Introduction
Pulmonary adenofibroma (PAF) is a rare benign tumor,
with ~40 cases reported worldwide since 1944(1). It is not listed in the 5th edition of
the World Health Organization (WHO) classification of thoracic
tumors (2). Although there is no
unified consensus, it is considered that PAF is a benign tumor
(3). Whether PAF and pulmonary
solitary fibrous tumor (PSFT) are homologous remains controversial,
but differential diagnosis is necessary (4). In most cases, computed tomography
(CT) imaging of PAF shows well-defined, homogeneous and solitary
nodules which cannot be easily differentiated from PSFT. To the
best of our knowledge, the present study is the first report of a
patient with PAF with liquefaction necrosis in the center of the
tumor on CT.
Case report
A 70-year-old man was hospitalized with an inguinal
hernia at The Sixth People's Hospital of Nantong (Nantong, China)
on July 5, 2021. The patient did not have any respiratory symptoms
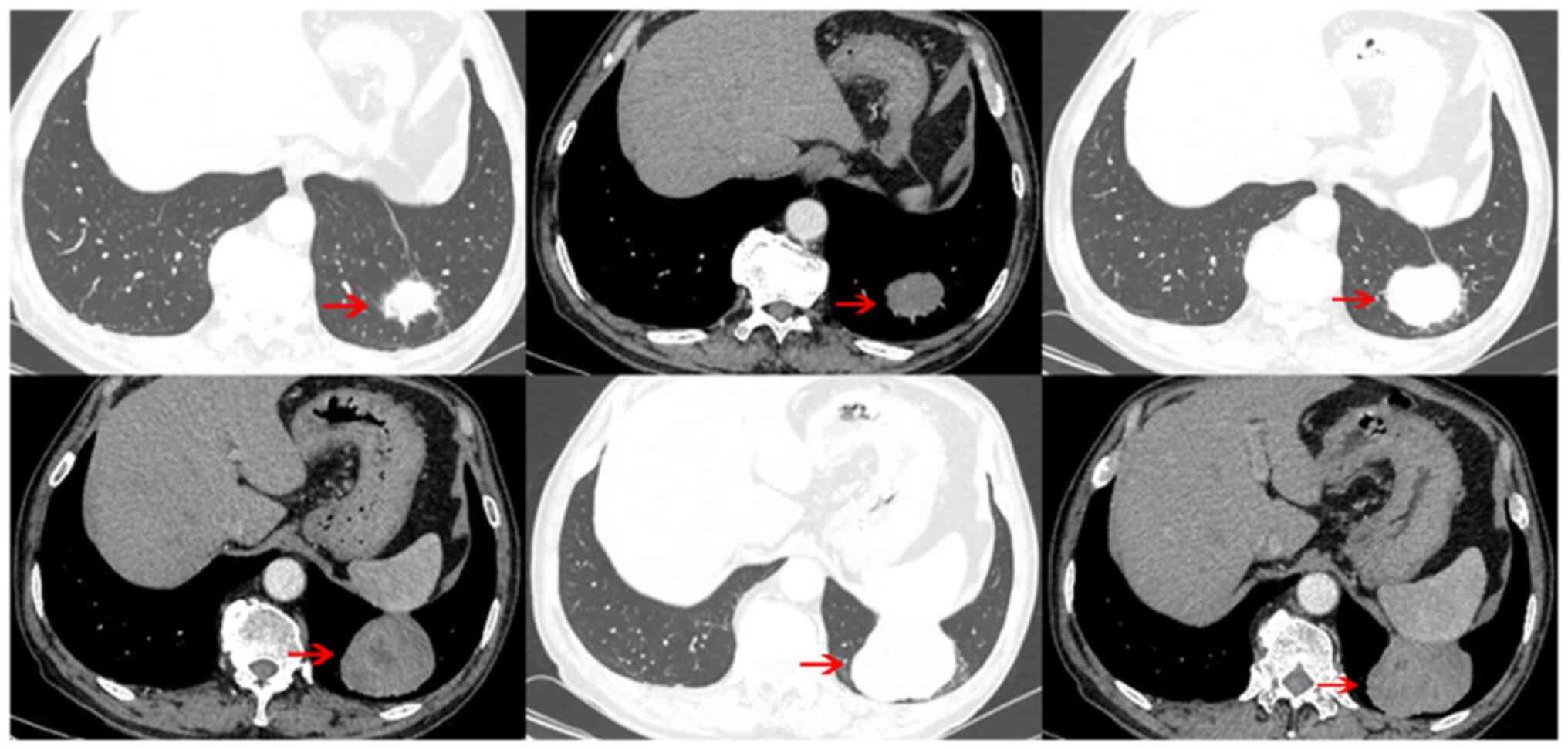
or discomfort. Chest CT scan revealed a tumor (~6.5x5.5x4.4 cm) in
the lower lobe of the left lung, characterized by uneven density
and unclear boundary with the pleura and diaphragm. A
contrast-enhanced scan demonstrated a slightly enhanced lesion with
liquefaction necrosis in the center (Fig. 1). The results of tumor markers,
including α fetoprotein, carcinoembryonic antigen, carbohydrate
antigen 125, carbohydrate antigen 19-9, carbohydrate antigen 72-4,
carbohydrate antigen 50, neuron specific enolase, cytokeratin 19
fragment, pro-gastrin-releasing peptide and serum ferritin, were
within the normal range. Initially, a benign lung tumor was
suspected although malignant tumor could not be ruled out. The
patient and his family refused needle biopsy and preferred surgical
resection of the tumor. Therefore, the patient underwent
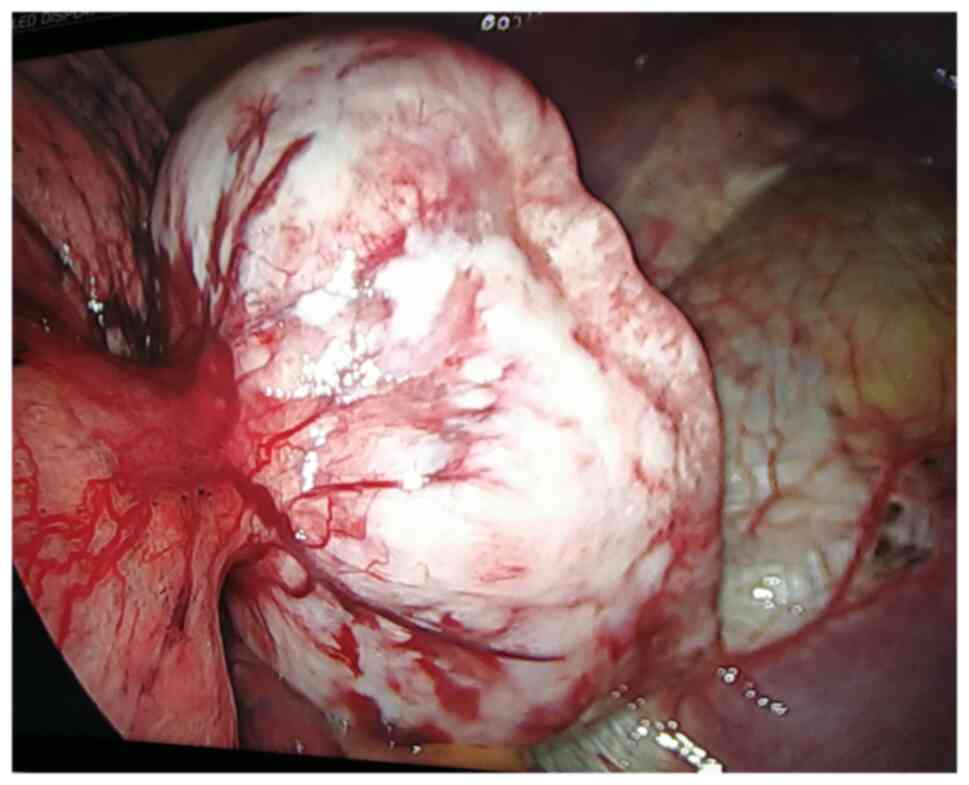
video-assisted thoracic surgery (VATS). An intraoperative
assessment revealed that the tumor was localized at the bottom of
the left lower lobe, with an intact capsule and smooth surface. The
tumor partly invaded the lung tissue and partly adhered to the
diaphragm (Fig. 2). Thus, wedge
resection of the left lung lower lobe was performed to ensure
complete tumor removal via VATS. The result of intraoperative
frozen section showed a benign tumor. Postoperative pathology
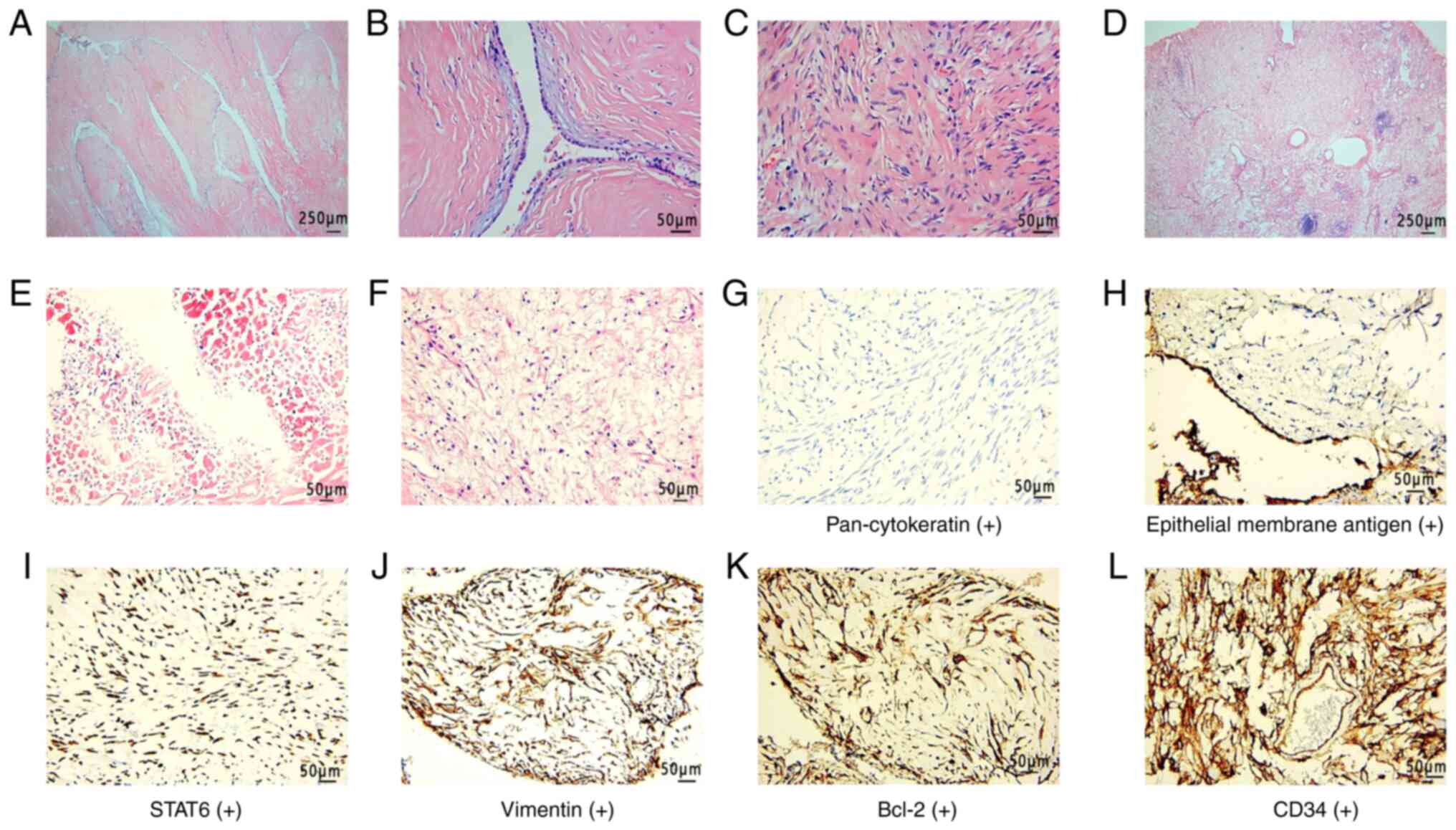
revealed that the tumor comprised epithelial and stromal spindle
cells. The cubic epithelial cells covered the tumor, forming
gland-like fissures. The central tissue of the tumor was decomposed
and liquefied, normal cell structure disappeared and a large number
of foam cells were seen. The epithelial cells only covered the
tumor surface and adenoid fissures. Foam cells were only visible in
part of the central liquefied necrotic area. The majority of the
tumor was composed of stromal cells. Due to the large size of the
tumor, some low-quality sections were generated during the
processing of specimen fixation, pathological sampling and paraffin
embedment. Therefore the analysis of the percentage of epithelial,
stromal and foam cells was affected by large errors.
Immunohistochemistry demonstrated that the epithelial component
stained positive for pan-cytokeratin and epithelial membrane
antigen and the stromal component stained positive for Bcl-2, CD34,
STAT6 and vimentin. The tumor showed negative staining for CD99,
estrogen and progesterone receptor, desmin, S100, smooth muscle
actin and thyroid transcription factor 1 markers (Fig. 3). Tumor tissues were fixed in 10%
neutral buffered formalin at 4˚C for 24 h. Sections were cut from
paraffin-embedded blocks at a thickness of 3 µm. At room
temperature, sections were stained with hematoxylin and eosin for 6
min for histopathological analysis. A Leica Bond MAX automated
immunostainer (Leica Biosystems) was used for immunostaining.
Paraffin-embedded sections were placed in xylene I and xylene II,
respectively, for 10 min. After removing excess fluid, sections
were placed in absolute ethanol I and absolute ethanol II,
respectively, for 2 min, 95% ethanol for 2 min, 75% ethanol for 2
min, distilled water for 2 min and phosphate buffered solution (PH
7.3). Slides were heated in elhylene diamine tetraacetic acid
antigen repair buffer (pH 8.0) for 20 min at 95˚C to retrieve the
antigens. Sections were rinsed with phosphate buffered solution (PH
7.3) three times after natural cooling (2 min per rinse). The
endogenous peroxidase was blocked with 3% hydrogen peroxide for 10
min at room temperature. Sections were incubated with primary
antibodies (ready-to-use) overnight at 4˚C. The endogenous
peroxidase was blocked with 3% hydrogen peroxide for 8 min at room
temperature. Subsequently, sections were incubated with the
secondary antibody (ready-to-use) for 8 min at room temperature.
Chromogen detection was performed using a DAB detection kit
(polymer) (ready-to-use; including endogenous peroxidase,
horseradish peroxidase-conjugated sheep anti-mouse or anti-rabbit
IgG, DAB substrate buffer and DAB chromogen). The primary
antibodies used were as follows: Monoclonal mouse anti-human
pan-cytokeratin (ready-to-use; cat. no. ZM-0069), monoclonal mouse
anti-human epithelial membrane antigen (ready-to-use; cat. no.
ZM-0095), monoclonal mouse anti-human Bcl-2 (ready-to-use; cat. no.
ZM-0536), monoclonal mouse anti-human CD34 (ready-to-use; cat. no.
ZM-0046), polyclonal rabbit anti-human STAT6 (ready-to-use; cat.
no. ZA-0647), monoclonal mouse anti-human vimentin (ready-to-use;
cat. no. ZM-0260), monoclonal mouse anti-human CD99 (ready-to-use;
cat. no. ZA-0577), monoclonal mouse anti-human estrogen receptor
(ready-to-use; cat. no. ZA-0102), monoclonal mouse anti-human
progesterone receptor (ready-to-use; cat. no. ZA-0255), monoclonal
mouse anti-human desmin (ready-to-use; cat. no. ZA-0610),
monoclonal mouse anti-human S100 (ready-to-use; cat. no. ZM-0224),
monoclonal mouse anti-human smooth muscle actin (ready-to-use; cat.
no. ZM-0003) and monoclonal mouse anti-human thyroid transcription
factor 1 markers (ready-to-use; cat. no. ZM-0270) (all purchased
from OriGene Technologies, Inc.). The secondary antibody Bond
Polymer Refine Detection (ready-to-use; cat. no. DS9800-CN) (Leica
Biosystems) was labeled with compact polymer. The sections were
observed using an Olympus BX53 light microscope (Olympus
Corporation; magnification, x40 or x200). The diagnosis of PAF was
based on the epithelial and stromal cells and adenoid structures.
Nine months after the operation, the patient was in good health,
with no recurrence or metastasis.
Discussion
Currently, the pathogenesis of PAF is unclear
(5). Clinical data has shown that
most patients with PAF are middle-aged (1). Because of the slow progression of
PAF, most patients do not show symptoms in the early stages. Suster
and Moran (3) demonstrated that
PAF is a type of immature hamartoma based on its inability to
differentiate into more specialized mature components, such as fat,
smooth muscle or cartilage. Fusco et al (6) proposed that PAF is not only a benign
tumor but a certain type of solitary fibrous tumor (SFT). By
contrast, Lindholm et al (7) suggested that PAF should be not
included in the SFT spectrum due to potential recurrence and
metastasis of SFT. However, careful analysis should be conducted to
distinguish PAF from PSFT because they exhibit a degree of
homology.
PAF comprises stromal and epithelial components and
is characterized by a biphasic growth pattern due to the growth of
these two components (8).
Histopathologically, PAF is characterized by the presence of
stromal cells and gland-like fissures covered with epithelial
cells. PSFT is an intermediate tumor and is listed as a tumor of
fibroblastic/myofibroblastic differentiation in the 5th edition of
WHO classification of thoracic tumors (2). PSFT has a similar stromal composition
to PAF. PSFT consists of dense and sparse areas of cells with
collagen fibers between the two areas (8). Based on these typical features, PAF
was diagnosed in the present case. Atypical histopathological
findings make it difficult to perform a differential diagnosis for
PAF and PSFT. Therefore, immunohistochemistry should be
performed.
In most cases, PAF manifests as well-circumscribed
isolated nodules in the peripheral lung (1). However, fat, cartilage or
calcification do not appear in the lesions (9). In the present case, the enhanced CT
scan showed mild or moderate enhancement because PAF primarily
comprises stromal and epithelial cells with limited blood supply.
In contrast-enhanced CT scan of PSFT, the cell-dense and
vascular-rich areas are significantly enhanced, while the
cell-sparse and low-vascular areas are not significantly enhanced
(10). Based on the aforementioned
facts, we hypothesized that PAF and PSFT present as liquefaction
necrosis of certain tumors due to large tumor size and poor blood
supply. There is evidence of cavitation in PAF in the center of the
tumor (8). These characteristics
make difficult to reach a conclusive diagnosis. Additionally, there
is a need to differentiate PAF from other malignant tumors such as
carcinosarcoma and pleuropulmonary blastoma on CT images (11). Due to non-specific imaging findings
of PAF, the diagnosis and differentiation from other tumors,
especially PSFT, is based on histopathology and
immunohistochemistry (12).
In addition, both PAFs and PSFTs contain stromal
elements, hence both may test positive for CD34, CD31, Bcl-2, CD99
and vimentin (6). PSFT is mainly
positive for STAT-6, CD34 and BCL-2 expression (13). On the other hand, PAF is typically
negative for STAT6 expression, and only a proportion of patients
show positivity for CD34 and Bcl-2. For example, Liang et al
(1) reported patients who showed
positive expression of CD34 (14/33) and Bcl-2 (11/26). In the study
by Lindholm et al (7), 13
patients with PAF showed no expression of CD34 and BCL-2. Analysis
of nuclear expression demonstrated that STAT6 is a highly sensitive
and specific marker of PSFT (14).
Nonetheless, Fusco et al (6) found that PAF is a tumor with the
molecular signature of SFT by finding a fusion gene of NAB2 exon 4
and STAT6 exon 2 in the stromal cells of both tumors. This finding
may explain the positive staining of STAT6 in 5 out of 7 patients
in their study. Similar results were extremely rare in previous
reports. Both the present case report and the case reported by
Sonokawa et al (15)
support the conclusion of Fusco et al.
Asymptomatic patients with small nodules do not
require treatment but regular follow-up (9). For patients with symptoms, large
tumors or difficult diagnosis lung biopsy can be performed
according to the situation and clinical considerations. VATS is an
appropriate approach for the diagnosis and treatment of PAF based
on the present case.
Although biliary adenofibroma is associated with
malignant transformation and lung metastasis (16), to the best of our knowledge there
is no report of PAF metastasis or recurrence.
CT manifestations of PAF are not specific. Large PAF
may present liquefaction necrosis due to poor central blood supply.
This requires PAF to be distinguished from PSFT and malignant
tumors on CT. Therefore, the final diagnosis is based on
histopathology and immunohistochemistry.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS drafted the manuscript. LS performed
histopathological and immunohistochemical examination of the tumor.
ZL collected patient data. RS, LS and ZL contributed to data
analysis, drafting and revision of the manuscript. RS and LS
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Sixth People's Hospital of Nantong (approval No. 2022007).
Patient consent for publication
The patient provided oral informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liang Z, Zhou P, Wang Y, Zhang Y, Li D, Su
X, Fan Y, Tang Y, Jiang L and Wang W: Pulmonary adenofibroma:
Clinicopathological and genetic analysis of 7 cases with literature
review. Front Oncol. 11(667111)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
WHO Classification of Tumours Editorial
Board. WHO classification of tumours. Thoracic tumours. 5th
edition. Vol. 5. Lyon: IARC Press, 2021.
|
|
3
|
Suster S and Moran CA: Pulmonary
adenofibroma: Report of two cases of an unusual type of
hamartomatous lesion of the lung. Histopathology. 23:547–551.
1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Erber R, Haller F, Hartmann A and Agaimy
A: Prominent entrapment of respiratory epithelium in primary and
metastatic intrapulmonary non-epithelial neoplasms: A frequent
morphological pattern closely mimicking adenofibroma and other
biphasic pulmonary lesions. Virchows Arch. 477:195–205.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Matsuda K, Nakajima W, Togashi T and Sano
Y: Pulmonary adenofibroma in a sika deer. J Vet Med Sci.
81:486–490. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fusco N, Guerini-Rocco E, Augello C,
Terrasi A, Ercoli G, Fumagalli C, Vacirca D, Braidotti P,
Parafioriti A and Jaconi M: , et al: Recurrent NAB2-STAT6
gene fusions and oestrogen receptor-α expression in pulmonary
adenofibromas. Histopathology. 70:906–917. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lindholm KE, Sansano-Valero I, Rodriguez
JL, Ramon Y and Moran CA: Pulmonary adenofibromas: A
clinicopathologic correlation of 13 cases. Am J Surg Pathol.
44:917–921. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hao J, Zhang C, Cao Q, Zou J and Wang C:
Pulmonary adenofibroma: Report of a case with multiple masses. Ann
Clin Lab Sci. 46:691–695. 2016.PubMed/NCBI
|
|
9
|
Wang Y, Xiao HL, Jia Y, Chen JH, He Y, Tan
QY and Zhang WG: Pulmonary adenofibroma in a middle-aged man:
Report of a case. Surg Today. 43:690–693. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
You X, Sun X, Yang C and Fang Y: CT
diagnosis and differentiation of benign and malignant varieties of
solitary fibrous tumor of the pleura. Medicine (Baltimore).
96(e9058)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Amer M, Abdeen Y, Shaaban H and
Alderink C: Solitary pulmonary adenofibroma in a middle-aged man
with bladder cancer. Lung India. 34:570–572. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rao N, Colby TV, Falconieri G, Cohen H,
Moran CA and Suster S: Intrapulmonary solitary fibrous tumors:
Clinicopathologic and immunohistochemical study of 24 cases. Am J
Surg Pathol. 37:155–166. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Olson NJ, Czum JM, de Abreu FB, Linos K
and Black CC: Synchronous pulmonary adenofibroma and solitary
fibrous tumor: Case report and review of the literature. Int J Surg
Pathol. 27:322–327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tan SY, Szymanski LJ, Galliani C, Parham D
and Zambrano E: Solitary fibrous tumors in pediatric patients: A
rare and potentially overdiagnosed neoplasm, confirmed by STAT6
immunohistochemistry. Pediatr Dev Pathol. 21:389–400.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sonokawa T, Enomoto Y, Kunugi S, Terasaki
Y and Usuda J: A case of pulmonary adenofibroma treated by
thoracoscopic resection. J Nippon Med Sch. 88:564–568.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Akin O and Coskun M: Biliary adenofibroma
with malignant transformation and pulmonary metastases: CT
findings. AJR Am J Roentgenol. 179:280–281. 2002.PubMed/NCBI View Article : Google Scholar
|