Introduction
Data increasingly points to the role of interleukin
6 (IL-6) in the etiology and progression of heart failure caused by
post-myocardial infarction (1-3).
The immediate and long-term consequences of cardiac insufficiency
and heart failure are of utmost importance to patients. Cardiac
remodeling processes differ significantly in extent between
individuals and are often limited (4,5).
Even though it remains unclear how the inflammatory response and
increased oxidative stress contributes to myocardial necrosis, it
is undeniable that both myocardial infarction (MI) and the gold
standard treatment, percutaneous coronary intervention,
cause IL-6 to increase in cardiac tissue, which increases its
vulnerability to permanent damage (6-8).
Current studies on ventricular remodeling indicate
that the ventricular remodeling mechanism involves changes in the
nervous and endocrine systems, the constellation of cytokines, the
transduction pathways of cellular signals and mutation patterns
(9,10). The TGFβ1-Smad3-MMP2/9 pathway has
been identified as a common pathway responsible for tissue fibrosis
(11). As a signal transduction
factor of TGFβ1, Smad protein is also involved in cardiac
development, cell proliferation, growth and apoptosis. Smad protein
can interact with other transcription factors and regulate its
activity through cytokines (12,13).
Myocardial injury severity determines the
development of ventricular remodeling, and rational drug treatment
is important for improving this pathological process (8,10).
For ventricular remodeling, current therapeutic drugs such as
β-blockers, inhibitors of the angiotensin-converting enzyme (ACE)
or antagonists of angiotensin II receptors, are often used together
(14,15). At present, the most commonly used
ACEIs are benazepril, captopril and ramipril. In addition, drugs
that inhibit ventricular remodeling also have calcium channel
blockers, which can selectively block Ca2+. The
Ca2+ channel on the cell membrane decreases the
intracellular Ca2+ concentration, which helps reverse
myocardial remodeling. Statins, including simvastatin, prevent
hypertrophy and fibrosis of cardiomyocytes. Cardiomyocytes are
inhibited from synthesizing RhoA and Rho-GTPase by statins, which
prevent the formation of fibroblasts, tissue fibrosis and cardiac
hypertrophy (8,16).
The majority of cardiology cases are revealed in
biologically older individuals, either because of chronology or
premature aging, such as autoimmune, chronic inflammatory or
musculoskeletal conditions. Almost a quarter of all patients with
heart attacks are over the age of 75 years old. In addition to
heart disease in elderly patients, a significant increase in
younger patients with heart damage due to comorbidities or
unhealthy lifestyle habits is expected to be observed. In older
patients, myocardial infarction, recurrences and heart failure will
be more common, and the prognosis will be worse compared with
younger patients, with a mortality rate of 20-25% in hospitals. In
addition, patient mortality increases almost exponentially with
increasing age (9,17,18).
Patients in their eighth decade of life have a mortality rate of
17%, and patients in their ninth decade of life have a mortality
rate of 33% (19). Therefore, it
is important to preserve the cardiac muscle following an
infarction. It is hypothesized that blocking IL-6 can prevent
myocyte senescence, thereby allowing repair, regeneration and
maintenance of cardiac tissue (20-22).
The majority of patients are multimorbid and
elderly, so combination therapy is often complicated and should be
avoided. Thus, more potent drugs are needed. Identifying new
pathophysiological patterns that could be used as therapeutical
targets is a need that has not been met. Consequently, the use of
IL-6 receptor inhibitors has been investigated as a possible
candidate for restoring cardiac function (23,24).
The present study examined myocardial remodeling induced by IL-6
receptor inhibitors, evidenced by reduction in infarction size,
collagen content and inflammation of the myocardium.
Materials and methods
Animal model
A total of 80 male Sprague-Dawley (SD) rats aged 5
months (average body weight, 300-330 g) were housed in a certified
SPF grade facility (Tongji University Laboratory Animal Center,
Shanghai, China) with individually ventilated cages and the
following conditions: Temperature, 20-24˚C; relative humidity,
30-70%; 12-h light-dark cycle (8:00 a.m. to 8:00 p.m.); and free
access to food and water. All experiments were performed in
accordance with the protocols approved by the Animal Care and Use
Committee at Tongji University. This research proposal was approved
by the Ethics Committee of Yangpu Hospital, School of Medicine,
Tongji University (approval no. LL-2021-SCI-006).
The myocardial infarction model was established in
rats by ligating the left anterior descending coronary artery
(LAD). SD rats were first fixed on the operating table and
anesthetized with an intraperitoneal injection of sodium
pentobarbital (formulated as a 2% solution at a dose of 35 mg/kg).
The skin was cut along the left iliac crest and the sternal xiphoid
line, muscles and the pericardium were separated. LAD was ligated
to a depth of 0.3-0.5 mm under aseptic conditions, without any
other injuries (intentional or unintentional) in LAD. V2 lead
electrocardiogram was performed postoperatively to determine
whether it demonstrated ST-segment elevation and gradual
pathological Q waves. The myocardial infarction model was validated
by observing the local myocardial color paleness and
echocardiography to demonstrate the local myocardial segmental
motility disturbances. The acute myocardial infarction (AMI)-rat
standard was successfully established using this model. Only AMI
rats that met all standard requirements (permanent LAD ligation and
proper LAD ligation confirmed by electrocardiography) were included
in the experimental groups.
Animal grouping
The rats were randomly divided into four groups: i)
Normal control group without any treatment, the N group (n=20); ii)
LAD ligation with an injection of normal saline, the LINS group
(n=20); iii) LAD ligation with an injection of IL-6 recombinant
protein (cat. no. PHC0066; Thermo Fisher Scientific, Inc.), the
LIIL group (n=20); and iv) LAD ligation with injection of IL-6
receptor inhibitor (Tocilizumab; cat. no. A2012; Selleck
Chemicals), the LIILR group (n=20). Before the treatments, humane
endpoint criteria were established, the parameters including
labored breathing, obvious loss of body weight (>25%),
prostration, unresponsiveness to external stimuli and a drop in
body temperature (±2˚C) (24).
When rats appeared with one or more of aforementioned reactions,
they were promptly euthanized. Animal care staff monitored the rats
daily, and no rats reached the humane endpoints before the end of
the present study. Overall, 10-50% of the rats died from myocardial
infarction during or after surgical operation and were immediately
euthanized with overdose of sodium pentobarbital by intraperitoneal
injection in conformity to their vital signs (apnea and cessation
of heartbeat). The anesthetic dose of sodium pentobarbital in rats
was 30 mg/kg, the euthanasia dose was 150 mg/kg (25).
Animal treatment in specific
groups
In the LINS group, the LAD was ligated. After the
successful preparation of the myocardial infarction model, the AMI
area was divided into four points and injected with 20 µl of
physiological NaCl each (a total of 80 µl) at a distance of 1-2 cm.
In the LIIL group and LIILR group, IL-6 recombinant protein (1.8
mg/kg) (26,27) or IL-6 receptor inhibitor (50 mg/kg)
(28), respectively, were
accordingly injected into the four points in the myocardial
infarction area.
Cardiac function test
The Acuson Sequoia C256 ultrasound systems (Siemens
AG) linear array probe was used at a frequency of 8 MHz and
detection depth of 2 cm to determine the heart's function at 1 and
4 weeks after surgery. Rats were anesthetized by intraperitoneal
injection of sodium pentobarbital. The rat's chest hair was scraped
off, the rat was fixed on its back and connected to the V2 lead
electrocardiogram. The probe was placed in the rat's chest, and the
two-dimensional ultrasound indicated the level of the short axis of
the fundus near the papillary muscle. Subsequently, M-mode
ultrasound was used to determine the left ventricular end-diastolic
diameter (LVEDD) and left ventricular end-systolic diameter (LVESD)
of each group of the left ventricular. The left ventricular
fractional shortening (LVFS) was calculated using the Teichholtz
formula (29),
[Volume=7D3/(2.4 + D), where D represents the
ventricular diameter] (30), and
values of the left ventricular ejection fraction (LVEF) were
calculated to assess cardiac function. Each set of data illustrated
the average of three consecutive cardiac cycles.
Histopathological studies
After anesthetizing rats with an excess of
pentobarbital, their hearts were removed by a thoracotomy and cut
along the ligature line to obtain the largest cross-sectional area
of the left ventricle. Sections were used for Masson staining,
hematoxylin and eosin (HE) staining and immunohistochemistry (IHC)
(27), while the remains were used
to measure myocardial inflammatory response indicators
myeloperoxidase (MPO), reactive oxygen species (ROS), interleukin 6
(IL-6), transforming growth factor β1 (TGFβ1) and matrix
metalloproteinase-2/9 (MMP2/9). The remaining hearts tissues were
harvested, homogenized and centrifuged (12,000 x g) at 4˚C for 10
min in 300 µl CTAB buffer [50 mM CTAB (cat. no. H5882;
MilliporeSigma) in 50 mM potassium phosphate buffer, pH 6.0]. The
tissue supernatant was harvested and used for protein quantitative
analysis with a Bicinchoninic Acid protein assay kit (cat. no.
23227; Thermo Fisher Scientific, Inc.). The tissue supernatant was
stored at -80˚C until used for ELISA assays. MPO in heart
homogenate was determined using rat-specific ELISA kits (cat. no.
HK105-01; Hycult Biotech Inc.). ROS was measured by rat ROS ELISA
kit (cat. no. LS-F9759-1; Lifespan Biosciences). Interleukin 6
(IL-6) was assayed by rat IL-6 ELISA (for lysates) kit (cat. no.
ERA32RB; Thermo Fisher Scientific, Inc.). TGFβ1 was determined by
TGFβ1 rat ELISA kit (cat. no. BMS623-3; Thermo Fisher Scientific,
Inc.). MMP2 was measured by rat MMP2 ELISA kit (cat. no.
LS-F32418-1; Lifespan Biosciences). MMP9 was assayed by rat MMP9
ELISA kit (cat. no. LS-F5605-1; Lifespan Biosciences). ELISA assays
were performed according to the manufacturer's instructions.
HE staining
HE staining was performed for pathological
evaluation (27). Briefly, the
tissue was fixed using a 10% formalin solution for 7 days at room
temperature. These samples were then paraffin-embedded, and cut
into 5-µm sections, placed on a slide and dried in a 60˚C oven for
30 min. The slide was deparaffinized using xylene, rehydrated using
100, 90, 80 and 70% alcohol, and washed under running tap water.
Subsequently, the slides were stained with hematoxylin at room
temperature for 10 min, and then washed in running tap water to
remove excess solution. Slides were differentiated in 1%
concentrated hydrochloric acid diluted in 70% ethanol for 30 sec
and washed again under running tap water for 1 min. The sections
were then counterstained with eosin for 2 min at room temperature,
and dehydrated very quickly through 95 and 100% alcohol, and
cleared by 100% xylene. Coverslips were then mounted on the slides
using resinous mounting medium. The images were collected using a
light microscope (BX43; Olympus Corporation).
Masson staining
Paraffin sections were prepared from animal
myocardial tissue. Masson staining was performed to evaluate the
size of the infarct and degree of fibrosis in the marginal zone.
The formalin-fixed, paraffin-embedded tissues of hearts were cut
into 5-µm sections and affixed on slides, dried in a 60˚C oven for
30 min, defaraffinized with xylene, rehydrated in a graded series
of alcohols (100, 90, 80 and 70%), and washed under running tap
water. Next, the sections were stained according to the
manufacturer's instructions of the Masson's trichrome staining kit
(cat. no. G1340; Beijing Solarbio Science & Technology Co.,
Ltd.). The sections were dehydrated very quickly through 95 and
100% alcohol, cleared by xylene and then coverslips were mounted on
glass slides using resinous mounting medium. Under the light
microscope (BX43; Olympus Corporation), the cardiomyocytes
presented with red cytoplasm, black nucleus and blue-green collagen
fibers. A total of three sections were selected from each animal.
The left ventricular collagen area ratio to the left ventricular
area was determined as myocardial infarct size under a low-power
field (magnification, x2.5). Local myocardial collagen and
inflammatory response were observed under a high power objective
lens (20X magnification objective). ImageJ software (National
Institutes of Health) was used to measure the infarct size or
fibrosis area and total area. Each tissue was measured with 5
different regions. The percentage of infarct size or fibrosis area
was calculated as the infarct size or fibrosis-positive area
divided by the total area.
IHC
Animal myocardial tissues were collected immediately
after the rats were sacrificed and fixed overnight with 4% neutral
formalin at room temperature and embedded in paraffin. The sections
were subjected to IHC to determine the levels and distributions of
IL-6 and TGFβ1. Sections (5-µm) were deparaffinized and rehydrated,
and then rinsed in distilled water for 5 min. Slides were
microwaved in 0.01 M citrate antigen retrieval buffer pH 6.0 until
boiling for at least 15 min, after which they were left to cool to
room temperature. The slides were washed with phosphate-buffered
saline (PBS) for 5 min and submerged in 3% hydrogen peroxide for 10
min at room temperature to block endogenous peroxidase, This was
followed by submersion in blocking reagent (cat. no. 20773-M;
MilliporeSigma) for 1 h at room temperature in a humidified,
light-protected chamber and then washing with PBS three times.
Next, sections were stained with primary and secondary antibodies.
The following primary antibodies were used: Rabbit anti-IL-6
(1:100; cat. no. ab6672; Abcam) and rabbit anti-TGFβ1 (1:100; cat.
no. ab92486; Abcam). Tissue sections were incubated with a primary
antibody overnight at 4˚C. Subsequently, slides were washed with
PBS and incubated with goat anti-rabbit IgG horseradish
peroxidase-conjugated secondary antibody (1:500; cat. no. sc-2004;
Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. Next,
the sections were washed in PBS and visualized with a DAB substrate
kit (cat. no. ab64238; Abcam) according to the manufacturer's
instructions. This was followed by incubation with hematoxylin for
3 min at room temperature and washing with PBS three times.
Finally, the sections were dehydrated very quickly using 95 and
100% alcohol and cleared using xylene, and then coverslips were
mounted on the slides using resinous mounting medium. Images were
observed using an Axio Observer Z1 microscope (Carl Zeiss AG).
Statistical analysis
The SPSS 22.0 software (IBM Corp.) was used for the
analyses. All data were expressed as mean ± standard deviation.
Comparisons of multiple groups were analyzed using one-way analysis
of variance (ANOVA) with Bonferroni's correction. GraphPad Prism
5.0 (GraphPad Software, Inc.) software was used to present
statistical results. Animal survival curves were assessed using the
Kaplan-Meier method. P<0.05 was considered to indicate a
statistically significant difference.
Results
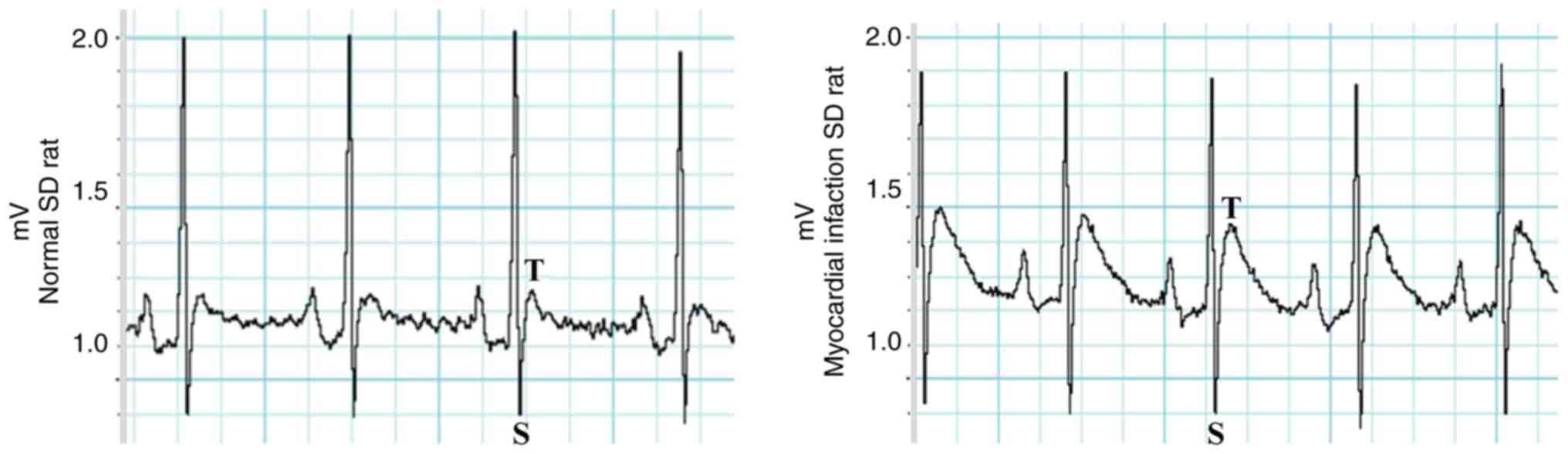
Standardization of the acute
myocardial infarction rat model
The standard AMI rat model and the postoperative
validation points were established. Electrocardiograms (ECGs) of
all AMI rats exhibited electrocardiographic ST-segment elevation,
as presented in Fig. 1.
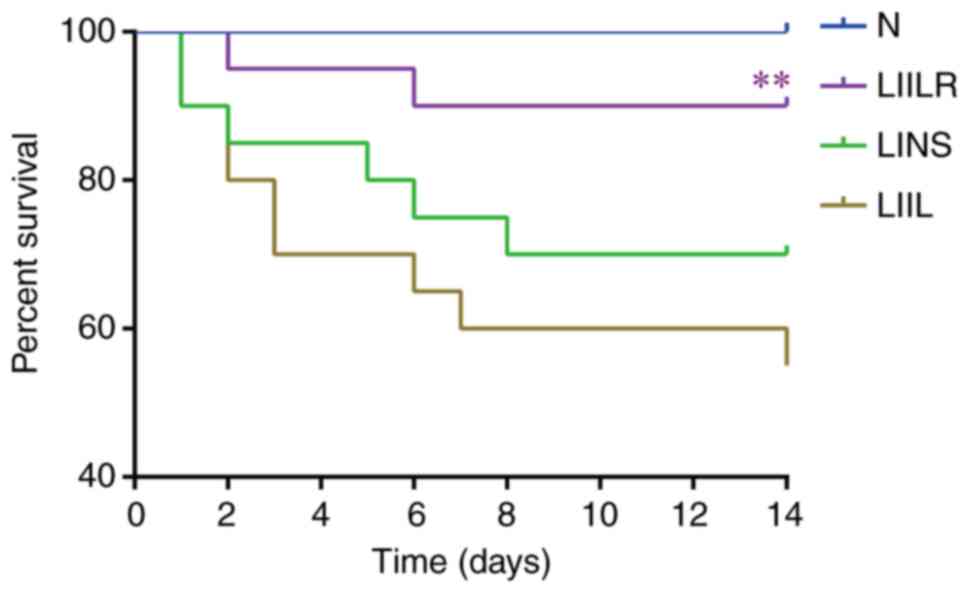
Survival curve of the animals
The survival of the rats was monitored for 2 weeks
after myocardial infarction modeling. Rat mortality was not
reported in the N group (survival rate was 100%). Compared with the
N group, LAD ligation and normal saline injection (LINS group)
resulted in high mortality, with a survival rate of 70%.
LAD-ligation and IL-6-injection (LIIL group) had the highest
mortality. The LIILR group had a significantly decreased mortality
rate (survival rate of 90%) compared with the LIIL group
(P=0.0068). The survival curves of each group are depicted in
Fig. 2.
Effect of IL-6 and its receptor
inhibitor on cardiac function
Changes in cardiac function were measured using an
Acuson Sequoia C256 ultrasound systems (Siemens AG) at 1 and 2
weeks after LAD ligation-induced myocardial infarction (Fig. 3). In the LINS group, the LVFS and
the LVEF demonstrated a gradual decline, with a progressive LVESD
and LVEDD increase compared with the N group. The LIIL group with
injection of IL-6 had a significantly decreased LVFS and LVEF at 1
and 2 weeks compared with the N group. In addition, the LIIL group
had a significantly increased LVESD and LVEDD compared with the N
group. In the LIILR group with injection of IL-6 receptor
inhibitors, LVFS and LVEF were significantly higher than those in
LINS group and LIIL group at 2 weeks. LVESD and LVEDD at 1 and 2
weeks in the LIILR group were lower compared with those in the LIIL
group (Fig. 3). These results
suggest that IL-6 receptor inhibitors can protect cardiac function
in myocardial infarction-induced cardiac damage.
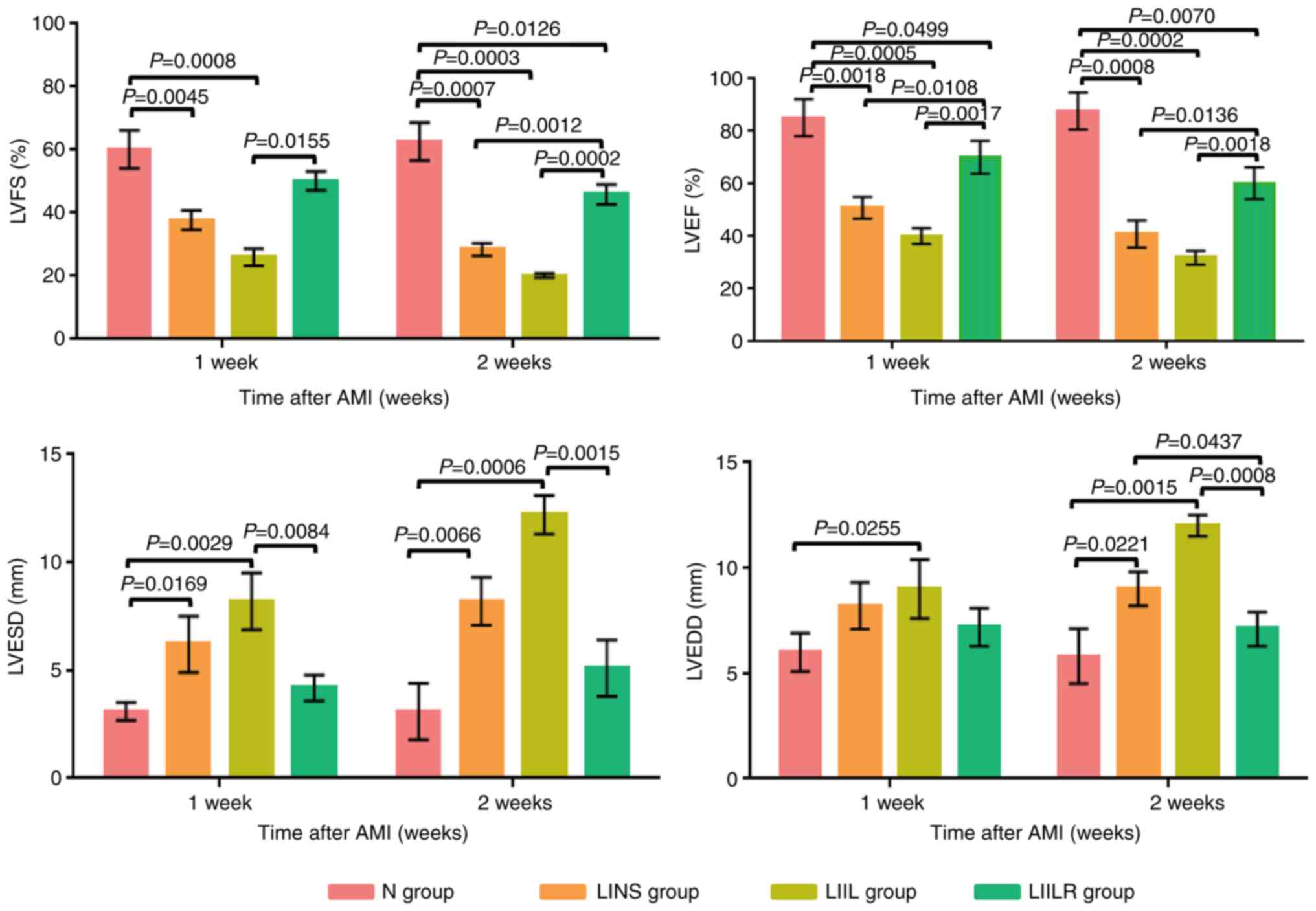 | Figure 3Changes in LVEF, LVFS, LVESD, and
LVEDD in groups 1 week and 2 weeks after myocardial infarction. N
group, n=20; LINS group, n=13; LIIL group, n=10; LIILR group, n=18.
LVEF, left ventricular ejection fraction; LVFS, left ventricular
fractional shortening; LVESD, left ventricle end systolic
dimension; LVEDD, left ventricle end diastolic dimension; N group,
normal control group; LINS group, LAD ligation with an injection of
normal saline group; LIIL group, LAD ligation with an injection of
IL-6 recombinant protein group; LIILR group, LAD ligation with
injection of IL-6 receptor inhibitor group; AMI, acute myocardial
infarction. |
Effects of IL-6 and its receptor
inhibitors on myocardial infarction size and collagen content
The myocardial infarction size and collagen content
were detected by Masson staining. The myocardial structure of the N
group was intact at low magnification. The infarct size was the
largest in the IL6 injection group (LIIL group), followed by the
ligation group (LINS group). The infarct size of the inhibitor
group (LIILR group) was significantly smaller compared with that of
the previous two groups (Fig.
4).
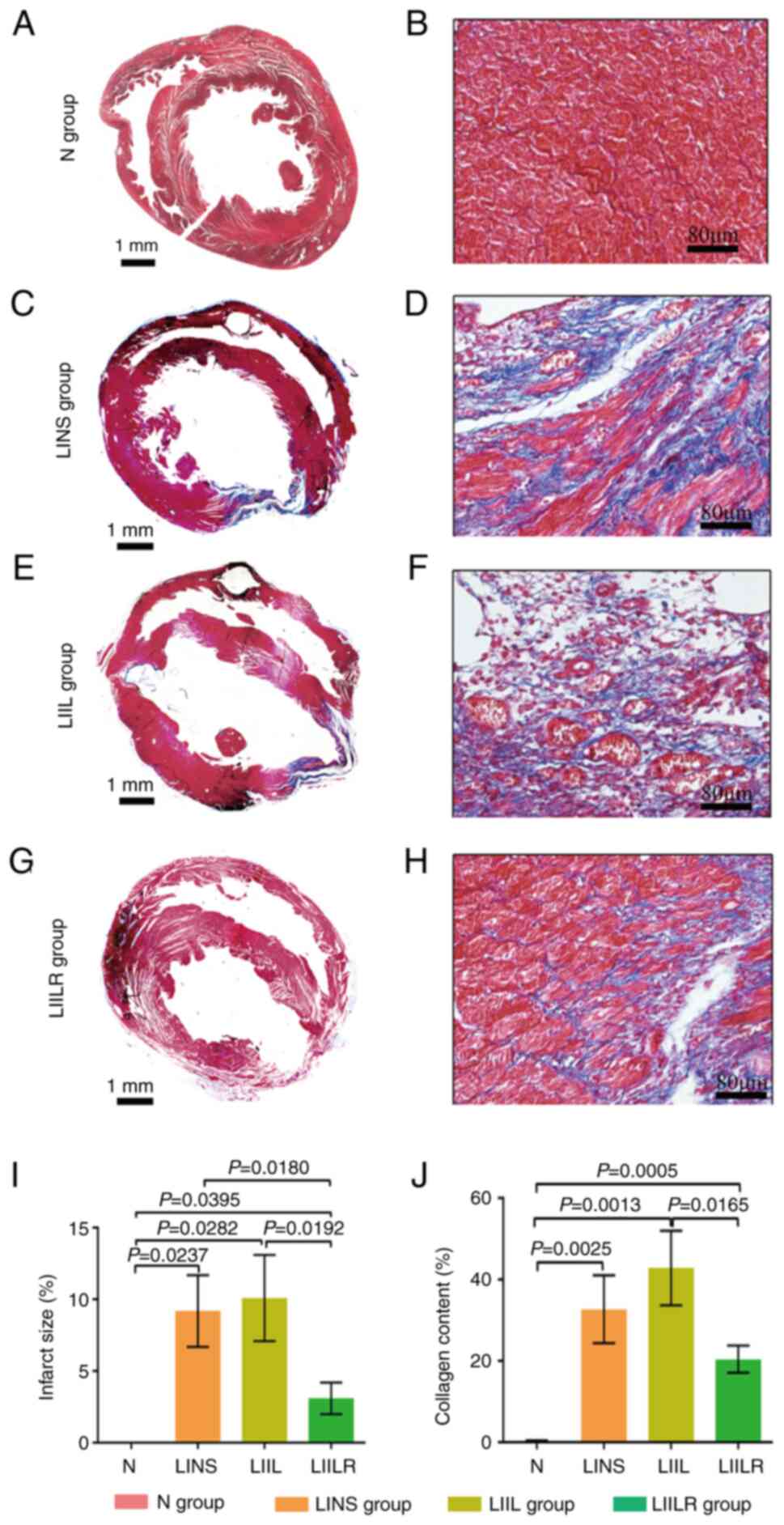 | Figure 4Overall Masson staining and infarct
size of the rat heart in the N group, and 2 weeks after myocardial
infarction in the LINS group, LIIL group and LIILR group. Masson
staining showing myocardial tissue fibrosis in rats. Cardiomyocytes
were stained in bright red, collagen in dark blue. (A) Overall
Masson staining of the N group. (B) Magnified Masson staining of
the N group. (C) Overall Masson staining of the LINS group. (D)
Overall Masson staining of the LINS group. (E) Magnified Masson
staining of the LIIL group. (F) Magnified Masson staining of the
LIIL group. (G) Magnified Masson staining of the LIILR group. (H)
Magnified Masson staining of the LIILR group. (I) Quantification of
infarct size percentage in each rat group. (J) Quantification of
collagen content percentage in each rat group. Left column scale
bar, 1 mm; right column scale bar, 80 µm. N group, n=20; LINS
group, n=13; LIIL group, n=10; LIILR group, n=18. N group, normal
control group; LINS group, LAD ligation with an injection of normal
saline; LIIL group, LAD ligation with an injection of IL-6
recombinant protein; LIILR group, LAD ligation with injection of
IL-6 receptor inhibitor group. |
Under a high-power polarizing microscope, the Masson
staining revealed cardiomyocytes in red and the collagen fibers in
blue-green, as presented in Fig.
4. There was no obvious collagen in the N group, and the
cardiomyocytes were bright red (Fig.
4B). The myocardial cells in the infarct border area of the
rats in the LINS group were disordered, the blue collagen fibers in
the interstitium increased, the myocardial bundle was segmented,
some collagen fibers were merged and the vacuolar changes were
observed locally (Fig. 4D). A
significant inflammatory response was observed in the
cardiomyocytes of the LIIL group. After treatment with IL-6, there
were more blue-green collagen fibers in the myocardial tissue,
collagen deposition increased significantly, the inflammatory
response was significantly aggravated and the myocardial structure
changed accordingly (Fig. 4F).
Treatment with IL-6 receptor inhibitors inhibited this phenomenon.
An infarcted area showed a weaker inflammatory response, with
reduced collagen (Fig. 4H). These
results suggest that IL-6 receptor inhibitors could attenuate the
myocardial infarction-induced fibrosis.
The quantitative analysis in Fig. 4 demonstrated that the collagen
content in the myocardium of each group was significantly higher
compared with that in the N group. Compared with the N group, the
collagen content of the LIIL group increased significantly after
the injection of IL-6. Injection of IL-6 receptor inhibitor
significantly reduced collagen content compared with the LIIL
group. These results indicated that IL-6 promoted myocardial
collagen deposition and increased fibrosis and inflammatory
response.
Effect of IL-6 and its receptor
inhibitor on myocardial tissue inflammation
A myocardial tissue inflammation index (MPO) and an
oxidative stress index (ROS) were measured to determine the degree
of inflammation in the heart. As presented in Fig. 5, ROS and MPO in the LINS group were
significantly higher compared with the N group. Injection of IL-6
promoted a further increase in the levels of ROS in the LIIL group
compared with the LINS group. In the LIILR group (injection of IL-6
receptor inhibitor), ROS and MPO were significantly lower compared
with the LIIL and LINS groups.
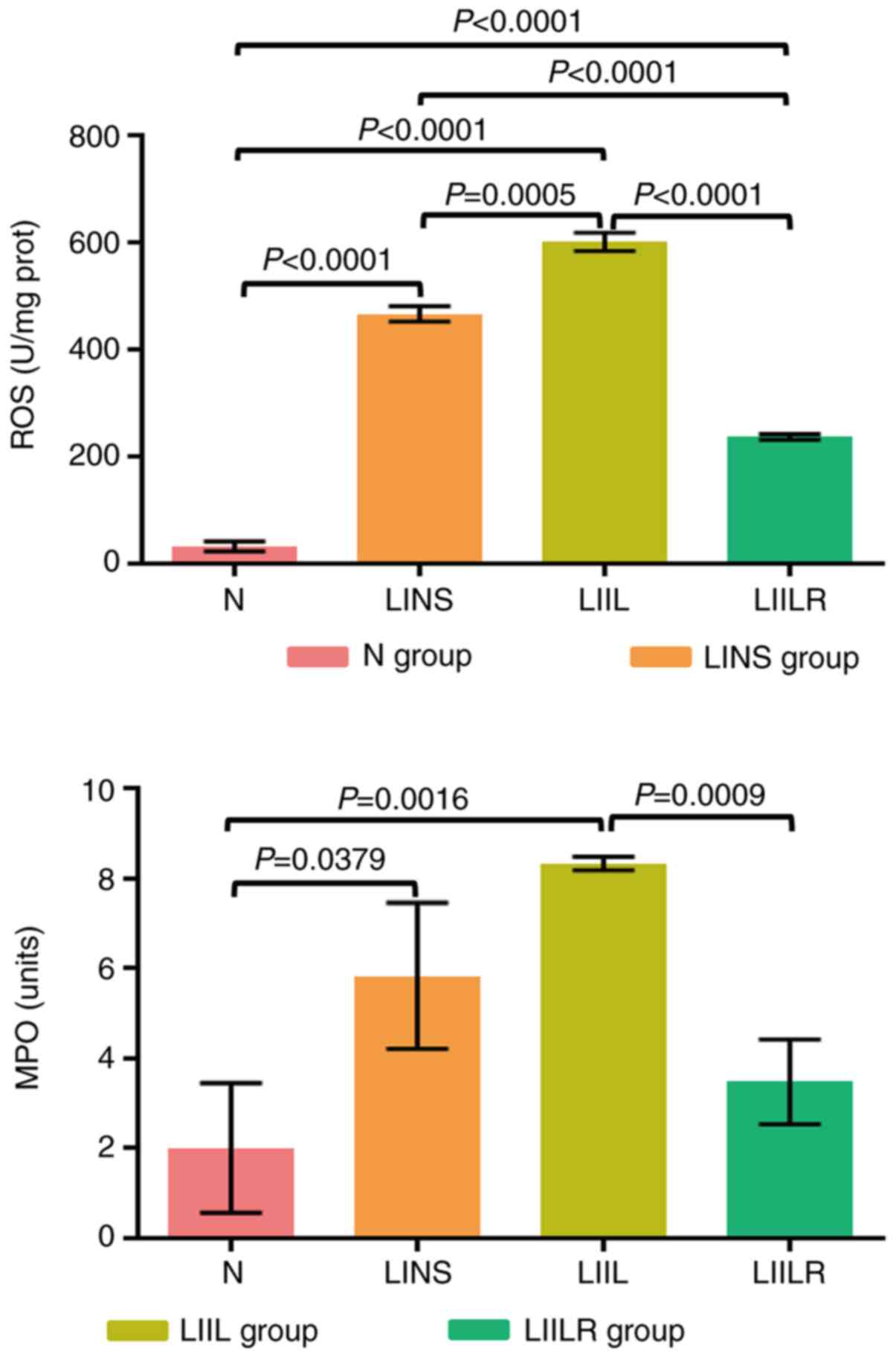 | Figure 5Detection of the myocardial
inflammatory response indicators MPO and ROS by enzyme-linked
immunosorbent assay (ELISA) and Chemiluminescence. N group, n=20;
LINS group, n=13; LIIL group, n=10; LIILR group, n=18. N group,
normal control group; LINS group, LAD ligation with an injection of
normal saline group; LIIL group, LAD ligation with an injection of
IL-6 recombinant protein group; LIILR group, LAD ligation with
injection of IL-6 receptor inhibitor group; ROS, reactive oxygen
species; MPO, myeloperoxidase. |
HE staining was applied to observe the local
inflammatory response of myocardial tissue (Fig. 6) under a high-power microscope. The
myocardial tissue of the N group revealed intact and well-defined
myocardial cells, while no obvious inflammatory reaction or
myocardial necrosis was observed. The LINS group revealed
leucocytes in particular infiltrated the infarcted area,
accompanied by cardiac myocyte loss. These pathological changes
were even more pronounced in the LIIL group, where numerous
inflammatory cells infiltrated the infarcted area. Injection of
IL-6 receptor inhibitors markedly reduced the local inflammatory
response and myocardial hemorrhage. This indicated that IL-6
injection significantly increased myocardial inflammation and
fibrosis. By contrast, treatment with an IL-6 receptor inhibitor
decreased both myocardial fibrosis and the inflammatory response.
This suggested that IL-6 promoted fibrosis and inflammation in the
infarcted myocardium, which could be partially reversed using IL-6
inhibitors.
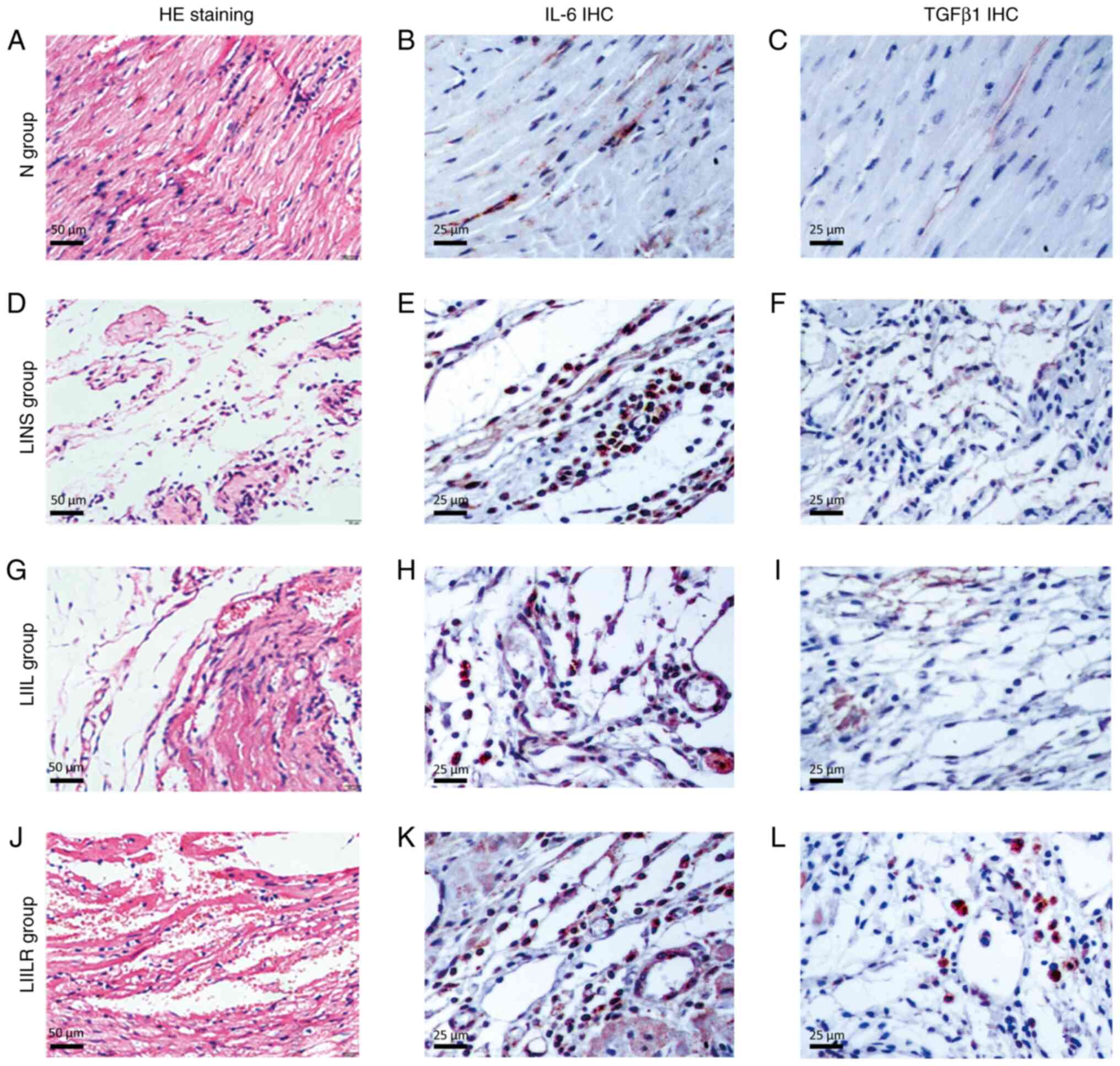 | Figure 6Histological and immunohistological
analyses. (A, D, G and J) HE staining shows the inflammatory
response of the cardiomyopathy in rats and myocardial necrosis. The
myocardial tissue of (A) the N group showed that the morphology of
myocardial cells was complete and the boundaries were clear. The
(D) LINS groups and (G) LIIL groups saw a large number of
inflammatory cells in particular infiltrate the infarcted area,
accompanied by removal of disrupted ventricular tissue. These
pathological phenomena were significantly reduced in the (J) LIILR
group, and local vascular reactivation and residual heart muscle
could be observed. Representative images of immunohistochemical
staining of IL-6 (brown blots) in heart tissues of rat at 2 weeks
are shown as (B), (E), (H) and (K), respectively. The number of
positive expression particles in the LIIL group was the largest,
followed by the LINS group and the LIILR, where they were
significantly reduced, and the N group, where it was rare. TGFβ1
immunized dyeing in myocardial tissue was sepia particles, mainly
distributed in the infarction region, the number of positive
expression particles was the largest in the (I) LIIL group,
followed by the (F) LINS group, (L) LIILR group significantly
reduced and the (C) N group was rare. HE, hematoxylin and eosin;
IHC, immunohistochemistry; N group, normal control group; LINS
group, LAD ligation with an injection of normal saline group; LIIL
group, LAD ligation with an injection of IL-6 recombinant protein
group; LIILR group, LAD ligation with injection of IL-6 receptor
inhibitor group. |
Effects of IL-6 and its receptor
inhibitors on the IL-6/TGFβ1-MMP signaling pathway in myocardial
tissue fibrosis
To investigate the local expression of the proteins
involved in the IL-6/TGFβ1-MMP signaling pathway analyzed, the
present study used immunohistochemical staining and observed the
local distribution of IL-6 and TGFβ1 in myocardial tissue. These
two factors were mainly expressed in inflammatory regions, vascular
endothelium and cardiomyocytes (brown, as presented by the arrows
in Fig. 6). Compared with the N
group, the number of IL-6 and TGFβ1-positive expression levels of
IL-6 and TGFβ1 in the LINS group were markedly higher. These
parameters were further increased in the LIIL group (injected with
IL-6), while IL-6 receptor inhibitor injection led to a marked
reduction of the observed expression of IL-6 and TGFβ1 (LIILR
group) (Fig. 6).
Effects of IL-6 and its receptor
inhibitors on the IL-6/TGFβ1-MMP signaling pathway in myocardial
tissue fibrosis
The effect of the IL-6/TGFβ1-MMP signaling pathway
on the fibrosis process was investigated in the infarcted
myocardium. First, treatment with IL-6 and its receptor inhibitors
was induced, followed by an ELISA analysis of IL-6 and TGFβ1 in the
infarcted myocardium. Ligation (induction of myocardial infarction)
significantly increased the expression levels of IL-6, TGFβ1 and
MMP-9 in the hearts of rats in the LINS group compared with the N
group. IL-6, TGFβ1, MMP-2 and MMP-9 in the LIIL group were further
increased after injection of IL-6. The expression levels of these
factors were significantly reduced after using IL-6 receptor
inhibitors (Fig. 7).
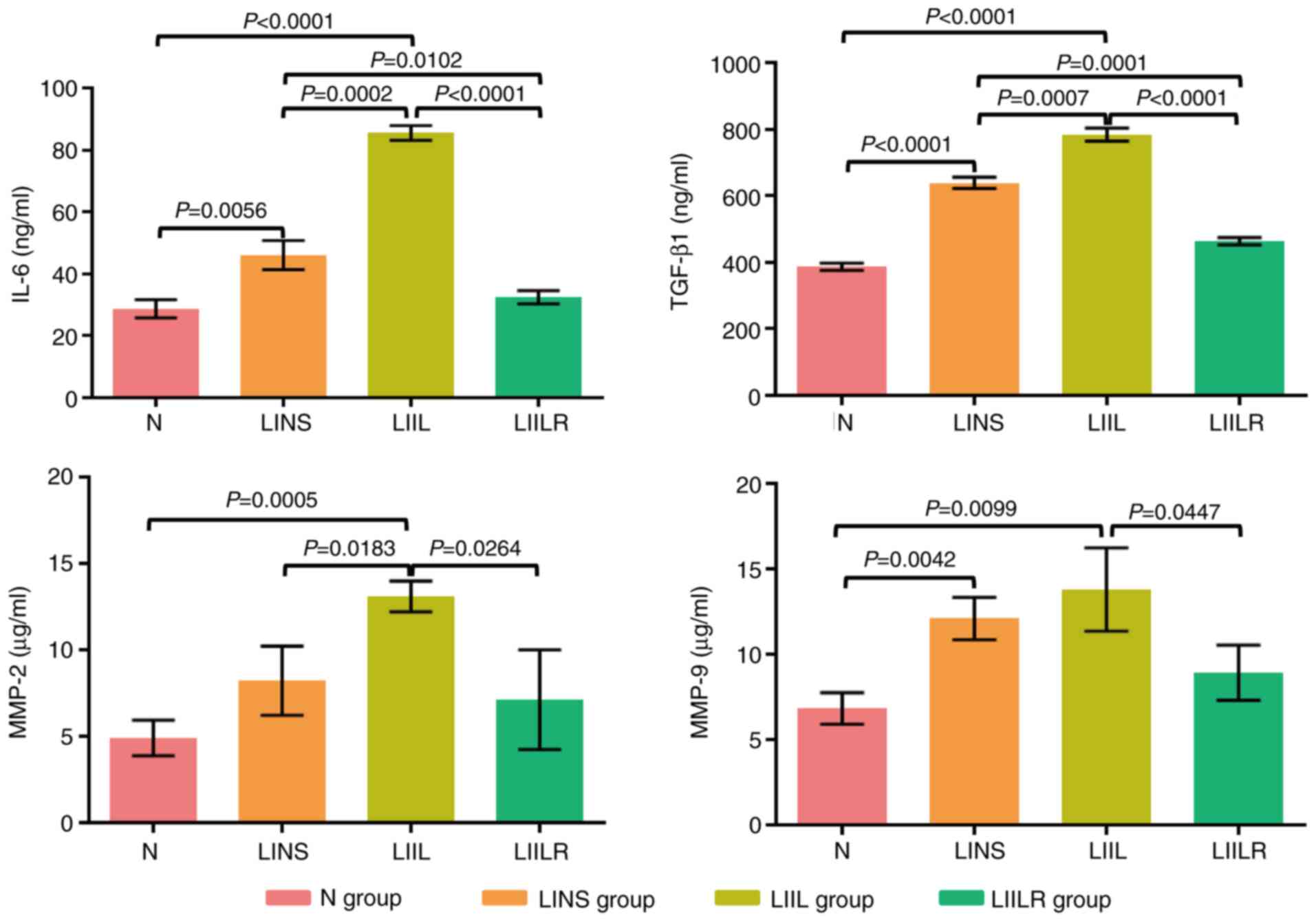 | Figure 7Detection of IL-6, TGFβ1, MMP-2 and
MMP-9 by ELISA. N group, n=20; LINS group, n=13; LIIL group, n=10;
LIILR group, n=18. N group, normal control group; LINS group, LAD
ligation with an injection of normal saline group; LIIL group, LAD
ligation with an injection of IL-6 recombinant protein group; LIILR
group, LAD ligation with injection of IL-6 receptor inhibitor
group. |
Discussion
Since the widespread use of cardiac reperfusion
therapy, the survival rate of patients with myocardial infarction
has significantly increased. Despite this, congestive heart failure
caused by remodeling of the is still a significant clinical problem
(31-34).
The prognosis of these patients is similar to those of numerous
patients suffering from end-stage malignancies (35,36).
The prognosis of patients with heart disease is heavily influenced
by left ventricular remodeling. Among its manifestations are
myocardial fibrosis, dilation of the ventricles and myocardial
dysfunction. An infarct-related remodeling may occur early (within
72 h) or late (after 72 h), which causes further progressive
expansion of the early infarct size, left ventricular dilatation,
left ventricular wall fibrosis and permanent collagen scarring.
Significantly prolonged remodeling causes fibrous tissue necrosis
and a large amount of necrotic material to be deposited, further
aggravating the process and resulting in a vicious cycle. In
clinical terms, this manifests as cardiac insufficiency (37).
The present study demonstrated that, compared with
the normal control group (N group), a typical left ventricular
remodeling occurred in the LINS group at postoperative week, with a
significant expansion of LVEDD. Consequently, left ventricular pump
function was also significantly reduced. After 2 weeks, the changes
in the LINS group were aggravated, causing the animal survival rate
to decrease further. In addition to observing the structural
remodeling of the left ventricle, the remodeling of myocardial
tissue structures in the left ventricle were observed, indicating
that myocardial necrosis, local inflammation and collagen
deposition were still occurring. The same left ventricular
remodelling processes were observed after IL-6 was injected into
the infarct area. The IL-6 inhibitor caused a notable decrease in
remodeling. These results indicated that IL-6 was an important
cytokine in cells, playing an important role in the occurrence and
development of left ventricular remodeling. The results also
confirmed that IL-6 antagonists, such as Tocilizumab, could vastly
improve the prognosis of patients with myocardial infarction by
inhibiting the overall cardiac infarct remodeling.
The present study observed that the mortality of the
infarcted rats in the LIIL group receiving IL-6 injection was
higher compared with that in the other groups, while the mortality
in the LIILR group injected with the IL-6 receptor inhibitor
decreased. Cardiac function (LVEF and LVFS) was progressively
decreased in rats after acute myocardial infarction. Infusion of
IL-6 further impaired cardiac function (LIIL group), while
injection of IL-6 receptor antagonist prevented heart function
decline of the infarcted rats (LIIL group), which suggested that
such an antagonistic mechanism was a potentially therapeutic
preventive target. This would be suitable for post-infarction
patients with high IL-6 levels.
In the present study, cardiovascular function was
similar between the LINS and LIIL groups, and left ventricular
remodeling developed similarly. LVEDD and LVESD increased in both
post-induced infarction (LAD ligation, LINS group) and the
IL-6-added group (LIIL group). As a result of the injection of IL-6
inhibitor (LIILR group), both LVEDD and LVESD demonstrated
measurable improvements in overall cardiac function after
myocardial infarction. Furthermore, was demonstrated that IL-6
played an important role in post-infarction patients, since
injection of IL-6 also led to typical post-infarction changes in
clinical outcomes that are associated with loss of cardiac
function.
Moreover, induced infarction through LAD ligation
increased the inflammation index (MPO) and oxidative stress index
(ROS). These changes were confirmed by pathological analysis, which
demonstrated a local inflammatory reaction and collagen formation
in the infarcted myocardium. Injection of IL-6 aggravated these
pathological changes and expanded the infarction area. Injection of
IL-6 receptor inhibitors significantly reduced local myocardial
inflammation and the collagen content. Therefore, the physiological
changes described as aforementioned are based on local histological
improvement due to IL-6 inhibition, suggesting that IL-6 inhibitors
could be of importance to post-MI patients. The treatment prevented
a rapid expansion of the potentially permanently destroyed
infarction area (thus losing cardiac function) and significantly
reversed the infarction-caused dilapidation of the cardiac tissue.
Several post-infarction syndromes result from this, such as
arrhythmia, Dressler's syndrome and cardiac insufficiency. The
severity of these conditions significantly impacts patient quality
of life (38,39).
The LAD ligation that caused the myocardial
infarction also caused an increase in the expression of local
myocardial inflammatory factors TGFβ1, MMP2 and MMP9. TGFβ1 is a
leading player in the apoptotic cascade (40,41).
Previous studies have indicated a close relationship between IL-6
and TGFβ1, where the latter induces phosphorylation of SMAD-2,
phosphorylated SMAD-2, and SMAD-4 formation in intestinal
epithelial cells (41,42). The complex downregulates IL-6
signaling (43). In the present
study, IL-6 significantly upregulated the expression of TGFβ1 in
infarcted hearts, while the injection of IL-6 receptor inhibitor
significantly reduced its expression, suggesting that TGFβ1 may be
involved as a notable gene downstream of IL-6.
Notably, IL-6 injections increased their levels
further, while adding IL-6 receptor inhibitors also decreased them.
In acute ischemia, these inflammatory factors activate fibroblasts,
enhancing their ability to synthesize and secrete collagen.
Furthermore, some fibroblasts undergo degeneration and necrosis,
releasing cellular debris, further inducing the formation of
collagen fibers and their deposition. Myocardial tissue remodeling
ends once the tissue environment balances inflammatory factors with
fibroblasts. Inhibition of this dynamic process and acceleration of
the time-to-balance result in less myocardium losing its original
state and thus less functional loss (42-45).
The present study revealed that injection of IL-6
receptor inhibitor led to a significant reduction in the observed
expression of IL-6 and TGFβ1 (LIILR group). Thus, the detrimental
effect of TGFβ1 was reduced by a reduction in the collagen content
in the damaged infarction area, avoiding further sequelae. In
another study, strictly linked to myocardial infarction,
non-alcoholic fatty liver disease (NAFLD), the fibrotic effect of
TGFβ1 is more pronounced, as evident from previous studies
(46-49)
Furthermore, in NAFLD, the pro-inflammatory cytokine IL-6 plays an
important role in increasing its serum concentration, likely
inducing the production of a large amount of collagen fiber and
their deposition via TGFβ1 (50-52).
It is TGFβ1 that subverts Th1 and Th2 differentiation in response
to IL-6, resulting in T cells. TGFβ1 in the context of an
inflammatory cytokine milieu supports de novo
differentiation of IL-17-producing T cells (53). Il-17 is also central to the
atherosclerosis process and is a potential driver of myocardial
infarction (54).
MMP belongs to the Zn-dependent protease family and
can degrade extracellular matrix (ECM) components. The tissue
inhibitor of metalloproteinase (TIMP) acts oppositely to MMP,
inhibiting the degradation and thus regulating the content of ECM.
The factors that influence the dynamic balance of MMP and TIMP are
generally considered to be mainly TNF-α, EGF and TGF-α.
Upregulation of the expression levels of these factors leads to an
increase in the ratio of MMP/TIMP. The degradation rate is greater
compared with the synthesis rate, resulting in a decrease in ECM,
which leads to interstitial filling of the fibers, interstitial
fibrosis and ultimately ventricular remodeling (11,31).
After treatment with IL-6, the level of MMP2/9 in the homogenate of
myocardial tissue also increased, and the IL-6 receptor inhibitor
treatment significantly reduced both indices and local tissue fiber
content. Similar changes were also observed. The present study
indicated that, unlike common tissue necrosis factors such as
TNF-α, EGF and TGF-α, the inflammatory mediator IL-6 was also a
principal factor affecting ECM in infarcted hearts. Degradation of
the ECM imbalance and the synthesis of ECM are key factors that
lead to organ fibrosis. Additionally, IL-6 may also affect infarct
remodeling by regulating MMP2/9.
In conclusion, the results of the present study
provided a novel therapeutic target for left ventricular remodeling
after clinical myocardial infarction.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shanghai Health
and Family Planning Commission Project (grant no. 201940057), the
Shanghai Yangpu District Health and Family Planning Commission Fund
for Hao Yi Shi Training Project (grant nos. 202056 and 2020-2023),
the Natural Science Foundation of Shanghai (grant no. 18ZR1436000)
and Major Program of National Key Research and Development Project
(grant no. 2020YFA0112604-02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XP and JW made substantial contributions to the
conception and design of the study, and the acquisition of the
experimental data. MW, HW and FC analyzed and interpreted the data.
SZ and XP performed the statistical analysis. XP, JW and EB
performed the M-mode ultrasound analysis. YZho and NC performed the
electrocardiogram analysis. XL and FC assisted with the IHC
staining. YZha and EB assisted with the ELISA analysis. XL, YZha
and HW drafted the manuscript. FC, XP and EB revised the manuscript
for important intellectual content. FC and XP confirm the
authenticity of all the raw data. All authors contributed to the
writing of the article and have read and approved the final
manuscript.
Ethics approval and consent to
participate
This research proposal was approved by the Ethics
Committee of Yangpu Hospital, School of Medicine, Tongji University
(approval no. LL-2021-SCI-006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iuchi A, Harada H and Tanaka T: IL-6
blockade for myocardial infarction. Int J Cardiol. 271:19–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ritschel VN, Seljeflot I, Arnesen H,
Halvorsen S, Weiss T, Eritsland J and Andersen GØ: IL-6 signalling
in patients with acute ST-elevation myocardial infarction. Results
Immunol. 4:8–13. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gabriel AS, Martinsson A, Wretlind B and
Ahnve S: IL-6 levels in acute and post myocardial infarction: Their
relation to CRP levels, infarction size, left ventricular systolic
function, and heart failure. Eur J Intern Med. 15:523–528.
2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Azevedo PS, Polegato BF, Minicucci MF,
Paiva SA and Zornoff LA: Cardiac Remodeling: Concepts, clinical
impact, pathophysiological mechanisms and pharmacologic treatment.
Arq Bras Cardiol. 106:62–69. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Möttönen MJ, Ukkola O, Lumme J, Kesäniemi
YA, Huikuri HV and Perkiömäki JS: Cardiac remodeling from middle
age to senescence. Front Physiol. 8(341)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ellis SG, Tendera M, de Belder MA, van
Boven AJ, Widimsky P, Janssens L, Andersen HR, Betriu A, Savonitto
S, Adamus J, et al: Facilitated PCI in patients with ST-Elevation
myocardial infarction. N Engl J Med. 358:2205–2217. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ohtsuka T, Hamada M, Inoue K, Ohshima K,
Suzuki J, Matsunaka T, Ogimoto A, Hara Y, Shigematsu Y and Higaki
J: Relation of circulating interleukin-6 to left ventricular
remodeling in patients with reperfused anterior myocardial
infarction: Relation of circulating interleukin-6 to left
ventricular remodeling in patients with reperfused anterior
myocardial infarction. Clin Cardiol. 27:417–420. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
González A, Ravassa S, López B, Moreno MU,
Beaumont J, San José G, Querejeta R, Bayés-Genís A and Díez J:
Myocardial remodeling in hypertension. Hypertension. 72:549–558.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Williams A, Kamper SJ, Wiggers JH, O'Brien
KM, Lee H, Wolfenden L, Yoong SL, Robson E, McAuley JH, Hartvigsen
J and Williams CM: Musculoskeletal conditions may increase the risk
of chronic disease: A systematic review and meta-analysis of cohort
studies. BMC Med. 16(167)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
French BA and Kramer CM: Mechanisms of
postinfarct left ventricular remodeling. Drug Discov Today Dis
Mech. 4:185–196. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Segura AM, Frazier OH and Buja LM:
Fibrosis and heart failure. Heart Fail Rev. 19:173–185.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nakao A, Imamura T, Souchelnytskyi S,
Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH,
Miyazono K and ten Dijke P: TGF-beta receptor-mediated signalling
through Smad2, Smad3 and Smad4. EMBO J. 16:5353–5362.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arrigo M, Jessup M, Mullens W, Reza N,
Shah AM, Sliwa K and Mebazaa A: Acute heart failure. Nat Rev Dis
Primers. 6(16)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mebazaa A, Pitsis AA, Rudiger A, Toller W,
Longrois D, Ricksten SE, Bobek I, De Hert S, Wieselthaler G,
Schirmer U, et al: Clinical review: Practical recommendations on
the management of perioperative heart failure in cardiac surgery.
Crit Care. 14(201)2010.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Hanif K, Bid HK and Konwar R: Reinventing
the ACE inhibitors: Some old and new implications of ACE
inhibition. Hypertens Res. 33:11–21. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rich MW, Chyun DA, Skolnick AH, Alexander
KP, Forman DE, Kitzman DW, Maurer MS, McClurken JB, Resnick BM,
Shen WK, et al: Knowledge Gaps in cardiovascular care of the older
adult population: A Scientific Statement From the American Heart
Association, American College of Cardiology, and American
Geriatrics Society. J Am Coll Cardiol. 67:2419–2140.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moffat K and Mercer SW: Challenges of
managing people with multimorbidity in today's healthcare systems.
BMC Fam Pract. 16(129)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Granger CB, Goldberg RJ, Dabbous O, Pieper
KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG,
Flather MD, et al: Predictors of hospital mortality in the global
registry of acute coronary events. Arch Intern Med. 163:2345–2353.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Siddiqi S and Sussman MA: Cardiac hegemony
of senescence. Curr Transl Geriatr Exp Gerontol Rep 2:
10.1007/s13670-013-0064-3, 2013.
|
|
21
|
Sussman MA and Anversa P: Myocardial aging
and senescence: Where have the stem cells gone? Annu Rev Physiol.
66:29–48. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Torella D, Rota M, Nurzynska D, Musso E,
Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA,
et al: Cardiac stem cell and myocyte aging, heart failure, and
insulin-like growth factor-1 overexpression. Circ Res. 94:514–524.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van Merode T, van de Ven K and van den
Akker M: Patients with multimorbidity and their treatment burden in
different daily life domains: A qualitative study in primary care
in the Netherlands and Belgium. J Comorb. 8:9–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Faustino-Rocha AI, Ginja M, Ferreira R and
Oliveira PA: Studying humane endpoints in a rat model of mammary
carcinogenesis. Iran J Basic Med Sci. 22:643–649. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mohamed AS, Hosney M, Bassiony H,
Hassanein SS, Soliman AM, Fahmy SR and Gaafar K: Sodium
pentobarbital dosages for exsanguination affect biochemical,
molecular and histological measurements in rats. Sci Rep.
10(378)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu Y, Wan Q, Shi L, Ou J, Li Y, He F, Wang
H and Gao J: Siwu granules and erythropoietin synergistically
ameliorated anemia in adenine-induced chronic renal failure rats.
Evid Based Complement Alternat Med. 2019(5832105)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kobara M, Noda K, Kitamura M, Okamoto A,
Shiraishi T, Toba H, Matsubara H and Nakata T: Antibody against
interleukin-6 receptor attenuates left ventricular remodelling
after myocardial infarction in mice. Cardiovasc Res. 87:424–430.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mochizuki D, Adams A, Warner KA, Zhang Z,
Pearson AT, Misawa K, McLean SA, Wolf GT and Nör JE: Anti-tumor
effect of inhibition of IL-6 signaling in mucoepidermoid carcinoma.
Oncotarget. 6:22822–22835. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Arora G, Morss AM, Piazza G, Ryan JW,
Dinwoodey DL, Rofsky NM, Manning WJ and Chuang ML: Differences in
left ventricular ejection fraction using teichholz formula and
volumetric methods by cmr: Implications for patient stratification
and selection of therapy. J Cardiovasc Magn Reson.
12(P202)2010.
|
|
30
|
Wandt B, Bojö L, Tolagen K and Wranne B:
Echocardiographic assessment of ejection fraction in left
ventricular hypertrophy. Heart. 82:192–198. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Konstam MA, Kramer DG, Patel AR, Maron MS
and Udelson JE: Left ventricular remodeling in heart failure
current concepts in clinical significance and assessment. JACC
Cardiovasc Imaging. 4:98–108. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eltzschig HK and Collard CD: Vascular
ischaemia and reperfusion injury. Br Med Bull. 70:71–86.
2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu N, Zhang X, Du S, Chen D and Che R:
Upregulation of miR-335 ameliorates myocardial ischemia reperfusion
injury via targeting hypoxia inducible factor 1-alpha subunit
inhibitor. Am J Transl Res. 10:4082–4094. 2018.PubMed/NCBI
|
|
34
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jones NR, Hobbs FR and Taylor CJ:
Prognosis following a diagnosis of heart failure and the role of
primary care: A review of the literature. BJGP Open.
1(bjgpopen17X101013)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Price JF: Congestive heart failure in
children. Pediatr Rev. 40:60–70. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Klaeboe LG and Edvardsen T:
Echocardiographic assessment of left ventricular systolic function.
J Echocardiogr. 17:10–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Malik J, Zaidi SMJ, Rana AS, Haider A and
Tahir S: Post-cardiac injury syndrome: An evidence-based approach
to diagnosis and treatment. Am Heart J Plus Cardiol Res Practice.
12(100068)2021.
|
|
39
|
Cahill TJ and Kharbanda RK: Heart failure
after myocardial infarction in the era of primary percutaneous
coronary intervention: Mechanisms, incidence and identification of
patients at risk. World J Cardiol. 9:407–415. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cao Y, Chen L, Zhang W, Liu Y,
Papaconstantinou HT, Bush CR, Townsend CM Jr, Thompson EA and Ko
TC: Identification of apoptotic genes mediating TGF-β/Smad3-induced
cell death in intestinal epithelial cells using a genomic approach.
Am J Physiol Gastrointest Liver Physiol. 292:G28–G38.
2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Walia B, Wang L, Merlin D and Sitaraman
SV: TGF-beta down-regulates IL-6 signaling in intestinal epithelial
cells: Critical role of SMAD-2. FASEB J. 17:2130–2132.
2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ge Q, Moir LM, Black JL, Oliver BG and
Burgess JK: TGFβ1 induces IL-6 and inhibits IL-8 release in human
bronchial epithelial cells: The role of Smad2/3. J Cell Physiol.
225:846–854. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ramesh S, Wildey GM and Howe PH:
Transforming growth factor beta (TGFbeta)-induced apoptosis: The
rise & fall of Bim. Cell Cycle. 8:11–17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Su JH, Luo MY, Liang N, Gong SX, Chen W,
Huang WQ, Tian Y and Wang AP: Interleukin-6: A Novel Target for
Cardio-Cerebrovascular Diseases. Front Pharmacol.
12(745061)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shinde AV and Frangogiannis NG:
Fibroblasts in myocardial infarction: A role in inflammation and
repair. J Mol Cell Cardiol. 70:74–82. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Venugopal H, Hanna A, Humeres C and
Frangogiannis NG: Properties and functions of fibroblasts and
myofibroblasts in myocardial infarction. Cells.
11(1386)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang JH, Zhao L, Pan X, Chen NN, Chen J,
Gong QL, Su F, Yan J, Zhang Y and Zhang SH: Hypoxia-stimulated
cardiac fibroblast production of IL-6 promotes myocardial fibrosis
via the TGF-β1 signaling pathway. Lab Invest. 96:839–852.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang L, Roh YS, Song J, Zhang B, Liu C,
Loomba R and Seki E: Transforming growth factor beta signaling in
hepatocytes participates in steatohepatitis through regulation of
cell death and lipid metabolism in mice. Hepatology. 59:483–495.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Okina Y, Sato-Matsubara M, Matsubara T,
Daikoku A, Longato L, Rombouts K, Thanh Thuy LT, Ichikawa H,
Minamiyama Y, Kadota M, et al: TGF-β1-driven reduction of
cytoglobin leads to oxidative DNA damage in stellate cells during
non-alcoholic steatohepatitis. J Hepatol. 73:882–895.
2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Xu F, Liu C, Zhou D and Zhang L:
TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J
Histochem Cytochem. 64:157–167. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tarantino G, Conca P, Riccio A, Tarantino
M, Di Minno MN, Chianese D, Pasanisi F, Contaldo F, Scopacasa F and
Capone D: Enhanced serum concentrations of transforming growth
factor-beta1 in simple fatty liver: Is it really benign? J Transl
Med. 6(72)2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Machado MV and Cortez-Pinto H:
Non-invasive diagnosis of non-alcoholic fatty liver disease. A
critical appraisal. J Hepatol. 58:1007–1019. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Brun P, Giron MC, Qesari M, Porzionato A,
Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R,
et al: Toll-like receptor 2 regulates intestinal inflammation by
controlling integrity of the enteric nervous system.
Gastroenterology. 145:1323–1333. 2013.PubMed/NCBI View Article : Google Scholar
|