on high- and low-fat diets.
Jpn J Cancer Res. 84:1010–1014. 1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Osman AM, Telity SA, Telity SA, Damanhouri
ZA, Al-Harthy SE, Al-Kreathy HM, Ramadan WS, Elshal MF, Khan LM and
Kamel F: Chemosensitizing and nephroprotective effect of
resveratrol in cisplatin-treated animals. Cancer Cell Int.
15(6)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tikoo K, Kumar P and Gupta J:
Rosiglitazone synergizes anticancer activity of cisplatin and
reduces its nephrotoxicity in 7, 12-dimethyl benz{a}anthracene
(DMBA) induced breast cancer rats. BMC Cancer.
9(107)2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cho JH, Shin JC, Cho JJ, Choi YH, Shim JH
and Chae JI: Esculetin (6,7-dihydroxycoumarin): A potential cancer
chemopreventive agent through suppression of Sp1 in oral squamous
cancer cells. Int J Oncol. 46:265–271. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Park C, Jin CY, Kim GY, Choi IW, Kwon TK,
Choi BT, Lee SJ, Lee WH and Choi YH: Induction of apoptosis by
esculetin in human leukemia U937 cells through activation of JNK
and ERK. Toxicol Appl Pharmacol. 227:219–228. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sakagami H: Apoptosis-inducing activity
and tumor-specificity of antitumor agents against oral squamous
cell carcinoma. Jpn Dent Sci Rev. 46:173–187. 2010.
|
|
25
|
Alsahafi E, Begg K, Amelio I, Raulf N,
Lucarelli P, Sauter T and Tavassoli M: Clinical update on head and
neck cancer: Molecular biology and ongoing challenges. Cell Death
Dis. 10(540)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wen Y and Grandis JR: Emerging drugs for
head and neck cancer. Expert Opin Emerg Drugs. 20:313–329.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kimura Y and Sumiyoshi M: Antitumor and
antimetastatic actions of dihydroxycoumarins (esculetin or
fraxetin) through the inhibition of M2 macrophage differentiation
in tumor-associated macrophages and/or G1 arrest in tumor cells.
Eur J Pharmacol. 746:115–125. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang G, Lu M, Yao Y, Wang J and Li J:
Esculetin exerts antitumor effect on human gastric cancer cells
through IGF-1/PI3K/Akt signaling pathway. Eur J Pharmacol.
814:207–215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li J, Li S, Wang X and Wang H: Esculetin
induces apoptosis of SMMC-7721 cells through
IGF-1/PI3K/Akt-mediated mitochondrial pathways. Can J Physiol
Pharmacol. 95:787–794. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee SY, Lim TG, Chen H, Jung SK, Lee HJ,
Lee MH, Kim DJ, Shin A, Lee KW, Bode AM, et al: Esculetin
suppresses proliferation of human colon cancer cells by directly
targeting β-catenin. Cancer Prev Res (Phila). 6:1356–1364.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim WK, Byun WS, Chung HJ, Oh J, Park HJ,
Choi JS and Lee SK: Esculetin suppresses tumor growth and
metastasis by targeting Axin2/E-cadherin axis in colorectal cancer.
Biochem Pharmacol. 152:71–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu X, Gu J and Qian H: Esculetin
attenuates the growth of lung cancer by downregulating Wnt targeted
genes and suppressing NF-κB. Arch Bronconeumol (Engl Ed).
54:128–133. 2018.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
33
|
Turkekul K, Colpan RD, Baykul T, Ozdemir
MD and Erdogan S: Esculetin inhibits the survival of human prostate
cancer cells by inducing apoptosis and arresting the cell cycle. J
Cancer Prev. 23:10–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang G, Xu Y and Zhou HF: Esculetin
inhibits proliferation, invasion, and migration of laryngeal cancer
in vitro and in vivo by inhibiting janus kinas (JAK)-signal
transducer and activator of transcription-3 (STAT3) activation. Med
Sci Monit. 25:7853–7863. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang J, Lu ML, Dai HL, Zhang SP, Wang HX
and Wei N: Esculetin, a coumarin derivative, exerts in vitro and in
vivo antiproliferative activity against hepatocellular carcinoma by
initiating a mitochondrial-dependent apoptosis pathway. Braz J Med
Biol Res. 48:245–253. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chang HT, Chou CT, Lin YS, Shieh P, Kuo
DH, Jan CR and Liang WZ: Esculetin, a natural coumarin compound,
evokes Ca(2+) movement and activation of Ca(2+)-associated
mitochondrial apoptotic pathways that involved cell cycle arrest in
ZR-75-1 human breast cancer cells. Tumour Biol. 37:4665–4678.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liang C, Ju W, Pei S, Tang Y and Xiao Y:
Pharmacological activities and synthesis of esculetin and its
derivatives: A mini-review. Molecules. 22(387)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chu S and Ferro TJ: Sp1: Regulation of
gene expression by phosphorylation. Gene. 348:1–11. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Safe S, Imanirad P, Sreevalsan S, Nair V
and Jutooru I: Transcription factor Sp1, also known as specificity
protein 1 as a therapeutic target. Expert Opin Ther Targets.
18:759–769. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Louie JS, Shukla R, Richards-Kortum R and
Anandasabapathy S: High-resolution microendoscopy in
differentiating neoplastic from non-neoplastic colorectal polyps.
Best Pract Res Clin Gastroenterol. 29:663–673. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shirmanova MV, Druzhkova IN, Lukina MM,
Dudenkova VV, Ignatova NI, Snopova LB, Shcheslavskiy VI, Belousov
VV and Zagaynova EV: Chemotherapy with cisplatin: Insights into
intracellular pH and metabolic landscape of cancer cells in vitro
and in vivo. Sci Rep. 7(8911)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Simons AL, Fath MA, Mattson DM, Smith BJ,
Walsh SA, Graham MM, Hichwa RD, Buatti JM, Dornfeld K and Spitz DR:
Enhanced response of human head and neck cancer xenograft tumors to
cisplatin combined with 2-deoxy-D-glucose correlates with increased
18F-FDG uptake as determined by PET imaging. Int J Radiat Oncol
Biol Phys. 69:1222–1230. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chirino YI, Hernández-Pando R and
Pedraza-Chaverri J: Peroxynitrite decomposition catalyst
ameliorates renal damage and protein nitration in cisplatin-induced
nephrotoxicity in rats. BMC Pharmacol. 4(20)2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Stewart DJ, Benjamin RS, Luna M, Feun L,
Caprioli R, Seifert W and Loo TL: Human tissue distribution of
platinum after cis-diamminedichloroplatinum. Cancer Chemother
Pharmacol. 10:51–54. 1982.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mora Lde O, Antunes LM, Francescato HD and
Bianchi Mde L: The effects of oral glutamine on cisplatin-induced
nephrotoxicity in rats. Pharmacol Res. 47:517–522. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tubaro A, Del Negro P, Ragazzi E, Zampiron
S and Della Loggia R: Anti-inflammatory and peripheral analgesic
activity of esculetin in vivo. Pharmacol Res Commun. 20 (Suppl
5):S83–S85. 1988.PubMed/NCBI View Article : Google Scholar
|
|
48
|
OECD: Test No. 423: Acute Oral
toxicity-Acute Toxic Class. Method. In: OECD Guidelines for the
Testing of Chemicals, Section 4. OECD Publishing, Paris, 2002.
|
|
49
|
Lee JH, Hwang EH and Lee SR: A study on
the radiosensitivity and chemosensitivity of A-253 cell line in
vitro. J Korean Acad Oral Maxillofac Radiol. 27:91–104. 1997.
|