Introduction
Mycoplasma pneumoniae (M. pneumoniae)
is globally recognized as an important pathogen leading to upper
and lower respiratory tract infection in human, particularly in
children (1). ~40% of children
over five years of age suffering from community-acquired pneumonia
(CAP) are caused by M. pneumonia (2). M. pneumoniae-induced pneumonia
(MPP) accounts for 30% of pneumonia (3). As many as 18% of pediatric pneumonia
patients need hospitalization (4).
Furthermore, it has been reported that M. pneumoniae
infection in children aged 5~15 years is closely linked with lobar
pneumonia, featuring as acute, severe and prone to complications
such as pleural effusion, atelectasis and extrapulmonary system
involvement (5). Currently,
macrolides, such as azithromycin (AZM), are recommended as the
first-line therapy for M. pneumoniae infection. However, the
therapeutic effect is limited due to the drug resistance.
The activation of systemic inflammatory response is
a critical characteristic for severe pneumonia, and the
dysregulation of inflammatory cytokines, including tumor necrosis
factor-α (TNF-α), interleukin (IL)-6 and IL-10, contributes to the
development of this disease (6).
Methylprednisolone (MP) is a synthetic glucocorticoid preparation
which inhibits immune and inflammatory response (7). A combined treatment of MP and AZM
provided improved results compared with AZM alone in terms of
faster improvement in clinical manifestations, lower clinical
symptom score, and attenuated laboratory and radiographic items in
refractory MPP of children, indicating that the combination of MP
and AZM may be a promising therapeutic strategy for children with
MPP (8).
At present, RNAomics, including microRNA (miRNA or
miR), piRNA, mRNA and lncRNA, have emerged as a research focus in
the post-genome era. miRNAs are a class of single-stranded
non-coding RNA with regulatory functions (9). It was demonstrated that dysregulated
miRNA expression is associated with lung-related diseases, and
miRNA is involved in the regulation of various biological behaviors
(10).
Existing studies have revealed a downregulated
expression of miR-499a-5p in clinical patients suffering from
hepatitis B virus or autism, and in in vivo models including
gliomas, lung adenocarcinoma and myocardial injury (11-15).
In particular, miR-499a-5p was declined in sepsis-induced lung
injury, and restoration of miR-499-5p protected the lung from
injury by inhibiting inflammation (16). Therefore, the involvement of
miR-499a-5p in inflammation- and infection-related diseases was
hypothesized (15,17). However, whether miR-499a-5p is also
downregulated in MPP remains unclear.
In the present study, M. pneumoniae strain
was utilized to infect A549 cells to mimic MPP in vitro, and
the role of miR-499a-5p in MPP was investigated for the first time.
In addition, the potential mechanism underlying combination of MP
and AZM in MPP was also explored.
Materials and methods
Clinical specimen collection
This study included hospitalized children aged 5-10
years who were diagnosed with MPP at Wuhan Fourth Hospital (Wuhan,
China) from January to December 2019. Children with compromised
immunity, or suffering from chronic lung diseases or asthma were
excluded from the present study. Peripheral blood samples were
obtained from 30 fasting patients with MPP (18 females and 12
males) and 30 fasting healthy volunteers (17 females and 13 males)
in the morning. All patients were in the acute stage of MPP and did
not take any medication prior to sample collection. The present
study was approved (approval no. 2018-KY-08) by the Ethics
Committee of Wuhan Fourth Hospital (Wuhan, China). Patients were
enrolled in the study after written informed consent was obtained
from their parents or guardians.
Cell culture and treatment
M. pneumoniae standard strain ATCC15531
[American Type Culture Collection (ATCC)] was cultured in
PPLO-yeast-extract-glucose-penicillin media containing 20% fetal
bovine serum. A549 cells were obtained from ATCC and cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Cell
culture conditions comprised humidified air, 5% CO2 and
37˚C.
To establish MPP model in vitro,
1x106 ccu M. pneumoniae suspensions were used to
infect 1x105 A549 cells for 4 h (18,19).
Then, the medium was refreshed to remove the unbound M.
pneumoniae. For treatment, A549 cells were treated with 20
µg/ml AZM (Pfizer Pharmaceuticals) alone or in combination with 400
µg/ml MP (MP sodium succinate; Pfizer, Inc.) 1 h prior to M.
pneumonia induction (20,21).
Cell counting kit (CCK)-8 assay
Cell proliferation was assessed by CCK-8 assay.
After the A549 cells seeded in a 96-well plate at a density
5x103 cells/well were infected with M. pneumonia
or treated with AZM and MP for 24, 48 and 72 h, respectively, 10 µl
of CCK-8 reagent (Beijing Solarbio Science & Technology Co.,
Ltd.) was added to each well for another 4 h incubation at 37˚C.
Absorbance at 450 nm was determined using a microplate reader
(ELx808; Omega Bio-Tek, Inc.).
Cytokines analysis
The production of C-reactive protein (CRP) and
inflammatory cytokines, including TNF-α (cat. no. DTA00D), IL-6
(cat. no. D6050), IL-10 (cat. no. D1000B) and CRP (cat. no. DCRP00)
in the cell culture medium were detected using their corresponding
ELISA assay kits (R&D Systems, Inc.) following the
manufacturer's protocol.
TUNEL assay
Cell apoptosis was determined using TUNEL assay
(cat. no. C1098, Beyotime Institute of Biotechnology). After
indicated treatment, A549 cells in a 24-well plate
(2x105 cells/well) were fixed in 4% paraformaldehyde for
25 min at room temperature and incubated in permeabilization
solution (0.1% Triton X-100 in 0.1% sodium citrate), followed by
incubation with 0.3% H2O2 in methanol at room
temperature for 20 min. Then, TUNEL detection solution was added to
cells for another 1 h of incubation at 37˚C, followed by incubation
with stop solution for 10 min at 37˚C. Finally, cells were
incubated with DAB solution at room temperature for 10 min in the
dark and counterstained with hematoxylin to stain the nuclei. After
mounting with neutral resin, cells in five fields of view were
randomly selected for observation under a light microscope (Olympus
Corporation).
Cell transfection
miR-499a-5p mimic (5'-UUAAGACUUGCAGUGAUGUUU-3') and
miR-499a-5p inhibitor (5'-AAACATCTCTGCAAGTCTTAA-3'), as well as
their negative controls (mimic control (5'-ACUACUGAGUGACAGUAGA-3')
and inhibitor control (5'-CAGUACUUUUGUGUAGUACAA-3'), were
synthesized by Guangzhou RiboBio Co., Ltd. A549 cells at the
logarithmic phase were seeded into six-well plates
(1.5x106 cells/well) and cultured overnight. When 80-90%
confluence was achieved, A549 cells were transfected with
miR-499a-5p mimic (50 nM), mimic control (50 nM), miR-499a-5p
inhibitor (50 nM) and inhibitor control (50 nM) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) in
line with the manufacturer's protocol, respectively. After 48 h of
transfection at 37˚C, A549 cells were harvested for further
experiments.
Western blotting
The total proteins were extracted from A549 cells
using RIPA buffer with protease inhibitors (Beyotime Institute of
Biotechnology). After determining the protein concentration with
BCA protein assay kit (Beyotime Institute of Biotechnology), the
proteins (30 µg) were electrophoresed via 10% SDS-PAGE and
transferred onto PVDF membrane (MilliporeSigma). After blocking
with 5% skimmed milk for 1 h at room temperature, the membranes
were incubated with primary antibodies against Bcl-2 (1:1,000; cat.
no. ab196495), Bax (1:1,000; cat. no. ab182733), cleaved caspase3
(1:500; cat. no. ab32042), caspase3 (1:500; cat. no. ab13847),
STAT3 (1:1,000; cat. no. ab68153) and GAPDH (1:1,000; cat. no.
ab181603) at 4˚C overnight. The aforementioned antibodies were all
purchased from Abcam. Then, these membranes were incubated with
HRP-conjugated goat anti-rabbit secondary antibody (1:5,000; cat.
no. sc-2004; Santa Cruz Biotechnology, Inc.) at room temperature
for 2 h. Enhanced chemiluminescence (MilliporeSigma) was used to
visualize reactive protein bands on X-ray film. The protein bands
were quantitatively analyzed by ImageJ v1.8.0 software (National
Institutes of Health).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from clinical samples or A549 cells was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). After measuring the purity at a
wavelength of 260 nm and concentration of RNA using NanoDrop 2000
(Thermo Fisher Scientific, Inc.), the extracted RNA was reversely
transcribed into cDNAs using PrimerScript reverse transcriptase kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol, followed by qPCR using SYBR Green kit (Qiagen GmbH) on an
ABI7500 real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows: 10
min initial denaturation at 94˚C, 15 sec denaturation at 94˚C and
30 sec of annealing at 55˚C (40 cycles) and final extension for 1
min at 72˚C. The values were calculated using the 2-ΔΔCq
method (22) and normalized to U6
or GAPDH expression. The sequences of the primers used were as
followed: miR-499a-5p forward, 5'-GCCGAGTTAAGACTTGCAGTGA-3' and
reverse, 5'-CTCAACTGGTGTCGTGGA-3'; STAT3 forward,
5'-CATCCTGAAGCTGACCCAGG-3' and reverse, 5'-TCCTCACATGGGGGAGGTAG-3';
GAPDH forward, 5'-AATGGGCAGCCGTTAGGAAA-3' and reverse,
5'-CGCGCCCAATACGACCAAATC-3'; and U6 forward,
5'-AACGCTTCACGAATTTGCGT-3' and reverse,
5'-CTCGCTTCGGCAGCACA-3'.
Bioinformatics
The potential binding site between STAT3 and
miR-499a-5p was predicted by Starbase v2.0 (https://starbase.sysu.edu.cn/).
Luciferase reporter assay
The STAT3 sequence including the putative binding
sites of miR-499a-5p was sub-cloned and inserted into the pmirGLO
vector (Promega Corporation) to form wild-type STAT3 (STAT3-WT) and
mutant STAT3 (STAT3-MUT) vectors. Then, A549 cells were
co-transfected with STAT3-WT or STAT3-MUT vector, as well as
miR-499a-5p mimic and mimic control using Lipofectamine®
2000 (Thermo Fisher Scientific, Inc.). After 48 h of transfection,
relative luciferase activity was detected using a dual-luciferase
reporter assay kit (Promega Corporation) and normalized to that of
Renilla.
Statistical analysis
All data were analyzed using SPSS software (version
22.0; IBM Corp.). Differences between two groups were determined
using unpaired Student's t-test, and comparison among groups was
analyzed using one-way ANOVA analysis followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of miR-499a-5p and
inflammatory cytokines between patients and volunteers with or
without MPP treatment
A total of 30 children with MPP and 30 healthy
volunteers were recruited in the present study (Table I). First, the peripheral blood from
children with MPP and healthy volunteers was collected for
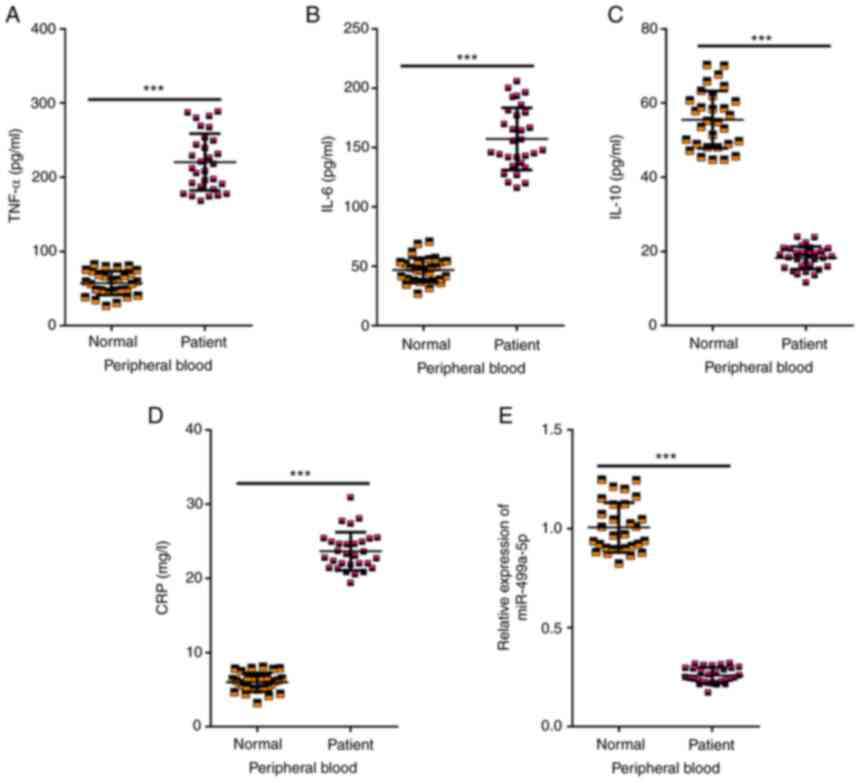
analysis. As revealed in Fig.
1A-D, the levels of TNF-α, IL-6 and CRP were significantly
increased while the level of IL-10 was significantly decreased in
patients compared with those in the healthy volunteers. In
addition, a downregulated expression of miR-499a-5p was detected in
peripheral blood of patients compared with the healthy volunteers
(Fig. 1E). The aforementioned
findings suggested that miR-499a-5p may be associated with the
development of MPP.
 | Table ICharacteristics of the subjects in
the present study. |
Table I
Characteristics of the subjects in
the present study.
|
Characteristics | Patients with MPP
(n=30) | Healthy volunteers
(n=30) |
|---|
| Sex | | |
|
Female | 18 (60%) | 17 (57%) |
|
Male | 12 (40%) | 13 (43%) |
| Age, years | | |
|
5-7 | 20 (67%) | 19 (58%) |
|
8-10 | 10 (33%) | 11 (42%) |
| Mean age,
years | 7.03±1.67 | 7.17±1.74 |
| CRP (mg/l) | 6.00±1.25 | 23.62±2.58 |
M. pneumoniae infection induces
inflammation and apoptosis of A549 cells
To establish MPP model in vitro,
1x106 ccu M. pneumoniae suspensions were used to
infect A549 cells for 4 h. It was revealed that the levels of
TNF-α, IL-6 and CRP were significantly elevated while the level of
IL-10 was significantly decreased in M. pneumoniae-infected
A549 cells (Fig. 2A-D), which were
consistent with the aforementioned results from clinical samples.
Furthermore, after M. pneumoniae infection, cell viability
was also significantly decreased (Fig.
2E). TUNEL assay also revealed the remarkably increased
apoptosis of M. pneumoniae-infected cells (Fig. 2F). Accordingly, the decreased
expression of Bcl-2, increased expression of Bax and cleaved
caspase-3 as well as unchanged level of caspase 3 were observed
(Fig. 2G).
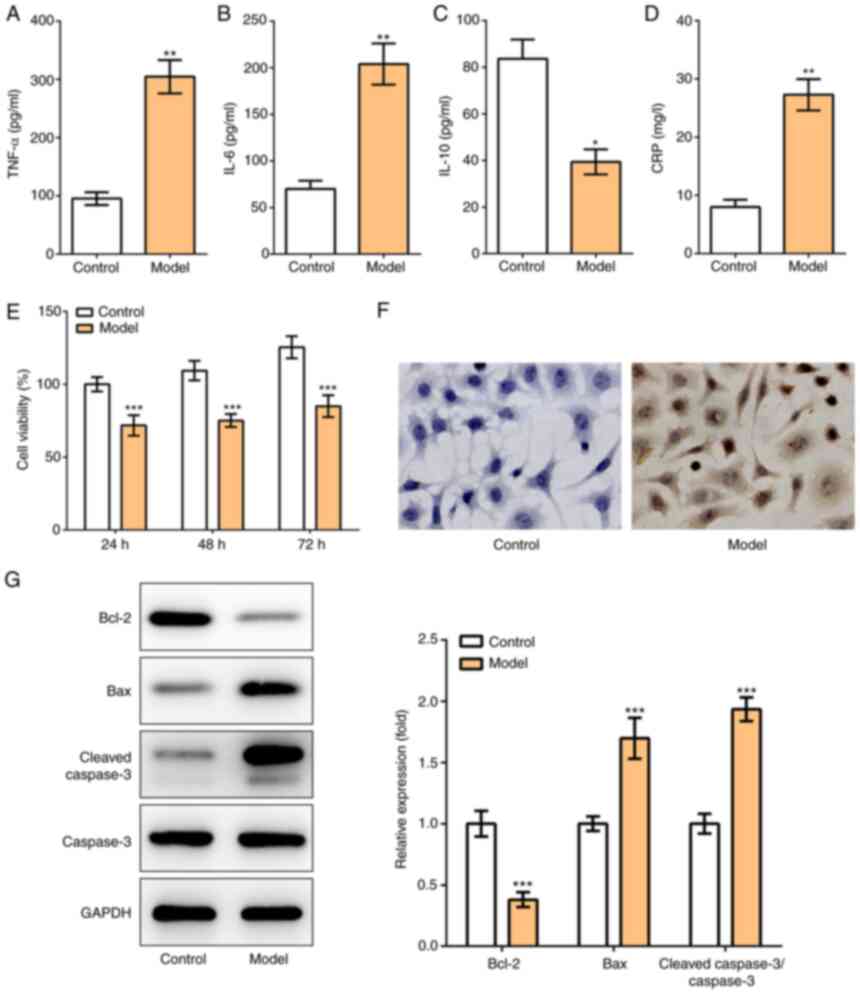 | Figure 2M. pneumoniae infection
induces inflammation and apoptosis of A549 cells. (A-D) A549 cells
were infected with M. pneumoniae to induce MPP model in
vitro, and the production of inflammatory cytokines, including
(A) TNF-α, (B) IL-6, (C) IL-10 and (D) CRP was determined using
corresponding ELISA kits. (E) Cell Counting Kit-8 assay was applied
to detect cell viability. (F) TUNEL assay was conducted to detect
cell apoptosis (magnification, x200). (G) The protein expression
levels of Bcl-2, Bax, cleaved caspase-3 and caspase-3 were detected
using western blotting. *P<0.05,
**P<0.01 and ***P<0.001 vs. control.
M. pneumoniae, Mycoplasma pneumoniae; MPP,
Mycoplasma pneumoniae-induced pneumonia; CRP, C-reactive
protein. |
Treatment with MP and AZM attenuates
inflammation and apoptosis in M. pneumoniae-infected A549
cells
After successful establishment of MPP model in
vitro, MP and AZM were used for treatment. Compared with the
model group, AZM or MP treatment reduced levels of inflammatory
factors in M. pneumoniae-infected A549 cells. Particularly,
the combination of AZM and MP exhibited a more pronounced
inhibition on inflammation (Fig.
3A-D). Additionally, CCK-8 and TUNEL assays showed that AZM
alone or the combination of MP promoted cell viability and
decreased cell apoptosis in M. pneumoniae-infected A549
cells (Fig. 3E and F). Western blot analysis also detected
the upregulated expression of Bcl-2 and the downregulated
expression of Bax and cleaved caspase-3 (Fig. 3G).
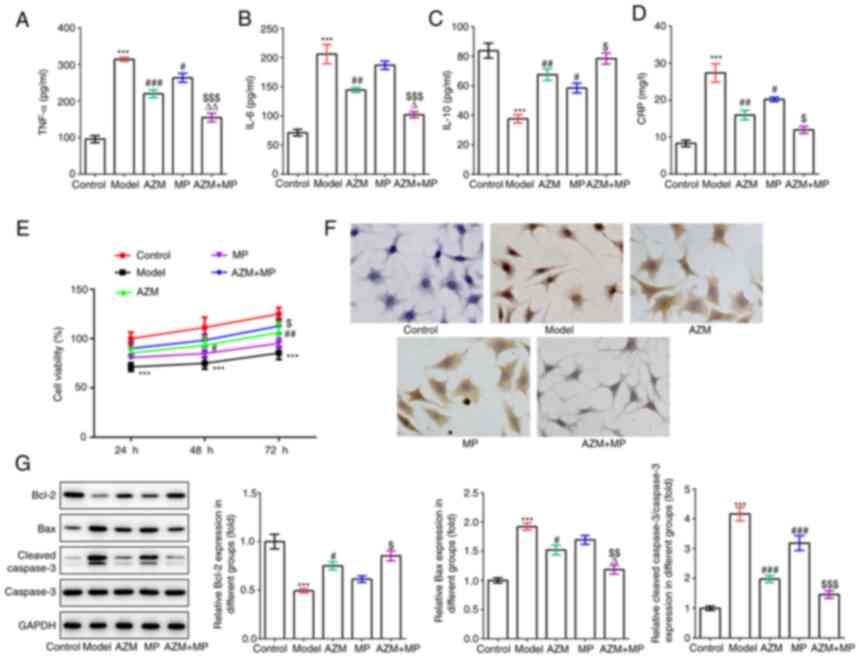 | Figure 3Treatment with MP and AZM attenuates
inflammation and apoptosis in M. pneumoniae-infected A549
cells. (A-D) The production of inflammatory cytokines, including
(A) TNF-α, (B) IL-6, (C) IL-10 and (D) CRP was determined using
corresponding ELISA kits in M. pneumoniae-induced pneumonia
model with or without AZM and MP treatment. (E) Cell Counting Kit-8
assay was applied to detect cell viability. (F) TUNEL assay was
conducted to detect cell apoptosis (magnification, x200). (G) The
protein expression levels of Bcl-2, Bax, cleaved caspase-3 and
caspase-3 were detected using western blotting.
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. model;
$P<0.05, $$P<0.01 and
$$$P<0.001 vs. MP; ∆P<0.05 and
∆∆P<0.01 vs. AZM. MP, methylprednisolone; AZM,
azithromycin; M. pneumoniae, Mycoplasma pneumoniae;
CRP, C-reactive protein. |
Effects of miR-449a-5p during MPP
treatment
To identify the explicit role of miR-499a-5p in MPP,
the expression level of miR-499a-5p in MPP model was also detected
in vitro. As revealed in Fig.
4A, there was a decrease in the expression of miR-499a-5p in
the model group and an icrease in the level of miR-499a-5p in the
AZM + MP group. Thus, miR-499a-5p may be involved in the
progression of MPP. To verify our hypothesis, A549 cells were
transfected with miR-499a-5p inhibitor to suppress the expression
of miR-499a-5p (Fig. 4B). In
addition, the transfected and untransfected A549 cells were
infected with M. pneumoniae and treated with AZM and MP, and
miR-499a-5p inhibitor greatly reversed the elevated miR-499a-5p
expression caused by AZM and MP treatment (Fig. 4C). Inhibition of miR-499a-5p
significantly reversed the promotive effect of combination
treatment of AZM and MP on cell viability (Fig. 4D). Furthermore, the inhibitory
effects of AZM and MP combination treatment on inflammatory
response and cell apoptosis were also retarded by miR-499a-5p
inhibitor (Fig. 4E-J).
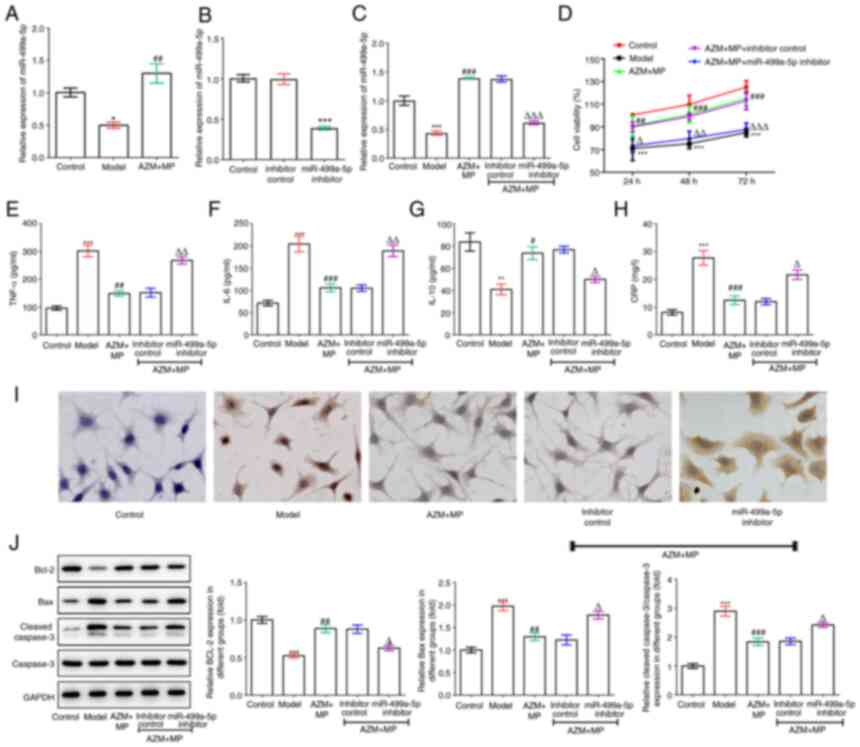 | Figure 4Effects of miR-449a-5p during MPP
treatment. (A) The expression level of miR-499a-5p was detected
using RT-qPCR in MPP model with AZM and MP treatment. (B) A549
cells were transfected with miR-499a-5p inhibitor or inhibitory
control and the expression level of miR-499a-5p was detected using
RT-qPCR. (C) The transfected and untransfected A549 cells were
infected with M. pneumoniae and treated with AZM and MP and
the expression level of miR-499a-5p was detected using RT-qPCR. (D)
Cell Counting Kit-8 assay was applied to detect cell viability.
(E-H) The production of inflammatory cytokines, including (E)
TNF-α, (F) IL-6, (G) IL-10 and (H) CRP was determined using
corresponding ELISA kits. (I) TUNEL assay was conducted to detect
cell apoptosis (magnification, x200). (J) The protein expression
levels of Bcl-2, Bax, cleaved caspase-3 and caspase-3 were detected
using western blotting. *P<0.05,
**P<0.01 and ***P<0.001 vs. control;
#P<0.05, ##P<0.01 and
###P<0.001 vs. model; ∆P<0.05,
∆∆P<0.01 and ∆∆∆P<0.001 vs. inhibitor
control. miR, microRNA; MPP, M. pneumoniae-induced
pneumonia; RT-qPCR, reverse transcription-quantitative PCR; AZM,
azithromycin; MP, methylprednisolone; M. pneumoniae,
Mycoplasma pneumoniae; CRP, C-reactive protein. |
miR-499a-5p targets and negatively
regulates STAT3
To further discover the functional mechanism of
miR-499a-5p, a potential binding site between STAT3 and miR-499a-5p
was identified through Starbase (Fig.
5A). Subsequently, this binding connection between STAT3 and
miR-499a-5p was verified by luciferase reporter assay (Fig. 5B and C). In addition, RT-qPCR and western
blotting assays revealed that both the mRNA and protein expression
of STAT3 were significantly decreased upon miR-499a-5p
overexpression but increased following miR-499a-5p inhibition
(Fig. 5D and E), indicating that miR-499a-5p could
target and negatively regulate STAT3. Furthermore, the mRNA and
protein expression of STAT3 were also elevated in M.
pneumoniae-infected A549 cells; however, the combination
treatment of AZM and MP inhibited the elevated STAT3, and this
inhibitory effect was then weakened by miR-499a-5p inhibitor
(Fig. 5F and G). These findings suggested that
miR-499a-5p targets and negatively regulates STAT3, which is
involved in the pathogenic mechanism and treatment of MPP.
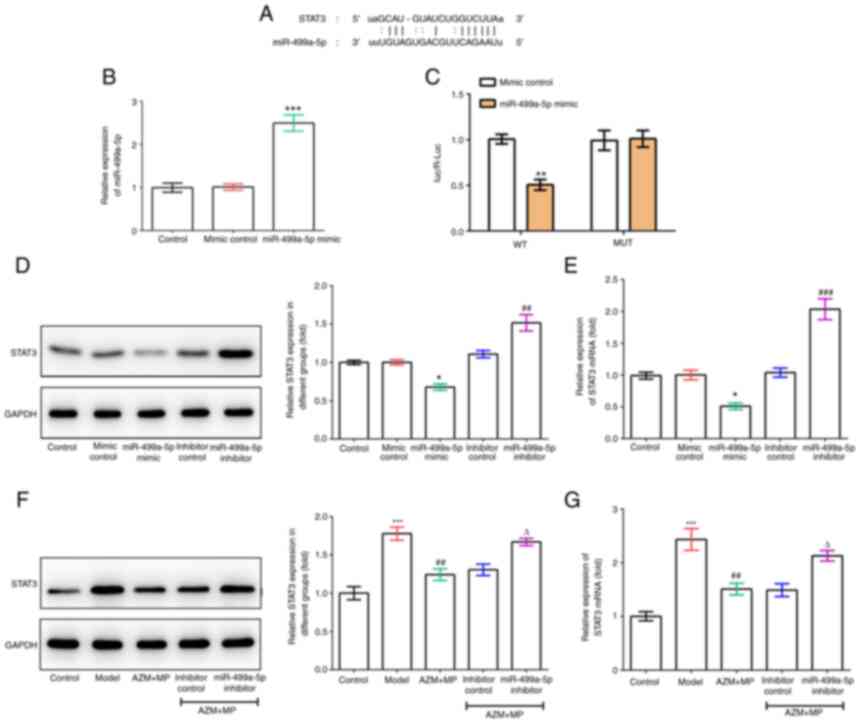 | Figure 5miR-499a-5p targets and negatively
regulates STAT3. (A) The potential binding site between miR-499a-5p
and STAT3 was predicted by StarBase. (B) A549 cells were
transfected with miR-499a-5p mimic and mimic control, then the
expression level of miR-499a-5p was detected by RT-qPCR. (C)
Luciferase reporter assay was conducted to identify the
relationship of miR-499a-5p and STAT3. (D and E) A549 cells were
transfected with miR-499a-5p mimic, miR-499a-5p inhibitor and their
controls, then the mRNA level and protein expression of STAT3 in
each group were detected using RT-qPCR and western blotting,
respectively. *P<0.05, **P<0.01 and
***P<0.001 vs. mimic control; ##P<0.01
and ###P<0.001 vs. inhibitor control. (F and G)
Combination treatment of AZM and MP was used in M.
pneumoniae-infected A549 cells with or without miR-499a-5p
inhibition and the mRNA and protein expression of STAT3 in each
group were detected using RT-qPCR and western blotting,
respectively. ***P<0.001 vs. control;
##P<0.01 vs. model; ∆P<0.05 vs. AZM +
MP + inhibitor control. miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; AZM, azithromycin; MP,
methylprednisolone; M. pneumoniae, Mycoplasma
pneumoniae. |
Discussion
M. pneumoniae is one of the important
pathogenic microorganisms responsible for respiratory tract
infection in humans, particularly in children. Attention has been
markedly paid to uncover the mechanism of AZM and MP in the
prevention and treatment of MPP. In the present study, MPP cell
models were established to study the effect of AZM and MP during
M. pneumoniae infection and to uncover the underlying
mechanism. It was identified that combination treatment with AZM
and MP was more effective against M. pneumoniae-induced
inflammatory, cell viability loss and apoptosis than treatment with
AZM or MP alone. Furthermore, the role of miR-499a-5p in M.
pneumoniae-infected A549 cells was characterized and it was
revealed that combination treatment with AZM and MP may exert its
function in M. pneumoniae-induced A549 cells by regulating
miR-499a-5p/STAT3 axis.
M. pneumoniae infection shows various
clinical manifestations, ranging from asymptomatic infection to
fatal pneumonia. In pneumonia pathogenesis, the imbalance between
pro-inflammatory cytokines such as TNF-α and IL-6 and
anti-inflammatory cytokines such as IL-10 is crucial in triggering
inflammatory response (23). A
previous study has demonstrated that M. pneumoniae infection
induced inflammation and innate immune cell activation in the lung
by activation of NLRP3 (NLR-family, leucine-rich repeat protein 3)
inflammasome complex (24).
Additionally, CRP is a non-specific inflammatory marker, and higher
CRP level is usually found in more severe patients with MPP
(25). In the present study, an
imbalance of inflammatory cytokines was also observed, as evidenced
by elevated TNF-α, IL-6 and CRP expression levels and reduced IL-10
level upon M. pneumoniae infection, leading to inflammatory
response in M. pneumoniae-infected A549 cells.
Cell apoptosis is another essential factor in the
pneumonia pathogenesis that contributes to the loss of defense
function of alveolar epithelial cells (26). It has been previously demonstrated
that M. pneumoniae contributes to DNA damage in host mucosal
epithelial cells, leading to cell apoptosis and necrosis through
the release of toxic metabolites (27). Therefore, in the present study, it
was also hypothesized that M. pneumoniae infection results
in cell apoptosis of A549 cells, which was then verified by
elevated apoptotic rate, decreased expression of Bcl-2 and
increased expression of Bax and cleaved caspase-3.
During the treatment of MPP, though macrolides, such
as AZM, are recommended as the first-line therapy, the therapeutic
effect is limited due to drug resistance. AZM alone is more likely
to cause refractory mycoplasma pneumonia. Furthermore, the children
with low immunity can also develop refractory mycoplasma pneumonia.
Therefore, based on the treatment of AZM, MP, as a potent
glucocorticoid, can effectively suppress the immune response of the
body, reduce alveolar edema, inhibit leukocyte phagocytosis and
infiltration, improve bronchial obstruction and block the disease
progress. In the present study, decreased levels of TNF-α, IL-6 and
CRP, and increased level of IL-10, as well as reduced level of cell
apoptosis, were found in the combination treatment of AZM and MP,
indicating that the combination therapy had a more effective
influence on blocking inflammatory response and suppressing
apoptosis in MPP.
Notably, it was also revealed that miR-499a-5p was
not only closely connected with the progression of MPP, but also
affected the effects of AZM and MP combination therapy. miR-499a-5p
inhibitor partly weakened the protection efficacy of the
combination treatment against cell viability loss, inflammation and
apoptosis. Just as aforementioned, miR-499a-5p was involved in
inflammation- and infection-related diseases. In addition,
miR-499a-5p exhibited a protective role against the progression of
multiple diseases by regulating its different targeted genes. For
instance, Gu et al (28)
uncovered that miR-499a-5p could inhibit the proliferation, and
enhance apoptosis of cervical cancer cells by targeting eIF4E; Zhao
et al (11) found a
decreased level of miR-499a-5p in damaged cardiomyocyte induced by
hypoxia-reoxygenation, and overexpression of miR-499a-5p could
effectively mitigate cardiomyocyte injury by reducing cell
apoptosis via directly targeting CD38. In the present study, STAT3
was demonstrated to be a direct target of miR-499a-5p. STAT3 is a
key signaling protein to facilitate multiple cellular processes,
including growth, differentiation and apoptosis (29). STAT3 is responsible for autosomal
dominant hyperimmunoglobulin E syndrome which is associated with
various infections such as lung and skin infection (30). It has been reported that M.
pneumoniae can induce airway mucus hypersecretion by regulating
STAT3-mediated signaling pathways (31). STAT3 activation was also observed
in A549 cells stimulated by lipid-associated membrane proteins from
M. pneumonia, and was closely associated with inflammation
(32). In the present study, it
was revealed that M. pneumoniae infection induced a higher
expression of STAT3, which was then attenuated upon the combination
treatment of AZM and MP. Considering the relationship between
miR-499a-5p and STAT3, these results indicated that miR-499a-5p may
be an important regulator for MPP formation and treatment.
However, there are certain limitations to the
present study. First of all, the effect of combined AZM and MP was
only investigated in an in vitro cell model, and an in
vivo experiment is needed to validate our conclusion. In
addition, miR-499a-5p/STAT3 axis is one of the potential regulatory
mechanisms involved in the protective effects of AZM and MP in MPP,
and more mechanisms of action need deep exploration.
In conclusion, all the results of the present study
support the idea that combination treatment of AZM and MP could
inhibit M. pneumoniae infection-induced inflammation, cell
viability loss and apoptosis partly by regulating miR-499a-5p/STAT3
axis. Consequently, combination treatment of AZM and MP will be an
alternative ideal therapeutic strategy for children with MPP in
clinical practice.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Scientific
Research Program of Wuhan Municipal Health and Family Planning
Commission (grant no. WZ18D02).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YC and CH designed the experiments. YC, SD, LT, HC
and JC conducted the experiments. YC, SD, LT and CH analyzed and
interpreted the data. YC drafted the manuscript. CH revised the
manuscript. All authors read and approved the final manuscript. YC
and SD confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
2018-KY-08) by the Ethics Committee of Wuhan Fourth Hospital
(Wuhan, China). Patients were enrolled in the study after written
informed consent was obtained from their parents or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang X, Chen X, Tang H, Zhu J, Zhou S, Xu
Z, Liu F and Su C: Increased frequency of Th17 cells in children
with Mycoplasma pneumoniae pneumonia. J Clin Lab Anal.
30:1214–1219. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Atkinson TP and Waites KB: Mycoplasma
pneumoniae infections in childhood. Pediatr Infect Dis J.
33:92–94. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cartner SC, Lindsey JR, Gibbs-Erwin J,
Cassell GH and Simecka JW: Roles of innate and adaptive immunity in
respiratory mycoplasmosis. Infect Immun. 66:3485–3491.
1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Waites KB and Talkington DF: Mycoplasma
pneumoniae and its role as a human pathogen. Clin Microbiol
Rev. 17:697–728. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ferwerda A, Moll HA and de Groot R:
Respiratory tract infections by Mycoplasma pneumoniae in
children: A review of diagnostic and therapeutic measures. Eur J
Pediatr. 160:483–491. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao S, Wang L, Zhu W and Jiang J:
Mycoplasma pneumonia infection and asthma: A clinical study. Pak J
Med Sci. 31:548–551. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Spiga F, Zhao Z and Lightman SL: Prolonged
treatment with the synthetic glucocorticoid methylprednisolone
affects adrenal steroidogenic function and response to inflammatory
stress in the rat. Brain Behav Immun. 87:703–714. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shan LS, Liu X, Kang XY, Wang F, Han XH
and Shang YX: Effects of methylprednisolone or immunoglobulin when
added to standard treatment with intravenous azithromycin for
refractory Mycoplasma pneumoniae pneumonia in children.
World J Pediatr. 13:321–327. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pu M, Chen J, Tao Z, Miao L, Qi X, Wang Y
and Ren J: Regulatory network of miRNA on its target: coordination
between transcriptional and post-transcriptional regulation of gene
expression. Cell Mol Life Sci. 76:441–451. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ebrahimi A and Sadroddiny E: MicroRNAs in
lung diseases: Recent findings and their pathophysiological
implications. Pulm Pharmacol Ther. 34:55–63. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao L, Wang B, Zhang W and Sun L: Effect
of miR-499a-5p on damage of cardiomyocyte induced by
hypoxia-reoxygenation via downregulating CD38 protein. J Cell
Biochem. 121:996–1004. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
He S, Li Z, Yu Y, Zeng Q, Cheng Y, Ji W,
Xia W and Lu S: Exosomal miR-499a-5p promotes cell proliferation,
migration and EMT via mTOR signaling pathway in lung
adenocarcinoma. Exp Cell Res. 379:203–213. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang M, Yang C, Liu X, Zheng J, Xue Y,
Ruan X, Shen S, Wang D, Li Z, Cai H and Liu Y: An upstream open
reading frame regulates vasculogenic mimicry of glioma via
ZNRD1-AS1/miR-499a-5p/ELF1/EMI1 pathway. J Cell Mol Med.
24:6120–6136. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ozkul Y, Taheri S, Bayram KK, Sener EF,
Mehmetbeyoglu E, Öztop DB, Aybuga F, Tufan E, Bayram A, Dolu N, et
al: A heritable profile of six miRNAs in autistic patients and
mouse models. Sci Rep. 10(9011)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Singh AK, Rooge SB, Varshney A, Vasudevan
M, Bhardwaj A, Venugopal SK, Trehanpati N, Kumar M, Geffers R,
Kumar V and Sarin SK: Global microRNA expression profiling in the
liver biopsies of hepatitis B virus-infected patients suggests
specific microRNA signatures for viral persistence and
hepatocellular injury. Hepatology. 67:1695–1709. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang W, Li J, Yao H and Li T: Restoring
microRNA-499-5p protects sepsis-induced lung injury mice via
targeting Sox6. Nanoscale Res Lett. 16(89)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jarret A, McFarland AP, Horner SM, Kell A,
Schwerk J, Hong M, Badil S, Joslyn RC, Baker DP, Carrington M, et
al: Hepatitis-C-virus-induced microRNAs dampen interferon-mediated
antiviral signaling. Nat Med. 22:1475–1481. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Lin Y, Tan D, Kan Q, Xiao Z and Jiang Z:
The protective effect of Naringenin on airway remodeling after
Mycoplasma pneumoniae infection by inhibiting
autophagy-mediated lung inflammation and fibrosis. Mediators
Inflamm. 2018(8753894)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu F, Zhang X, Zhang B, Mao W, Liu T, Sun
M and Wu Y: TREM1: A positive regulator for inflammatory response
via NF-κB pathway in A549 cells infected with Mycoplasma
pneumoniae. Biomed Pharmacother. 107:1466–1472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zerin T, Kim YS, Hong SY and Song HY:
Protective effect of methylprednisolone on paraquat-induced A549
cell cytotoxicity via induction of efflux transporter,
P-glycoprotein expression. Toxicol Lett. 208:101–107.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kitsiouli E, Antoniou G, Gotzou H,
Karagiannopoulos M, Basagiannis D, Christoforidis S, Nakos G and
Lekka ME: Effect of azithromycin on the LPS-induced production and
secretion of phospholipase A2 in lung cells. Biochim Biophys Acta.
1852:1288–1297. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi S, Liu X and Li H: Downregulation of
caspase3 alleviates Mycoplasma pneumoniae induced apoptosis
in alveolar epithelial cells. Mol Med Rep. 16:9601–9606.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Segovia JA, Chang TH, Winter VT, Coalson
JJ, Cagle MP, Pandranki L, Bose S, Baseman JB and Kannan TR: NLRP3
is a critical regulator of inflammation and innate immune cell
response during Mycoplasma pneumoniae infection. Infect
Immun. 86:e00548–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu LQ, Wang ZH and Yao HY: Hepatocyte
growth factor can guide treatment of Mycoplasma pneumoniae
pneumonia in children. Exp Ther Med. 19:3432–3438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang C, Dong WB, Zhao S, Li QP, Kang L,
Lei XP, Guo L and Zhai XS: Construction of p66Shc gene interfering
lentivirus vectors and its effects on alveolar epithelial cells
apoptosis induced by hyperoxia. Drug Des Devel Ther. 10:2611–2622.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun G, Xu X, Wang Y, Shen X, Chen Z and
Yang J: Mycoplasma pneumoniae infection induces reactive
oxygen species and DNA damage in A549 human lung carcinoma cells.
Infect Immun. 76:4405–4413. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gu X, Dong M, Liu Z, Yang J and Shi Y:
MiR-499a-5p inhibits proliferation, invasion, migration, and
epithelial-mesenchymal transition, and enhances radiosensitivity of
cervical cancer cells via targeting eIF4E. Onco Targets Ther.
13:2913–2924. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guanizo AC, Fernando CD, Garama DJ and
Gough DJ: STAT3: A multifaceted oncoprotein. Growth Factors.
36:1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Deverrière G, Lemée L, Grangé S, Boyer S,
Picard C, Fischer A and Marguet C: Life-threatening Pneumopathy and
U urealyticum in a STAT3-deficient hyper-IgE syndrome patient.
Pediatrics. 139(e20160845)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hao Y, Kuang Z, Jing J, Miao J, Mei LY,
Lee RJ, Kim S, Choe S, Krause DC and Lau GW: Mycoplasma
pneumoniae modulates STAT3-STAT6/EGFR-FOXA2 signaling to induce
overexpression of airway mucins. Infect Immun. 82:5246–5255.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi SY, Lim JW, Shimizu T, Kuwano K, Kim
JM and Kim H: Reactive oxygen species mediate Jak2/Stat3 activation
and IL-8 expression in pulmonary epithelial cells stimulated with
lipid-associated membrane proteins from Mycoplasma
pneumoniae. Inflamm Res. 61:493–501. 2012.PubMed/NCBI View Article : Google Scholar
|