Introduction
Moyamoya syndrome (MMS) is a moyamoya vascular
disease that occurs in patients with a number of underlying
diseases, including sickle cell anemia (1), Fanconi anemia (2) and iron deficiency anemia (3). Among all the blood disorders, MMS is
more commonly associated with sickle cell anemia. Non-deformable
erythrocytes, especially sickle cells, obstruct the blood flow in
the vasa vasorum, thus leading to vessel wall ischemia and
subsequent intimal proliferation of the internal carotid artery,
thereby leading to occlusion (4).
The reduction in blood flow from anemia, along with fewer deformed
blood cells, may also lead to progressive endothelial proliferation
and subsequent vascular occlusion (5). Symptom progression has been reported
in most patients with MMS within 5 years and may lead to transient
ischemic attack (TIA), ischemic stroke or cerebral hemorrhage
(6). Without appropriate
treatment, the prognosis of patients with MMS is poor (7). Therefore, identifying the etiology of
MMS along with prompt diagnosis and treatment is essential to
improve patient outcomes. Although a variety of hematological
disorders have been identified as causes of MMS (1-3),
it is unclear whether thalassemia is a cause of MMS. The present
study describes the case of a 43-year-old man with α-thalassemia
who manifested moyamoya vessels with a ruptured aneurysm bleeding
into the ventricle.
Case report
A 43-year-old male was admitted to the Xiaolan
People's Hospital of Zhongshan (Zhongshan, China) due to the sudden
manifestation of headache, dizziness and nausea. The patient was
generally healthy, with a family history of anemia, no family
history of spontaneous intracerebral hemorrhage or aneurysm, and no
any history of hypertension, diabetes or any other chronic disease.
Physical examination was unremarkable except for a stiff neck, an
overgrown maxilla and a prominent forehead. Routine blood analysis
revealed a hemoglobin level of 94 g/l (normal range, 110-160 g/l),
a mean corpuscular volume of 63 fl (normal range, 82-92 fl) and a
mean corpuscular hemoglobin content of 18.2 pg (normal range, 27-31
pg). All remaining laboratory examinations were normal. Head
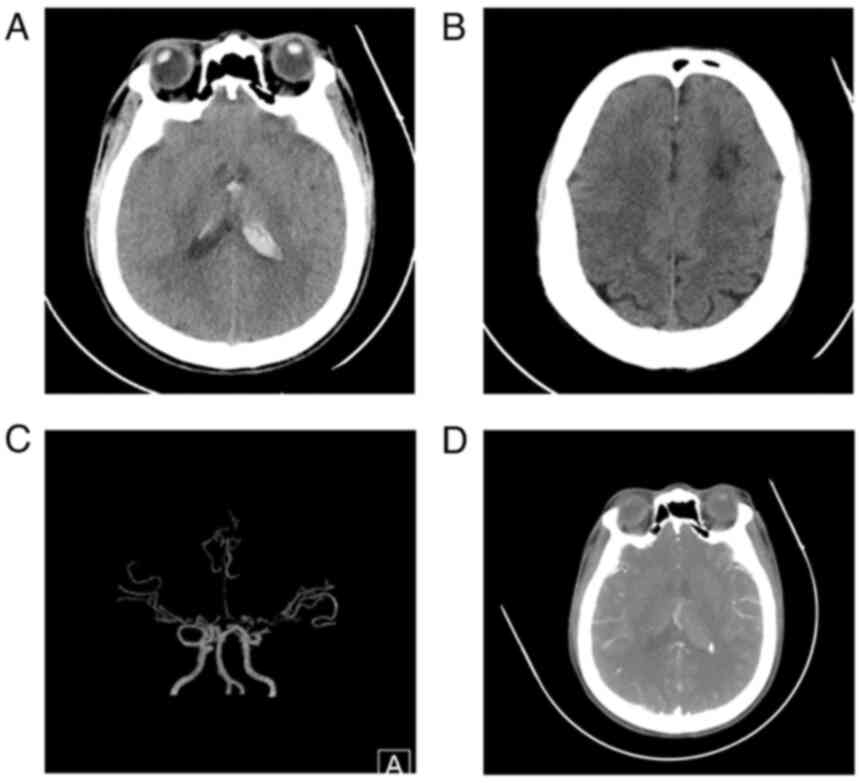
computed tomography (CT) revealed a hemorrhage from the ventricular
system and localized white matter hypodensity in the left frontal
lobe (Fig. 1A and B). CT angiography indicated bilateral
internal carotid artery terminal segments, bilateral anterior
cerebral arteries, bilateral middle cerebral artery stenosis with
moyamoya vessels and subependymal nodular dense opacities in the
posterior horn of the left lateral ventricle (Fig. 1C and D). Other disorders associated with MMS,
such as vasculitis (8), autoimmune
disease (9), infection (10) and thrombophilia (11), were excluded through medical
history, physical examination and relevant blood tests. The patient
was duly diagnosed with moyamoya disease (MMD) with aneurysm
rupture and bleeding. The cause of the anemia was unknown.
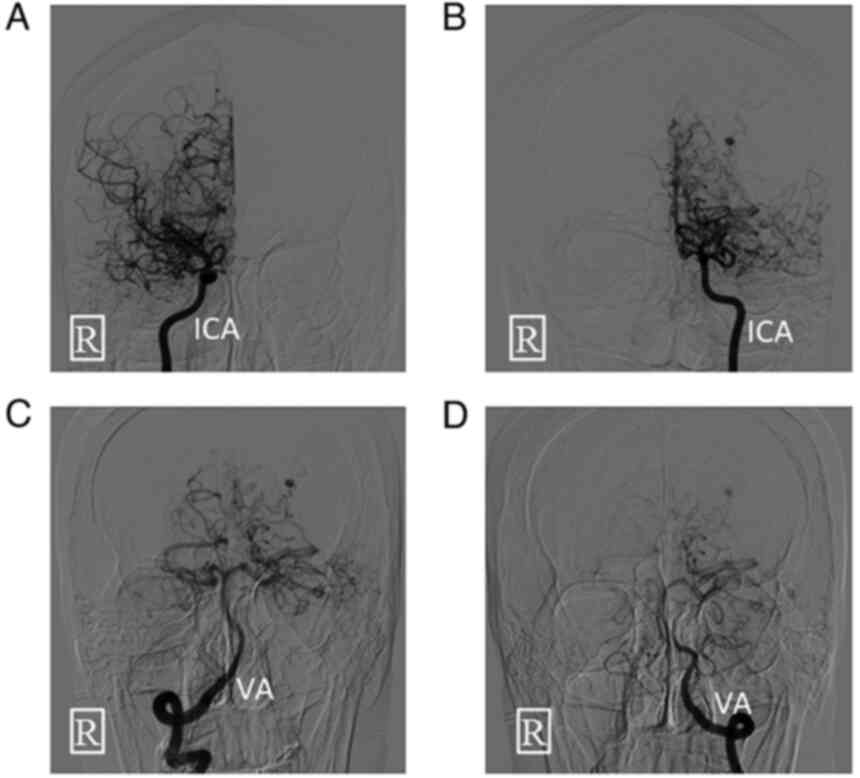
Digital subtraction angiography was performed on the
day of admission (Fig. 2) followed
by cerebral aneurysm embolization. During surgery, a Marathon
microcatheter was used to superselect the origin of the left
posterolateral choroidal artery. The aneurysm and parent artery
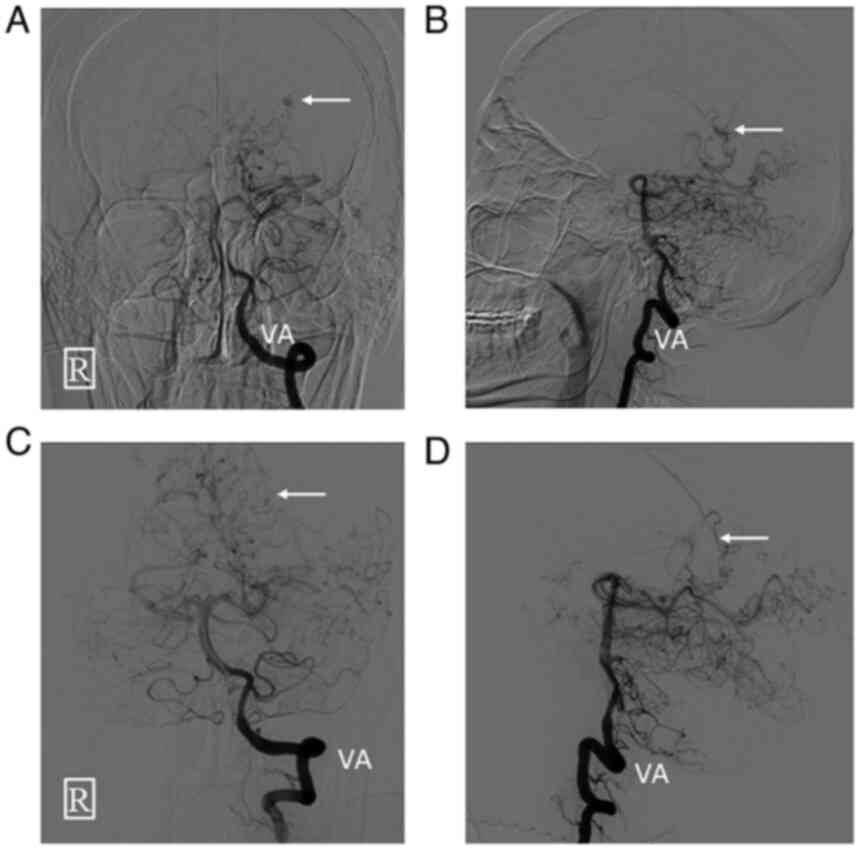
were then occluded with Glubran glue. Postoperative angiography
showed that the aneurysm and the left posterolateral choroidal
artery were completely occluded and no longer visualized (Fig. 3).
The patient was subsequently diagnosed with
α-thalassemia with a -SEA/-α3.7 genotype by
genetic testing. The patient was given intermittent lumbar
puncture, analgesic (0.3 g/bid ibuprofen) and fluid infusion
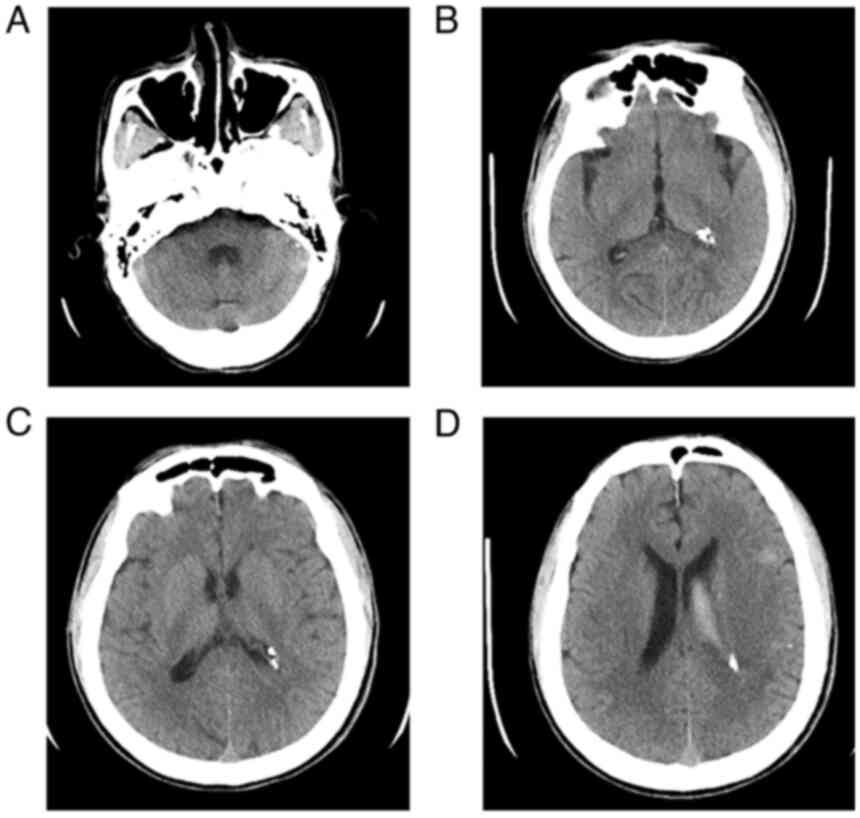
(normal saline and 5% glucose solution). At 18 days after surgery,
CT scans revealed that the ventricles were unobstructed, whereas
strip-like and nodular dense opacities were newly evident in the
left ventricle, which were consistent with the postoperative
changes (Fig. 4). The condition of
the patient improved and they were discharged from the hospital.
According to the usual MMS treatment procedures of the Xiaolan
People's Hospital of Zhongshan, long-term strict follow-up was
planned, and left superficial temporal artery and middle cerebral
artery bypass will be performed at 3 months post-surgery. Before
bypass surgery, whole-brain CT perfusion (CTP) will be performed to
evaluate whether to adjust the surgical plan. During follow-up, if
the patient has a rapid decrease in hemoglobin or rapid enlargement
of the spleen, a blood transfusion or splenectomy will be performed
after a comprehensive evaluation. At 1 month post-discharge, CT
confirmed that each ventricle was unobstructed, the
intraventricular hemorrhage had been completely absorbed and an
embolic glue artifact appeared in the left ventricle (Fig. S1).
Discussion
α-thalassemia is a single-gene genetic disease with
a high global incidence. Approximately 5% of the global population
carry mutations in the α-globin gene (12). High incidence areas include
tropical and subtropical regions such as southern China, Southeast
Asia, the Mediterranean region, India, the Middle East and Africa
(13,14). In China, epidemiological surveys
show that the highest rates of α-thalassemia mutation occur in the
Guangdong (12.70%), Guangxi (19.11%) and Hainan (45.04%) provinces
(15). In China, Japan and South
Korea, the annual incidence of newly diagnosed cases can be as high
as 6.03 per 100,000 individuals (16). To the best of our knowledge, there
is no report of any demographic research on MMD in the Guangxi and
Guangdong provinces. MMS manifests in a variety of ways, including
TIA, reversible ischemic neurological deficit, ischemic stroke,
hemorrhagic stroke, epilepsy, cognitive impairment, involuntary
movements and headache (17,18).
The present patient was born in Guangxi, China, which is an area
with a high incidence of α-thalassemia. Causes of MMS such as
atherosclerosis, neurofibromatosis (multiple), intracranial tumors,
radiation injury and hyperthyroidism were excluded through
examination of the patient's medical history, laboratory tests and
radiographic imaging. To the best of our knowledge, there are few
reports relating to MMS associated with α-thalassemia.
Thalassemia is a common and autosomal recessive
genetic disease with a high incidence (19). According to the type of globin
synthesis disorder, thalassemia can be divided into α-thalassemia
and β-thalassemia (19). Mutations
or deletions of the globin gene in thalassemia result in an
imbalance in globin synthesis, premature destruction of erythrocyte
precursors in the bone marrow, hemolysis of erythrocytes in the
peripheral blood and the appearance of abnormal erythrocytes
(20). Endothelial damage,
platelet activation, increased plasma microparticles, impaired
nitric oxide bioavailability, increased blood oxidants, loss of
erythrocyte deformability and phosphatidylserine exposure on the
outer leaflet of the red blood cell membrane lead to a
hypercoagulable state (21-23).
Anemia and hypercoagulability may lead to tissue hypoxia,
endothelial hypertrophy and microvascular stenosis (23,24).
MMS is occasionally reported in β-thalassemia. In a previous study,
among 13 patients with β-thalassemia and MMS, 7 patients had
non-transfusion-dependent thalassemia, whereas 3 patients had
hemoglobin E (HbE)-β thalassemia (4). In another recent study, a patient
with HbE-β-thalassemia was diagnosed with MMS at the age of 9
years, and magnetic resonance imaging showed the presence of
multiple old lacunar infarcts in both cerebral hemispheres
(25). The patient's age at onset
was much younger than previously reported (25). Several studies have shown that
multiple cerebral infarction and large artery occlusion in
β-thalassemia-related MMS are associated with a chronic
hypercoagulable state (4,24,25).
As α-thalassemia and β-thalassemia share similar pathogenesis, we
hypothesize that the left frontal cerebral infarction and large
artery occlusion in the present study were related to the anemia
and hypercoagulable state. The establishment of an α-thalassemia
animal model is expected to further clarify the mechanism of
α-thalassemia-induced MMS and provide a theoretical basis for the
clinical diagnosis and treatment of α-thalassemia-related MMS.
To the best of our knowledge, there is currently no
definite and effective drug for MMS (26). Cases of MMS complicated by aneurysm
rupture should be treated surgically as soon as possible to prevent
rebleeding (27). In the present
case, the aneurysm was located at the distal end of the
posterolateral choroidal artery and had caused hemorrhage in the
ventricular system after rupture. Emergency embolization of the
aneurysm effectively prevented rebleeding. Intracranial and
extracranial revascularization surgery is the main treatment for
MMS and can effectively prevent ischemic stroke (28,29).
In recent years, the efficacy of extracranial and intracranial
revascularization for reducing the risk of bleeding has gradually
been confirmed (30,31). Intracranial and extracranial
revascularization procedures include direct techniques (e.g.,
external carotid artery-to-internal carotid artery bypass) and
indirect techniques (e.g., encephaloduroarteriosynangiosis,
omental-cranial transposition, encephalo-myo-synangiosis and
encephaloduroarteriomyosynangiosis) (16). Compared to indirect techniques,
direct techniques have the advantage that they can re-establish
blood flow immediately once the anastomosis is created. Since the
present patient had MMS with a ruptured aneurysm, the left
superficial temporal artery was bypassed and a middle cerebral
artery bypass was performed after the acute phase of intracranial
hemorrhage. Whole-brain CTP can reveal subtle hemodynamic changes
that can effectively guide surgery for MMD, evaluate changes in the
postoperative cerebral perfusion status and can be used for disease
follow-up (32). Whole-brain CTP
will be performed on the present patient prior to surgery to assess
the cerebral hemodynamic status and thus decide whether to adjust
the surgical plan.
In conclusion, α-thalassemia may represent a
causative factor for MMS. To the best of our knowledge, there is
currently no definite and effective drug for MMS. Patients with MMS
should undergo extracranial and intracranial revascularization as
soon as possible.
Supplementary Material
Head computed tomography performed 1
month after discharge showing that each ventricle is unobstructed
and that the intraventricular hemorrhage is completely absorbed.
The fourth (A) and the third ventricle (B) are unobstructed. (C and
D) The lateral ventricle is unobstructed, with embolic glue
artifacts in the left ventricle.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and MZ confirm the authenticity of all the raw
data. JZ and XZ designed the study and drafted the manuscript. MZ
and YS collected and analyzed the clinical data. XZ critically
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Informed consent for participation in the study or
use of the medical data was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this manuscript and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamani M, Obaid EF and Hemida AH: Moyamoya
syndrome in a 32-year-old male with sickle cell anemia. Cureus.
12(e10001)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al-Hawsawi ZM, Al-Zaid MA, Barnawi AI and
Yassine SM: Fanconi anemia associated with moyamoya disease in
Saudi Arabia. Saudi Med J. 36:233–235. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Meena SS, Ramkumar TV, Sharma S, Aneja S
and Kumar A: Moyamoya syndrome associated with severe iron
deficiency anemia in a young child. Pediatr Hematol Oncol.
29:368–371. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Das S, Dubey S, Acharya M, Chatterjee S,
Lahiri D, Das G, Ray BK and Kraemer M: Thalassemia and moyamoya
syndrome: Unfurling an intriguing association. J Neurol.
266:2838–2847. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bersano A, Guey S, Bedini G, Nava S, Hervé
D, Vajkoczy P, Tatlisumak T, Sareela M, van der Zwan A, Klijn CJ,
et al: Research progresses in understanding the pathophysiology of
moyamoya disease. Cerebrovasc Dis. 41:105–118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang L, Xing W and Zhang J: Recurrent
transient ischemic attacks in a moyamoya syndrome patient with
ultra-long imaging follow-up. Quant Imaging Med Surg. 12:2144–2152.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu XJ, Zhang D, Wang S, Zhao YL, Teo M,
Wang R, Cao Y, Ye X, Kang S and Zhao JZ: Clinical features and
long-term outcomes of moyamoya disease: A single-center experience
with 528 cases in China. J Neurosurg. 122:392–399. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shindo T, Ito M, Sugiyama T, Okuyama T,
Kono M, Atsumi T and Fujimura M: Diagnostic value of vessel wall
imaging to determine the timing of extracranial-intracranial bypass
for moyamoya syndrome associated with active Sjögren's syndrome: A
case report. J Neurol Surg A Cent Eur Neurosurg: Apr 22, 2022 (Epub
ahead of print). doi: 10.1055/a-1832-3269.
|
|
9
|
Santoro JD, Lee S, Wang AC, Ho E, Nagesh
D, Khoshnood M, Tanna R, Durazo-Arvizu RA, Manning MA, Skotko BG,
et al: Increased autoimmunity in individuals with down syndrome and
moyamoya disease. Front Neurol. 12(724969)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nagel MA, Niemeyer CS and Bubak AN:
Central nervous system infections produced by varicella zoster
virus. Curr Opin Infect Dis. 33:273–278. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Salih MA, Murshid WR, Al-Salman MM,
Abdel-Gader AG, Al-Jarallah AA, Alorainy IA, Hassan HH, Kentab AY,
Van Maldergem L, Othman SA, et al: Moyamoya syndrome as a risk
factor for stroke in Saudi children. Novel and usual associations.
Saudi Med J. 27 (Suppl 1):S69–S80. 2006.PubMed/NCBI
|
|
12
|
Piel FB and Weatherall DJ: The
α-thalassemias. N Engl J Med. 371:1908–1916. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Modell B and Darlison M: Global
epidemiology of haemoglobin disorders and derived service
indicators. Bull World Health Organ. 86:480–487. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chui DH: Alpha-thalassaemia and population
health in Southeast Asia. Ann Hum Biol. 32:123–130. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shang X, Peng Z, Ye Y, Asan Zhang X, Chen
Y, Zhu B, Cai W, Chen S, Cai R, et al: Rapid targeted
next-generation sequencing platform for molecular screening and
clinical genotyping in subjects with hemoglobinopathies.
EBioMedicin. 23:150–159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Berry JA, Cortez V, Toor H, Saini H and
Siddiqi J: Moyamoya: An update and review. Cureus.
12(e10994)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Newman S, Boulter JH, Malcolm JG, Pradilla
I and Pradilla G: Outcomes in patients with moyamoya syndrome and
sickle cell disease: A systematic review. World Neurosurg.
135:165–170. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang H, Zheng L and Feng L: Epidemiology,
diagnosis and treatment of moyamoya disease. Exp Ther Med.
17:1977–1984. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wu H, Huang Q, Yu Z and Zhong Z: Molecular
analysis of alpha- and beta-thalassemia in Meizhou region and
comparison of gene mutation spectrum with different regions of
Southern China. J Clin Lab Anal. 35(e24105)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang Y, Long Y, Deng D, Liu Z, Liang H,
Sun N, Xu Y, Lai Y and Cheng P: Alterations of anticoagulant
proteins and soluble endothelial protein C receptor in thalassemia
patients of Chinese origin. Thromb Res. 172:61–66. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gallagher PG: Disorders of erythrocyte
hydration. Blood. 130:2699–2708. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Litvinov RI and Weisel JW: Role of red
blood cells in haemostasis and thrombosis. ISBT Sci Ser.
12:176–183. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bhattacharyya M, Kannan M, Chaudhry VP,
Mahapatra M, Pati H and Saxena R: Hypercoagulable state in five
thalassemia intermedia patients. Clin Appl Thromb Hemost.
13:422–427. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Doctor PN, Choudhari A, Verma M and
Merchant RH: Moyamoya syndrome in hemoglobin E-beta thalassemia: A
rare presentation and association. J Postgrad Med. 64:240–242.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zahra A, Al-Abboh H, Habeeb Y and Adekile
A: Moyamoya syndrome in a child with HbEβ-thalassemia. Clin Case
Rep. 10(e05536)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jang DK, Lee KS, Rha HK, Huh PW, Yang JH,
Park IS, Ahn JG, Sung JH and Han YM: Bypass surgery versus medical
treatment for symptomatic moyamoya disease in adults. J Neurosurg.
127:492–502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao X, Wang X, Wang M, Meng Q and Wang C:
Treatment strategies of ruptured intracranial aneurysms associated
with moyamoya disease. Brit J Neurosurg. 35:209–215.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kuroda S and Houkin K: Bypass surgery for
moyamoya disease: Concept and essence of sugical techniques. Neurol
Med Chir (Tokyo). 52:287–294. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ren B, Zhang ZS, Liu WW, Bao XY, Li DS,
Han C, Xian P, Zhao F, Wang H, Wang H and Duan L: Surgical outcomes
following encephaloduroarteriosynangiosis in adult moyamoya disease
associated with type 2 diabetes. J Neurosurg. 125:308–314.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Miyamoto S, Yoshimoto T, Hashimoto N,
Okada Y, Tsuji I, Tominaga T, Nakagawara J and Takahashi JC: JAM
Trial Investigators. Effects of extracranial-intracranial bypass
for patients with hemorrhagic moyamoya disease: Results of the
Japan adult moyamoya trial. Stroke. 45:1415–1421. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wan M and Duan L: Recent progress in
hemorrhagic moyamoya disease. Br J Neurosurg. 29:189–191.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Han Q, Yao F, Zhang Z and Huang Y:
Evaluation of revascularization in different Suzuki stages of
ischemic moyamoya disease by whole-brain CT perfusion. Front
Neurol. 12(683224)2021.PubMed/NCBI View Article : Google Scholar
|