Introduction
Prostate cancer (PC) is one of the most commonly and
frequently diagnosed malignant solid tumours in men. It is the
second most diagnosed cancer worldwide, representing one of the
major causes of death among men in both industrialized countries
and developing countries according to recently published data, with
increases in cases of urinary tract carcinomas, such as penile
carcinoma, having been identified among the developing countries of
Africa, Asia and South America (1,2). The
progression of PC worldwide is expected to grow to almost 2.3
million new cases, and 740,000 deaths, by 2040(1). In Romania, PC is the second most
common diagnosed malignancy, with high incidence numbers compared
with other neoplastic diseases (3), and the second most common cause of
death by cancer in men.
During the course of PC diagnosis, several
laboratory and clinical tests are routinely performed. Screening
tests are frequently used, including the test for prostate-specific
antigen (PSA). Despite its low sensitivity, this screening test is
widely used (4,5) in detecting PC when a 4 ng/ml cut-off
point is used. Furthermore, if the PSA value of the patients falls
within 4-10 ng/ml, also known as the ‘borderline’ or ‘grey-level’,
this poses serious concerns in terms of making the correct
diagnosis (6). Therefore, a
combination of several other diagnostic tests are recommended, such
as digital rectal examination (DRE), prostate health index, the 4k
score, IsoPSA™ (Cleveland Diagnostics) and imaging testing
(7). Considering all these tests,
expanding the pool of biomarkers that contribute to the early and
accurate detection of PC would be of great interest for
researchers, medical staff, and people at risk (8).
In the present study, the possibility of using
glutathione-S-transferase gene P1 (GST-P1), a genetic marker
involved in carcinogen detoxification, antineoplastic product
activation and metabolism of chemotherapeutic agents (9), in patients that are in the ‘grey
area’ of the PSA values was evaluated.
Materials and methods
Patient study
This observational, retrospective study was
conducted on consecutive patients that presented either for control
examination or due to lower urinary tract symptoms (LUTS) at the
Urology Clinic of County Hospital of Constanta between January 2018
and January 2020. A total of 80 patients that met the inclusion
criteria of having a PSA value between 4 and 10 ng/ml were
recruited.
Ultrasound control was conducted in all patients,
with the prostatic volume measured by DRE Afiniti 30-Philips
Ultrasound Machine with a C9-4v transducer probe. For all patients
with abnormal prostate volumes, the PSA level was evaluated using
the electrochemiluminescent immunoassay method (Cobas
INTEGRA® 411 Analyzer). Transrectal ultrasonography with
prostate biopsy was also performed. On the extracted tissue, GST-P1
gene expression was analysed, and histopathological examination was
performed to confirm the diagnosis. The histopathological
examination (hematoxylin-eosin staining) was considered as being
the golden standard for PC diagnosis.
Isolation of genomic DNA from harvested tissue was
performed with the aid of a QIAamp DNA mini kit from Qiagen GmbH,
which combines the selective property of links on a silicon
membrane with a flexible elution volume of 20-100 µl. Isolation of
genomic DNA was performed from small amounts of tumour tissue
biopsies (<10 mg), which were transferred immediately after
harvesting to cryotubes with DNA/RNA shield solution (Zymo Research
Corp.) to preserve the integrity of the genetic material. Sodium
bisulfite conversion of genomic DNA was performed using an EpiTect
Bisulfite Kit (Qiagen GmbH), and subsequently, methylation-specific
PCR was performed using a CpG WIZ GST-P1 Amplification Kit (Merck
KGaA; see below for further details).
According to the results of the histopathological
examination, patients were divided into two groups: Patients with
PC and patients with benign tumours, or benign prostatic
hyperplasia (BPH; control group). The results from the two groups
were compared to identify possible differences in age, prostate
volume, PSA value, environment, LUTS and GST-P1 methylation status.
The diagnostic accuracy of GST-P1 methylation status in these
particular patients for whom the PSA values were inconclusive was
evaluated.
The index test (GST-P1 methylation
status)
The index test (GST-P1 methylation status) can be
methylated or unmethylated. Methylation-specific PCR for GST-P1 was
performed using a CpG WIZ GSTpi Amplification Kit (Merck KGaA),
according to the manufacturer's instructions. Concerning the
protocol, the U Primer Set was defined as that which annealed to
unmethylated DNA that has undergone a chemical modification, the M
Primer Set was that which annealed to methylated DNA, and the W
Primer Set was that which served as a control for efficiency of
chemical modification. The primer sequence was not provided by the
manufacturer, which only specified that the amplified region is
defined as the sequence between the 3'-nucleotide of the sense
primer and the complement of the 3'-nucleotide of the anti-sense
primer for each gene promoter. The nucleotide numbering system was
the one used in the GenBank submission, identified as AY324387 for
GSTpi. For each experiment, the controls provided by the test were
used, namely U control DNA and M control DNA, which were amplified
with their corresponding primer set and served as the controls for
unmethylated and methylated DNA, respectively, and untreated W
genomic control DNA, which was amplified with the W primer set and
served as a control for the efficiency of chemical modification.
The PCR products were electrophoresed on a 2% agarose gel and
visualized with ethidium bromide. Finally, a negative PCR control
(i.e., no DNA) was performed for each set of primers (Figs. S1 and S2).
The specificity and sensitivity of the test were
determined, to yield positive and negative predictive values of the
test. 95% confidence intervals (CI) were calculated to quantify the
statistical precision of the measurements (10). For comparing continuous variables,
the mean and the standard deviation (mean ± SD) are presented, and
comparisons were made using Student's t-test for independent
variables. For comparing proportions, in the case of dichotomous
variables, the χ2 test was used. The summary data for
these variables are presented as proportions. To determine the
relationship between PSA values and the GST-P1 methylation status,
a point-biserial correlation was used. This method represents a
special case of Pearson's product moment correlation applied to a
dichotomous and a continuous variable, as described in IBM
documentation for SPSS (v.19.0). P<0.05 was considered to
indicate a statistically significant difference.
The study received the ethical committee approval
(no. 446/30.03.2018) of the Ethical Committee for Clinical Studies
of the Emergency County Hospital Constanta. Procedures at all
stages of the study were carried out in compliance with the
principles of the Declaration of Helsinki. Informed consent forms
were received from all participants prior to their enrolment in the
study group.
Results
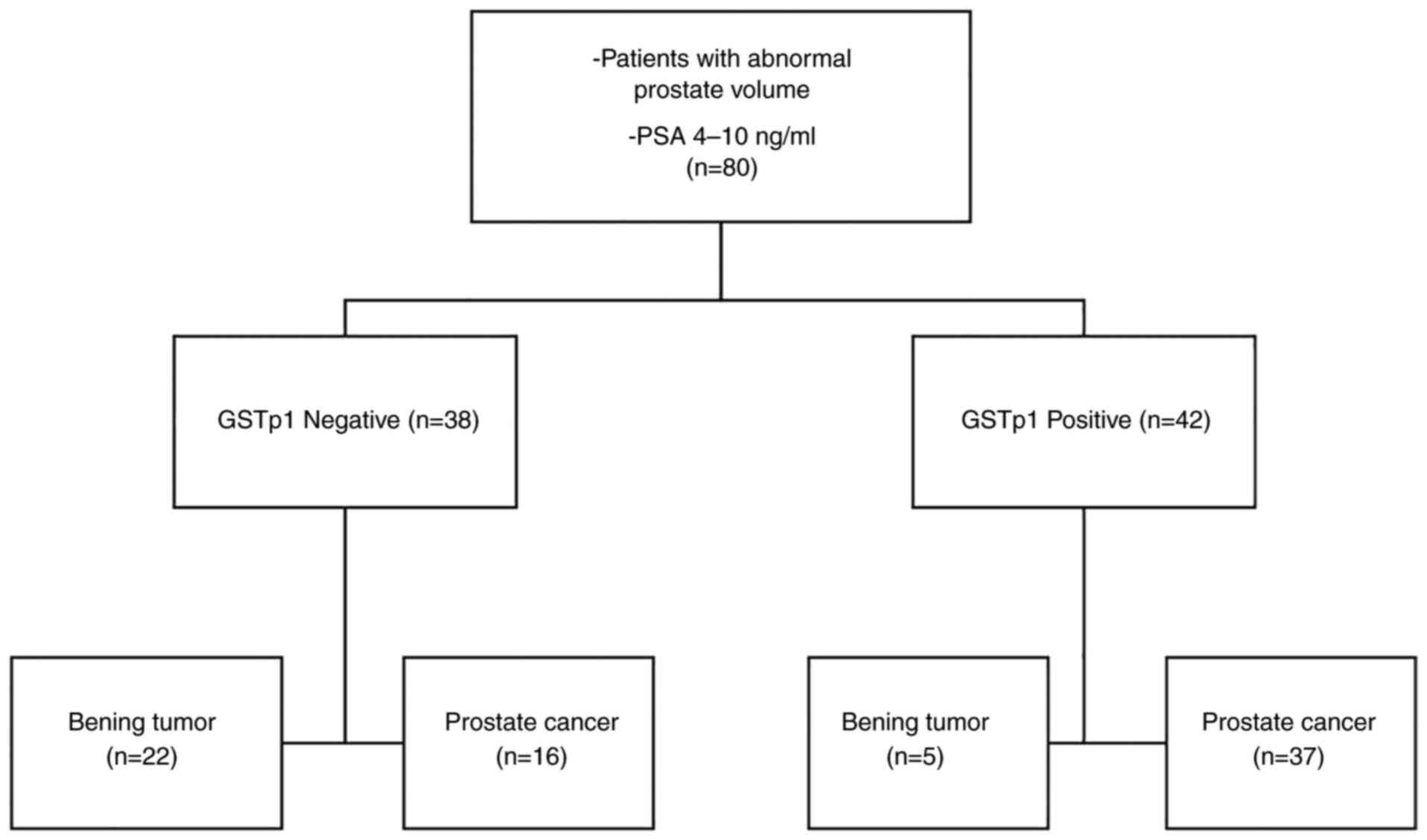
The total number of patients was 80. As the present
study was a retrospective study, tests were performed on all of the
patients, with no dropouts. The main characteristic of the sample
group of patients was that all the participants had PSA values
between 4 and 10 ng/ml. The results of the test are detailed in
Fig. 1.
Subsequently, the characteristics of patients with
PC and those with a benign tumour, or BPH, were analysed (Table I). Patients diagnosed with PC
tended to be older (70.02 years; SD=8.7) compared with patients
with BPH (64.07 years; SD=8.9), and these patients also came
predominantly from urban areas, i.e., a higher percentage of
patients from urban areas were diagnosed with PC. All other
measured parameters, including prostate volume, LUTS and PSA
values, were found not to have statistically significant
differences (all P-values ≥0.5). DRE raised the suspicion of PC in
69.8% of the patients diagnosed with PC, but also raised the
suspicion of malign tumour in 29.6% of the patients with a BPH.
 | Table IDescriptive statistics of the sample
(n=80). |
Table I
Descriptive statistics of the sample
(n=80).
| Variable | Prostate cancer
(n=53) | Benign tumour
(n=27) | P-value |
|---|
| Mean age ± SD
(years) | 70.02±8.70 | 64.07±8.90 | 0.005a,b |
| Mean prostate volume
± SD | 46.579±13.025 | 42.226±13.029 | 0.162b |
| PSA value
(ng/ml) | 7.08±1.81 | 7.13±1.87 | 0.91b |
| Environment
(urban/rural) | 31/22 | 8/19 | 0.015a,c |
| LUTS
(present/absent) | 22/31 | 16/11 | 0.133c |
| Suspicion at digital
rectal exam (yes/no) | 37/16 | 8/19 | 0.001a,c |
| GST-P1 expression
(positive/negative) | 37/16 | 5/22 | 0.001a,c |
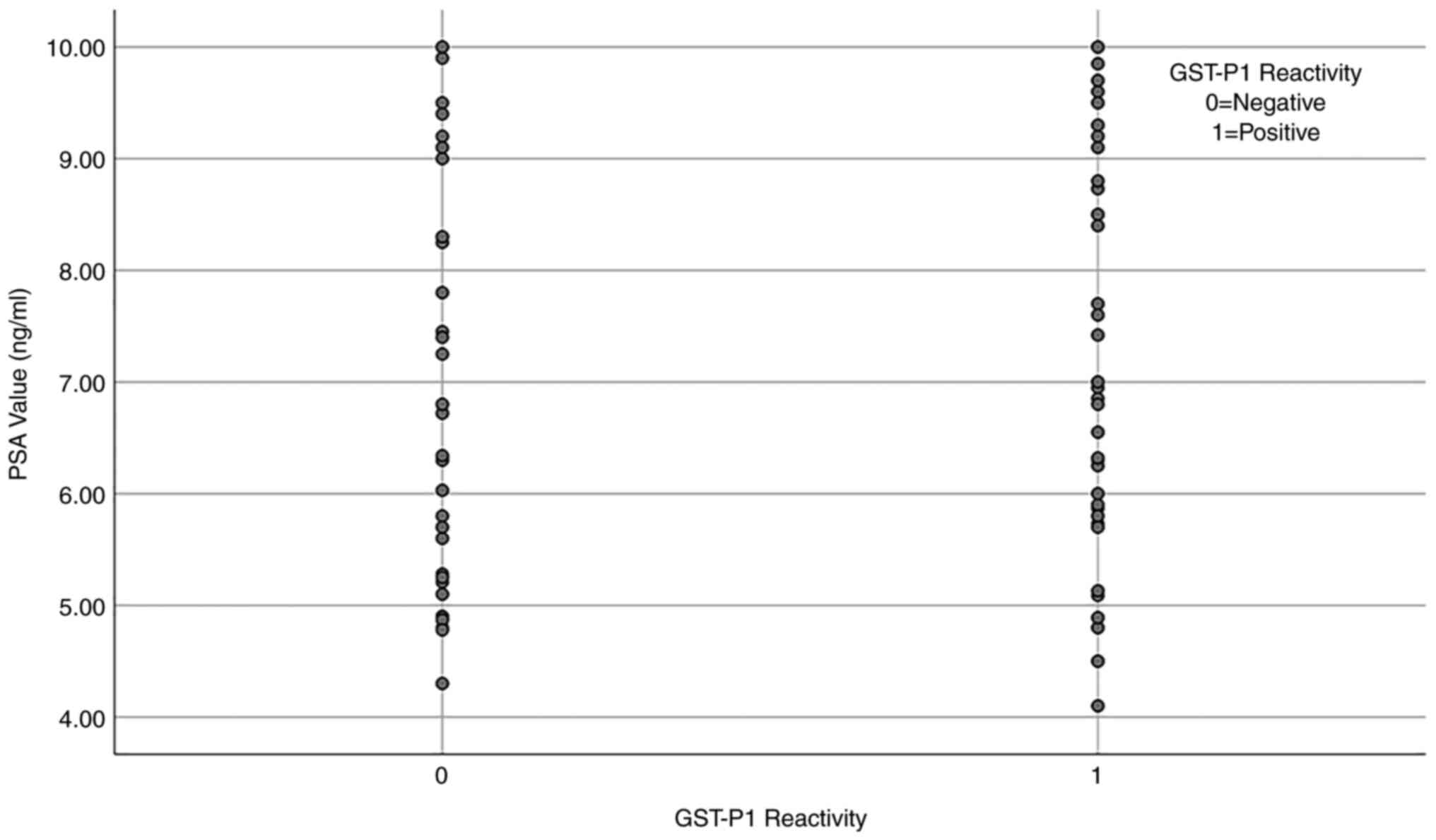
A point-biserial correlation analysis was performed
to determine the relationship between PSA values and GST-P1
methylation status. A positive correlation was identified, although
this was not found to be statistically significant
(rpb=0.081; n=80; p=0.473) (Fig. 2).
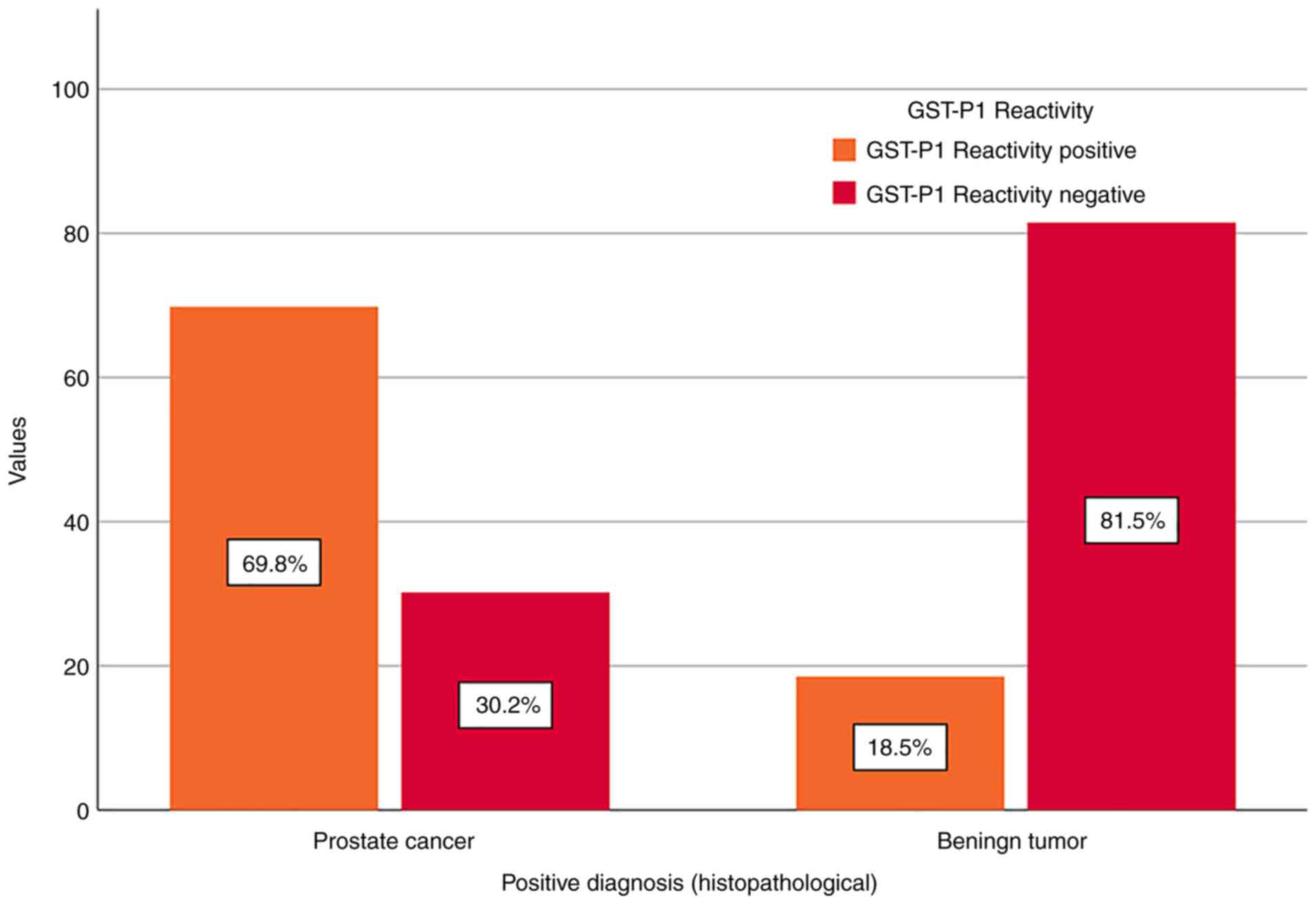
Furthermore, more detailed attention was paid to the
results for GST-P1 reactivity in patients within the grey area of
PSA values. Among the 53 patients diagnosed with PC, 69.8% (n=37)
were GST-P1-positive, whereas, among the 27 patients diagnosed with
BPH, 18.5% (n=5) were GST-P1-positive. The calculated accuracy of
the test was 73.75%, as it correctly identified 37 patients with PC
and 22 patients with BPH (Fig.
3).
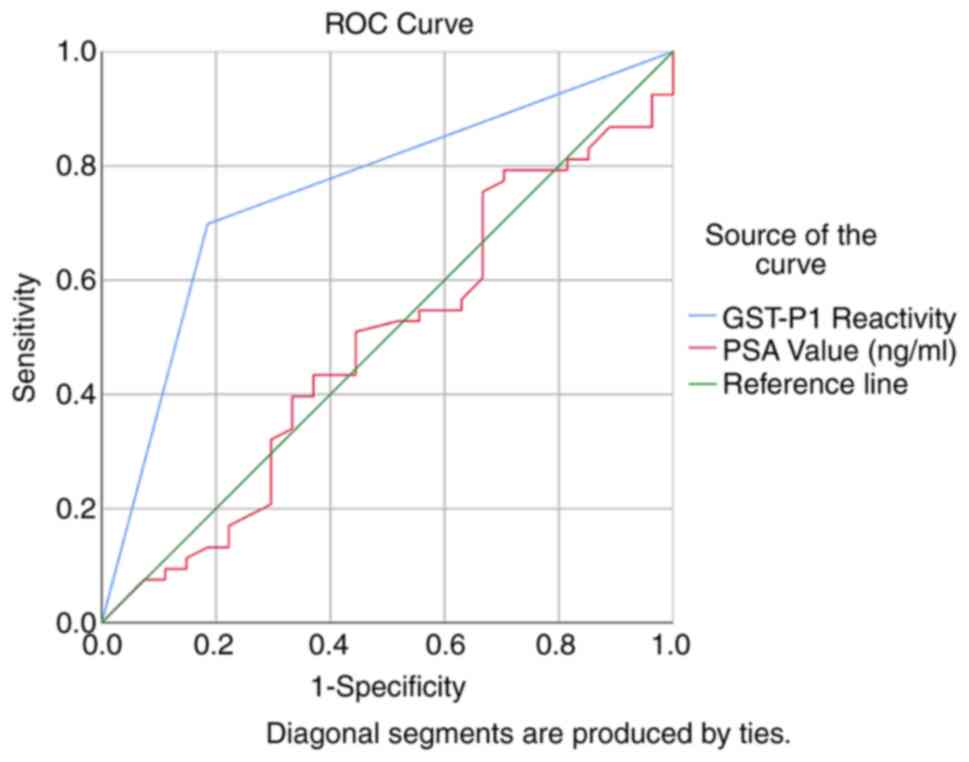
The calculated sensitivity for diagnosing PC in
patients with PSA values between 4 and 10 ng/ml was 69.81% (95% CI,
55.66-81.66%), and the specificity was 81.48% (95% CI,
61.92-93.70%) (Table II). At the
same time, based on the prevalence given by the study population,
the positive predictive value was determined to be 88.1% (95% CI,
74.37-96.02%), and the negative predictive value had a lower value
of 57.89% (95% CI, 40.82-73.69%). The receiver operating
characteristic (ROC) curve was subsequently drawn for GST-P1 and
PSA for the diagnosis of PC (Fig.
4).
 | Table IIScreening test results. |
Table II
Screening test results.
| Variable | Value | 95% CI |
|---|
| Sensitivity | 69.81% | 55.66-81.66% |
| Specificity | 81.48% | 61.92-93.70% |
| AUC | 0.76 | 0.65-0.85 |
| Positive likelihood
ratio | 3.77 | 1.68-8.48 |
| Negative likelihood
ratio | 0.37 | 0.24-0.58 |
| Disease
prevalence | 66.25% | 54.81-76.45% |
| Positive predictive
value | 88.10% | 74.37-96.02% |
| Negative predictive
value | 57.89% | 40.82-73.69% |
Discussion
The present study aimed to evaluate the potential of
using the GST-P1 gene as a biomarker for the diagnosis of PC in
patients for which the PSA value is inconclusive, i.e., within the
‘grey area’, defined as values between 4 and 10 ng/ml. The results
of the analysis indicate that GST-P1 has good potential to
discriminate between patients with PC or BPH. The calculated
sensitivity was 69.81%, whereas the specificity of the test was
81.48%, with a positive predictive value of 88.1% and a negative
predictive value of 57.89%. These results suggest that the
evaluation of GST-P1 in patients for which the PSA is inconclusive
may prove to be useful for diagnosing the presence or absence of
PC, allowing for a faster detection time and treatment
initiation.
Methylation of the GST-P1 gene represents the most
common genetic alteration that is reported in PC (11,12),
being observed in >90% of cases of PC, whereas it is seldom
observed in benign prostate tissue (13). A recently published systematic
review and meta-analysis (14)
estimated that the incidence of GST-P1 methylation was higher in
patients with PC than in those without, with an odds ratio (OR) of
18.58 (95% CI, 9.6-35.35; P<0.001). The detection of GST-P1 was
considered in several studies as a non-invasive diagnostic tool for
early detection of PC (15,16),
being evaluated within meta-analysis (17). The results tend to vary a lot, and,
as determined by Wu et al (17), the pooled specificity of GST-P1 was
found to be excellent (89%; 95% CI, 80-95%) with a lower
sensitivity, of 63% (95% CI, 50-75%). Another meta-analysis that
analysed >35 studies which focused on the usefulness of GST-P1
in PC diagnosis (18) concluded
that the sensitivity for GST-P1 (on biopsies) was 81.7±8.3%, and
the specificity was 95.8±0.6%.
Another recent study suggested that GST-P1 may be
involved in the development and progression of various types of
cancers, fincluding lung cancer, colorectal cancer, gastric cancer,
and even metabolic diseases, with these roles being evaluated in
recent works (19).
Although, in general, research conducted previously
has been carried out on participants that were evaluated for the
presence of PC (and thus the characteristics of the test were
applicable to the general population), the particularity of our
study was the fact that it was focused solely on patients for which
the PSA is inconclusive (within the range of 4-10 ng/ml). This
might explain the lower value of the specificity when compared with
other studies, and also could account for the higher value of the
sensitivity.
Another major difference, which, in the context of
screening purposes may be a limitation of our study, refers to the
method of measuring the methylation status of GST-P1, which was
executed by DNA genomic isolation from the harvested tissue.
Previously published studies (16,20-22)
have indicated that there is a correlation between the detection of
GST-P1 from tissue samples and the methylation status examined from
urine samples, within various limits. Other studies showed
significant differences in the sensitivity and specificity of
GST-P1 for PC, depending on the testing method (23); therefore, new research on the
potential of GST-P1 usage as a screening test in patients within
the ‘grey-area’ of PSA values could bring valuable new information
for the development of novel methods of identifying patients with
PC. Another possible limitation of the present study was the
absence of other methods for determining the level of GST-P1
expression (i.e., immunohistochemistry).
The usage of genetic markers for the diagnosis of
oncological conditions is increasing, as their potential to serve
this purpose is very promising. In the present study, the potential
of GST-P1 marker usage was evaluated in the diagnosis of PC in
patients for which the PSA values were uncertain (within the ‘grey
area’). The results indicated a good sensitivity of 69.8% and a
good specificity of 81.48%, when compared with the golden standard
of diagnosis-histopathological examination. These results have the
potential of sustaining the use of this diagnosis method in
patients for which the suspicion of PC exists, but the PSA values
are inconclusive.
Supplementary Material
GST-P1: 2% Agarose gel analysis of
GST-P1-methylation-specific PCR. L, lane: 100 bp DNA ladder. Lane
1: Wild-type primers with wild-type DNA control; lane 2:
Unmethylated primers with unmethylated DNA control; lane 3:
Methylated primers with methylated DNA control; lanes 4--6:
Experimental sample 1 with wild-type, unmethylated and methylated
primers; lanes 7-9: Experimental sample 2 with wild-type,
unmethylated and methylated primers; lanes 10-12: Experimental
sample 3 with wild-type, unmethylated and methylated primers.
GST-P1, glutathione S-transferase gene P1.
GST-P1: 2% Agarose gel analysis of
GST-P1-methylation-specific PCR. Lane 2: wild-type primers with
wild-type DNA control; lane 3: Unmethylated primers with
unmethylated DNA control; lane 4: Methylated primers with
methylated DNA control; lanes 5-7: Experimental sample 4 with
wild-type, unmethylated and methylated primers; lanes 8-10:
Experimental sample 5 with wild-type, unmethylated and methylated
primers; lanes 11-13: Experimental sample 6 with wild-type,
unmethylated and methylated primers; lane L: 50-100 bp DNA ladder.
GST-P1, glutathione S-transferase gene P1.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MS, VB, DOC, AIS, CT and FV contributed to the
conception and design of this study. MS, APS, LM, AM, CB and DS
were responsible for the data collection and analysis. MS, AIS,
APS, CT and DOC oversaw drafting the manuscript. LM, DS, VB, AM,
CT, CB and FV revised the manuscript critically for important
intellectual content. All authors read and approved the final
version of the manuscript. MS and FV confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of the Clinical County Emergency Hospital ‘St. Andrew’
Constanta (approval no. 446, approval date: 30.03.2018). The
written informed consent was obtained from all subjects. The
research was carried out respecting all the international and
national regulations and in agreement with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Culp MB, Soerjomataram I, Efstathiou JA,
Bray F and Jemal A: Recent global patterns in prostate cancer
incidence and mortality rates. Eur Urol. 77:38–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iorga L, Dragos Marcu R, Cristina Diaconu
C, Maria Alexandra Stanescu A, Pantea Stoian A, Liviu Dorel
Mischianu D, Surcel M, Bungau S, Constantin T, Boda D, et al:
Penile carcinoma and HPV infection (review). Exp Ther Med.
20:91–96. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chirilă S, Rugină S and Broască V:
Neoplastic diseases incidence in constanta county during 2007-2012.
ARS Medica Tomitana. 20:211–214. 2015.
|
|
4
|
Ankerst DP and Thompson IM: Sensitivity
and specificity of prostate-specific antigen for prostate cancer
detection with high rates of biopsy verification. Arch Ital Urol
Androl. 78:125–129. 2006.PubMed/NCBI
|
|
5
|
Ashley T: Using predictive value,
sensitivity and specificity to interpret laboratory tests: PSA for
the diagnosis of prostate cancer. J Insur Med. 37:261–263.
2005.PubMed/NCBI
|
|
6
|
Ross T, Ahmed K, Raison N, Challacombe B
and Dasgupta P: Clarifying the PSA grey zone: The management of
patients with a borderline PSA. Int J Clin Pract. 70:950–959.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mottet N, van den Bergh RCN, Briers E, Van
den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N,
Gandaglia G, Gillessen S, et al: EAU-EANM-ESTRO-ESUR-SIOG
guidelines on prostate cancer-2020 update. Part 1: Screening,
diagnosis, and local treatment with curative intent. Eur Urol.
79:243–262. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brooks D, Olver IN and Esterman AJ: Beyond
PSA testing for prostate cancer. Med J Aust. 208:426–427.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moyer AM, Salavaggione OE, Wu TY, Moon I,
Eckloff BW, Hildebrandt MA, Schaid DJ, Wieben ED and Weinshilboum
RM: Glutathione s-transferase p1: Gene sequence variation and
functional genomic studies. Cancer Res. 68:4791–801.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kornbrot D: Point biserial correlation.
In: Encyclopedia of Statistics in Behavioral Science. Everitt BS
and Howell DC (eds). John Wiley & Sons, Ltd., Hoboken NJ,
2005.
|
|
11
|
Henrique R and Jerónimo C: Molecular
detection of prostate cancer: A role for GSTP1 hypermethylation.
Eur Urol. 46:660–669. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Santric V, Djokic M, Suvakov S,
Pljesa-Ercegovac M, Nikitovic M, Radic T, Acimovic M, Stankovic V,
Bumbasirevic U, Milojevic B, et al: GSTP1 rs1138272 polymorphism
affects prostate cancer risk. Med Lith. 56(128)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bott SRJ, Williamson M and Kirby RS:
Chapter 11-Genetic changes and their prognostic significance in
prostate cancer. In: Prostate Cancer. Mydlo JH and Godec CJ (eds).
Academic Press, Oxford, pp101-112, 2003.
|
|
14
|
Zhou XL, Jiao DC, Dou MM, Chen JJ, Li ZN,
Li YH, Liu J and Han X: . Association of glutathione-S-transferase
p1 gene promoter methylation and the incidence of prostate cancer:
A systematic review and meta-analysis. J Cancer Res Clin Oncol.
145:1939–1948. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Crocitto LE, Korns D, Kretzner L, Shevchuk
T, Blair SL, Wilson TG, Ramin SA, Kawachi MH, Smith SS, et al:
Prostate cancer molecular markers GSTP1 and hTERT in expressed
prostatic secretions as predictors of biopsy results. Urology.
64:821–825. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hoque MO, Topaloglu O, Begum S, Henrique
R, Rosenbaum E, Van Criekinge W, Westra WH and Sidransky D:
Quantitative methylation-specific polymerase chain reaction gene
patterns in urine sediment distinguish prostate cancer patients
from control subjects. J Clin Oncol. 23:6569–6575. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu TY, Giovannucci E, Welge J, Mallick P,
LeMasters G, Tang WY and Ho S: Abstract 2797: Measurement of GST-P1
methylation in body fluids and prostate cancer diagnosis: A
meta-analysis. Cancer Res. 70 (Suppl 8):2797. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Van Neste L, Herman JG, Otto G, Bigley JW,
Epstein JI and Van Criekinge W: The Epigenetic promise for prostate
cancer diagnosis. Prostate. 72:1248–1261. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cui J, Li GQ, Yin J, Li LW, Tan Y, Wei HR,
Liu B, Deng L, Tang J, Chen Y and Yi L: GSTP1 and cancer:
Expression, methylation, polymorphisms and signaling (review). Int
J Oncol. 56:867–878. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jerónimo C, Usadel H, Henrique R, Silva C,
Oliveira J, Lopes C and Sidransky D: Quantitative GSTP1
hypermethylation in bodily fluids of patients with prostate cancer.
Urology. 60:1131–1135. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cairns P, Esteller M, Herman JG,
Schoenberg M, Jeronimo C, Sanchez-Cespedes M, Chow NH, Grasso M, Wu
L, Westra WB and Sidransky D: Molecular detection of prostate
cancer in urine by GSTP1 hypermethylation. Clin Cancer Res.
7:2727–2730. 2001.PubMed/NCBI
|
|
22
|
Voinea F, Mazilu L, Micu IS, Suceveanu AP,
Iliescu M, Dumitru A, Constantin VD, Paunica I and Suceveanu AI:
Modern approaches for antiandrogen-resistant prostate cancer
therapy. J Mind Med Sci. 8(10)2021.
|
|
23
|
Woodson K, O'Reilly KJ, Hanson JC, Nelson
D, Walk EL and Tangrea JA: The usefulness of the detection of GSTP1
methylation in urine as a biomarker in the diagnosis of prostate
cancer. J Urol. 179:508–511. 2008.PubMed/NCBI View Article : Google Scholar
|