Introduction
Chronic obstructive pulmonary disease (COPD) is a
serious lung condition that is characterized by persistent
respiratory symptoms and progressive airflow obstruction (1-3).
COPD is the third leading cause of mortality worldwide with a
global prevalence of 4.2% according to the World Health
Organization (1-3).
In total, 25% of patients with COPD are diagnosed into the acute
exacerbation (AE)-COPD category, which is characterized by more
severe respiratory impairment, increased mucus production and worse
overall quality of life (4). This
in turn worsens the mortality rates among patients with AE-COPD
compared with patients with stable COPD (S-COPD) (4). Therefore, early diagnosis and
continuous monitoring of disease progression is essential for the
timely intervention against the acute exacerbation of COPD
(5,6). There is a significant demand to
develop novel biomarkers for COPD for predicting the risk of acute
exacerbation and monitoring disease severity to implement accurate
and personalized management strategies with aims of improving the
prognosis of COPD.
Cell division cycle 42 (CDC42) is a key regulator of
several cellular processes, including cell proliferation, division,
migration, morphogenesis and establishment of epithelial polarity
(7-16).
It has been previously reported that CDC42 can regulate the
physiology of macrophages, recruitment of T cells during
inflammation and lung injury (7-16).
In addition, CDC42 has been found to facilitate the recruitment,
migration, adhesion and retention of macrophages through a number
of signaling pathways such as mTOR-CDC42 signaling and PI3Kδ-CDC42
signaling (8-11,17).
Previous studies have shown that CDC42 can inhibit the
differentiation of naive T cells into Th1 and Th17 cells to further
promote cytokine secretion and exocytosis (12,13).
Furthermore, CDC42 can promote endothelial regeneration and
vascular repair after inflammatory lung injury (14-16).
Emerging evidence has also suggested that changes in the macrophage
phenotype, recruitment of T cells associated with inflammation and
lung injury can occur during COPD pathogenesis (1).
Based on these previous observation, it can
hypothesized that CDC42 can possibly serve as a novel biomarker for
COPD. Therefore, the present study aimed to measure the expression
levels of CDC42 in blood samples derived from patients with AE-COPD
and S-COPD in addition to healthy control (HCs) individuals. The
objective was to evaluate the potential association of CDC42
expression with the risk acute exacerbation of COPD, inflammation
and disease severity.
Materials and methods
Patient recruitment
The present study was approved by the Institutional
Review Board of the Central Hospital of Wuhan. A total of 60
patients with AE-COPD and 60 with S-COPD were enrolled in the
Central Hospital of Wuhan (Wuhan, China) between May 2019 and
November 2020. All patients, aged >18 years, were diagnosed with
COPD according to the Global Initiative for Chronic Obstructive
Lung Disease (GOLD) criteria (18). AE-COPD is characterized by the
acute worsening of respiratory symptoms, resulting in the need for
additional therapy. The most common symptoms of exacerbation
include increased dyspnea, airway inflammation, mucus production
and marked gas trapping (18). By
contrast, patients with S-COPD were clinically stable for ≥3 months
without exacerbations. In the present study, patients were
classified into the AE-COPD or S-COPD cohorts according to disease
status at first admission. The AE-COPD patients denoted those who
initially came to the Central Hospital of Wuhan with symptoms of
exacerbation including increased dyspnea, airway inflammation,
mucus production or marked gas trapping. The S-COPD patients
denoted those who initially came to the Central Hospital of Wuhan
for reexamination without symptoms of exacerbation in the previous
three or more months. This classification was not altered after
study enrollment, regardless of any changes in disease status, to
avoid overlapping the cases between both cohorts. In both patients
with AE-COPD and S-COPD the exclusion criteria were as follows: i)
Pregnant or lactating women; ii) patients with COPD with asthma;
iii) patients with anaphylactic diseases (e.g., allergic asthma);
iv) patients with other respiratory diseases (e.g., active
tuberculosis); v) patients with lung cancer; or vi) patients with
other malignancies (such as liver cancer, colorectal cancer,
gastric carcinoma or hematologic malignancies). Additionally, 60
healthy individuals, who visited the hospital for physical
examination were recruited and allocated into the HCs group during
the same period. All individuals in the HCs group had no obvious
abnormalities in their physical examination and no history of COPD,
asthma, respiratory diseases, autoimmune diseases, inflammatory
diseases or malignancies. All subjects provided written informed
consent.
The mean ages of the HC group, patients with S-COPD
and patients with AE-COPD were 66.1±6.4, 67.3±7.1 and 66.7±7.2
years, respectively (Table I). In
terms of sex, there were 24 (40%) females and 36 (60%) males in the
HC group, 18 (30%) females and 42 (70%) males in the S-COPD group
and 16 (26.7%) females and 44 (73.3%) males in the AE-COPD
group.
 | Table ICharacteristics of patients with COPD
and HCs. |
Table I
Characteristics of patients with COPD
and HCs.
| Parameter | HCs (N=60) | Stable-COPD
(N=60) | Acute
exacerbation-COPD (N=60) | Statistic
(F/χ2/Z/H) | P-value |
|---|
| Age, years | 66.1±6.4 | 67.3±7.1 | 66.7±7.2 | 0.394 | 0.675 |
| Sex | | | | 2.646 | 0.266 |
|
Female | 24 (40.0) | 18 (30.0) | 16 (26.7) | | |
|
Male | 36 (60.0) | 42 (70.0) | 44 (73.3) | | |
| BMI,
kg/m2 | 22.8±2.6 | 22.1±2.6 | 22.5±3.0 | 0.999 | 0.370 |
| Family history of
COPD | 10 (16.7) | 20 (33.3) | 17 (28.3) | 4.550 | 0.103 |
| History of
smoking | 17 (28.3) | 33 (55.0) | 28 (46.7) | 9.095 | 0.011 |
| History of
hypertension | 24 (40.0) | 30 (50.0) | 35 (58.3) | 4.045 | 0.132 |
| History of
hyperlipidemia | 16 (26.7) | 14 (23.3) | 15 (25.0) | 0.178 | 0.915 |
| History of diabetes
mellitus | 10 (16.7) | 11 (18.3) | 14 (23.3) | 0.922 | 0.631 |
| FEV1/forced volume
vital capacity, % | 82.2
(79.8-84.2) | 61.3
(57.6-65.2) | 60.7
(54.9-65.8) | 119.447 | <0.001 |
| FEV1, predicted
% | 99.2
(95.7-100.6) | 74.6
(57.4-82.5) | 57.1
(46.6-81.4) | 121.708 | <0.001 |
| Global Initiative
for Chronic | | | | -1.980 | 0.048 |
| Obstructive Lung
Disease stage | | | | | |
|
Stage I | - | 27 (45.0) | 16 (26.7) | | |
|
Stage
II | - | 22 (36.7) | 28 (46.6) | | |
|
Stage
III | - | 11 (18.3) | 16 (26.7) | | |
Data and blood sample collection
The demographic features and medical history of
patients were recorded after enrollment. Forced expiratory volume
in 1 sec (FEV1) and forced vital capacity (FVC) were
recorded following the pulmonary function test (PFT) (19). FEV1 (% predicted) and
FEV1/FVC ratio were calculated based on the
FEV1 and FVC values. Based on the FEV1 (%
predicted), airflow obstruction severity was classified according
to the GOLD criteria (https://goldcopd.org/archived-reports/) as follows: i)
GOLD stage 1, FEV1 (% predicted) ≥80%; ii) GOLD stage 2,
FEV1 (% predicted)=50-79%; iii) GOLD stage 3,
FEV1 (% predicted)=30-49%; and iv) GOLD stage 4,
FEV1 (% predicted) <30%.
In addition, peripheral blood samples were collected
from all 180 participants in the present study. The serum samples
and peripheral blood mononuclear cells (PBMCs) were collected by
centrifugal separation and density gradient separation,
respectively. In detail, peripheral blood samples were naturally
coagulated for about 20 min at room temperature and centrifuged at
650 x g for 10 min using a refrigerated centrifuge (4˚C) (Thermo
Fisher Scientific, Inc.). The supernatant serum was collected
carefully and stored at -70˚C. PBMCs were separated following
gradient centrifugation of the blood over Ficoll-Hypaque density
gradient (Biochrom, Ltd.). PBMCs were used to measure the
expression levels of CDC42 using reverse transcription-quantitative
PCR (RT-qPCR). The levels of TNF-α, IL-1β, IL-6a and IL-17 secreted
were assessed in the serum samples using the corresponding human
ELISA kits.
RT-qPCR
RT-qPCR was performed for the quantitative analysis
of CDC42 expression in PBMCs. Total RNA was extracted from PBMCs
using PureZOL RNA isolation reagent (Bio-Rad Laboratories, Inc.).
Subsequently, total RNA was reverse transcribed into complementary
DNA using the iScript™ Reverse Transcription Supermix kit
(denaturation at 65˚C for 5 min, reverse transcriptase at 37˚C for
5 min and inactivation at 85˚C for 5 sec) (Bio-Rad Laboratories,
Inc.). qPCR was performed using the TB Green Premix DimerEraser™
kit (Takara Bio, Inc.). The primer sequences used for CDC42
detection were the same as those previously reported (20). In detail, the primers for human
CDC42 were: Forward, 5'-GGCGATGGTGCTGTTGGTAA-3' and reverse,
5'-GCGGTCGTAATCTGTCATAATCCT-3'. GAPDH was used as an internal
control. Primers for human GAPDH were: Forward,
5'-GAGTCCACTGGCGTCTTCAC-3' and reverse,
5'-ATCTTGAGGCTGTTGTCATACTTCT-3'. The thermocycling conditions of
qPCR were: Initial denaturation at 95˚C for 30 sec, followed by 40
cycles of 95˚C for 5 sec, 55˚C for 30 sec and 72˚C for 30 sec,
which were performed in an ABI 7500 real-time PCR system. The
relative CDC42 expression was calculated using the
2-ΔΔCq method (21).
ELISA assay
Commercial ELISA kits (cat. nos. DTA00D, DLB50,
D6050 and D1700; R&D Systems Inc.) were purchased to detect the
serum levels of TNF-α, IL-1β, IL-6 and IL-17. All procedures,
including sample and reagent preparation, assay procedure and
calculation of the results were implemented according to the
protocols of the kits.
Statistical analysis
Data were presented as N (%), the mean ± standard
deviation (SD) or median (interquartile range); P-value represents
the significance of the results; r represents linear coefficient; H
and Z represent the statistic of nonparametric rank sum test; F
represents the statistic of ANOVA; χ2 represents the
statistic of chi-square test. Data analysis and graph plotting were
performed using SPSS 26.0 (IBM Corp.) and GraphPad Prism 7.01
(GraphPad Software Inc.) software, respectively. Wilcoxon rank sum
test, χ2 test, one-way ANOVA, Kruskal-Wallis and Dunn's
tests were used to compare the differences amongst groups. Multiple
comparisons were corrected by Bonferroni's post hoc test.
Spearman's rank correlation test was performed to evaluate the
association between the variables. Receiver operating
characteristic (ROC) curve analysis was used to evaluate the
performance of the variables in distinguishing different subjects.
Based on ROC analysis, the best statistical cut-off value of CDC42
expression level was calculated, which corresponded to the point at
which the sum of false-positives and false-negatives was less than
any other point. Sensitivity and specificity for selected cut-off
points were then assessed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients COPD and
the HC group
There were no differences in terms of age and sex
among the three groups (Table I).
Additionally, the proportion of subjects with a history of smoking
among the HC, S-COPD and AE-COPD groups varied significantly
(P<0.05; Table I).
FEV1/FVC ratio and FEV1 (% predicted) also
differed significantly among the three groups (both P<0.001 vs.
HC; Table I). Furthermore, there
was a significant association between the distribution of GOLD
stages and the proportion of patients in the S-COPD and AE-COPD
groups (P<0.05; Table I). In
terms of the secreted levels of inflammatory cytokines TNF-α,
IL-1β, IL-6 and IL-17, they were higher in AE-COPD group compared
with S-COPD group, higher in AE-COPD group compared with HC group
and higher in S-COPD group compared with HC group (all P<0.05;
Fig. S1).
Expression levels of CDC42 among the
HC, S-COPD and AE-COPD groups
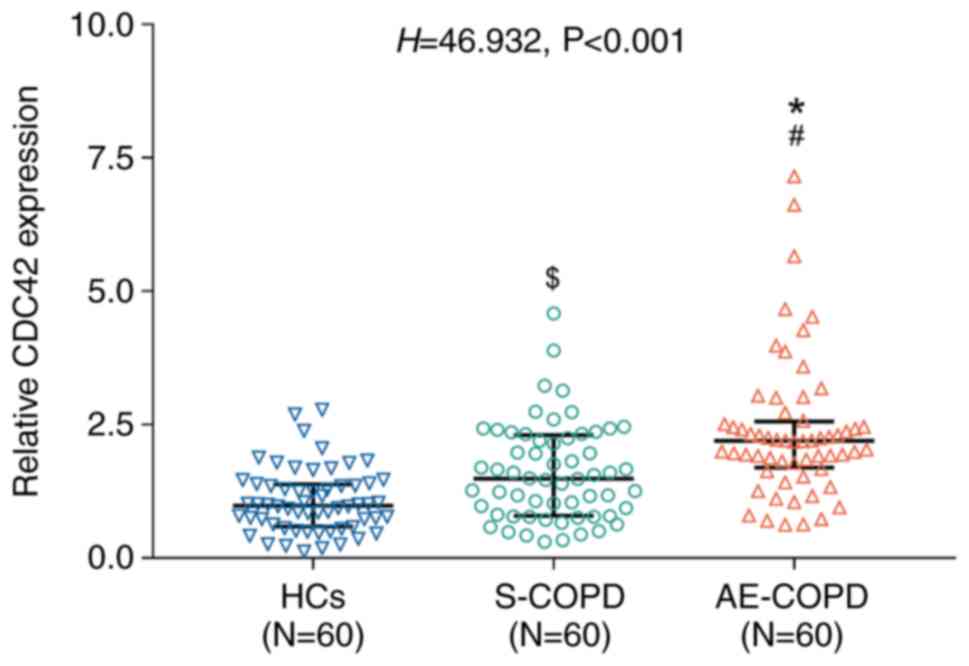
The expression levels of CDC42 varied significantly
among the HC, S-COPD and AE-COPD groups (P<0.001; Fig. 1). Specifically, CDC42 expression
was the highest in the AE-COPD group, followed by the S-COPD group
and those in the HC group exhibited the lowest expression levels of
CDC42 (Fig. 1). Comparisons
between the sub-groups revealed that the expression of CDC42 was
significantly higher in the AE-COPD group compared with that in the
HC group (P<0.001) and that in the S-COPD group (P<0.01).
Furthermore, the CDC42 expression level was higher in patients with
S-COPD compared with that in the HC group (P<0.05; Fig. 1).
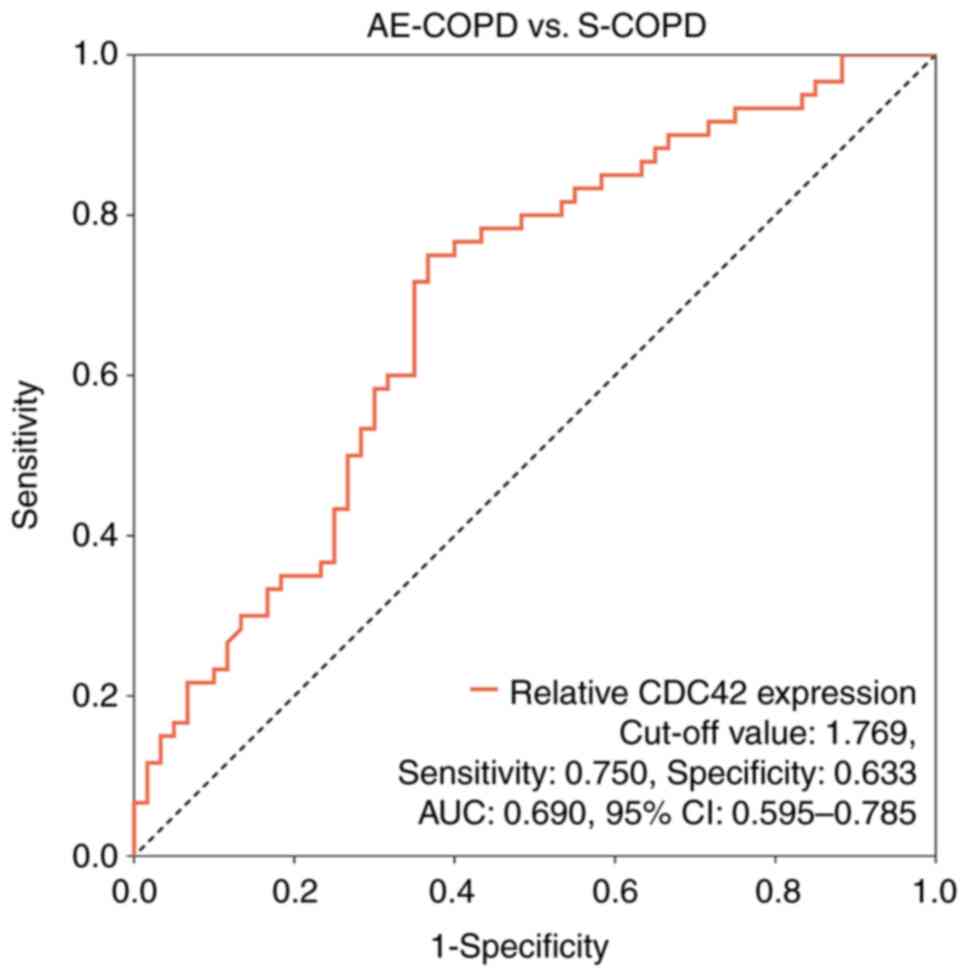
Additionally, ROC curve analysis was performed to
assess the viability of using CDC42 expression to differentiate
patients with AE-COPD to those with S-COPD. According to ROC
analysis, CDC42 exhibited potential for distinguishing patients
with AE-COPD from S-COPD, yielding an area under the curve (AUC)
value of 0.690 (95% confidence interval=0.595-0.785; Fig. 2). At the optimal cut-off point, the
AUC for CDC42 expression was 1.769, with a sensitivity and
specificity of 0.750 and 0.633, respectively (Fig. 2).
Association of CDC42 expression with
disease severity among patients with COPD
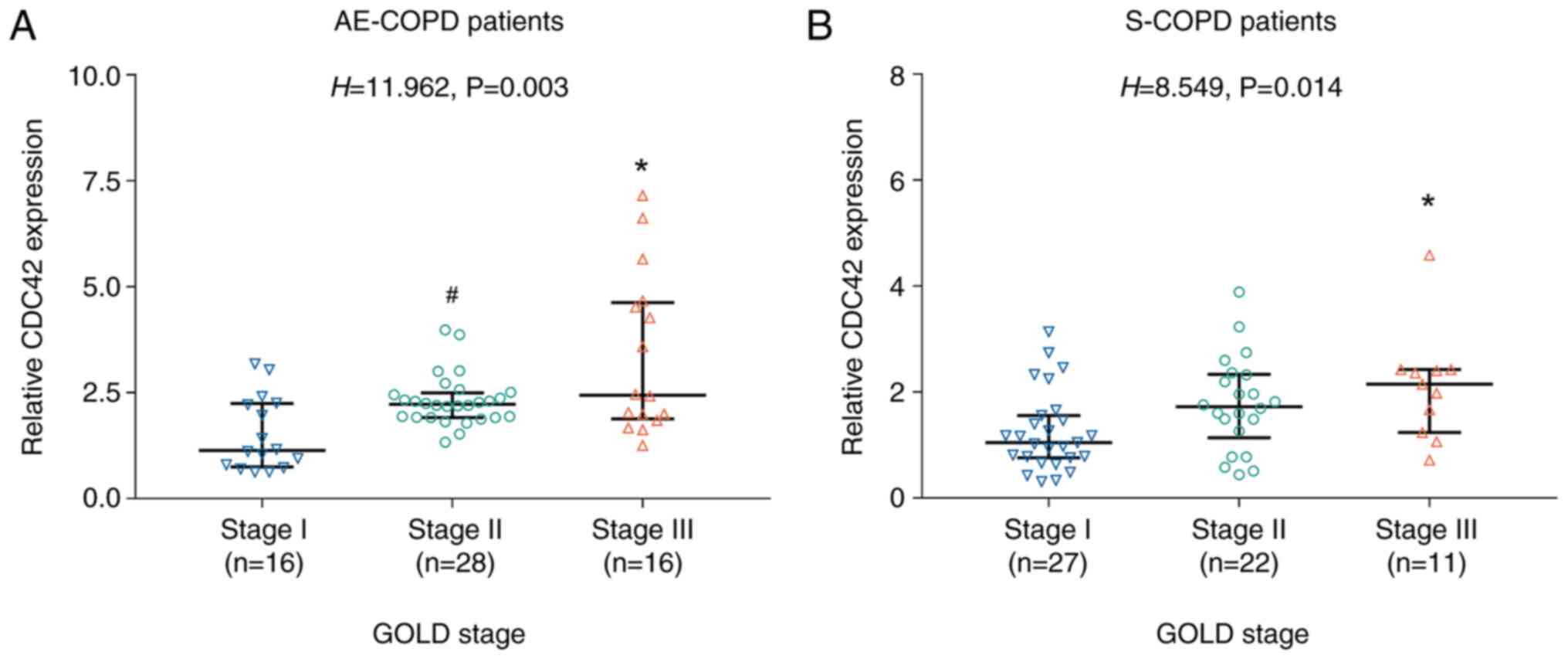
Subsequently, the potential association between
CDC42 expression and COPD severity was evaluated. The results
showed that CDC42 expression was highest in GOLD stage III,
followed by GOLD stage II, and lowest in GOLD stage I AE COPD
patients (P=0.003; Fig. 3A).
Consistently, this trend was also observed in S-COPD patients
(P=0.014; Fig. 3B).
Correlation between CDC42 expression
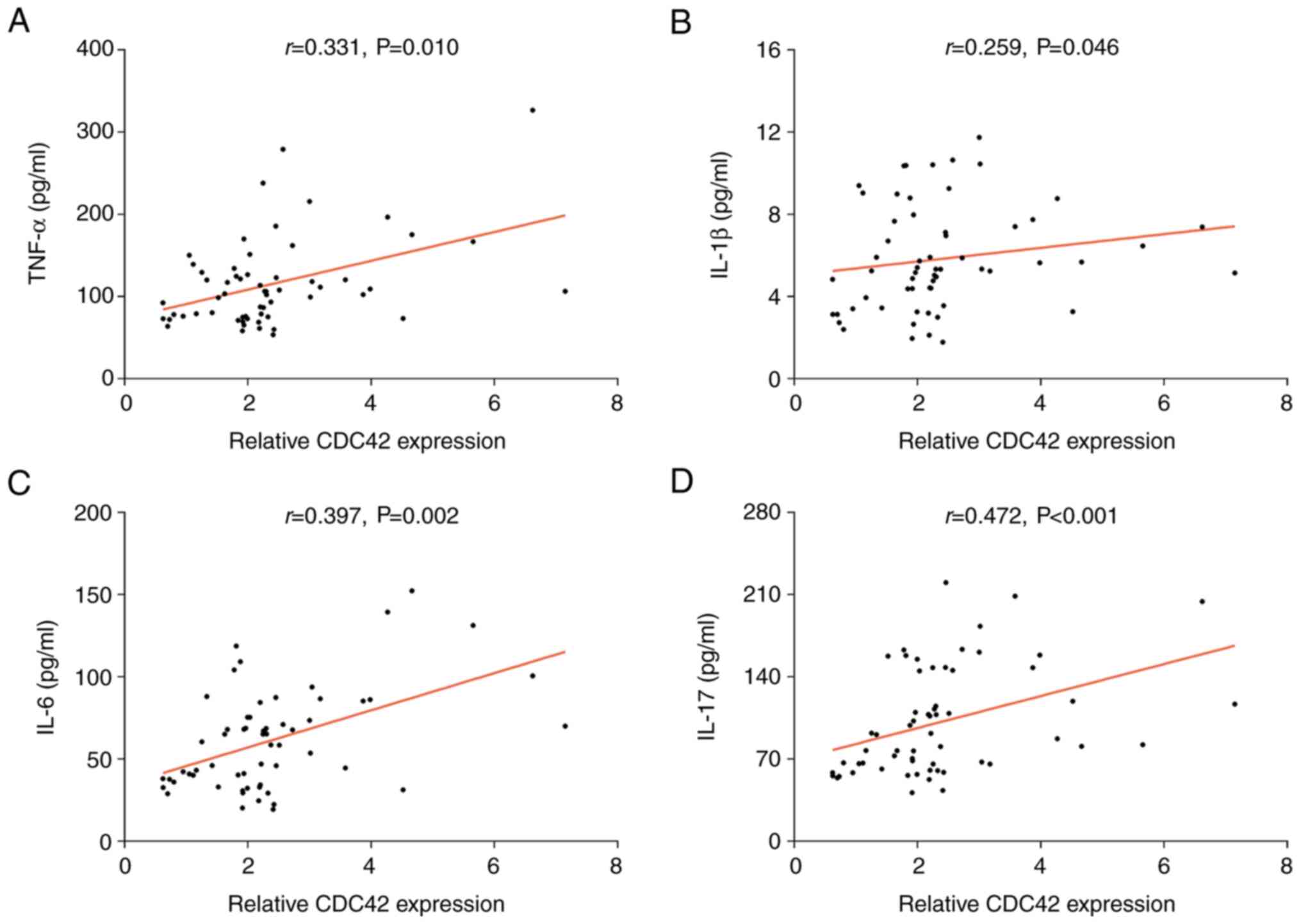
and indicators of inflammation
The expression of CDC42 was found to be positively
correlated with the serum levels of TNF-α (r=0.331; P<0.05),
IL-1β (r=0.259; P<0.05), IL-6 (r=0.397; P<0.01) and IL-17
(r=0.472; P<0.001) in patients with AE-COPD (Fig. 4). Furthermore, CDC42 expression was
positively correlated with TNF-α (r=0.377; P<0.01) and IL-17
(r=0.280; P<0.05) levels, but not with those of IL-1β and IL-6,
in patients with S-COPD (Fig.
S2A-D). Additionally, CDC42 expression was positively
correlated with the secreted levels of TNF-α (r=0.308; P<0.05;
Fig. S2E), but not with those of
IL-1β, IL-6 and IL-17 in the HC group (Fig. S2F-H). Finally, if patients with
S-COPD and AE-COPD were combined into one COPD group and compared
with the HC group, the levels of TNF-α, IL-1β, IL-6, and IL-17 were
all found to be significantly higher in patients with COPD compared
with those in the HC group (all P<0.001; Table SI).
Discussion
The present study demonstrated that CDC42 expression
recovered phagocytosis of alveolar macrophages and is proliferated
in a mouse model of COPD (22).
The present study demonstrated that the expression levels of CDC42
in patients with AE-COPD were higher compared with those in
patients with S-COPD. Furthermore, CDC42 expression could
distinguish patients with AE-COPD from those with S-COPD. Previous
studies demonstrated that CDC42 could serve a protective role in
the lungs against inflammation-mediated vascular endothelial injury
by inhibiting the p21-activated kinase/Akt pathway, whereby CDC42
expression was upregulated in the lung injury model (14,23).
Additionally, lung injury serves a crucial role in the occurrence
and development of COPD (1,14,23).
Therefore, CDC42 expression may be increased in patients with COPD
due to an as yet unknown compensatory mechanism. In addition,
patients with AE-COPD typically experienced longer disease
durations compared with those in patients with stable COPD, such
that prolonged disease may enhance endothelial dysfunction and
disease severity (24). Since
CDC42 has been demonstrated to exert a protective effect against
endothelial injury, CDC42 expression was higher in patients with
AE-COPD compared with that in patients with S-COPD.
To date, studies on the possible association between
CDC42 expression and COPD severity remain limited. In the present
study, CDC42 expression in patients with AE-COPD was found to be
associated with increased GOLD staging in both patients with S-COPD
and those with AE-COPD. This could be due to the fact that CDC42
upregulation is associated with enhanced inflammation as a result
of increased macrophage activation and promotion of Th cell
differentiation (8-13).
Emerging evidence has suggested that inflammation
can aggravate lung injury and is therefore positively associated
with GOLD stage (4). This is may
explain why increased CDC42 expression was associated with GOLD
stage in patients with COPD in the present study. Based on the
aforementioned findings, CDC42 expression may be increased in a
compensatory manner in the lung injury model. Furthermore, lung
injury could result in reduced FEV1, leading to
upgrading to higher GOLD COPD stages (25). Consequently, CDC42 expression could
be positively associated with GOLD stage in this manner.
Previous studies revealed that the expression levels
of CDC42 were positively associated with the levels of inflammatory
cytokines in lung injury (14,23).
The present study showed that CDC42 expression in patients with
AE-COPD was positively correlated with the serum levels of
inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-17. However, these
correlations were relatively weak in patients with S-COPD, possibly
owing to the fact that CDC42 could induce the differentiation of T
cells into Th1 or Th17 cells to promote the recruitment, migration,
adhesion and retention of macrophages (11). Furthermore, it has been previously
reported that CDC42 expression is positively associated with IL-6
(primarily secreted by macrophages), TNF-α (which is primarily
secreted by Th1 and Th17 cells), IL-1 (primarily secreted by Th1
cells) and IL-17 (primarily secreted by Th17 cells) (8-13,26-29).
However, the present study has several limitations.
The present study only included 120 patients with COPD, which is a
relatively small sample size. In addition, since the present study
was a single-center study, the possibility of selection bias could
not be excluded. Furthermore, the molecular mechanism underlying
the effects of CDC42 on the regulation of physiological changes in
macrophages, recruitment of inflammation-related T cells and lung
injury in COPD should be investigated further. In the present
study, the expression levels of CDC42 were only assessed at one
single time point. Therefore, changes in the expression levels of
CDC42 for longer periods in association with COPD progression
require further investigation. The present study was designed to
explore the preliminary clinical value of CDC42 in patients with
COPD. To translate this into clinical practice a series of further
studies in the real-world setting are required. The following types
of studies should be applied: i) Multicenter studies with larger
sample sizes should be performed to verify its potential clinical
value; ii) a study aiming to build models for predicting AE-COPD
should be performed; and iii) multi-timepoint monitoring should be
conducted in future studies to reveal the value of CDC42 in
monitoring COPD progression and treatment response. The present
study is a case-controlled study, which lacked scheduled follow-up
data in the protocol. Therefore, the potential accuracy of CDC42 as
a prognostic marker require further investigation.
In conclusion, the present study revealed that CDC42
expression was associated with an increased risk of acute
exacerbation, inflammation and COPD severity, which provides a
potential novel biomarker for COPD.
Supplementary Material
Comparison of the levels of
inflammatory cytokines. Comparison of (A) TNF-α, (B) IL-1β, (C)
IL-6 and (D) IL-17 among HCs, patients with S-COPD and patients
with AE-COPD. CDC42, cell division cycle 42; COPD, chronic
obstructive pulmonary disease; S-COPD, stable COPD; AE-COPD, acute
exacerbation COPD; HCs, health controls. *P<0.05
AE-COPD vs. HCs, #P<0.05 AE-COPD vs. S-COPD,
$P<0.05 S-COPD vs. HCs.
Correlation between CDC42 expression
and the levels of inflammatory cytokines. Correlation of CDC42
expression with (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-17 in
patients with stable COPD. Correlation between CDC42 expression and
the levels of (E) TNF-α, (F) IL-1β, (G) IL-6 and (H) IL-17 in HCs.
CDC42, cell division cycle 42; COPD, chronic obstructive pulmonary
disease S-COPD, stable COPD; HCs, health controls.
Comparison of CDC42 expression and
inflammatory cytokines between patients with COPD and HCs.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The Wuhan Municipal
Health Commission (grant no. WZ18Q14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to the study conception and
design. Material preparation, data collection and analysis were
performed by XM, FY and HZ. XM and HZ confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was performed in line with the
principles of the Declaration of Helsinki. Ethics approval was
granted by the Institutional Review Board of The Central Hospital
of Wuhan, Tongji Medical College, Huazhong University of Science
and Technology (Wuhan, China). All individuals in the present study
signed the informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hattab Y, Alhassan S, Balaan M, Lega M and
Singh AC: Chronic obstructive pulmonary disease. Crit Care Nurs Q.
39:124–130. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the global
burden of disease study 2010. Lancet. 380:2095–2128.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
GBD 2017 Disease and Injury Incidence and
Prevalence Collaborators. Global, regional, and national incidence,
prevalence, and years lived with disability for 354 diseases and
injuries for 195 countries and territories, 1990-2017: a systematic
analysis for the global burden of disease study 2017. Lancet.
392:1789–1858. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ming X, Duan W and Yi W: Long non-coding
RNA NEAT1 predicts elevated chronic obstructive pulmonary disease
(COPD) susceptibility and acute exacerbation risk, and correlates
with higher disease severity, inflammation, and lower miR-193a in
COPD patients. Int J Clin Exp Pathol. 12:2837–2848. 2019.PubMed/NCBI
|
|
5
|
Heaney LG and McGarvey LP: Personalised
medicine for asthma and chronic obstructive pulmonary disease.
Respiration. 93:153–161. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lowe KE, Regan EA, Anzueto A, Austin E,
Austin JHM, Beaty TH, Benos PV, Benway CJ, Bhatt SP, Bleecker ER,
et al: COPDGene((R)) 2019: Redefining the diagnosis of chronic
obstructive pulmonary disease. Chronic Obstr Pulm Dis. 6:384–399.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zheng C, Wang Y, Yang L, Zhou S, Gao Y, Li
F, Feng Y, Wang Z, Zhan L, Yan Q, et al: Cell division cycle 42
plays a cell type-specific role in lung tumorigenesis. Sci Rep.
7(10407)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang B, Zhang J, Xia L, Luo J, Zhang L,
Xu Y, Zhu X and Chen G: Inhibition of CDC42 reduces macrophage
recruitment and suppresses lung tumorigenesis in vivo. J Recept
Signal Transduct Res. 41:504–510. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou J, Dehne N and Brüne B: Nitric oxide
causes macrophage migration via the HIF-1-stimulated small GTPases
Cdc42 and Rac1. Free Radic Biol Med. 47:741–749. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nikolic DM, Gong MC, Turk J and Post SR:
Class A scavenger receptor-mediated macrophage adhesion requires
coupling of calcium-independent phospholipase A(2) and
12/15-lipoxygenase to Rac and Cdc42 activation. J Biol Chem.
282:33405–33411. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shiraishi A, Uruno T, Sanematsu F,
Ushijima M, Sakata D, Hara T and Fukui Y: DOCK8 protein regulates
macrophage migration through Cdc42 protein activation and LRAP35a
protein interaction. J Biol Chem. 292:2191–2202. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kalim KW, Yang JQ, Li Y, Meng Y, Zheng Y
and Guo F: Reciprocal regulation of glycolysis-driven Th17
pathogenicity and regulatory T cell stability by Cdc42. J Immunol.
200:2313–2326. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen L, Collado K and Rastogi D:
Contribution of systemic and airway immune responses to pediatric
obesity-related asthma. Paediatr Respir Rev. 37:3–9.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lv J, Zeng J, Guo F, Li Y, Xu M, Cheng Y,
Zhang L, Cai S, Chen Y, Zheng Y and Hu G: Endothelial Cdc42
deficiency impairs endothelial regeneration and vascular repair
after inflammatory vascular injury. Respir Res.
19(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu GD, Chen YH, Tong WC, Cheng YX, Zhang
L, Zhang L and Cai SX: The comparison between the vascular
endothelial cells special cdc42-deficient heterozygous mice and the
non-knockout mice on lung tissue pathological change and
vasopermeability in acute lung injury. Nan Fang Yi Ke Da Xue Xue
Bao. 31:995–998. 2011.PubMed/NCBI(In Chinese).
|
|
16
|
Xing J, Wang Q, Coughlan K, Viollet B,
Moriasi C and Zou MH: Inhibition of AMP-activated protein kinase
accentuates lipopolysaccharide-induced lung endothelial barrier
dysfunction and lung injury in vivo. Am J Pathol. 182:1021–1030.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang S and Gong F: Cdc42 participates in
the occurrence of chronic obstructive pulmonary disease by
regulating migration of inflammatory cells. Minerva Med.
110:477–480. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vogelmeier CF, Criner GJ, Martinez FJ,
Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M,
Fabbri LM, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive lung disease 2017 report.
GOLD executive summary. Am J Respir Crit Care Med. 195:557–582.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cho O, Oh YT, Chun M, Noh OK and Heo JS:
Prognostic implication of FEV1/FVC ratio for limited-stage small
cell lung cancer. J Thorac Dis. 10:1797–1805. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun N, Ye L, Chang T and Li X and Li X:
microRNA-195-Cdc42 axis acts as a prognostic factor of esophageal
squamous cell carcinoma. Int J Clin Exp Pathol. 7:6871–6879.
2014.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hu ST, Xia Q, Zeng XL, Bao HR and Liu XJ:
Effects of PI3Kδ-RhoA pathway on phagocytosis defect of alveolar
macrophages in a mouse model of chronic obstructive pulmonary
disease. Zhonghua Jie He He Hu Xi Za Zhi. 40:520–526.
2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
23
|
Yu Y, Jing L, Zhang X and Gao C:
Simvastatin attenuates acute lung injury via regulating CDC42-PAK4
and endothelial microparticles. Shock. 47:378–384. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Theodorakopoulou MP, Alexandrou ME,
Bakaloudi DR, Pitsiou G, Stanopoulos I, Kontakiotis T and Boutou
AK: Endothelial dysfunction in COPD: A systematic review and
meta-analysis of studies using different functional assessment
methods. ERJ Open Res. 7:2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rushton L: Chronic obstructive pulmonary
disease and occupational exposure to silica. Rev Environ Health.
22:255–272. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dinarello CA: The IL-1 family of cytokines
and receptors in rheumatic diseases. Nat Rev Rheumatol. 15:612–632.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chang SH: T helper 17 (Th17) cells and
interleukin-17 (IL-17) in cancer. Arch Pharm Res. 42:549–559.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu P, Jia S, Lou Y, He K and Xu LX:
Cryo-thermal therapy inducing MI macrophage polarization created
CXCL10 and IL-6-rich pro-inflammatory environment for
CD4+ T cell-mediated anti-tumor immunity. Int J
Hyperthermia. 36:408–420. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Katz S, Zsiros V and Kiss AL: Under
inflammatory stimuli mesenteric mesothelial cells
transdifferentiate into macrophages and produce pro-inflammatory
cytokine IL-6. Inflamm Res. 68:525–528. 2019.PubMed/NCBI View Article : Google Scholar
|