|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer Statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hanker AB, Sudhan DR and Arteaga CL:
Overcoming endocrine resistance in breast cancer. Cancer Cell.
37:496–513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
El Sayed R, El Jamal L, El Iskandarani S,
Kort J, Abdel Salam M and Assi H: Endocrine and targeted therapy
for hormone-receptor-positive, HER2-Negative advanced breast
cancer: Insights to sequencing treatment and overcoming resistance
based on clinical trials. Front Oncol. 9(510)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nagaraj G and Ma CX: Clinical challenges
in the management of hormone receptor-positive, human epidermal
growth factor receptor 2-Negative metastatic breast cancer: A
literature review. Adv Ther. 38:109–136. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baselga J, Im SA, Iwata H, Cortés J, De
Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, et
al: Buparlisib plus fulvestrant versus placebo plus fulvestrant in
postmenopausal, hormone receptor-positive, HER2-negative, advanced
breast cancer (BELLE-2): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 18:904–916.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Musolino A, Campone M, Neven P, Denduluri
N, Barrios CH, Cortes J, Blackwell K, Soliman H, Kahan Z, Bonnefoi
H, et al: Phase II, randomized, placebo-controlled study of
dovitinib in combination with fulvestrant in postmenopausal
patients with HR(+), HER2(-) breast cancer that had progressed
during or after prior endocrine therapy. Breast Cancer Res.
19(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dimitrakopoulos FI, Kottorou A and Tzezou
A: Endocrine resistance and epigenetic reprogramming in estrogen
receptor positive breast cancer. Cancer Lett. 517:55–65.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guo P, Chen W, Li H, Li M and Li L: The
histone acetylation modifications of breast cancer and their
therapeutic implications. Pathol Oncol Res. 24:807–813.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sabnis GJ, Goloubeva O, Chumsri S, Nguyen
N, Sukumar S and Brodie AM: Functional activation of the estrogen
receptor-α and aromatase by the HDAC inhibitor entinostat
sensitizes ER-negative tumors to letrozole. Cancer Res.
71:1893–1903. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Clements ME, Holtslander L, Edwards C,
Todd V, Dooyema SD, Bullock K, Bergdorf K, Zahnow CA, Connolly RM
and Johnson RW: HDAC inhibitors induce LIFR expression and promote
a dormancy phenotype in breast cancer. Oncogene. 40:5314–5326.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Munster PN, Thurn KT, Thomas S, Raha P,
Lacevic M, Miller A, Melisko M, Ismail-Khan R, Rugo H, Moasser M
and Minton SE: A phase II study of the histone deacetylase
inhibitor vorinostat combined with tamoxifen for the treatment of
patients with hormone therapy-resistant breast cancer. Br J Cancer.
104:1828–1835. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wardley AM, Stein R, McCaffrey J, Crown J,
Malik Z, Barrett-Lee DR and Lee GT: Phase II data for entinostat, a
class 1 selective histone deacetylase inhibitor, in patients whose
breast cancer is progressing on aromatase inhibitor therapy. J Clin
Oncol. 28 (Suppl 15)(S1052)2010.
|
|
14
|
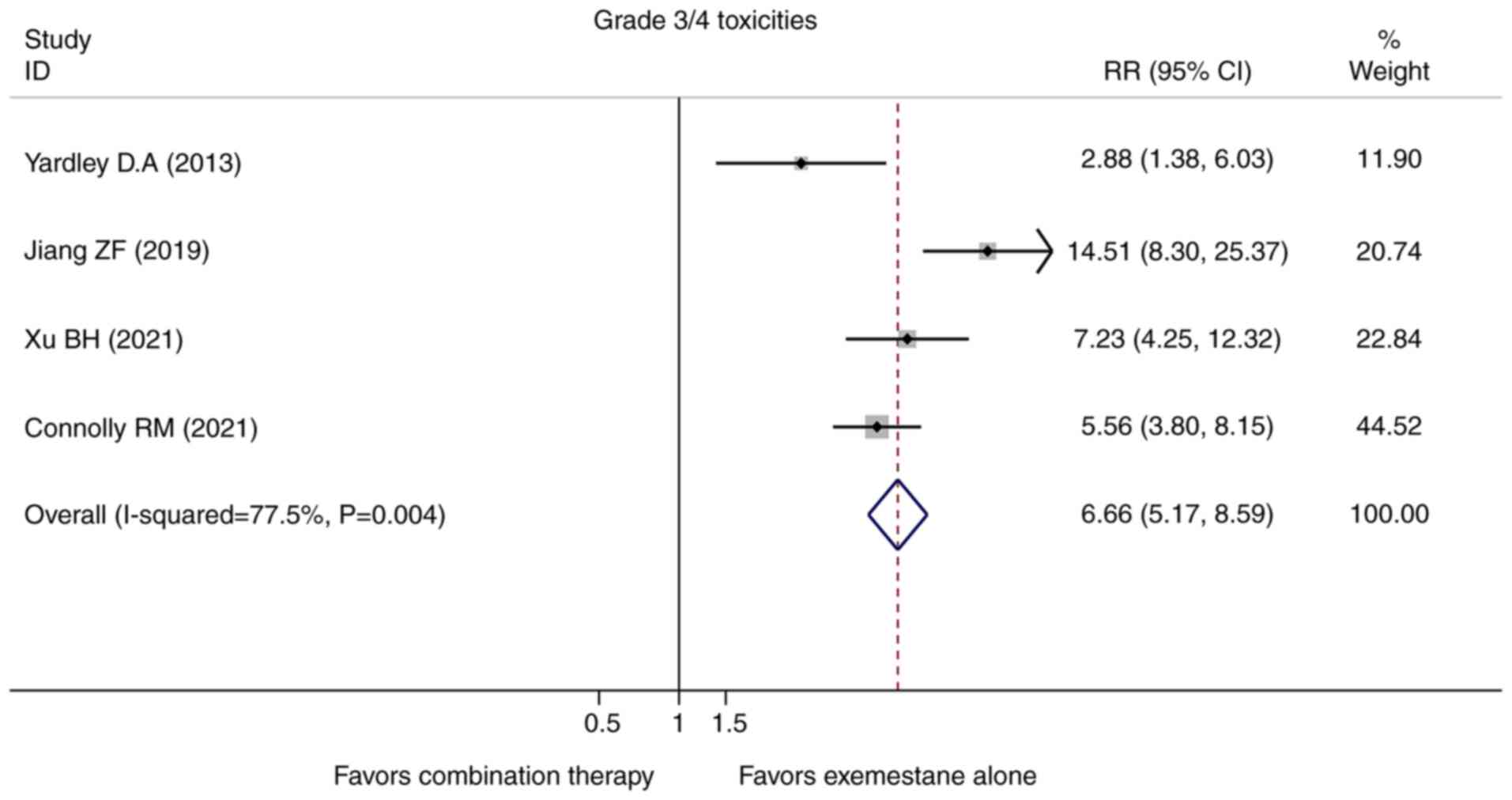
Yardley DA, Ismail-Khan RR, Melichar B,
Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee
MJ and Trepel JB: Randomized phase II, double-blind,
placebo-controlled study of exemestane with or without entinostat
in postmenopausal women with locally recurrent or metastatic
estrogen receptor-positive breast cancer progressing on treatment
with a nonsteroidal aromatase inhibitor. J Clin Oncol.
31:2128–2135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Connolly RM, Zhao F, Miller KD, Lee MJ,
Piekarz RL, Smith KL, Brown-Glaberman UA, Winn JS, Faller BA,
Onitilo AA, et al: E2112: Randomized phase III trial of endocrine
therapy plus entinostat or placebo in hormone receptor-positive
advanced breast cancer. A Trial of the ECOG-ACRIN cancer research
group. J Clin Oncol. 39:3171–3181. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu B: A Randomized Controlled Phase III
Trial of Entinostat, a Class I Selective Histone Deacetylase
Inhibitor, in Combination with Exemestane in Patients with Hormone
Receptor Positive Advanced Breast Cancer. Annual San Antonio Breast
Cancer Symposium, San Antonio, TX, 2021.
|
|
19
|
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui
S, Wang S, Ouyang Q, Yin Y, Geng C, et al: Tucidinostat plus
exemestane for postmenopausal patients with advanced, hormone
receptor-positive breast cancer (ACE): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 20:806–815.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Szostakowska M, Trębińska-Stryjewska A,
Grzybowska EA and Fabisiewicz A: Resistance to endocrine therapy in
breast cancer: Molecular mechanisms and future goals. Breast Cancer
Res Treat. 173:489–497. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rani A, Stebbing J, Giamas G and Murphy J:
Endocrine resistance in hormone receptor positive breast
cancer-from mechanism to therapy. Front Endocrinol (Lausanne).
10(245)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
André F, Ciruelos EM, Juric D, Loibl S,
Campone M, Mayer IA, Rubovszky G, Yamashita T, Kaufman B, Lu YS, et
al: Alpelisib plus fulvestrant for PIK3CA-mutated, hormone
receptor-positive, human epidermal growth factor
receptor-2-negative advanced breast cancer: Final overall survival
results from SOLAR-1. Ann Oncol. 32:208–217. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dent S, Cortés J, Im YH, Diéras V, Harbeck
N, Krop IE, Wilson TR, Cui N, Schimmoller F, Hsu JY, et al: Phase
III randomized study of taselisib or placebo with fulvestrant in
estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced
breast cancer: The SANDPIPER trial. Ann Oncol. 32:197–207.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yardley DA, Liggett W, Mainwaring M,
Castrellon A, Blakely L, Hemphill B, Anz B III, Young RR, Shastry
M, DeBusk LM, et al: A Phase II open label study of everolimus in
combination with endocrine therapy in resistant hormone
Receptor-Positive HER2-Negative advanced breast cancer. Clin Breast
Cancer. 20:89–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiménez-Garduño AM, Mendoza-Rodríguez MG,
Urrutia-Cabrera D, Domínguez-Robles MC, Pérez-Yépez EA,
Ayala-Sumuano JT and Meza I: IL-1β induced methylation of the
estrogen receptor ERα gene correlates with EMT and chemoresistance
in breast cancer cells. Biochem Biophys Res Commun. 490:780–785.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang F and Cui Y: Dysregulation of DNA
methylation patterns may identify patients with breast cancer
resistant to endocrine therapy: A predictive classifier based on
differentially methylated regions. Oncol Lett. 18:1287–1303.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hii LW, Chung FF, Soo JS, Tan BS, Mai CW
and Leong CO: Histone deacetylase (HDAC) inhibitors and doxorubicin
combinations target both breast cancer stem cells and non-stem
breast cancer cells simultaneously. Breast Cancer Res Treat.
179:615–629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu Z, Zou J, Li S, Topper MJ, Tao Y, Zhang
H, Jiao X, Xie W, Kong X, Vaz M, et al: Epigenetic therapy inhibits
metastases by disrupting premetastatic niches. Nature. 579:284–290.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seidman AD, Bordeleau L, Fehrenbacher L,
Barlow WE, Perlmutter J, Rubinstein L, Wedam SB, Hershman DL, Hayes
JF, Butler LP, et al: National cancer institute breast cancer
steering committee working group report on meaningful and
appropriate end points for clinical trials in metastatic breast
cancer. J Clin Oncol. 36:3259–3268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wiegering A, Uthe FW, Hüttenrauch M,
Mühling B, Linnebacher M, Krummenast F, Germer CT, Thalheimer A and
Otto C: The impact of pyrvinium pamoate on colon cancer cell
viability. Int J Colorectal Dis. 29:1189–1198. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mazumder A, Shiao S and Haricharan S: HER2
activation and endocrine treatment resistance in HER2-negative
breast cancer. Endocrinology. 162(bqab153)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bender LM and Nahta R: Her2 cross talk and
therapeutic resistance in breast cancer. Front Biosci.
13:3906–3912. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Saatci O, Huynh-Dam KT and Sahin O:
Endocrine resistance in breast cancer: From molecular mechanisms to
therapeutic strategies. J Mol Med (Berl). 99:1691–1710.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu YS, Wong A and Kim HJ: Ovarian function
suppression with luteinizing hormone-releasing hormone agonists for
the treatment of hormone receptor-positive early breast cancer in
premenopausal women. Front Oncol. 11(700722)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schmid P, Untch M, Kossé V, Bondar G,
Vassiljev L, Tarutinov V, Lehmann U, Maubach L, Meurer J,
Wallwiener D and Possinger K: Leuprorelin acetate every-3-months
depot versus cyclophosphamide, methotrexate, and fluorouracil as
adjuvant treatment in premenopausal patients with node-positive
breast cancer: The TABLE study. J Clin Oncol. 25:2509–2515.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang P, Li CZ, Jiao GM, Zhang JJ, Zhao
HP, Yan F, Jia SF, Hu BS and Wu CT: Effects of ovarian ablation or
suppression in premenopausal breast cancer: A meta-analysis of
randomized controlled trials. Eur J Surg Oncol. 43:1161–1172.
2017.PubMed/NCBI View Article : Google Scholar
|