Introduction
Lymphatic malformation (LM) is one of the slow-flow
vascular anomalies caused by abnormal embryologic development of
the lymphatic system. LM manifests before the age of 2 years in
>90% of patients, and has been called ‘lymphangioma’ or ‘cystic
hydroma’ in the past (1-3).
It most commonly occurs in the neck, followed by the trunk and
extremities. However, intra-abdominal LM is rare, only accounting
for 5-10% of cases (4-6).
Abdominal pain and other gastrointestinal symptoms such as
abdominal distension, vomiting and constipation are present in
children with abdominal LM (4,6,7),
which is different from the painless LM mass typical in the more
common sites (2,8). LMs are classified by size as
macrocystic, microcystic or mixed patterns, which is of therapeutic
importance (2). In the abdomen,
the majority of LM are classified as a macrocystic lesion (6-8),
arising from mesentery, retroperitoneum or solid organs (4,8,9).
Historically, surgical resection has been the
standard treatment regardless of location (1,2,7).
Percutaneous sclerotherapy as a minimally invasive method by
image-guidance has been preferred treatment applied for LM with
good outcomes over the past years (1,2,6-8).
However, surgical resection for intra-abdominal LM, especially with
laparoscopy, has some advantages. In The Second Affiliated Hospital
Of Xi'an Jiaotong University (Xi'an, China), laparoscopic surgery
has been widely used in the pediatric population, and so the
present retrospective analysis of intra-abdominal LM treated with
laparoscopy was undertaken to provide pediatric surgeons with more
experience in diagnosis and treatment of this lesion.
Materials and methods
Study population
A total of 13 patients with intra-abdominal LM were
collected retrospectively from electronic medical records of The
Second Affiliated Hospital Of Xi'an Jiaotong University (Xi'an,
China) of patients from March 2017 to June 2021. Overall, 10
children were included in the study; one child who was simply
monitored and two children who underwent sclerotherapy were
excluded. Clinical data were reviewed including present and past
history of illness, demographic characteristics, imaging choice for
diagnosis, operative data and postoperative follow-up. Written
informed consent was obtained from parents of children. The study
was approved by the Ethics Committee of The Second Affiliated
Hospital Of Xi'an Jiaotong University (approval number:
2022205).
Laparoscopic technique
Laparoscopy was performed with patients under
general anesthesia in the supine position. At first, a 5-mm
umbilical trocar was inserted with the open method (10). Pneumoperitoneum was established and
maintained at 8-12 mmHg by CO2 gas according to the
child's age and weight. Subsequently, the next two 5-mm (or 3-mm)
trocars were inserted according to lesion location.
After confirmation of the origin and involvement of
the lymphatic cysts, the surgeon decided how to finish the
resection. LMs can contain huge cysts that block sight and be
difficult to extract via the small incisions created by the
trocars. Therefore, large cysts were punctured with Veress needle
guided by laparoscopy and lymphatic fluid was suctioned clear
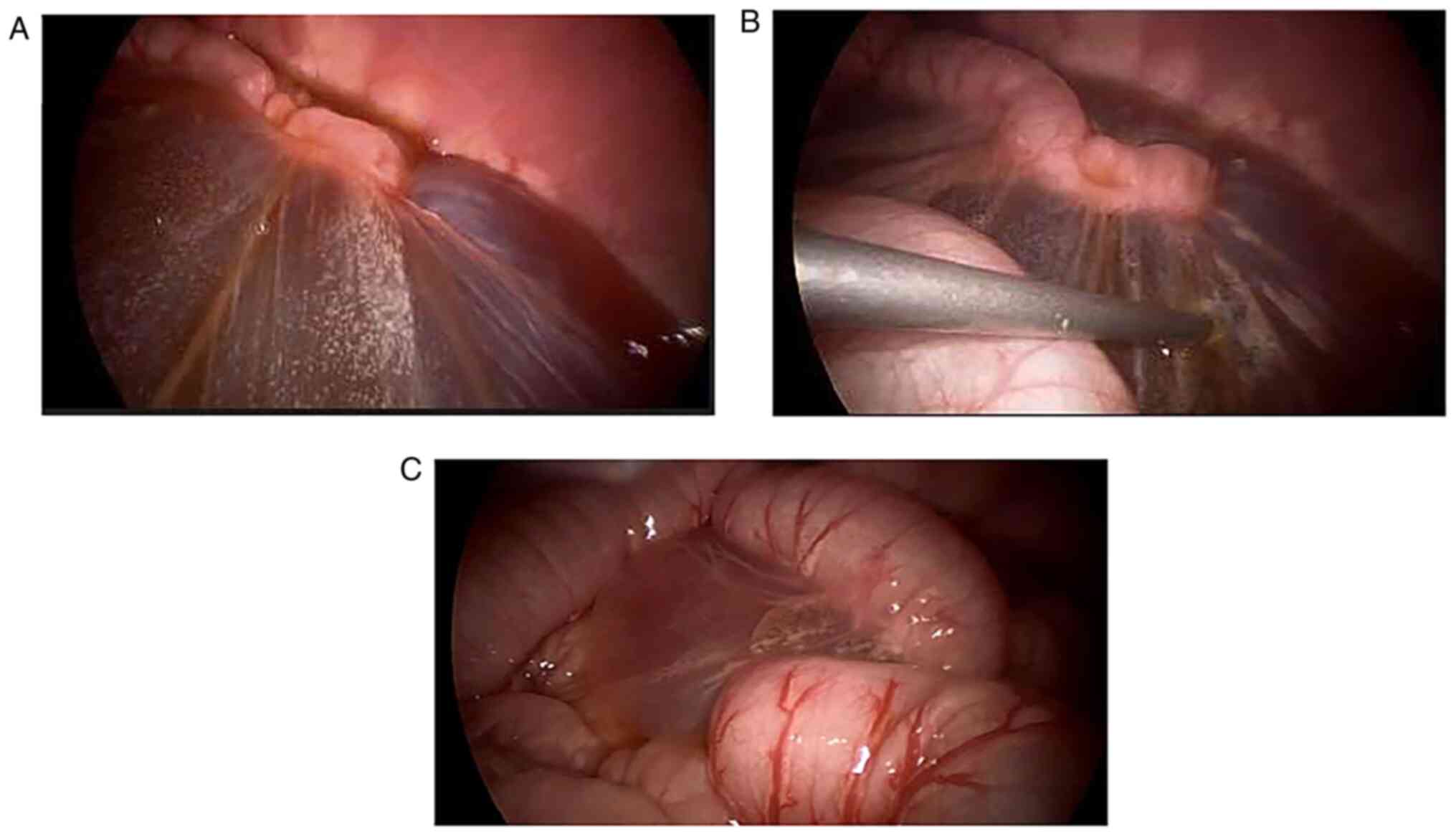
(Fig. 1) to extracorporeal
container. The lymphatic cysts, mesentery or adjacent tissues were
then resected. Complete resection of the LM and intestinal
anastomosis after partial resection of the small intestine were
performed by exteriorization of the small bowel via the enlarged
umbilical trocar port. Nearly complete resection was made in
instances of severe adhesion to important tissues or organs, and
the cyst wall was cauterized by electrocoagulation.
Results
Clinical characteristics of
patients
Overall, 10 patients with intra-abdominal LM
underwent laparoscopic surgery, and their characteristics are
presented in Table I. The male to
female ratio was 1:1, and the average age at operation was 55
months (range, 1-94 months). All of them were referral patients
with 20% (2/10) diagnosed with LM, half of whom presented to the
emergency department because of acute abdomen. Subsequently, one
patient was diagnosed on prenatal ultrasound, one infant presented
with restlessness and the other eight patients suffered from
abdominal pain accompanied by vomiting or fever. The referral
diagnosis was obtained from the medical records or description by
the parents. The interval from primary diagnosis to surgery ranged
from 5 days to 21 months (median, 24 days).
 | Table IPatient characteristics and
preoperative assessment. |
Table I
Patient characteristics and
preoperative assessment.
| Patient no. | Sex | Age, months | Weight, kg | Emergency
experience | Referral
diagnosis | Symptoms | Duration, days | Preoperative
imaging | Size, cm |
|---|
| 1 | Male | 37 | 16.5 | Yes | Peritoneal
effusion | Abdominal pain,
fever | 7 | US + CT | 12 |
| 2 | Male | 77 | 19 | Yes | Lymphangioma | Abdominal pain | 639 | US + CT | 7.8 |
| 3 | Male | 54 | 18 | Yes | Intestinal
duplication | Abdominal pain,
vomiting | 9 | US | 2.8 |
| 4 | Female | 94 | 20 | No | Omental cyst | Abdominal pain | 93 | US + MRI | 4.6 |
| 5 | Female | 53 | 16 | Yes | Mesenteric cyst | Abdominal pain,
fever | 5 | US + CT | 9.9 |
| 6 | Male | 1 | 5 | No | Lymphatic
malformation | Prenatal
examination | 45 | US + MRI | 10 |
| 7 | Female | 74 | 19 | No | Giant mass | Abdominal pain | 36 | US + MRI | 12.7 |
| 8 | Female | 78 | 24 | Yes | Intra-abdominal
cyst | Abdominal pain,
vomiting | 29 | US + MRI | 11.8 |
| 9 | Female | 73 | 21 | No | Intra-abdominal
cyst | Abdominal pain | 19 | US + CT | 9.6 |
| 10 | Male | 9 | 10 | No | Intra-abdominal
cyst | Restless | 6 | US + MRI | 13.8 |
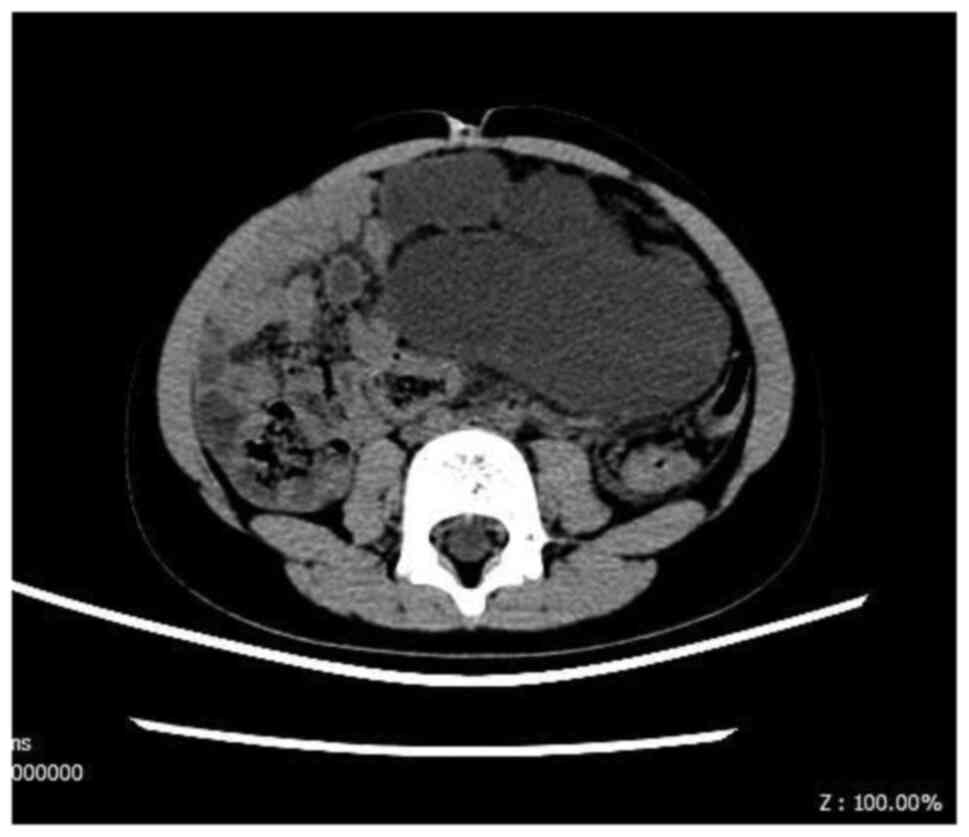
Ultrasound as the first choice was performed in all
of the patients to identify the lesion. Computed tomography
(Fig. 2) and magnetic resonance
imaging were performed for further information in four and five
cases, respectively. Overall, 40% (4/10) of the patients were
diagnosed with LM preoperatively without any biopsies; three
mesenteric cysts, two intra-abdominal cysts and one intestinal
duplication were suspected in the remaining six patients
preoperatively. By imaging, LM size ranged from 2.8-13.8 cm (mean,
9.5 cm).
Surgical outcome
The final diagnosis was confirmed by intraoperative
findings and postoperative histopathology. As presented in Table II, LMs were located in the
mesentery in 70% (7/10) patients, other locations included
retroperitoneum and greater omentum. Macrocystic LM was diagnosed
in nine patients by intraoperative and imaging findings after final
diagnosis.
 | Table IIIntraoperative and postoperative
data. |
Table II
Intraoperative and postoperative
data.
| Patient no. | Location | Type | Laparoscopic
approach | Mode of
operation | Operative duration,
min | Intraoperative
bleeding, ml | Final
diagnosis | Follow-up,
months |
|---|
| 1 | Greater
omentum | Macrocystic | Yes | Lesion with partial
greater omentum | 165 | 80 | Omental LM with
hemorrhage | 60 |
| 2 | Mesocolon | Macrocystic | Yes | Lesion with
segmental colon resection | 135 | <5 | Mesenteric LM with
infection | Missing visit |
| 3 | Mesoileum | Mixed | Yes | Lesion excision
only | 85 | <5 | Mesenteric LM with
hemorrhage | 50 |
| 4 | Mesocolon | Macrocystic | Yes | Lesion excision
only | 65 | <5 | Mesenteric LM | 46 |
| 5 | Mesocolon | Macrocystic | Yes | Lesion excision
only | 80 | <5 | Mesenteric LM with
infection | 36 |
| 6 | Mesocolon | Macrocystic | Yes | Lesion excision
only | 95 | <5 | Mesenteric LM | 17 |
| 7 |
Retroperitoneum | Macrocystic | Converting to
laparotomy | Lesion with
segmental colon resection | 310 | 20 | Retroperitoneal LM
with hemorrhage | 11 |
| 8 | Mesoileum | Macrocystic | Yes | Lesion with
segmental ileum resection | 100 | <5 | Mesenteric LM | 48 |
| 9 | Jejunum- ileum
mesentery | Macrocystic | Yes | Lesion with
segmental jejunum- ileum resection | 120 | <5 | Mesenteric LM | 30 |
| 10 |
Retroperitoneum | Macrocystic | Converting to
laparotomy | Lesion with
segmental colon to ileum resection | 260 | 10 | Retroperitoneal LM
with infection | 19 |
Laparoscopic resection of LMs was completed in eight
patients; however, two patients were converted to laparotomy
because of the retroperitoneal origin of huge LMs that involved the
colon. In three of the eight patients who underwent complete
laparoscopic resection, an assisting and enlarged incision via
umbilical trocar site to finish the extraperitoneal intestinal
anastomosis. Incomplete excision of the LM was performed in patient
7 because of severe adhesion to the inferior vena cava. The
residual cyst wall was cauterized carefully to avoid recurrence.
Complete resection was performed in the other nine patients. The
mean duration of operative time was 106 min (range, 65-165 min) in
eight patients with complete laparoscopic resection. Total blood
loss during the operation was very small. Intracystic hemorrhage
and infection was confirmed by intraoperative findings and
histopathology in 60% (6/10) patients. No complications or
mortality occurred after surgery. The mean follow-up period was 35
months (range, 11-61 months). Patients were asymptomatic and no
recurrence was identified by ultrasound from 1 to 12 months after
surgery except for missing one.
Discussion
Intra-abdominal LM is rare in children and
challenging for surgeons to differentiate from other cystic lesions
(8). Surgical excision is
indicated for patients with LM who have complications such as
infection or hemorrhage (2).
Laparoscopic surgery is a minimally invasive method for treating
intra-abdominal LM, and the rapid development of laparoscopic
technique has benefitted patients with LM (11). Because of less intraoperative blood
loss, postoperative pain and scarring, laparoscopic surgery has
more advantages compared with conventional open surgery (11).
The majority of LMs are noted in the first few years
of life, and grow proportionately with the growth of children
(2). Intra-abdominal LMs are
usually diagnosed with acute gastrointestinal symptoms because of
complications of large LMs at the mean age of 5 years (4), which is similar to the results of the
present study. Female patients outnumbered male in some previous
studies (4,6); male predominance was reported in
other studies (7,12,13),
but there was no sex predilection in the present cases, which may
be because of the small number of patients included.
The majority of patients of intra-abdominal LM are
reported to have acute gastrointestinal symptoms (4,6,13),
which differed from the majority of LMs presenting as a painless
mass (1,2,8)
diagnosed clinically and radiologically (7,14).
Although varied symptoms were present in 80-96% of patients,
abdominal pain was the most common symptom of intra-abdominal LMs
(4,6,7,12) as
in the present study. Macrocystic LMs were the most common pattern
of intra-abdominal LMs although combined and microcystic were also
identified in the abdomen (4,6,7,9,13).
Except for symptoms from mass effect and volvulus, complications
including intracystic hemorrhage and infection occurred in 60% of
the present patients and may be the main cause of acute abdomen
(3). Therefore, the diagnosis was
challenging preoperatively and misdiagnosis occurred in ~1/3 of
patients with intra-abdominal LM (4).
On imaging, cystic lesions such as teratoma, enteric
duplication cyst and ovarian cyst can be confused with
intra-abdominal LMs (8). To avoid
radiation, ultrasound is the first choice to evaluate
intra-abdominal LMs in children, which typically shows multilocular
cystic masses. Further evaluation with cross-sectional imaging
provides additional information on diagnosis and location of the
lesion. However, although imaging findings play an important role
in the diagnosis of intra-abdominal LMs, there is no highly
specific radiological presentation that makes the definitive
diagnosis (4,8).
Percutaneous sclerotherapy by image-guidance has
been preferred as primary treatment modality for LM over the past
years (1,2,15).
However, repeated sclerotherapy with residual lesions (6,7,13)
cannot replace surgery as the first treatment modality because of
frequent recurrence (3,16-18).
The final diagnosis was usually corrected by intraoperative
findings and the histopathology of lesions (8,19),
which makes resection important (4,11,12,16,17,20).
Surgical resection is a potential curative procedure for LM
(19), and the laparoscopic
approach can be performed to find the location and dissect
primarily without large scars (4,11).
In fact, it is also challenging to remove
intra-abdominal LMs completely because of the involvement of
neighboring structures such as blood vessels and the alimentary
tract (4,17), and recurrence may result after
incomplete resection (3).
Therefore, segmental resection of the intestine is usually
necessary to achieve complete the resection of and decrease the
potential recurrence of LMs (3,4,20). A
total of five patients in the present study underwent intestinal
anastomosis after segmental intestinal resection, which was common
(84% of patients) in a previous study (4) and in case reports (11,16-18).
In some circumstances, LM cannot be resected completely because of
the involvement of vital structures, and percutaneous sclerotherapy
may be suitable for recurrence after surgical resection (6).
Ileus and recurrence are the frequent postoperative
complications of surgical resection (3,4).
With the exception of residual lesion in one patient who underwent
incomplete resection, there were no instances of recurrence or
ileus in our patients during the mean follow-up period of 35
months. Conversion to laparotomy occurred in patients from the
current study who had large retroperitoneal LM, suggesting that
these larger-volume lesions had more involvement of adjacent organs
and tissues (9). It is worthwhile
to perform laparoscopic exploration to confirm the origin and
extent of the lesion as well as the possibility of laparoscopic
resection. Furthermore, the classification of mesenteric LM by
radiological information would give surgeons guidance in
preoperative planning (4).
Compared with adults, pediatric laparoscopic surgery
of intra-abdominal LM is challenging because of the smaller space
in children. Puncturation and aspiration guided by laparoscopy is
recommended to minimize the lesion and to provide more space for
laparoscopic resection because of benign cysts (3). After the removal of lesions,
segmental intestinal resection and anastomosis of can be finished
by expanding the umbilical trocar incision in some cases. Complete
resection can be performed by laparoscopy without relapse during
the follow-up. This approach was proved to be effective for
treating intra-abdominal LM in the present study.
To the best of our knowledge, this is the largest
study of laparoscopic resection of intra-abdominal LM in the
pediatric population. However, there were limitations to the
current study, as in the majority of case series: Small number of
patients, retrospective review and no controls because of the
rarity of intra-abdominal LMs.
In summary, intra-abdominal LM in pediatric
population of the present study was diagnosed by initial acute
gastrointestinal symptoms, mainly onset of abdominal pain at the
mean age of 5 years. Macrocytic LM was the most frequent type of
intra-abdominal LM and was effectively resected laparoscopically.
Complete resection was associated with a lower recurrence, which
was usually accompanied by segmental intestinal resection.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and JF participated in study design and data
collection, carried out the initial analysis and drafted the
article. QY and WG analyzed and interpreted the patient data
regarding the technique and outcome of laparoscopic surgery, and
revised the manuscript. PL and XG conceptualized and designed the
study, and performed critical revisions of the article. QL and JF
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from parents
of children. The study was approved by the Ethics Committee of The
Second Affiliated Hospital Of Xi'an Jiaotong University (approval
no. 2022205).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elluru RG, Balakrishnan K and Padua HM:
Lymphatic malformations: Diagnosis and management. Semin Pediatr
Surg. 23:178–185. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kulungowski AM and Patel M: Lymphatic
malformations. Semin Pediatr Surg. 29(150971)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Makni A, Chebbi F, Fetirich F, Ksantini R,
Bedioui H, Jouini M, Kacem M and Ben Safta Z: Surgical management
of intra-abdominal cystic lymphangioma. Report of 20 cases. World J
Surg. 36:1037–1043. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim SH, Kim HY, Lee C, Min HS and Jung SE:
Clinical features of mesenteric lymphatic malformation in children.
J Pediatr Surg. 51:582–587. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kronfli AP, McLaughlin CJ, Moroco AE and
Grant CN: Lymphatic malformations: A 20-year single institution
experience. Pediatr Surg Int. 37:783–790. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Russell KW, Rollins MD, Feola GP, Arnold
R, Barnhart DC and Scaife ER: Sclerotherapy for intra-abdominal
lymphatic malformations in children. Eur J Pediatr Surg.
24:317–321. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Madsen HJ, Annam A, Harned R, Nakano TA,
Larroque LO and Kulungowski AM: Symptom resolution and volumetric
reduction of abdominal lymphatic malformations with sclerotherapy.
J Surg Res. 233:256–261. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Michael LF, White CL, Oliveri B, Lee EY
and Restrepo R: Intraabdominal lymphatic malformations: Pearls and
pitfalls of diagnosis and differential diagnoses in pediatric
patients. AJR Am J Roentgenol. 208:637–649. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Poroes F, Petermann D, Andrejevic-Blant S,
Labgaa I and Di Mare L: Pediatric cystic lymphangioma of the
retroperitoneum: A case report and review of the literature.
Medicine (Baltimore). 99(e20827)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McHoney M, Kiely EM and Mushtaq I: Color
Atlas of Pediatric Anatomy, Laparoscopy, and Thoracoscopy. Springer
Berlin Heidelberg, Berlin, Heidelberg, 2017.
|
|
11
|
Zhuo CH, Shi DB, Ying MG, Cheng YF, Wang
YW, Zhang WM, Cai SJ and Li XX: Laparoscopic segmental colectomy
for colonic lymphangiomas: A definitive, minimally invasive
surgical option. World J Gastroenterol. 20:8745–8750.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Takiff H, Calabria R, Yin L and Stabile
BE: Mesenteric cysts and intra-abdominal cystic lymphangiomas. Arch
Surg. 120:1266–1269. 1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chaudry G, Burrows PE, Padua HM, Dillon
BJ, Fishman SJ and Alomari AI: Sclerotherapy of abdominal lymphatic
malformations with doxycycline. J Vasc Interv Radiol. 22:1431–1435.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thomas DM, Wieck MM, Grant CN, Dossa A,
Nowicki D, Stanley P, Zeinati C, Howell LK and Anselmo DM:
Doxycycline sclerotherapy is superior in the treatment of pediatric
lymphatic malformations. J Vasc Interv Radiol. 27:1846–1856.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Acord M, Srinivasan AS and Cahill AM:
Percutaneous treatment of lymphatic malformations. Tech Vasc Interv
Radiol. 19:305–311. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shayesteh S, Salimian KJ, Fouladi DF,
Blanco A, Fishman EK and Kawamoto S: Intra-abdominal lymphangioma:
A case report. Radiol Case Rep. 16:123–127. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gasparella P, Singer G, Castellani C,
Sorantin E, Haxhija EQ and Till H: Giant lymphatic malformation
causing abdominal compartment syndrome in a neonate: A rare
surgical emergency. J Surg Case Rep. 2020(rjaa252)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cauley CE, Spencer PJ, Sagar P and
Goldstein AM: Giant mesenteric lymphatic malformation presenting as
small bowel volvulus. J Surg Case Rep. 2013(rjt083)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lal A, Gupta P, Singhal M, Sinha SK, Lal
S, Rana S and Khandelwal N: Abdominal lymphatic malformation:
Spectrum of imaging findings. Indian J Radiol Imaging. 26:423–428.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sriram G, Zendejas B, Vargas SO and Chen
C: Colonic mesenteric lymphatic malformation presenting as an
intraabdominal abscess in an infant: A case report. Int J Surg Case
Rep. 39:154–158. 2017.PubMed/NCBI View Article : Google Scholar
|