Introduction
Sepsis is a life-threatening immune response caused
by a dysregulated reaction to infection, and can lead to organ
dysfunction (1). The kidney is one
of the most commonly affected organs during sepsis, which can
result in sepsis-associated acute kidney injury (S-AKI). This
condition contributes to the morbidity and the high mortality rate
of sepsis, which is close to 50% within 90 days of intensive care
admission (2,3). Although the pathophysiological
mechanisms underlying S-AKI remain to be fully elucidated, it is
widely accepted that deleterious inflammatory cascade responses
during sepsis can contribute to AKI (1,4).
Moreover, innate immunity-mediated inflammation and the consequent
oxidative stress and apoptosis are closely associated with AKI
damage of renal tubular epithelial cells (RTECs) (1,4).
Toll-like receptors (TLRs), which are activated
following lipopolysaccharide (LPS) binding, induce downstream
inflammatory signaling cascades and therefore serve a key role in
innate immunity (5). Previous
studies have demonstrated that LPS significantly enhances the
expression of Rho-associated protein kinase (ROCK)1 and ROCK2 in
human umbilical vein endothelial cells (6) and retinal pigment epithelium
(7). ROCK2 is an important
regulator of the inflammatory response and serves a role in sepsis.
For example, TNF and heterogeneous nuclear ribonucleoprotein
L-related immunoregulatory long non-coding RNA aggravates
sepsis-induced acute lung injury by regulating ROCK2 expression
(8). Another study reported that
ROCK inhibitors can potentially be used to overcome sepsis-induced
deleterious effects in the brain (9). In a mouse model of sepsis, ROCK2 was
involved in the effect of heparin sodium on endotoxin-induced lung
vascular leakages (10). However,
whether ROCK2 serves a role in S-AKI is unclear.
The NF-κB signaling pathway has long been considered
a prototypical proinflammatory signaling pathway and it serves an
important role in the pathogenesis of organ injury induced by
sepsis (11). Furthermore, ROCK2
exhibits its inducible effect on the inflammatory response via
NF-κB signaling pathway activation (12). In the present study, the role of
ROCK2 in LPS-induced RTEC inflammation and apoptosis as well as the
underlying mechanisms were investigated.
Materials and methods
Cell culture and treatment
The human RTEC HK-2 cell line was purchased from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. Cells were cultured in Keratinocyte Serum-Free medium
(K-SFM; cat. no. 17005-042; Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and gentamicin/amphotericin solution (500X; Gibco; Thermo
Fisher Scientific, Inc.) in a 37˚C humidified atmosphere with 5%
CO2 and 95% air.
For establishment of the S-AKI cell model, HK-2
cells were stimulated with 10 µg/ml LPS (Sigma-Aldrich; Merck KGaA)
for 24 h at 37˚C (13). Phorbol
12-myristate 13-acetate (PMA; DC Chemicals) is an activator of
NF-κB (14). To confirm whether
PMA induces cytotoxicity to HK-2 cells, the cell culture medium of
HK-2 cells was replaced with fresh complete culture medium
containing different concentrations (5, 10, 20, 40 and 80 ng/ml) of
PMA for 24 h at 37˚C. For further NF-κB activation, the cell
culture medium of LPS-induced HK-2 cells was replaced with fresh
medium containing 20 ng/ml PMA for 24 h at 37˚C (15). The cell culture medium of
LPS-induced HK-2 cells was replaced with fresh medium containing
0.1% DMSO as a solvent control for 24 h at 37˚C.
Cell transfection
pGPU6 plasmids (cat. no. C02001; Shanghai GenePharma
Co., Ltd.) containing short hairpin (sh)RNA-ROCK2#1
(5'-ATCAGACAGCATCCTTTCT-3') and #2 (5'-GCAAATCTGTTAATACTCG-3') or
the scrambled shRNA-negative control (shRNA-NC;
5'-GGACTACTCTAGACGTATA-3') were designed by Shanghai GenePharma
Co., Ltd. All plasmids (50 nM) were transfected into cells using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) for
24 h at 37˚C. Transfection efficiency was evaluated after 48 h of
transfection at 37˚C via reverse transcription-quantitative PCR
(RT-qPCR) and western blotting.
Cell viability assay
Cell viability was quantified using an MTT Assay kit
(cat. no. ab211091; Abcam). HK-2 cells were seeded into 96-well
plates at a density of 1x105 cells/well and then
cultured in complete K-SFM with LPS and PMA or DMSO. After
incubation for 24 h at 37˚C, the medium was replaced with 50 µl
serum-free K-SFM and 50 µl MTT reagent was added. After incubation
for 3 h at 37˚C, 150 µl DMSO was added to each well and the cells
were incubated at 37˚C for a further 15 min on an orbital shaker.
Subsequently, the optical density at 590 nm was determined using a
microplate reader (Multiskan FC Microplate Photometer; cat. no.
51119180; Thermo Fisher Scientific, Inc.).
Lactate dehydrogenase (LDH) assay
An LDH Assay kit (cat. no. ab65393; Abcam) was used
to assess the level of cell plasma membrane damage or cytotoxicity.
Briefly, HK-2 cells were seeded into 96-well plates at a density of
5x104 cells/well and were then cultured in complete
K-SFM with LPS and PMA or DMSO. Following incubation for 24 h at
37˚C, the cell culture medium was centrifuged at 600 x g at 4˚C for
10 min and the supernatant was transferred into another 96-well
plate. Subsequently, 100 µl LDH reaction mix was added to each well
and incubated at room temperature for 30 min. The optical density
at 490 nm was determined using a microplate reader (Multiskan FC
Microplate Photometer; Thermo Fisher Scientific, Inc.).
ELISA
Inflammatory cytokine levels, including TNF-α, IL-6
and IL-1β in the cell supernatant were quantified using ELISA kits
for TNF-α (cat. no. ab181421; Abcam), IL-6 (cat. no. ab178013;
Abcam) and IL-1β (cat. no. ab100562; Abcam) according to the
manufacturer's protocol.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) and was
subsequently quantified using a BCA Protein Concentration Assay kit
(Beyotime Institute of Biotechnology). Equal quantities of total
protein (50 µg) were separated via SDS-PAGE on 10-12% gels.
Separated proteins were electro-transferred onto a PVDF membrane
(MilliporeSigma). Subsequently, membranes were blocked with 5%
non-fat milk at room temperature for 2 h and were then incubated
with primary antibodies overnight at 4˚C. Following the primary
incubation, membranes were incubated with an HRP goat anti-rabbit
IgG secondary antibody (cat. no. ab6721; 1:10,000; Abcam) at 37˚C
for 1 h. Proteins were visualized using an ECL reagent kit
(Shanghai Yeasen Biotech Co., Ltd.) and were semi-quantified using
ImageJ software (1.46r; National Institutes of Health). The rabbit
primary antibodies used were as follows: ROCK2 (cat. no. ab125025;
1:10,000), Bcl-2 (cat. no. ab32124; 1:1,000), Bax (cat. no.
ab32503; 1:1,000), cleaved caspase 3 (cat. no. ab32042; 1:500),
caspase 3 (cat. no. ab32351; 1:5,000), cleaved poly (ADP-ribose)
polymerase (PARP; cat. no. ab32064; 1:1,000), PARP (cat. no.
ab191217; 1:1,000), phosphorylated (p)-NF-κB p65 (cat. no. ab76302;
1:1,000), NF-κB p65 (cat. no. ab32536; 1:1,000), NLR family pyrin
domain containing 3 (NLRP3; cat. no. ab263899; 1:1,000),
apoptosis-associated speck-like protein containing a CARD (ASC;
cat. no. ab283684; 1:1,000) (all from Abcam), caspase 1 p20 (cat.
no. 22915-1-AP; 1:1,000; ProteinTech Group, Inc.), procaspase 1
(cat. no. ab207802; 1:1,000), kidney injury molecule-1 (KIM-1; cat.
no. ab228973), neutrophil gelatinase-associated lipocalin (NGAL;
cat. no. ab125075; 1:1,000) and GAPDH (cat. no. ab9485; 1:2,500)
(all from Abcam).
RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Reverse transcription was performed using a 5X All-In-One RT
Master Mix first strand cDNA synthesis kit (Applied Biological
Materials, Inc.) according to the manufacturer's protocol. qPCR was
performed using a QuantiTect SYBR Green PCR Kit (Qiagen AB; 10 µl
reaction volume) and the Roche LightCycler® 480 System
(Roche Diagnostics GmbH). The following thermocycling conditions
were used: Pre-denaturation at 95˚C for 10 sec; followed by 40
cycles of denaturation at 95˚C for 15 sec and annealing at 75˚C for
20 sec; and a final extension of 10 min at 72˚C. The mRNA
expression levels were quantified using the 2-ΔΔCq
method (16) and normalized to
GAPDH. The following primers were used: ROCK2 forward (F),
5'-TCCCGATAACCACCCCTCTT-3' and reverse (R),
5'-CCAAGGAATTTAAGCCATCCACT-3'; TNF-α F, 5'-GCTGCACTTTGGAGTGATCG-3'
and R, 5'-CTTGTCACTCGGGGTTCGAG-3'; IL-6 F,
5'-AGTGAGGAACAAGCCAGAGC-3' and R, 5'-AGCTGCGCAGAATGAGATGA-3'; IL-1β
F, 5'-CAGAAGTACCTGAGCTCGCC-3' and R, 5'-AGATTCGTAGCTGGATGCCG-3';
and GAPDH F, 5'-AATTCCATGGCACCGTCAAG-3' and R,
5'-TGGACTCCACGACGTACTC-3'.
TUNEL assay
Apoptosis was detected using an In Situ Cell
Death Detection kit (cat no. 11684817910; Roche Diagnostics GmbH)
according to the manufacturer's protocol. Briefly, cultured cells
were fixed with 4% formaldehyde at room temperature for 30 min.
Next, the cells were incubated with 1% Triton X-100. Subsequently,
the cells were mixed with 50 µl TUNEL reaction mixture containing
biotin-11-dUTP and TdT Enzyme, and then with streptavidin
fluorescein at 37˚C for 30 min. The nuclei were counterstained
using 4',6-diamidino-2-phenylindole at room temperature for 10 min
in the dark. Slides were mounted using glycerol. TUNEL-positive
cells were determined in three random fields of view using an
Olympus BX60 fluorescence microscope (Olympus Corporation) equipped
with a digital charge-coupled device. Apoptosis (%) was quantified
as follows: Number of TUNEL-positive nuclei/total number of cells
x100.
Statistical analysis
Experiments were performed in triplicate at minimum.
Data are presented as the mean ± SD and were analyzed using
GraphPad Prism 8.0 (GraphPad Software, Inc.). For statistical
analysis, pairwise comparisons between two groups were analyzed
using the unpaired Student's t-test. One-way ANOVA followed by
Tukey's post hoc test was used for comparisons between >2
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
ROCK2 knockdown protects HK-2 cells
against LPS-induced damage
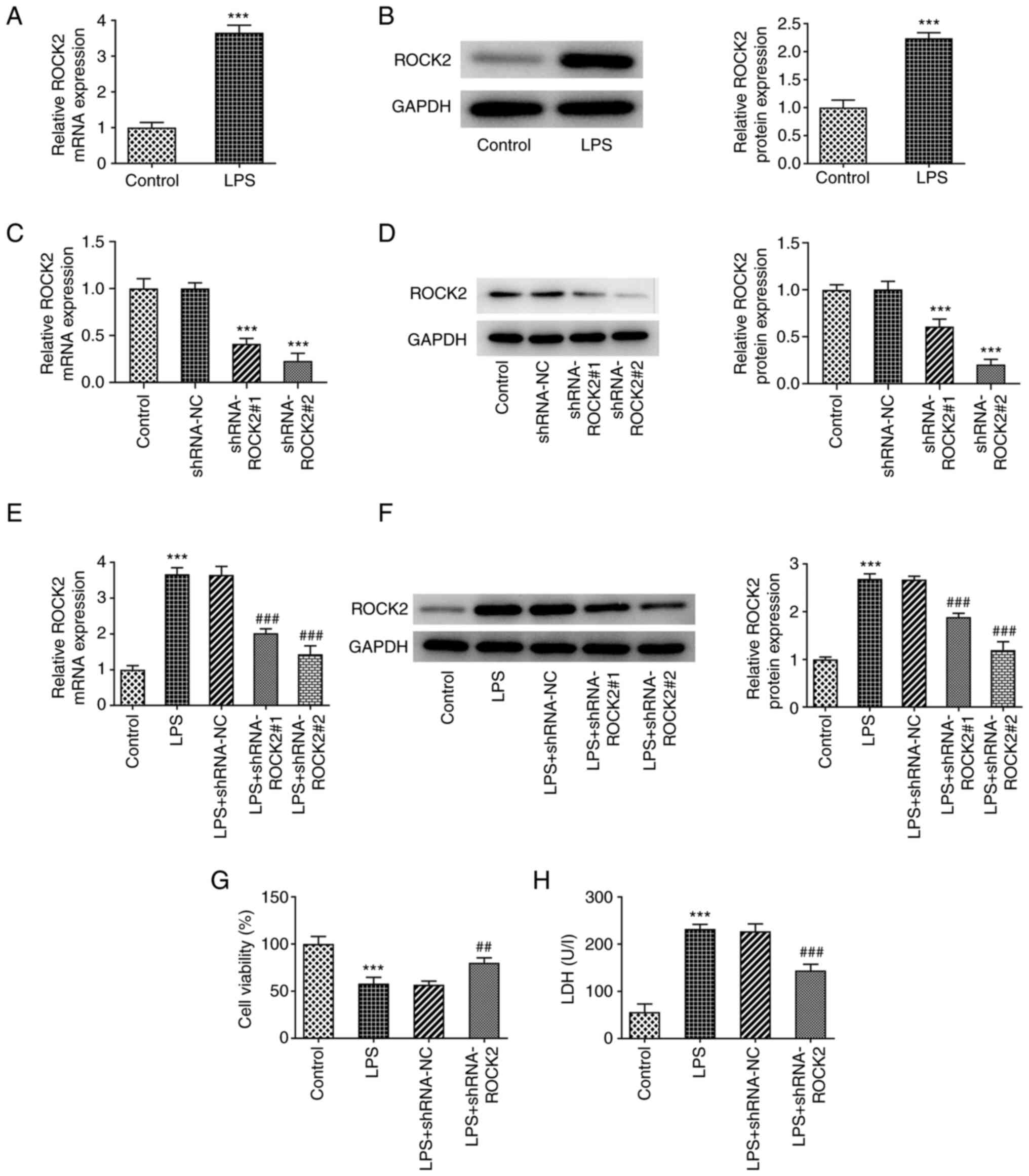
ROCK2 expression levels in HK-2 cells following LPS
stimulation were investigated. As presented in Fig. 1A and B, both ROCK2 mRNA and protein expression
levels in HK-2 cells were significantly increased by LPS, which
indicated the potential role of ROCK2 in LPS-induced HK-2 injury.
Subsequently, HK-2 cells and LPS-induced HK-2 cells were all
respectively transfected with shRNA-ROCK2 to silence ROCK2
expression. The transfection efficiency was determined at the mRNA
and protein expression levels and shRNA-ROCK2#2 was selected for
subsequent experiments based on its greater efficiency compared
with shRNA-ROCK2#1, as demonstrated in Fig. 1C-F. Moreover, cell viability was
determined. The results presented in Fig. 1G and H demonstrated that LPS resulted in a
significant decrease in cell viability and significant increase in
LDH activity compared with the control, which suggested that LPS
may be cytotoxic to HK-2 cells. However, LPS-treated HK-2 cells
that were transfected with shRNA-ROCK2 exhibited significantly
increased cell viability along with significantly decreased LDH
activity compared with the LPS + shRNA-NC group. These results
indicated the inhibitory effect of ROCK2 silencing on LPS
cytotoxicity on HK-2 cells.
ROCK2 knockdown protects HK-2 cells
against LPS-induced inflammation and apoptosis
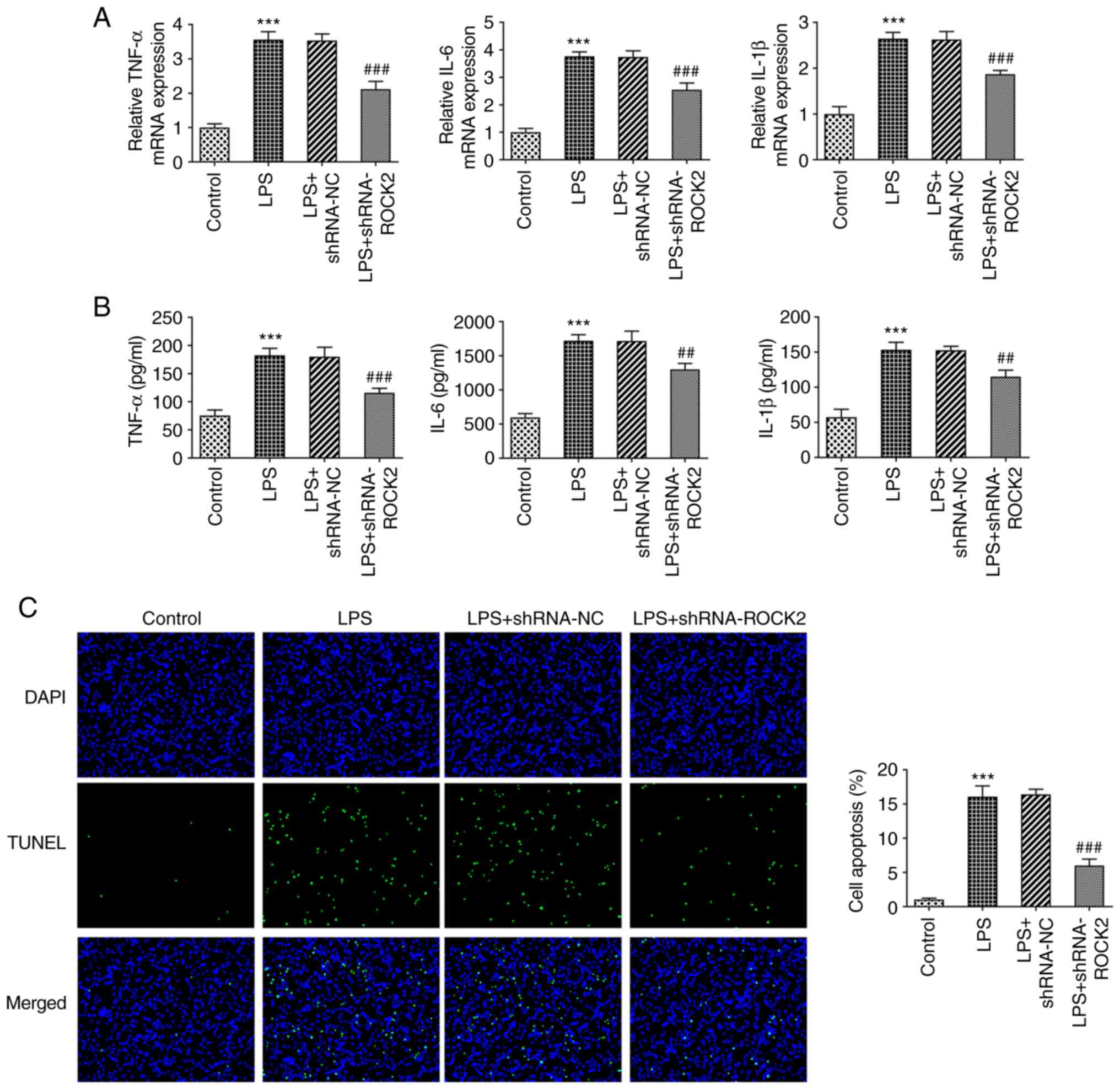
As observed in Fig.
2A and B, both concentrations
and mRNA expression levels of pro-inflammatory factors TNF-α, IL-6
and IL-1β were significantly enhanced when treated with LPS
compared with control HK-2 cells. However, this increase was
significantly inhibited by ROCK2 knockdown. Furthermore, as
presented in Fig. 2C, LPS
treatment also resulted in a significantly increased apoptosis rate
in HK-2 cells compared with the control; whereas ROCK2 knockdown
significantly reduced apoptosis caused by LPS in the LPS +
shRNA-ROCK2 group compared with the LPS + shRNA-NC group. Similar
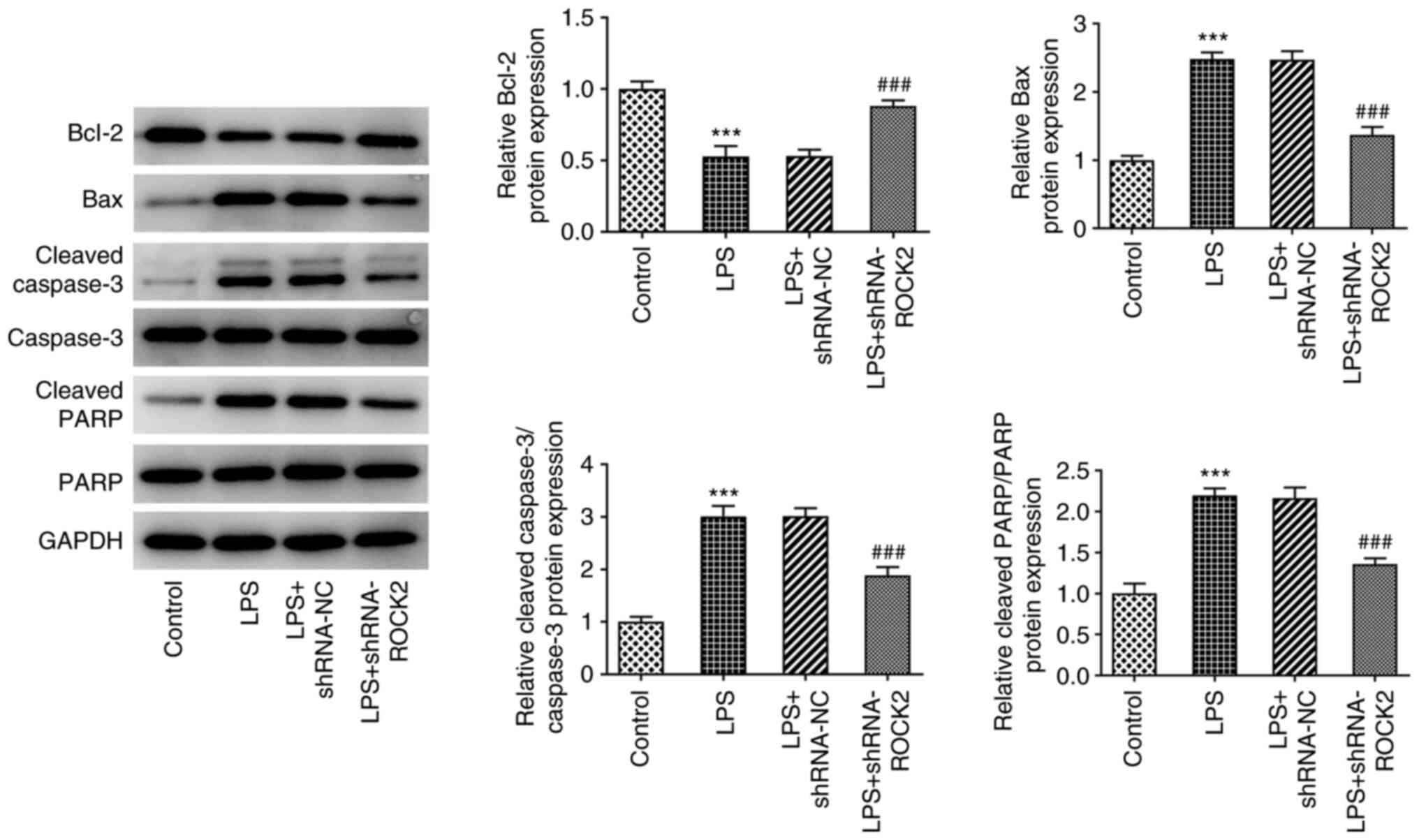
results are demonstrated in Fig.
3, whereby LPS treatment resulted in a significant reduction in
the protein expression levels of the anti-apoptotic protein Bcl-2,
but a significant increase in the expression levels of the
proapoptotic proteins Bax, cleaved caspase-3 and cleaved PARP
compared with the control. However, ROCK2 knockdown in LPS-induced
HK-2 cells significantly increased Bcl-2 protein expression levels
and reduced Bax, cleaved caspase-3 and cleaved PARP protein
expression levels compared with the LPS + shRNA-NC group. These
results indicated that ROCK2 knockdown may suppress LPS-induced
inflammation and apoptosis in HK-2 cells.
ROCK2 knockdown inhibits the
activation of NF-κB/NLRP3 signaling induced by LPS in HK-2
cells
To investigate whether ROCK2 knockdown exerted the
aforementioned effects on LPS-induced HK-2 cells by regulating the
NF-κB signaling pathway, the protein expression levels of NF-κB and
its associated downstream proteins were analyzed. As presented in
Fig. 4A, the results from the
western blotting analysis demonstrated that the protein expression
levels of p-NF-κB p65, NLRP3, ASC and caspase-1 p20 were
significantly increased when treated with LPS compared with the
control, which indicated the activation of the NF-κB/NLRP3
signaling pathway. Subsequently, an NF-κB activator, PMA, was used
to treat HK-2 cells at different concentrations and the results
demonstrated that PMA inhibited the HK-2 cells viability from 20 to
80 ng/ml while the inhibition effect of 20 ng/ml PMA on cell
viability was the smallest (Fig.
S1). To avoid significant damage to HK-2 cells, 20 ng/ml PMA
was used to treat LPS-induced ROCK2 knocked-down HK-2 cells. As
presented in Fig. 4B, compared
with the LPS + shRNA-ROCK2 group and DMSO + LPS + shRNA-ROCK2
group, PMA treatment significantly increased the protein expression
levels of p-NF-κB p65, NLRP3, ASC and caspase-1 p20. This result
indicated that the inhibitory effect of ROCK2 knockdown on
LPS-induced NF-κB/NLRP3 signaling pathway activation may be
partially reversed by PMA.
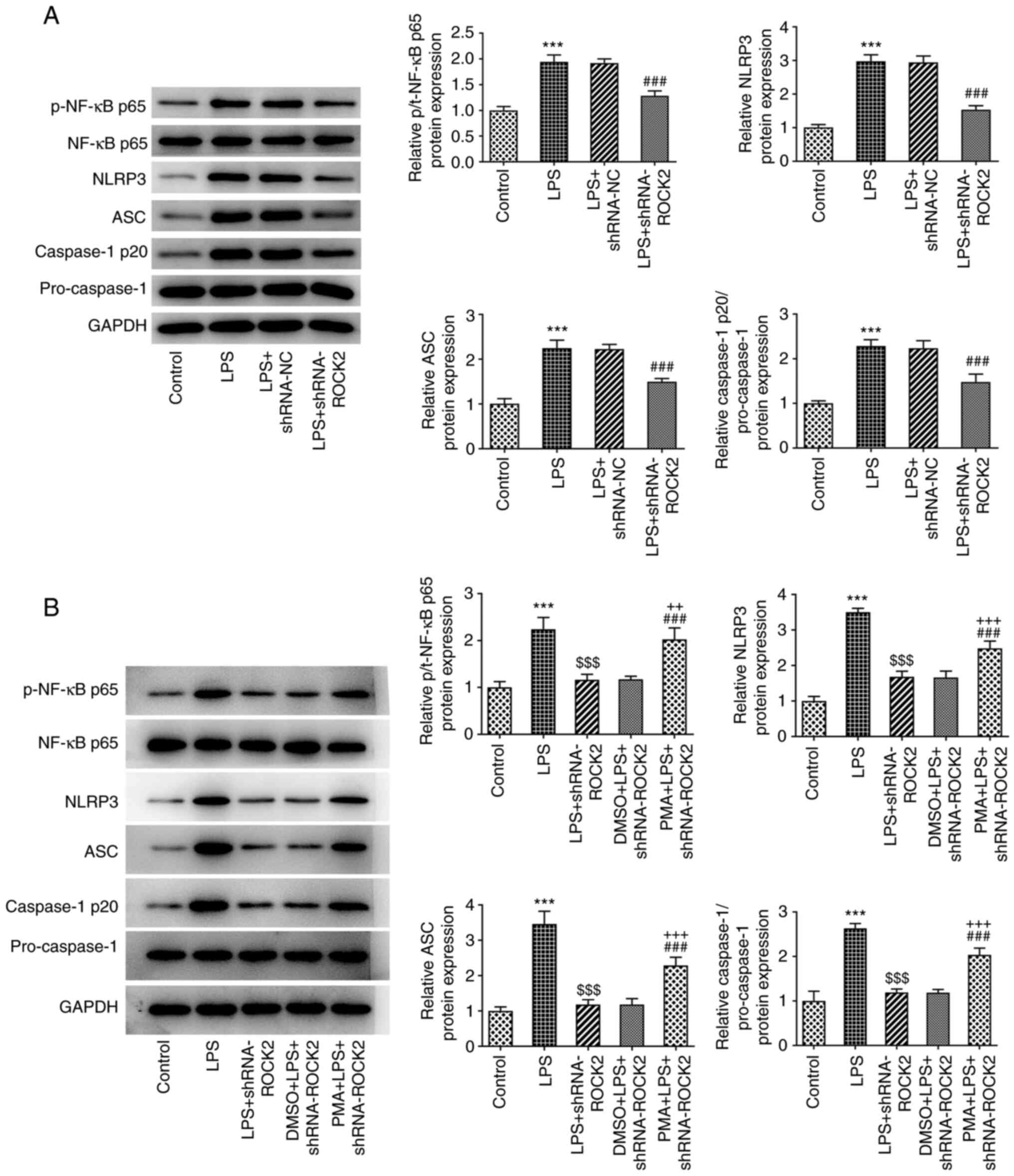 | Figure 4Effect of ROCK2 silencing on the
NF-κB/NLRP3 pathway in LPS-induced HK-2 cells. (A) The protein
expression of p-NF-κB p65/NF-κB p65, NLRP3, ASC, caspase-1
p20/pro-caspase-1 in HK-2 cells in different groups was measured
using western blotting. (B) PMA was used to treat LPS-induced,
shRNA-ROCK2-transfected HK-2 cells or untransfected cells, then the
protein expression levels of p-NF-κB p65/NF-κB p65, NLRP3, ASC,
caspase-1 p20/pro-caspase-1 in HK-2 cells in different groups were
measured using western blotting. ***P<0.001 vs.
control; $$$P<0.001 vs. LPS; ###P<0.001
vs. LPS + shRNA-ROCK2 or LPS + shRNA-NC; ++P<0.01 and
+++P<0.001 vs. DMSO + LPS + shRNA-ROCK2. ROCK2,
Rho-associated protein kinase 2; LPS, lipopolysaccharide; sh, short
hairpin; NC, negative control; NLRP3, NLR family pyrin domain
containing 3; ASC, apoptosis-associated speck-like protein
containing a CARD; PMA, phorbol 12-myristate 13-acetate; p,
phosphorylated; t, total. |
Activation of NF-κB reverses the
effect of ROCK2 knockdown on LPS-treated HK-2 cells
The role of PMA in ROCK2 knockdown on LPS-induced
HK-2 cell injury was further explored. The results in Fig. 5A and B demonstrated that PMA led to a
significant decrease in cell viability and an increase in LDH
activity in the PMA + LPS + shRNA-ROCK2 group compared with the
DMSO + LPS + shRNA-ROCK2 group. Moreover, as presented in Fig. 5C and D, the LPS-stimulated increase in the
concentration and mRNA expression levels of TNF-α, IL-6 and IL-1β
in HK-2 cells, which were reduced by shRNA-ROCK2, were subsequently
significantly enhanced by PMA treatment. Furthermore, PMA
significantly enhanced the apoptotic rate of HK-2 cells in the PMA
+ LPS + shRNA-ROCK2 group compared with the DMSO + LPS +
shRNA-ROCK2 group (Fig. 5E). The
effect of ROCK2 knockdown on the protein expression levels of
apoptosis-associated proteins, including Bcl-2, Bax, cleaved
caspase-3 and cleaved PARP in LPS-induced HK-2 cells, was
significantly reversed by PMA (Fig.
6A). The expression levels of KIM-1 and NGAL were significantly
increased in LPS-induced HK-2 cells, which was lessened by ROCK2
depletion, and PMA reduced the aforementioned protein expression
compared with the DMSO + LPS + shRNA-ROCK2 group (Fig. 6B). These results indicated that PMA
may inhibit the protective effect of ROCK2 knockdown on LPS-induced
HK-2 cell injury.
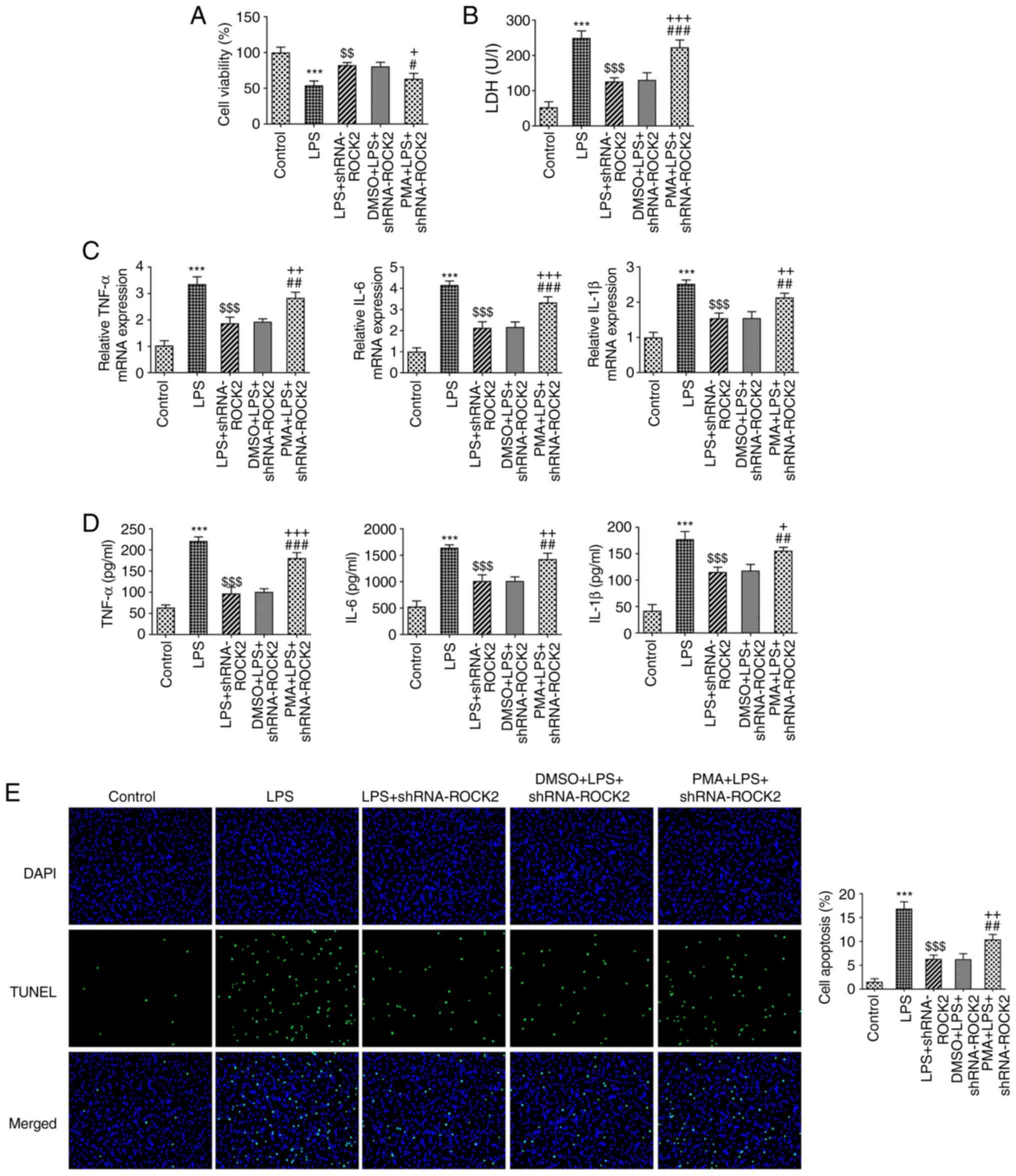 | Figure 5PMA reverses the effect of ROCK2
silencing on the viability, inflammation and apoptosis of
LPS-induced HK-2 cells. (A) Cell viability was measured using MTT
assay. (B) LDH production was measured using a commercially
available LDH release assay kit. (C) Reverse
transcription-quantitative PCR was utilized to measure the mRNA
level of TNF-α, IL-6 and IL-1β in HK-2 cells. (D) ELISA was used to
determine the production of TNF-α, IL-6 and IL-1β. (E) TUNEL
staining was utilized to measure HK-2 apoptotic rate.
***P<0.001 vs. control; $$P<0.01 and
$$$P<0.001 vs. LPS; #P<0.05,
##P<0.01 and ###P<0.001 vs. LPS +
shRNA-ROCK2; +P<0.05, ++P<0.01 and
+++P<0.001 vs. DMSO + LPS + shRNA-ROCK2. ROCK2,
Rho-associated protein kinase 2; LPS, lipopolysaccharide; sh, short
hairpin; LDH, lactate dehydrogenase; PMA, phorbol 12-myristate
13-acetate. |
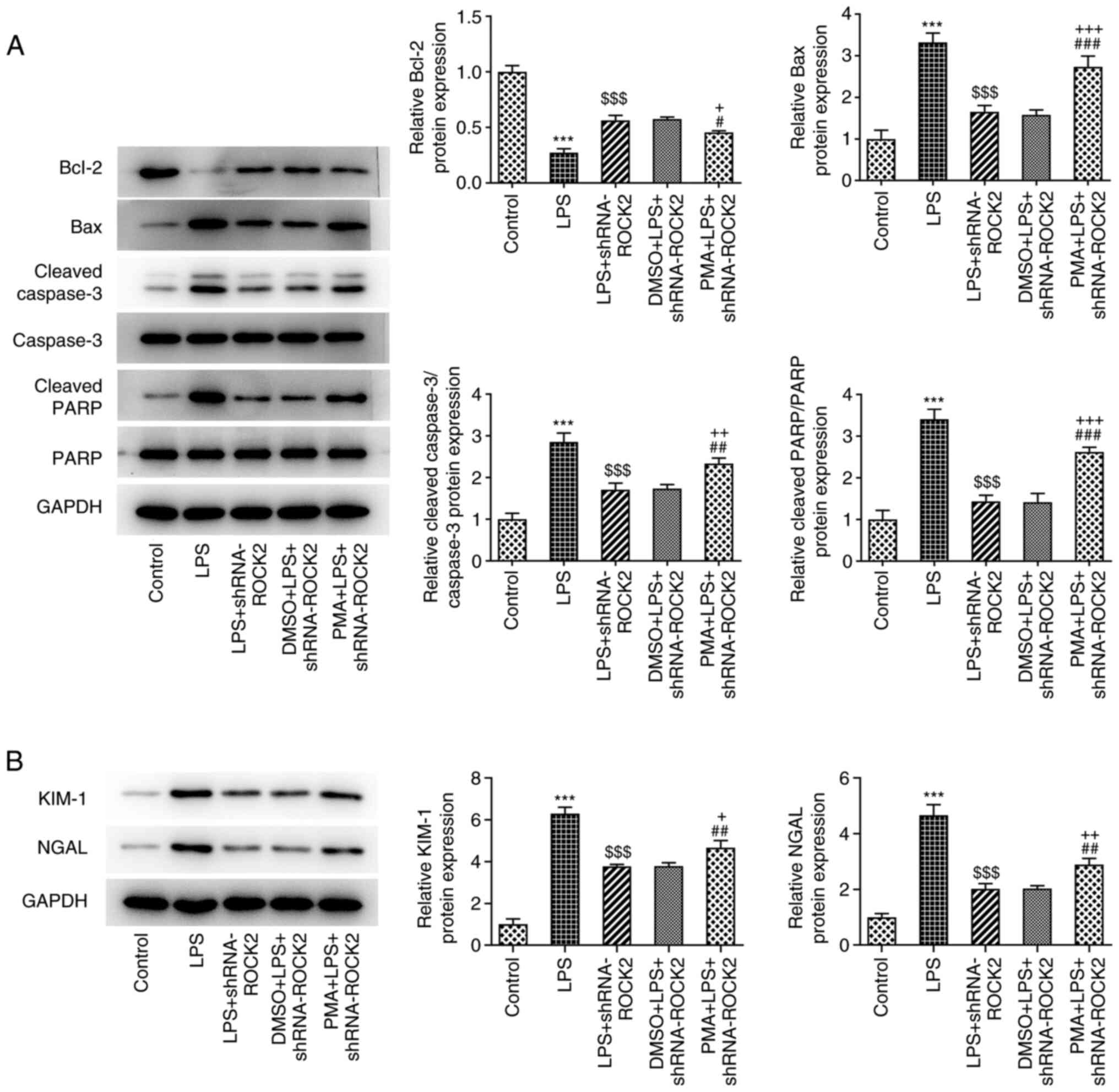 | Figure 6PMA reverses the effect of ROCK2
silencing on apoptosis related protein expression in LPS-induced
HK-2 cells. (A) Protein expression of Bcl-2, Bax, cleaved caspase
3/caspase 3 and cleaved PARP/PARP was measured using western
blotting. (B) Protein expression levels of KIM-1 and NGAL was
measured using western blotting. ***P<0.001 vs.
control; $$$P<0.001 vs. LPS; #P<0.05,
##P<0.01 and ###P<0.001 vs. LPS +
shRNA-ROCK2; +P<0.05, ++P<0.01 and
+++P<0.001 vs. DMSO + LPS + shRNA-ROCK2. ROCK2,
Rho-associated protein kinase 2; LPS, lipopolysaccharide; sh, short
hairpin; PARP, poly (ADP-ribose) polymerase; KIM-1, kidney injury
molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; PMA,
phorbol 12-myristate 13-acetate. |
Discussion
AKI is a common complication in patients in the
intensive care unit (ICU) and ~50% of AKI cases can be attributed
to sepsis, as observed in critically ill children in Southern India
in a prospective observational study from June 2010 to March
2011(17). Sepsis and AKI
synergistically increase the morbidity and mortality of patients in
the ICU. Therefore, a more complete molecular understanding of
renal tubular injury is important for the discovery of novel S-AKI
therapeutics. The results of the present study demonstrated that
ROCK2 knockdown markedly attenuated LPS-induced HK-2 cell injury.
Furthermore, PMA, an activator of NF-κB, significantly reversed the
effects of ROCK2 knockdown. Therefore, ROCK2 inhibition may serve
as an important target for the prevention of S-AKI. A previous
study indicated that Rho kinase inhibition attenuates renal injury
in LPS-treated mice (18).
However, Rho kinase includes ROCK1 and ROCK2, and isoform-specific
regulation remains largely unknown (18). Nozaki et al (19) demonstrated that the Rho-kinase
inhibitor fasudil alleviates cisplatin nephrotoxicity in the
kidney; however, the disease model and interventions were all
different compared with the present study in which cell model was
constructed and ROCK2 was knocked down. The present study confirmed
the regulatory role of ROCK2 in LPS-induced HK-2 cell injury.
LPS is a TLR4 agonist that can stimulate an
immediate and robust inflammatory response, which can induce the
activation of the innate immune system in human sepsis (5). LPS is widely used to induce cells to
construct sepsis cell models (20). In the present study, it was
demonstrated that LPS notably increased ROCK2 expression levels in
HK-2 cells. LPS has previously been reported to upregulate ROCK2
expression levels in pulmonary microvascular endothelial cells
(21) and brain microvascular
endothelial cells (22). In the
kidney tissues of sepsis animal models and LPS-induced HK-2 cells,
ROCK2 expression is also enhanced (23).
Previous research has reported that the RhoA/ROCK
signaling pathway serves an important role in the regulation of the
inflammatory response (24). In
sepsis, inhibition of the RhoA/ROCK2 signaling pathway is
implicated in alleviating sepsis-associated injury (24-26).
In the present study, it was hypothesized that the downregulation
of ROCK2 would reduce LPS-induced HK-2 cell injury. Therefore,
ROCK2 expression in HK-2 cells was inhibited using shRNA-ROCK2. The
results demonstrated that ROCK2 knockdown significantly improved
cell viability, in addition to reducing the levels of LDH activity
and of pro-inflammatory cytokines, apoptosis rate and the
expression levels of proapoptotic proteins in LPS-induced HK-2
cells, all of which indicated the potential suppression of HK-2
cell injury. Although the pathogenesis of AKI caused by sepsis
still remains unclear, the theory of tubular apoptosis and
inflammation has been widely accepted (27). Therefore, ROCK2 knockdown may serve
as a potential therapeutic approach for alleviating S-AKI.
NF-κB regulates the inflammatory response and has
been reported to serve an important role in the pathogenesis of
organ injury induced by sepsis (11). The results of the present study
demonstrated reduced protein expression levels of p-NF-κB p65,
NLRP3, ASC and caspase-1 p20 upon ROCK2 knockdown in LPS-induced
HK-2 cells. This result was similar to that of a previous study,
which reported that the downregulation of ROCK2 alleviates
ethanol-induced cerebral nerve injury partly through the
suppression of the NF-κB signaling pathway (28). NLRP3 mediates the cleavage and
maturation of proinflammatory cytokines, such as IL-1β, which
results in a complex network of cellular responses that trigger
local and systemic inflammatory reactions (29,30).
NF-κB signaling is a prerequisite for the activation of the NLRP3
inflammasome (31). It has
previously been reported that inhibition of the NF-κB signaling
pathway contributes to the protective effect of dexmedetomidine on
LPS-induced AKI in vivo (32). The present study therefore
hypothesized that ROCK2 knockdown may exhibit a protective effect
against S-AKI via the inhibition of NF-κB activation. Therefore,
PMA, an activator of NF-κB, was introduced into cells, and the
results demonstrated that PMA significantly reversed the effect of
ROCK2 knockdown on HK-2 cells. These results demonstrated the role
of NF-κB inactivation in ROCK2 knockdown-mediated S-AKI
alleviation. Activation of the NLRP3 inflammasome in tubular cells
has been associated with the pathogenesis of AKI (30,33).
Renal ischemia/reperfusion (I/R) injury activates the NLRP3 and
nuclear factor erythroid 2-related factor 2 (Nrf2) signaling
pathways and MCC950 (an NLRP3 inhibitor) can suppress the NLRP3
signaling pathway and protect against I/R-induced renal injury
(34). Propofol was indicated to
increase Nrf2 expression and decrease NLRP3 expression in a
ventilator-induced lung injury (VILI) mouse model, and activation
of Nrf2 or inhibition of NLRP3 could reduce the pro-inflammatory
factors in lung tissues in VILI mice (35). The present study indicated that LPS
induction activated the NLRP3 pathway. ROCK2 knockdown could
alleviate LPS-induced inflammatory injury and apoptosis of HK-2
cells by suppressing the NLRP3 pathway. Therefore, it was
speculated that regulating the NLRP3 signaling pathway could affect
the biological behaviors of HK-2 cells induced by LPS. In addition,
an inhibitor of the NLRP3 pathway should be applied in further
experiments.
In conclusion, the results of the present study
demonstrated that ROCK2 knockdown inhibited tubular apoptosis and
decreased inflammation by decreasing the NF-κB/NLRP3 activation in
S-AKI. These results also indicated that pharmacological inhibition
of ROCK2 may be a potential therapeutic approach for the treatment
of S-AKI. However, further research is needed to investigate the
effect of ROCK2 inhibition on S-AKI using other renal tubular
epithelial cells (rat NRK-52E cells and mouse TCMK-1 cells) and
animal models.
Supplementary Material
PMA reduces the viability of HK-2
cells. The viability of HK-2 cells treated with different
concentrations of PMA was detected using MTT assay.
*P<0.05, **P<0.01 and
***P<0.001 vs. control. PMA, phorbol 12-myristate
13-acetate.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY designed the study, performed the experiments,
interpreted the data and critically revised the manuscript. XQ
performed the experiments, analyzed the data and drafted the
manuscript. LY and XQ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellomo R, Kellum JA and Ronco C: Acute
kidney injury. Lancet. 380:756–766. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Peerapornratana S, Manrique-Caballero CL,
Gómez H and Kellum JA: Acute kidney injury from sepsis: Current
concepts, epidemiology, pathophysiology, prevention and treatment.
Kidney Int. 96:1083–1099. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Järvisalo MJ, Hellman T and Uusalo P:
Mortality and associated risk factors in patients with blood
culture positive sepsis and acute kidney injury requiring
continuous renal replacement therapy-A retrospective study. PLoS
One. 16(e0249561)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Poston JT and Koyner JL: Sepsis associated
acute kidney injury. BMJ. 364(k4891)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lim KH and Staudt LM: Toll-like receptor
signaling. Cold Spring Harb Perspect Biol.
5(a011247)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Qi Y, Liang X, Hu X, He H, Tang L and Yao
W: Tetrahydroxystilbene glucoside protects against LPS-induced
endothelial dysfunction via inhibiting RhoA/ROCK signaling and
F-actin remodeling. Gen Physiol Biophys. 39:407–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gao Z, Li Q, Zhang Y, Gao X, Li H and Yuan
Z: Ripasudil alleviated the inflammation of RPE cells by targeting
the miR-136-5p/ROCK/NLRP3 pathway. BMC Ophthalmol.
20(134)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen H, Hu X, Li R, Liu B, Zheng X, Fang
Z, Chen L, Chen W, Min L and Hu S: LncRNA THRIL aggravates
sepsis-induced acute lung injury by regulating miR-424/ROCK2 axis.
Mol Immunol. 126:111–119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jianjun Z, Baochun Z, Limei M and Lijun L:
Exploring the beneficial role of ROCK inhibitors in sepsis-induced
cerebral and cognitive injury in rats. Fundam Clin Pharmacol.
35:882–891. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Han J, Ding R, Zhao D, Zhang Z and Ma X:
Unfractionated heparin attenuates lung vascular leak in a mouse
model of sepsis: Role of RhoA/Rho kinase pathway. Thromb Res.
132:e42–e47. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1(a001651)2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagai Y, Matoba K, Kawanami D, Takeda Y,
Akamine T, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K and
Nishimura R: ROCK2 regulates TGF-β-induced expression of CTGF and
profibrotic genes via NF-κB and cytoskeleton dynamics in mesangial
cells. Am J Physiol Renal Physiol. 317:F839–F851. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shi C, Zhao Y, Li Q and Li J: lncRNA
SNHG14 plays a role in sepsis-induced acute kidney injury by
regulating miR-93. Mediators Inflamm. 2021(5318369)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xin F, Wang H, Yuan F and Ding Y:
Platelet-rich plasma combined with alendronate reduces pain and
inflammation in induced osteoarthritis in rats by inhibiting the
nuclear factor-kappa B signaling pathway. Biomed Res Int.
2020(8070295)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen H, Lin W, Lin P, Zheng M, Lai Y, Chen
M, Zhang Y, Chen J, Lin X, Lin L, et al: IL-10 produces a dual
effect on OGD-induced neuronal apoptosis of cultured cortical
neurons via the NF-κB pathway. Aging (Albany NY). 11:10796–10813.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krishnamurthy S, Narayanan P, Prabha S,
Mondal N, Mahadevan S, Biswal N and Srinivasan S: Clinical profile
of acute kidney injury in a pediatric intensive care unit from
Southern India: A prospective observational study. Indian J Crit
Care Med. 17:207–213. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Meyer-Schwesinger C, Dehde S, von Ruffer
C, Gatzemeier S, Klug P, Wenzel UO, Stahl RA, Thaiss F and Meyer
TN: Rho kinase inhibition attenuates LPS-induced renal failure in
mice in part by attenuation of NF-kappaB p65 signaling. Am J
Physiol Renal Physiol. 296:F1088–F1099. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nozaki Y, Kinoshita K, Hino S, Yano T,
Niki K, Hirooka Y, Kishimoto K, Funauchi M and Matsumura I:
Signaling Rho-kinase mediates inflammation and apoptosis in T cells
and renal tubules in cisplatin nephrotoxicity. Am J Physiol Renal
Physiol. 308:F899–F909. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dickson K and Lehmann C: Inflammatory
response to different toxins in experimental sepsis models. Int J
Mol Sci. 20(4341)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang J, Ruan F and Zheng Z: Ripasudil
attenuates lipopolysaccharide (LPS)-mediated apoptosis and
inflammation in pulmonary microvascular endothelial cells via
ROCK2/eNOS signaling. Med Sci Monit. 24:3212–3219. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Feng S, Zou L, Wang H, He R, Liu K and Zhu
H: RhoA/ROCK-2 pathway inhibition and tight junction protein
upregulation by catalpol suppresses lipopolysaccaride-induced
disruption of blood-brain barrier permeability. Molecules.
23(2371)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan XX, Zheng AD, Zhang ZE, Pan GC and
Zhou W: Protective effect of pantoprazole against sepsis-induced
acute lung and kidney injury in rats. Am J Transl Res.
11:5197–5211. 2019.PubMed/NCBI
|
|
24
|
Chen T, Guo Q, Wang H, Zhang H, Wang C,
Zhang P, Meng S, Li Y, Ji H and Yan T: Effects of esculetin on
lipopolysaccharide (LPS)-induced acute lung injury via regulation
of RhoA/Rho kinase/NF-кB pathways in vivo and in vitro. Free Radic
Res. 49:1459–1468. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wei F, Liu S, Luo L, Gu N, Zeng Y, Chen X,
Xu S and Zhang D: Anti-inflammatory mechanism of ulinastatin:
Inhibiting the hyperpermeability of vascular endothelial cells
induced by TNF-α via the RhoA/ROCK signal pathway. Int
Immunopharmacol. 46:220–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu P, Xiao Z, Yan H, Lu X, Zhang X, Luo
L, Long C and Zhu Y: Baicalin suppresses Th1 and Th17 responses and
promotes Treg response to ameliorate sepsis-associated pancreatic
injury via the RhoA-ROCK pathway. Int Immunopharmacol.
86(106685)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ozkok A and Edelstein CL: Pathophysiology
of cisplatin-induced acute kidney injury. Biomed Res Int.
2014(967826)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li X, Tong J, Liu J and Wang Y:
Down-regulation of ROCK2 alleviates ethanol-induced cerebral nerve
injury partly by the suppression of the NF-κB signaling pathway.
Bioengineered. 11:779–790. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Q, Ou Y, Hu G, Wen C, Yue S, Chen C,
Xu L, Xie J, Dai H, Xiao H, Zhang Y and Qi R: Naringenin attenuates
non-alcoholic fatty liver disease by down-regulating the
NLRP3/NF-κB pathway in mice. Br J Pharmacol. 177:1806–1821.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin Q, Li S, Jiang N, Shao X, Zhang M, Jin
H, Zhang Z, Shen J, Zhou Y, Zhou W, et al: PINK1-parkin pathway of
mitophagy protects against contrast-induced acute kidney injury via
decreasing mitochondrial ROS and NLRP3 inflammasome activation.
Redox Biol. 26(101254)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Meng L, Li L, Lu S, Li K, Su Z, Wang Y,
Fan X, Li X and Zhao G: The protective effect of dexmedetomidine on
LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB
and PI3K/Akt/mTOR pathways. Mol Immunol. 94:7–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lin Q, Li S, Jiang N, Jin H, Shao X, Zhu
X, Wu J, Zhang M, Zhang Z, Shen J, et al: Inhibiting NLRP3
inflammasome attenuates apoptosis in contrast-induced acute kidney
injury through the upregulation of HIF1A and BNIP3-mediated
mitophagy. Autophagy. 17:2975–2990. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Su Y, Wang Y, Liu M and Chen H: Hydrogen
sulfide attenuates renal I/R-induced activation of the inflammatory
response and apoptosis via regulating Nrf2-mediated NLRP3 signaling
pathway inhibition. Mol Med Rep. 24(518)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ruan H, Li W, Wang J, Chen G, Xia B, Wang
Z and Zhang M: Propofol alleviates ventilator-induced lung injury
through regulating the Nrf2/NLRP3 signaling pathway. Exp Mol
Pathol. 114(104427)2020.PubMed/NCBI View Article : Google Scholar
|