Introduction
Non-alcoholic fatty liver disease (NAFLD),
pathogenically reflected more accurately as metabolic-associated
fatty liver disease (1,2), has been recognized as the most common
chronic liver disease, affecting one-quarter of the population
worldwide. While the general incidence of liver-related outcomes in
NAFLD is only 0.97/1,000 person-years, the health burden of NAFLD
remains heavy in consideration of its huge patient population
(3). The spectrum of NAFLD ranges
from simple NAFL to non-alcoholic steatohepatitis (NASH) to
NAFLD-related cirrhosis (NAFLD-cirrhosis). NASH is characterized by
histological hepatic steatosis, lobular inflammation and cell
injury; NAFLD-cirrhosis (characterized by increased liver hardness,
formation of pseudolobules and even increased portal vein pressure)
has a relatively poor prognosis with higher risk for cardiovascular
disease and hepatic cell cancer (4-6).
Despite the rising prevalence of NAFLD, its onset and progression
remains unclear; it is most likely to have multiple etiologies,
intimately linked to metabolic abnormalities such as obesity,
hypertension, dyslipidemia and type 2 diabetes (T2DM) (7). The widely accepted mechanisms include
inflammation, oxidative stress, insulin resistance, dyslipidemia
and obesity (8,9). A number of studies suggest that
intestinal microbiota serve an important role in the development of
NAFLD (10,11), which works through the regulation
of gut barrier and liver inflammation response via Toll-like
receptor (TLR)4 signaling (12),
which enables the release of TNF-α and the activation of hepatic
stellate cells (13).
As the metabolites of gut bacteria, short-chain
fatty acids (SCFAs) are small molecular compounds with fewer than
six carbon atoms; they are mainly produced from fermentation of
dietary fibers by gut bacteria (14). The most abundant SCFAs are acetate,
propionate and butyrate, which account for >90% of those present
in the intestines (15); most
(90-95%) SCFAs are absorbed in the colon. SCFAs not only provide
energy for intestinal epithelium, but they also perform a number of
biological functions, such as the regulation of immunity,
lipometabolism and glycometabolism (16). As the liver is linked to the
intestines through the hepatic portal circulation, gut-derived
SCFAs will arrive to the liver first, and could be recognized as a
type of signal molecule connecting gut dysbiosis to the development
of NAFLD. In vitro and animal studies have previously
demonstrated that SCFAs participate in nutrient absorption and
insulin sensitivity through activating G-protein coupled receptors
(GPCRs) (17,18). Furthermore, SCFAs may serve roles
in suppressing the immune response and reducing liver inflammation
through histone deacetylases (HDACs), and then downgrade the number
of regulatory T cells (19). SCFAs
also modulate the production of several inflammatory cytokines such
as TNF-α, which is one of the terminal products of the TLR4 signal
pathway, as well as the key to liver inflammation and fibrosis
(enhancing the mRNA expression of tissue inhibitor of
metalloproteinase 1 in activated hepatic stellate cells and
suppressing apoptosis of hepatic stellate cells) (20).
Previous studies have reported the content
alterations of SCFAs in feces in patients of NAFLD (21,22).
However, clinical evidence of SCFAs associated with the severity of
NAFLD remains inadequately documented in humans. To the best of our
knowledge, no previous study has explored the association between
plasma SCFAs and TNF-α. In this retrospective cross-sectional
study, plasma concentrations of general SCFAs were measured in
healthy control (HC) individuals and patients with NAFLD of
distinct stages for a better understanding of the progression of
NALFD. In addition, correlation analysis between plasma SCFAs and
TNF-α in circulation was conducted. Multiple linear stepwise
regression analysis was used to look for variables that affected
TNF-α in circulation. This study may provide insight into the
potential interplay of cytokines and microbial metabolites in the
development of NAFLD.
Materials and methods
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Sixth People's Hospital of Chengdu (Chengdu, China; reference
no. 2020-L-004; December 2, 2020) and conformed to the ethical
guidelines of The Declaration of Helsinki (2000) of the World
Medical Association. Written informed consent was obtained from all
participants.
Patients
Finally, 71 patients with NAFLD and 9 HC volunteers
who came to the hospital for routine health assessment were
consecutively included between May 2018 and March 2019 at The Sixth
People's Hospital of Chengdu (Chengdu, China). Study participants
of both sexes were all >18 years old. All HC volunteers were
individuals without evidence or history of liver or metabolic
diseases. Patients with NAFLD met the diagnostic criteria for the
Prevention and Treatment for Non-Alcoholic Fatty Liver Disease of
the Chinese Society of Hepatology, Chinese Medical Association,
updated in 2018(5). Patients with
NASH (n=20) were screened out from normal NAFL (n=27) based on
elevated transaminases [alanine transaminase (ALT) or aspartate
transaminase (AST) >45 U/l] and abnormal medical images
(inflammation or swelling of the liver according to color doppler
ultrasound, computed tomography or magnetic resonance imaging). If
there was any doubt about the stage of disease, a liver biopsy was
performed by the clinical team, comprising a clinical doctor, a
radiology expert and a pathology doctor, all of whom had >10
years of work experience in their respective fields.
NAFLD-cirrhosis (n=24) was defined as the appearance of features in
radiology or endoscopy [hepatatrophia, widened portal vein,
varicose esophageal vein or liver stiffness measurement ≥15.0 kPa
from Fibroscan (FibroScan®502 CAP™; Shenzhen Echosens
Medical Equipment Co., Ltd.)] on the basis of NAFLD. All subjects
with the following conditions were excluded from the study:
Significant alcohol consumption (males, >30 g/day; females,
>20 g/day), chronic hepatitis B or hepatitis C infection,
autoimmune liver diseases, drug-induced liver disease, cancer,
diabetes mellitus or any other disease associated with the liver,
or if they had been treated with antibiotics or probiotics within
the 6 months before inclusion. Written informed consent was
obtained from each participant.
Blood sample collection and assay
For each patient, blood samples of 5 ml were
collected in EDTA tubes for blood routine test using a CAL8000
blood analysis pipeline (Shenzhen Mindray Bio-medical Electronics
Co., Ltd) and 5 ml blood samples were collected by common yellow
tubes for blood biochemical assay using the Roche Cobas c702
Automatic Biochemical Analyzer (Roche Diagnostics). Another 10 ml
of blood sample was collected using a heparin-coated
anticoagulation tube for the detection of SCFAs and TNF-α. All
blood samples were collected from the elbow vein one day following
inclusion with overnight fasting for 12 h. Blood routine test and
blood biochemical test were performed immediately after receiving
the samples by the Clinical Laboratory Center of The Sixth People's
Hospital of Chengdu. The samples for the detection of SCFAs and
TNF-α were ultracentrifuged at 1,500 x g for 5-10˚C for 10 min for
separating plasma and immediately stored at -20˚C and detected
within 7 days. The plasma concentrations of the three main SCFAs
(acetate, propionate and butyrate) were measured using an Agilent
8890B-5977B gas chromatograph (Shanghai Majorbio Bio-Pharm
Technology Co., Ltd.). The concentration of plasma TNF-α was
measured by the Clinical Laboratory Center of The Sixth People's
Hospital of Chengdu (Chengdu, China) using ELISA, with a testing
kit (cat. no. M-KMLJ61715; Nanjing Camilo Biological Engineering
Co., Ltd). The fibrosis-4 (FIB-4) index was the square root of [age
(years) x AST (U/l)]/[platelet count (109/l) x ALT
(U/l)].
Statistical analysis
All statistical analyses were performed using SPSS
statistical software (version 22.0; IBM Corp). All data
corresponded to a normal or approximate normal distribution, which
was checked by the Kolmogorov-Smirnov test, and all data were
expressed as the mean ± standard error of the mean. Qualitative
variables were compared using χ2 test or Fisher's
exact test. Comparisons between quantitative variables were made
using one-way ANOVA; Bonferroni's post-hoc test was performed for
multiple comparisons. Spearman's analysis and Pearson's correlation
analysis were used to estimate the correlations between SCFAs and
TNF-α. A multiple linear stepwise regression model was computed to
investigate predictor variables that had a significant influence on
TNF-α in the circulation. P<0.05 was considered to indicate a
statistically significant difference.
Results
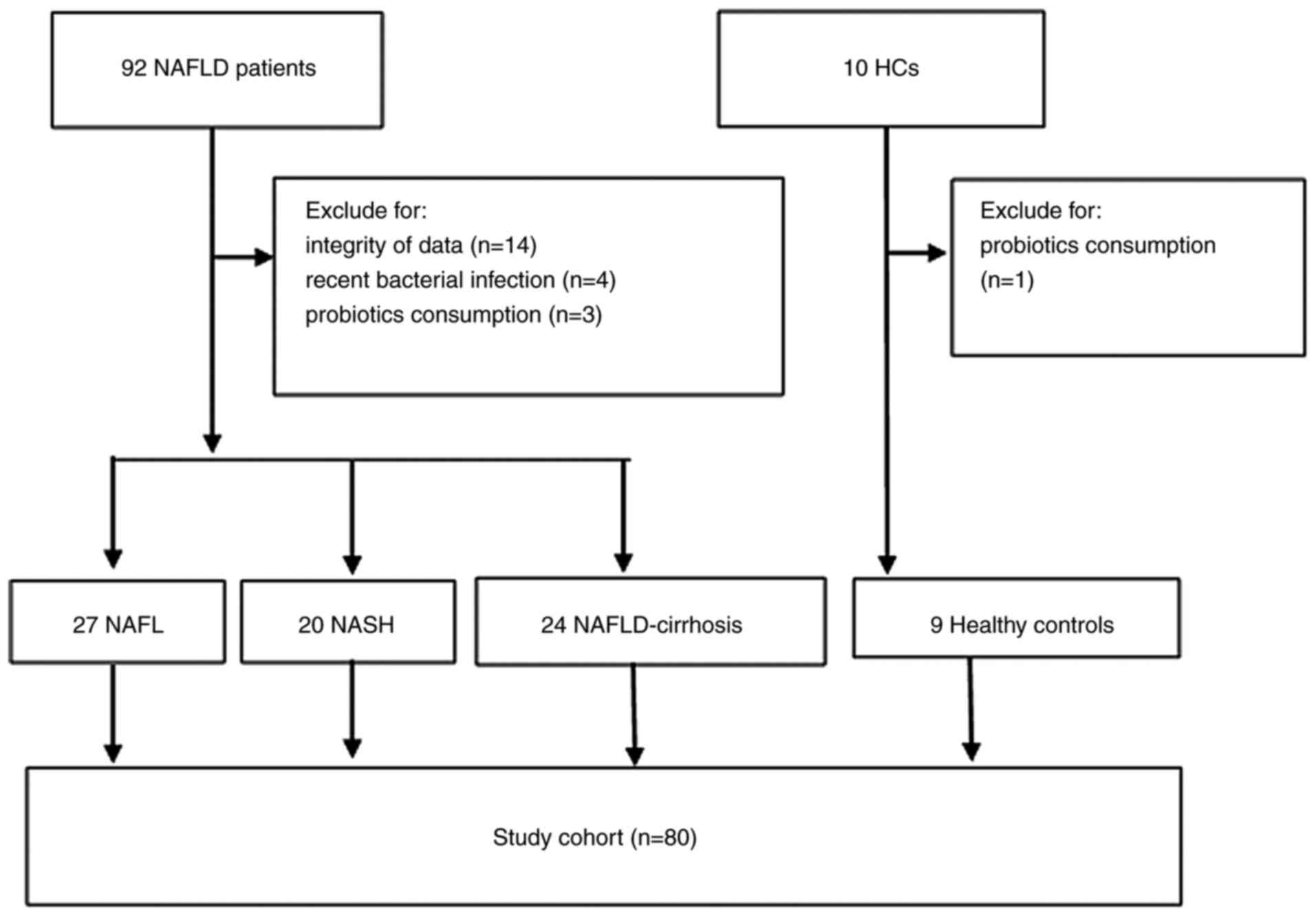
Recruitment of participants
The medical records of 92 patients with NAFLD and 10
HC volunteers were reviewed. In total, 22 patients were excluded
from the study due to lacking complete data (n=14 NAFLD), history
of recent bacterial infection (n=4 NAFLD) or consumption of
probiotics (n=3 NAFLD; n=1 HC). Finally, 71 patients with NAFLD
(including 27 patients with NAFL, 20 patients with NASH and 24
patients with NAFLD-cirrhosis) and 9 HCs were included in this
retrospective study for analysis. A total of 11 patients underwent
liver biopsy for the diagnosis of NASH. An overview of the study
participants is depicted in Fig.
1.
Baseline characteristics of the study
cohort
The baseline characteristics of the 80 participants
were summarized in Table I. The
mean age of the whole study population was 49.98±9.46 years and
43.75% of the participants were males. The four groups were
comparable in terms of age and sex. Patients with NAFL, NASH and
NAFLD-cirrhosis were more likely to be obese compared with the HC
group (P<0.001); they were also more likely to have T2DM,
hypertension or dyslipidemia compared with the HC group, though not
statistically significant. The concentrations of ALT, AST and
alkaline phosphatase, FIB-4 index and TNF-α were significantly
elevated in the NASH and NAFLD-cirrhosis groups compared with the
HC and NAFL groups (P<0.05).
 | Table IBaseline characteristics of the
patient cohort. |
Table I
Baseline characteristics of the
patient cohort.
| Characteristic | HC (n=9) | NAFL (n=27) | NASH (n=20) | NAFLD-cirrhosis
(n=24) |
|---|
| Age, years | 53.78±6.96 | 47.56±10.01 | 52.50±9.76 | 49.17±8.91 |
| Male sex | 4 (44.44) | 12 (44.44) | 11 (55.00) | 8 (33.33) |
| BMI,
kg/m2 | 22.46±1.76 |
27.40±3.17a |
28.81±3.34a |
28.29±2.47a |
| T2DM | 0 (0.00) | 5 (18.52) | 4 (20.00) | 6 (25.00) |
| Hypertension | 0 (0.00) | 4 (14.81) | 3 (15.00) | 4 (16.67) |
| Dyslipidemia | 0 (0.00) | 9 (33.33) | 7 (35.00) | 8 (33.33) |
| ALT, U/l | 26.89±9.35 | 38.04±12.06 |
108.35±40.99a,b |
59.71±30.38c,d |
| AST, U/l | 24.22±7.41 | 30.00±7.71 |
67.70±29.85a,b |
58.63±23.26a,b |
| ALP, U/l | 55.00±17.08 | 61.11±17.81 |
118.50±65.09b,e |
135.79±55.87a,b |
| FIB-4 index | 1.11±0.43 | 1.57±0.69 |
2.63±0.72a,b |
3.36±0.59a,b |
| TNF-α, pg/ml | 0.050±0.011 | 0.062±0.035 |
0.158±0.032a,b |
0.251±0.022a,b |
Plasma SCFA levels vary with the
development of NAFLD
Comparisons of the plasma concentrations of acetate,
propionate, butyrate and the sum of the three were displayed in
Fig. 2. Although not statistically
significant, acetate, propionate, butyrate and the combined SCFA
levels were increased in patients with NAFL compared with those in
the HCs. The concentrations of acetate, propionate, butyrate and
the total three SCFAs were significantly decreased in
NAFLD-cirrhosis compared with NAFL (P<0.001; Fig. 2A-D). The concentrations of acetate
(P<0.05), propionate (P<0.05) and the total three SCFAs
(P<0.01) in NASH were significantly decreased compared with
those in NAFL (Fig. 2A, B and D).
Although not statistically significant, the concentration of
butyrate in the patients with NASH was lower compared with that of
the patients with NAFL (Fig. 2C).
In addition, the concentration of butyrate was significantly lower
in patients with NAFLD-cirrhosis compared with that in patients
with NASH (P<0.01; Fig. 2C);
the concentrations of acetate, propionate and total SCFAs were also
lower in patients with NAFLD-cirrhosis compared with those in
patients with NASH, but no significant differences were identified
(Fig. 2A, B and D).
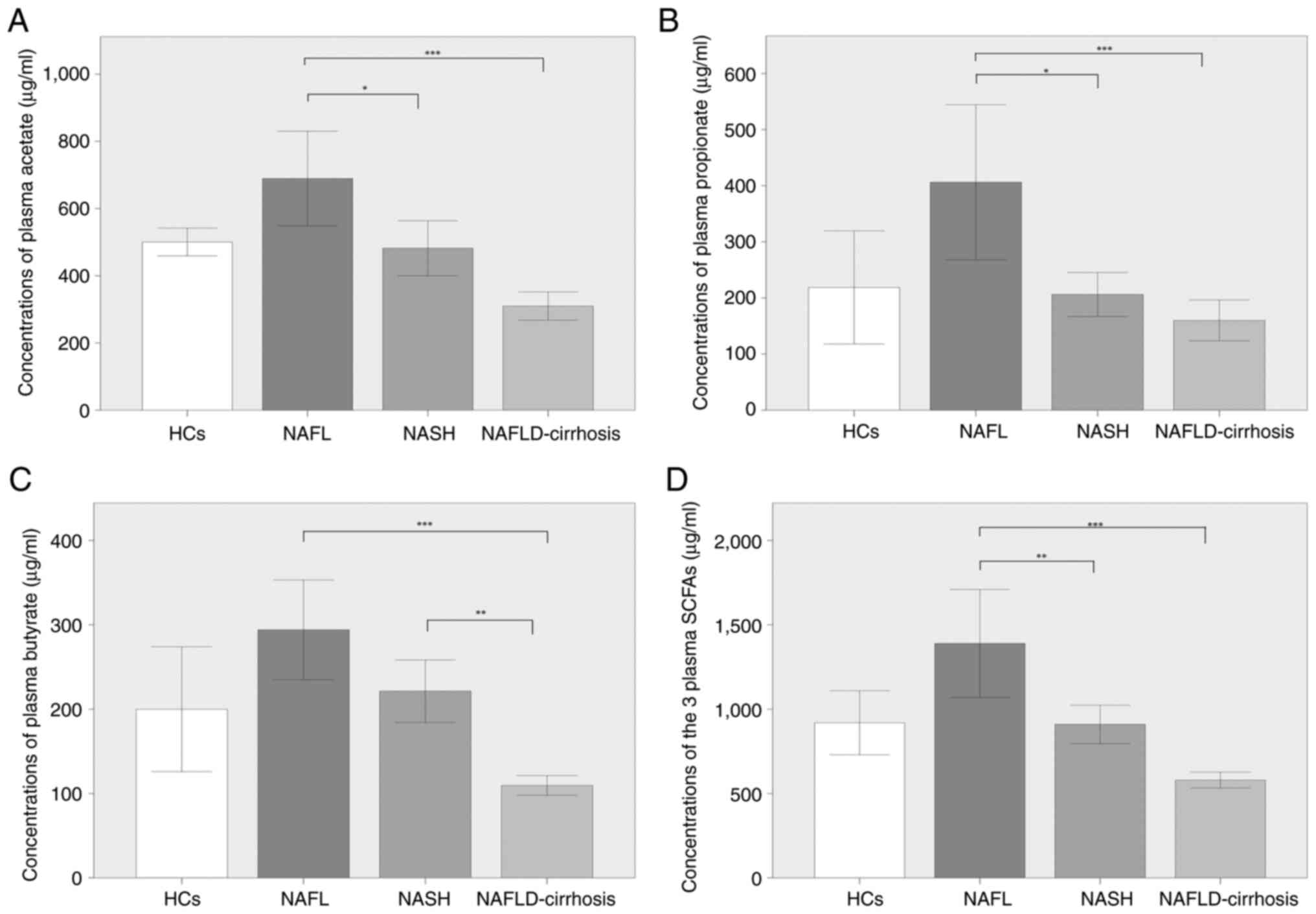 | Figure 2Comparison of the plasma
concentrations of acetate, propionate, butyrate and the total of
the three SCFAs in patients with NAFL, NASH and NALFD-cirrhosis and
participants with HCs. (A) Concentrations of acetate in HCs
(500.59±53.48 µg/ml), and in patients with NAFL (689.28±355.86
µg/ml), NASH (482.13±174.61 µg/ml) and NAFLD-cirrhosis
(309.93±99.83 µg/ml). (B) Concentrations of propionate in HCs
(218.76±131.06 µg/ml) and in patients with NAFL (406.24±349.64
µg/ml), NASH (206.16±83.86 µg/ml) and NAFLD-cirrhosis (159.99±86.42
µg/ml). (C) Concentrations of butyrate in HCs (199.91±96.30 µg/ml)
and in patients with NAFL (294.13±149.36 µg/ml), NASH (221.39±79.06
µg/ml) and NAFLD-cirrhosis (109.52±124.65 µg/ml). (D)
Concentrations of the total three SCFAs in HCs (919.27±247.06
µg/ml) and in patients with NAFL (1,389.64±809.15 µg/ml), NASH
(909.68±243.02 µg/ml) and NAFLD-cirrhosis (579.44±112.09 µg/ml).
*P<0.05, **P<0.01 and
***P<0.001. HCs, healthy controls; NAFLD,
non-alcoholic fatty liver disease; NAFLD-cirrhosis, NAFLD-related
cirrhosis; NASH, non-alcoholic steatohepatitis; SCFAs, short-chain
fatty acids. |
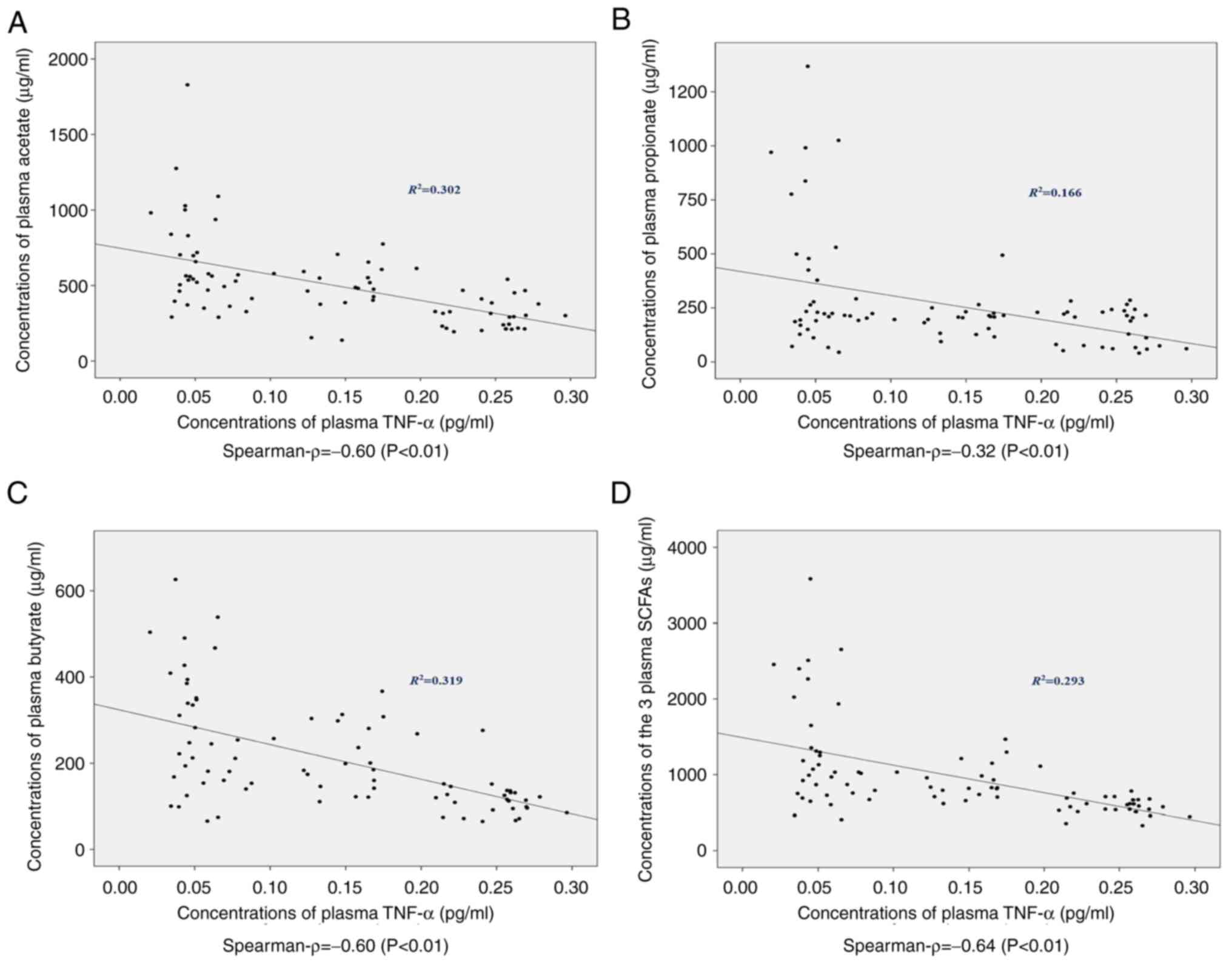
Concentrations of plasma SCFAs are
negatively correlated with peripheral TNF-α plasma
concentration
The concentrations of plasma TNF-α in the different
groups are presented in Table I.
The levels of TNF-α were comparable in HCs and patients with NAFL
(P=0.268), whereas the levels were significantly increased in NASH
and NAFLD-cirrhosis patients compared with the other two groups
(P<0.001). The associations between plasma SCFAs and peripheral
TNF-α were analyzed. Significant negative correlations were
observed between the concentrations of TNF-α and acetate
(R2=0.302; ρ=-0.60; P<0.01), propionate
(R2=0.166; ρ=-0.32; P<0.01), butyrate
(R2=0.319; ρ=-0.60; P<0.01) and the total three SCFAs
(R2=0.293; ρ=-0.64; P<0.01) in this study (Fig. 3).
NAFLD progression and the reduction in
the total level of the three SCFAs are independent variables to
predict elevated TNF-α in circulation
A multiple linear stepwise regression model was
computed to investigate predictor variables that may have a
significant influence on the level of plasma TNF-α concentration
(Table II). Age, sex, NAFLD
stage, BMI, T2DM, hypertension, dyslipidemia, elevated
aminotransferases, FIB-4, acetate, propionate, butyrate and the
total three SCFAs were entered into the regression model. The model
was statistically significant, with adjusted R2=0.871, F
(3,76)=178.998 and P<0.001. NAFLD stage, T2DM and the total
three SCFAs fitted well. TNF-α was calculated as follows:
TNF-α=-0.028 + (0.073 x NAFLD stage)-(0.017 x T2DM)-(0.0001 x the
total three SCFAs). The NAFLD stage was observed to have a
significantly positive impact on the concentration of plasma TNF-α
(β=0.849; P<0.001), whereas the total three SCFAs had
significantly negative influence (β=-0.189; P<0.001). Therefore,
the development of NAFLD and the decline of the total SCFAs in
plasma were recognized as independent risk factors associated with
the elevated TNF-α level in circulation.
 | Table IIMultiple linear stepwise regression
analysis for prediction the independent risk variables of plasma
TNF-α. |
Table II
Multiple linear stepwise regression
analysis for prediction the independent risk variables of plasma
TNF-α.
| | TNF-α
concentration |
|---|
| Variable | Unstandardized
β | SE (β) | Standardized β | P-value | VIF | 95% CI |
|---|
| Constant | -0.028 | 0.015 | - | 0.06 | - | -0.057-0.001 |
| Age | 0.000 | 0.000 | 0.050 | 0.285 | 1.249 | -0.000-0.001 |
| Sex | 0.004 | 0.008 | 0.023 | 0.598 | 1.104 | -0.011-0.019 |
| BMI | -0.001 | 0.001 | 0.911 | 0.548 | 1.505 | -0.003-0.002 |
| NAFLD stage | 0.073 | 0.004 | 0.849 | <0.001 | 1.241 | 0.066-0.081 |
| T2DM | -0.017 | 0.009 | -0.076 | 0.068 | 1.026 | -0.035-0.001 |
| Hypertension | -0.001 | 0.011 | -0.004 | 0.933 | 1.100 | -0.022-0.021 |
| Dyslipidemia | -0.006 | 0.008 | -0.034 | 0.453 | 1.157 | -0.023-0.010 |
| Acetate | -0.000 | 0.000 | -0.108 | 0.156 | 3.328 | -0.000-0.000 |
| Propionate | -0.000 | 0.000 | -0.035 | 0.662 | 3.786 | -0.000-0.000 |
| Butyrate | -0.000 | 0.000 | -0.058 | 0.458 | 3.485 | -0.000-0.000 |
| Total main
SCFAs | -0.0001 | 0.000 | -0.189 | <0.001 | 1.212 | -0.000-0.000 |
| Elevated
aminotransferases | -0.013 | 0.010 | -0.072 | 0.214 | 1.931 | -0.032-0.007 |
| FIB-4 | -0.000 | 0.006 | -0.003 | 0.965 | 3.397 | -0.013-0.012 |
Discussion
In the present study, plasma concentrations of SCFAs
and TNF-α, a key product of the TLR4 signaling pathway in liver
inflammation, were analyzed in 71 patients with NAFLD and 9 HC
patients. Although not statistically significant, plasma SCFA
levels were elevated in patients with NAFL compared with those in
HCs, and plasma SCFAs were reduced in NASH and NAFLD-cirrhosis
patients compared with those in patients with NAFL. In addition,
the decrease in SCFAs was negatively related to the elevated TNF-α
in plasma, particularly acetate and butyrate. The progression of
NAFLD and the decline in total SCFA levels were identified as
independent risk variables associated with the elevation of TNF-α
in circulation.
Currently, there is still no established and
effective medical treatment for NAFLD. Recent evidence suggested
that supplementation of dietary fibers was beneficial to the
metabolism of patients with NAFLD (23). As the main products of dietary
fibers in the bowel, SCFAs participate in the development of NAFLD
through several ways. For example, in the intestinal cavity, SCFAs
stimulate secretions of peptide YY and the gut-derived hormone
glucagon-like peptide 1 (GLP-1) through GPCR41 and GPCR43(18). Peptide YY slows intestinal
transport and increases satiety. GLP-1 promotes the development of
NAFLD by regulating fatty acid oxidation and insulin sensitivity
(24). In the circulation, SCFAs
directly regulate liver metabolism. For example, they affect the
expression of peroxisome proliferator-activated receptor α
target genes through AMP-activated protein kinase (AMPK)
phosphorylation, which reduces free fatty acid (FFA) from adipose
tissue to the liver (25,26). Previous studies showed that a
rectal infusion of acetate and propionate achieved 40% reduction in
serum FFA levels (27). It was
reported that serum FFA contributed 60% of fatty acids to the newly
synthesized triglyceride in the liver (28). However, the role of SCFAs in the
development of NAFLD remains controversial. One published study
reported higher concentrations of fecal SCFAs in patients with NAFL
compared with those in HCs owing to the predominance of
SCFA-producing families and bacterium, such as Bifidobacterium
bifidum, Butyrivibrio, Megasphaera and Prevotella (29); another study suggested that the
reduction in plasma SCFAs was associated with more advanced liver
fibrosis or cirrhosis (30).
Therefore, it is necessary to investigate SCFA levels in blood and
stool with regard to the changes along the NAFLD spectrum. The
present study found that the plasma levels of acetate, propionate,
butyrate and the total of the three SCFAs combined were increased
in patients with NAFL compared with those in HCs, although this
result was not statistically significant, whereas the levels were
significantly decreased in patients with NAFLD-cirrhosis compared
with NAFL. The levels were also significantly lower in patients
with NASH compared with NAFL, except for butyrate; however, the
concentration of butyrate in patients with NAFLD-cirrhosis was
lower compared with that in patients with NASH. Taken together,
these data suggest that the plasma levels of SCFAs may increase in
early stage NAFLD (although this requires further verification),
and the concentrations subsequently decrease in the advanced stages
of NAFLD. One explanation hypothesized by us is that SCFAs may
serve a protective role and a phenomenon like ‘decompensation’
happens when inflammation and fibrosis occur. Another explanation
is that SCFAs vary with the change of SCFA-producing microbiota in
the intestine. As aforementioned, patients with NAFL are
characterized with a higher abundance of SCFA-producing bacterium.
For example, Blautia obeum may be the most reliable
biological marker for the differentiation between mild NASH and
NAFLD (31). The populations of
main SCFA-producing bacterial phyla, such as Firmicutes and
Bacteroidetes, are significantly reduced in the advanced
stages of cirrhosis (30). It may
be cautiously hypothesized that the alteration of intestinal
microflora with the development of NAFLD may be another
manifestation of the ‘two hits’ or ‘multiple hits’ theory,
according to which lipotoxicity of adipose tissue, gut microbiome,
microbiota-related metabolites, dietary components and genetic
pathways evolved as crucial factors in the pathogenesis of NAFLD
(32). The present results
obtained with the specimens of human subjects suggested that
maintaining plasma SCFA levels may be beneficial for advanced
NAFLD, such as NASH and NAFLD-cirrhosis, but not for NAFL.
It is well documented that macrophages serve a major
role in inflammatory responses in the pathology of NASH and
NAFLD-related cirrhosis (33,34).
TNF-α is one of the most important pro-inflammatory cytokines
secreted by macrophages. Pathogen- or damage-associated molecular
substances, e.g., lipopolysaccharide (LPS), stimulate the canonical
NF-κB signaling pathway by binding TLR4(35). NF-κB activation induces the
transcription of a variety of inflammatory cytokines and
chemokines, including TNF-α. TNF-α may then act as a mediator of
hepatotoxicity, inflammation and NAFLD development. Once produced,
TNF-α enhances the mRNA expression levels of tissue inhibitor of
metalloproteinase 1 in activated hepatic stellate cells and
suppresses the induction of apoptosis, and then promotes the
process of hepatocyte injury (36). Increased TNF-α expression and that
of its receptors have been reported in patients with NASH (37). Notably, high basal TNF-α levels
could be a risk factor of developing NAFLD, even in healthy
individuals (38). A number of
previous studies have identified TNF-α as a predictor of NASH and
liver cirrhosis (20,36,38).
As SCFAs act as inflammation inhibitors in respiratory infections
and in kidney injury (39,40), it may be assumed that SCFAs may
induce a similar anti-inflammatory effect in NAFLD.
Results from the present study demonstrated that the
level of TNF-α was increased with NAFLD progression, which was
consistent with the aforementioned previous studies. In addition,
significant negative correlations between SCFAs and TNF-α were
identified and supported by multiple linear stepwise regression
model calculations, with an adjusted R2=0.871, which
means that an 87.1% change of TNF-α in circulation may be explained
by variables in this model. Rather than age, sex, BMI, metabolic
disorders, abnormal liver function, FIB-4 or any single SCFA alone,
the progression of NAFLD and decreased total SCFA concentration in
the blood were recognized as independent risk factors associated
with the elevation of TNF-α in circulation. However, the
correlation coefficients between TNF-α and acetate, propionate and
butyrate were not the same, ρ was -0.60, -0.32 and -0.60,
respectively. It may be hypothesized that this apparent discrepancy
arises from that acetate and butyrate are incorporated in lipid
metabolism, whereas propionate is mainly involved in
gluconeogenesis (26). For
instance, acetate increases the expression of genes for fatty acid
oxidation by activating AMPK (26). Butyrate, on the one hand,
suppresses the immune response and reduces liver inflammation
through HDACs; on the other hand, it also ameliorates liver
inflammation by promoting the expression of tight junction protein
in the colon (41). The
correlation results between SCFAs and TNF-α from the present study
were also consistent with recent findings in studies using animals.
For example, oral supplementation of acetate decreased macrophage
aggregation and TNF-α level in methionine- and choline-deficient
diet-induced NASH mice (42).
Treatment with butyrate for 6 weeks reduced hepatic injury by
downregulation of TNF-α in mice with NASH (41). In addition, the differences between
the correlation coefficients in the present study may help us
search for more representative biological markers of NAFLD. On the
whole, the putative protective affection of SCFAs may be achieved
through alleviating inflammation, in which TNF-α serves a role.
There are several limitations to the present study.
First, the study was cross-sectional research and it was limited by
sample size. Continuous investigations were lacking and baseline
data of participants may have been confounded by the patients'
condition at the time of enrollment. It might thus be difficult to
distinguish cause from effect. However, considering the relatively
large number of risk factors and the good stability of the
regression model, the research results were still worthy of
attention. Second, owing to the retrospective nature of the study,
dietary composition and microbiome analysis were not performed, and
additional samples (such as blood, stool, liver tissue and
intestinal tissue) could not be obtained for further investigation.
Therefore, further investigations are required, which may include a
prospective exploration of the intestinal flora, tight junction
proteins and their associations with SCFAs at different stages of
NAFLD. In addition, the concentration of LPS in blood and the
association between LPS and SCFAs can be investigated. As NAFLD
progresses, downregulated SCFA levels may increase the
concentration of LPS in the blood due to damage to the intestinal
barrier and production of more gram-negative bacteria. The
expression levels of TLR-4 and NK-κB in liver can also be examined,
as LPS may activate hepatic stellate cells by TLR-4, and thus
promote the release of inflammatory factors, such as TNF-α, through
the NF-κB signaling pathway. Furthermore, hepatocyte apoptosis and
production of reactive oxygen species can be investigated, which
may be associated with the functions of TNF-α in NAFLD progression
(35,36,43-45).
Finally, in the present study, elevated serum transaminases and
abnormal medical images were used for the diagnosis of NASH
(details were provided in the methods section), but not all
patients underwent liver biopsy to confirm the diagnosis of NASH
due to invasive examination.
The present study revealed variations in plasma SCFA
concentrations within the spectrum of NAFLD, as well as significant
negative correlations between SCFAs and peripheral TNF-α, which may
be beneficial for a better understanding of the pathogenesis of
NAFLD. The results may provide a glimpse into the potential links
between intestinal microecology, inflammatory response and the
development of NAFLD. In addition, they may provide a new way to
explore disease pathogenesis in terms of disease spectrum. Further
research is needed, such as analysis of the intestinal
microecology, signaling pathways and functions involved in TNF-α.
Additional SCFA supplementation for NASH and NAFLD-cirrhosis may
serve as a potential therapeutic strategy, which needs further
research and verification.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by The Chinese Foundation for
Hepatitis Prevention and Control (grant no. TQGB20180125).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QX and JX designed and performed the study together,
and they are responsible for data analysis, writing and revising
the paper. QX, JX, YL and TZ are responsible for data collection
and detection of samples. XC and ZJZ were responsible for data
analysis, interpretation of the data, obtaining ethics approval and
confirming the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Sixth People's Hospital of Chengdu (Chengdu, China; reference
no. 2020-L-004; December 2, 2020). Written informed consent was
obtained from all participants for the use of their samples for
detection and publication of their relevant data.
Patient consent for publication
All participants in this study provided written
informed consent for the use of their samples and publication of
their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eslam M, Sanyal AJ and George J:
International Consensus Panel. MAFLD: A consensus-driven proposed
nomenclature for metabolic associated fatty liver disease.
Gastroenterology. 158:1999–2014.e1. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eslam M, Newsome PN, Sarin SK, Anstee QM,
Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour
JF, Schattenberg JM, et al: A new definition for metabolic
dysfunction-associated fatty liver disease: An international expert
consensus statement. J Hepatol. 73:202–209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Männistö VT, Salomaa V, Färkkilä M, Jula
A, Männistö S, Erlund I, Sundvall J, Lundqvist A, Perola M and
Åberg F: Incidence of liver-related morbidity and mortality in a
population cohort of non-alcoholic fatty liver disease. Liver Int.
41:2590–2600. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Calzadilla Bertot L and Adams LA: The
natural course of non-alcoholic fatty liver disease. Int J Mol Sci.
17(774)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
National Workshop on Fatty Liver and
Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese
Medical Association; Fatty Liver Expert Committee, Chinese Medical
Doctor Association. Guidelines of prevention and treatment for
nonalcoholic fatty liver disease: A 2018 update. Zhonghua Gan Zang
Bing Za Zhi. 26:195–203. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
6
|
Li B, Zhang C and Zhan YT: Nonalcoholic
fatty liver disease cirrhosis: A review of its epidemiology, risk
factors, clinical presentation, diagnosis, management, and
prognosis. Can J Gastroenterol Hepatol.
2018(2784537)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cobbina E and Akhlaghi F: Non-alcoholic
fatty liver disease (NAFLD)-pathogenesis, classification, and
effect on drug metabolizing enzymes and transporters. Drug Metab
Rev. 49:197–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sangouni AA and Ghavamzadeh S: A review of
synbiotic efficacy in non-alcoholic fatty liver disease as a
therapeutic approach. Diabetes Metab Syndr. 13:2917–2922.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tarantino G, Citro V and Capone D:
Nonalcoholic fatty liver disease: A challenge from mechanisms to
therapy. J Clin Med. 9(15)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Safari Z and Gérard P: The links between
the gut microbiome and non-alcoholic fatty liver disease (NAFLD).
Cell Mol Life Sci. 76:1541–1558. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Astbury S, Atallah E, Vijay A, Aithal GP,
Grove JI and Valdes AM: Lower gut microbiome diversity and higher
abundance of proinflammatory genus Collinsella are associated with
biopsy-proven nonalcoholic steatohepatitis. Gut Microbes.
11:569–580. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Miura K and Ohnishi H: Role of gut
microbiota and Toll-like receptors in nonalcoholic fatty liver
disease. World J Gastroenterol. 20:7381–7391. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Csak T, Ganz M, Pespisa J, Kodys K,
Dolganiuc A and Szabo G: Fatty acid and endotoxin activate
inflammasomes in mouse hepatocytes that release danger signals to
stimulate immune cells. Hepatology. 54:133–144. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Silva YP, Bernardi A and Frozza RL: The
role of short-chain fatty acids from gut microbiota in gut-brain
communication. Front Endocrinol (Lausanne). 11(25)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chan JC, Kioh DY, Yap GC, Lee BW and Chan
EC: A novel LCMSMS method for quantitative measurement of
short-chain fatty acids in human stool derivatized with
12C- and 13C-labelled aniline. J Pharm Biomed
Anal. 138:43–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Canfora EE, Meex RCR, Venema K and Blaak
EE: Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev
Endocrinol. 15:261–273. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Koh A, De Vadder F, Kovatcheva-Datchary P
and Bäckhed F: From dietary fiber to host physiology: Short-chain
fatty acids as key bacterial metabolites. Cell. 165:1332–1345.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aragonès G, González-García S, Aguilar C,
Richart C and Auguet T: Gut microbiota-derived mediators as
potential markers in nonalcoholic fatty liver disease. Biomed Res
Int. 2019(8507583)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schilderink R, Verseijden C and de Jonge
WJ: Dietary inhibitors of histone deacetylases in intestinal
immunity and homeostasis. Front Immunol. 4(226)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stojsavljević S, Gomerčić Palčić M,
Virović Jukić L, Smirčić Duvnjak L and Duvnjak M: Adipokines and
proinflammatory cytokines, the key mediators in the pathogenesis of
nonalcoholic fatty liver disease. World J Gastroenterol.
20:18070–18091. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Michail S, Lin M, Frey MR, Fanter R, Paliy
O, Hilbush B and Reo NV: Altered gut microbial energy and
metabolism in children with non-alcoholic fatty liver disease. FEMS
Microbiol Ecol. 91:1–9. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rau M, Rehman A, Dittrich M, Groen AK,
Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P and
Geier A: Fecal SCFAs and SCFA-producing bacteria in gut microbiome
of human NAFLD as a putative link to systemic T-cell activation and
advanced disease. United European Gastroenterol J. 6:1496–1507.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kundi ZM, Lee JC, Pihlajamäki J, Chan CB,
Leung KS, So SSY, Nordlund E, Kolehmainen M and El-Nezami H:
Dietary fiber from oat and rye brans ameliorate western
diet-induced body weight gain and hepatic inflammation by the
modulation of short-chain fatty acids, bile acids, and tryptophan
metabolism. Mol Nutr Food Res. 65(e1900580)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Svegliati-Baroni G, Saccomanno S,
Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G,
Pacetti D, Vivarelli M, Nicolini D, et al: Glucagon-like peptide-1
receptor activation stimulates hepatic lipid oxidation and restores
hepatic signalling alteration induced by a high-fat diet in
nonalcoholic steatohepatitis. Liver Int. 31:1285–1297.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He J, Zhang P, Shen L, Niu L, Tan Y, Chen
L, Zhao Y, Bai L, Hao X, Li X, et al: Short-Chain fatty acids and
their association with signalling pathways in inflammation, glucose
and lipid metabolism. Int J Mol Sci. 21(6356)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
den Besten G, Bleeker A, Gerding A, van
Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen
AK, Reijngoud DJ and Bakker BM: Short-Chain fatty acids protect
against high-fat diet-induced obesity via a PPARγ-Dependent switch
from lipogenesis to fat oxidation. Diabetes. 64:2398–2408.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Donnelly KL, Smith CI, Schwarzenberg SJ,
Jessurun J, Boldt MD and Parks EJ: Sources of fatty acids stored in
liver and secreted via lipoproteins in patients with nonalcoholic
fatty liver disease. J Clin Invest. 115:1343–1351. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Wolever TM, Brighenti F, Royall D, Jenkins
AL and Jenkins DJ: Effect of rectal infusion of short chain fatty
acids in human subjects. Am J Gastroenterol. 84:1027–1033.
1989.PubMed/NCBI
|
|
29
|
de la Cuesta-Zuluaga J, Mueller NT,
Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM and
Escobar JS: Metformin is associated with higher relative abundance
of mucin-degrading akkermansia muciniphila and several short-chain
fatty acid-producing microbiota in the gut. Diabetes Care.
40:54–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Juanola O, Ferrusquía-Acosta J,
García-Villalba R, Zapater P, Magaz M, Marín A, Olivas P, Baiges A,
Bellot P, Turon F, et al: Circulating levels of butyrate are
inversely related to portal hypertension, endotoxemia, and systemic
inflammation in patients with cirrhosis. FASEB J. 33:11595–11605.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aoki R, Onuki M, Hattori K, Ito M, Yamada
T, Kamikado K, Kim YG, Nakamoto N, Kimura I, Clarke JM, et al:
Commensal microbe-derived acetate suppresses NAFLD/NASH development
via hepatic FFAR2 signalling in mice. Microbiome.
9(188)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tilg H, Adolph TE and Moschen AR: Multiple
parallel hits hypothesis in nonalcoholic fatty liver disease:
Revisited after a decade. Hepatology. 73:833–842. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mridha AR, Wree A, Robertson AAB, Yeh MM,
Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou
GN, et al: NLRP3 inflammasome blockade reduces liver inflammation
and fibrosis in experimental NASH in mice. J Hepatol. 66:1037–1046.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Alisi A, Carpino G, Oliveira FL, Panera N,
Nobili V and Gaudio E: The role of tissue macrophage-mediated
inflammation on NAFLD pathogenesis and its clinical implications.
Mediators Inflamm. 2017(8162421)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Robinson SM and Mann DA: Role of nuclear
factor kappa B in liver health and disease. Clin Sci (Lond).
118:691–705. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tomita K, Tamiya G, Ando S, Ohsumi K,
Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, et al:
Tumour necrosis factor alpha signalling through activation of
Kupffer cells plays an essential role in liver fibrosis of
non-alcoholic steatohepatitis in mice. Gut. 55:415–424.
2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Alaaeddine N, Sidaoui J, Hilal G, Serhal
R, Abedelrahman A and Khoury S: TNF-α messenger ribonucleic acid
(mRNA) in patients with nonalcoholic steatohepatitis. Eur Cytokine
Netw. 23:107–111. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Seo YY, Cho YK, Bae JC, Seo MH, Park SE,
Rhee EJ, Park CY, Oh KW, Park SW and Lee WY: Tumor necrosis
factor-α as a predictor for the development of nonalcoholic fatty
liver disease: A 4-year follow-up study. Endocrinol Metab (Seoul).
28:41–45. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Machado MG, Sencio V and Trottein F:
Short-chain fatty acids as a potential treatment for infections: A
closer look at the lungs. Infect Immun. 89(e0018821)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang S, Lv D, Jiang S, Jiang J, Liang M,
Hou F and Chen Y: Quantitative reduction in short chain fatty
acids, especially butyrate, contributes to the progression of
chronic kidney disease. Clin Sci (Lond). 133:1857–1870.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang T, Yang H, Heng C, Wang H, Chen S, Hu
Y, Jiang Z, Yu Q, Wang Z, Qian S, et al: Amelioration of
non-alcoholic fatty liver disease by sodium butyrate is linked to
the modulation of intestinal tight junctions in db/db mice. Food
Funct. 11:10675–10689. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Deng M, Qu F, Chen L, Liu C, Zhang M, Ren
F, Guo H, Zhang H, Ge S, Wu C and Zhao L: SCFAs alleviated
steatosis and inflammation in mice with NASH induced by MCD. J
Endocrinol. 245:425–437. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liedtke C and Trautwein C: The role of TNF
and Fas dependent signaling in animal models of inflammatory liver
injury and liver cancer. Eur J Cell Biol. 91:582–589.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li KZ, Liao ZY, Li YX, Ming ZY, Zhong JH,
Wu GB, Huang S and Zhao YN: A20 rescues hepatocytes from apoptosis
through the NF-κB signaling pathway in rats with acute liver
failure. Biosci Rep. 39(BSR20180316)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
García-Ruiz I, Rodríguez-Juan C,
Díaz-Sanjuan T, del Hoyo P, Colina F, Muñoz-Yagüe T and
Solís-Herruzo JA: Uric acid and anti-TNF antibody improve
mitochondrial dysfunction in ob/ob mice. Hepatology. 44:581–591.
2006.PubMed/NCBI View Article : Google Scholar
|