Introduction
Oxaliplatin (OXA) is a third-generation
platinum-based chemotherapeutic drug, which has a broad spectrum of
anticancer activity (1). With the
increasing clinical application of OXA, inevitable adverse
reactions have been reported (2).
Peripheral neuropathic pain is the major side effect affecting
85-90% of patients following OXA treatment and this can last for
months or years (3,4). In clinical practice, all types of
analgesics have little effect in mitigating this type of pain
(5,6). Severe neuropathic pain may lead to
dose reduction or even discontinuation of OXA chemotherapy, which
is unfavorable for tumor control and survival (7). Therefore, the study of the
pathological mechanism of OXA-induced neuropathy and the
development of related analgesics have important clinical
significance.
The spinal cord is involved in the processing of
nociceptive information by receiving and integrating information
from the peripheral nervous system and transmitting this
information to the brain (8). OXA
may induce pathophysiological changes in the spinal cord, including
the release of pro-inflammatory cytokines and increase of oxidative
stress (9). Pro-inflammatory
cytokines are mainly released by activated astrocytes (10). During the development of pain,
astrocytes become reactive and last longer and undergo hyperplasia
and hypertrophy within several days after injury (11). Activated astrocytes release
numerous pro-inflammatory mediators and activate intracellular
signaling to maintain the pain process (12). The increased oxidative stress
activates a variety of transcription factors, leading to the
differential expression of genes involved in inflammatory pathways
and triggering the inflammatory response (13). Therefore, the inhibition of the
inflammatory response mediated by activated astrocytes and the
increased oxidative stress can be potential therapeutic targets for
the treatment of OXA-induced neuropathic pain.
Resveratrol (Res) is a natural polyphenolic
flavonoid, which has antioxidative, anticancer and
antiproliferative effects (14).
Although Res has multiple beneficial effects, it induces harmful
effects at high concentration, such as pro-oxidant effect (15). However, low dose administration of
Res (1 mg/kg) did not adversely affect animals' health (16). Res has been reported to be a
powerful antioxidant with remarkable anti-inflammatory properties
(17). Furthermore, a pre-clinical
study has demonstrated that Res has a beneficial effect on the
management of pathological pain (18). In a neuropathic mouse model of
chronic constriction injury of the sciatic nerve, Res treatment
repressed the expression of pro-inflammatory cytokines, including
TNF-α, IL-1β and IL-6(19). In a
complete Freund's adjuvant-induced joint inflammatory pain C57BL/6
mouse model, Res treatment reduced the spinal cord expression of
NF-κB, TNF-α and IL-1β, and alleviated inflammatory pain (18). In a spared nerve injury rat model,
Res suppressed microglia-mediated neuroinflammation and relieved
neuropathic pain (20). In line
with these findings, a previous study has demonstrated that Res can
alleviate bone cancer pain (19).
The present study aimed to investigate the effect and the mechanism
of action of Res on OXA-induced pathological pain.
Materials and methods
Animals and drug administration
A total of 36 Male Sprague-Dawley rats weighing
180-200 g (6-8 weeks old) were purchased from Hubei Province
Experimental Animal Center. Animals were housed in a
temperature-controlled room (22±1˚C) and 55±5% humidity with a
12/12 h light-dark cycle regime and access to water and food ad
libitum. All efforts were made to minimize the number of
animals used and their suffering.
OXA (Selleck Chemicals) was dissolved in 5% glucose
solution (21). Res
(3,5,40-trihydroxystilbene; Sigma-Aldrich; Merck KGaA) was
dissolved in DMSO and diluted with 0.9% NaCl. Rats were randomly
divided into four groups: Control, Res, OXA and OXA + Res. Each
group contained six rats. For OXA treatment, the rats from the OXA
and OXA + Res groups received intraperitoneal injection of 4 mg/kg
OXA once daily for 5 consecutive days. The rats from the Control
and Res groups received intraperitoneal injection of the same
volume of 5% glucose solution once daily for 5 consecutive days.
The pain behavioral test was performed 6 days after the first OXA
injection. At 7 days after the first OXA injection, the rats from
the Res and OXA + Res groups were intrathecally injected with Res
(1 mg/kg). The Control and OXA groups were intrathecally injected
with the same volume of vehicle (DMSO and 0.9% NaCl). Intrathecal
injection was performed as previously described (22,23).
Briefly, rats were anesthetized through an intraperitoneal
injection of 50 mg/kg sodium pentobarbital and the skin was
sterilized with 75% alcohol. Then, a 25-µl Hamilton syringe with a
30-gauge needle was held at an angle of ~20˚ above the vertebral
column. The needle was inserted into the intervertebral space
between L5 and L6. A puncture stimulating a tail-flick reaction was
considered as successful. The Res solution (10 µl) was injected and
the needle was left in the dosing position for >30 sec after
injection. Subsequently, the mechanical allodynia assay was
performed at 0, 2, 4 and 6 h after Res administration.
Antibodies and reagents
Anti-NF-κB p65 mouse monoclonal antibody (cat. no.
BF8005) and β-actin rabbit antibody (cat. no. AF7018) were obtained
from Affinity Biosciences. GFAP rabbit polyclonal antibody (cat.
no. A0237), IL-1β rabbit monoclonal antibody (cat. no. A19635),
TNF-α rabbit polyclonal antibody (cat. no. A0277) and COX-2 rabbit
polyclonal antibody (cat. no. A1253) were purchased from ABclonal
Biotech Co., Ltd.. The Reactive Oxygen Species Assay kit (cat. no.
S0033S) and Mito-Tracker Red CMXRos (C1049B) were obtained from
Beyotime Biotechnology. H&E staining solution (cat. no. BL735B)
was purchased from Biosharp Life Sciences. Res (cat. no. 501-36-0)
was purchased from Sigma. OXA (cat. no. 61825-94-3) was obtained
from Shanghai Aladdin Biochemical Technology Co., Ltd. The
secondary antibodies used for western blotting were HRP Goat
Anti-Rabbit IgG (H+L) (AS014) and HRP Goat Anti-Mouse IgG (H+L)
(AS014), purchased from ABclonal Technology. The secondary
antibodies used for immunofluorescence analysis were Goat
anti-mouse IgG H&L (FITC) (ab6785), Goat Anti-Rabbit IgG
H&L (TRITC; cat. no. ab6718) and Goat Anti-Rabbit IgG H&L
(FITC) (ab6717), purchased from Abcam.
Mechanical allodynia assay
The paw withdrawal threshold (PWT) was determined
using the modified up-down method (24). Rats were accustomed individually
for 30 min in a transparent plastic box with a 5x5 cm wire mesh
grid floor. Subsequently, the von Frey filaments (0.4-26 g;
Stoelting Co.) were used to stimulate the hind paw. The filaments
were pressed vertically against the mid-plantar surface of the hind
paw. The force caused filaments bent and this state was maintained
for 3-5 s with a 2-min interval between two stimulations. A
positive response was defined as paw withdrawal was observed within
3 sec of the stimulated hind paw. Six measurements were taken for
each rat. If a positive response was obtained, a lower level of von
Frey hair was used; conversely, the next higher force was used. The
pattern of the positive and negative withdrawal response was
converted to PWT. After the behavioral test, three rats were
perfused and fixed for morphological analysis, including H&E
staining and immunofluorescence. The other three rats were
sacrificed and the spinal cord were collected for western blot
analysis.
Cell culture and Res treatment
C6 glial cells (Jennio Biotech Co., Ltd.) were
cultured in DMEM supplemented with 10% fetal bovine serum, 50 U/ml
penicillin and 50 µg/ml streptomycin (all Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2. For ROS and
mitochondrial membrane potential assessment, C6 glial cells
(1.5x105) were seeded on a 24-well plate and incubation
with 5 ng/µl TNF-α at 37˚C for 4 h. Subsequently, cells were
treated with 0 and 1 µM Res at 37˚C for 24 h and then the cells
were used for ROS assessment and mitochondrial membrane potential
detection. TNF-α was diluted with 0.9% NaCl. Res was dissolved in
DMSO (Beyotime Biotechnology).
H&E staining
After 5 days of OXA administration and subsequent
Res treatment, the rats from the Control, OXA and OXA + Res groups
(n=3/group) were deeply anesthetized with 60 mg/kg sodium
pentobarbital and perfused transcardially with saline containing
heparin. The clearing of the liver (red to light brown in color) is
an indicator of a good perfusion. Then switched perfusate to 4%
paraformaldehyde (PFA, 0.1 M phosphate buffer, pH 7.4) and perfused
until the animal body was stiff and rigid. After perfusion was
complete, spinal cords were removed and post-fixed in 4% PFA (0.1 M
phosphate buffer, pH 7.4) for 12 h at 4˚C and embedded in paraffin.
Subsequently, the tissues were cut into 4-µm sections using a
microtome (RM 2165; Leica Microsystems GmbH). The sections were
stained using the standard H&E method. Briefly, paraffin
sections were treated with xylene (5 min, two times), 100% ethanol
(10 min, two times), 90% ethanol (10 min), 70% ethanol (10 min) for
dewaxing and rinsing with tap water for 2 min. Then the sections
were dyed with hematoxylin solution for 3 min and washed with tap
water for 10 sec. The sections were stained with eosin for 3 min
and washed with tap water for 10 sec. The dehydration and
transparent treatment were conducted by putting the slices into 80%
ethanol (5 min), 90% ethanol (5 min), 95% ethanol (5 min), 100%
ethanol (5 min, two times), xylene (5 min, times). Finally, the
sections were sealed with neutral balsam and observed using a
fluorescence microscope (Olympus IX73; Olympus). H&E images
were analyzed using the ImageJ 1.51j8 software (National Institutes
of Health). The scoring criteria of inflammation cell infiltration
(25) is: 0 (normal); 1
(lymphocyte infiltration around meninges and blood vessels); 2,
1-10 lymphocytes in a field); 3 (11-100 lymphocytes in a field); 4
(>100 lymphocytes in a field).
Immunofluorescence analysis
For immunofluorescence analysis, the paraffin
sections were treated with xylene (5 min, two times), 100% ethanol
(10 min, two times), 90% ethanol (10 min), 70% ethanol (10 min) for
dewaxing and rinsing with ddH2O (5 min, two times). Then
the sections were conducted to antigen retrieval (Improved Citrate
Antigen Retrieval Solution, P0083, Beyotime Biotechnology). Immerse
the slices in antigen retrieval solution and heat at 95-100˚C for
20 min. The antigen retrieval solution was preheated to 95-100˚C
before use. Then cooled to room temperature and washed 1-2 times
with distilled water for 3-5 min. After incubation with 3% hydrogen
peroxide for 10 min, immunostaining was performed. The tissue
sections were blocked with goat serum (Gibco, 10%, room
temperature) for 1 h and then incubated with primary antibody
overnight at 4˚C. Subsequently, the sections were incubated with
fluorescent secondary antibody at room temperature for 1 h and
observed under a fluorescence microscope (Olympus IX73; Olympus
Corporation). The fluorescence intensities were analyzed using
ImageJ 1.51j8 (National Institutes of Health). The following
primary antibodies were used: Anti-IL-1β (1:100), anti-GFAP
(1:100), anti-COX-2 (1:100) and anti-NF-κB (1:100).
Western blot analysis
6 h after the Res treatment, three rats were
euthanized with an overdose of pentobarbital sodium (100~150 mg/kg)
by intraperitoneal injection and sacrificed through decapitation.
Lumber spinal cord samples were collected and homogenized in RIPA
lysis buffer containing 1% protease inhibitors (Sigma-Aldrich;
Merck KGaA). After centrifugation at 12,000 g, 4˚C for 20 min, the
supernatant was collected. For cells, after treatment, the cells in
the cell culture dish were collected in a centrifuge tube. The
cells were lysed in ice-cold RIPA buffer, after cell lysis, one
third volume of 4x sample loading buffer was added and heated in
boiling water bath for 10 min and ultrasonic treatment of 10~15
sec. After centrifugation at 12,000 g, 4˚C for 20 min, the
supernatant was collected. Protein concentration (tissue and cells)
was quantified using a BCA analysis kit (Beyotime Biotechnology).
Protein lysates (40 µg/lane) were separated on 10% SDS-PAGE and
transferred to 0.22 µm PVDF membranes. Membranes were blocked with
5% milk in TBST (0.1% Tween-20) for 1 h at room temperature. Then
the membranes were incubated with specific primary antibodies at
4˚C overnight and HRP-conjugated secondary antibodies in TBST
(1:5,000) at room temperature for 1 h. Protein bands were
visualized using ECL detection reagent (Biosharp Life Sciences) and
detected with an iBright 1500 instrument (Invitrogen; Thermo Fisher
Scientific, Inc.). The grey values of western blot bands were
analyzed using ImageJ 1.51j8 software (National Institutes of
Health). β-actin was used as a loading control. The following
primary antibodies were used: Anti-IL-1β (1:1,000), anti-GFAP
(1:1,000), anti-COX-2 (1:1,000), anti-NF-κB (1:1,000), anti-TNF-α
(1:1,000) and anti-β-actin (1:1,000).
Reactive oxygen species (ROS)
assessment
Reactive Oxygen Species Assay Kit uses fluorescent
probe DCFH-DA for ROS detection. The DCFH-DA was diluted with
serum-free medium at 1:1,000 to a final concentration of 10 µM. The
cell culture medium was removed and diluted DCFH-DA was added.
Cells were incubated at 37˚C for 30 min. After incubation, cells
were washed three times with serum-free medium to adequately remove
DCFH-DA that did not enter the cell. Then the cells were placed
under a fluorescence microscope (Olympus IX73; Olympus) for
observation. The fluorescence intensities were analyzed using
ImageJ 1.51j8 software.
Mitochondrial membrane potential
detection
Following Res treatment, the cell culture medium is
removed and the Mito-Tracker Red CMXRos working solution was added,
and the cells are incubated at 37˚C for 20 min. Then the
Mito-Tracker Red CMXRos working solution was removed and the fresh
cell culture medium was added. The cells were observed by
fluorescence microscope (Olympus IX73; Olympus). The fluorescence
intensities were analyzed using ImageJ 1.51j8 software (National
Institutes of Health).
Molecular docking
The X-ray crystal structure of COX-2 was obtained
from the Protein Data Bank (PDB ID: 5f19; rcsb.org/structure/5F19). The structure of Res was
downloaded from the PubChem database (pubchem.ncbi.nlm.nih.gov/compound/445154) and
optimized using ChemBio3D Ultra 14.0 software (PerkinElmer
Informatics). Auto Dock Vina 1.2.0 software (Center For
Computational Structural Biology) was used to dock conformation
between COX-2 and Res. PyMOL 2.2.3 (PyMOL by Schrödinger, DeLano
Scientific LLC; pymol.org/installers/) was used to visualize the
conformation.
Statistical analysis
All statistical analyses were performed using SPSS
21.0 statistics software (IBM Corp.). Data obtained through H&E
staining, immunofluorescence and western blotting were analyzed
using one-way analysis of variance followed by Tukey's test and
presented as the mean ± SD. PWT data were analyzed using unpaired
Student's t-test and presented as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference. All
the experiments were repeated three times.
Results
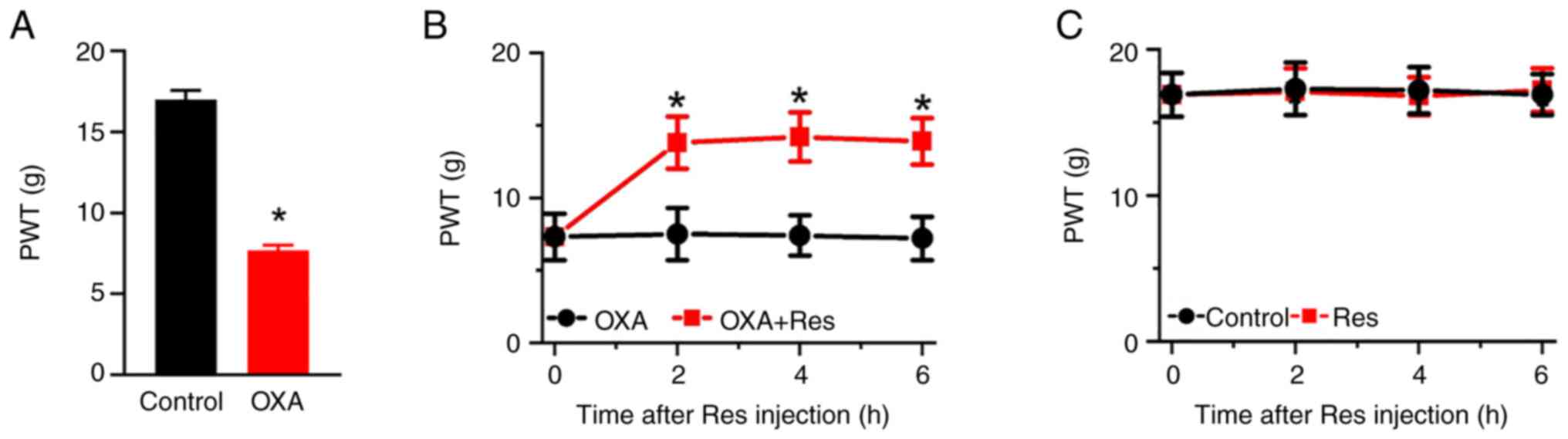
Res treatment relieves OXA-induced
mechanical allodynia
To investigate the effect of Res on neuropathic
pain, an OXA-induced neuropathic pain rat model was established by
once-daily intraperitoneal injection of OXA for 5 consecutive days.
On day 6 after the OXA administration, the PWT value indicating
mechanical pain sensitivity was recorded. The PWT values in the OXA
group were significantly decreased compared with those in the
Control group (P<0.05; Fig.
1A). The PWT values of the Control and OXA groups were 17.0±0.6
and 7.7±0.3, respectively. Res was intrathecally injected into the
lumbar spinal cord of OXA rats. The PWT values of OXA-induced rats
were significantly increased at 2-6 h after Res treatment (Fig. 1B). The PWT values of the OXA and
OXA + Res groups at post-treatment time points of 2, 4 and 6 h were
7.5±1.8 vs. 13.8±1.8 (P<0.05 vs. OXA group), 7.4±1.4 vs.
14.2±1.7 (P<0.05 vs. OXA group) and 7.2±1.5 vs. 13.9±1.6
(P<0.05 vs. OXA group), respectively. However, Res treatment had
no significant effect on the mechanical pain behavior of control
rats (Fig. 1C).
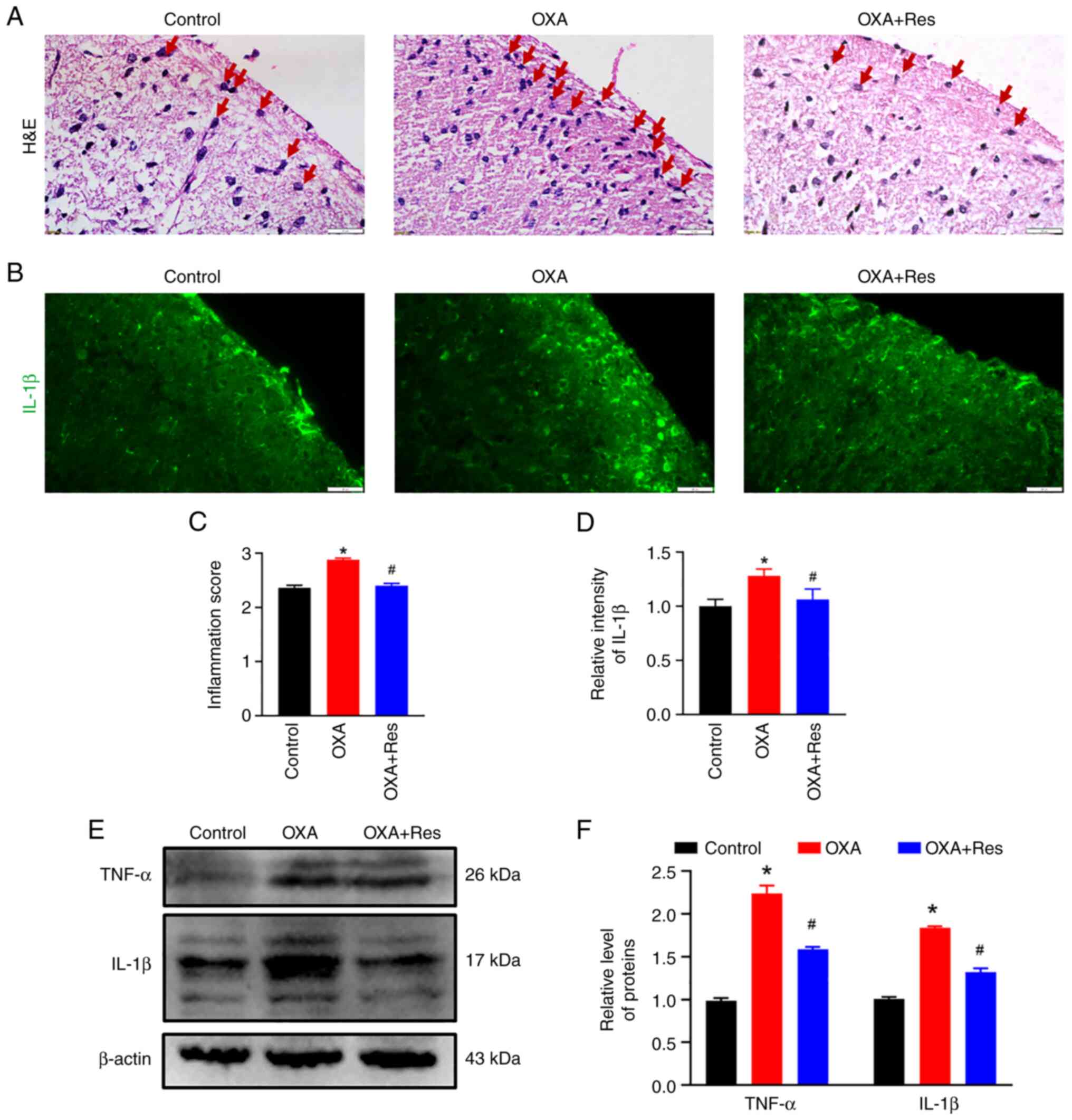
Res decreases OXA-induced spinal
inflammation
Histological characterization was performed to
analyze spinal inflammation. Subsequent to OXA administration,
severe infiltration of inflammatory cells (red arrow) was observed
in the spinal dorsal horn, while Res treatment decreased the
inflammatory response induced by OXA administration (Fig. 2A). The relative inflammation scores
in the OXA and OXA + Res groups were 2.9±0.03 (P<0.05 vs.
Control; Fig. 2C) and 2.5±0.04
(P<0.05 vs. OXA group; Fig.
2C), respectively. Pro-inflammatory cytokines, such as TNF-α
and IL-1β, promote the inflammatory reaction and are associated
with the process of pathological pain (26). OXA enhanced the fluorescence
intensities of spinal IL-1β, while this increase was reduced by Res
treatment (Fig. 2B). The relative
intensities of the OXA and OXA + Res groups were 1.28±0.06
(P<0.05 vs. Control) and 1.06±0.09 (P<0.05 vs. OXA group;
Fig. 2D), respectively. Western
blot analysis indicated upregulation of spinal TNF-α and IL-1β
protein expression in the OXA group (Fig. 2E). The relative gray values of
TNF-α and IL-1β in the OXA group were 2.24±0.09 and 1.84±0.02,
respectively (P<0.05 vs. Control; Fig. 2F). Following Res treatment, the
levels of TNF-α and IL-1β were decreased to 1.59±0.02 and
1.32±0.04, respectively (P<0.05 vs. OXA group; Fig. 2F).
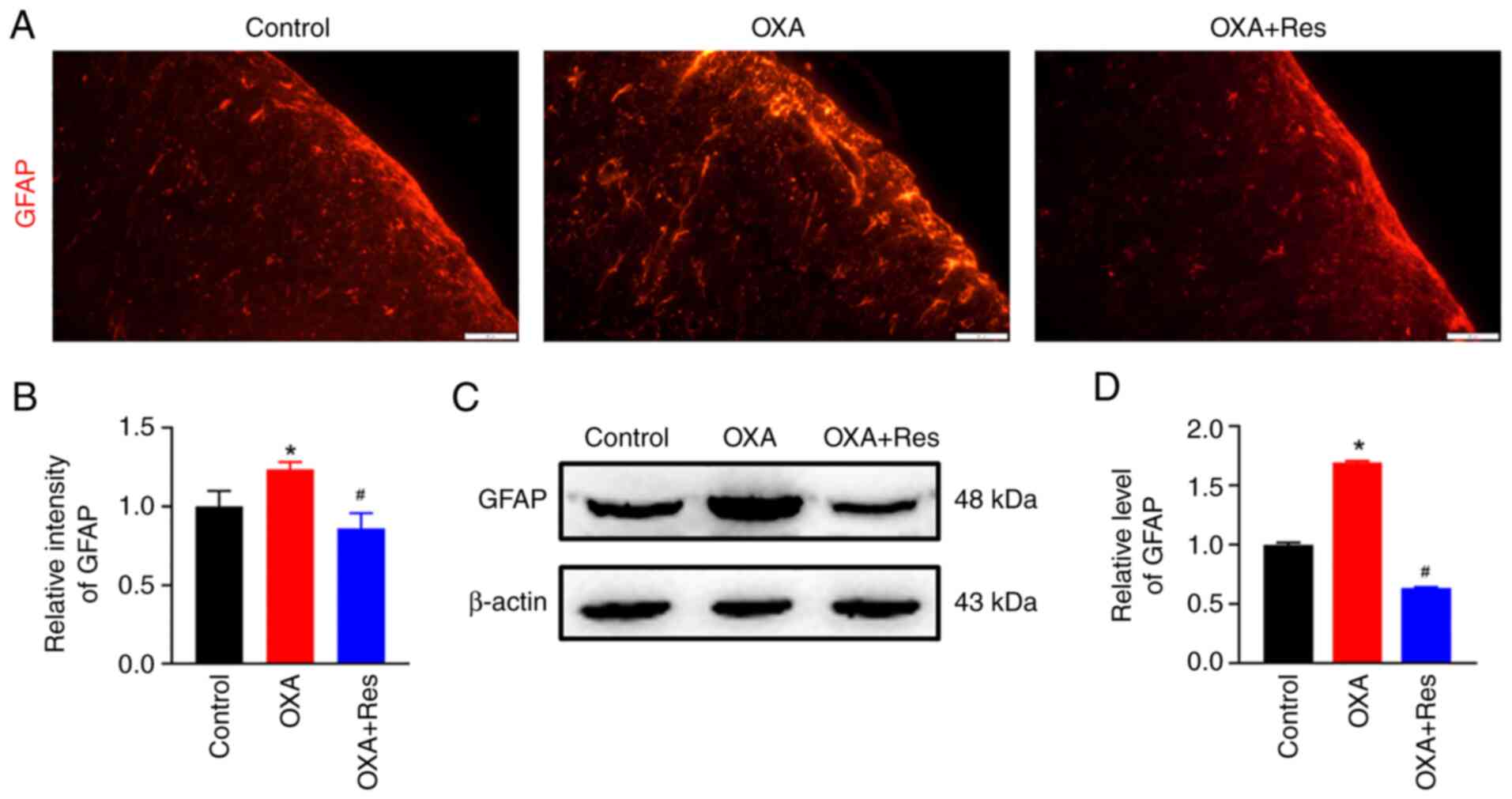
Res inhibits spinal astrocyte
activation
Localization, distribution and expression of the
astrocytic marker GFAP were measured using an immunofluorescence
assay and western blot analysis. The fluorescence intensity of
spinal GFAP was increased in the OXA group, while it was decreased
in the OXA + Res group compared with the Control group (Fig. 3A). The relative fluorescence
intensities of the OXA and OXA + Res groups were 1.23±0.05
(P<0.05 vs. Control; Fig 3B)
and 0.86±0.09 (P<0.05 vs. OXA group; Fig. 3B), respectively. Western blot
analysis indicated that relative GFAP expression was increased to
1.70±0.01 in the OXA group (P<0.05 vs. Control; Fig. 3C and D), whereas Res treatment reduced the
relative spinal GFAP expression in the OXA + Res group with the
relative grey value decreased to 0.63±0.01 (P<0.05 vs. OXA
group; Fig. 3C and D).
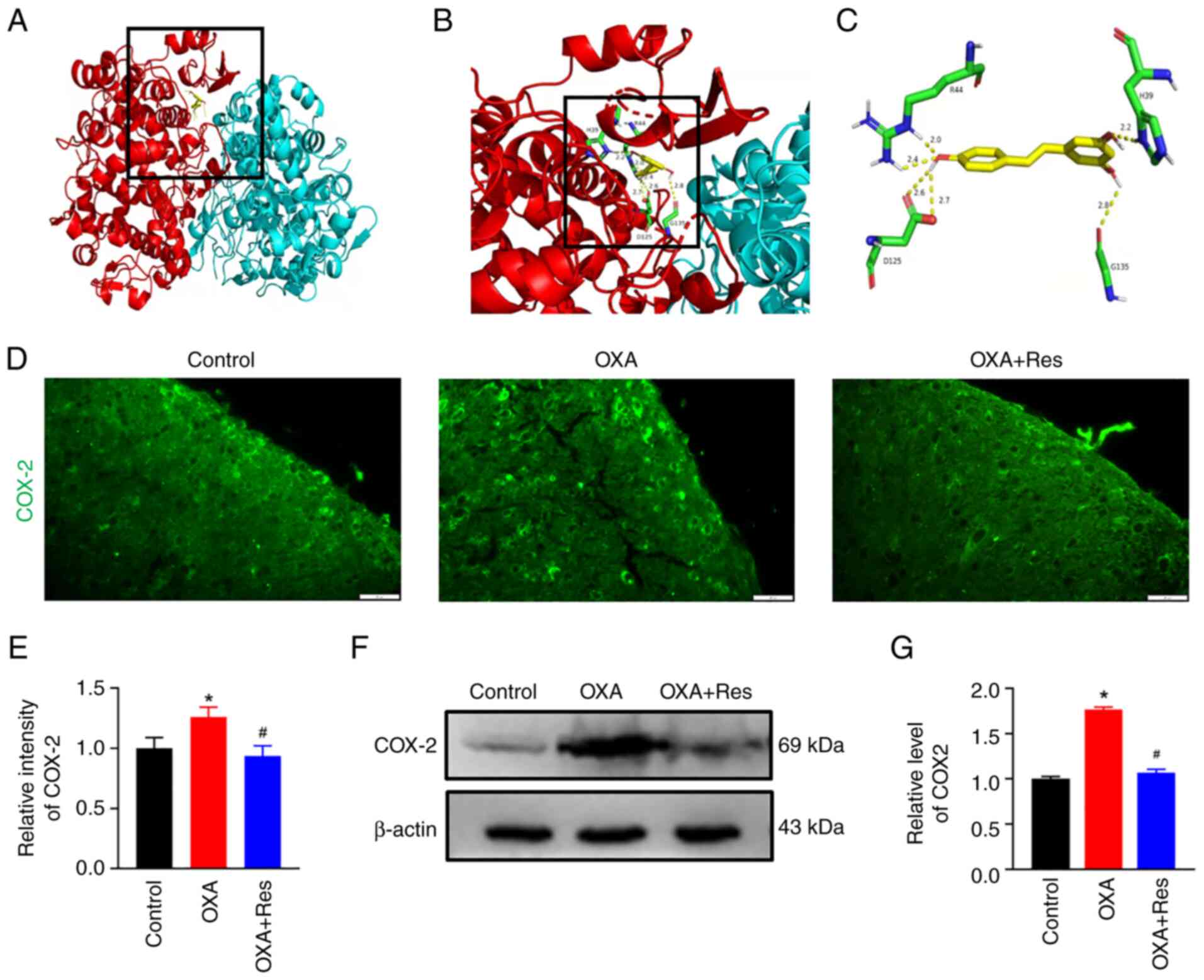
Res reduces COX-2 expression
COX-2 is involved in inflammatory responses and
COX-2 inhibitors are used as nonsteroidal anti-inflammatory drugs
(22). To identify if Res is a
potential COX-2 inhibitor, a molecular docking assay was performed
on the X-ray crystal structures of COX-2 and the ligand Res
(Fig. 4A-C). Auto Dock data showed
that Res formed six hydrogen bonds with COX-2 at residues H39, R44,
D125 and G135. H39 and R44 belong to the epidermal growth
factor-like domain of COX-2 and D125 and G135 belong to the
C-terminal globular catalytic domain of COX-2. An
immunofluorescence assay and western blot analysis were used to
detect spinal COX-2 location and expression, respectively. The
fluorescence intensity of spinal COX-2 was increased in the OXA
group with a relative fluorescence intensity of 1.26±0.08
(P<0.05 vs. Control; Fig. 4D
and E). Res exerted an inhibitory
effect on COX-2 expression, which showed a relative fluorescence
intensity of 0.93±0.09 (P<0.05 vs. OXA; Fig. 4D and E). Western blot analysis indicated that
COX-2 expression was upregulated in the OXA group (Fig. 4F) showing a relative gray value of
1.76±0.03 (P<0.05 vs. Control; Fig.
4G). The present data indicated that spinal COX-2 was
upregulated after OXA administration. The upregulated COX-2
expression was reduced following Res treatment (Fig. 4F) with a relative gray value
decreased to 1.06±0.04 (P<0.05 vs. OXA; Fig. 4G).
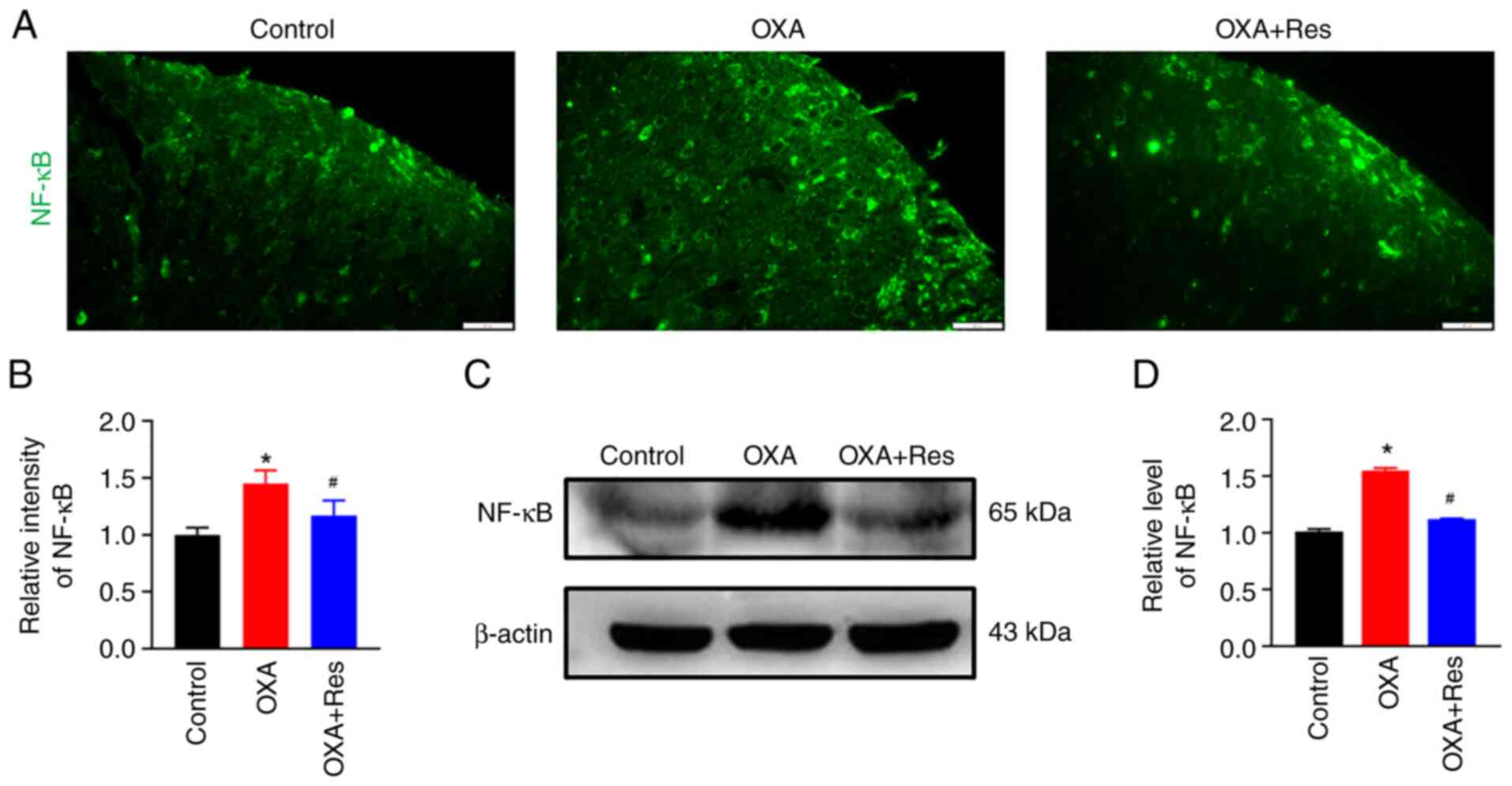
Res decreases NF-κB expression
The effect of Res on NF-κB was detected using an
immunofluorescence assay and western blot analysis. The relative
fluorescence intensity of spinal NF-κB in the OXA group was
significantly increased to 1.45±0.12, while Res treatment in the
OXA + Res group reduced the increase in relative fluorescence
intensity to 1.17±0.13 (P<0.05 vs. OXA; Fig. 5A and B). The relative expression levels of the
spinal NF-κB protein were significantly increased to 1.55±0.02 in
the OXA group (P<0.05 vs. Control; Fig. 5C and D), whereas these were significantly
decreased to 1.12±0.01 in the OXA + Res group compared with the OXA
group (P<0.05; Fig. 5C and
D).
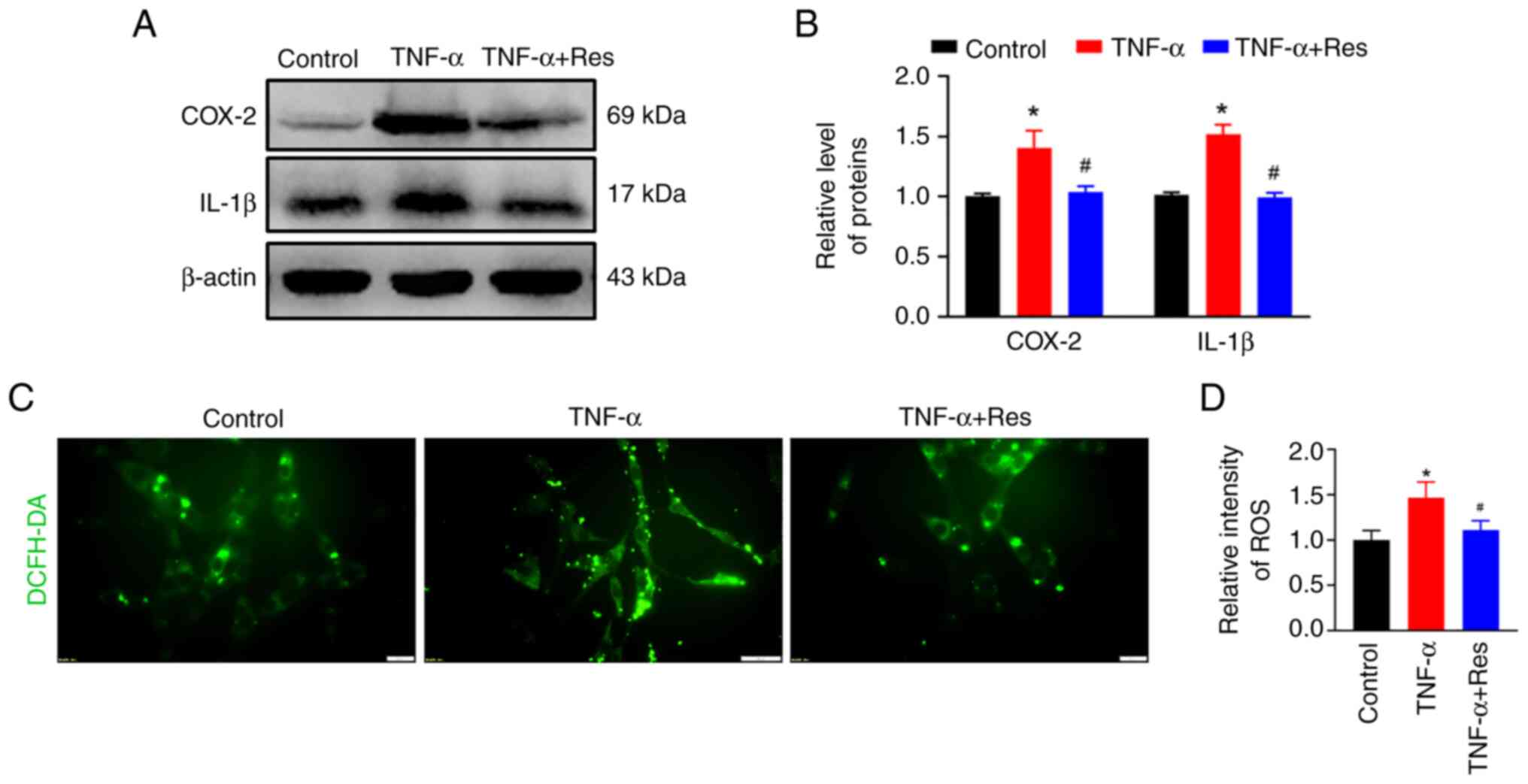
Confirmation of the anti-inflammatory
effect of Res in C6 cells
C6 rat glioma cells were treated with TNF-α to
induce inflammation. Western blot analysis indicated an increase in
COX-2 and IL-1β expression in TNF-α-treated cells that was
significantly reduced in the TNF-α + Res group (Fig. 6A). The relative expression levels
of COX-2 and IL-1β in the TNF-α-treated group were 1.49±0.32 and
1.51±0.08, respectively (P<0.05 vs. Control; Fig. 6B), while in the TNF-α + Res group
these were 1.04±0.05 and 0.99±0.04, respectively (P<0.05 vs.
TNF-α; Fig. 6B). The ROS
production marked by DCFH-DA was used to analyze the effect of Res
on ROS production. Res treatment reduced the TNF-α-induced high ROS
levels (Fig. 6C) and the relative
ROS fluorescence intensities in the TNF-α and TNF-α + Res groups
were 1.47±0.17 (P<0.05 vs. Control; Fig. 6D) and 1.11±0.10 (P<0.05 vs.
TNF-α; Fig. 6D), respectively.
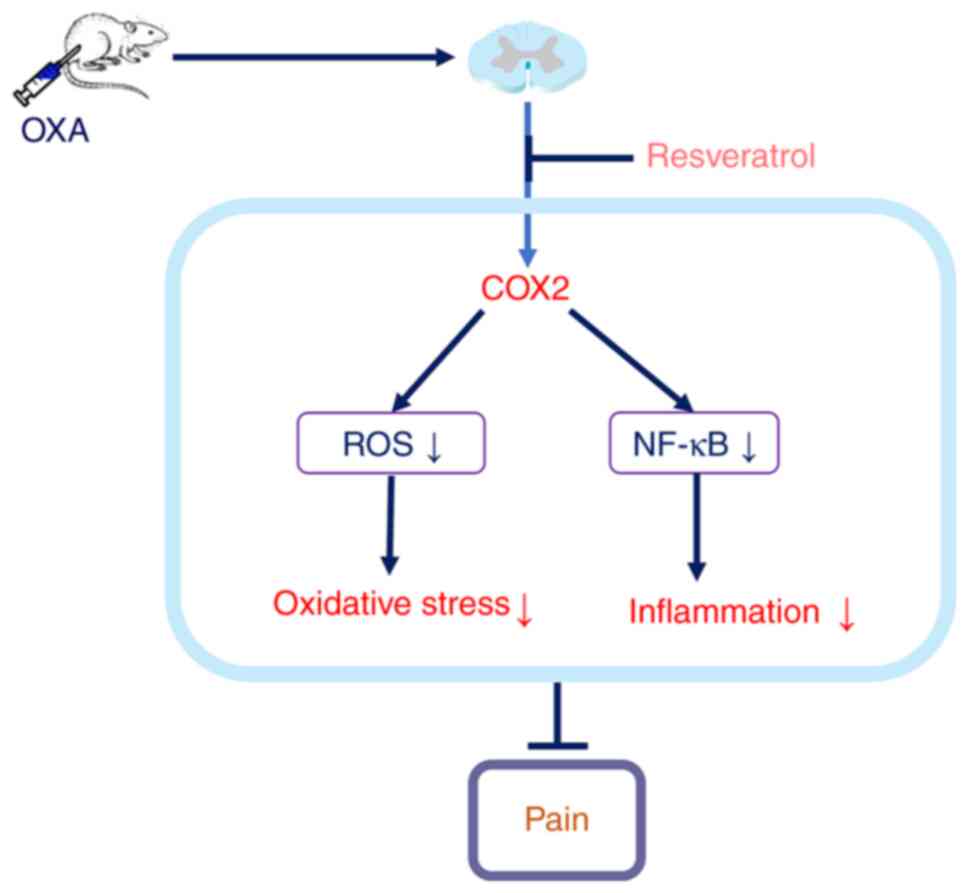
The present results demonstrated that intrathecal
injection of Res inhibited COX-2 expression, reduced high ROS
levels, decreased spinal inflammation and relieved OXA-induced
neuropathic pain (Fig. 7).
Discussion
According to the global cancer statistics produced
by the International Agency for Research on Cancer, there are ~19.3
million new cancer cases and ~10.0 million cancer deaths worldwide
in 2020. Among them, ~4.57 million new cancer cases occurred in
China, accounting for 23.7% of the cancer cases worldwide, ranking
first in the world (27).
Chemotherapy is a type of drug treatment used in numerous types of
cancer to kill cancer cells and shrink tumor size. OXA is a
platinum-based chemotherapeutic drug used to treat several types of
cancer and has certain commonly reported side effects, including
peripheral neuropathy (2,28). OXA-induced neuropathy is manifested
following multiple chemotherapy cycles and is characterized by
paresthesia and pain (29,30). The severity of this condition may
require a reduction in the dose of OXA or even discontinuation of
OXA chemotherapy, which is unfavorable for tumor control and
survival (31). In the present
study, once-daily intraperitoneal injection of OXA for 5
consecutive days in rats increased pain sensitivity, spinal
inflammation reaction and oxidative stress. This suggested that
spinal cord inflammation and increased oxidative stress were
involved in the OXA-induced neuropathic pain. OXA is considered to
upregulate oxidative stress-related genes (32) and induce overproduction of free
radicals, such as ROS (33). COX-2
is an important enzyme that is involved in free radical scavenging
(34). In the present study, the
spinal COX-2 protein expression was upregulated following OXA
treatment.
Spinal cord inflammation, a cardinal feature of
pain, is characterized by activated glial cells and increased
production of inflammatory mediators (35). TNF-α and IL-1β are the most potent
and studied inflammatory cytokines expressed in the microglia cells
and astrocytes of the spinal cord (36). NF-κB is the main regulator of the
inflammatory response by activating a variety of transcription
factors, such as TNF-α and IL-1β (37). In the present study, OXA
administration induced the upregulation of the astrocytic marker
GFAP, inflammation-related factor NF-κB, TNF-α and IL-1β. COX-2
operates by regulating NF-κB signaling. Treatment with COX-2
inhibitor celecoxib decreases NF-κB expression in a dose and
time-dependent manner. Furthermore, COX-2 regulates E-cadherin
expression through the NF-κB/Snail signaling pathway in gastric
cancer (38,39). Therefore, we hypothesized that
COX-2 mediates the inflammatory process by regulating NF-κB
signaling.
Res has an antioxidant effect and, as a free radical
scavenger, reduces the content of free radicals and inhibits the
production of ROS (40). Through
3D molecular docking technology, the molecular structure of Res can
be accurately linked to the molecular structure of COX-2,
indicating that Res can target the site of COX action, thereby
inhibiting epoxidation (41). Res
can also reduce mitochondrial damage by regulating mitochondrial
membrane potential, inhibiting mitochondrial lipid peroxidation and
regulating mitochondrial gene expression (42). Mitochondrial function damage can
cause a sharp rise in the level of ROS, thereby accelerating nerve
cell apoptosis (43). In the
present study, Res reduced ROS generation and the inflammatory
reaction through the inhibition of COX-2. In conclusion, the
present study revealed that intrathecal injection of Res could
inhibit COX-2 expression, reduce ROS levels, decrease spinal
inflammation and relieve OXA-induced neuropathic pain.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81971066, 81901149 and
32100823), Research Project of Hubei Provincial Department of
Education (grant no. Q20212804) and Hubei University of Science and
Technology Program (grant nos. 2020TD02 and BK202116).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZBD, YJW, WJW, JW, BJW, HLZ, MX and LL performed
experiments, collected and analyzed data and drafted the
manuscript. ZBD and YJW confirm the authenticity of all the raw
data. LL designed experiments, analyzed data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures in the present study
were performed in compliance with the local and international
guidelines on ethical use of animals and all efforts were made to
minimize the number of animals used and their sufferings. The
animal experiments were approved by the Experimental Animal Ethics
Committee of Hubei University of Science and Technology (approval
no. 2020-01-900; Xianning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yi Y, Li L, Song F, Li P, Chen M, Ni S,
Zhang H, Zhou H, Zeng S and Jiang H: L-tetrahydropalmatine reduces
oxaliplatin accumulation in the dorsal root ganglion and
mitochondria through selectively inhibiting the
transporter-mediated uptake thereby attenuates peripheral
neurotoxicity. Toxicology. 459(152853)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kang L, Tian Y, Xu S and Chen H:
Oxaliplatin-induced peripheral neuropathy: Clinical features,
mechanisms, prevention and treatment. J Neurol. 268:3269–3282.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Takeshita E, Ishibashi K, Koda K, Oda N,
Yoshimatsu K, Sato Y, Oya M, Yamaguchi S, Nakajima H, Momma T, et
al: The updated five-year overall survival and long-term
oxaliplatin-related neurotoxicity assessment of the FACOS study.
Surg Today. 51:1309–1319. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Furgała-Wojas A, Kowalska M, Nowaczyk A,
Fijałkowski Ł and Sałat K: Comparison of bromhexine and its active
metabolite-ambroxol as potential analgesics reducing
oxaliplatin-induced neuropathic pain-pharmacodynamic and molecular
docking studies. Curr Drug Metab. 21:548–561. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kong VKF and Irwin MG: Adjuvant analgesics
in neuropathic pain. Eur J Anaesthesiol. 26:96–100. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tuttle AH, Tohyama S, Ramsay T, Kimmelman
J, Schweinhardt P, Bennett GJ and Mogil JS: Increasing placebo
responses over time in U.S. clinical trials of neuropathic pain.
Pain. 156:2616–2626. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang J, Zhang XS, Tao R, Zhang J, Liu L,
Jiang YH, Ma SH, Song LX and Xia LJ: Upregulation of CX3CL1
mediated by NF-κB activation in dorsal root ganglion contributes to
peripheral sensitization and chronic pain induced by oxaliplatin
administration. Mol Pain. 13(1744806917726256)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shigematsu N, Kawashiri T, Kobayashi D,
Shimizu S, Mine K, Hiromoto S, Uchida M, Egashira N and Shimazoe T:
Neuroprotective effect of alogliptin on oxaliplatin-induced
peripheral neuropathy in vivo and in vitro. Sci Rep.
10(6734)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang P, Li X, Hu D, Lai Q, Wang Y, Ma X,
Xu Q, Li W, Huang J and He J: Peripheral neural interface. Adv Exp
Med Biol. 1101:91–122. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang X, Guan Z, Wang X, Sun D, Wang D, Li
Y, Pei B, Ye M, Xu J and Yue X: Curcumin alleviates
oxaliplatin-induced peripheral neuropathic pain through inhibiting
oxidative stress-mediated activation of NF-κB and mitigating
inflammation. Biol Pharm Bull. 43:348–355. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Menyhárt Á, Frank R, Farkas AE, Süle Z,
Varga VÉ, Nyúl-Tóth Á, Meiller A, Ivánkovits-Kiss O, Lemale CL,
Szabó Í, et al: Malignant astrocyte swelling and impaired glutamate
clearance drive the expansion of injurious spreading depolarization
foci. J Cereb Blood Flow Metab. 24(271678X211040056)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li D, Liu N, Zhao HH, Zhang X, Kawano H,
Liu L, Zhao L and Li HP: Interactions between Sirt1 and MAPKs
regulate astrocyte activation induced by brain injury in vitro and
in vivo. J Neuroinflammation. 14(67)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eto K, Kim SK, Takeda I and Nabekura J:
The roles of cortical astrocytes in chronic pain and other brain
pathologies. Neurosci Res. 126:3–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shaito A, Posadino AM, Younes N, Hasan H,
Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH,
Nasrallah GK and Pintus G: Potential adverse effects of
resveratrol: A literature review. Int J Mol Sci.
21(2084)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Salehi B, Mishra AP, Nigam M, Sener B,
Kilic M, Sharifi-Rad M, Fokou PVT, Martins N and Sharifi-Rad J:
Resveratrol: A double-edged sword in health benefits. Biomedicines.
6(91)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wilson T, Knight TJ, Beitz DC, Lewis DS
and Engen RL: Resveratrol promotes atherosclerosis in
hypercholesterolemic rabbits. Life Sci. 59:PL15–PL21.
1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu D, Li Y, Zhang B, Wang Y, Liu Y, Luo Y,
Niu W, Dong M, Liu M, Dong H, et al: Resveratrol alleviate hypoxic
pulmonary hypertension via anti-inflammation and anti-oxidant
pathways in rats. Int J Med Sci. 13:942–954. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ma Y, Liu S, Shu H, Crawford J, Xing Y and
Tao F: Resveratrol alleviates temporomandibular joint inflammatory
pain by recovering disturbed gut microbiota. Brain Behav Immun.
87:455–464. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tao L, Ding Q, Gao C and Sun X:
Resveratrol attenuates neuropathic pain through balancing
pro-inflammatory and anti-inflammatory cytokines release in mice.
Int Immunopharmacol. 34:165–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Shi Y, Huang Y, Liu W, Cai G,
Huang S, Zeng Y, Ren S, Zhan H and Wu W: Resveratrol mediates
mechanical allodynia through modulating inflammatory response via
the TREM2-autophagy axis in SNI rat model. J Neuroinflammation.
17(311)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hao M, Tang Q, Wang B, Li Y, Ding J, Li M,
Xie M and Zhu H: Resveratrol suppresses bone cancer pain in rats by
attenuating inflammatory responses through the AMPK/Drp1 signaling.
Acta Biochim Biophys Sin (Shanghai). 52:231–240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ding H, Chen J, Su M, Lin Z, Zhan H, Yang
F, Li W, Xie J, Huang Y, Liu X, et al: BDNF promotes activation of
astrocytes and microglia contributing to neuroinflammation and
mechanical allodynia in cyclophosphamide-induced cystitis. J
Neuroinflammation. 17(19)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hylden JL and Wilcox GL: Intrathecal
morphine in mice: A new technique. Eur J Pharmacol. 67:313–316.
1980.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luo H, Liu L, Zhao JJ, Mi XF, Wang QJ and
Yu M: Effects of oxaliplatin on inflammation and intestinal floras
in rats with colorectal cancer. Eur Rev Med Pharmacol Sci.
24:10542–10549. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song S, Guo R, Mehmood A, Zhang L, Yin B,
Yuan C, Zhang H, Guo L and Li B: Liraglutide attenuate central
nervous inflammation and demyelination through AMPK and
pyroptosis-related NLRP3 pathway. CNS Neurosci Ther. 28:422–434.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Matsuda M, Huh Y and Ji RR: Roles of
inflammation, neurogenic inflammation, and neuroinflammation in
pain. J Anesth. 33:131–139. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Austin PJ, Wu A and Moalem-Taylor G:
Chronic constriction of the sciatic nerve and pain hypersensitivity
testing in rats. J Vis Exp. 13(3393)2012.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Forstenpointner J, Oberlojer VC,
Naleschinski D, Höper J, Helfert SM, Binder A, Gierthmühlen J and
Baron R: A-fibers mediate cold hyperalgesia in patients with
oxaliplatin-induced neuropathy. Pain Pract. 18:758–767.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee JH and Kim W: The role of satellite
glial cells, astrocytes and microglia in oxaliplatin-induced
neuropathic pain. Biomedicines. 8(324)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
DiAntonio A: Axon degeneration:
Mechanistic insights lead to therapeutic opportunities for the
prevention and treatment of peripheral neuropathy. Pain. 160 (Suppl
1):S17–S22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lu Y, Lin Y, Huang X, Wu S, Wei J and Yang
C: Oxaliplatin aggravates hepatic oxidative stress, inflammation
and fibrosis in a non-alcoholic fatty liver disease mouse model.
Int J Mol Med. 43:2398–2408. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Agnes JP, Santos VW, das Neves RN,
Gonçalves RM, Delgobo M, Girardi CS, Lückemeyer DD, Ferreira MA,
Macedo-Júnior SJ, Lopes SC, et al: Antioxidants improve
oxaliplatin-induced peripheral neuropathy in tumor-bearing mice
model: Role of spinal cord oxidative stress and inflammation. J
Pain Aug. 22:996–1013. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Amić A, Marković Z, Marković JM, Jeremić
S, Lučić B and Amić D: Free radical scavenging and COX-2 inhibition
by simple colon metabolites of polyphenols: A theoretical approach.
Comput Biol Chem. 65:45–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hellenbrand DJ, Quinn CM, Piper ZJ,
Morehouse CN, Fixel JA and Hanna AS: Inflammation after spinal cord
injury: A review of the critical timeline of signaling cues and
cellular infiltration. J Neuroinflammation. 18(284)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Teixeira-Santos L, Albino-Teixeira A and
Pinho D: Neuroinflammation, oxidative stress and their interplay in
neuropathic pain: Focus on specialized pro-resolving mediators and
NADPH oxidase inhibitors as potential therapeutic strategies.
Pharmacol Res. 162(105280)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gebremedhn EG, Shortland PJ and Mahns DA:
The incidence of acute oxaliplatin-induced neuropathy and its
impact on treatment in the first cycle: A systematic review. BMC
Cancer. 18(410)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Carothers AM, Davids JS, Damas BC and
Bertagnolli MM: Persistent cyclooxygenase-2 inhibition
downregulates NF-{kappa}B, resulting in chronic intestinal
inflammation in the min/+ mouse model of colon tumorigenesis.
Cancer Res. 70:4433–4442. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen Z, Liu M, Liu X, Huang S, Li L, Song
B, Li H, Ren Q, Hu Z, Zhou Y and Qiao L: COX-2 regulates E-cadherin
expression through the NF-κB/Snail signaling pathway in gastric
cancer. Int J Mol Med. 32:93–100. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Song Q, Feng YB, Wang L, Shen J, Li Y, Fan
C, Wang P and Yu SY: COX-2 inhibition rescues depression-like
behaviors via suppressing glial activation, oxidative stress and
neuronal apoptosis in rats. Neuropharmacology.
160(107779)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Meng T, Xiao D, Muhammed A, Deng J, Chen L
and He J: Anti-inflammatory action and mechanisms of resveratrol.
Molecules. 26(229)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shamsara J and Shahir-Sadr A: Developing a
CoMSIA Model for Inhibition of COX-2 by resveratrol derivatives.
Iran J Pharm Res. 15:459–469. 2016.PubMed/NCBI
|
|
43
|
Ito J, Shirasuna K, Kuwayama T and Iwata
H: Resveratrol treatment increases mitochondrial biogenesis and
improves viability of porcine germinal-vesicle stage
vitrified-warmed oocytes. Cryobiology. 93:37–43. 2020.PubMed/NCBI View Article : Google Scholar
|