1. Introduction
General features of GMA
Adsorptive granulocyte and monocyte apheresis (GMA)
is an extracorporeal apheresis technique that selectively removes
about 65% of activated granulocytes and 55% of
monocytes/macrophages with a small number of platelets, from the
peripheral blood. Depletion of these activated cells could be
considered a valid treatment option for several immune-related
diseases, which do not respond to conventional treatments. Indeed,
inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-8,
IL-23, tumor necrosis factor (TNF)-α and others, are mostly
produced by myeloid lineage leukocytes (1). GMA is usually performed once a week
for 5-10 time sessions in a clinical setting, depending on the
severity of the patient's disease and their response to treatment
(2). This technique consists of a
column filled with cellulose acetate (CA) beads, which selectively
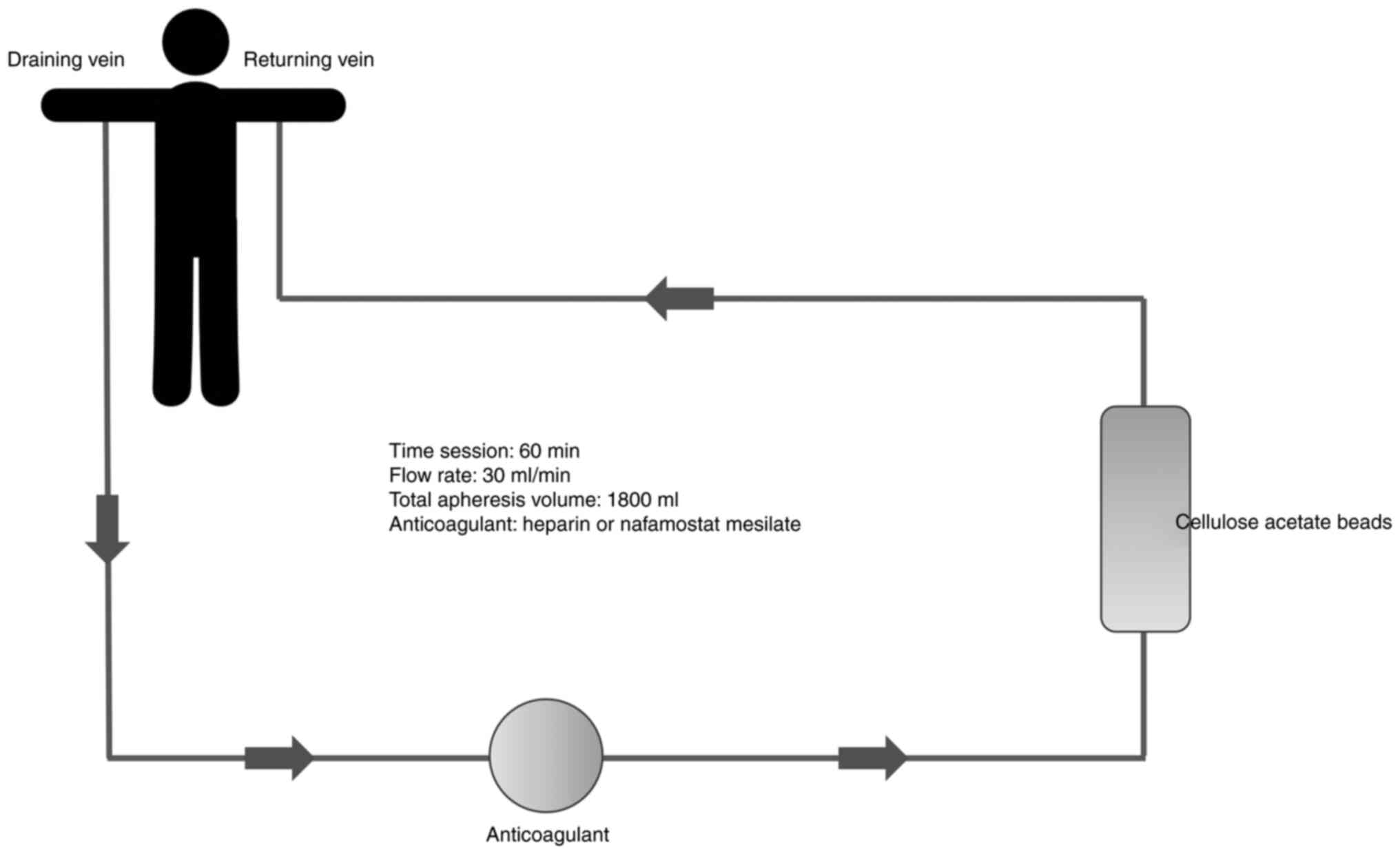
deplete activated myeloid lineage leukocytes (1). In each session of 60 min, 1,800 ml of
blood is drained from the cubital vein of one arm, at a flow rate
of 30 ml/min, through the GMA column and returned to the cubital
vein of the contralateral arm (3).
Anticoagulants must be used in patients undergoing GMA therapy,
either nafamostat mesylate or heparin (4) (Fig.
1).
The first application of GMA was to treat ulcerative
colitis (UC), although nowadays it is applicable in several skin
diseases. These include generalized pustular psoriasis (GPP),
pyoderma gangrenosum (PG), palmoplantar pustular psoriasis (PPP),
Behcet's disease (BD), Sweet's syndrome (SS), adult-onset Still's
disease (AOSD), impetigo herpetiformis (IH), reactive arthritis
(ReA), PG, acne and hidradenitis suppurativa (PASH) syndrome,
cutaneous allergic vasculitis (CAV) and systemic lupus
erythematosus (SLE) (1). This
therapeutical device has been increasingly used in dermatology in
recent years due to its non-pharmacological feature, which makes
GMA suitable for various settings and patients, especially with
chronic disorders.
Mechanism of action
GMA has several effects, including granulocyte
removal, reducing levels of pro-inflammatory cytokines, promoting
anti-inflammatory molecules and stimulating specific cells with an
immunomodulatory role, such as myeloid-derived suppressor cells
(MDSC) and regulatory B-cells and T-cells. Nevertheless, not all
the specific mechanisms of this apheresis technique have been
completely understood yet (3,5).
Selective granulocytes/monocytes
removal
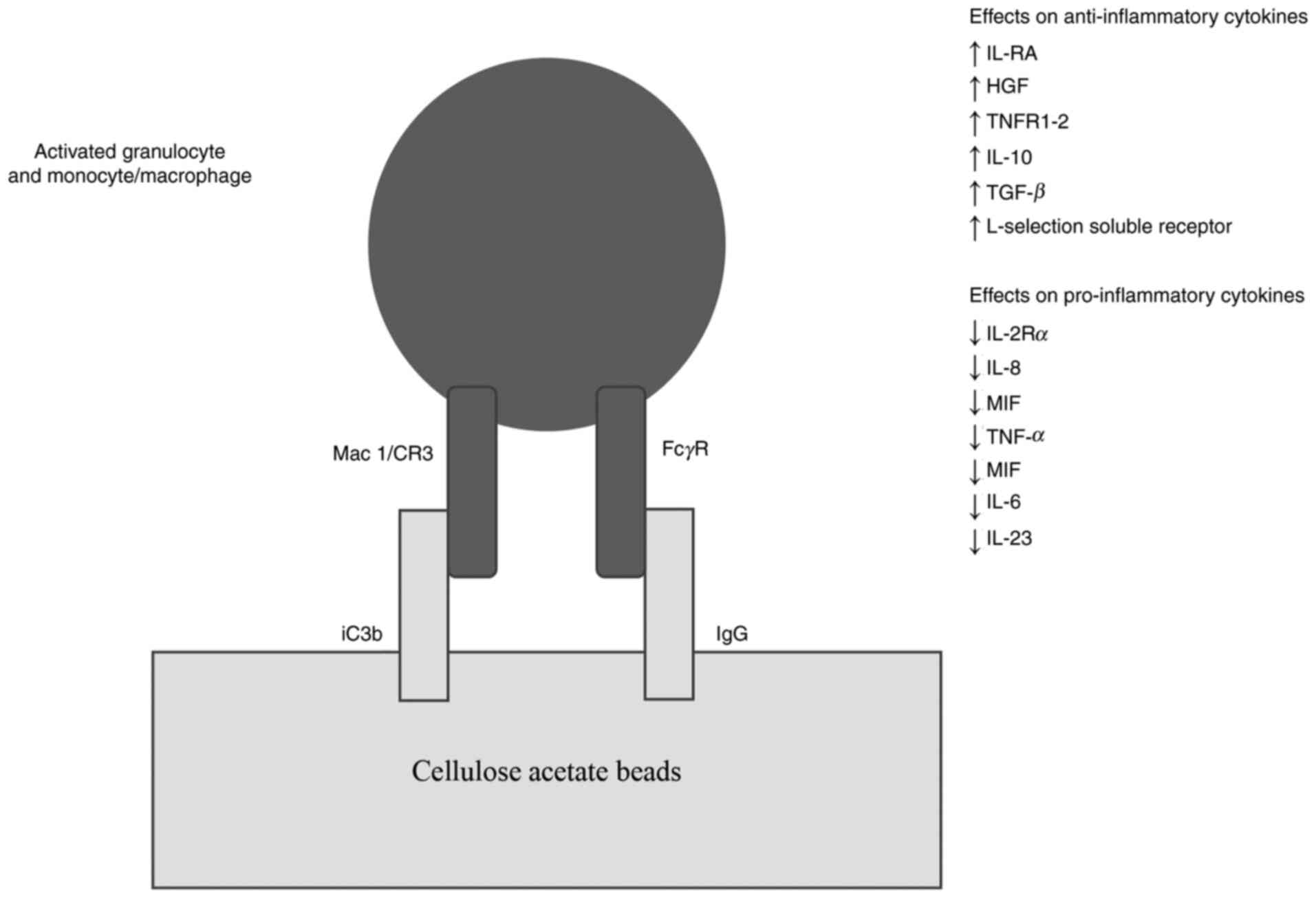
Activated granulocytes and monocytes/macrophages
express Mac-1, a cell-surface adhesive molecule that belongs to the
integrin family. CA beads in the GMA column activate and absorb
complement component iC3b, a ligand for Mac-1. Thus, the column
selectively traps activated granulocytes by binding Mac-1 expressed
on the granulocytes to iC3b on the beads (2). Furthermore, immunoglobulin G (IgG) on
the CA beads mediates the adsorption through Fcγ receptors on the
myeloid lineage cells (Fig. 2).
GMA also removes CD11b+ activated neutrophils, reducing their
infiltration into the inflamed regions (5,6).
Yokoyama et al observed a decrease in peripheral CD14(+)
CD16(+) monocytes (pro-inflammatory phenotype) and an increase in
circulating levels of the CD4+ CD25high+/FOXP3 phenotype
(functional regulatory T cells) in patients' blood after GMA
treatment. This effect is significant since T regs actively
suppress inflammatory responses by producing anti-inflammatory
cytokines such as IL-10, IL-35 and transforming growth factor
(TGF)-β (7). Despite removing
granulocytes, peripheral leukocyte count after the entire process
remains basically unchanged. In addition, it was noticed a
significant reduction in CD10+ (mature and activated) and an
increase in CD10-(immature, naive) granulocytes, provided by the
bone marrow, which are physiologically less inflammatory (1,6).
Effects on pro-inflammatory and
anti-inflammatory cytokines
In vitro studies on whole human blood showed
that activated granulocytes and monocytes produce high levels of
IL-1 Receptor Antagonist (IL-1Ra), Hepatocyte Growth Factor (HGF),
soluble TNF Receptors I e II and L-selectin soluble receptor. The
quantities of these anti-inflammatory cytokines were directly
proportional to the number of cells that adhered to the carriers;
when these cytokines reach the patient's circulation, they
contribute to resolve inflammation (1). GMA also reduces the levels of IL-2Rα,
IL-8 and Macrophage Migration Inhibitory Factor (MIF), a soluble
lymphokine. This effect is significant because the IL-2/IL-2Rα axis
is crucial for T cells differentiation and expansion while IL-8 is
a potent chemotactic factor for granulocytes. MIF regulates the
migration of macrophages and promotes the pro-inflammatory function
of immune cells. In patients with Inflammatory Bowel Disease (IBD),
GMA also contributes in regulating the inflammatory process by
decreasing the levels of TNF-α, IL-1β, IL-6(2). A recent in vitro study by
Nishise et al, showed that CA beads inhibit IL-23, released
from adsorbed granulocytes and monocytes (Fig. 2). IL-23 may promote the increase of
IL-17, which is involved in the pathogenesis of various autoimmune
diseases, autoinflammation and malignant neoplasms (8).
Stimulation of immunomodulatory
cells
As explained previously, iC3b is selectively
absorbed by the GMA column. iC3b, as a complement activation
fragment, contributes to the development of MDSC. This kind of
cells are involved in an immunomodulatory activity: they can
express immunosuppressive molecules like Arginase 1, Inducible
Nitric Oxide Synthase (iNOS), IL-10 and TGFβ, and they promote the
differentiation of CD4+ T cells to T regs and suppress T cell
response. T regs migrate from peripheral blood to local tissue to
solve inflammation. Finally, the contact between neutrophils and CA
beads stimulates the production of apoptotic cells, which re-enter
the patient's bloodstream and raise the levels of regulatory
B-cells (1,3).
Safety profile
One of the most important features of GMA is its
safety profile (5). Data from
clinical practice confirmed that no serious adverse events have
been observed in patients treated with GMA. Domènech et al
(1) have shown that more than a
half of the reported events were related to the difficulty in
performing blood access and adequate flow rate, elevation of venous
pressure, coagulation and blood return problems. The most common
clinical adverse events reported by patients were just mild ones,
such as headache, fever, feeling of weakness and chills. Although
GMA is a therapy targeting neutrophils, increased risk of infection
has never been raised, since it does not cause any immunodeficiency
(1). In conclusion, patients'
perceptions about this technique are overall positive due to the
procedure's convenience, as reported by Rodriguez-Lago et al
(9).
GMA vs conventional therapies:
effectiveness, cost and safety
Sparse studies are available in Europe comparing GMA
with conventional medications. Tominaga et al (10) underlined the equivalent efficacy of
GMA to the corticosteroids in UC patients. Nevertheless, the
authors observed minor safety concerns, reported as adverse events,
in GMA (P<0.001) and a better safety profile albeit a major
average medical cost (P<0.05). In another study by Panés et
al (11), the average annual
cost per patient with UC treated with corticosteroids was 6740
euros and with 5 GMA sessions was 6959 euros. Moreover, the
proportion of patients achieving clinical remission with GMA was
22.5% higher. Overall, a new course of corticosteroids and surgery
was avoided in 18.5 and 4% of patients treated with GMA,
respectively. Yoshino et al (12) described two groups of UC patients
positive for Cytomegalovirus (CMV) after antiviral therapy. In the
first group, 11 patients were treated with GMA, while the second
group of 9 subjects took immunosuppressive therapies (IMT). As a
result, 54.5% (6/11) of the GMA group achieved clinical remission,
against 44.4% (4/9) of the IMT group. GMA did not induce CMV
reactivation because it removes granulocytes and
monocytes/macrophages, which CMV infects latently. In conclusion,
the authors showed that GMA is safe and effective for this class of
patients. To our knowledge, no studies comparing GMA and
conventional treatments in dermatological diseases have been
published.
2. Application of GMA in neutrophilic
dermatoses
Neutrophilic dermatoses consist of a heterogeneous
group of inflammatory skin conditions, characterized by the
presence of a non-infectious infiltrate of mature neutrophilic
leukocytes on histopathology. Clinical cutaneous features are
heterogeneous, including vesiculo-pustules, papules, plaques,
nodules, or ulcerations. In some cases, there could also be an
extracutaneous involvement (13,14).
Generalized Pustular Psoriasis
Psoriasis is an immune-mediated, chronic,
inflammatory disease, defined by keratoderma and hyperproliferation
of keratinocytes. Its pathogenesis primarily involves T helper
lymphocytes and their related cytokines, although neutrophils could
be variably expressed in psoriasis lesions, contributing to its
definition (15,16) There are five types of psoriasis:
psoriasis vulgaris, arthropathic psoriasis, psoriatic erythroderma,
guttate psoriasis, pustular psoriasis (17). GPP is an unusual and severe variant
of pustular psoriasis, clinically characterized by sterile pustules
overlying painful, erythematous skin (18). Histologically, pustular psoriasis
has spongiform pustules of Kogoj into the epidermis, formed by
neutrophil infiltration. Moreover, the epidermis is characterized
by an absent granular layer, parakeratosis, Munro's microabscesses,
suprapapillary thinning and psoriasiform hyperplasia, while the
dermis is characterized by dilated blood vessels with fewer
neutrophils (18). Triggering
factors of GPP include pyrogallic acid, infections, pregnancy,
drugs, hypocalcemia (19). The
mechanisms underlying the pathogenesis of GPP currently remain
unclear (11). Certain monogenic
autoinflammatory disorders clinically present as generalized
variants of pustular psoriasis (CAMPS- CARD14-mediated
pustular psoriasis-DIRA, DITRA-deficiency of IL-36 receptor
antagonist-, those related to mutations in AP1S3) (18). First-line treatment options for
adult GPP include retinoids, cyclosporine (CsA) and methotrexate
(MTX). Second-line therapies include adalimumab (ADA), etanercept,
topical agents and phototherapy. More recently, GMA has been
employed in cases recalcitrant to other medications, or in special
populations, such as very young or old patients, pregnant and those
infected with hepatitis (18).
Kanekura et al (20) showed
that pustular psoriasis was dramatically ameliorated by GMA
therapy, while psoriasis vulgaris responded minimally to this
treatment. This aspect may be due to fewer infiltrated neutrophils
in this pathology. In the largest study, Ohnishi et al
(21) described 22 patients
treated with GMA: 16 patients obtained effective response after the
whole treatment, 5 patients maintained an unchanged condition and
only in one case there was a worsening of the disease. Moreover,
the majority of these subjects took concomitant therapies. Filosa
and Filosa (13) observed that
very few patients reported slight side effects (e.g. headache,
dizziness, light headedness on standing, chills and feeling of
weakness); one patient developed an allergic reaction to nafamostat
mesylate, while another one developed pemphigoid. However, most of
these effects are related to the use of anticoagulant, which is
indispensable for the procedure (22). Overall, relapse of GPP after a
complete course of therapy was seen in 6 patients. For patients who
had a recurrence or did not respond to regular GMA, further
sessions were disposed, also with an intensive regime (proposing
therapy twice a week), obtaining remission in all patients
(23-25).
Mizutani et al (26)
described two cases in which patients initially received regular
GMA (5 sessions, once a week), and intensive GMA (5 sessions, twice
a week) upon recurrence; the authors observed that increasing the
number of sessions weekly, better results have been achieved in
terms of clinical response. Furthermore, the same authors reported
a case of a patient with GPP, who was pregnant during both regular
and intensive GMA therapy. Her children were born with no anomalies
but with low birth weights and at 33 and 36 weeks of pregnancy
(19). Patients' features and
results of GMA efficacy for treating GPP are shown in Table I (17,20,21,23,25-39).
 | Table IGeneralized pustular psoriasis:
Demographics and clinical course. |
Table I
Generalized pustular psoriasis:
Demographics and clinical course.
| First author | Reporting year | No. of
patients | Age, years | Sex | Response to
GMA | (Refs.) |
|---|
| Kanekura T | 2003 | 1 | 62 | M | Yes | (20) |
| Ohnishi H | 2018 | 22 | 32-78 | 13F, 9M | 16 Yes, 6 No | (21) |
| Shukuya R | 2011 | 2 | 26-68 | 2 F | Yes | (23) |
| Suzuki A | 2012 | 3 | 36-71 | 1F, 2M | Yes | (25) |
| Mizutani Y | 2020 | 2 | 31-77 | 2 F | Yes | (26) |
| Fujii A | 2017 | 1 | 79 | F | Yes | (27) |
| Sakanoue M | 2013 | 4 | 37-59 | 3F, 1M | Yes | (28) |
| Ikeda S | 2013 | 15 | 50-13 | 4F, 11M | 12 Yes, 3 No | (29) |
| Fujii A | 2019 | 1 | 43 | M | Yes | (30) |
| Koike Y | 2017 | 1 | 13 | M | Yes | (31) |
| Shindo E | 2019 | 1 | 34 | F | Yes | (17) |
| Fujisawa T | 2013 | 1 | 60 | F | Yes | (32) |
| Tominaga C | 2015 | 1 | 78 | F | Yes | (33) |
| Fujisawa T | 2011 | 3 | 61-64 | 1F, 2M | Yes | (34) |
| Fujisawa T | 2015 | 3 | 31-63 | 2F, 1M | Yes | (35) |
| Furusawa K | 2012 | 1 | 42 | F | Yes | (36) |
| Mabuchi T | 2014 | 1 | 54 | F | Yes | (37) |
| Seishima M | 2008 | 1 | 44 | F | Yes | (38) |
| Sugiura K | 2014 | 1 | 65 | F | Yes | (39) |
Pyoderma Gangrenosum
PG is an inflammatory disease, clinically
characterized by painful skin ulcerations, especially on lower
legs, with erythematous and undermined borders and histologically
defined by the presence of a neutrophilic infiltrate in the dermis.
PG is mostly associated with UC and Crohn's disease. However, other
possible associations may include rheumatoid arthritis and
hematological malignancies (40).
Five clinical variants are currently recognized: classic or
ulcerative, bullous, pustular, vegetative, and peristomal types
(41). Treatment of PG usually
comprise topical therapy (e.g. topical corticosteroids or topical
tacrolimus), systemic treatment (e.g. oral corticosteroids, CsA,
tacrolimus, colchicine, dapsone, MTX, intravenous immunoglobulin)
and targeted therapy, including TNF-α inhibitors, anti IL-1 and
IL-12/23 antagonists (42).
Alternatively to current existing therapies, GMA is an effective
option with minimal side effects, especially for steroid and
immunosuppressant-resistant PG (43). Forty-nine cases of GMA application
for PG have been summarized in Table
II (28,43-62).
In the main case series, Higashi et al (44) reported a complete response in eight
patients, a nearly complete response in three patients and a
partial response in two patients. Skin lesions remained stable in
four cases, while a disease progression was observed in two cases.
The same authors reported that in 12 patients, treatment outcomes
were assessed 2 months after the final GMA session. During that
post-GMA period, skin ulcers continued to shrink in eight patients
and remained unchanged in three. Sakanoue et al (28) described an excellent response in
three patients and a good response in one patient, all after 10
sessions of GMA, performed weekly. A moderate response was observed
in two patients who underwent 5 time-sessions GMA, once a week.
Russo et al (43) wrote the
first case report in Europe, discussing the application of 10
sessions of GMA, scheduled weekly, for a patient with PG, whose
ulcer started resolving after the 6th treatment. This patient did
not experience any side effects or relapse of the disease during a
6-months follow up period. Moreover, Ikeda et al (45) showed an amelioration in patients'
Dermatology Life Quality Index (DLQI), reflecting better daily
function and quality of life after a course of GMA. Very few side
effects occurred after GMA: Ishikawa et al (47) reported a case of a patient who
developed a mild headache, while Higashi et al (44) described two episodes of non-serious
transient hypertension and a failure in blood drainage for five
patients, for whom however GMA was continued by decreasing the
blood flow rate and changing the limb position. Overall, a relapse
of PG after GMA was seen in four patients.
 | Table IIPyoderma gangrenosum: Demographics
and clinical course. |
Table II
Pyoderma gangrenosum: Demographics
and clinical course.
| First author | Reporting year | No. of
patients | Age, years | Sex | Response to
GMA | (Refs.) |
|---|
| Sakanoue M | 2013 | 6 | 21-76 | 1F, 5M | Yes | (28) |
| Russo I | 2016 | 1 | 73 | M | Yes | (43) |
| Higashi Y | 2021 | 19 | 21-79 | 11F, 8M | 13 Yes, 6 No | (44) |
| Ikeda K | 2011 | 1 | 36 | F | Yes | (45) |
| Ohmori T | 2003 | 1 | 19 | M | Yes | (46) |
| Ishikawa H | 2004 | 1 | 30 | M | Yes | (47) |
| Yoneda K | 2005 | 1 | 39 | F | Yes | (48) |
| Yanar-Fujisawa
R | 2005 | 1 | 31 | F | Yes | (49) |
| Seishima M | 2007 | 1 | 29 | F | Yes | (50) |
| Fujino Y | 2008 | 1 | 55 | F | Yes | (51) |
| Kawakami T | 2009 | 1 | 19 | M | Yes | (52) |
| Doi R | 2010 | 1 | 19 | M | Yes | (53) |
| Kobayashi S | 2011 | 1 | 29 | M | Yes | (54) |
| Ohno M | 2016 | 1 | 36 | F | Yes | (55) |
| Okada M | 2017 | 1 | 71 | F | Yes | (56) |
| Yamashita A | 2017 | 1 | 30 | F | Yes | (57) |
| Tominaga K | 2020 | 1 | 57 | M | Yes | (58) |
| Shibuya T | 2020 | 1 | 50 | F | Yes | (59) |
| Kanekura T | 2005 | 2 | 44-67 | 2M | Yes | (60) |
| Kanekura T | 2002 | 1 | 38 | M | Yes | (61) |
| Kawai M | 2021 | 1 | 18 | F | Yes | (62) |
| Kawakami T | 2009 | 1 | 19 | M | Yes | (52) |
Palmoplantar pustular psoriasis
PPP is a chronic inflammatory disease, clinically
characterized by erythema, scales and sterile pustules on the palms
and soles. There is a higher prevalence between females, especially
those who smoke (63).
Histologically, neutrophilic infiltration destroys epidermal
microarchitecture and after the evacuation of the pus, pustules in
PPP leave a visible cavity behind. When they are not evacuated,
pustules dry up and form brownish scabs that subsequently exfoliate
(19). PPP is usually resistant to
treatment, with high rates of recurrence. A lot of systemic drugs
have been tested, including colchicine, itraconazole, alitretinoin
and biologics (64). Another
therapeutic choice for PPP is GMA, as described by several authors.
In the largest case series, Sakanoue et al (28) performed 5 sessions of GMA, once a
week, resulting in two cases with excellent outcomes, seven cases
with a good response, three with a moderate response and two
patients were unresponsive. None of the patients reported side
effects. Another report by Fujisawa et al (65) described an excellent response in
three patients after 5 sessions of GMA, scheduled weekly. Kawakami
et al (66) assessed the
effect of the GMA at the end of treatment and after 3 months of
follow up. In all patients GMA was conducted once a week, for 5
consecutive weeks. One patient showed a remarkable improvement
immediately after GMA and two patients achieved the same result at
a 3-month follow-up. Deterioration of skin symptoms was noted in
two patients, at the follow up visit. The majority of patients were
treated with several therapies before GMA, without efficacy.
Patients' clinical features and results after therapy are shown in
Table III (28,38,65-67).
 | Table IIIPalmoplantar pustular psoriasis:
Demographics and clinical course. |
Table III
Palmoplantar pustular psoriasis:
Demographics and clinical course.
| First author | Reporting year | No. of
patients | Age, years | Sex | Response to
GMA | (Refs.) |
|---|
| Sakanoue M | 2013 | 14 | 35-77 | 8 F, 6 M | 12 Yes, 2 No | (28) |
| Seishima M | 2008 | 1 | 66 | M | Yes | (38) |
| Fujisawa T | 2014 | 3 | 28-64 | 1 F, 2 M | Yes | (65) |
| Kawakami H | 2019 | 5 | 48-77 | 5 F | 4 Yes, 1 No | (66) |
| Kanekura T | 2004 | 1 | 57 | F | Yes | (67) |
Behcet disease
BD is a multisystem inflammatory chronic disorder,
clinically characterized by recurrent oral and genital aphthosis,
severe uveitis, cutaneous lesions such as erythema nodosum and
pustules, in addition to multi-organ involvement and arthritis. The
majority of patients are from Japan, the Middle East or the
Mediterranean basin and the peak incidence is in the age between 20
and 35(68). HLA-B51 is the allele
with the best-known role in the pathogenesis, even if environmental
triggers and immune cells and cytokines could be involved too
(69). Histological features of
cutaneous lesions could be a leukocytoclastic vasculitis with
fibrinoid necrosis of postcapillary venules, a neutrophilic
vascular reaction, or a lymphocytic perivasculitis (70). Pharmacological agents used to treat
BD include colchicine, dapsone, corticosteroids and
immunosuppressants such as azathioprine (AZA), MTX and CsA. The
efficacy of TNF-α inhibitors in BD has been reported recently. In
pregnant women, these systemic agents raise fetal risks such as
teratogenicity, stillbirth and spontaneous abortion (68). GMA could be a valid treatment
option in BD, since neutrophils are involved in its
etiopathogenesis, as shown in Table
IV (28,71,72).
In their case series, Sakanoue et al (28) performed GMA five times, once a
week, for the majority of patients. After this treatment, two
patients had an excellent response, three a good one, one of them
showed moderate results and in two cases there were no changes,
compared with the beginning. Only one patient underwent GMA for 10
times, weekly, with a final moderate response to therapy. None of
the patients experienced side effects. Different therapies were
prescribed previously to GMA, including colchicine, loxoprofen,
mefenamic acid and prednisolone (PSL). Higashi et al
(71) reported a case of a
39-year-old woman, who was found to be pregnant during the therapy.
She had no complications related to GMA and delivered a healthy
newborn, underlining the lack of negative effects of this therapy.
Finally, Kanekura et al (72) described two patients, a 21-year-old
man and a woman of 50 years, successfully treated with GMA. They
underwent GMA 5 times and 8 times weekly, respectively. Both skin
lesions and pain improved dramatically at the end of the therapy,
and no side effects were experienced.
 | Table IVBehcet disease: Demographics and
clinical course. |
Table IV
Behcet disease: Demographics and
clinical course.
| First author | Reporting year | No. of
patients | Age, years | Sex | Response to
GMA | (Refs.) |
|---|
| Sakanoue M | 2013 | 9 | 18-74 | 8 F, 1M | 7 Yes, 2 No | (28) |
| Higashi Y | 2013 | 1 | 39 | F | Yes | (71) |
| Kanekura T | 2004 | 2 | 21-50 | 1F, 1M | Yes | (72) |
Sweet's syndrome
SS is an acute febrile neutrophilic dermatosis
characterized by different clinical features, including fever,
neutrophilia and tender erythematous skin lesions, asymmetrically
distributed on face, neck and upper extremities. Classical SS has a
worldwide distribution, usually affecting middle-aged women. It may
be associated with infection of the upper respiratory or
gastrointestinal tract, and with IBD (73). Histopathological diagnostic
criteria include a dense neutrophilic infiltrate in the upper
dermis. Occasionally, eosinophiles, lymphocytes or histiocytes may
also be present (73). Systemic
corticosteroids, colchicine and potassium iodide are considered as
first-line treatments for SS. Second-line therapies include
indomethacin, clofazimine, CsA and dapsone. The use of biologic
agents has also been described (74). GMA may be a useful
non-pharmacologic tool for SS, with no safety concerns. Fujii et
al (75) reported a case of a
55-year-old woman affected by SS, previously treated with PSL,
nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine, who
underwent GMA for three times, once a week, showing resolution of
symptoms after the first session. This patient did not have any
relapse of the disease during a 4 month's follow-up. Similarly,
Sakanoue et al (28)
described a 65-year-old male patient for whom GMA was performed 5
times, weekly, with good final response. He was previously treated
with loxoprofen, without efficacy. No adverse effect was reported
in both cases.
Adult-onset Still's disease
AOSD is a systemic inflammatory disorder of unknown
etiology, characterized by a high spiking fever, transient skin
rash, polyarthralgia, and hyperferritinemia. Other frequently
observed clinical features include sore throat, hepatomegaly,
splenomegaly, lymphadenopathy and serositis. Neutrophilic
leukocytosis and granulocytosis are important diagnostic criteria
too. This disease occurs worldwide and usually affects young adults
(76). At present, AOSD
therapeutic strategy aims to prevent organ damage and
life-threatening complications and minimize adverse effects of
treatment. However, therapies of AOSD remain largely empirical,
lacking controlled clinical trials (77). Various drugs including NSAIDs,
corticosteroids, MTX and other disease-modifying anti-rheumatic
drugs (DMARDs) and biologic agents do not always guarantee complete
remission of AOSD (76). Kanekura
et al (78) suggested that
GMA represents a promising treatment modality for AOSD, based on
the positive outcome they obtained in the present case. More
specifically, a 33-year-old woman was treated with GMA for 5 times,
once a week, concomitantly taking corticosteroids and meloxicam.
Both her laboratory findings and symptoms improved a lot after this
treatment, fever decreased remarkably and skin lesions became faint
in color and smaller in size. Furthermore, arthralgia dramatically
improved. No adverse effects were observed and the patient suffered
no relapse for the following 22-month period.
Impetigo Herpetiformis
IH is a rare systemic inflammatory disease occurring
in pregnancy and it is considered as a subtype of GPP. IH's disease
severity ranges from ‘severe’, a state sometimes accompanied by
impaired placental function or electrolyte abnormalities, to
‘mild’, characterized by pustular skin eruptions (79). This condition mostly occurs in the
third trimester of pregnancy and usually resolves after delivery.
However, there is the possibility of recurrence in the following
pregnancies (80). Patients with
IH sometimes experience intrauterine growth restriction (IUGR),
possibly due to lower oxygen and nutrition intake from the inflamed
placenta (79). Conventional
treatment for IH comprises topical steroid application or oral
steroid administration. Second-choices therapeutic options include
CsA, phototherapy and anti TNF-α drugs. Current reports indicate
that GMA is useful in IH treatment to improve skin eruption and
reduce placental inflammation and thus ameliorate IUGR (79,81,82).
Iwasaki A (79) described a case
of a 33-year-old woman with IH at 30 week's gestation of her first
pregnancy. She was previously treated with topical and systemic
steroids, then she performed 2 GMA sessions at 7-day intervals
concomitantly with oral PSL. After the second GMA, skin eruption
improved rapidly and almost resolved. The patient reported no
relapse of the disease in the following months. Another case,
observed by Fujii et al (81), involved a 28-year-old woman at her
first pregnancy. Clinically, she experienced IH at 25 weeks of
gestation and had a homozygous CARD14 mutation. Two GMA
sessions were performed: after the first one, skin lesions
immediately improved and pustules disappeared. Following the second
course, performed after 7 days, the eruption completely
disappeared. In a report by Saito-Sasaki et al (82), a 30-year-old woman affected by IH
at 10 weeks into her fourth pregnancy, was initially treated with
methylprednisolone and CsA. She totally performed 14 courses of
GMA, since IH relapsed twice in the meantime. Once she completed
all the sessions, skin lesions disappeared. None of the three
patients experienced side effects related to GMA therapy.
3. GMA in other skin disorders
Reactive arthritis
A triad of symptoms characterizes ReA, previously
known as Reiter's syndrome: oligoarthritis of large joints,
urethritis in men and cervicitis in women and conjunctivitis,
usually occurring in young adults some weeks after a urogenital or
gastrointestinal infection (83).
Mucocutaneous features, including circinate balanitis, keratoderma
blenorrhagicum, ulcerative vulvitis, nail changes, oral lesions,
are often associated concomitantly or sequentially (84). Because patients test negative for
rheumatoid factor, ReA is classified as a seronegative
spondyloarthropathy. There are cases of familial aggregation of ReA
which may be related to its association with HLA-B27(84). NSAIDs are first-line drugs for the
management of this disease. DMARDs, such as sulfasalazine, are
effective for peripheral manifestations, while most experts
consider glucocorticoids use in ReA contraindicated, except for an
occasional intra-articular injection (83). As ReA is attributable to activated
neutrophils and it is histologically similar to pustular psoriasis
in its prominent neutrophil infiltration, therapy with GMA may be
useful for treating this disease, as reported by Yoshifuku et
al (85). Particularly, the
authors described a case of a 73-year-old man with scaly,
coalescent erythematous macules with ulcers on penis and scrotum
and a scaly erythematous plaque on his right hand. Moreover, he
suffered from lumbar pain and multiple arthralgia, ulcer of the
corneal epithelium and conjunctivitis and sterile urethritis. He
did several treatments before apheresis, including ceftriaxone
sodium, cefepime dihydrochloride, vancomycin hydrochloride and
diclofenac sodium. The thrice-daily use of diclofenac sodium
suppositories, which was required to ease the patient's pain before
GMA therapy, was discontinued one week after the introduction of
GMA. After the treatment, skin lesions improved dramatically and
articular pain decreased. Ocular involvement and urethritis also
ameliorated. The patient experienced no adverse effect related to
GMA.
PASH syndrome
PASH syndrome is a recently proposed disease entity,
belonging to the spectrum of autoinflammatory syndromes, similar to
pyogenic sterile arthritis, pyoderma gangrenosum and acne (PAPA)
syndrome and aseptic abscesses syndrome. However, in contrast to
these two disorders, PASH syndrome shows a clear predilection for
the skin and lacks arthritis and visceral involvement (86). Both PG and hidradenitis suppurativa
(HS), are included in the spectrum of neutrophilic dermatoses. HS
is a chronic-relapsing, debilitating inflammatory disease of the
hair follicles that usually presents after puberty and affects
apocrine gland-bearing skin (most frequently the axillae as well as
the inguinal and anogenital regions). It is clinically
characterized by recurrent, painful, deep-seated nodules that
usually end in abscesses and sinus tracts with suppuration and
hypertrophic scarring (87).
Treatment of PASH syndrome could be challenging. Systemic
glucocorticosteroids, AZA, CsA, dapsone and isotretinoin, may fail
to control the disease satisfactorily. Biologic agents targeting
IL-1 and TNF-α have been recommended to treat PASH syndrome, but
their efficacy has not been well established because of its rarity
(87). The efficacy of GMA on PASH
syndrome has been observed in two case reports. Hatanaka et
al (88) described a woman
with a 15-years history of disease. Different therapies were
prescribed prior to GMA, including systemic and topical
antibiotics, oral PSL, and frequent surgical incisions. For this
patient, GMA was performed twice a week for a total of five
sessions; five additional sessions were scheduled at 7-day
intervals. After the final session, a remarkable improvement of
skin lesions was noticed and no relapse occurred during the
following four years. Mizutani et al (89) reported a case of a male adolescent
with clinical manifestations of PASH syndrome for two years, who
failed to respond to treatments with oral PSL, CsA, dapsone and
minocycline. For this reason, he received GMA sessions weekly for
10 consecutive weeks with a consequent great improvement of his
condition. However, pustule formation did not completely disappear
and thus ADA was administered.
Cutaneous allergic vasculitis
CAV is a disorder characterized by inflammation of
small vessels, especially post capillary venules. Histologically,
blood vessel necrosis is found with fibrinoid material deposits and
inflammatory cellular infiltrate, nuclear dust, and erythrocyte
extravasation (90). The most
common clinical presentation of CAV consists of palpable purpura of
the lower extremities. Less frequently, it reveals itself as
nodular erythema, livedo racemosa, and punched-out ulcers (91). CAV may be idiopathic or may have a
defined cause such as infection, medication, connective tissue
disease, or malignancy. Extracutaneous disease or systemic
vasculitis could also be related (92). An isolated episode of CAV
associated with a known inciting factor may be managed by removal
or treatment of the trigger, along with symptomatic measures.
First-line systemic treatments for chronic, idiopathic CAV include
colchicine or dapsone, used singly or in combination. Recurrent,
chronic, or severely symptomatic CAV that does not respond to the
aforementioned therapies may require initiation of an
immunosuppressive agent such as AZA, mycophenolate mofetil, MTX,
CsA, or rituximab (92). When
these treatments are partially successful, GMA could be considered
a therapeutic option. Kanekura et al (93) described a 49-year-old woman
affected by CAV with intractable leg ulcers, which responded well
to GMA therapy. GMA was carried out five times, once a week,
simultaneously with loxoprofen. Ulcers were covered by regenerated
skin at the end of all five treatment sessions, without relapse
during a 5-months follow up period. No adverse event was reported.
Also, Sakanoue et al (28)
treated a case of CAV performing GMA, with an excellent response at
the end of five sessions, scheduled once a week.
Systemic Lupus Erythematosus
SLE is a chronic autoimmune disease characterized by
the production of autoantibodies directed against nuclear and
cytoplasmic antigens, which may affect any organ. Skin is the most
affected part of the body, especially in areas exposed to light,
and main cutaneous manifestations comprises malar rash and discoid
lesions. Other clinical features include photosensitivity, oral
ulcers, arthritis, serositis, renal findings (persistent
proteinuria, hematuria, cellular casts), neurologic disorders
(seizures or psychosis), hematologic findings (thrombocytopenia,
leukopenia, lymphopenia, or anemia). As far as laboratory findings
concerned, clinicians should test for antinuclear antibodies (ANA),
and if the result is positive, they should search for
antigen-specific ANA, such as those targeting double-stranded DNA
(dsDNA) or ribonucleoprotein complexes (Ro/SSA, La/SSB, Smith, and
RNP), collectively referred to as extractable nuclear antigens. SLE
is more common between adult women, with a higher peak of
prevalence among African Americans. Several medications are used to
treat SLE, including glucocorticoids, antimalarial agents, NSAIDs,
immunosuppressive agents, and B cell-targeting biologics. However,
the most important drug to treat SLE is hydroxychloroquine
(94,95). Kanekura et al (96) posited that SLE patients could also
benefit from GMA treatment. The authors described a male patient of
22-year-old, showing malar and discoid rash, leukocytopenia with
lymphocytopenia, positive antibodies for dsDNA and Smith antigen
(Sm) and ANA. He was previously treated using systemic
corticosteroids. A total of five GMA sessions were scheduled once a
week, without discontinuing patient's therapy with oral PSL. After
the whole treatment, his skin rashes ameliorated dramatically and
laboratory exams too. Demographic features and the therapeutic
response of patients with SS, AOSD, IH, ReA, PASH syndrome, CAV and
SLE are summarized in Table V
(28,75,78,79,81,82,85,88,89,93,96).
 | Table VOther diseases: Demographics and
clinical course. |
Table V
Other diseases: Demographics and
clinical course.
| Disease | First author | Reporting year | No. of
patients | Age (yr) | Sex | Response to
GMA | (Refs.) |
|---|
| SS | Sakanoue M | 2013 | 1 | 65 | M | Yes | (28) |
| | Fujii A | 2017 | 1 | 55 | F | Yes | (75) |
| AOSD | Kanekura T | 2004 | 1 | 33 | F | Yes | (78) |
| IH | Iwasaki A | 2018 | 1 | 33 | F | Yes | (79) |
| | Fujii K | 2020 | 1 | 28 | F | Yes | (81) |
| | Saito-Sasaki N | 2017 | 1 | 30 | F | Yes | (82) |
| ReA | Yoshifuku A | 2011 | 1 | 73 | M | Yes | (85) |
| PASH Syndrome | Hatanaka M | 2021 | 1 | 34 | F | Yes | (88) |
| | Mizutani Y | 2017 | 1 | 18 | M | Yes | (89) |
| CAV | Sakanoue M | 2013 | 1 | 34 | F | Yes | (28) |
| | Kanekura T | 2006 | 1 | 49 | F | Yes | (93) |
| SLE | Kanekura T | 2004 | 1 | 22 | M | Yes | (96) |
4. Conclusion
GMA is considered a promising and innovative
treatment option for skin diseases linked to activated neutrophils
and it represents an effective alternative to currently existing
therapies, with minimal side effects compared to other systemic
therapies. This review collected available publications regarding
the effect of GMA in dermatologic disorders. The majority of
patients underwent GMA treatments five or ten times, once a week,
in relation to the severity of their disease and clinical response.
Concomitant GMA therapy with other drugs (e.g. corticosteroids,
CsA, etretinate) might shorten the time to remission and might
increase the healing rate (62).
Furthermore, GMA is useful to reduce systemic inflammation, not
only to improve skin eruption, but also to reduce placental
inflammation and thus ameliorate IUGR as reported in different
cases of IH in pregnant women, who gave birth to healthy newborns
(79,81,82).
Another advantage of this technique concerns its safety profile, in
contrast to multiple adverse events reported with conventional and
biologic drugs. Despite the higher cost of GMA, compared with
traditional medication, this therapeutical option could be
cost-effective on a long-term perspective, decreasing
hospitalization, surgery and reducing the overall cost of medical
services. More research is needed before GMA would be accepted as
first-line therapy, especially for particular groups of patients,
such as pregnant women, children and adolescents. Moreover, it is
sometimes difficult to estimate the effects of GMA alone since, in
many cases, it was used in combination with other therapies.
Another major issue of most studies is the short follow-up period.
Further trials are required to evaluate GMA's safety and optimal
therapeutic regimens for achieving long-lasting effects. On the
basis of our results, we strongly suggest that this technique is a
valuable choice for patients with intractable steroid and
immunosuppressant-resistant skin diseases attributable to activated
granulocytes. We hope that this non-pharmacological option could be
applied as a first-line treatment to other chronic diseases for
different medical purposes in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LG, GM and MA made substantial contributions to
conception and design, interpretation of data, participated in
drafting the article and gave final approval of the version to be
submitted and any revised version. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Domènech E, Grífols JR, Akbar A and
Dignass AU: Use of granulocyte/monocytapheresis in ulcerative
colitis: A practical review from a European perspective. World J
Gastroenterol. 27:908–918. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kanekura T: Clinical and immunological
effects of adsorptive myeloid lineage leukocyte apheresis in
patients with immune disorders. J Dermatol. 45:943–950.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kanekura T, Hiraishi K, Kawahara K,
Maruyama I and Kanzaki T: Granulocyte and monocyte adsorption
apheresis (GCAP) for refractory skin diseases caused by activated
neutrophils and psoriatic arthritis: Evidence that GCAP removes
Mac-1-expressing neutrophils. Ther Apher Dial. 10:247–256.
2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen XL, Mao JW and Wang YD: Selective
granulocyte and monocyte apheresis in inflammatory bowel disease:
Its past, present and future. World J Gastrointest Pathophysiol.
11:43–56. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cuadrado E: Granulocyte/monocyte apheresis
as immunotherapic tool: Cellular adsorption and immune modulation.
Autoimmun Rev. 8:292–296. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hanai H, Takeda Y, Eberhardson M, Gruber
R, Saniabadi AR, Winqvist O and Lofberg R: The mode of actions of
the Adacolumn therapeutic leucocytapheresis in patients with
inflammatory bowel disease: A concise review. Clin Exp Immunol.
163:50–58. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yokoyama Y, Fukunaga K, Fukuda Y, Tozawa
K, Kamikozuru K, Ohnishi K, Kusaka T, Kosaka T, Hida N, Ohda Y, et
al: Demonstration of low-regulatory CD25High+CD4+ and
high-pro-inflammatory CD28-CD4+ T-Cell subsets in patients with
ulcerative colitis: Modified by selective granulocyte and monocyte
adsorption apheresis. Dig Dis Sci. 52:2725–2731. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nishise S, Abe Y, Nomura E, Sato T, Sasaki
Y, Iwano D, Yoshizawa K, Yagi M, Sakuta K and Ueno Y: Effect of
cellulose acetate beads on interleukin-23 release. Ther Apher Dial.
20:354–359. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rodríguez-Lago I, Benítez JM,
García-Sánchez V, Gutiérrez A, Sempere L, Ginard D, Barreiro-de
Acosta M and Cabriada JL: Granulocyte and monocyte apheresis in
inflammatory bowel disease: The patients' point of view.
Gastroenterol Hepatol. 41:423–431. 2018.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
10
|
Tominaga K, Nakano M, Hoshino M, Kanke K
and Hiraishi H: Efficacy, safety and cost analyses in ulcerative
colitis patients undergoing granulocyte and monocyte adsorption or
receiving prednisolone. BMC Gastroenterol. 13(41)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Panés J, Guilera M, Ginard D, Hinojosa J,
González-Carro P, González-Lara V, Varea V, Domènech E and Badia X:
Treatment cost of ulcerative colitis is apheresis with Adacolumn
cost-effective? Dig Liver Dis. 39:617–625. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yoshino T, Nakase H, Matsuura M, Matsumura
K, Honzawa Y, Fukuchi T, Watanabe K, Murano M, Tsujikawa T,
Fukunaga K, et al: Effect and safety of granulocyte-monocyte
adsorption apheresis for patients with ulcerative colitis positive
for cytomegalovirus in comparison with immunosuppressants.
Digestion. 84:3–9. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Filosa A and Filosa G: Neutrophilic
dermatoses: A broad spectrum of disease. G Ital Dermatol Venereol.
153:265–272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nelson CA, Stephen S, Ashchyan HJ, James
WD, Micheletti RG and Rosenbach M: Neutrophilic dermatoses:
Pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis,
and Behçet disease. J Am Acad Dermatol. 79:987–1006.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 61:496–509. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Krueger JG and Bowcock A: Psoriasis
pathophysiology: Current concepts of pathogenesis. Ann Rheum Dis.
64 (Suppl 2):ii30–ii36. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shindo E, Shikano K, Kawazoe M, Yamamoto
T, Kusunoki N, Hashimoto Y and Nanki T: A case of generalized
pustular psoriasis caused by hydroxychloroquine in a patient with
systemic lupus erythematosus. Lupus. 28:1017–1020. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hoegler KM, John AM, Handler MZ and
Schwartz RA: Generalized pustular psoriasis: A review and update on
treatment. J Eur Acad Dermatol Venereol. 32:1645–1651.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Navarini AA, Burden AD, Capon F, Mrowietz
U, Puig L, Köks S, Kingo K, Smith C and Barker JN: ERASPEN Network.
European consensus statement on phenotypes of pustular psoriasis. J
Eur Acad Dermatol Venereol. 31:1792–1799. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kanekura T, Yoshii N, Yonezawa T, Kawabata
H, Saruwatari H and Kanzaki T: Treatment of pustular psoriasis with
granulocyte and monocyte adsorption apheresis. J Am Acad Dermatol.
49:329–332. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ohnishi H, Kadowaki T, Mizutani Y, Nishida
E, Tobita R, Abe N, Yamaguchi Y, Eto H, Honma M, Kanekura T, et al:
Genetic background and therapeutic response in generalized pustular
psoriasis patients treated with granulocyte and monocyte adsorption
apheresis. Eur J Dermatol. 28:108–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sawada K, Ohdo M, Ino T, Nakamura T,
Numata T, Shibata H, Sakou J, Kusada M and Hibi T: Safety and
tolerability of nafamostat mesilate and heparin as anticoagulants
in leukocytapheresis for ulcerative colitis: Post Hoc analysis of a
large-scale, prospective, observational study. Ther Apher Dial.
20:197–204. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shukuya R, Hasegawa T, Niwa Y, Okuma K and
Ikeda S: Granulocyte and monocyte adsorption apheresis for
generalized pustular psoriasis. J Dermatol. 38:1130–1134.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugiura K: The genetic background of
generalized pustular psoriasis: IL36RN mutations and CARD14
gain-of-function variants. J Dermatol Sci. 74:187–192.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Suzuki A, Haruna K, Mizuno Y, Kuwae Y, Ono
Y, Okumura K, Negi O, Kon Y, Takeuchi K, Takamori K, et al:
Successful treatment of three cases of generalized pustular
psoriasis with granulocyte and monocyte adsorption apheresis. Ther
Apher Dial. 16:445–448. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mizutani Y, Fujii K, Kawamura M, Inoue M,
Mizutani YH, Matsuyama K, Doi T, Nagaya S and Seishima M: Intensive
granulocyte and monocyte adsorption apheresis for generalized
pustular psoriasis. J Dermatol. 47:1326–1329. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fujii A, Ohnishi H and Seishima M:
Generalized pustular psoriasis with IL-36 Receptor antagonist
mutation successfully treated with granulocyte and monocyte
adsorption apheresis accompanied by reduced serum IL-6 level. Ther
Apher Dial. 22:92–93. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sakanoue M, Takeda K, Kawai K and Kanekura
T: Granulocyte and monocyte adsorption apheresis for refractory
skin diseases due to activated neutrophils, psoriasis, and
associated arthropathy. Ther Apher Dial. 17:477–483.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ikeda S, Takahashi H, Suga Y, Eto H, Etoh
T, Okuma K, Takahashi K, Kanbara T, Seishima M, Morita A, et al:
Therapeutic depletion of myeloid lineage leukocytes in patients
with generalized pustular psoriasis indicates a major role for
neutrophils in the immunopathogenesis of psoriasis. J Am Acad
Dermatol. 68:609–617. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fujii A, Fujii K and Seishima M:
Generalized pustular psoriasis with CARD14 Variant c.526G>C
(p.Asp176His) successfully treated with granulocyte and monocyte
adsorption apheresis. Ther Apher Dial. 23:298–299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Koike Y, Okubo M, Kiyohara T, Fukuchi R,
Sato Y, Kuwatsuka S, Takeichi T, Akiyama M, Sugiura K and Utani A:
Granulocyte and monocyte apheresis can control juvenile generalized
pustular psoriasis with mutation of IL36RN. Br J Dermatol.
177:1732–1736. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fujisawa T, Moriya C, Shibuya Y, Kanoh H
and Seishima M: Combination therapy of infliximab and
granulocyte/monocyte adsorption apheresis for refractory pustular
psoriasis with psoriatic arthritis. Acta Derm Venereol. 93:364–365.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tominaga C, Yamamoto M, Imai Y and
Yamanishi K: A case of old age-onset generalized pustular psoriasis
with a deficiency of IL-36RN (DITRA) treated by granulocyte and
monocyte apheresis. Case Rep Dermatol. 7:29–35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fujisawa T, Murase K, Okumura Y, Kanoh H,
Doi T, Yoshida S, Ogura S and Seishima M: Generalized pustular
psoriasis successfully treated with granulocyte and monocyte
adsorption apheresis. Ther Apher Dial. 15:374–378. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fujisawa T, Suzuki S, Mizutani Y, Doi T,
Yoshida S, Ogura S and Seishima M: Granulocyte and monocyte
adsorption apheresis for generalized pustular psoriasis:
Therapeutic outcomes in three refractory patients. Ther Apher Dial.
19:336–341. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Furusawa K, Hasegawa T and Ikeda S:
Immunosuppressant and infliximab-resistant generalized pustular
psoriasis successfully treated with granulocyte and monocyte
adsorption apheresis. Ther Apher Dial. 16:379–380. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mabuchi T, Manabe Y, Yamaoka H, Ota T,
Kato M, Ikoma N, Kusakabe Y, Komaba H and Ozawa A: Case of
generalized pustular psoriasis with end-stage renal disease
successfully treated with granulocyte monocyte apheresis in
combination with hemodialysis. J Dermatol. 41:521–524.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Seishima M, Mizutani Y, Shibuya Y,
Nagasawa C and Aoki T: Efficacy of granulocyte and monocyte
adsorption apheresis for pustular psoriasis. Ther Apher Dial.
12:13–18. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sugiura K, Haruna K, Suga Y and Akiyama M:
Generalized pustular psoriasis caused by deficiency of
interleukin-36 receptor antagonist successfully treated with
granulocyte and monocyte adsorption apheresis. J Eur Acad Dermatol
Venereol. 28:1835–1836. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Maverakis E, Marzano AV, Le ST, Callen JP,
Brüggen MC, Guenova E, Dissemond J, Shinkai K and Langan SM:
Pyoderma gangrenosum. Nat Rev Dis Primers. 6(81)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ahronowitz I, Harp J and Shinkai K:
Etiology and management of pyoderma gangrenosum: A comprehensive
review. Am J Clin Dermatol. 13:191–211. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Alavi A, French LE, Davis MD, Brassard A
and Kirsner RS: Pyoderma Gangrenosum: An update on pathophysiology,
diagnosis and treatment. Am J Clin Dermatol. 18:355–372.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Russo I, Miotto S, Colpo A, Marson P,
Tison T, Ferrazzi A and Alaibac M: Successful treatment of pyoderma
gangrenosum with granulocyte and monocyte adsorption apheresis. Int
Wound J. 14:282–284. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Higashi Y, Ibusuki A, Baba N, Hatanaka M,
Tada KI and Kanekura T: Granulocyte and monocyte adsorptive
apheresis for pyoderma gangrenosum. Ther Apher Dial. 26:450–455.
2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ikeda K, Hamada T, Otsuka M and Iwatsuki
K: Beneficial effects of neutrophil-targeted therapy for pyoderma
gangrenosum associated with ulcerative colitis. Eur J Dermatol.
21:804–805. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ohmori T, Yamagiwa A, Nakamura I,
Nishikawa K and Saniabadi AR: Treatment of pyoderma gangrenosum
associated with Crohn's disease. Am J Gastroenterol. 98:2101–2102.
2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ishikawa H, Kumano T, Suzuki Y, Mabe K,
Suzuki T, Momma S and Momma T: A case of successful treatment with
granulocytapheresis (GCAP) for pyoderma gangrenosum complicating
ulcerative colitis. Jap J Clin Dermatol. 58:1099–1101. 2004.
|
|
48
|
Yoneda K, Chino Y, Kamei K, Yamada T,
Nagura K and You M: Four Cases of pyoderma gangrenosum associated
with ulcerative colitis. Jap J Clin Dermatol. 59:263–266. 2005.
|
|
49
|
Yanaru-Fujisawa R, Matsumoto T, Nakamura
S, Kochi S, Iida M, Kohda F, Hirahashi M, Yao T and Mibu R:
Granulocyte apheresis for pouchitis with arthritis and pyoderma
gangrenosum after restorative proctocolectomy for ulcerative
colitis: A case report. Inflamm Bowel Dis. 11:780–781.
2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Seishima M, Mizutani Y, Shibuya Y,
Nagasawa C and Aoki T: Efficacy of granulocyte and monocyte
adsorption apheresis for three cases of refractory pyoderma
gangrenosum. Ther Apher Dial. 11:177–182. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fujino Y, Suzuki Y, Kohama R, Omoya T,
Kitazoe K, Nakamoto J, Aoki H, Yano M, Sikiji T and Satake N: A
case of Pyoderma Gangrenosum successfully treated by
granulocytapheresis and steroid therapy. Tokushima J Med. 30:29–32.
2008.
|
|
52
|
Kawakami T, Yamazaki M and Soma Y:
Reduction of interleukin-6, interleukin-8, and
anti-phosphatidylserine-prothrombin complex antibody by granulocyte
and monocyte adsorption apheresis in a patient with pyoderma
gangrenosum and ulcerative colitis. Am J Gastroenterol.
104:2363–2364. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Doi R, Haga T, Fujita A, Saito C, Takeuchi
S, Matsuoka A, Kawakami T, Soma Y and Kouro T: Rinsho Derma.
52:585–587. 2010.
|
|
54
|
Kobayashi S, Takeshita T and Furue M: A
case of Pyoderma Gangrenosum with Ulcerative Colitis successfully
treated with Granulocytapheresis, Skin grafting and Steroid
therapy. Nishi Nihon Hifuka. 73:474–477. 2011.
|
|
55
|
Ohno M, Koyama S, Ohara M, Shimamoto K,
Kobayashi Y, Nakamura F, Mitsuru K and Andoh A: Pyoderma
gangrenosum with ulcerative colitis successfully treated by the
combination of granulocyte and monocyte adsorption apheresis and
corticosteroids. Intern Med. 55:25–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Okada M, Okawa T, Takashima R and
Higashiyama M: A case of successful treatment with
granulocytapheresis for pyoderma gangrenosum complicating
ulcerative colitis. Skin Research. 16:150–154. 2017.
|
|
57
|
Yamashita A, Nakayama C, Tashiro J and
Miwa J: Ulcerative colitis accompanied by pyoderma gangrenosum
successfully treated with granulocyte monocyte apheresis: A case
report. Prog Dig Endosc. 90:130–131. 2017.
|
|
58
|
Tominaga K, Kamimura K, Sato H, Ko M,
Kawata Y, Mizusawa T, Yokoyama J and Terai S: Cytapheresis for
pyoderma gangrenosum associated with inflammatory bowel disease: A
review of current status. World J Clin Cases. 8:2092–2101.
2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shibuya T, Haga K, Saeki M, Haraikawa M,
Tsuchihashi H, Okahara K, Nomura O, Fukushima H, Murakami T,
Ishikawa D, et al: Pyoderma gangrenosum in an ulcerative colitis
patient during treatment with vedolizumab responded favorably to
adsorptive granulocyte and monocyte apheresis. J Clin Apher.
35:488–492. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kanekura T, Kawahara K, Maruyama I and
Kanzaki T: Treatment of pyoderma gangrenosum with granulocyte and
monocyte adsorption apheresis. Ther Apher Dial. 9:292–296.
2005.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kanekura T, Maruyama I and Kanzaki T:
Granulocyte and monocyte adsorption apheresis for pyoderma
gangrenosum. J Am Acad Dermatol. 47:320–321. 2002.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kawai M, Kawanami C, Fukuda A and Seno H:
Pyoderma gangrenosum with primary sclerosing cholangitis-associated
colitis successfully treated with concomitant granulocyte and
monocyte adsorption apheresis with corticosteroids. Clin J
Gastroenterol. 14:1561–1566. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Murakami M and Terui T: Palmoplantar
pustulosis: Current understanding of disease definition and
pathomechanism. J Dermatol Sci. 98:13–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mrowietz U, Bachelez H, Burden AD, Rissler
M, Sieder C, Orsenigo R and Chaouche-Teyara K: Secukinumab for
moderate-to-severe palmoplantar pustular psoriasis: Results of the
2PRECISE study. J Am Acad Dermatol. 80:1344–1352. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Fujisawa T, Tawada C, Mizutani Y, Doi T,
Yoshida S, Ogura S and Seishima M: Efficacy of granulocyte and
monocyte adsorption apheresis for treatment of palmoplantar
pustulosis. Ther Apher Dial. 18:238–243. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kawakami H, Nagaoka Y, Hirano H, Matsumoto
Y, Abe N, Tsuboi R, Kanno Y and Okubo Y: Evaluation of the efficacy
of granulocyte and monocyte adsorption apheresis on skin
manifestation and joint symptoms of patients with pustulotic
arthro-osteitis. J Dermatol. 46:144–148. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kanekura T, Kawabata H, Maruyama I and
Kanzaki T: Treatment of psoriatic arthritis with granulocyte and
monocyte adsorption apheresis. J Am Acad Dermatol. 50:242–246.
2004.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Bulur I and Onder M: Behçet disease: New
aspects. Clin Dermatol. 35:421–434. 2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Tong B, Liu X, Xiao J and Su G:
Immunopathogenesis of Behcet's Disease. Front Immunol.
10(665)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Alpsoy E, Zouboulis CC and Ehrlich GE:
Mucocutaneous lesions of Behcet's disease. Yonsei Med J.
48:573–585. 2007.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Higashi Y, Shimokawa M, Kawai K and
Kanekura T: Granulocyte and monocyte adsorption apheresis for
Behçet's disease in a pregnant woman. J Dermatol. 40:1042–1044.
2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kanekura T, Gushi A, Iwata M, Fukumaru S,
Sakamoto R, Kawahara K, Maruyama I and Kanzaki T: Treatment of
Behçet's disease with granulocyte and monocyte adsorption
apheresis. J Am Acad Dermatol. 51 (2 Suppl):S83–S87.
2004.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Villarreal-Villarreal CD, Ocampo-Candiani
J and Villarreal-Martínez A: Sweet Syndrome: A review and update.
Actas Dermosifiliogr. 107:369–378. 2016.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
74
|
Cohen PR: Sweet's syndrome-a comprehensive
review of an acute febrile neutrophilic dermatosis. Orphanet J Rare
Dis. 2(34)2007.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Fujii A, Mizutani Y, Hattori Y, Takahashi
T, Ohnishi H, Yoshida S and Seishima M: Sweet's syndrome
successfully treated with granulocyte and monocyte adsorption
apheresis. Case Rep Dermatol. 9:13–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gerfaud-Valentin M, Jamilloux Y, Iwaz J
and Sève P: Adult-onset Still's disease. Autoimmun Rev. 13:708–722.
2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Giacomelli R, Ruscitti P and Shoenfeld Y:
A comprehensive review on adult onset Still's disease. J Autoimmun.
93:24–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kanekura T, Terasaki K, Higashi Y, Yoshii
N, Kawahara K, Maruyama I and Kanzaki T: Improvement of adult
Still's disease with granulocyte and monocyte adsorption apheresis.
Clin Exp Dermatol. 29:410–412. 2004.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Iwasaki A, Kawakami H and Okubo Y:
Granulocyte/Monocyte adsorption apheresis as a novel therapeutic
approach in the treatment of an impetigo herpetiformis case. Ther
Apher Dial. 22:414–416. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Namazi N and Dadkhahfar S: Impetigo
herpetiformis: Review of pathogenesis, complication, and treatment.
Dermatol Res Pract. 2018(5801280)2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Fujii K, Takahashi T, Matsuyama K, Fujii
A, Mizutani Y, Ohnishi H and Seishima M: Impetigo herpetiformis
with a CARD14 Thr79Ile variant successfully treated with
granulocyte and monocyte adsorption apheresis. J Dermatol.
47:e84–e85. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Saito-Sasaki N, Izu K, Sawada Y, Hino R,
Nakano R, Shimajiri S, Nishimura I, Nakamura H, Sugiura K and
Nakamura M: Impetigo herpetiformis complicated with intrauterine
growth restriction treated successfully with granulocyte and
monocyte apheresis. Acta Derm Venereol. 97:410–411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Selmi C and Gershwin ME: Diagnosis and
classification of reactive arthritis. Autoimmun Rev. 13:546–549.
2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wu IB and Schwartz RA: Reiter's syndrome:
the classic triad and more. J Am Acad Dermatol. 59:113–121.
2008.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Yoshifuku A, Oyama K, Ibusuki A, Kawasaki
M, Sakanoue M, Matsushita S, Kawai K, Kawahara K, Maruyama I and
Kanekura T: Granulocyte and monocyte adsorption apheresis as an
effective treatment for Reiter disease. Clin Exp Dermatol.
37:241–244. 2012.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Braun-Falco M, Kovnerystyy O, Lohse P and
Ruzicka T: Pyoderma gangrenosum, acne, and suppurative hidradenitis
(PASH)-a new autoinflammatory syndrome distinct from PAPA syndrome.
J Am Acad Dermatol. 66:409–415. 2012.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Cugno M, Borghi A and Marzano AV: PAPA,
PASH and PAPASH Syndromes: Pathophysiology, presentation and
treatment. Am J Clin Dermatol. 18:555–562. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Hatanaka M, Fujii K and Kanekura T:
Successful treatment of pyoderma gangrenosum, acne, and suppurative
hidradenitis syndrome with granulocyte and monocyte adsorption
apheresis. J Dermatol. 48:376–377. 2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Mizutani Y, Okano T, Takahashi T, Ohnishi
H, Ohara O, Sano A and Seishima M: Pyoderma gangrenosum, acne and
suppurative hidradenitis syndrome treated with granulocyte and
monocyte adsorption apheresis. Acta Derm Venereol. 97:275–276.
2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Tosca N and Stratigos JD: Possible
pathogenetic mechanisms in allergic cutaneous vasculitis. Int J
Dermatol. 27:291–296. 1988.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chen KR and Carlson JA: Clinical approach
to cutaneous vasculitis. Am J Clin Dermatol. 9:71–92.
2008.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Goeser MR, Laniosz V and Wetter DA: A
practical approach to the diagnosis, evaluation, and management of
cutaneous small-vessel vasculitis. Am J Clin Dermatol. 15:299–306.
2014.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kanekura T, Yoshii N, Kawahara K, Maruyama
I and Kanzaki T: Granulocyte and monocyte adsorption apheresis for
cutaneous allergic vasculitis. Ther Apher Dial. 10:287–290.
2006.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Kiriakidou M and Ching CL: Systemic lupus
erythematosus. Ann Intern Med. 172:ITC81–ITC96. 2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Fortuna G and Brennan MT: Systemic lupus
erythematosus: Epidemiology, pathophysiology, manifestations, and
management. Dent Clin North Am. 57:631–655. 2013.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kanekura T, Hashiguchi T, Mera Y, Katahira
A, Nakamura I, Maruyama I and Kanzaki T: Improvement of SLE skin
rash with granulocyte and monocyte adsorption apheresis.
Dermatology. 208:79–80. 2004.PubMed/NCBI View Article : Google Scholar
|