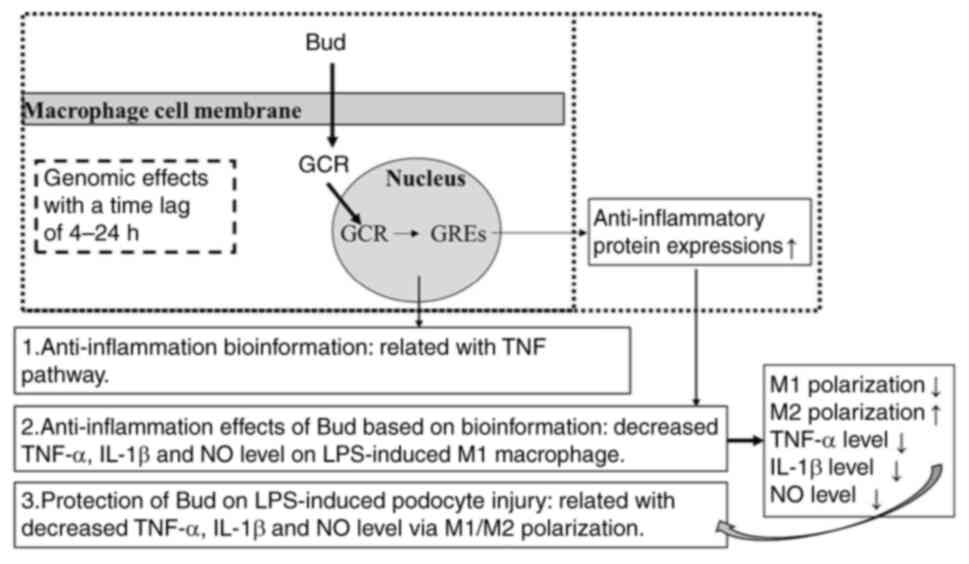
Introduction
For the first 40 years of the 20th century, when
asthma occurred, the treatments were given by injection or oral
administration of the adrenergic agonist epinephrine and the
phosphodiesterase inhibitor theophylline (1). In 1930, epinephrine became available
as an aerosol. To date, repurposing drugs as inhaled therapies in
asthma has made progress in drug delivery to the action site. More
importantly, inhaled therapies permit the prophylactic treatment of
asthma. As a nonhalogenated corticosteroid, budesonide (Bud) was
developed in the early 1970s and was available as an inhaled
steroid by 1982(1). It is now one
of the most widely used inhaled medicines in lung diseases such as
asthma and chronic obstructive pulmonary disease (COPD) worldwide
(2).
As a repurposing drug, Bud showed its efficacy again
in a recent double-blind, random, placebo-controlled phase 2b trial
in patients with immunoglobulin A nephropathy (IgAN) (3). IgAN is the commonest type of
glomerulonephritis in Asia and the western world (4). The mainstay of therapy is therefore
optimized supportive care, i.e., measures that lower blood
pressure, reduce proteinuria and help to decrease nonspecific
insults to the kidneys (4).
Glomerular podocyte injury is a key factor associated with
proteinuria in IgAN (5). Clinical
research shows that Bud is effective in the treatment of patients
with IgAN at high risk of progression in terms of reducing
proteinuria and preserving renal function over 24 months of therapy
(6). However, whether Bud can
reduce proteinuria by protecting podocytes in vitro and
in vivo remains unclear.
Complex factors are involved in the development and
progression of IgAN. Autoimmunity and inflammation are considered
to be the basic mechanisms; however, the exact pathogenesis remains
to be elucidated (7). To explore a
possible protective effect of Bud on podocyte injury under
inflammatory stress (8), the Gene
Expression Omnibus (GEO) was used in the present study to identify
the induced anti-inflammatory genes in humans involved in
transcription and signaling following inhalation of Bud (9). Differentially expressed genes (DEGs)
were identified between the volunteers who inhaled Bud or placebo
randomly (9). The results showed
that, based on downregulated DEGs, the TNF signaling pathway served
an important role when Bud exerted anti-inflammatory effects (The
results are shown in Table I).
 | Table IThe 10 KEGG pathways based on
downregulated DEGs. |
Table I
The 10 KEGG pathways based on
downregulated DEGs.
| Term | Description | Count in gene
set | Gene ratio | P-value |
|---|
| hsa04668 | TNF signaling
pathway | 10 | 3.257 |
1.966x10-4 |
| hsa05146 | Amoebiasis | 9 | 2.932 |
9.259x10-4 |
| hsa04670 | Leukocyte
transendothelial migrationa | 9 | 2.932 |
1.573x10-3 |
| hsa00280 | Valine, leucine and
isoleucine degradation | 5 | 1.629 |
1.222x10-2 |
| hsa04068 | FoxO signaling
pathwaya | 8 | 2.606 |
1.429x10-2 |
| hsa05222 | Small cell lung
cancer | 6 | 1.954 |
2.330x10-2 |
| hsa05169 | Epstein-Barr virus
infection | 7 | 2.280 |
2.949x10-2 |
| hsa04152 | AMPK signaling
pathway | 7 | 2.280 |
3.053x10-2 |
| hsa05200 | Pathways in
cancer | 14 | 4.560 |
3.785x10-2 |
| hsa05160 | Hepatitis C | 7 | 2.280 |
4.228x10-2 |
The authors' previous in vitro study suggests
that increased TNF-α protein expression in polarized macrophages is
related to podocyte injury (10).
With lipopolysaccharide (LPS)-induced RAW264.7 macrophages, the
effects of Bud on the protein expression of two markers of
classically activated macrophages (M1), inducible nitric oxide
synthase (iNOS) and TNF-α and two markers of alternatively
activated macrophages (M2), mannose receptor (CD206) and arginase
(Arg)-1, were identified (10) and
the effects of Bud on LPS-induced podocyte injury by modulating
M1/M2 polarization were further explored in the present study
(Fig. 1).
Materials and methods
Microarray material and microarray
data
GEO (http://www.ncbi.nlm.nih.gov/geo/) is an international
public repository that archives and freely distributes
high-throughput gene expression and other functional genomics
datasets (11). A gene expression
dataset, namely, GSE83233 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE83233)
‘An inhaled dose of budesonide induces genes involved in
transcription and signaling in human airways’, was downloaded from
GEO [GPL15207 platforms, (PrimeView) Affymetrix Human Gene
Expression Array]. In the study (9), healthy male, nonsmoker, nonallergic
volunteers (age 18-50 years) with normal lung function were
recruited into this prospective, double-blind, placebo-controlled,
randomized, two-period crossover study involving an initial
screening visit, followed by two intervention visits. Participants
were screened at visit one to fulfil the study eligibility
criteria. At visit two, volunteers were randomized to receive
inhaled Bud (1,600 µg) or placebo, both via Turbuhaler. Then, at
two to three weeks later, at visit three, participants received
either Bud or placebo, as appropriate, to complete both study arms.
At 6 h after placebo or Bud inhalation, bronchial biopsies were
obtained via bronchoscopy (9). The
study protocol and consent form were approved by the Conjoint
Health Research Ethics Board at the University of Calgary and
Alberta Health Services and the ethical approval number was
23241.
Identification of DEGs
The DEGs in the Bud and placebo specimens were
selected from GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r), which is a
platform for examining DEGs across experimental conditions by
comparing multiple datasets in GEO series. The genes with multiple
probes were averaged and the probes that lacked gene symbols were
removed. The DEGs with a |logFC (fold change)| >0 and P<0.01
were screened out and represented statistical significance.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of
DEGs
The database for annotation, visualization and
integrated discovery (DAVID), which can be freely accessed
(http://david.ncifcrf.gov), is a web-based online
bioinformatics resource that aims to provide tools for the
functional interpretation of large lists of genes and proteins
(12). In addition, GO, a
significant bioinformatics tool, enables the annotation of genes
according to biological processes (13). The KEGG is a knowledge-based
platform for the systematic analysis of gene functions, linking
genomic information with higher-order functional information
(14,15). The DAVID online database was used
to study the functions of DEGs. P<0.05 was considered to
indicate a statistically significant difference. ImageGP
(http://www.ehbio.com/ImageGP/) was used
to draw enrichment plots for GO and KEGG.
Cell source and culture
A mouse macrophage RAW264.7 cell line was purchased
from Procell Life Science & Technology Co., Ltd. According to
the instructions, the cells were maintained in RPMI-1640 medium
(Beijing Solarbio Science & Technology Co., Ltd.) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Shanxi
MiniBio Technology Co., Ltd.) in a 5% CO2 incubator at
37˚C. The medium was replaced the next day.
A conditionally immortalized mouse MPC-5 podocyte
cell line was purchased from Fuheng Biology Company and cultured in
RPMI-1640 medium supplemented with 10% FBS and recombinant IFN-γ
(cat. no. G1021; APeXBIO Technology LLC) at 33˚C. Podocytes were
reseeded and cultured in RPMI-1640 medium with 10 mg/ml type-I
collagen (BD Biosciences) at 37˚C without IFN-γ for 7-15 days
before testing.
Cytotoxicity of Bud and LPS on
RAW264.7 and MPC-5 cells
RAW264.7 cells and MPC-5 cells were seeded into
96-well plates separately at a density of 1x106 cells/ml
and cultured in 10% FBS RPMI-1640 medium for 24 h at 37˚C.
Following another 24 h treatment with Bud (cat. no. 200721527;
Chiatai Tianqing Pharmaceutical Group Co., Ltd.) at 5, 10, 20, 40
and 80 µM (16,17), LPS at 0.02, 0.10, 0.50, 2.50 and
12.5 µg/ml at 37˚C (18-20).
The supernatants were removed and each well was washed with PBS
before the addition of 10% FBS RPMI-1640 medium and 10 µl CCK-8
reagent (Shanxi MiniBio Technology Co., Ltd.). Cell viability was
determined by measuring the absorbance at 450 nm using a
microporous plate reader (Model 550; Bio-Rad Laboratories, Inc.)
after an incubation period of 2 h at 37˚C. The average optical
density was determined by examining six wells per group.
Establishment of LPS-induced M1
polarization
Based on the cytotoxicity results of LPS on RAW264.7
cells. LPS (0.10 µg/ml) was used to induce macrophage polarization
to the M1 subtype for 24 h at 37˚C. The nitric oxide (NO) level was
tested with the Griess assay (21). Nitrite, a stable end-product of NO
metabolism, was measured using the Griess reaction. Culture media
of the RAW 264.7 cells (100 µl) was mixed with an equal volume of
Griess reagent (Yantai Science & Biotechnology Co. Ltd.),
followed by spectrophotometric measurement at 540 nm. Nitrite
concentrations in the culture media were determined by comparison
with a sodium nitrite standard curve. All experiments were repeated
three times.
Effects of Bud on iNOS, TNF-α, Arg-1
and CD206 protein expression in M1 macrophages
The protein expression levels of iNOS, TNF-α, Arg-1
and CD206 at different concentrations of Bud (5, 10, 20 µM) in 0.10
µg/ml LPS-cultured macrophages were tested by western blotting.
RPMI-1640 was used as the normal control, LPS was used as the model
control and 10 µM curcumin (cat. no. C7090; Beijing Solarbio
Science & Technology Co., Ltd.; purity: 95.0%) was used as the
positive control (22). The
treated cells (1x106 cells/ml) were removed from the
culture media and lysed with RIPA lysis buffer from Beijing
Solarbio Science & Technology Co., Ltd. for 30 min. The protein
concentrations were determined using a BCA Protein Assay kit from
Beijing Solarbio Science & Technology Co., Ltd. Samples
containing 50 µg of protein were resolved by 10% SDS-PAGE
electrophoresis and transferred to polyvinylidene fluoride
membranes (MilliporeSigma) in a buffer tank with platinum wire
electrodes. After immersing the membranes into 5% nonfat dried milk
(diluted in 0.1% (v/v) Tween-20 PBS) for 2 h at room temperature to
block nonspecific binding, the membranes were incubated overnight
with a primary antibody against iNOS at a 1:2,000 dilution (cat.
no. 18985-1-AP; ProteinTech Group, Inc.), a primary antibody
against TNF-α (cat. no. bs-2081R; Bioss) at a 1:1,000 dilution, a
primary antibody against CD206 (cat. no. bs-21473R; Bioss) at a
1:1,000 dilution and a primary antibody against Arg-1 (cat. no.
16001-1-AP; ProteinTech Group, Inc.) at a 1:5,000 dilution at 4˚C.
The membranes were then washed three times (10 min each) and
incubated with the corresponding secondary IgG conjugated to HRP
antibody (cat. no. SA00001-2; ProteinTech Group, Inc.) at room
temperature for 1 h. The results were finally analyzed by the
Quantity One analysis system (Bio-Rad Laboratories, Inc.). GAPDH at
a dilution of 1:5,000 (cat. no. 10494-1-AP; ProteinTech Group,
Inc.) was used as the internal loading control.
Effects of Bud on TNF-α, IL-1β and NO
levels in M1 macrophages
RAW264.7 cells (1x106 cells/ml) were
treated with 0.10 µg/ml LPS for 2 h and then treated with Bud (5,
10, 20 µM) in LPS-cultured macrophages for another 22 h at 37˚C.
RPMI-1640 was designated the normal control for all the
above-tested groups and the curcumin group was designated the
positive control (22). Cell
supernatants were then harvested and centrifuged at 1,500 x g for
10 min at 4˚C. TNF-α levels were determined using an ELISA kit
(cat. no. MM-0132M1; Jiangsu Meimian Industrial Co., Ltd.). IL-1β
levels were tested with an ELISA kit (cat. no. MM-0040M1; Jiangsu
Meimian Industrial Co., Ltd.). The absorbance was measured using a
microplate reader. Each sample underwent repeated testing four
times.
The NO levels of the normal control, curcumin
control and Bud at 5, 10 and 20 µM were also tested with the Griess
assay (21). All experiments were
repeated three times.
Collection of the supernatant from
macrophages (23)
RAW264.7 cells were seeded into six-well plates and
cultured in normal control, LPS model and curcumin positive, Bud 5,
10, 20 µM at 37˚C, respectively. The supernatants were
collected 24 h later, centrifuged at 1,500 x g for 15 min at 4˚C
and labeled as (Nor)MS, (LPS)MS, (Cur)MS, (Bud5)MS, (Bud10)MS and
(Bud20)MS. The collected supernatants were centrifuged for the
second time at 1,500 x g for 10 min at 4˚C, filtered with a 0.22 µm
sterile membrane and stored at -80˚C for further use.
Determination of LPS-induced podocyte
injury (24)
Podocytes (5x105 cells/ml) were cultured
with normal medium, 0.1 µg/ml LPS, 10 µM curcumin, 5 µM Bud, 10 µM
Bud, or 20 µM Bud and (Nor)MS, (LPS)MS, (Cur)MS, (Bud5)MS,
(Bud10)MS, or (Bud20)MS for 22 h at 37˚C. Podocytes were then
seeded into 96-well plates. The cells were subsequently treated
with bromodeoxyuridine (BrdU) reagent during the final 2 h of
incubation. The BrdU cell proliferation assay kit (cat. no. 2750;
MilliporeSigma) was performed according to the manufacturer's
protocol. The survival rate of the normal group was 100% and the
survival rate of cells in the other groups was compared with that
of the normal group. The mean was obtained by examining six wells
per group.
Statistical analysis
SPSS 19.0 software (IBM Corp.) was used for
statistical analysis. All the data were expressed as the mean ±
standard deviation (SD) of the mean. A two independent sample
unpaired Student's t-test was used to analyze differences between
two groups. One-way analysis of variance with Bonferroni's posttest
was used when more than two groups were present. P<0.05 was
considered to indicate a statistically significant difference.
Results
DEGs results
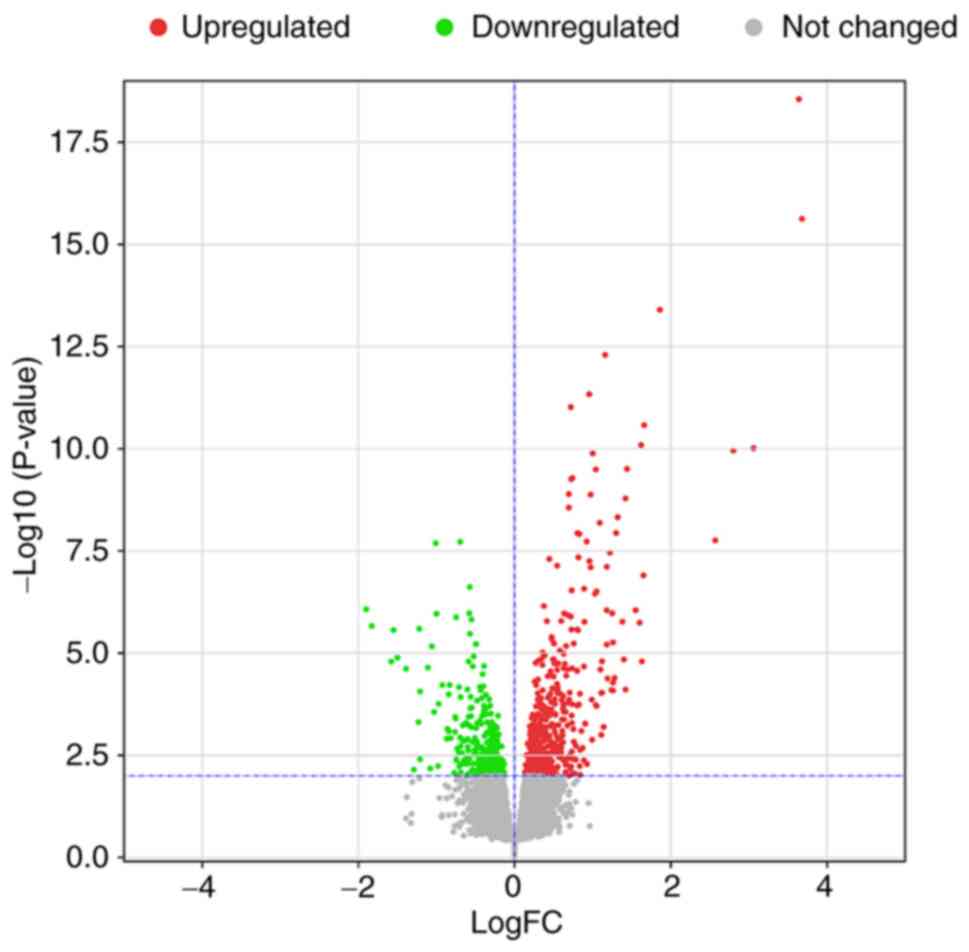
After standardization of the microarray data, all
DEGs between the Bud group and placebo group in GSE83233 were
identified, as shown in Fig. 2.
The dataset consisted of a total of 832 significant DEGs [|logFC
(fold change)| >0 and P<0.01], of which 311 DEGs were
downregulated and 521 were upregulated.
GO and KEGG results
Functional and pathway enrichment analyses were
conducted on DEGs for further analyses of biological
classification. Based on total DEGs, the numbers of significant
cell component (CC), molecular function (MF), biological process
(BP) and KEGG pathways were 22, 34, 142 and 37, respectively
(P<0.05). The top 20 CC, MF, BP and KEGG terms are shown
separately in Fig. 3. The results
indicated that the main CC terms included the nucleus, cytoplasm
and plasma membrane. The MF terms were mainly related to protein
binding, transcription factor activity, sequence-specific DNA
binding, etc. The main BP terms included positive/negative
regulation of transcription from RNA polymerase II promoter and
positive/negative regulation of transcription, DNA-templated and
inflammatory response. KEGG results showed that a number of
pathways were involved in the inflammatory response, such as the
forkhead box subgroup O (FoxO) signaling pathway and the TNF
signaling pathway.
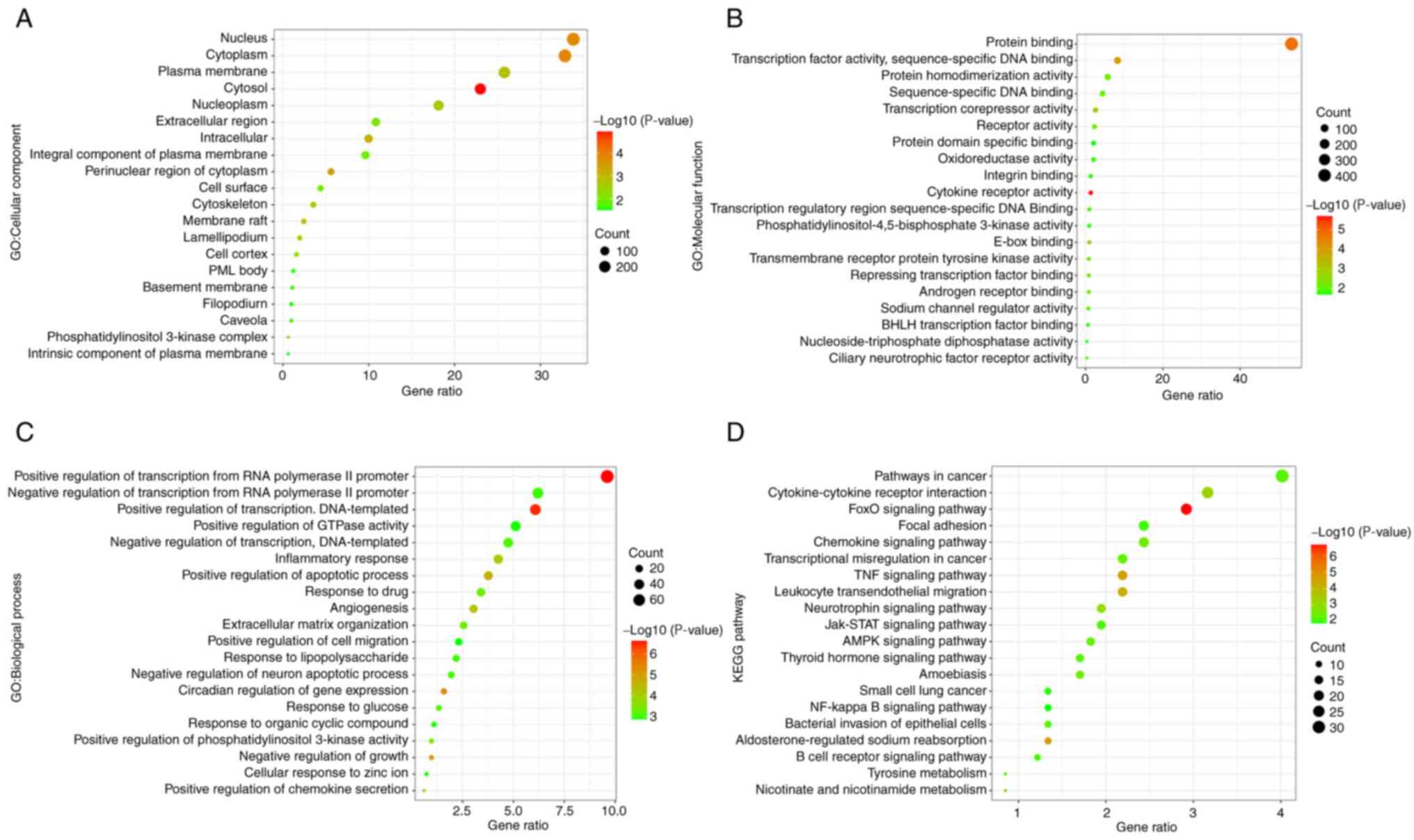 | Figure 3GO and KEGG analysis. The top 20
items in (A) CC, (B) MF and (C) BP. (D) The top 20 items in KEGG
are all ranked according to their P-value. Based on total DEGs, the
total numbers of significant CC, MF, BP and KEGG pathway were 22,
34, 142 and 37, respectively (P<0.05). GO, Gene Ontology; KEGG,
Kyoto Encyclopedia of Genes and Genomes; CC, cell component; MF,
molecular function; BP, biological processes. |
Based on upregulated DEGs, there were 18 significant
KEGG items (P<0.05) among the 31 items in Table II. The FoxO signaling pathway had
the lowest P-value and the second highest gene ratio in KEGG. Based
on downregulated DEGs, further KEGG study showed that there was a
total of 16 items in which the 10 had significant differences
(P<0.05) in Table I. The TNF
signaling pathway had the lowest P-value and the second highest
gene ratio.
 | Table IIThe 18 KEGG pathways based on
upregulated DEGs. |
Table II
The 18 KEGG pathways based on
upregulated DEGs.
| Term | Description | Count in gene
set | Gene ratio | P-value |
|---|
| hsa04068 | FoxO signaling
pathwaya | 16 | 3.101 |
1.948x10-5 |
| hsa04960 |
Aldosterone-regulated sodium
reabsorption | 8 | 1.550 |
1.888x10-4 |
| hsa04060 | Cytokine-cytokine
receptor interaction | 19 | 3.682 |
6.581x10-4 |
| hsa04722 | Neurotrophin
signaling pathway | 11 | 2.132 |
4.605x10-3 |
| hsa04062 | Chemokine signaling
pathway | 14 | 2.713 |
6.059x10-3 |
| hsa05100 | Bacterial invasion
of epithelial cells | 8 | 1.550 |
1.145x10-2 |
| hsa00760 | Nicotinate and
nicotinamide metabolism | 5 | 0.969 |
1.251x10-2 |
| hsa04710 | Circadian
rhythm | 5 | 0.969 |
1.578x10-2 |
| hsa05202 | Transcriptional
misregulation in cancer | 12 | 2.326 |
1.677x10-2 |
| hsa04064 | NF-kappa B
signaling pathway | 8 | 1.550 |
1.997x10-2 |
| hsa04662 | B cell receptor
signaling pathway | 7 | 1.357 |
2.156x10-2 |
| hsa04670 | Leukocyte
transendothelial migrationa | 9 | 1.744 |
2.916x10-2 |
| hsa04910 | Insulin signaling
pathway | 10 | 1.938 |
3.079x10-2 |
| hsa04923 | Regulation of
lipolysis in adipocytes | 6 | 1.163 |
3.150x10-2 |
| hsa04015 | Rap1 signaling
pathway | 13 | 2.519 |
3.476x10-2 |
| hsa04630 | Jak-STAT signaling
pathway | 10 | 1.938 |
4.038x10-2 |
| hsa04360 | Axon guidance | 9 | 1.744 |
4.809x10-2 |
| hsa04666 | Fc gamma R-mediated
phagocytosis | 7 | 1.357 |
4.971x10-2 |
Cytotoxicity results of Bud and
LPS
Fig. 4A shows that
Bud inhibited the growth of RAW 264.7 cells in the range of 40-80
µM (cell viability at 40 µM: 90.5±4.5 and 80 µM: 83.8±8.0%) and had
significant differences compared with 0 µM Bud (100.3±4.0%; P=0.009
and P=0.006). Fig. 4A also shows
that the viability of MPC-5 cells treated with 80 µM Bud was
(86.3±7.5)%, which was significantly different from that of cells
treated with 0 µM Bud (101.4±9.7%; P=0.005). The above results
suggested that Bud at 5, 10 and 20 µM could be used to test its
effects on macrophage polarization and podocyte injury.
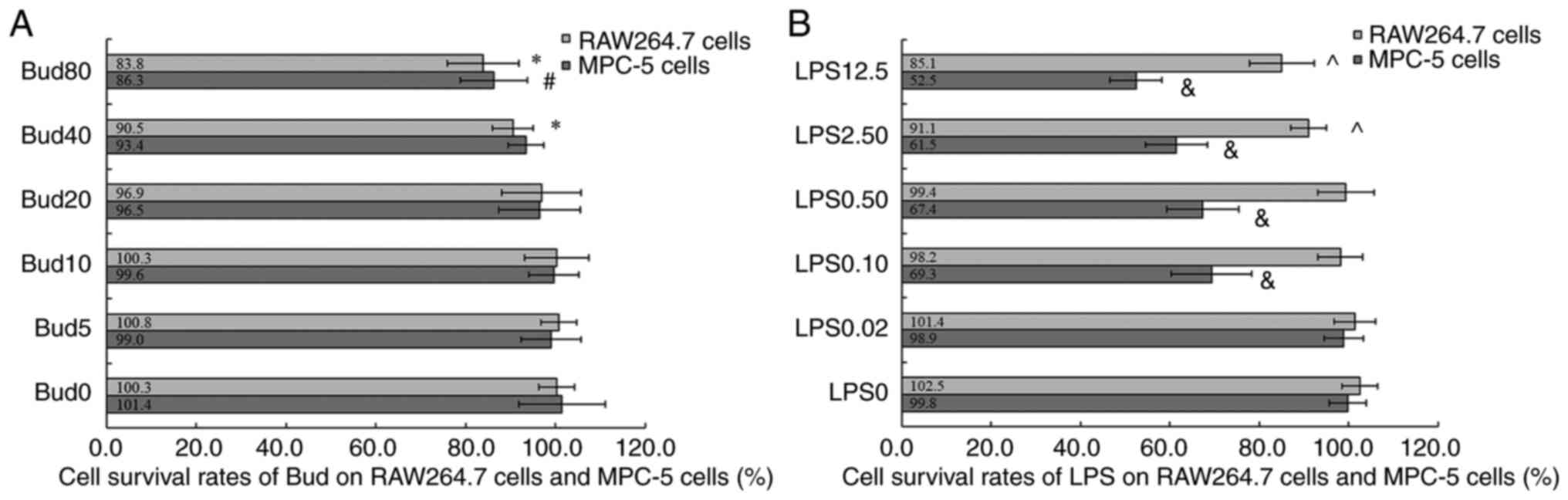 | Figure 4Cytotoxicity of Bud and LPS on
RAW264.7 macrophage cells and MPC-5 podocyte cells. (A) Bud. (B)
LPS. Values are expressed as the mean ± SD (n=6).
*P<0.05, vs. Bud0 in RAW264.7 cells.
#P<0.05, vs. Bud0 in MPC-5 cells.
^P<0.05, vs. LPS0 in RAW264.7 cells.
&P<0.05, vs. LPS0 in MPC-5 cells. Bud,
budesonide; LPS, lipopolysaccharide; Bud0, Bud 0 µM; Bud5, Bud 5
µM; Bud10, Bud 10 µM; Bud20, Bud 20 µM; Bud40, Bud 40 µM; Bud80,
Bud 80 µM; LPS0, lipopolysaccharide 0 µg/ml; LPS0.02,
lipopolysaccharide 0.02 µg/ml; LPS0.10, lipopolysaccharide 0.10
µg/ml; LPS0.50, lipopolysaccharide 0.50 µg/ml; LPS2.50,
lipopolysaccharide 2.50 µg/ml; LPS12.5, lipopolysaccharide 12.5
µg/ml. |
Fig. 4B shows that
LPS inhibited the growth of RAW 264.7 cells at 2.50 µg/ml and 12.5
µg/ml (cell viability: 91.1±4.0 and 85.1±7.2%) and had a
significant difference compared with 0 µg/ml LPS (P=0.010 and
P=0.008). According to the above results, LPS at 0.02, 0.10 and
0.50 µg/ml could be used on RAW 264.7 cells to induce M1
polarization.
The cell survival rates of MPC-5 cells treated with
different concentrations of LPS are shown in Fig. 4B. The results showed that compared
with 0 µg/ml LPS, LPS had significant cytotoxicity on MPC-5 cells
at 0.10, 0.50 µg/ml, 2.50 and 12.5 µg/ml (four: P<0.01). In this
case, 0.10, 0.50, 2.50 and 12.5 µg/ml LPS could be used on MPC-5
cells to establish a podocyte injury model.
Establishment of LPS-induced M1
polarization
Compared with NO production in 0 µg/ml LPS (normal
control; 3.3±0.3 µM), NO production significantly increased after
macrophages were cultured with 0.10 µg/ml LPS for 24 h (77.6±1.5
µM; P<0.001).
When Bud was added to RAW264.7 cells and cultured
for 24 h separately, the results showed that 5, 10 and 20 µM Bud
had no significant effects on NO production compared with the
normal control (4.0±0.1, 4.5±0.6, 4.0±0.2 µM; three: P=NS).
Additionally, compared with the normal control, curcumin did not
increase NO production significantly (4.3±0.2 µM; P=NS).
Effects of Bud on iNOS, TNF-α, Arg-1
and CD206 protein expression in M1 macrophages
Fig. 5A and
B shows the CD206, Arg-1, TNF-α
and iNOS protein bands of the normal, LPS model, curcumin and Bud
at three different concentrations.
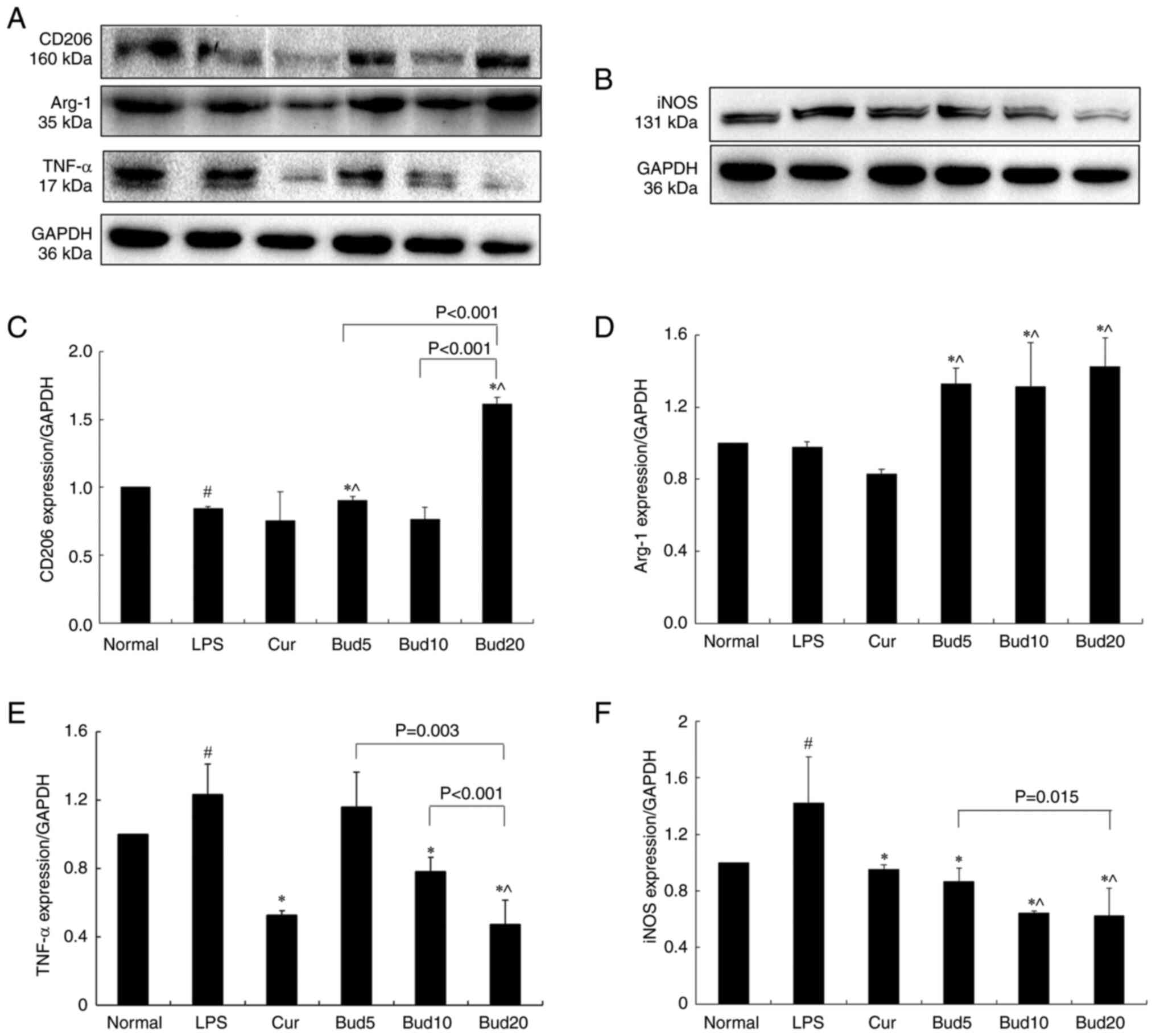 | Figure 5Effects of Bud on CD206, Arg-1, TNF-α
and iNOS protein expression in LPS-induced RAW264.7 cells. (A)
CD206, Arg-1 and TNF-α protein expression. (B) iNOS protein
expression. The results of CD206, Arg-1, TNF-α and iNOS are
represented in (C), (D), (E) and (F), respectively. All results
were expressed as a ratio with respect to the control and
represented as the mean ± SD (n=3). #P<0.05, LPS vs.
normal. *P<0.05, Cur and Bud vs. LPS. ^P<0.05, Bud
vs. normal. Bud, budesonide; CD206, mannose receptor; Arg-1,
arginase-1; iNOS, inducible nitric oxide synthase; LPS,
lipopolysaccharide; Bud5, Bud 5 µM; Bud10, Bud 10 µM; Bud20, Bud 20
µM; Cur, curcumin. |
Compared with the normal control, LPS significantly
increased TNF-α and iNOS protein expression (P=0.025; P=0.025)
while decreasing Arg-1 and CD206 protein expression (CD206:
P<0.001). As the positive control, curcumin significantly
decreased TNF-α and iNOS protein expression compared with the LPS
model (P=0.004; P=0.013). The above results are shown in Fig. 5.
The results also showed the effects of 5, 10 and 20
µM Bud on CD206, Arg-1, TNF-α and iNOS protein expression, as shown
in Fig. 5C-F. Compared with the
LPS model, 5 µM Bud significantly increased Arg-1 and CD206
expression (P<0.001; P=0.002) and decreased iNOS expression
(P=0.021) and 10 µM Bud significantly increased Arg-1 expression
(P=0.030) and decreased TNF-α and iNOS expression (P=0.024;
P=0.002). Among the three concentrations, Bud at 20 µM not only
showed a significant effect on the above four protein expression
levels when compared with the LPS model (CD206: P<0.001, Arg-1:
P<0.001, TNF-α: P<0.001 and iNOS: P=0.004) but also showed
significantly improved effects on CD206, TNF-α and iNOS than the
other two concentrations (all: P<0.05).
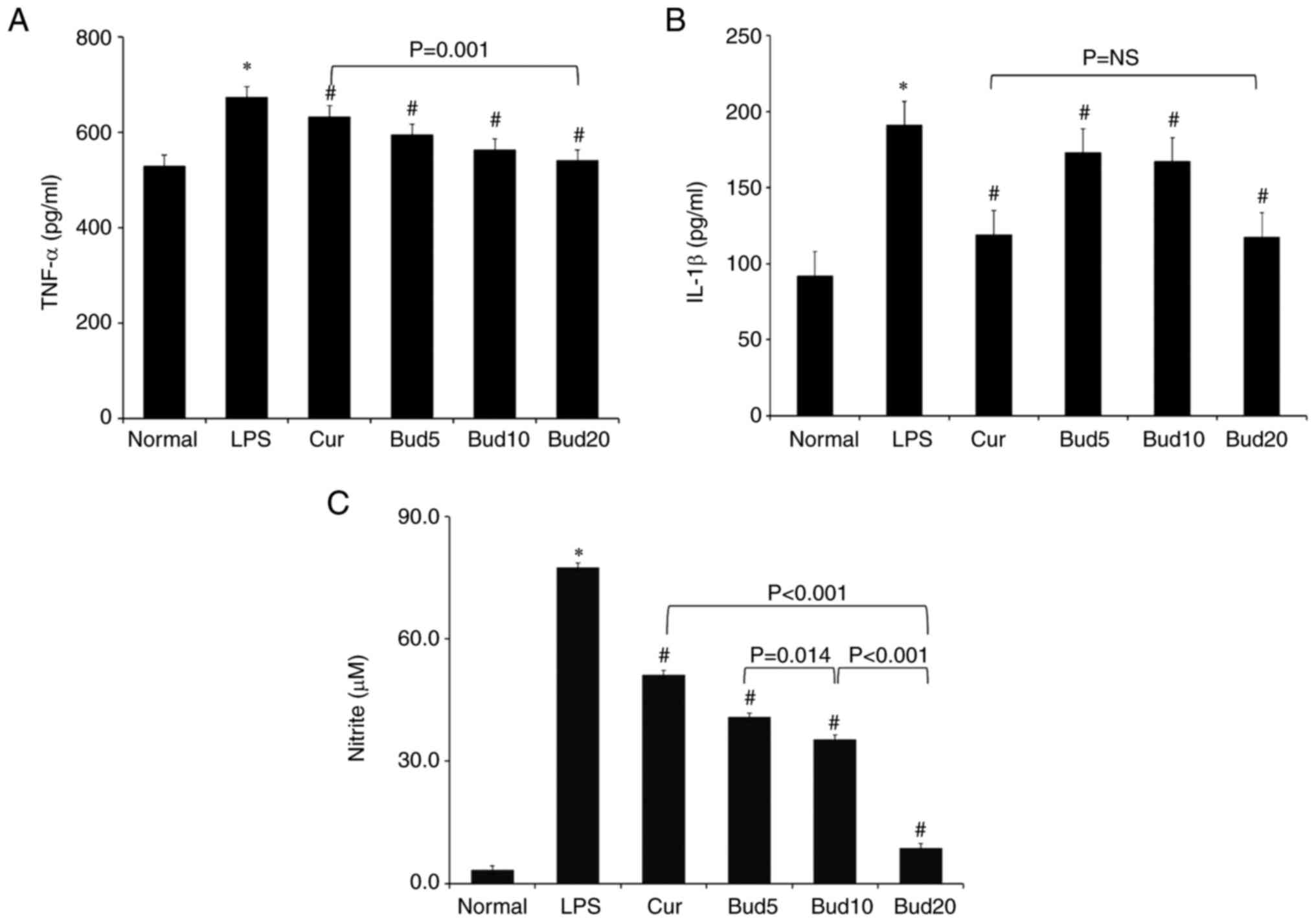
Effects of Bud on TNF-α, IL-1β and NO
levels in M1 macrophages
The 0.10 µg/ml LPS significantly stimulated TNF-α
levels (677.5±23.5 pg/ml vs. 530.8±27.0 pg/ml; P=0.003), IL-1β
levels (188.8±14.6 pg/ml vs. 89.4±6.7 pg/ml; P<0.001) and NO
production (77.6±1.5 µM vs. 3.3±0.3 µM; P<0.001) compared with
the normal group. When compared with the LPS group, the positive
curcumin significantly decreased the TNF-α level to (627.2±23.3
pg/ml; P=0.039), significantly decreased the IL-1β level to
(122.6±6.0 pg/ml; P=0.006) and significantly decreased NO
production to (51.2±1.0 µM; P<0.001).
Compared with the LPS group, Bud at 5, 10 and 20 µM
all showed significant decreasing effects on IL-1β levels and NO
production (six: P<0.05). The decreasing effects of Bud on NO
production occurred in a significant dose-dependent manner
(40.8±1.2 µM NO production at 5 µM vs. 35.4±0.7 µM NO production at
10 µM vs. 8.8±0.3 µM NO production at 20 µM; three: P<0.05).
Additionally, compared with the LPS group, Bud at 10 and 20 µM
showed significant effects on decreasing TNF-α levels (P=0.026;
P<0.001).
Bud at 20 µM showed a significantly improved
decreasing effect on NO production and TNF-α levels than curcumin
(P<0.001 and P=0.001), but Bud at 20 µM and curcumin did not
show a significant difference in decreasing IL-1β levels (P=NS).
All of the above results are shown in Fig. 6.
The results of Bud on LPS-induced
podocyte injury
Fig. 7 shows that
the MPC-5 cell survival rates between normal control and LPS model
were significantly different (P<0.001). Compared with LPS,
(LPS)MS further decreased the cell survival rate significantly
(54.6±5.2% vs. 66.1±7.5%; P=0.001).
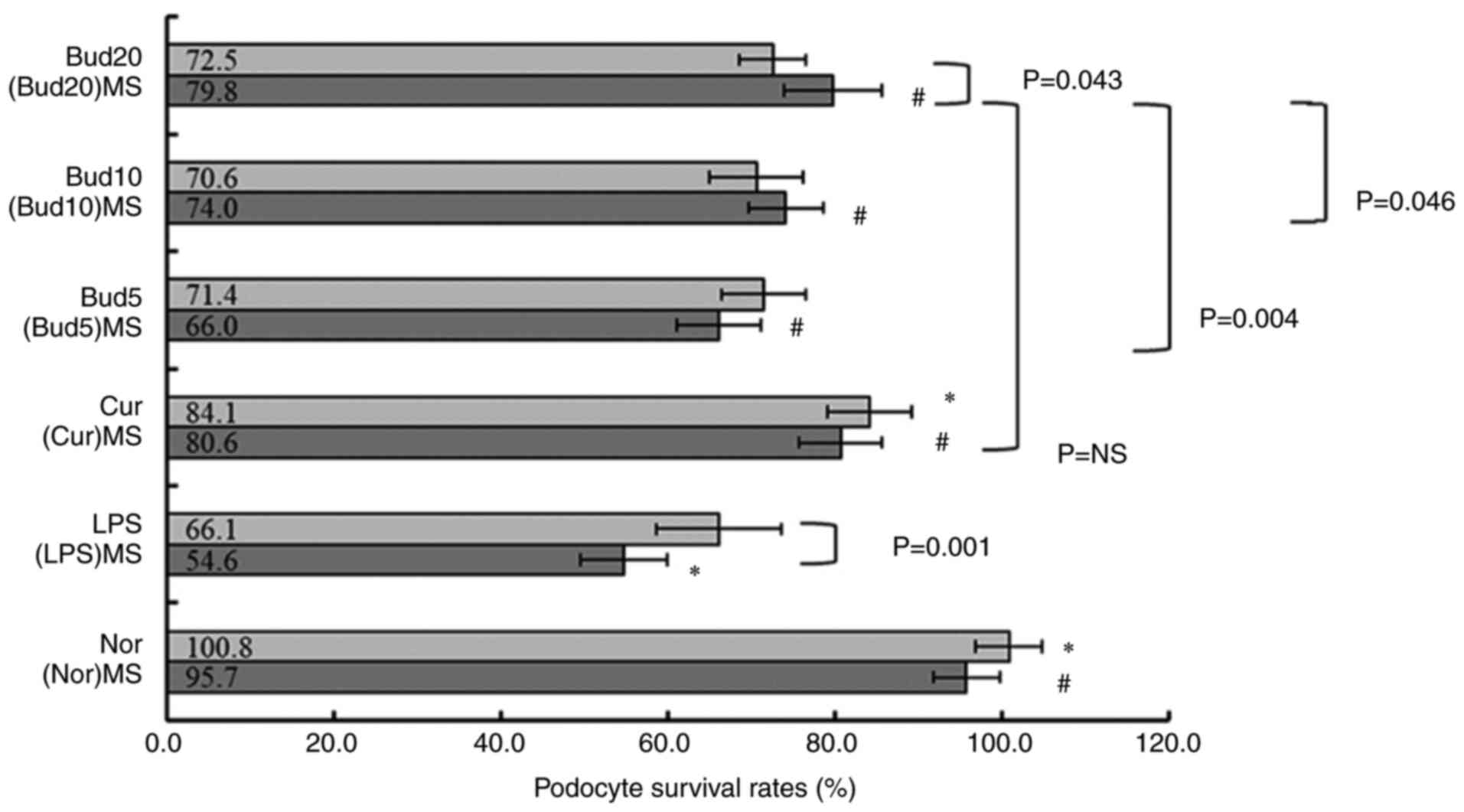 | Figure 7Cell survival rates of LPS-treated
and macrophage supernatant-treated podocytes treated with Bud.
Values are expressed as the mean ± SD of the mean (n=6).
*P<0.05, vs. LPS. #P<0.05, vs. LPS(MS).
LPS, lipopolysaccharide; Bud, budesonide; Nor, normal control;
(Nor)MS, supernatants from normal-cultured macrophages; (LPS)MS,
supernatants from LPS-cultured macrophages; Cur, curcumin, positive
control; (Cur)MS, supernatants from Cur 10 µM and LPS-cultured
macrophages; Bud5, Bud 5 µM; (Bud5)MS, supernatants from Bud 5 µM
and LPS-cultured macrophages; Bud10, Bud 10 µM; (Bud10)MS,
supernatants from Bud 10 µM and LPS-cultured macrophages; Bud20,
Bud 20 µM; (Bud20)MS, supernatants from Bud 20 µM and LPS-cultured
macrophages. |
Additionally, compared with LPS, curcumin
significantly increased the cell survival rate (84.1±5.0%;
P=0.003), but Bud at 5, 10 and 20 µM did not show any significant
influence on the cell survival rate under LPS stress (three:
P=NS).
Compared with (LPS)MS, (Cur)MS (80.6±4.9%; P=0.001)
and (Bud5)MS (66.0±5.0%; P=0.027), (Bud10)MS (74.0±4.5%; P=0.002),
(Bud20)MS (79.8±5.8%; P=0.001) all showed significantly increased
effects on cell survival rates. The increasing effect on the cell
survival rate of (Bud20)MS was significantly improved compared with
that of (Bud10)MS and (Bud5)MS (P=0.046; P=0.004), but there was no
significant difference compared with that of (Cur)MS (P=NS).
Discussion
Glucocorticoids, including Bud, exert their
activities by two main mechanisms of action: the classic genomic
effects with a time lag of 4-24 h (25) and secondary nongenomic effects with
a rapid action (25,26). The genomic effects develop in the
cytoplasm and Bud binds to a specific receptor, forming a complex
that enters the nucleus. This complex binds to specific
glucocorticoid response elements in genes and increases the
expression of anti-inflammatory proteins (25).
Based on the above pharmacological activities in
Fig. 1, the bioinformation results
in Fig. 3 could be understood
easily: The highly regulated gene ratios in the nucleus, cytoplasm,
plasma membrane and nucleoplasm in CC and the main regulated MFs
include protein binding, transcription factor activity,
sequence-specific DNA binding, protein homodimerization activity
and receptor activity.
In vitro research shows that transcriptomic
changes in response to glucocorticoid exposure are similar in
airway smooth muscle derived from donors with fatal asthma and
donors without asthma, with enriched ontological pathways that
included cytokine- and chemokine-related categories (27). A clinical study shows that an
inhaled dose of Bud induced genes involved in transcription to
enhance anti- and proinflammatory effector genes (9). With GSE83233 information, the present
study confirmed the relationships among the positive/negative
regulation of transcription from RNA/DNA and the inflammatory
response in BP results.
As an anti-inflammatory agent, Bud participates in a
number of inflammatory pathways, such as NF-κB signaling in
cigarette smoke-induced airway inflammation rats (28), FoxO and PI3K-Akt signaling in
primary human bronchial epithelial cells and the TNF signaling
pathway (29). All these
inflammatory signals are shown in Fig.
3D. The present study indicates that the TNF signaling pathway
is important for the anti-inflammatory effects of Bud.
Clinically, montelukast sodium plus inhaled Bud has
efficacy in pediatric asthma (30), in children with cough variant
asthma (31) and in elderly
patients with asthma (32). All
these efficacies are related to the effects of Bud on decreasing
TNF-α levels (30-32).
Animal research shows that Bud markedly attenuates pathological
injury in mice with acute lung injury (ALI) and reduced TNF-α and
IL-1β levels in the bronchoalveolar lavage fluid (BALF) and serum
of mice with ALI. Additionally, Bud obviously reduced the numbers
of macrophages in the BALF of mice with ALI (33).
Macrophages are the most abundant immune cells in
the lung (~70% of all immune cells) and they serve important roles
in environmental allergen-induced airway inflammation in asthma.
Activated macrophages commonly exist in two distinct subtypes: the
protective roles of M2 macrophages and pathogenic roles of M1
macrophages have been discussed in a number of lung diseases,
including asthma and COPD (34,35).
Macrophage polarization to the M1 subtype is an important
specifying feature of inflammation, which is involved in the
progression of pulmonary inflammation and secretion of TNF-α
(36).
To date, some research on airway inflammation, M1/M2
macrophage polarization and secretion of TNF-α have been reported
in asthmatic and COPD animal models and in vitro (37-39).
Some in vitro M1/M2 research is also performed in human
primary monocyte-derived macrophages (40) and RAW264.7 cells (35,41).
According to the current in vivo and in vitro M1/M2
research in lung diseases, iNOS (36) and TNF-α (36,39,40)
are important indicators of the M1 subtype and Arg-1(36) and CD206 (35,36)
are indicators of the M2 subtype.
The present study confirmed that the increased
anti-inflammatory protein expression of Bud in LPS-induced
macrophages was related to its regulatory effects on M1/M2
polarization. To the best of the authors' knowledge, the present
study is the first to explore the effects of Bud on Arg-1 and CD206
protein expression. Bud further decreased TNF-α, IL-1β and NO
levels via M1/M2 polarization.
As aforementioned, podocyte injury is a key factor
associated with proteinuria in IgAN (5), in which inflammation-induced podocyte
injury serves an important role (42). To establish an in vitro
podocyte injury model, the specific LPS concentration able to
induce podocyte injury was determined with a CCK-8 assay before
sampling and testing. According to studies, podocyte injury can be
induced by LPS at concentrations ranging from 0.1 to 10 µg/ml and
the survival rate of MPC-5 ranges from 25 to 50% (18-20).
As a positive control in the present study, curcumin showed
significant protection against 0.1 µg/ml LPS-induced podocyte
injury.
Curcumin has been reported to directly protect
against podocyte injury in vitro and in vivo. The
podocyte-protective effect of curcumin and its effects on the NF-κB
pathway has been confirmed in a doxorubicin-induced conditionally
immortalized mouse podocyte cell line (43). In another in vitro model,
curcumin reverses angiotensin II-induced podocyte injury in a
dose-dependent manner (44).
Curcumin inhibits podocyte cell apoptosis and accelerates cell
autophagy in diabetic nephropathy by regulating
Beclin1/UVRAG/Bcl2(45). Unlike
curcumin, Bud had no direct protective effect on podocyte injury
induced by LPS according to the BrdU assay.
The CCK-8 assay has been used to determine the
proliferation of podocyte cells, including the MPC-5 cell line
(46) and human podocytes
(47). BrdU is a nucleoside analog
of thymidine and its incorporation into DNA during replication
within S-phase of the cell cycle is used to quantify cell
proliferation (48). In rats
administered BrdU to detect cellular proliferation in kidney
injury, immunohistochemical analysis indicates that the number of
BrdU-positive cells is increased following kidney injury (49). In the study of NG2-lineage cells
transdifferentiation following podocyte depletion, the BrdU assay
has also used to detect cell proliferation (50). The GO functional enrichment
analysis in the present study showed that positive/negative
regulation of transcription of DNA-templated were highly enriched
in BP. Nucleus and nucleoplasm were highly enriched in CC and
sequence-specific DNA binding was highly enriched in MF.
A recent identification of the miRNA-mRNA network
and immune-related gene signatures in IgAN by integrated
bioinformatics analysis shows that infiltrating immune cells may
serve significant roles in IgAN pathogenesis and that the numbers
of activated macrophages increase in IgAN (51). It is reported that renal NOD-like
receptor, pyrin domain-containing 3 (NLRP3) inflammasome expression
is significantly increased in IgAN patients compared with normal
control tissues (52).
Dys-glycosylated IgA1 isolated from IgAN patient serum can induce
NLRP3 expression in podocytes and initiate the interaction between
podocytes and macrophage differentiation (52).
A series of cell experiments in the coculture of
macrophages and podocytes (23) or
isolated culture (53) examine
their interaction. The present study showed that (LPS)MS, the
isolated culture of LPS-treated macrophages, induced podocyte
injury. Additionally, podocyte injury was more severe in (LPS)MS
than in LPS.
A mechanistic study demonstrated that Tim-3
aggravates podocyte injury by promoting macrophage activation via
the TNF-α pathway (54). Apart
from TNF-α, a number of inflammatory factors, including IL-1β
(55) and NO (53), take part in the inflammatory
process and contribute to podocyte injury via macrophage
polarization. The present study confirmed that Bud and curcumin
indirectly protected against podocyte injury by modulating M1/M2
polarization via a reduction in TNF-α, NO and IL-1β levels. Since a
number of gene expression changes occur in human airways following
Bud inhalation, the above effects observed in vitro may be
relevant to IgAN treatment of Bud (29).
Based on the foregoing, there may be a connection
between the protection of Bud in podocyte injury and the regulation
of Bud on macrophage polarization via the decreased TNF-α, NO and
IL-1β levels.
As an inflammatory cytokine, TNF-α triggers the
expression of not only inflammatory molecules but also cell
adhesion molecules, including vascular cell adhesion molecule-1
(VCAM-1) (56). VCAM-1 helps
regulate inflammation-associated vascular adhesion and the
transendothelial migration of leukocytes, such as macrophages and T
cells (56). Chronic epithelial
immune activation leads to structural changes in the airways. These
structural changes are driven by cell-autonomous JNK signaling and
the activity of its downstream-acting transcription factor FoxO
(57). This may explain the
simultaneous leukocyte transendothelial migration and FoxO
signaling pathway presented in Tables
I and II.
Long-term use of glucocorticoids in patients will
increase the risk of bone fractures (58). The FoxO signaling pathway might be
associated with the adverse effects of osteoporosis (59,60).
The FoxO family (FoxOs), including FoxO1, FoxO3 and FoxO4, serve
important roles in a number of diseases, such as osteoporosis and
osteoarthritis. Research supports the contention that FoxOs
contribute to the deleterious effects of reactive oxygen species on
the skeleton (59). Inhibiting the
transcription of FoxO activated by oxidative stress through
antioxidative stress and positively regulating the Wnt signaling
pathway can improve the osteogenic differentiation and bone
formation of bone tissue (60). It
might be useful to further explore the influence of Bud on FoxOs:
which one takes part in anti-inflammatory action and which one
causes side effects.
Bud has been one of the most widely used lung
medicines worldwide for a number of years (2). Other than being used to treat the
indications it was originally approved for in clinics, Bud has
provided possibilities for IgAN treatment in recent years (61). IgAN is the most common type of
glomerulonephritis in the world (62). IgAN patients will develop
proteinuria, which will continue to worsen. Podocyte injury under
inflammatory stress is a key factor associated with proteinuria in
IgAN patients (5). Based on a
microarray dataset GSE83233, the TNF-α pathway was identified as an
important signal when Bud exerts its anti-inflammatory effects.
With an in vitro LPS-induced podocyte injury model, the
present study showed that the protective effect of Bud on podocyte
injury under inflammatory stress was related to its modulation of
macrophage polarization. An in vivo study of Bud on renal
injury of IgAN should be conducted in future research.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Beijing Bethune
Charitable Foundation (grant no. SCZ425FN) and Wu Jieping Medical
Foundation (grant no. 320.6750.2021-08-11). The funders had no role
in study design, data collection and analysis, decision to publish,
or preparation of the manuscript.
Availability of data and materials
The in silico datasets generated and/or
analyzed during the current study are available in the GEO
repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE83233.
The in vitro datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
HL and YL designed the study and drafted the
manuscript. XZ performed the experiments and prepared the
manuscript. GW analyzed and interpreted the data. DS, YF and CZ
analyzed the data. YZ performed the statistical analysis. HL and YL
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson SD: Repurposing drugs as inhaled
therapies in asthma. Adv Drug Deliv Rev. 133:19–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tashkin DP, Lipworth B and Brattsand R:
Benefit: Risk profile of budesonide in obstructive airways disease.
Drugs. 79:1757–1775. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fellström BC, Barratt J, Cook H, Coppo R,
Feehally J, de Fijter JW, Floege J, Hetzel G, Jardine AG, Locatelli
F, et al: Targeted-release budesonide versus placebo in patients
with IgA nephropathy (NEFIGAN): A double-blind, randomised,
placebo-controlled phase 2b trial. Lancet. 389:2117–2127.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Floege J, Rauen T and Tang SCW: Current
treatment of IgA nephropathy. Semin Immunopathol. 43:717–728.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chang M, Yang B, Li L, Si Y, Zhao M, Hao
W, Zhao J and Zhang Y: Modified huangqi chifeng decoction
attenuates proteinuria by reducing podocyte injury in a rat model
of immunoglobulin a nephropathy. Front Pharmacol.
12(714584)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ismail G, Obrişcă B, Jurubiţă R, Andronesi
A, Sorohan B, Vornicu A, Sinescu I and Hârza M: Budesonide versus
systemic corticosteroids in IgA Nephropathy: A retrospective,
propensity-matched comparison. Medicine (Baltimore).
99(e21000)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang S, Dong L, Pei G, Jiang Z, Qin A, Tan
J, Tang Y and Qin W: High neutrophil-to-lymphocyte ratio is an
independent risk factor for end stage renal diseases in IgA
nephropathy. Front Immunol. 12(700224)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wakashin H, Heymann J, Roshanravan H,
Daneshpajouhnejad P, Rosenberg A, Shin MK, Hoek M and Kopp JB:
APOL1 renal risk variants exacerbate podocyte injury by increasing
inflammatory stress. BMC Nephrol. 21(371)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leigh R, Mostafa MM, King EM, Rider CF,
Shah S, Dumonceaux C, Traves SL, McWhae A, Kolisnik T, Kooi C, et
al: An inhaled dose of budesonide induces genes involved in
transcription and signaling in the human airways: Enhancement of
anti- and proinflammatory effector genes. Pharmacol Res Perspect.
4(e00243)2016.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Li Y, Zheng D, Shen D, Zhang X, Zhao X and
Liao H: Protective effects of two safflower derived compounds,
kaempferol and hydroxysafflor yellow A, on hyperglycaemic
stress-induced podocyte apoptosis via modulating of macrophage
M1/M2 polarization. Immunol Res. 2020(2462039)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Natour B, Rankin R, McKenna R, McMillan
H, Zhang SD, About I, Khan AA, Galicia JC, Lundy FT and El-Karim
IA: Identification and validation of novel biomarkers and
therapeutics for pulpitis using connectivity mapping. Int Endod J.
54:1571–1580. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sherman BT, Hao M, Qiu J, Jiao X, Baseler
MW, Lane HC, Imamichi T and Chang W: DAVID: A web server for
functional enrichment analysis and functional annotation of gene
lists (2021 update). Nucleic Acids Res. 50:W216–W221.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
The Gene Ontology Consortium. The gene
ontology resource: 20 years and still GOing strong. Nucleic Acids
Res. 47(D1):D330–D338. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Deng X, Gao J and Zhao F: Identification
of differentially expressed genes and pathways in kidney of
ANCA-associated vasculitis by integrated bioinformatics analysis.
Ren Fail. 44:204–216. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res. 47:D590–D595. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Trybus E, Krol G, Obarzanowski T, Trybus
W, Kopacz-Bednarska A, Obarzanowski M and Krol T: In vivo and in
vitro studies on multidirectional mechanism of anti-allergic
activity of budesonide. J Physiol Pharmacol. 68:907–919.
2017.PubMed/NCBI
|
|
17
|
Kim HY, Cheon JH, Lee SH, Min JY, Back SY,
Song JG, Kim DH, Lim SJ and Han HK: Ternary nanocomposite carriers
based on organic clay-lipid vesicles as an effective colon-targeted
drug delivery system: Preparation and in vitro/in vivo
characterization. J Nanobiotechnology. 18(17)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu G, Mo L, Wu C, Shen X, Dong H, Yu L,
Pan P and Pan K: The miR-15a-5p-XIST-CUL3 regulatory axis is
important for sepsis-induced acute kidney injury. Ren Fail.
41:955–966. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peng Y, Liu L, Wang Y, Yao J, Jin F, Tao
T, Yuan H, Shi L and Lu S: Treatment with toll-like receptor 2
inhibitor ortho-vanillin alleviates lipopolysaccharide-induced
acute kidney injury in mice. Exp Ther Med. 18:4829–4837.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang W, Qi R, Li T, Zhang X, Shi Y, Xu M
and Zhu T: Kidney organoids as a novel platform to evaluate
lipopolysaccharide-induced oxidative stress and apoptosis in acute
kidney injury. Front Med (Lausanne). 8(766073)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ying ZH, Li HM, Yu WY and Yu CH: Iridin
prevented against lipopolysaccharide-induced inflammatory responses
of macrophages via inactivation of PKM2-mediated glycolytic
pathways. J Inflamm Res. 14:341–354. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tong C, Wu H, Gu D, Li Y, Fan Y, Zeng J
and Ding W: Effect of curcumin on the non-alcoholic steatohepatitis
via inhibiting the M1 polarization of macrophages. Hum Exp Toxicol.
40 (12_suppl):S310–S317. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang C, Yue Y, Huang S, Wang K, Yang X,
Chen J, Huang J and Wu Z: M2b macrophages stimulate
lymphangiogenesis to reduce myocardial fibrosis after myocardial
ischaemia/reperfusion injury. Pharm Biol. 60:384–393.
2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang HX, Yuan J, Li YF and Li RS:
Thalidomide decreases high glucose-induced extracellular matrix
protein synthesis in mesangial cells via the AMPK pathway. Exp Ther
Med. 17:927–934. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ponticelli C and Locatelli F:
Glucocorticoids in the treatment of glomerular diseases: Pitfalls
and pearls. Clin J Am Soc Nephrol. 13:815–822. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nuñez FJ, Johnstone TB, Corpuz ML,
Kazarian AG, Mohajer NN, Tliba O, Panettieri RA Jr, Koziol-White C,
Roosan MR and Ostrom RS: Glucocorticoids rapidly activate cAMP
production via Gαs to initiate non-genomic
signaling that contributes to one-third of their canonical genomic
effects. FASEB J. 34:2882–2895. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kan M, Koziol-White C, Shumyatcher M,
Johnson M, Jester W, Panettieri RA Jr and Himes BE: Airway smooth
muscle-specific transcriptomic signatures of glucocorticoid
exposure. Am J Respir Cell Mol Biol. 61:110–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang H, Liu B, Jiang S, Wu JF, Qi CH,
Mohammadtursun N, Li Q, Li L, Zhang H, Sun J and Dong JC: Baicalin
ameliorates cigarette smoke-induced airway inflammation in rats by
modulating HDAC2/NF-κB/PAI-1 signalling. Pulm Pharmacol Ther.
70(102061)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mostafa MM, Rider CF, Shah S, Traves SL,
Gordon PMK, Miller-Larsson A, Leigh R and Newton R:
Glucocorticoid-driven transcriptomes in human airway epithelial
cells: Commonalities, differences and functional insight from cell
lines and primary cells. BMC Med Genomics. 12(29)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Y and Wang H: Efficacy of
montelukast sodium chewable tablets combined with inhaled
budesonide in treating pediatric asthma and its effect on
inflammatory factors. Pharmazie. 74:694–697. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen L, Huang M and Xie N: The effect of
montelukast sodium plus budesonide on the clinical efficacy,
inflammation, and pulmonary function in children with cough variant
asthma. Am J Transl Res. 13:6807–6816. 2021.PubMed/NCBI
|
|
32
|
Zhang Y and Li B: Effects of montelukast
sodium plus budesonide on lung function, inflammatory factors, and
immune levels in elderly patients with asthma. Ir J Med Sci.
189:985–990. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dong L, Zhu YH, Liu DX, Li J, Zhao PC,
Zhong YP, Chen YQ, Xu W and Zhu ZQ: Intranasal application of
budesonide attenuates lipopolysaccharide-induced acute lung injury
by suppressing nucleotide-binding oligomerization domain-like
receptor family, pyrin domain-containing 3 inflammasome activation
in mice. J Immunol Res. 2019(7264383)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lu J, Xie L and Sun S: The inhibitor
miR-21 regulates macrophage polarization in an experimental model
of chronic obstructive pulmonary disease. Tob Induc Dis.
19(69)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen Z, Wu H, Shi R, Fan W, Zhang J, Su W,
Wang Y and Li P: miRNAomics analysis reveals the promoting effects
of cigarette smoke extract-treated Beas-2B-derived exosomes on
macrophage polarization. Biochem Biophys Res Commun. 572:157–163.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Su JC, Zhang Y, Chen C, Zhu YN, Ye YM, Sun
YK, Xiang SY, Wang Y, Liu ZB and Zhang XF: Hydrogen regulates the
M1/M2 polarization of alveolar macrophages in a rat model of
chronic obstructive pulmonary disease. Exp Lung Res. 47:301–310.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lin YC, Lin YC, Tsai ML, Tsai YG, Kuo CH
and Hung CH: IL-33 regulates M1/M2 chemokine expression via
mitochondrial redox-related mitophagy in human monocytes. Chem Biol
Interact. 359(109915)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li Q, Lu L, Li X and Lu S: Long non-coding
RNA NKILA alleviates airway inflammation in asthmatic mice by
promoting M2 macrophage polarization and inhibiting the NF-κB
pathway. Biochem Biophys Res Commun. 571:46–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu Y, Gao X, Miao Y, Wang Y, Wang H,
Cheng Z, Wang X, Jing X, Jia L, Dai L, et al: NLRP3 regulates
macrophage M2 polarization through up-regulation of IL-4 in asthma.
Biochem J. 475:1995–2008. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shang Y, Sun Y, Xu J, Ge X, Hu Z, Xiao J,
Ning Y, Dong Y and Bai C: Exosomes from mmu_circ_0001359-modified
ADSCs attenuate airway remodeling by enhancing FoxO1
signaling-mediated M2-like macrophage activation. Mol Ther Nucleic
Acids. 19:951–960. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Han J, Pang X, Zhang Y, Peng Z, Shi X and
Xing Y: Hirudin protects against kidney damage in
streptozotocin-induced diabetic nephropathy rats by inhibiting
inflammation via P38 MAPK/NF-κB pathway. Drug Des Devel Ther.
14:3223–3234. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fan HY, Wang XK, Li X, Ji K, Du SH, Liu Y,
Kong LL, Xu JC, Yang GQ, Chen DQ and Qi D: Curcumin, as a
pleiotropic agent, improves doxorubicin-induced nephrotic syndrome
in rats. J Ethnopharmacol. 250(112502)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yu N, Yang L, Ling L, Liu Y, Yu Y, Wu Q,
Gu Y and Niu J: Curcumin attenuates angiotensin II-induced podocyte
injury and apoptosis by inhibiting endoplasmic reticulum stress.
FEBS Open Bio. 10:1957–1966. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang P, Fang J, Zhang J, Ding S and Gan
D: Curcumin inhibited podocyte cell apoptosis and accelerated cell
autophagy in diabetic nephropathy via regulating
Beclin1/UVRAG/Bcl2. Diabetes Metab Syndr Obes. 13:641–652.
2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shi L, Xiao C, Zhang Y, Xia Y, Zha H, Zhu
J and Song Z: Vitamin D/vitamin D receptor/Atg16L1 axis maintains
podocyte autophagy and survival in diabetic kidney disease. Ren
Fail. 44:694–705. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li M, Liu X and Zhang Z: Hyperglycemia
exacerbates cadmium-induced glomerular nephrosis. Toxicol Ind
Health. 37:555–563. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wadey KS, Somos A, Cross SJ, Reolizo LM,
Johnson JL and George SJ: Monitoring cellular proliferation,
migration, and apoptosis associated with atherosclerosis plaques in
vitro. Methods Mol Biol. 2419:133–167. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu QZ, Chen XD, Liu G and Guan GJ:
Identification and isolation of kidney-derived stem cells from
transgenic rats with diphtheria toxin-induced kidney damage. Exp
Ther Med. 12:1651–1656. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Suzuki T, Eng DG, McClelland AD, Pippin JW
and Shankland SJ: Cells of NG2 lineage increase in glomeruli of
mice following podocyte depletion. Am J Physiol Renal Physiol.
315:F1449–F1464. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wei SY, Guo S, Feng B, Ning SW and Du XY:
Identification of miRNA-mRNA network and immune-related gene
signatures in IgA nephropathy by integrated bioinformatics
analysis. BMC Nephrol. 22(392)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Peng W, Pei GQ, Tang Y, Tan L and Qin W:
IgA1 deposition may induce NLRP3 expression and macrophage
transdifferentiation of podocyte in IgA nephropathy. J Transl Med.
17(406)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ji L, Chen Y, Wang H, Zhang W, He L, Wu J
and Liu Y: Overexpression of Sirt6 promotes M2 macrophage
transformation, alleviating renal injury in diabetic nephropathy.
Int J Oncol. 55:103–115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang H, Xie T, Li D, Du X, Wang T, Li C,
Song X, Xu L, Yi F, Liang X, et al: Tim-3 aggravates podocyte
injury in diabetic nephropathy by promoting macrophage activation
via the NF-κB/TNF-α pathway. Mol Metab. 23:24–36. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liao H, Li Y, Zhang X, Zhao X, Zheng D,
Shen D and Li R: Protective effects of thalidomide on
high-glucose-induced podocyte injury through in vitro modulation of
macrophage M1/M2 differentiation. J Immunol Res.
2020(8263598)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kong DH, Kim YK, Kim MR, Jang JH and Lee
S: Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in
immunological disorders and cancer. Int J Mol Sci.
19(1057)2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wagner C, Uliczka K, Bossen J, Niu X, Fink
C, Thiedmann M, Knop M, Vock C, Abdelsadik A, Zissler UM, et al:
Constitutive immune activity promotes JNK- and FoxO-dependent
remodeling of Drosophila airways. Cell Rep.
35(108956)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Caramori G, Ruggeri P, Arpinelli F, Salvi
L and Girbino G: Long-term use of inhaled glucocorticoids in
patients with stable chronic obstructive pulmonary disease and risk
of bone fractures: A narrative review of the literature. Int J
Chron Obstruct Pulmon Dis. 14:1085–1097. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Almeida M and Porter RM: Sirtuins and
FoxOs in osteoporosis and osteoarthritis. Bone. 121:284–292.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Long Z, Wu J, Xiang W, Zeng Z, Yu G and Li
J: Exploring the mechanism of icariin in osteoporosis based on a
network pharmacology strategy. Med Sci Monit.
26(e924699)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pattrapornpisut P, Avila-Casado C and
Reich HN: IgA nephropathy: Core curriculum 2021. Am J Kidney Dis.
78:429–441. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Barbour SJ, Coppo R, Zhang H, Liu ZH,
Suzuki Y, Matsuzaki K, Katafuchi R, Er L, Espino-Hernandez G, Kim
SJ, et al: Evaluating a new international risk-prediction tool in
IgA nephropathy. JAMA Intern Med. 179:942–952. 2019.PubMed/NCBI View Article : Google Scholar
|