Introduction
Asthma is a prevalent chronic disease in children
(1). Increased prevalence of
pediatric asthma has been observed in a number of studies conducted
in China (2,3). The management goals of asthma in
children include improved control of symptoms, normal activity
maintenance, decreased risk of lung growth impairment and symptom
exacerbation and decreased treatment-associated adverse effects
(4).
According to the World Health Organization 2019
report (5), ~262 million
individuals globally have chronic airway inflammation and asthma.
Among these, ~5.1 million are children younger than 18 years old.
Patients with asthma experience regular symptom exacerbations that
affect quality of life. The first-line medication for treatment of
asthma is inhaled corticosteroids (ICSs); however, these do not
prevent symptoms in ~40% of patients (5).
The long-acting anticholinergic bronchodilator drug
tiotropium taken once a day has shown to be efficacious as an
add-on treatment to ICS in adolescents as well as adults (6). The Global Initiative for Asthma
guidelines recommend tiotropium as an additional therapy under
steps 4 and 5 as part of the stepwise methodology for patients with
asthma aged ≥12 years (4).
In children aged 6-11 years, the Global Initiative
for Asthma guideline advises therapy with low dose ICS followed by
stepwise increase in ICS dosage and/or add-on maintenance
treatment, such as long-acting β-agonist (LABA) or leukotriene
receptor antagonist (LTRA), as necessary to treat uncontrolled
asthma (4). China released its
first children's asthma action plan in 2017 to improve asthma
control (7). In China, children
with asthma exhibit poorer compliance than adults with asthma.
Moreover, the prescribing doctor may not show consistent adherence
to therapy guidelines or provide proper treatment or enough
education to pediatric patients with asthma, worsening the problem
of poor asthma control (8,9).
Exacerbations of asthma symptoms are associated with
higher morbidity and mortality risk, as well as greater treatment
cost. The exacerbation risk is raised with decreased lung function
and frequent exacerbations may result in persistent asthma in
pediatric patients and increased chronic obstructive pulmonary
disease risk during adulthood (6,9,10).
Children with asthma exhibit increased comorbidity rates, including
depression and behavioral problems, that increase with severity of
the asthma (11). Moreover, poor
control of asthma increases the risk of sleep interference, such as
awakening at night, resulting in missed school days. Therefore, it
is necessary to increase patient adherence by improved
communication and scrutinizing add-on treatment choices for
treating asthma that are controlled sub-optimally (9). It is important to ensure potential
new treatments are effective and safe. Although the Global
Initiative for Asthma guidelines included tiotropium as one of the
add-on therapies under step 4, available evidence for efficacy and
safety is insufficient (4,9).
The present prospective study aimed to assess the
efficacy and safety of tiotropium add-on treatment administered at
5 µg dose for 12 weeks in children (age, 6-11 years old) with
severe and mild symptomatic asthma.
Materials and methods
Inclusion/exclusion criteria
The patients enrolled in the present study were
children with severe and mild symptomatic asthma aged 6-11 years.
Children received ICS (200 µg budesonide; Budecort; Cipla Ltd.),
ICS + LABA (formoterol fumarate at standard pediatric dose;
Perforomist®; Viatris Inc.), ICS + LTRA (5 mg
montelukast; Almont; Dr. Reddy Laboratories) or ICS (400 µg
budesonide; Budecort; Cipla Ltd.) + >2 controllers (LABA, LTRA
and/or theophylline; Alergin®; Cipla Ltd.) for ≥1 month
and had a score of ≥1.5 based on Asthma Control
Questionnaire-Interviewer-Administered (ACQ-IA) (12). Moreover, participants exhibited
pre-bronchodilator tested forced expiratory volume in 1 sec
(FEV1) at 60-90% of predicted normal logs in addition to
≥12% FEV1 reversibility 15-30 min post-administration of
200 µg salbutamol (Ventolin® HFA; GlaxoSmithKline
PLC).
Children with recent respiratory infection or any
significant disorder other than asthma were excluded.
Intervention
The present study divided 144 children were into
treatment groups and control group (n=72/) based on whether they
received tiotropium or not, respectively. In addition to ICS with
≥1 controller treatment, children in treatment group received
tiotropium (5 µg once daily; 2 doses of 2.5 µg each; Tiova Inhaler;
Cipla Ltd.) for 12 weeks with 3-week follow-up (13). The children in the control group
received ICS with ≥1 controller treatment for 12 weeks and did not
receive tiotropium. These participants were also followed-up for 3
weeks. Salbutamol was used as a rescue treatment as necessary.
Clinical characteristics and outcome
measurement
Asthma monitoring devices (e-diaries with integrated
peak flow meters; Smart Peak Flow; BMedical Pvt. Ltd.) were
utilized for measurement of lung function. Demographic and clinical
data were gathered from medical records.
The primary outcome was peak FEV1 change
from the baseline 3 h post-administration of tiotropium
[FEV1(0-3 h)]. Secondary outcomes were trough
FEV1 change from the baseline (10 min before next dose
administration of tiotropium, i.e. at the dosing interval end),
peak forced vital capacity (FVC) change from baseline 3 h
post-administration [FVC(0-3 h)], trough FVC change,
mean ACQ-IA score and weekly mean rescue treatment usage, peak
expiratory flow (PEF) and symptom-free days.
Other outcomes were mean forced expiratory flow
(FEF) at 25-75% of FVC (FEF25-75%) for every time-point
(during the morning) during 12 weeks of treatment, peak
FEV1(0-3 h), as well as trough of FEV change % and
predicted response at week 12.
Respiratory system-associated adverse
effects
Time to first asthma exacerbation (unusual increase
in ≥1 asthma symptom for ≥2 days and/or ≥30% decrease in morning
PEF for ≥2 days) and first severe exacerbation (asthma
deterioration requiring systemic CS therapy for ≥3 days) were
recorded. All outcome measures were evaluated in the morning
(unless required to monitor in the evening) using spirometry lung
function measurements. The same method was used in both centers at
which the patients enrolled. Adverse and side effects were noted
for 30 days following final administration of tiotropium for
assessment of safety.
Statistical analysis
Categorical variables are expressed as frequency (%)
and continuous variables are expressed as the mean ± standard
deviation (mean of 3 repeats). Distribution of continuous and
ordinal data were checked for normality using Kolmogorov-Smirnov
test. Mann-Whitney U-test was used for non-normal data. The
Student's t-test (Unpaired test for between groups and paired test
for within groups) was used for continuous and ordinal variables
and χ2-test was used for categorical variables for
statistical analysis. Analysis of all data was performed using IBM
SPSS Statistics (version 21.0; IBM Corp.). P<0.05 was considered
to indicate a statistically significant difference. Dunnett's
multiple comparisons test was used for post hoc analysis;
q>2.233 was considered to indicate significance.
Results
Study population
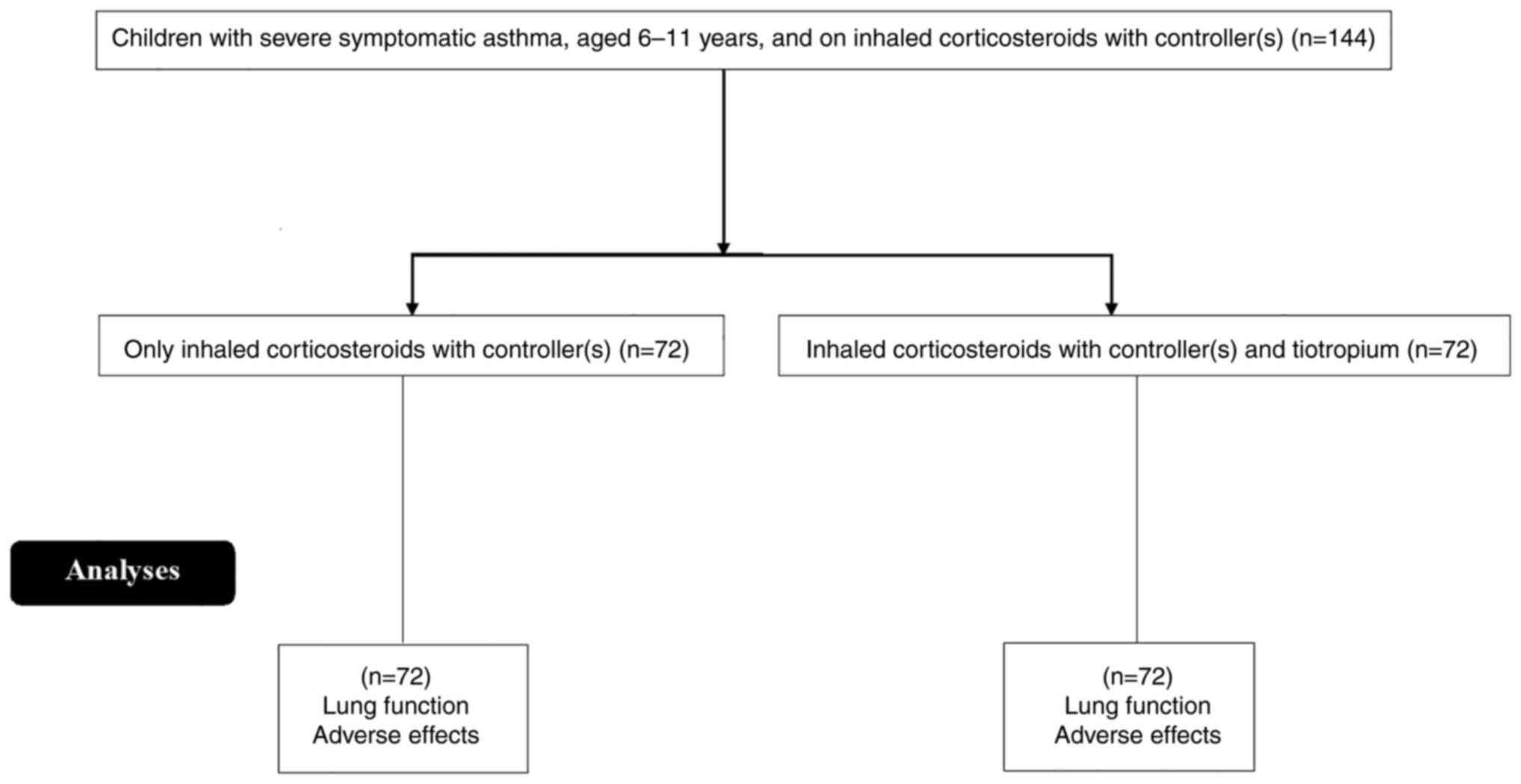
In this study, 144 children were enrolled in the
Department of Pediatrics of the First Affiliated Hospital of
Xinjiang Medical University (Urumqi, China), and the First People's
Hospital of Kashi (Kashi, China) from February 12, 2017, to August
16, 2019. Among these children, 72 were in the tiotropium therapy
group (treatment group) and 72 in the control group (Fig. 1).
Baseline characteristics
The demographic and clinical characteristics were
not significantly different between the two groups (Table I). The majority of children were
male (63%) and aged 9-11 years old (69%), with a mean age of
8.90±1.80 years. All subjects in the treatment groups and control
group started and ended the study at the same time.
 | Table IBaseline demographic and clinical
characteristics. |
Table I
Baseline demographic and clinical
characteristics.
| | Group | |
|---|
| Characteristic | Treatment group (5
µg; n=72) | Control group
(n=72) | P-value | 95% CI | Df |
|---|
| Median age (range),
years | 9.30
(6.00-10.90) | 9.20
(6.00-11.00) | 0.2146 (Mann-Whitney
U-test) | N/A | N/A |
| Age in years, n
(%) | | | | | |
|
6-<9 | 21(29) | 24.00 (33.00) | 0.7192
(χ2-test) | 0.6281 to 1.3070 | 1 |
|
9-11 | 51(71) | 48.00 (67.00) | | | |
| Sex, n (%) | | | | | |
|
Male | 46(64) | 45.00 (63.00) | 0.6828
(χ2-test) | 0.7323 to 1.4500 | 1 |
|
Female | 26(36) | 27.00 (37.00) | | | |
| Body mass index,
kg/m2 | 18.21±4.11 | 17.61±3.89 | 0.3698 (t-test) | -1.9180 to
0.7184 | 71 |
| Asthma duration,
years | 5.11±2.48 | 4.92±2.32 | 0.6357 (t-test) | -0.9812 to
0.6012 | 71 |
| FEV1,
ml | 1584.00±366.00 | 1546.00±348.00 | 0.5242
(t-test) | -155.6600 to
79.6570 | 71 |
| FEV1, %
predicted | 79.11±12.59 | 78.81±12.11 | 0.8843
(t-test) | -4.3700 to
3.7700 | 71 |
| FEV1, %
reversibility | 27.21±13.81 | 26.81±13.91 | 0.8628
(t-test) | -4.9660 to
4.1660 | 71 |
| FVC, ml | 2081.00±483.00 | 2008.00±451.00 | 0.3502
(t-test) | -226.9500 to
80.9520 | 71 |
| FVC, %
predicted | 91.69±14.61 | 90.10±13.81 | 0.5033
(t-test) | -6.2740 to
3.0940 | 71 |
|
FEF25-75%, l/s | 1.30±0.69 | 1.31±0.71 | 0.9318
(t-test) | -0.2207 to
0.2407 | 71 |
|
FEF25-75%, % predicted | 59.79±22.11 | 61.31±24.61 | 0.6972
(t-test) | -6.1870 to
9.2270 | 71 |
| Mean weekly
evening | 232.00±68.00 | 228.00±69.00 | 0.7266
(t-test) | -26.5690 to
18.5690 | 71 |
| PEF, l/min | | | | | |
| Median ACQ-IA score
(range) | 2.08
(1.55-2.62) | 2.14
(1.59-2.58) | 0.3903
(Mann-Whitney U-test) | N/A | N/A |
| ICS dosage, µg | 457.00±246.00 | 474.00±243.00 | 0.6772
(t-test) | -63.5560 to
97.5560 | 71 |
| Add-on maintenance
treatment prior to study, n (%) | | | | | |
|
LABA | 53(74) | 54.00(75) | 0.8487
(χ2-test) | 0.6680 to
1.3930 | 1 |
|
LTRA | 61(85) | 61.00(85) | 0.9999
(χ2-test) | 0.6350 to
1.5750 | 1 |
| Add-on maintenance
treatment during study, n (%) | | | | | |
|
LABA | 51(71) | 54(75) | 0.7076
(χ2-test) | 0.6350 to
1.2810 | 1 |
|
LTRA | 60(83) | 61(85) | 0.8201
(χ2-test) | 0.6178 to
1.4620 | 1 |
Outcome measures
The primary outcome, FEV1(0-3 h), was
significantly improved at week 12 in the treatment group compared
with that of the control group (Table
II; P<0.0001; df, 71; t-test). Regarding secondary outcomes,
trough FEV1 showed significant improvement with
tiotropium compared with control group (Table II; P<0.0001; df, 71; t-test).
Moreover, significant differences were noted for peak FVC(0-3
h) (Table II; P<0.0001;
df, 71; t-test), as well as trough FVC (Table II; P=0.0051; df, 71; t-test) at
week 12 between the treatment group and control group. ACQ-IA
scores demonstrated similar improvement in asthma control
[decreased from baseline in both groups (Table II; P<0.0001; Mann-Whitney
U-test] with significant differences between treatment group and
control group at week 12 (Table
II; P=0.1159; Mann-Whitney U-test). The mean weekly rescue
treatment usage, PEF measurement and symptom-free days (Table II; all P<0.0001; all df, 71;
t-test) showed significant improvements in treatment group compared
with control group.
 | Table IIPrimary and secondary outcome
measures at week 12. |
Table II
Primary and secondary outcome
measures at week 12.
| | Group | |
|---|
| Outcome
measure | Treatment group (5
µg; n=72) | Control group
(n=72) | P-value | 95% CI | Df |
|---|
| Peak FEV1(0-3
h), ml | 384.00±31.00 | 248.00±28.00 | <0.0001 | -145.73000 to
-126.20000 | 71 |
| Peak FEV1(0-3
h), % predicted | 17.40±7.30 | 11.50±5.80 | <0.0001 | -8.07200 to
-3.72800 | 71 |
| Trough
FEV1, ml | 224.00±28.00 | 140.00±31.00 | <0.0001 | -93.73200 to
-74.26800 | 71 |
| Trough
FEV1, % predicted | 9.30±4.20 | 5.80±3.40 | <0.0001 | -4.75900 to
-2.24100 | 71 |
| Peak FVC1(0–3
h), ml | 268.00±34.00 | 241.00±32.00 | <0.0001 | -37.87700 to
-16.12300 | 71 |
| Trough FVC, ml | 153.00±29.00 | 139.00±30.00 | 0.0051 | -23.72100 to
-4.27900 | 71 |
|
FEF25-75%, ml/sec | 389.00±36.00 | 116.00±27.00 | <0.0001 | -283.48000 to
-262.52000 | 71 |
| ACQ-IA score | 0.95±0.04 | 1.02±0.04 | <0.0001 | 0.05682 to
0.08318 | 71 |
| Mean weekly rescue
treatment usage | 0.29±0.08 | 0.36±0.09 | <0.0001 | 0.04195 to
0.09805 | 71 |
| Mean weekly peak
PEF (evening), l/min | 4.12±3.56 | 7.46±3.29 | <0.0001 | 2.21100 to
4.46900 | 71 |
| Mean weekly
symptom-free days | 0.19±0.04 | 0.16±0.04 | <0.0001 | -0.04318 to
-0.01682 | 71 |
FEF25-75% showed significant improvement
in the treatment group compared with control group at week 12
(Table II; P<0.0001; df, 71;
t-test). At week 12, peak FVC(0-3 h) % predicted
response and trough FEV1 % predicted response (Table II; both P<0.0001; both df, 71;
t-test), were statistically different between the two groups.
Respiratory system-associated adverse
effects
A total of 3 children (4%) in the treatment group
and 4 children (6%) in the control group experienced severe
exacerbation of asthma during the study period (P=0.6984; df, 1;
χ2-test). During the study period, ≥1 episode of
exacerbation was seen in 17 children (24%) in the treatment group
and 23 children (32%) in the control group (P=0.3522; df, 1;
χ2-test) (data not shown).
Side effects
The number of children reporting side effects in the
treatment group (n=30; 42%) was less than that in the control group
(n=35; 49%; Table III). The
number of serious side effects requiring hospitalization was 2 (3%;
asthma and respiratory tract infection) in the treatment group and
1 (1%; asthma) in the control group, which was comparable between
the groups. There was no discontinuation of treatment and no fatal
events occurred in either group. Side effects on the cardiovascular
and digestive systems in the treatment group were not reported.
 | Table IIISide effects reported <30 days
after last dosage administration. |
Table III
Side effects reported <30 days
after last dosage administration.
| | Group | |
|---|
| Side effect | Total (n=144) | Treatment group (5
µg; n=72) | Control group
(n=72) | P-value | 95% CI | Df |
|---|
| Any, n (%) | 65(45) | 30(42) | 35(49) | 0.5030 | 0.6214-1.2130 | 1 |
| Serious, n (%) | 3(2) | 2(3) | 1(1) | 0.5596 | 0.5930-3.0410 | 1 |
| Asthma, n (%) | 27(19) | 12(17) | 15(21) | 0.6694 | 0.5486-1.3690 | 1 |
| Decreased PEF rate,
n (%) | 18(13) | 8(11) | 10(14) | 0.8011 | 0.5076-1.5080 | 1 |
| Respiratory tract
infection, n (%) | 10(7) | 5(7) | 5(7) | 0.9999 | 0.5259-1.9020 | 1 |
| Nasopharyngitis, n
(%) | 7(5) | 3(4) | 4(6) | 0.6984 | 0.3559-2.0340 | 1 |
| Xerostomia, n
(%) | 1(1) | 1(1) | 0 (0) | 0.3156 | 1.7080-2.3760 | 1 |
| Oropharyngeal
candidiasis, n (%) | 2(1) | 1(1) | 1(1) | 0.9999 | 0.2476-4.0390 | 1 |
Discussion
In the present prospective study enrolling
6-11-year-old children with severe and mild asthma, tiotropium (5
µg) add-on to ICS with ≥1 controller treatment demonstrated
improvement in lung function. Significant improvement in peak
FEV1(0-3 h) primary outcome was observed in the
treatment group compared with the control. The secondary outcomes,
such as trough FEV1 change, peak FEV1(0-3h) %
predicted response and trough FEV1 % predicted response,
as well as FEF25-75%, showed significant statistical
differences between the two groups (all P<0.0001). Children who
received tiotropium add-on to ICS with ≥1 controller treatment
showed notable efficacy profiles of tiotropium for the management
of asthma. The positive effect of tiotropium as an add-on therapy
to ICS was shown in clinical trials performed in adults and
children by Kerstjens et al (14) and Hamelmann et al (15), respectively. The United States Food
and Drug Administration has approved tiotropium for children >6
years old with asthma. The present study observed children in China
aged 6-11 years with severe and mild asthma treated with tiotropium
add-on to standard therapy. The present study supports evidence
from randomized trials in children and adolescents in demonstrating
the safety and efficacy of tiotropium in severe asthma in a
real-world setting (6,16).
The peak and trough FEV1 change showed
significant improvement following tiotropium treatment in children
with severe and mild asthma. The outcomes of the present study are
in line with those of randomized control trial conducted on
children (9) and adolescents
(15). However, the improvement of
primary outcome is not in line with another study performed on
adolescents (15). Here,
tiotropium add-on to ICS with ≥1 controller treatment improved lung
function of 6-11-year-old children with severe and mild asthma.
ACQ-IA score after 12 weeks was not improved among
children who received tiotropium (treatment group) compared with
the control group, but >70% of children in both groups
demonstrated improvement compared with baseline. These results are
in agreement with studies of children and adolescents (9,15).
Although similar improvement in control of asthma in both groups
was observed, the number of respiratory system-associated adverse
events and side effects (asthma and PEF rate decrease) were found
to be lower in the tiotropium group. The results of respiratory
system-associated adverse events and side effects are in agreement
with studies of children and adolescents (9,15).
Tiotropium add-on to ICS with ≥1 controller treatment demonstrated
improvement in asthma control in 6-11-year-old children with severe
and mild asthma.
Improvement of FEF25-75% in the group
treated with tiotropium add-on to ICS was statistically significant
compared with control. FEF25%-75% associated with small
airways and is a sensitive indicator of symptomatic asthma
(13,17); the aforementioned improvement in
FEV1 showed the efficacy of tiotropium add-on to ICS
with ≥1 controller treatment in 6-11-year-old children experiencing
severe and mild symptomatic asthma.
Significant improvements were noted in peak
FEV1(0-3 h) and trough FVC changes. Moreover, the mean
weekly rescue treatment usage, PEF and symptom-free days showed
improvements in both groups with statistically significant
differences. These results are in agreement with those of the
randomized trials in children (6,9,16).
Children who received tiotropium add-on to ICS with ≥1 controller
treatment showed comparable tolerability profiles.
The efficacy and safety parameters of ICS +
tiotropium in contrast to ICS + LABA are available in other studies
(14,18). In a randomized trial (14) on adult patients with moderate
asthma, tiotropium showed comparable efficacy and tolerability to
LABA (salmeterol). To the best of our knowledge, however, few
studies have investigated the efficacy of ICS + LABA vs. ICS +
anticholinergic drugs in children (9,15).
Tiotropium add-on to ICS with ≥1 controller treatment appears to be
efficacious and safe in severe and mild asthmatic children.
Poor adherence to treatment is one of the common
issues in children that leads to poor asthma control (19,20).
Administering asthma treatments once rather than twice per day
improves adherence (21).
Once-daily tiotropium may be advantageous during step-wise approach
of asthma therapy in children (age, 6-11 years) with severe and
mild asthma (4), especially when
LABA is ineffective or not suitable (22). Tiotropium add-on to ICS with ≥1
controller treatment has good adherence and efficacy in children
with severe and mild asthma.
A limitation of the present study is the accuracy of
treatment usage/adherence and recall bias. The present study was a
multi-center observational study with a small sample size and the
results are not generalizable. The short study period limited
analysis of seasonal/severe exacerbation, asthma outcome and
deterioration. Additionally, short duration and small sample size
may overestimate effects of therapy (type-I error). Nonetheless,
the present data support evidence from a randomized trial (6) in children and adolescents and
demonstrate the safety and efficacy of tiotropium against severe
and mild asthma in a real-world setting.
In conclusion, tiotropium (5 µg) administered
once/day as an add-on treatment to ICS with ≥1 controller treatment
in children (age, 6-11 years) with severe or mild symptomatic
asthma was found to be efficacious and safe. This treatment may
have good adherence, improve lung function and improve asthma
control in 6-11-year-old children with severe or mild asthma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AA and BWMEMYSF were project administrators,
contributed equally to the acquisition of resources, as well as to
the methodology design, conceptualization, literature review,
visualization and validation of the study. PX contributed to the
investigation, data curation, formal analysis and literature
review, and drafted and edited the manuscript for intellectual
content. All authors agree to be accountable for all aspects of
work ensuring integrity and accuracy. All authors have read and
approved the final manuscript. AA, BWMEMYSF and PX confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This was a prospective cohort study. The ethics
committee of the Xinjiang Medical University approved the study
(approval no. XMU1454; 11 February 2016) and the Helsinki
Declaration (2008) guidelines were followed. Patient
confidentiality was strictly maintained. Written informed consent
was obtained from the parents/guardians of each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
AA, ORCID no. 0000-0003-1654-569X; BWMEMYSF, ORCID
no. 0000-0001-8310-9236; PX, ORCID no. 0000-0002-7413-5077.
References
|
1
|
Global Asthma Network: The Global Asthma
Report 2018. http://globalasthmareport.org/Global%20Asthma%20Report%202018.pdf.
Accessed April 22, 2022.
|
|
2
|
Xiang L, Zhao J, Zheng Y, Liu H, Hong J,
Bao Y, Chen A, Deng L, Ji W, Zhong N and Shen K: Uncontrolled
asthma and its risk factors in Chinese children: A cross-sectional
observational study. J Asthma. 53:699–706. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li F, Zhou Y, Li S, Jiang F, Jin X, Yan C,
Tian Y, Zhang Y, Tong S and Shen X: Prevalence and risk factors of
childhood allergic diseases in eight metropolitan cities in China:
A multicenter study. BMC Public Health. 11(437)2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Global Initiative for Asthma: Global
strategy for asthma management and prevention (2021 update).
https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
Accessed April 22, 2022.
|
|
5
|
World Health Organization: World health
statistics overview 2019. https://apps.who.int/iris/bitstream/handle/10665/311696/WHO-DAD-2019.1-eng.pdf.
Accessed June 6, 2022.
|
|
6
|
Szefler SJ, Murphy K, Harper T III, Boner
A, Laki I, Engel M, El Azzi G, Moroni-Zentgraf P, Finnigan H and
Hamelmann E: A phase III randomized controlled trial of tiotropium
add-on therapy in children with severe symptomatic asthma. J
Allergy Clin Immunol. 140:1277–1287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu K, Xiang L and Shen K: Efficacy of
Chinese Children's asthma action plan in the management of children
with asthma. Allergy Asthma Proc. 41:e3–e10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Anderson WC III and Szefler SJ: New and
future strategies to improve asthma control in children. J Allergy
Clin Immunol. 136:848–859. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Montella S, Baraldi E, Bruzzese D, Mirra
V, Di Giorgio A and Santamaria F: group of Primary Care
Pediatricians. What drives prescribing of asthma medication to
preschool wheezing children? A primary care study. Pediatr
Pulmonol. 48:1160–1170. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Belgrave DC, Buchan I, Bishop C, Lowe L,
Simpson A and Custovic A: Trajectories of lung function during
childhood. Am J Respir Crit Care Med. 189:1101–1109.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lange P, Celli B, Agustí A, Boje Jensen G,
Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez-Camblor
P, et al: Lung-function trajectories leading to chronic obstructive
pulmonary disease. N Engl J Med. 373:111–122. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Murray CS, Foden P, Sumner H, Shepley E,
Custovic A and Simpson A: Preventing severe asthma exacerbations in
children. A randomized trial of mite-impermeable bedcovers. Am J
Respir Crit Care Med. 196:150–158. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hamelmann E, Bernstein JA, Vandewalker M,
Moroni-Zentgraf P, Verri D, Unseld A, Engel M and Boner AL: A
randomised controlled trial of tiotropium in adolescents with
severe symptomatic asthma. Eur Respir J. 49(1601100)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kerstjens HA, Casale TB, Bleecker ER,
Meltzer EO, Pizzichini E, Schmidt O, Engel M, Bour L, Verkleij CB,
Moroni-Zentgraf P and Bateman ED: Tiotropium or salmeterol as
add-on therapy to inhaled corticosteroids for patients with
moderate symptomatic asthma: Two replicate, double-blind,
placebo-controlled, parallel-group, active-comparator, randomised
trials. Lancet Respir Med. 3:367–376. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hamelmann E, Bateman ED, Vogelberg C,
Szefler SJ, Vandewalker M, Moroni-Zentgraf P, Avis M, Unseld A,
Engel M and Boner AL: Tiotropium add-on therapy in adolescents with
moderate asthma: A 1-year randomized controlled trial. J Allergy
Clin Immunol. 138:441–450.e8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vogelberg C, Szefler SJ, Vrijlandt EJLE,
Boner AL, Engel M, El Azzi G, Vulcu SD, Moroni-Zentgraf PM,
Eickmeier O and Hamelmann EH: Tiotropium add-on therapy is safe and
reduces seasonal worsening in paediatric asthma patients. Eur
Respir J. 53(1801824)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rao DR, Gaffin JM, Baxi SN, Sheehan WJ,
Hoffman EB and Phipatanakul W: The utility of forced expiratory
flow between 25% and 75% of vital capacity in predicting childhood
asthma morbidity and severity. J Asthma. 49:586–592.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Szefler SJ, Goldstein S, Vogelberg C,
Bensch GW, Given J, Jugovic B, Engel M, Moroni-Zentgraf PM, Sigmund
R and Hamelmann EH: Forced expiratory flow (FEF25-75%) as a
clinical endpoint in children and adolescents with symptomatic
asthma receiving Tiotropium: A post hoc analysis. Pulm Ther.
6:151–158. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kew KM, Evans DJ, Allison DE and Boyter
AC: Long-acting muscarinic antagonists (LAMA) added to inhaled
corticosteroids (ICS) versus addition of long-acting beta2-agonists
(LABA) for adults with asthma. Cochrane Database Syst Rev.
2015(CD011438)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Burgess S, Sly P and Devadason S:
Adherence with preventive medication in childhood asthma. Pulm Med.
2011(973849)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Navaratnam P, Friedman HS and Urdaneta E:
The impact of adherence and disease control on resource use and
charges in patients with mild asthma managed on inhaled
corticosteroid agents. Patient Prefer Adherence. 4:197–205.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Price D, Robertson A, Bullen K, Rand C,
Horne R and Staudinger H: Improved adherence with once-daily versus
twice-daily dosing of mometasone furoate administered via a dry
powder inhaler: A randomized open-label study. BMC Pulm Med.
10(1)2010.PubMed/NCBI View Article : Google Scholar
|