Introduction
Breast cancer is the most common malignancy among
women worldwide. In the US, approximately 284,200 new cases of
breast cancer were diagnosed, accounting for 30% of female cancer
cases in 2021(1). Despite
improvements in cancer treatment, the relapse and metastasis of
breast cancer still present great difficulties for patients, most
of whom are hormone receptor-positive.
Actually, for hormone receptor-positive advanced
breast cancer (ABC) patients who do not have symptomatic visceral
disease, endocrine therapy (ET) is the preferred first-line therapy
(2,3). More importantly, ET is often
continued in the second- and third-line setting, with chemotherapy
deferred until the tumor becomes endocrine resistance and/or a
visceral crisis is imminent. Although more than 70% of patients
with ABC are hormone receptor-positive and candidates for ET, the
clinical benefit will eventually diminish as endocrine resistance
develops. Thus, a rapidly growing body of research has been
undertaken to identify novel drugs or treatment strategies that
could specifically reverse endocrine resistance.
For years, endocrine agents (such as exemestane or
fulvestrant) that lack cross-resistance with existing treatments
are given to the patient after progression on prior endocrine
therapy, thereby delaying the use of cytotoxic agents and
maintaining quality of life (4).
After several intracellular pathways were found to promote
resistance to anti-estrogen therapy, novel endocrine drug
combinations began to reform treatment schema and expand
therapeutic options (5-7).
Moreover, epigenetic modifications including DNA methylation and
histone modifications, which both lead to chromatin remodeling,
also contribute to endocrine resistance (8). It is widely known that histone
deacetylases (HDACs) are proteins required for control of gene
expression and exert an anti-proliferative effect and promote
apoptosis (9). Entinostat, a class
I selective oral HDAC inhibitor, has been shown to increase
expression of both the estrogen receptor (ER) and the enzyme
aromatase in a dose-dependent manner in vitro, which then
sensitizes breast cancer cells to estrogen and subsequent
inhibition by the aromatase inhibitor letrozole (10). Furthermore, entinostat was also
able to epigenetically induce leukemia inhibitory factor receptor
(LIFR) expression and activate a pro-dormancy program in breast
cancer cells, thereby slowing breast cancer cell proliferation and
reducing primary tumor growth in vivo (11). Thus, recently, several clinical
trials have been initiated to assess the efficacy of HDAC
inhibitors to restore sensitivity of breast cancer to hormone
manipulation (12,13). In a randomized phase II trial
(ENCORE 301, NCT00676663), entinostat in combination with
exemestane was compared to exemestane plus placebo in hormone
receptor-positive ABC patients who had received prior endocrine
therapy. The ENCORE 301 trial demonstrated that entinostat
significantly improved survival in hormone receptor-positive,
HER2-negative ABC patients who exhibited progression on previous
non-steroidal aromatase inhibitor (NSAI) therapy when combined with
exemestane (14). Interestingly,
E2112, a randomized phase III trial which closely mirrored the
design of the ENCORE 301 trial, failed to replicate the promising
results observed in the previous trial (15). In this regard, whether the
combination of an HDAC inhibitor and exemestane provides survival
benefit to the patients in this setting remains controversial.
Hence, we performed a meta-analysis of phase II and phase III
randomized controlled trials (RCTs) comparing the efficacy and
toxicity of an HDAC inhibitor plus exemestane with exemestane alone
in those hormone receptor-positive ABC patients who progressed on
prior endocrine therapy.
Materials and methods
Literature search and inclusion
criteria
All the phase II or phase III randomized controlled
trials (RCTs) which compared HDAC inhibitors plus exemestane with
exemestane alone in hormone receptor-positive ABC which progressed
on previous endocrine therapy from January 1, 1990 to December 31,
2021 from Pubmed, Cochrane library and Embase database were
searched. We also searched for relevant ongoing studies in the
ClinicalTrials.gov network (https://clinicaltrials.gov). In addition, posters and
abstracts of the annual meeting of European Society of Medical
Oncology (ESMO) and San Antonio Breast Cancer Symposium (SABCS) in
the past 10 years were also scanned using the relevant keywords.
The search algorithm was ‘[((breast OR mammary) AND (carcinoma OR
neoplasm OR tumor OR cancer) AND (metastatic OR advanced OR
relapse*) AND (pretreat*)) AND ((histone deacetylase inhibitors) OR
(HDAC inhibitors))]’. All the reference lists of the included
articles were scanned as well.
Our inclusion criteria were: i) prospective phase II
or phase III RCTs; ii) RCTs comparing HDAC inhibitors plus
exemestane with exemestane single agent in hormone
receptor-positive ABC that progressed on previous endocrine
therapy; iii) sufficient data for extraction, stratification, and
calculation of the study was available. Comments, noncomparative
studies, case reports, review articles were excluded.
Quality assessment
Two reviewers carefully evaluated the quality and
the eligibility of the studies independently. Disagreements were
resolved by discussion with a third reviewer. The quantitative
Jadad scale was used to assess study quality: i) whether the trial
reported an appropriate randomization method (0-2 scores); ii)
whether the report included an appropriate blinding method (0-2
scores); iii) whether the report included an account of the number
of withdrawals or dropouts.
Data extraction
The following useful information was extracted with
a custom-made spreadsheet and were checked for accuracy: authors'
names, date of publication, study design and allocated patient
details in both the experimental and control group, main patient
characteristics (patient no., median age, race, sex, menopausal
status, hormone receptor and HER2 status, line of therapy,
sensitivity to prior endocrine therapy), and data such as regimens,
drug dose, progression-free survival (PFS), overall survival (OS),
overall response rate (ORR), clinical benefit rate (CBR) and
corresponding pooled risk ratio (RR), pooled hazard ratio (HR), and
95% confidence interval (CI).
Statistically analysis
In order to appraise PFS and OS, HR and 95% CI were
employed. Similarly, RR and 95% CI were used to evaluate the ORR,
CBR and adverse effects (AEs). HRs, RRs and 95% CIs were collected
from the original publications directly if they were reported. In
addition, χ2-based Q-test was used to
estimate the heterogeneity between the groups (16). Heterogeneity was considered to
exist at Pheterogeneity <0.1 or I2
>50%, and a random effect model was used. Otherwise, a fixed
effect model was used (17).
Moreover, publication bias was evaluated using funnel plot and
regression test, according to the method reported by Begg and
Egger. All the calculations were performed using the STATA version
12.0 software (Stata Corp.). Of note, HR >1 suggests more PFS or
OS events in the combination group compared with exemestane
single-agent group; RR >1 suggests more relevant AEs and better
treatment response in the combination group, and vice versa.
Results
Study search and eligibility
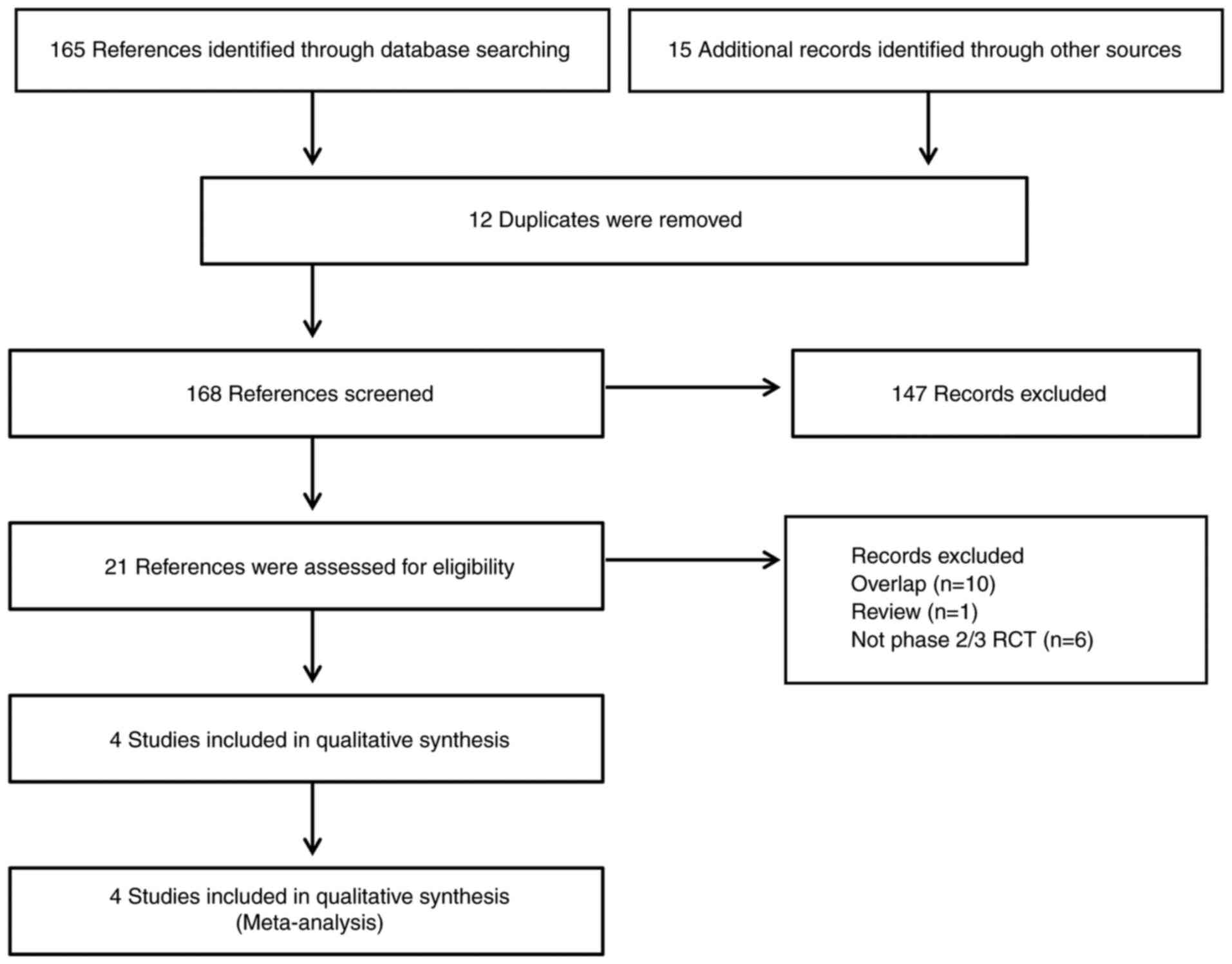
As shown in Fig. 1,
the search strategy yielded 165 records from Pubmed, Embase,
Cochrane library and conference abstracts; 15 more results were
added manually from other sources. Subsequently, 12 duplicates were
removed. We scanned the titles and abstracts of the 168 records
remaining, and then 21 references were accessed for eligibility.
Finally, only 4 studies (14,15,18,19)
that fulfilled the eligibility criteria were included in our
qualitative synthesis.
Study characteristics and
patients
A total of 1,457 hormone receptor-positive ABC
patients who had progressed on prior endocrine therapy were
eligible for this systematic review and meta-analysis. Of these,
848 patients received HDAC inhibitor and exemestane combination
therapy, and 609 patients received placebo plus exemestane therapy
(exemestane single-agent group). The characteristics of these
studies are listed in Table I. In
the four eligible studies, one was a phase II RCT and three were
phase III RCTs. Concerning the HDAC inhibitors, entinostat was
administered in 3 studies and tucidinostat was given in one study.
As shown in Table II, the
majority of the included patients were HER2-negative and
postmenopausal. Of note, all the premenopausal or perimenopausal
patients concurrently received ovarian function suppression (OFS)
with a luteinizing-hormone-releasing-hormone agonist (LHRHa).
 | Table ICharacteristics of the four trials
eligible for the present meta-analysis. |
Table I
Characteristics of the four trials
eligible for the present meta-analysis.
| First author | Year | Phase | Randomized | Double-blind | Regimens | Jadad score | (Refs.) |
|---|
| Yardley et
al | 2013 | II | Yes | Yes |
Entinostat+Exemestane/Placebo+Exemestane | 3 | (14) |
| Jiang et
al | 2019 | III | Yes | Yes |
Tucidinostat+Exemestane/Placebo+Exemestane | 3 | (19) |
| Xu | 2021 | III | Yes | Yes |
Entinostat+Exemestane/Placebo+Exemestane | 3 | (18) |
| Connolly et
al | 2021 | Ⅲ | Yes | Yes |
Entinostat+Exemestane/Placebo+Exemestane | 3 | (15) |
 | Table IIPatient characteristics and outcomes
in the present meta-analysis. |
Table II
Patient characteristics and outcomes
in the present meta-analysis.
| First author | No. of
patients | Menopausal
status | HER2 status | ORR (doublet agents
vs. single-agent) | Median PFS (doublet
agents vs. single-agent, months) | Median OS (doublet
agents vs. single-agent, months) | (Refs.) |
|---|
| Yardley et
al | 130 | Post (100%) | Negative
(90.8%) | 6.3 vs. 4.6% | 4.3 vs. 2.3 | 28.1 vs. 19.8 | (14) |
| Jiang et
al | 365 | Post (100%) | Negative | 18 vs. 9% | 7.4 vs. 3.8 | NR | (19) |
| Xu | 354 | Pre, Peri, and
Post | Negative | 17.4 vs. 10.9% | 6.32 vs. 3.72 | NR | (18) |
| Connolly et
al | 608 | Pre, Peri, and
Post- | Negative | 5.8 vs. 5.6% | 3.3 vs. 3.1 | 23.4 vs. 21.7 | (15) |
Progression-free and overall
survival
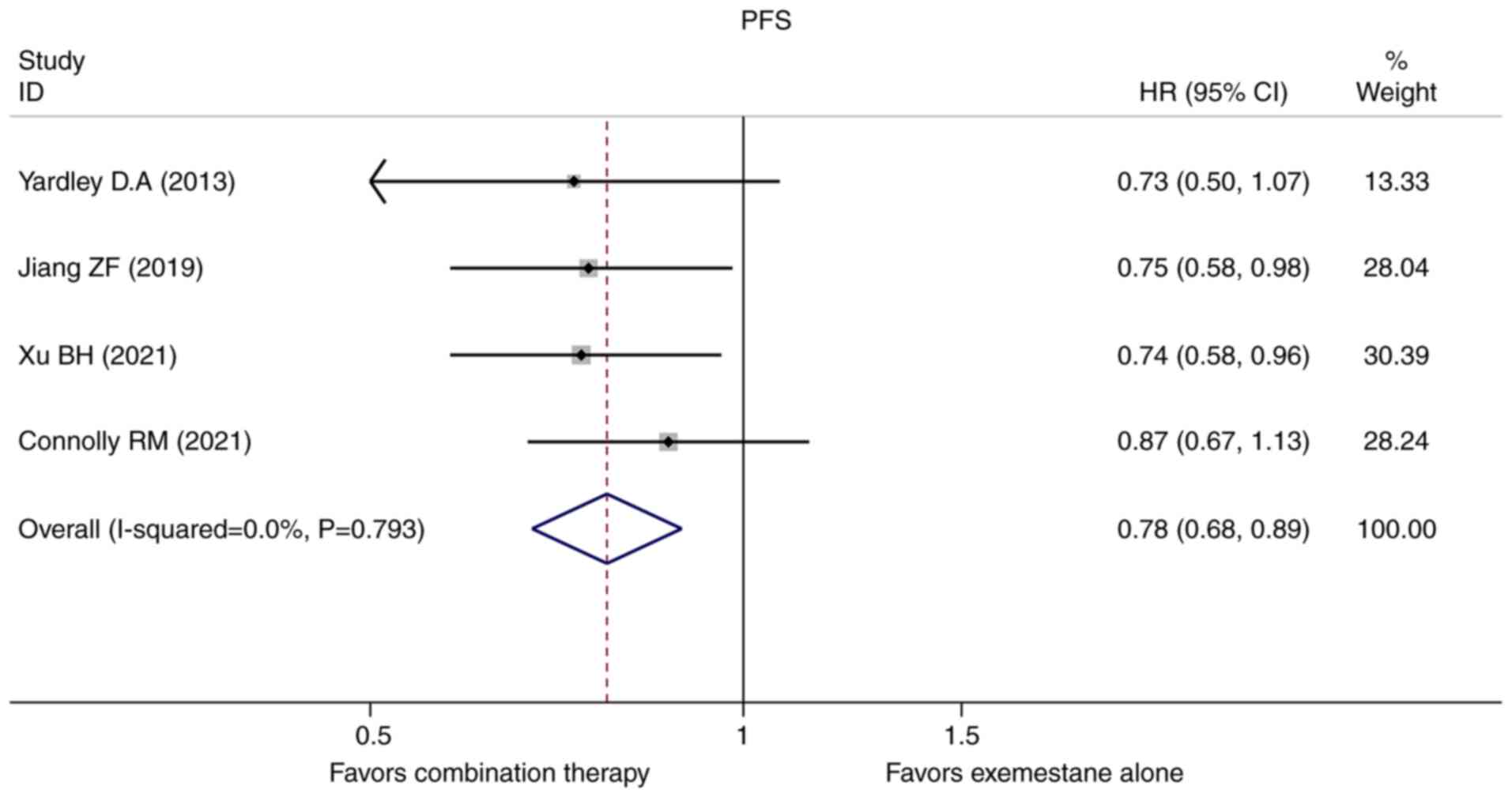
In this meta-analysis, the HRs and 95% CIs for PFS
were extracted directly in all the four original studies included.
A fixed-effect model was used to evaluate the pooled PFS because no
obvious heterogeneity existed. Pooled HR for PFS was 0.776 (95%
CI=0.675-0.892, P=0.000, Fig. 2).
Our results demonstrated that HDAC inhibitors plus exemestane
combination therapy was associated with a 22.4% reduction in
disease progression when compared with exemestane alone. In terms
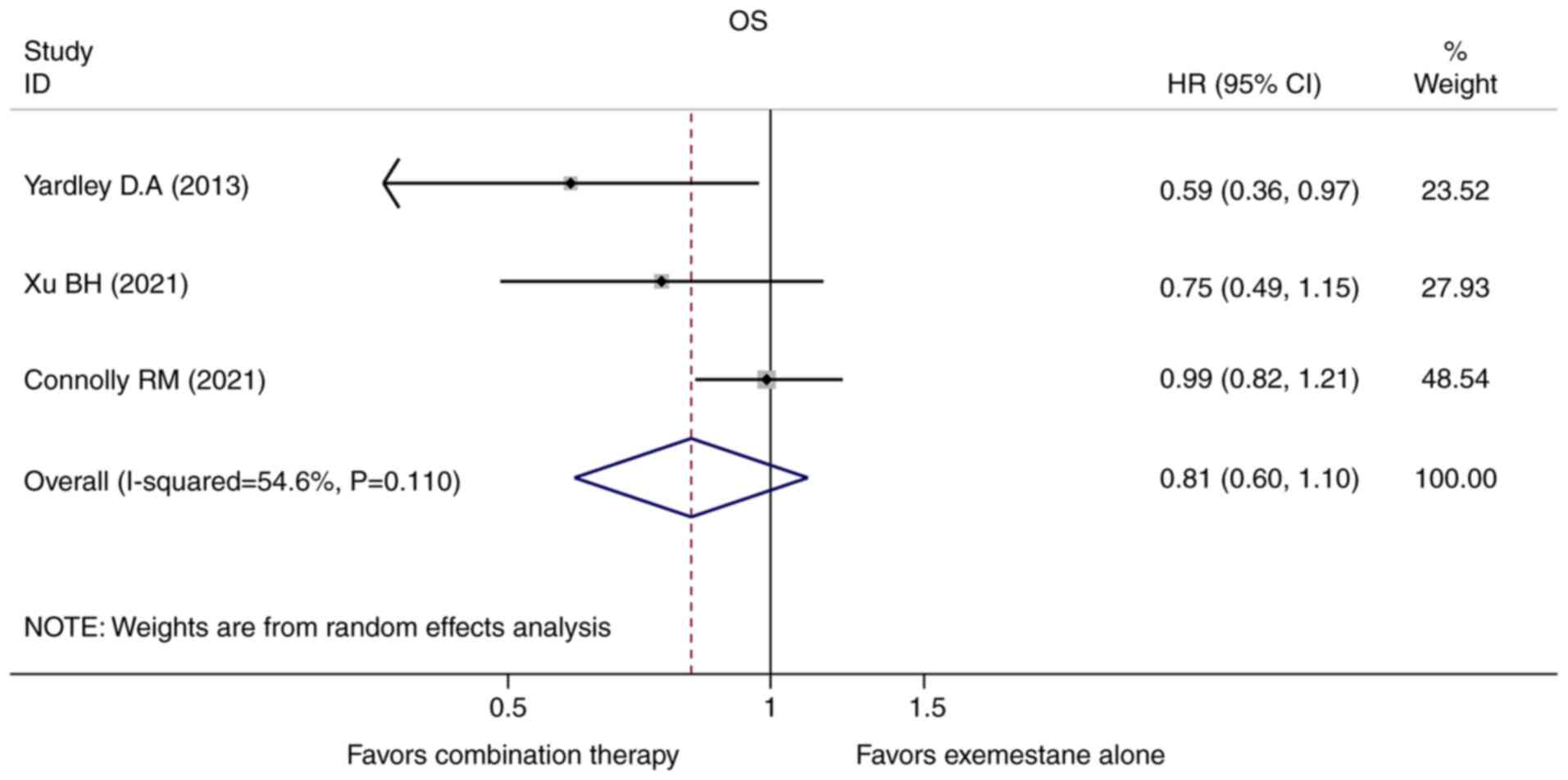
of OS, only three studies provided the HRs and the corresponding
95% CIs. A random-effect model was used to calculate the pooled HR
and 95% for OS because a statistically significant heterogeneity
existed (Pheterogeneity=0.110,
I2=54.6%). We found the combination of an HDAC inhibitor
plus exemestane was insufficient to prolong OS of the hormone
receptor-positive ABC patients who progressed after endocrine
therapy when compared with exemestane alone (HR=0.811, 95%
CI=0.596-1.104, P=0.183, Fig.
3).
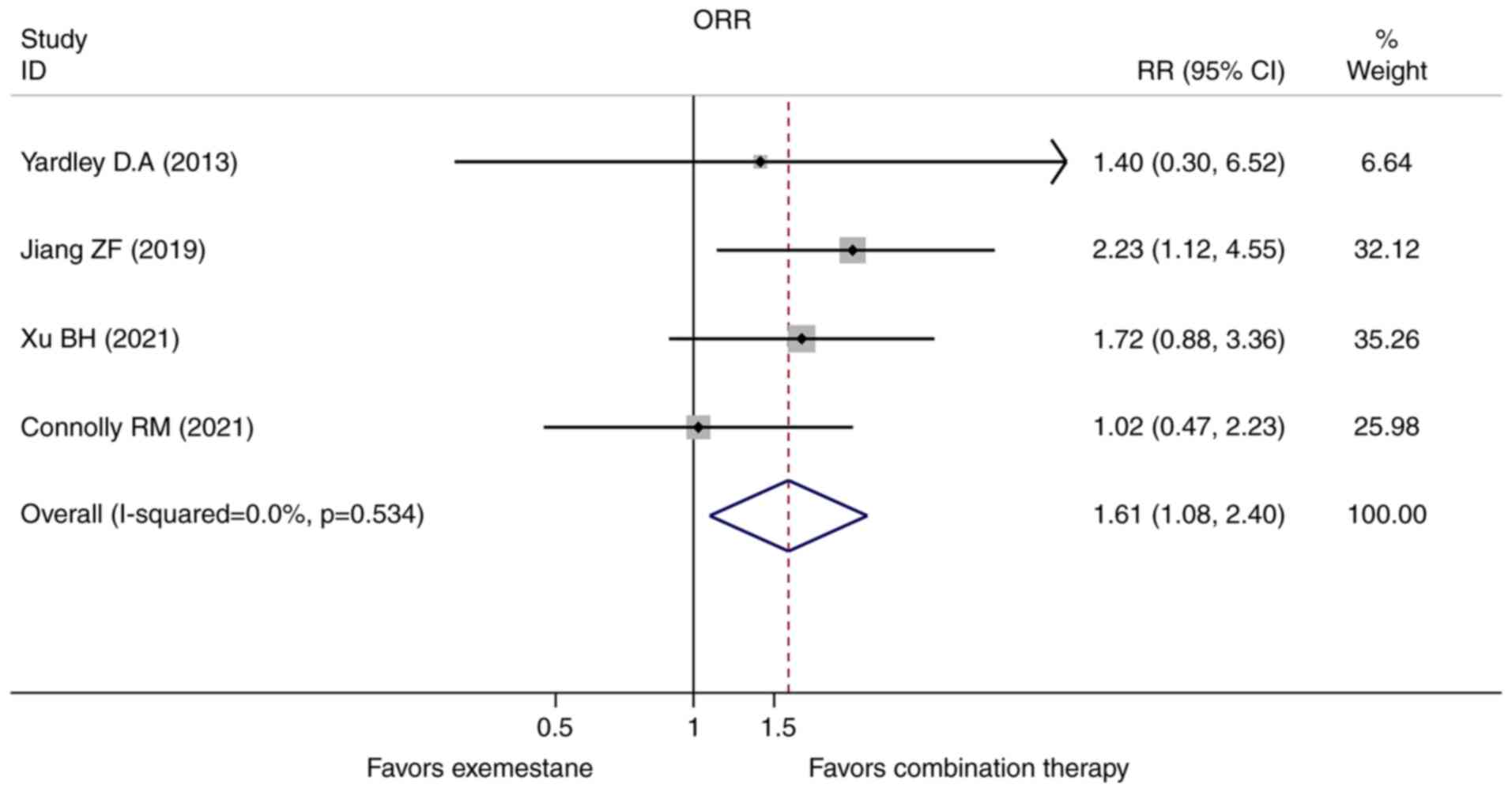
Overall response rate
The ORR data was reported directly in all the four
studies. The heterogeneity test indicated no significant
heterogeneity found, thus the pooled RR was calculated using
fixed-effect model subsequently. Consistent with the PFS, the
combination group also exhibited improved ORR compared with the
exemestane single-agent in the hormone receptor-positive advanced
breast cancer patients who progressed on prior endocrine therapy
(RR=1.612, 95% CI=1.085-2.396, P=0.018, Fig. 4).
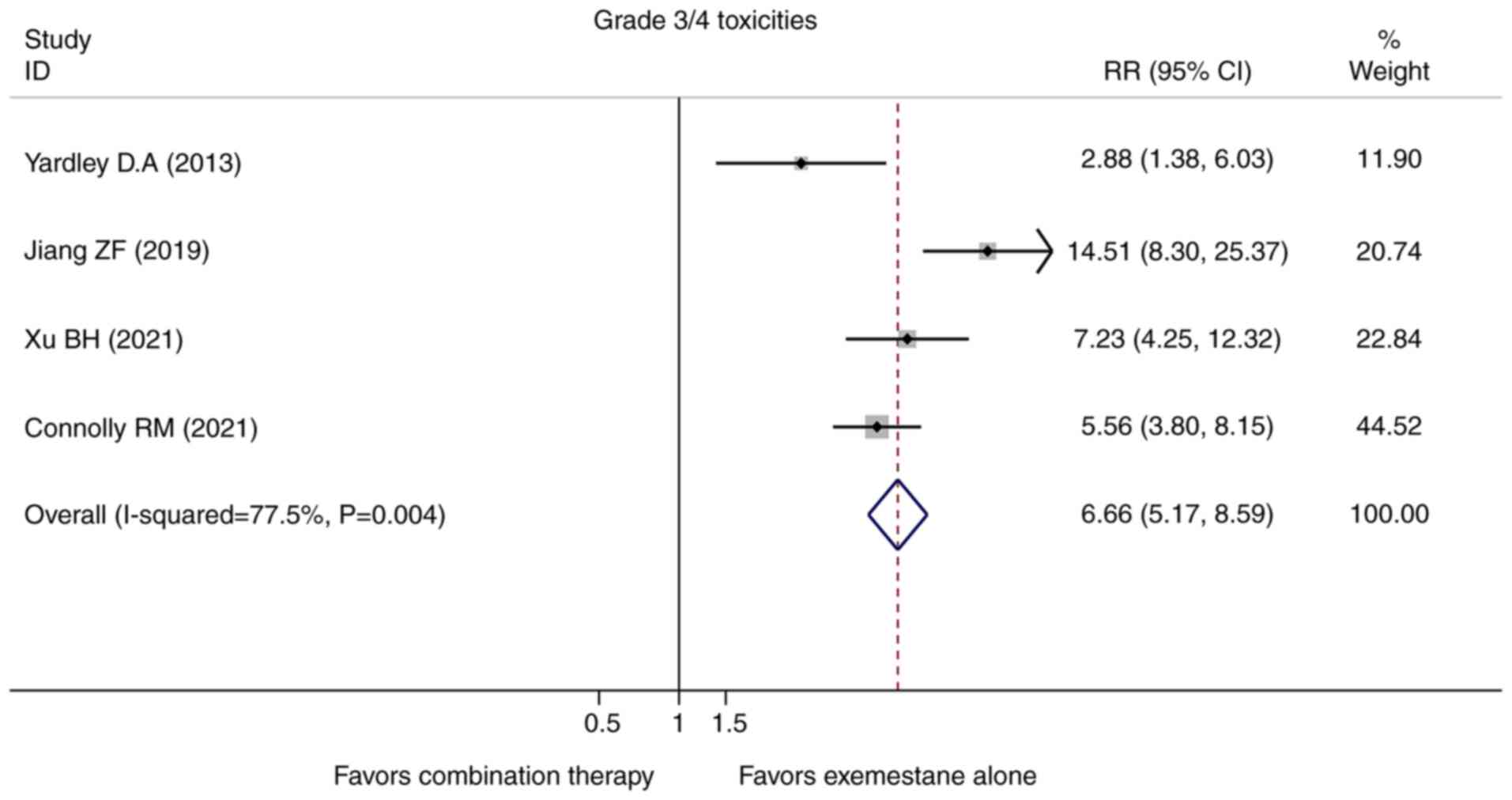
Toxicities
With respect to the toxicities and safety, more side
effects and drug-related treatment withdrawals were observed in the
combination group in the majority of the included studies. All four
studies included in this meta-analysis reported the grade 3/4
toxicities, and the pooled analysis showed that the combination
group developed more grade 3/4 toxicities although most of them
were asymptomatic (RR=6.663, 95% CI=5.166-8.595, P=0.000, Fig. 5). Importantly, few patients
experienced a neutropenic fever even though the predominant
treatment-related adverse effects in the combination group were
hematological side effects such as neutropenia and anemia.
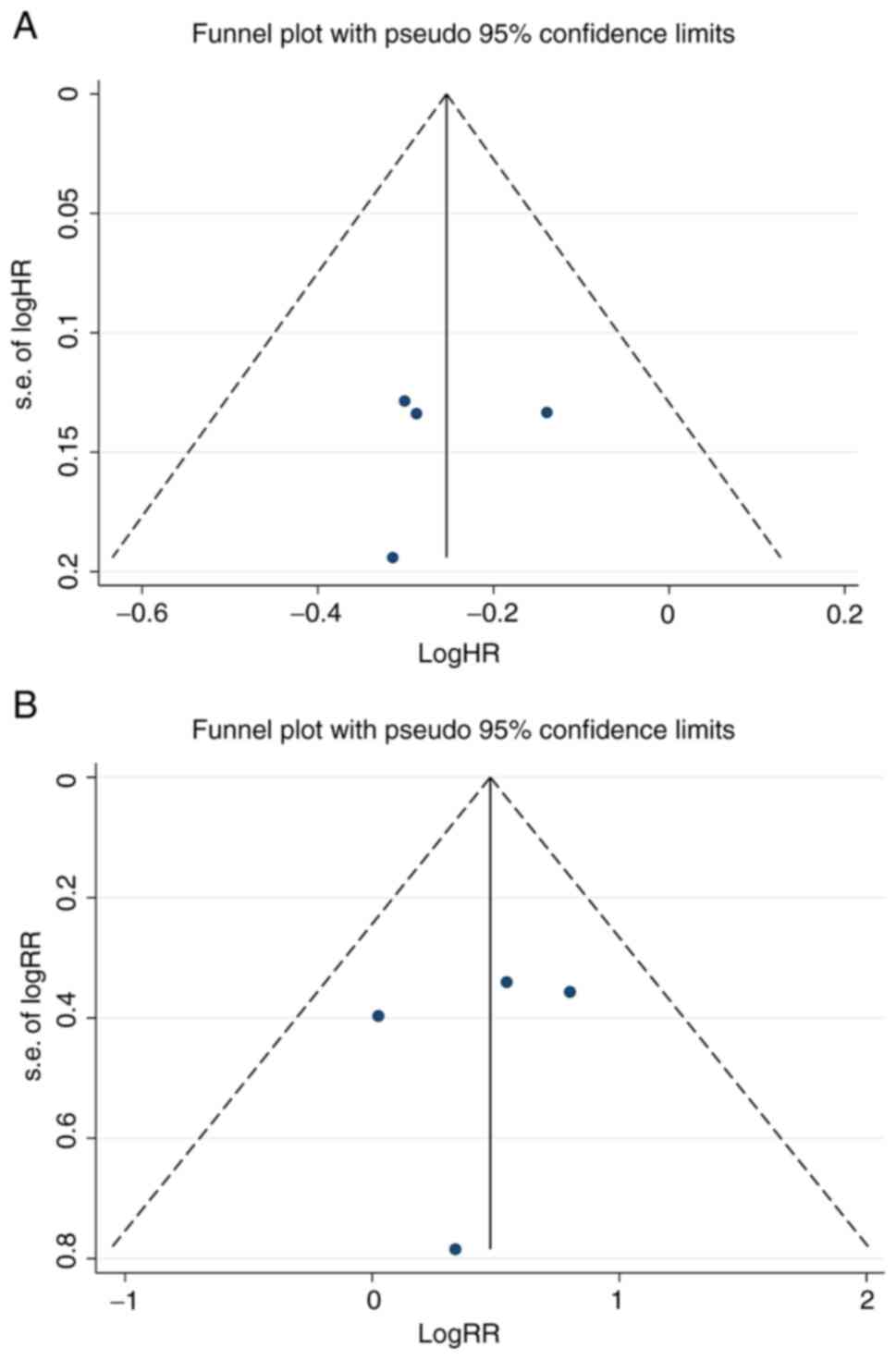
Publication bias
We also evaluated the publication bias using several
methods. The results of the Begg's test and the Egger's test showed
that no obvious publication bias existed (PFS: Begg's test:
P=1.000, Egger's test: P=0.719; ORR: Begg's test: P=0.734, Egger's
test: P=0.718). As shown in Fig.
6A and B, symmetric funnel
plots of the trials included in this meta-analysis suggested no
evidence of publication bias.
Discussion
Despite the great advances that have been made in
diagnostics and treatments for breast carcinoma, a substantial
proportion of patients are still diagnosed with advanced or
metastatic disease at initial presentation or develop disease
relapse. In fact, approximately 70-75% patients with advanced
breast cancer (ABC) are hormone receptor-positive, and endocrine
therapy is the recommend treatment because of the excellent
clinical efficacy and manageable safety profile (20). Unfortunately, a significant number
of patients inevitably develop endocrine resistance, which remains
a major clinical challenge.
In the past few decades, the treatment landscape for
hormone receptor-positive/HER2-negative ABC has evolved rapidly.
Accumulating preclinical evidence has demonstrated that resistance
to endocrine therapy is a multi-step procedure in which many
cellular and molecular parameters are involved (21). Increasing clinical trials aimed to
evaluate the feasibility of rational combinations of endocrine and
non-endocrine agents targeting the endocrine resistance-related
signaling pathway have been initiated; even those have only modest
activity when used alone, to abrogate endocrine resistance
(22-24).
Additionally, several studies have also suggested the critical role
of epigenetic modifications in the development of hormone
resistance (25,26). Additionally, various inhibitors
targeting DNA methylation and histone deacetylation enzyme are now
attracting particular attention as well (8). It is reported that quisinostat, a
class I and II HDAC, potentiated doxorubicin-induced cytotoxicity
in both breast cancer stem cells (CSCs) and non-CSCs derived from
various breast cancer cell lines in vitro (27). Moreover, researchers have also
demonstrated that low-dose entinostat adjuvant therapy was able to
inhibit metastases by disturbing the metastatic niche in a
xenograft model of breast cancer (28). Although HDAC inhibitors act as
promising cancer-treatment candidates in preclinical studies, their
therapeutic value in hormone receptor-positive ABC patients
resistant to traditionally endocrine agents remain unclear. The
results of randomized controlled trials evaluating the potential
role of HDAC inhibitors in hormone receptor-positive ABC are
inconsistent.
The aim of this meta-analysis was to determine the
clinical activity and safety of HDAC inhibitors in combination with
endocrine agents such as exemestane in treating breast cancer
patients who suffered relapse after previous endocrine therapy. Our
results demonstrated that the combination of an HDAC inhibitor plus
exemestane appears to be more efficacious compared with exemestane
alone. A longer PFS was shown in the combination group with a 22.4%
relative reduction in the risk of disease progression (HR=0.776,
95% CI=0.675-0.892, P=0.000, Fig.
2). Consistent with the PFS, ORR was also relatively higher in
the combination group (RR=1.612, 95% CI=1.085-2.396, P=0.018,
Fig. 4). However, the pooled
analysis revealed that the OS was similar in both groups (HR=0.811,
95% CI=0.596-1.104, P=0.183, Fig.
3). Indeed, OS can be challenging to assess in metastatic
breast cancer (MBC), given that patients may receive multiple
subsequent therapies after progression that can affect OS, thereby
confounding its reliability as the most robust end point. And PFS
is currently regarded as the most appropriate primary endpoint in
clinical trials that focus on treatment algorithms for hormone
receptor-positive/HER2-negative MBC population. Therefore, it is no
surprise that the significantly improved PFS did not translate into
clinical benefit of OS in the present meta-analysis (29). Taken together, our results clearly
indicate that the combinatorial therapy of an HDAC inhibitor and
exemestane is a promising strategy to overcome endocrine
resistance.
Actually, marginal survival gains of cytotoxic drugs
or targeted agents are often offset by persistent treatment-related
adverse effects in cancer (30).
In this meta-analysis, as compared with single-agent exemestane,
the incidence of treatment-related grade 3/4 adverse events was
more common in the combination group (Fig. 4). The predominant treatment-related
adverse events in the combination group were hematological
toxicities including neutropenia, thrombopenia and anemia. Although
the improved efficacy came at a cost of a modest increase in
toxicities, the combination of an HADC inhibitor administered
together with exemestane was associated with a manageable safety
profile.
Honestly, our work has several important limitations
and these results should be interpreted cautiously. First, this
meta-analysis has several sources of heterogeneity: different
treatment regimens, various prior endocrine agents and different
patient selections that inevitably introduced possible elements of
bias to the pooled analyses. For example, some patients included in
our meta-analysis had HER2 overexpression and were premenopausal
(14). It is well-known that HER2
activation has been identified as one of the most important
underlying mechanisms of endocrine resistance across a range of
experimental model systems and clinical trials (31). HER2 is found to crosstalk with many
well-defined oncogenic pathways closely related to endocrine
resistance. Therefore, HER2-positive patients and those anti-HER2
therapies themselves will definitely confound the pooled results in
turn (32,33). Moreover, the menopausal status also
contributed to the heterogeneity. For the perimenopausal and
premenopausal patients included, although LHRHa was given
simultaneously with the endocrine agents, the possibility that the
administered LHRHa had impact on the final estimation could not be
ruled out. After all, LHRHa, which was able to achieve OFS by
sustained suppression of the release of follicle-stimulating
hormone and luteinizing hormone from the pituitary (34), was reported as an effective
endocrine approach in breast cancer (35,36).
Second, the surgery, radiotherapy, line of therapy in the advanced
setting, metastatic sites, tumor burden, prior chemotherapy
regimens, as well as anti-estrogen treatment drugs and sequence in
these eligible studies were also heterogeneous. In addition, the
sensitivities to the most recent endocrine therapy were also not
homogeneous. Not even to mention that some patients received prior
CDK4/6 inhibitors or fulvestrant, and whether the subsequent
treatments including HDAC inhibitors could provide therapeutic
possibility for this population remain unknown. Third, not all of
the included studies administered the same HDAC inhibitor to the
patients. Among the four studies included, entinostat was
administrated in three studies, and tucidinostat was administrated
in one study. With regret, we were not able to perform subgroup
analysis based on endocrine resistance status, different HDAC
inhibitors, prior anti-estrogen agents and metastatic sites,
because only four studies were available in this meta-analysis with
fewer studies reporting these results in the subgroups. Finally,
the present meta-analysis was not conducted based on the individual
patient data but on the HRs or RRs and their corresponding 95% CIs
of each study. Despite all these limitations, our study is a
meaningful meta-analysis to evaluate the role of the combination of
an HDAC inhibitor plus exemestane in hormone receptor-positive ABC
that progressed on previous endocrine therapy.
In conclusion, our meta-analysis revealed that HDAC
inhibitors in combined with exemestane were associated with a
superior clinical benefit with more frequent adverse effects when
compared to exemestane alone in the hormone receptor-positive ABC
patients refractory to previous endocrine agents. Further studies
which focus on the discovery of new potentially predictive
biomarkers capable of identifying the most appropriate population
who will definitely benefit from HDAC inhibitor-based combination
strategy are urgently needed.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China grant (nos. 81860467 and 82060482).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX, WJ and YC made substantial contributions to the
conception and design of the current meta-analysis. WJ and YG wrote
the manuscript. Literature search, quality assessment, data
extraction, analysis and interpretation were performed by CG, WL,
LL and YG. LL and WJ confirmed the authenticity of all the raw
data. YG and LL revised the manuscript and polished the language.
All authors read and approved the final manuscript. All authors
agreed to be accountable for all aspects of the work and ensuring
that questions related to the accuracy or integrity of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer Statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hanker AB, Sudhan DR and Arteaga CL:
Overcoming endocrine resistance in breast cancer. Cancer Cell.
37:496–513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
El Sayed R, El Jamal L, El Iskandarani S,
Kort J, Abdel Salam M and Assi H: Endocrine and targeted therapy
for hormone-receptor-positive, HER2-Negative advanced breast
cancer: Insights to sequencing treatment and overcoming resistance
based on clinical trials. Front Oncol. 9(510)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nagaraj G and Ma CX: Clinical challenges
in the management of hormone receptor-positive, human epidermal
growth factor receptor 2-Negative metastatic breast cancer: A
literature review. Adv Ther. 38:109–136. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baselga J, Im SA, Iwata H, Cortés J, De
Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, et
al: Buparlisib plus fulvestrant versus placebo plus fulvestrant in
postmenopausal, hormone receptor-positive, HER2-negative, advanced
breast cancer (BELLE-2): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 18:904–916.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Musolino A, Campone M, Neven P, Denduluri
N, Barrios CH, Cortes J, Blackwell K, Soliman H, Kahan Z, Bonnefoi
H, et al: Phase II, randomized, placebo-controlled study of
dovitinib in combination with fulvestrant in postmenopausal
patients with HR(+), HER2(-) breast cancer that had progressed
during or after prior endocrine therapy. Breast Cancer Res.
19(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dimitrakopoulos FI, Kottorou A and Tzezou
A: Endocrine resistance and epigenetic reprogramming in estrogen
receptor positive breast cancer. Cancer Lett. 517:55–65.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guo P, Chen W, Li H, Li M and Li L: The
histone acetylation modifications of breast cancer and their
therapeutic implications. Pathol Oncol Res. 24:807–813.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sabnis GJ, Goloubeva O, Chumsri S, Nguyen
N, Sukumar S and Brodie AM: Functional activation of the estrogen
receptor-α and aromatase by the HDAC inhibitor entinostat
sensitizes ER-negative tumors to letrozole. Cancer Res.
71:1893–1903. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Clements ME, Holtslander L, Edwards C,
Todd V, Dooyema SD, Bullock K, Bergdorf K, Zahnow CA, Connolly RM
and Johnson RW: HDAC inhibitors induce LIFR expression and promote
a dormancy phenotype in breast cancer. Oncogene. 40:5314–5326.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Munster PN, Thurn KT, Thomas S, Raha P,
Lacevic M, Miller A, Melisko M, Ismail-Khan R, Rugo H, Moasser M
and Minton SE: A phase II study of the histone deacetylase
inhibitor vorinostat combined with tamoxifen for the treatment of
patients with hormone therapy-resistant breast cancer. Br J Cancer.
104:1828–1835. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wardley AM, Stein R, McCaffrey J, Crown J,
Malik Z, Barrett-Lee DR and Lee GT: Phase II data for entinostat, a
class 1 selective histone deacetylase inhibitor, in patients whose
breast cancer is progressing on aromatase inhibitor therapy. J Clin
Oncol. 28 (Suppl 15)(S1052)2010.
|
|
14
|
Yardley DA, Ismail-Khan RR, Melichar B,
Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee
MJ and Trepel JB: Randomized phase II, double-blind,
placebo-controlled study of exemestane with or without entinostat
in postmenopausal women with locally recurrent or metastatic
estrogen receptor-positive breast cancer progressing on treatment
with a nonsteroidal aromatase inhibitor. J Clin Oncol.
31:2128–2135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Connolly RM, Zhao F, Miller KD, Lee MJ,
Piekarz RL, Smith KL, Brown-Glaberman UA, Winn JS, Faller BA,
Onitilo AA, et al: E2112: Randomized phase III trial of endocrine
therapy plus entinostat or placebo in hormone receptor-positive
advanced breast cancer. A Trial of the ECOG-ACRIN cancer research
group. J Clin Oncol. 39:3171–3181. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu B: A Randomized Controlled Phase III
Trial of Entinostat, a Class I Selective Histone Deacetylase
Inhibitor, in Combination with Exemestane in Patients with Hormone
Receptor Positive Advanced Breast Cancer. Annual San Antonio Breast
Cancer Symposium, San Antonio, TX, 2021.
|
|
19
|
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui
S, Wang S, Ouyang Q, Yin Y, Geng C, et al: Tucidinostat plus
exemestane for postmenopausal patients with advanced, hormone
receptor-positive breast cancer (ACE): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 20:806–815.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Szostakowska M, Trębińska-Stryjewska A,
Grzybowska EA and Fabisiewicz A: Resistance to endocrine therapy in
breast cancer: Molecular mechanisms and future goals. Breast Cancer
Res Treat. 173:489–497. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rani A, Stebbing J, Giamas G and Murphy J:
Endocrine resistance in hormone receptor positive breast
cancer-from mechanism to therapy. Front Endocrinol (Lausanne).
10(245)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
André F, Ciruelos EM, Juric D, Loibl S,
Campone M, Mayer IA, Rubovszky G, Yamashita T, Kaufman B, Lu YS, et
al: Alpelisib plus fulvestrant for PIK3CA-mutated, hormone
receptor-positive, human epidermal growth factor
receptor-2-negative advanced breast cancer: Final overall survival
results from SOLAR-1. Ann Oncol. 32:208–217. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dent S, Cortés J, Im YH, Diéras V, Harbeck
N, Krop IE, Wilson TR, Cui N, Schimmoller F, Hsu JY, et al: Phase
III randomized study of taselisib or placebo with fulvestrant in
estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced
breast cancer: The SANDPIPER trial. Ann Oncol. 32:197–207.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yardley DA, Liggett W, Mainwaring M,
Castrellon A, Blakely L, Hemphill B, Anz B III, Young RR, Shastry
M, DeBusk LM, et al: A Phase II open label study of everolimus in
combination with endocrine therapy in resistant hormone
Receptor-Positive HER2-Negative advanced breast cancer. Clin Breast
Cancer. 20:89–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiménez-Garduño AM, Mendoza-Rodríguez MG,
Urrutia-Cabrera D, Domínguez-Robles MC, Pérez-Yépez EA,
Ayala-Sumuano JT and Meza I: IL-1β induced methylation of the
estrogen receptor ERα gene correlates with EMT and chemoresistance
in breast cancer cells. Biochem Biophys Res Commun. 490:780–785.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang F and Cui Y: Dysregulation of DNA
methylation patterns may identify patients with breast cancer
resistant to endocrine therapy: A predictive classifier based on
differentially methylated regions. Oncol Lett. 18:1287–1303.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hii LW, Chung FF, Soo JS, Tan BS, Mai CW
and Leong CO: Histone deacetylase (HDAC) inhibitors and doxorubicin
combinations target both breast cancer stem cells and non-stem
breast cancer cells simultaneously. Breast Cancer Res Treat.
179:615–629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu Z, Zou J, Li S, Topper MJ, Tao Y, Zhang
H, Jiao X, Xie W, Kong X, Vaz M, et al: Epigenetic therapy inhibits
metastases by disrupting premetastatic niches. Nature. 579:284–290.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seidman AD, Bordeleau L, Fehrenbacher L,
Barlow WE, Perlmutter J, Rubinstein L, Wedam SB, Hershman DL, Hayes
JF, Butler LP, et al: National cancer institute breast cancer
steering committee working group report on meaningful and
appropriate end points for clinical trials in metastatic breast
cancer. J Clin Oncol. 36:3259–3268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wiegering A, Uthe FW, Hüttenrauch M,
Mühling B, Linnebacher M, Krummenast F, Germer CT, Thalheimer A and
Otto C: The impact of pyrvinium pamoate on colon cancer cell
viability. Int J Colorectal Dis. 29:1189–1198. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mazumder A, Shiao S and Haricharan S: HER2
activation and endocrine treatment resistance in HER2-negative
breast cancer. Endocrinology. 162(bqab153)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bender LM and Nahta R: Her2 cross talk and
therapeutic resistance in breast cancer. Front Biosci.
13:3906–3912. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Saatci O, Huynh-Dam KT and Sahin O:
Endocrine resistance in breast cancer: From molecular mechanisms to
therapeutic strategies. J Mol Med (Berl). 99:1691–1710.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu YS, Wong A and Kim HJ: Ovarian function
suppression with luteinizing hormone-releasing hormone agonists for
the treatment of hormone receptor-positive early breast cancer in
premenopausal women. Front Oncol. 11(700722)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schmid P, Untch M, Kossé V, Bondar G,
Vassiljev L, Tarutinov V, Lehmann U, Maubach L, Meurer J,
Wallwiener D and Possinger K: Leuprorelin acetate every-3-months
depot versus cyclophosphamide, methotrexate, and fluorouracil as
adjuvant treatment in premenopausal patients with node-positive
breast cancer: The TABLE study. J Clin Oncol. 25:2509–2515.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang P, Li CZ, Jiao GM, Zhang JJ, Zhao
HP, Yan F, Jia SF, Hu BS and Wu CT: Effects of ovarian ablation or
suppression in premenopausal breast cancer: A meta-analysis of
randomized controlled trials. Eur J Surg Oncol. 43:1161–1172.
2017.PubMed/NCBI View Article : Google Scholar
|