Introduction
Spontaneous rupture of the kidney is a rare but
clinically critical event (1).
According to its location, spontaneous rupture of the kidney may be
categorized as renal parenchyma rupture, renal collecting system
rupture or mixed rupture (2).
Spontaneous rupture of the renal parenchyma is uncommon and its
incidence is lower compared with that of renal pelvis rupture
(3). The main causes of renal
parenchyma rupture are malignancies, tuberculosis, abscesses,
calculi, hemophilia and polycystic kidneys (4-6).
Formation of benign and malignant tumors appear to be the most
common cause of spontaneous rupture of the renal parenchyma
(7). However, selective incidences
of renal parenchyma rupture caused by kidney stones have been
reported (1,8). The present report describes a rare
case of renal parenchyma rupture caused by stones, where a large
hematoma was discovered on the affected side of the patient and
subsequently misdiagnosed as a malignancy for treatment. The
present report discusses the diagnosis and treatment of this case.
It was emphasized that: i) Spontaneous renal rupture may occur in
patients with long-term kidney calculi with a history of
extracorporeal shockwave lithotripsy (ESWL); and ii) in an
emergency with a ruptured kidney, rapid and accurate preoperative
diagnosis is important for the selection of treatment and surgical
protocol for patients.
Case report
In November 2020, a 67-year-old male presented to
the Affiliated Hospital of Yangzhou University (Yangzhou, China)
with pain on the left flank and oliguria. Apart from this, the
patient had no other symptoms or complaints. The patient had a
history of left kidney calculi for more than ten years, he had
undergone ESWL twice a year for kidney calculi in 2018 and 2019.
The patient's blood pressure on admission was 133/71 mmHg. Physical
examination revealed pain on percussion in the left renal area but
no palpation on the mass.
The routine blood test and coagulation function test
were conducted using the XN-3000 automatic blood cell analyzer and
automatic coagulation analyzer, respectively (Sysmex Corporation).
A Roche moduladp was used for other biochemical tests. Laboratory
analysis revealed a hemoglobin of 89 g/l (normal, 115-150 g/l),
elevated creatine of 163.8 µmol/l (normal, 41-81 µmol/l), potassium
of 5.42 mmol/l (normal, 3.5-5.3 mmol/l), leukocyte count of
13.95x109/l (normal, 3.5-9.5x109/l) and blood
glucose of 6.77 mmol/l (normal, 3.89-6.11 mmol/l).
The patient underwent ultrasound examination with a
Philips IU Elite (Philips Healthcare). The kidney was detected
using a convex curved probe at 33.5 MHz. Ultrasonography revealed
heterogeneous echo area in lower pole of left kidney, which
suggested the possibility of renal cell carcinoma rupture.
Subsequently, a plain and enhanced CT scan was performed with a
SOMATOM Definition AS 64-slice spiral CT machine (Siemens
Healthineers). The scan range was from the upper kidney to the
lower kidney. The scanning parameters were set as follows: Pitch,
0.9375:1; layer thickness, 5 mm; layer spacing, 5 mm; tube voltage,
120 kV; and tube current, 160 mA. The CT enhancement scan was
performed using iohexol (Cytiva) 1.5 ml/kg at an injection rate of
4.0 ml/sec. The arterial phase was scanned 25 to 30 sec after
contrast medium injection, the venous phase was scanned 60 to 70
sec and the renal pelvis filling phase was scanned 120 to 180 sec.
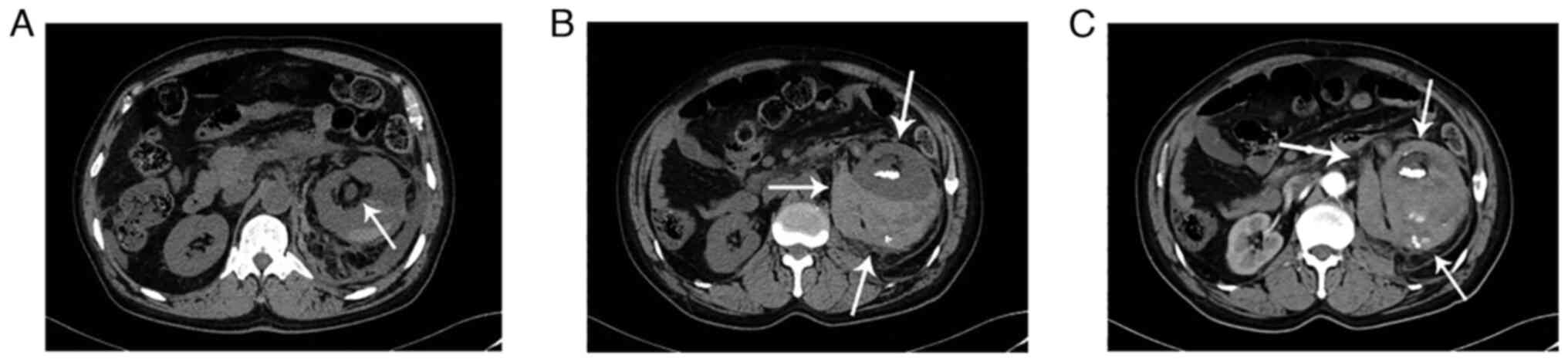
The results revealed that a strip of high-density shadow with a
width of ~0.9 cm could be seen in the left ventral ureter and the
upper urinary tract was dilated with effusion (Fig. 1A). An abnormally large mass in the
left renal area measured ~9.4x8.9x11.5 cm in size (Fig. 1B). Multiple nodular high-density
shadows were also observed in the left renal pelvis and calyces
(Fig. 1B). The enhancement was
uneven in the arterial phase according to contrast-enhanced CT,
similar to that in the renal parenchyma, but less intense in the
venous phase (Fig. 1C). According
to the ultrasound, CT imaging findings and clinical manifestations,
the preoperative diagnosis was as follows: i) Hemorrhage of the
left renal cell carcinoma with hematoma formation; and ii) calculus
of the left ureter, renal pelvis and calyceal, accompanied by
dilation and hydronephrosis of the urinary system.
After admission, the patient was given fluid
resuscitation and empirical anti-infection treatment. Subsequently,
the patient underwent radical nephrectomy, with resection of the
left kidney, surrounding fat tissues and a substantial proportion
of the ureter. To verify the preoperative diagnosis of spontaneous
rupture of the kidney caused by a tumor, the surgically removed
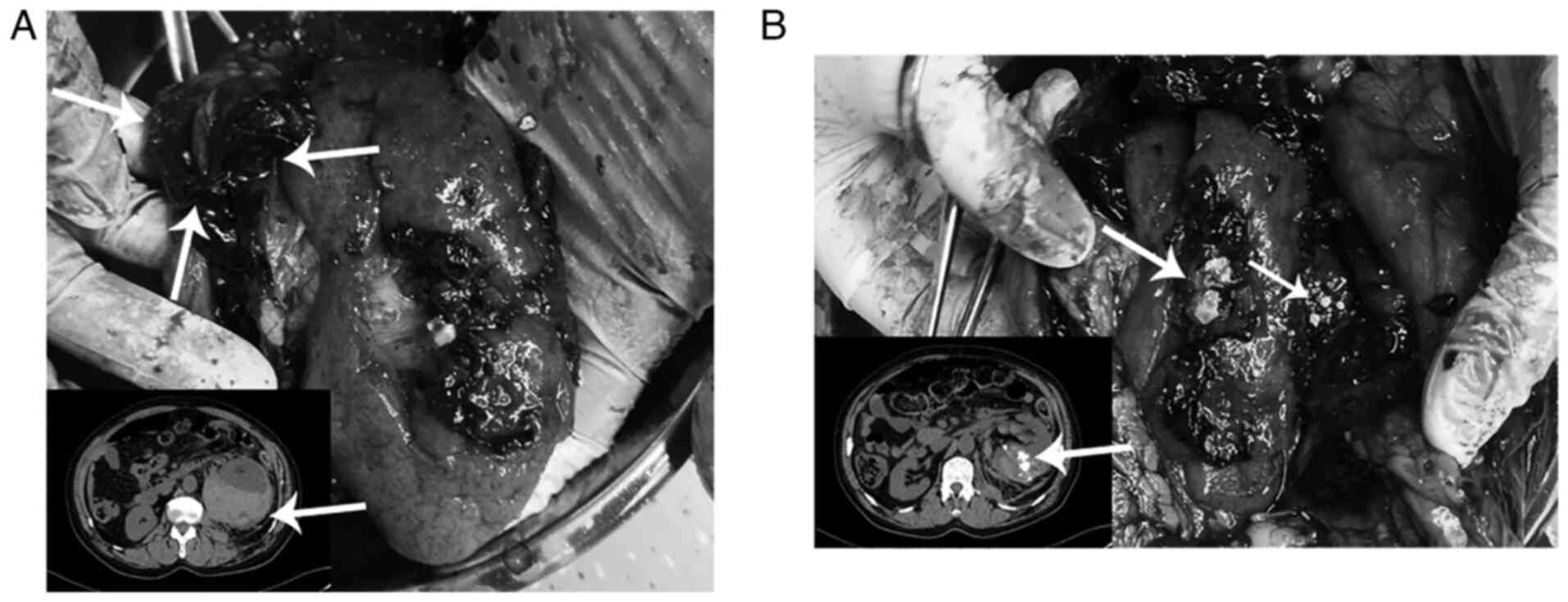
kidney was examined pathologically. There was a large hematoma in
the lower pole of the left kidney and the left renal cortex was
thin (Fig. 2A). In addition,
prominent stones were present in the stellate fracture of the
dorsal renal parenchyma. It was also observed that numerous yellow
stones were mixed with perirenal blood clots outside the renal
capsule (Fig. 2B). The renal
tissues were stained with H&E after operation. The specific
steps were as follows: i) The fixation solution was 10% formalin
solution, 20 volumes of the sample, and fixing was performed at
room temperature for 24 h; ii) dehydration was carried out by using
an alcohol sample with low concentration to high concentration,
then the tissue block was placed in a xylene clearing agent, and
the alcohol was replaced; iii) the tissue was placed in the melted
paraffin and blocks were automatically formed after the paraffin
was cooled; iv) 5-mm slices were cut with a microtome; v) dyeing: H
staining for 5 min and E staining for 3 min at room temperature;
vi) repeated alcohol dehydration as in step ii; and vii) sealing.
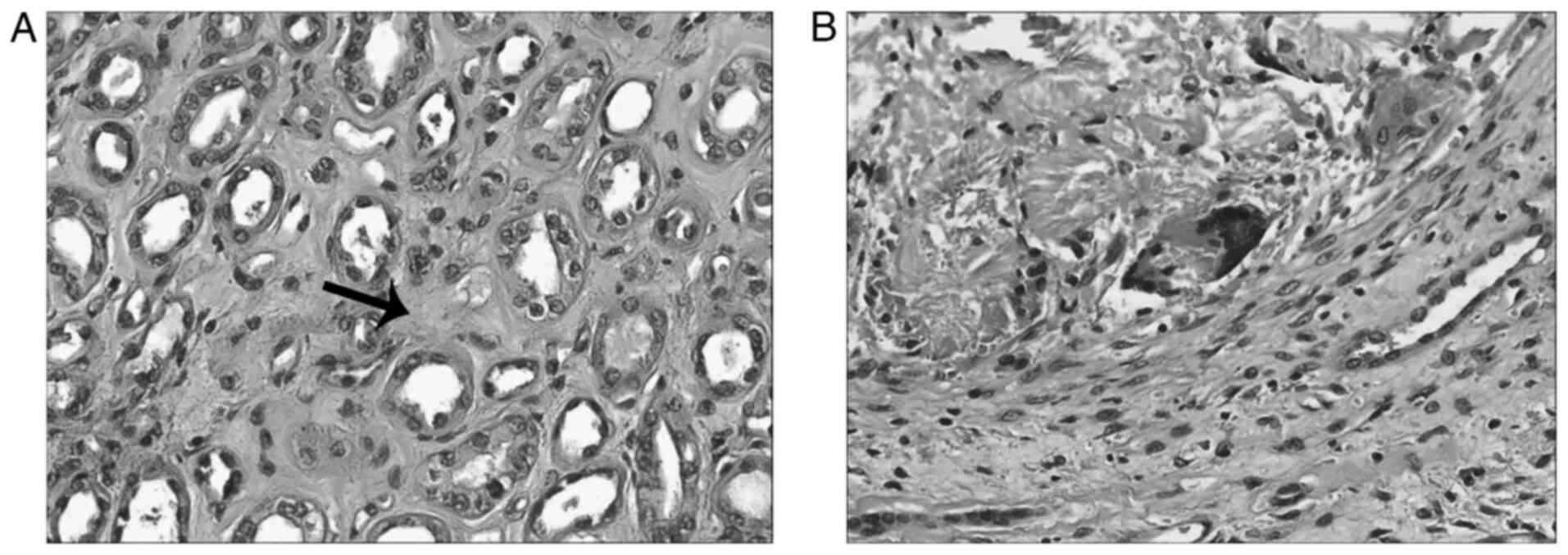
The sections were examined using a light microscope. Postoperative
pathology indicated that the lesions were consistent with
intrarenal stones, where giant cell reaction to stones, renal
interstitial atrophy (Fig. 3A) and
degeneration were observed (Fig.
3B). However, tumor, infection and vascular disease could not
be observed.
After the operation, the patient's vital signs were
stable and he returned to the general ward. The surgical drain was
removed on day 5, while intravenous antibiotics were continued
until day 7. The patient also received symptomatic treatment such
as analgesia, fluid replacement and cough relief. The patient was
discharged from hospital 10 days after the operation. We conducted
regular follow-up visits were performed and the last follow-up
visit was in April 2022. The patient recovered well and no other
diseases occurred during the follow-up visit.
Discussion
Non-traumatic spontaneous renal rupture is a rare
but critical clinical event that frequently leads to dilemmas
regarding diagnosis and treatment (9). The incidence of spontaneous renal
parenchyma rupture is lower compared with that of renal pelvic
rupture (8). The key words
‘spontaneous renal parenchymal rupture’ and ‘case report’ were
searched in PubMed and Google Scholar for published case reports.
The inclusion criteria were as follows: i) Case reports; ii) renal
parenchyma rupture was required to be mentioned in the abstract;
iii) detailed clinical information of patients was included.
Finally, 10 articles were included in the study. A total of 15
cases of renal parenchyma rupture were reported in these 10
articles (Table I) (1,6,8,10-16).
Among them, Durak et al (6)
reported an autopsy case in an elderly Turkish male. Among the
previously reported patients, males appeared to be at a slightly
higher risk, with the male to female ratio of 8:7 and a mean age of
49±23 years. In these previous reports, infection was the main
cause of spontaneous renal rupture in female patients. Spontaneous
rupture of the kidney occurs indirectly or directly as a result of
kidney atrophy and abscess formation caused by inflammation
(10,13). By contrast, renal tumors were the
cause of rupture in >50% of the male patients (5). In addition, renal parenchyma rupture
caused by renal cancer may lead to fatal hemorrhage (1,6,10).
Abdominal pain is a common feature in all patients with ruptured
renal parenchyma due to irritation of the retroperitoneal nerve
plexus and emesis in certain patients (17). Hematuria may also occur, but it is
not a characteristic symptom of non-traumatic renal rupture and its
appearance is mainly dependent on potential diseases (10). These symptoms are similar to acute
abdominal pain and require differentiation from other
gastrointestinal diseases (14).
The appearance of shock symptoms is a risk signal for spontaneous
renal rupture (18). Even if
prompt surgery is performed, the patient may finally succumb to
this condition due to organ failure (1). CT examination of the retroperitoneal
space is currently the most important means for diagnosing
spontaneous renal rupture (17).
Typical CT findings include high-density masses within the renal
capsule with exudation into the perirenal space (19). Strip-like and high-density
infiltration of perinephric fat is a common manifestation of renal
hemorrhage (20). The CT findings
in 10 of the 15 cases revealed perirenal hematomas, suggesting the
possibility of renal rupture. However, it remains difficult to
accurately make an etiological diagnosis, since an accurate
diagnosis prior to surgery was only made in two cases (10,15).
Faced with this situation, clinicians first consider the
possibility of a tumor, which is the most common etiology found
during histological evaluation after spontaneous renal rupture
surgery (3,17). A plain and enhanced CT examination
of the patient was performed in the present report. A CT scan is
able to detect, quantify the extent of and locate perirenal
hemorrhage (21). In the present
report, CT suggested a possible renal tumor. A preoperative
diagnosis of renal cancer-associated hemorrhage can be readily
detected using CT, which requires urgent surgery due to the
immediate risk of mortality (14).
 | Table IReported cases of spontaneous rupture
of renal parenchyma. |
Table I
Reported cases of spontaneous rupture
of renal parenchyma.
| Author (year) | Age, years/sex | Clinical
symptoms | CT description | Therapeutic
method | Causes of
rupture | (Refs.) |
|---|
| Councill WA and
Councill WA Jr (1950) | 48/F | Right lumbago with
vomiting | Not performed | Pyelotomy and
lithotomy | Incarcerated calculi
in lower segment of large calyx of kidney | (8) |
| Miyamoto H and Usuda
K (1994) | 3/M | Left abdominal
pain | Left hydronephrosis
with perirenal uroma | Left pyeloplasty | Renal rupture caused
by elevated renal pelvis pressure | (11) |
| Szentgyorgyi E et
al (1994) | 67/F | Abdominal pain and
fever | Extracapsular
hematoma of left kidney | Nephrectomy | Aposthematous
pyeloneplritis without obstruction | (10) |
| Szentgyorgyi E et
al (1994) | 69/F | Abdominal pain and
fever | Not performed | Nephrectomy | Acute purulent
pyelonephritis | (10) |
| Szentgyorgyi E et
al (1994) | 80/F | Pain in the left
lower abdomen | Heterogeneous
infiltration around kidney | Nephrectomy | Chronic
pyelonephritis and a cortical cyst | (10) |
| Szentgyorgyi E et
al (1994) | 70/M | Left renal colic | Not performed | Radical
nephrectomy | Adenopapillary cancer
with ecchymosis and necrosis | (10) |
| Szentgyorgyi E et
al (1994) | 41/M | Abdominalgia | Suspected renal
tumor | Radical
nephrectomy | Renal cancer with
hemorrhage | (10) |
| Wakasugi E et
al (1996) | 47/F | Abdominalgia | Left renal hemorrhage
with hematoma | Nephrectomy | The formation of
renal abscess | (12) |
| Altınoluk et
al (2012) | 25/F | Abdominal pain and
fever | Low-density mass
around left kidney | Nephrectomy | Xanthogranulomatous
pyelonephritis | (13) |
| Durak et al
(2014) | 79/M | - | Not performed | - | Renal-cell
carcinoma | (6) |
| Sudusinghe et
al (2018) | 48/M | Left lumbar
colic | Left perirenal
hematoma | Nephrectomy | Acquired renal
cystic disease | (14) |
| Zhang et al
(2019) | 57/F | Fever with
edema | Bilateral perirenal
hematoma | Methylprednisolone
pulse therapy | Microscopic
polyangiitis | (15) |
| Chiancone et
al (2021) | 64/M | Acute left flank
pain and massive haematuria | Left kidney rupture
and a left pelvic ureteral stone | Nephrectomy | Intrarenal
hypertension aft ureteral calculi obstruction | (1) |
| Yavuzsan et
al (2021) | 20/M | Abdominalgia | Mesenteric vascular
injury | Nephrectomy | Hydronephrosis and
increased abdominal pressure | (16) |
| Yavuzsan et
al (2021) | 38/M | Abdominalgia | Perirenal hematoma
formation | Radical
nephrectomy | Multiple renal
cysts, adenomas and small renal cancers | (16) |
It should be noted that a diagnosis made based on
imaging examination alone should not be regarded as sufficient. The
particularity of the present case was identified by comparing the
medical records with other reported cases of renal parenchyma
rupture (1,6,8).
Renal parenchymal rupture is a life-threatening emergency, where
the most common symptoms are intense sharp pain, hemorrhagic shock
and a palpable abdominal mass (17). In the present report, the patient
exhibited no symptoms other than left lower back pain and oliguria.
The first suspicion may be upper urinary tract obstruction caused
by urinary calculi. However, CT suggested a perirenal hematoma most
likely caused by the rupture of renal cancer, which is the most
common condition clinically. An important feature that was
overlooked in the present case was that the patient had developed
multiple stones in his left kidney over several years and had
undergone four ESWL procedures within 2 years. Therefore, in the
present case, the risk of spontaneous rupture of the renal
parenchyma was high. No significant lumbago or hematuria were
detected in the patient prior to the first ESWL in 2018. The
long-term presence of calculi during this period may lead to
hematuria that cannot be recognized by naked eyes or compensated
mild hydronephrosis, although the patient has no obvious symptoms
(14). In addition, infection may
occur (17). Since these
aforementioned symptoms resolved, the patient ignored the stones
that caused them. According to his description, no tumor was found
in the left kidney at the last preoperative examination of ESWL in
2019. We cannot rule out tumorigenesis after 2019. Tumors may arise
due to irritation caused by the stones or spontaneously in the
absence of precipitating factors. Among all cases reported, renal
parenchyma rupture was caused by kidney stones in two cases. In one
case, severe hydronephrosis was caused by the obstruction of
ureteral calculi in the pelvic region, where the patient delayed
medical treatment for 4 months due to the coronavirus disease 2019
outbreak (6). This delay may have
induced chronic tubulointerstitial nephritis or rupture of the
collecting system (1). Sudden
increases in the renal venous pressure are most likely to be the
physiological cause of parenchymal rupture. The characteristics in
the other case was similar to those in the patient in the present
report (8). Comparison of the two
cases indicated that both patients had kidney calculi for several
years, such that the stones grew to >2 cm in size. Long-term
friction and adverse reactions to stones rendered the renal cortex
thinner and weaker. Upper urinary tract obstruction caused by
ureteral calculi and ureteropelvic junction stenosis may have led
to increased renal pressure, changes in the normal renal
morphological structure and weakening of the renal parenchyma,
undoubtedly increasing the risk of renal parenchyma rupture
(22). A medical history review
revealed that the patient in the present report had undergone ESWL
twice a year for kidney calculi in 2018 and 2019. The patient's
ESWL surgeries were performed at an external hospital, which means
that a series of patient examination reports before and after each
ESWL were not available. It is a limitation of this report. In a
follow-up phone call to the patient, he stated that no tumor was
found during the routine pre-operative examination of the stones
and that he was discharged from the hospital after the symptoms had
resolved post-operatively. After ESWL, the patient's lower back
pain was markedly alleviated and no bleeding or urinary system
infection occurred. Hematuria occurred every time after surgery and
the hematuria disappeared at an average of 3 days. Re-examination
using B ultrasonography indicated that the stones were well cleared
after ESWL. Acute renal rupture has been previously reported to
occasionally occur after ESWL (23,24).
The renal tissue can be damaged when a kidney calculus is broken by
a clinical dose of shockwaves. One of the initial signs of tissue
damage is hematuria (25). This
type of early stage typically occurs in the renal medulla. With
increases in energy, the vascular lesions extend to the kidney
surface and then into the cortex, producing lesions and
intraparenchymal hemorrhage in the kidney (26). Fibrous scar tissue formation after
segmental kidney injury in the hemorrhagic lesion area may induce
ischemia, leading to increased tissue fragility (27-29).
This type of injury was reported to be markedly associated with the
number of ESWL admissions (29).
Renal pathology, such as intraoperative renal mucosal injury,
continuous high-pressure reperfusion after lithotripsy and possible
renal tumors, are also risk factors for renal rupture (30). In conclusion, the patient's left
kidney was already at risk prior to rupture. When the posture was
altered, stones in his left kidney may have protruded from the
renal parenchyma, leading to renal rupture.
Management of patients with spontaneous renal
rupture is based on the underlying etiology and the hemodynamic
state of the patient (14). In the
majority of cases, severe bleeding requires open surgical
intervention to prevent patient mortality. When CT confirms the
existence of perirenal hemorrhage, immediate surgical exploration
should be performed (16). Based
on the exploratory results, nephrectomy is almost always required
for patients with renal tumors, renal carbuncles, hydronephrosis or
severe cicatricial nephrosclerosis (10,13,16).
Conservative non-surgical management has also been described in
patients with hemodynamic stability and no evidence of continuous
blood loss. This method is mainly used for patients with chronic
hemodialysis and the possible pathology is acquired renal cystic
disease (14). Due to the present
patient's potential for renal tumors and poor preoperative imaging
sensitivity to confirm the presence of a tumor within the hematoma,
this patient required frequent CT monitoring every 3-6 months
post-operatively to exclude the possibility of a potential renal
malignancy. Although conservative treatment could have been
attempted to remove the stone and repair the kidney, due to the
erroneous diagnosis, the patient's left kidney was removed as a
precaution. It was emphasized that pre-operative diagnosis served a
decisive role in the selection of surgical methods in the present
case, which provides evidence for the spontaneous rupture of renal
parenchyma caused by kidney calculi. In addition, the molecular
clues of spontaneous renal rupture were explored, which revealed no
common consensus and provides a niche for research in the
future.
In conclusion, the present case was reported to draw
attention to the possibility of spontaneous renal rupture caused by
kidney calculi and avoid similar misdiagnoses. Particular attention
should be paid to patients with long-term kidney calculi who have a
history of ESWL. In such cases, the possibility of renal parenchyma
rupture should be evaluated. In addition, it would be suggested
that benign urinary system diseases be considered during early
diagnosis and treatment.
Acknowledgements
The authors would like to thank Dr Xingjun He and Mr
Shengqi Zheng from the Department of Urology, Affiliated Hospital
of Yangzhou University (Yangzhou, China) for their guidance and
help in writing and revising the manuscript. Dr Xingjun also
performed the patient's surgery and participated in the patient's
postoperative management.
Funding
Funding: The present report was financially supported by the
National Natural Science Foundation of China (grant no. 82002675),
Jiangsu Natural Science Research of Colleges and
Universities-General Project (grant no. 20KJB320014), Jiangsu
Science and Technology Program-Youth Fund Project (grant no.
BK2020938), Yangzhou Key Research & Development-Social
Development Project (grant no. YZ2020110), Yangzhou Soft Science
Research Program (grant no. YZ2020258), Jiangsu Postdoctoral
Research Funding Program (grant no. 2020Z268) and Yangzhou
University High-level Talent Research Start-up Fund (grant no.
2019LYF).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the present
report.
Authors' contributions
GY and XP contributed to the conception of the
study, collected, analyzed and interpreted data from the literature
and the data corresponding to the patient, and critically revised
the manuscript. YL, LQ, HT, ZZ, JL, XW, QF and FT contributed to
the conception of the study, performed the literature research and
drafted the manuscript. All authors have read and approved the
final manuscript. GY and XP confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Affiliated Hospital of Yangzhou University
(Yangzhou, China).
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the details of his medical case and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chiancone F, Meccariello C, Ferraiuolo M,
De Marco GP, Fedelini M, Langella NA and Fedelini P: A rare case of
spontaneous parenchymal kidney explosion in a patient with ureteral
obstruction caused by a single stone. Urologia. 88:386–388.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ozawa M, Ishii T, Suenaga S, Suzuki H and
Tsuchiya N: Spontaneous renal rupture treated by transcatheter
arterial embolization: A case report. Nihon Hinyokika Gakkai
Zasshi. 108:158–161. 2017.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
3
|
Gershman B, Kulkarni N, Sahani DV and
Eisner BH: Causes of renal forniceal rupture. BJU Int.
108:1909–1911. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Satoh S, Okuma A, Fujita Y, Tamaka M and
Nakano H: Spontaneous rupture of the renal pelvis during pregnancy:
A case report and review of the literature. Am J Perinatol.
19:189–195. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jalbani IK, Khurrum M and Aziz W:
Spontaneous rupture of pyonephrosis leading to pyoperitoneum. Urol
Case Rep. 26(100928)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Durak D, Eren F, Inanir NT, Eren B, Cetin
S and Gundogmus UN: Spontaneous rupture of a renal cell carcinoma
associated with fatal bleeding. Maedica (Bucur). 9:275–277.
2014.PubMed/NCBI
|
|
7
|
Kim WB, Lee ES, Doo SW, Yang WJ, Song YS
and Noh H: Spontaneously ruptured renal cell carcinoma during
hemodialysis in two patients with end-stage renal disease. Korean J
Urol. 52:865–867. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Councill WA and Councill WA Jr:
Spontaneous rupture of the renal parenchyma associated with renal
lithiasis. J Urol. 63:441–445. 1950.
|
|
9
|
Howalt JS and Squires JW: Spontaneous
rupture of the kidney. A case of atraumatic retroperitoneal
bleeding. Am J Surg. 123:484–488. 1972.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Szentgyorgyi E, Kondas J, Varga S,
Lorinczy D, Regos I and Kun I: Spontaneous rupture of the kidney: A
report on 5 cases. Int Urol Nephrol. 26:133–140. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Miyamoto H and Usuda K: Spontaneous
rupture of the hydronephrotic renal parenchyma associated with
urinoma in a child. Hinyokika Kiyo. 40:419–421. 1994.PubMed/NCBI(In Japanese).
|
|
12
|
Wakasugi E, Kato Y, Yano H, Kanbara N and
Kurita T: Spontaneous renal rupture due to xanthogranulomatous
pyelonephritis; a case report. Hinyokika Kiyo. 42:47–50.
1996.PubMed/NCBI(In Japanese).
|
|
13
|
Altınoluk B, Sayar H, Özkaya M, Sahinkanat
T and Malkoc O: Xanthogranulomatous pyelonephritis presented with
spontaneous kidney rupture in a young woman. Eur J Gen Med.
9:138–141. 2012.
|
|
14
|
Sudusinghe D, Wijayaratne D, Beligaswatta
C and Gunawansa N: Wunderlich syndrome; spontaneous atraumatic
rupture of the kidney: A case report. Arch Clin Nephrol. 4:026–028.
2018.
|
|
15
|
Zhang MY, Yang DP, Zhou JK and Yang XY:
Spontaneous rupture of kidneys triggered by microscopic
polyangiitis. Int J Clin Exp Med. 12:2883–2887. 2019.
|
|
16
|
Yavuzsan AH, Baloglu IH, Albayrak AT,
Bursali K and Demirel HC: Spontaneous kidney rupture: Two case
reports with unusual presentations. Cureus.
13(e15332)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang JQ, Fielding JR and Zou KH: Etiology
of spontaneous perirenal hemorrhage: A meta-analysis. J Urol.
167:1593–1596. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Grubb SM, Stuart JI and Harper HM: Sudden
onset flank pain: Spontaneous renal rupture. Am J Emerg Med.
35:1787.e1–1787.e3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Joachim GR and Becker EL: Spontaneous
rupture of the kidney. Arch Intern Med. 115:176–183.
1965.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Belkin BA and Vine HS: Spontaneous renal
rupture: Evaluation by computerized tomography. J Urol.
138:120–122. 1987.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sagel SS, Siegel MJ, Stanley RJ and Jost
RG: Detection of retroperitoneal hemorrhage by computed tomography.
AJR Am J Roentgenol. 129:403–407. 1977.PubMed/NCBI View Article : Google Scholar
|
|
22
|
McDougal WS, Kursh ED and Persky L:
Spontaneous rupture of the kidney with perirenal hematoma. J Urol.
114:181–184. 1975.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jeon BH, Jang JH, Oh JH, Oh SY, Lee SJ,
Kim SE, Kim CW, Choe JW and Lee KJ: Kidney rupture after
extracorporeal shockwave lithotripsy: Report of a case. J Emerg
Med. 37:13–14. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Seddiki A, Thomas J, Tobelem G, Ferriere
X, Bellahouel S, Trouiller D and Arvis G: A rare complication of
extracorporeal shock wave lithotripsy: Rupture of the kidney.
Apropos of a case. J Urol (Paris). 97:224–227. 1991.PubMed/NCBI(In French).
|
|
25
|
Chaussy C, Schuller J, Schmiedt E, Brandl
H, Jocham D and Liedl B: Extracorporeal shock-wave lithotripsy
(ESWL) for treatment of urolithiasis. Urology. 23:59–66.
1984.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Skolarikos A, Alivizatos G and de la
Rosette J: Extracorporeal shock wave lithotripsy 25 years later:
Complications and their prevention. Eur Urol. 50:981–990.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Williams CM and Thomas WC Jr: Permanently
decreased renal blood flow and hypertension after lithotripsy. N
Engl J Med. 321:1269–1270. 1989.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bataille P, Cardon G, Bouzernidj M, El
Esper N, Pruna A, Ghazali A, Westeel PF, Achard JM and Fournier A:
Renal and hypertensive complications of extracorporeal shock wave
lithotripsy: Who is at risk? Urol Int. 62:195–200. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Juan YS, Chuang SM, Wu WJ, Shen JT, Li CC,
Wang CJ and Huang CH: Evaluation of intrarenal blood flow by
Doppler ultrasonography immediately after extracorporeal shock wave
lithotripsy on hydronephrotic kidney. Kaohsiung J Med Sci.
21:412–417. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang H, Zhuang G, Sun D, Deng T and Zhang
J: Spontaneous rupture of the renal pelvis caused by upper urinary
tract obstruction: A case report and review of the literature.
Medicine (Baltimore). 96(e9190)2017.PubMed/NCBI View Article : Google Scholar
|