Introduction
The loss of dopaminergic neurons and formation of
Lewy bodies in the substantia nigra are the main pathological
characteristics of Parkinson's disease (PD) (1). Studies have found that aging,
heredity, epigenetic changes, and mitochondrial dysfunction are
principal factors promoting the occurrence of PD (2,3). At
present, the specific molecular mechanisms of dopaminergic neuron
degeneration in the substantia nigra are not yet fully understood.
Recent studies have shown that neuroinflammation is involved in the
occurrence and development of PD (4) and that neuroinflammation and
oxidative stress damage mediated by neuroglial activation play an
important role in the degeneration of dopaminergic neurons
(5,6). Our previous study revealed that
lipopolysaccharide (LPS)-activated microglia could release
pro-inflammatory factors, which resulted in inflammatory and
oxidative injury in dopaminergic neurons, leading to impaired cell
proliferation and increased apoptosis (7). Therefore, the identification of
molecular targets to reduce the death of dopaminergic neurons
caused by inflammation or oxidative injury may help slow down the
progression of PD.
Sirtuin 3 (SIRT3), a mitochondrial protein with
deacetylase activity, has a number of biological functions and
participates in the regulation of inflammatory damage, oxidative
stress, reactive oxygen species (ROS) clearance and mitochondrial
dysfunction (8,9). SIRT3 plays a pivotal role in the
occurrence and development of a variety of neurodegenerative
diseases (10,11). SIRT3 knockdown suppresses the
protective effects of dioscin on β-amyloid (Aβ) oligomer-mediated
ROS production, cell apoptosis and neurotoxicity in an Alzheimer's
disease (AD) model (12).
Mitochondrial SIRT3 has been shown to exert neuroprotective effects
in Huntington's disease by improving mitochondrial dynamics and
oxidative challenges (13).
Inflammatory injury and mitochondrial dysfunction can facilitate
ROS generation and the irreversible destruction of protein and DNA
structure. Neurons are particularly susceptible to mitochondrial
dysfunction, and the elevated ROS level promotes neuronal death and
protein deposition, thus accelerating the progression of
neurodegenerative diseases (14).
SIRT3 exerts a neuroprotective effect by eliminating mitochondrial
ROS, inhibiting inflammatory cytokine expression and cell
apoptosis, regulating energy metabolism and the balance of
mitochondrial respiratory electron transport chain, and increasing
adenosine triphosphate (ATP) production (15,16).
The newly synthesized rhamnoside derivative PL171 can rescue
Aβ42 oligomer-associated cell senescence, mitochondrial
dysfunction and oxidative stress injury by activating SIRT3 in an
AD cell model (17). By improving
mitochondrial function, SIRT3 protects parvalbumin and calretinin
interneurons against Aβ-mediated degeneration and dysfunction in
amyloid precursor protein and presenilin 1 (APP/PS1) double
transgenic AD model mice, thus repressing neuronal network
hyperactivity (18). Zhang et
al (19) proposed that
ε-viniferin could enhance SIRT3-mediated forkhead box O3 (FOXO3)
deacetylation, increase ATP production, decrease ROS production and
alleviate mitochondrial depolarization, thus inhibiting
rotenone-induced neuronal cell apoptosis. Oxidative stress injury
and mitochondrial dysfunction caused by abnormal aggregation of Aβ,
α-synuclein, or Huntington's disease in different target neurons
can result in severe neuron loss (20-22),
which is the common pathogenesis of different neurodegenerative
diseases. SIRT3 promotes the survival of target neurons, such as
dopaminergic or cholinergic neurons (23). Thus, SIRT3 could be used as a
potential intervention target for dopaminergic neuron survival
promotion.
A previous study demonstrated that SIRT3 expression
in the brain tissue of PD mice is reduced, and that SIRT3 gene
deletion significantly exacerbates the degeneration of dopaminergic
neurons in the substantia nigra and decreases the expression of
mitochondrial antioxidant enzyme superoxide dismutase 2 (SOD2),
suggesting that SIRT3 plays a protective role in
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced
dopaminergic neurotoxicity by scavenging free radicals in
mitochondria (11). SIRT3 also
exerts a protective effect on dopaminergic neurons by enhancing the
activities of mitochondrial citrate synthase and isocitrate
dehydrogenase (24). Theacrine
inhibits dopaminergic neuron apoptosis by directly increasing SIRT3
activity, which has been shown to restore mitochondrial functions
and reduce ROS accumulation in multiple animal or cell models of PD
(25). In addition, SIRT3
expression has also been shown to be reduced in an MPTP-induced PD
cell model, with SIRT3 overexpression inhibiting neuronal cell
apoptosis (26). Our previous
study found that microglia activation-induced oxidative stress
could downregulate the protein levels of SIRT3 and SOD2 in
dopaminergic neurons (7). The
aforementioned results suggested that SIRT3 was associated with the
occurrence and development of PD, although the detailed mechanism
of action has not been fully explored. It is also unclear whether
SIRT3 can inhibit the microglia activation-mediated death of
dopaminergic neurons.
A number of studies have revealed that SIRT3
expression can promote mitochondrial autophagy or inhibit
nucleotide-binding oligomerization domain, leucine rich repeat and
pyrin domain-containing protein 3 (NLRP3) inflammasome activation
in various cell types (27-29).
Targeting SIRT3 to improve mitophagy and alleviate senescence in
bone marrow mesenchymal stem cells may be a potential therapeutic
strategy for alleviating senile osteoporosis caused by advanced
glycation end products (27).
SIRT3 overexpression has been found to prominently facilitate wound
healing speed under high glucose conditions by activating mitophagy
(28). SIRT3 overexpression can
also activate mitochondrial autophagy and reduce mitochondrial
damage, as well as cardiomyocyte apoptosis; on the contrary, SIRT3
deficiency can inhibit mitophagy by reducing FOXO3A deacetylation
and Parkin expression, and contribute to the development of
diabetic cardiomyopathy (29).
Zheng et al (30) have
demonstrated that SIRT3 upregulation significantly attenuates Ti
particle-induced osteogenic suppression by improving osteogenesis
and inhibiting the NLRP3 inflammasome in vivo and in
vitro. SIRT3 overexpression can markedly weaken the activation
of NLRP3 inflammasome in human vascular endothelial cells and delay
the progression of vascular inflammation (31). However, whether SIRT3 expression in
dopaminergic neurons can alleviate the inflammation and oxidative
stress injury caused by microglia activation through the
mitophagy-NLRP3 inflammasome pathway remains unknown.
The aim of the present study was to explore whether
SIRT3 expression exerts a protective effect against cytotoxicity
resulting from microglia activation in dopaminergic neuronal cells
(DACs) by improving mitochondrial function. In addition, whether
the mitophagy-NLRP3 inflammasome pathway was involved in this SIRT3
neuroprotection was also investigated.
Materials and methods
Cell culture
The MN9D mouse midbrain dopaminergic cell line was
sourced from the American Type Culture Collection and maintained in
high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 100 µg/ml streptomycin and 100 U/ml
penicillin (Invitrogen; Thermo Fisher Scientific, Inc.) in an
incubator with an atmosphere of 5% CO2 at 37˚C. The
murine microglial cell line BV-2 (cat. no. GDC0311; China Center
for Type Culture Collection) was cultured in flasks containing DMEM
supplemented with 10% FBS at 37˚C with 5% CO2.
Transwell co-culture system
A total of 2x105 DACs (MN9D cells) were
cultured in 6-well plates in the lower chamber of a Transwell
co-culture system (cat. no. 3412; Corning, Inc.), with
2x105 microglia (BV-2 cells) in the upper chamber. LPS
(1 µg/ml, MilliporeSigma) was added to the microglia layer. Cell
migration was observed using an Olympus IX71 microscope
(magnification, x100; Olympus Corporation). DACs and microglia
shared the same DMEM medium with FBS at 37˚C for 48 h, although
direct cell-to-cell interaction was unlikely, as two types of cells
were physically separated by a 0.4 µm polycarbonate membrane.
Experimental groups
Experiments were conducted using the following
groups: i) Blank, DACs were co-cultured with microglia for 48 h;
ii) control, DACs were co-cultured with microglia exposed to 1
µg/ml LPS for 48 h; iii) SIRT3 or Vector, DACs in the chamber were
pretreated with SIRT3-adenoviral or empty vector (Vigene
Biosciences, Inc.) for 6 h respectively, and DACs were then
co-cultured with microglia stimulated with 1 µg/ml LPS for 48 h;
iv) siSIRT3 or Scrambled, DACs in the chamber were pretreated with
SIRT3 small interfering RNA (siRNA) or negative control siRNA
(Santa Cruz Biotechnology, Inc.) for 6 h respectively, then
co-cultured with microglia exposed to 1 µg/ml LPS for 48 h.
Adenovirus infection and siRNA
transfection
A recombinant adenoviral vector overexpressing SIRT3
(Ad-SIRT3-GFP; cat. no. VH820507) and a non-targeting adenoviral
vector (Ad-GFP) were purchased premade from Vigene Biosciences,
Inc. SIRT3 siRNA (cat. no. sc-61556) and negative control siRNA
(cat. no. sc-37007) were purchased from Santa Cruz Biotechnology,
Inc. For overexpression of SIRT3, when DACs reached a ~50%
confluence, cells were infected with Ad-SIRT3-GFP (SIRT3 group) or
Ad-GFP (Vector group) at a multiplicity of infection of 100, after
6 h, the cells were switched to fresh medium and incubated for an
additional 24 h. For SIRT3 knockdown, when DACs reached ~60%
confluence, the cells were transfected with 50 nM SIRT3 siRNA
(siSIRT3 group) or negative control siRNA (Scrambled group) using
Lipofectamine® 2000 (cat. no. 11668019; Invitrogen;
Thermo Fisher Scientific, Inc.) at 37˚C for 6 h according to the
manufacturer's instructions. After transfection, the serum-free
transfection mixture was replaced with DMEM + 10% FBS and cells
were cultured for an additional 24 h. SIRT3 and SOD2 gene
expression in DACs was evaluated using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting. After verifying the overexpression and knockdown
of SIRT3, the cells were used for subsequent experiments.
Evaluation of intracellular ROS
production
The fluorescent probe dihydroethidium (cat. no.
S0063) and the hydrogen peroxide assay kit (cat. no. S0038) were
obtained from Beyotime Institute of Biotechnology and used to
assess the intracellular accumulation of superoxide anion and
hydrogen peroxide, respectively, according to the manufacturer's
instructions. The fluorescence intensity was measured using a plate
reader (Thermo Fisher Scientific, Inc.). These experiments were
carried out as previously described (7).
RT-qPCR
TRIzol® (Thermo Fisher Scientific, Inc.)
was used to extract total RNA from DACs according to the
manufacturer's protocol. RNA concentration was detected using a
NanoDrop™ 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). cDNA synthesis was performed from 1.5 µg of RNA
using HiScript 1st Strand cDNA Synthesis Kit (cat. no. R111-02;
Vazyme Biotech Co., Ltd.) according to the manufacturer's protocol.
RT-qPCR was performed for target genes using SYBR Green PCR kit
(cat. no. Q111-02; Vazyme Biotech Co., Ltd.), as per the
manufacturer's instructions. The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 5 min; followed by 40
cycles of denaturation at 95˚C for 10 sec, annealing at 60˚C for 30
sec and extension at 72˚C for 10 sec. GAPDH served as the internal
reference gene and the relative mRNA expression was measured using
the 2-ΔΔCq method (32). The primer sequences used were as
follows: SIRT3 forward, 5'-ATGCCTGAAGACAGCTCCAACAC-3' and reverse,
5'-AGACATCCCTGGTCAGCCTTTCC-3'; SOD2 forward,
5'-TAAGGAGAAGCTGACAGCCGTGT-3' and reverse,
5'-AGAGCAGGCAGCAATCTGTAAGC-3'; LC3 forward,
5'-CAGCCACACCCTTTCACT-3' and reverse, 5'-GTCAGCAACCCCTGGAC-3';
Parkin forward, 5'-AAACAAGCAACCCTCACCT-3' and reverse,
5'-GGCACTCACCACTCATCC-3'; p62 forward, 5'-CAGCACAGGCACAGAAGA-3' and
reverse, 5'-GTCCCACCGACTCCAAG-3'; cytochrome c oxidase IV
(COX-IV) forward, 5'-CTACCCCTTGCCTGATGTG-3' and reverse,
5'-TGGATGCGGTACAACTGAA-3'; and GAPDH forward,
5'-TGTTTCCTCGTCCCGTAGA-3' and reverse,
5'-ATCTCCACTTTGCCACTGC-3'.
Cell apoptosis
A total of 2x105 DACs were collected by
centrifugation at 168 x g for 5 min at room temperature, and
Annexin V-FITC binding solution was used to resuspend the cell
pellet gently. Next, 20 µg/ml Annexin V-FITC and 20 µg/ml propidium
iodide (PI) were added to the suspension in sequence and mixed
gently, incubated for 20 min at room temperature (20-25˚C) in the
dark, then placed in icy water for 5 min. Detection was finished
within 1 h from staining and was carried out using a flow cytometer
(FACSCalibur™; BD Biosciences). CellQuest Pro software
(version 5.1.0; BD Biosciences) was used for analysis. The Annexin
V-FITC/PI assay kit (cat. no. 556547) was purchased from BD
Bioscience. Experimental procedures were performed as previously
described (7).
Cell cycle distribution analysis
A total of 2x105 DACs were collected by
centrifugation at 168 x g for 5 min at room temperature, washed
with ice-cold PBS, then fixed with ice-cold 70% ethanol (cat. no.
459836; MilliporeSigma) overnight in a freezer at 4˚C. The fixed
cells were centrifuged at 1,000 x g for 5 min to collect the
pellet, washed with ice-cold PBS and resuspended with staining
buffer containing 50 µg/ml PI, 100 µg/ml RNase A, and 0.2% Triton
X-100. The cell suspension was incubated at 37˚C for 30 min in the
dark. Flow cytometry (FACSCalibur; BD Biosciences) was performed to
evaluate cell cycle distribution within 24 h from staining.
CellQuest Pro software (version 5.1.0; BD Biosciences) was used for
analysis. The cell cycle analysis kit (cat. no. C1052) was
purchased from Beyotime Institute of Biotechnology.
Mitochondrial membrane potential (ΔΨm)
measurement
Tetraethylbenzimidazolylcarbocyanine iodide (JC-1,
10 µg/ml; cat. no. C2006; Beyotime Institute of Biotechnology) was
added to the 6-well plates with 2x105 DACs and incubated
at 37˚C for 20 min. Next, the cells were collected by
centrifugation at 168 x g for 5 min at room temperature, the
supernatant was discarded, and the cells were washed with JC-1
staining buffer. The cell suspension was centrifuged at 168 x g for
5 min at room temperature and pellets were resuspended with JC-1
staining buffer again. The fluorescence intensity was detected
promptly at dual excitation wavelengths (shift from 490 to 525 nm)
and dual emission wavelengths (shift from 530 to 590 nm) using an
Olympus IX71 fluorescence microscope (Olympus Corporation;
magnification, x100). There is an inverse relationship between the
degree of mitochondrial depolarization and the ratio value of
red/green fluorescence intensity (7).
Mitochondrial permeability transition
pore (mPTP) opening assay
A total of 2x105 DACs were incubated with
5 µM calcein-AM (cat. no. sc-203865; Santa Cruz Biotechnology,
Inc.) and 5 µM CoCl2 (cat. no. 60818; MilliporeSigma)
simultaneously for 15 min at 37˚C. Cells were centrifuged at 168 x
g for 5 min at room temperature and washed, and cell pellets were
resuspended in 0.4 ml PBS. Flow cytometry (FACSCalibur; BD
Biosciences) was then performed to detect the mitochondrial
calcein-AM fluorescence. CellQuest Pro software (version 5.1.0; BD
Biosciences) was used for analysis. The maximum excitation and
emission wavelengths of calcein-AM are 494/517 nm. There is an
inverse correlation between the number of mPTP opening and the
calcein-AM fluorescence intensity (7).
Mitochondrial and cytosolic protein
extraction
Mitochondrial and cytosolic protein extractions were
performed using Cell Mitochondria Isolation Kit (cat. no. C3601;
Beyotime Institute of Biotechnology). DACs were collected and
resuspended in mitochondrial separation reagent containing 1 mM
PMSF. After incubation on ice for 15 min, the cell suspension was
homogenized, then centrifuged at 1,000 x g for 10 min at 4˚C. The
supernatant was then centrifuged at 11,000 x g for 10 min at 4˚C.
The precipitation was blended with mitochondrial lysate solution to
obtain mitochondrial proteins. The supernatant was centrifuged at
12,000 x g for 20 min at 4˚C to obtain cytosolic proteins.
Western blotting
The treated cells were harvested and completely
lysed with RIPA lysis buffer (cat. no. P0013B), and a BCA protein
assay kit (cat. no. P0012; Beyotime Institute of Biotechnology) was
used to determine the total protein level in the sample. Cell
protein extracts (20 µg) were separated using 10-15% SDS-PAGE and
transferred to PVDF membranes. After blocking with 5% non-fat milk
powder (cat. no. P0216; Beyotime Institute of Biotechnology) at
room temperature for 1.5 h, the following primary antibodies were
added and incubated overnight at 4˚C: Anti-SIRT3 (cat. no. 5490),
anti-cytochrome c (Cyt c, cat. no. 4272), anti-Bax (cat. no.
2772), anti-Bcl-2 (cat. no. 3498), anti-cleaved caspase-3 (cat. no.
9664), anti-COX-IV (cat. no. 4844) and anti-caspase-1 (cat. no.
24232) (all from Cell Signaling Technology, Inc. and diluted
1:1,000); anti-cyclophilin D (CypD, cat. no. ab167513), anti-LC3
(cat. no. ab192890), anti-Beclin-1 (cat. no. ab207612) and
anti-NLPR3 (cat. no. ab270449) (all from Abcam and diluted
1,1,000); anti-SOD2 (cat. no. 24127-1-AP; 1:1,000) and
anti-GAPDH (cat. no. 10494-1-AP; 1:2,000) (both from ProteinTech
Group, Inc.). Subsequently, HRP-conjugated goat anti-rabbit IgG
(H+L) secondary antibody (cat. no. SA00001-2; 1:5,000; ProteinTech
Group) was added at room temperature for 2 h. The bands were
visualized using an enhanced chemiluminescence reagent kit (cat.
no. P0018M; Beyotime Institute of Biotechnology). The intensity of
the optical bands was quantified using AlphaEaseFC software
(version 4.0.0; Alpha Innotech Corporation).
Detection of caspase-3 and caspase-9
enzyme activities
The spectrophotometric method (at a wavelength of
405 nm) was used to measure the caspase-3 and caspase-9 enzyme
activities in the cell lysate. The caspase-3 and caspase-9 testing
kits (cat. nos. C1116 and C1158) were purchased from Beyotime
Institute of Biotechnology.
Detection of IL-1β and IL-18
levels
IL-1β and IL-18 levels in the cell lysate were
determined using the spectrophotometric method (at a wavelength of
450 nm), according to the manufacturer's instructions. The IL-1β
and IL-18 ELISA kits (cat. nos. PI301 and PI553) were purchased
from Beyotime Institute of Biotechnology.
Statistical analysis
SPSS software (version 20.0; IBM Corporation) was
used to analyze the data. The minimal number of independent
experiments performed for the different assays was four.
Statistical significance of the differences between groups was
determined using one-way ANOVA followed by the least significant
difference (three groups) or Tukey's post hoc test (four groups).
P<0.05 was considered to indicate a statistically significant
difference.
Results
SIRT3 overexpression mitigates
microglia activation-mediated cytotoxicity in DACs
Our previous studies confirmed that inflammatory
factors released from activated microglia could promote apoptosis
through the mitochondrial pathway and markedly reduce SIRT3 gene
expression in DACs (7,16). In the present study, whether SIRT3
expression played a neuroprotective role in DACs was further
examined.
As shown in Fig.
1A, the mRNA levels of SIRT3 and SOD2 in DACs transfected with
SIRT3 plasmid (SIRT3 group) increased ~7-fold, compared with those
in the vector or control groups. Moreover, the protein levels of
SIRT3 and SOD2 in the SIRT3 group were significantly higher than
those in other two groups (Fig.
1B).
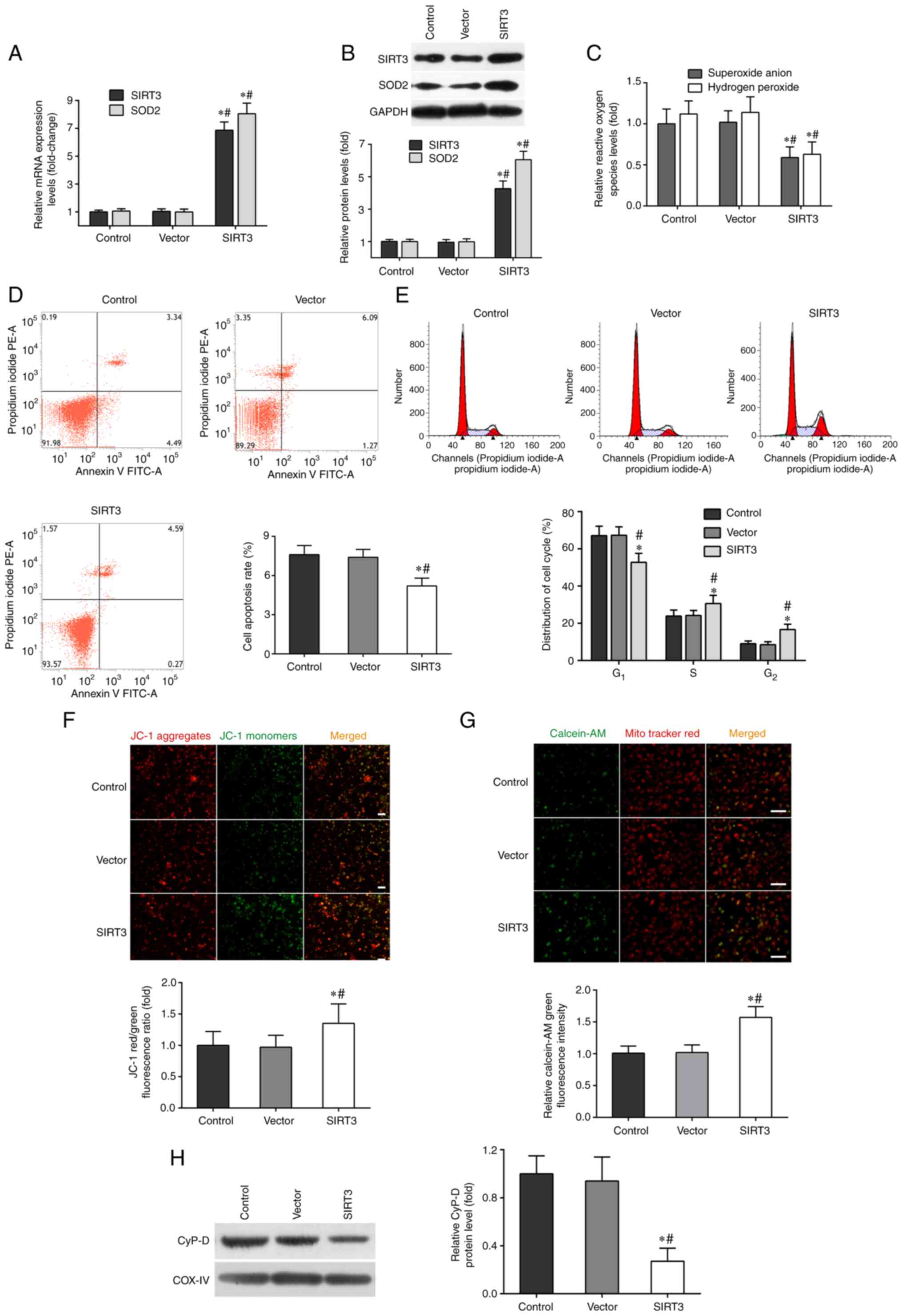 | Figure 1SIRT3 overexpression mitigates
microglia-mediated cytotoxicity in DACs. DACs were incubated with
SIRT3 adenoviral vector or non-targeting empty vector, then
co-cultured with activated-microglia for 48 h. The microglia were
stimulated with 1 µg/ml lipopolysaccharide. (A) The mRNA expression
levels of SIRT3 and SOD2 in DACs were measured using reverse
transcription-quantitative PCR. (B) The protein expression of SIRT3
and SOD2 in DACs was determined using western blotting. (C) The
fluorescent probe dihydroethidium was used to detect intracellular
superoxide anion levels, and a hydrogen peroxide assay kit was used
to detect intracellular hydrogen peroxide levels in DACs. (D) Cell
apoptosis rate and (E) cell cycle distribution of DACs were
measured using flow cytometry. (F) The fluorescence probe JC-1 was
used to detect the loss of mitochondrial membrane potential. Scale
bar, 100 µm. (G) Mitochondrial calcein-AM green fluorescence
intensity was examined using flow cytometry to evaluate the number
of the membrane permeability transport pores opening in DACs.
MitoTracker (red) staining indicates the localization of
mitochondria. Scale bar, 50 µm. (H) Western blotting was performed
to detect the protein levels of mitochondrial cyclophilin D in
DACs. Data in A, B, C, F, G and H are shown as fold change over the
Control group. Data are presented as mean ± S.D. n=4.
*P<0.05 vs. Control; #P<0.05 vs.
Vector. SIRT3, sirtuin 3; DAC, dopaminergic neuronal cell; SOD2,
superoxide dismutase 2; JC-1, tetraethylbenzimidazolylcarbocyanine
iodide. |
SIRT3 overexpression also inhibited superoxide anion
and hydrogen peroxide production in DACs induced by microglia
activation, compared with the control and vector groups (Fig. 1C). The results of flow cytometry
showed that SIRT3 overexpression in DACs could significantly
decrease their apoptotic rate (Fig.
1D), increase the frequency of S and G2/M phase
cells, and reduce the number of G0/G1 phase
cells (Fig. 1E). JC-1 and
calcein-AM fluorescent probe assays indicated that SIRT3
overexpression in DACs reduced ΔΨm depolarization (Fig. 1F) and microglia activation-induced
mPTP opening (Fig. 1G). SIRT3
overexpression also significantly inhibited the protein expression
of CypD in the SIRT3 group (Fig.
1H). These results revealed that SIRT3 overexpression in DACs
could relieve microglia activation-induced neuron injury.
Microglia activation-induced
cytotoxicity is increased following SIRT3 knockdown
The next experiments aimed to determine whether
SIRT3 knockdown could exacerbate microglia activation-induced
cytotoxicity in DACs. As shown in Fig.
2A, the mRNA levels of SIRT3 and SOD2 in DACs transfected with
SIRT3 siRNA (siSIRT3 group) were decreased by ~70% compared with
those in the scrambled (transfected with negative control siRNA) or
control groups. The protein levels of SIRT3 and SOD2 in the siSIRT3
group were also significantly lower than those in the other two
groups (Fig. 2B).
 | Figure 2SIRT3 knockdown by siRNA transfection
impairs DAC function. DACs were treated with SIRT3 siRNA (siSIRT3
group) or scrambled siRNA, then co-cultured with
activated-microglia for 48 h. Microglia were exposed to 1 µg/ml
lipopolysaccharide. (A) Reverse transcription-quantitative PCR was
performed to measure the mRNA expression of SIRT3 and SOD2 in DACs.
(B) The protein levels of SIRT3 and SOD2 in DACs were determined
using western blotting. (C) The fluorescent probe dihydroethidium
was used to detect intracellular superoxide anion level, and a
hydrogen peroxide assay kit was used to detect intracellular
hydrogen peroxide level in DACs. (D) Cell apoptosis rate and (E)
cell cycle distribution of DACs in the Control, Scrambled and
siSIRT3 groups were measured using flow cytometry. (F) The
fluorescence probe JC-1 was used to detect the loss of
mitochondrial membrane potential. Scale bar, 100 µm. (G)
Mitochondrial calcein-AM green fluorescence intensity was examined
using flow cytometry to evaluate the number of the membrane
permeability transport pores opening in DACs. MitoTracker (red)
staining indicates the localization of mitochondria. Scale bar, 50
µm. (H) Protein level of mitochondrial cyclophilin D in DACs was
determined using western blotting. Data in A, B, C, F, G and H are
shown as fold change over Control group. Data are presented as mean
± S.D. n=4. *P<0.05 vs. Control;
#P<0.05 vs. Scrambled. SIRT3, sirtuin 3; DAC,
dopaminergic neuronal cell; si/siRNA, small interfering RNA; SOD2,
superoxide dismutase 2; JC-1, tetraethylbenzimidazolylcarbocyanine
iodide. |
SIRT3 silencing further promoted intracellular
microglia activation-induced ROS production in the siSIRT3 group
(Fig. 2C). The flow cytometry
results demonstrated that SIRT3 knockdown in DACs significantly
increased their apoptotic rate (Fig.
2D), decreased the frequency of cells in the S and
G2 phases, and increased the number of cells in the
G0/G1 phase (Fig. 2E). JC-1 and calcein-AM fluorescent
probe assays indicated that SIRT3 knockdown in DACs further
enhanced microglia activation-induced ΔΨm depolarization (Fig. 2F) and mPTP opening (Fig. 2G). siSIRT3 transfection also
promoted the protein expression of CypD in the siSIRT3 group
(Fig. 2H). In conclusion, these
data provided evidence that SIRT3 knockdown in DACs exacerbated
microglia activation-induced cell damage.
SIRT3-induced neuroprotection in DACs
is associated with the mitochondrial apoptotic pathway
In order to study the molecular mechanisms
underlying SIRT3-induced neuroprotection in DACs against microglia
activation-mediated cytotoxicity, ELISA and western blotting were
performed to measure the levels of several key proteins in the
mitochondrial apoptotic signaling pathway. Compared with the vector
or control group, SIRT3 overexpression significantly decreased the
protein levels of cytoplasmic Cyt C, Bax and total cleaved
caspase-3 in SIRT3-overexpressing DACs, and increased the protein
levels of mitochondrial Cyt C and total Bcl-2 (Fig. 3A). By contrast, compared with the
control or scrambled group, SIRT3 knockdown significantly increased
the expression of cytoplasmic Cyt C, Bax and total cleaved
caspase-3 in siSIRT3 group DACs, and decreased the expression of
mitochondrial Cyt C and total Bcl-2 (Fig. 3B).
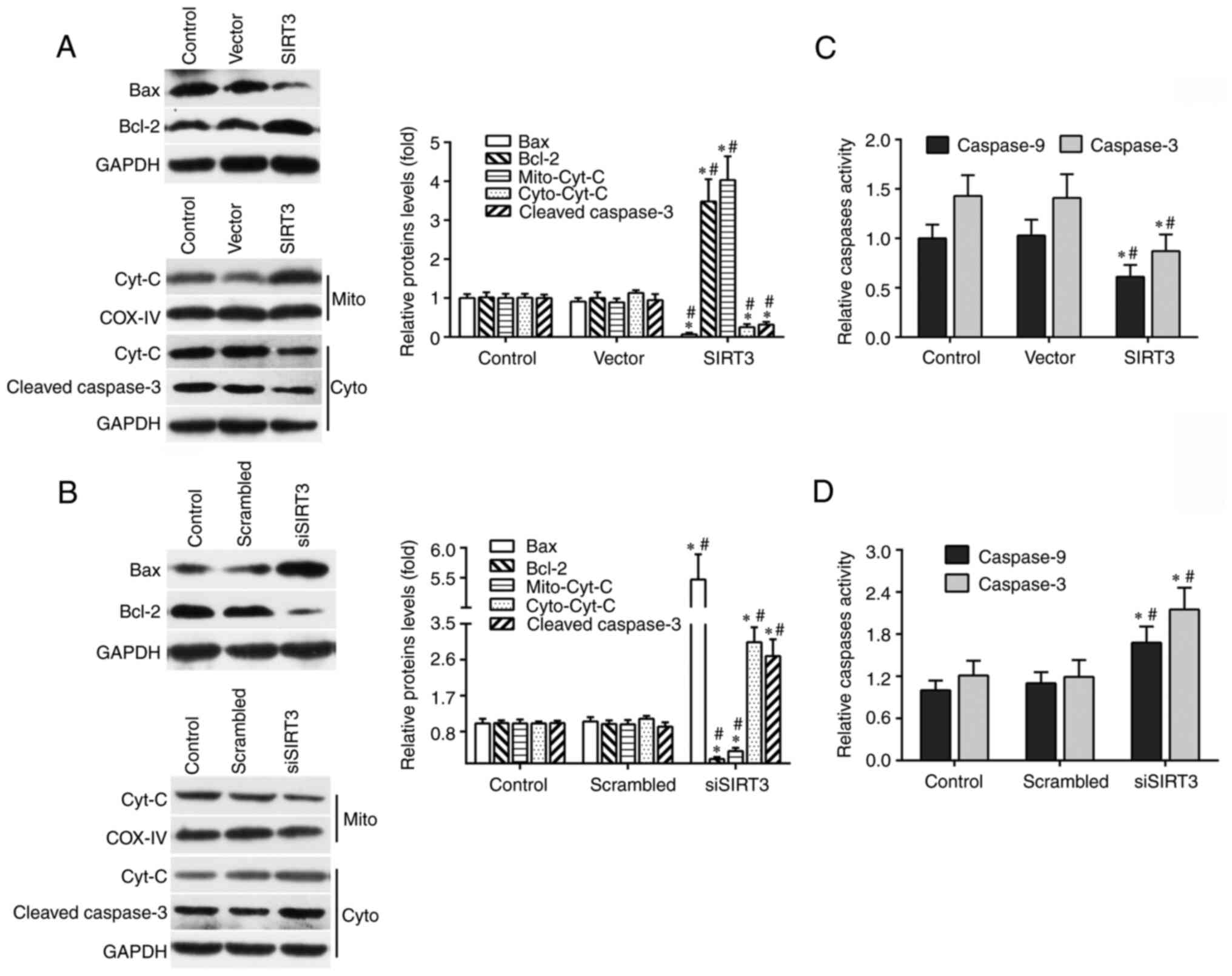 | Figure 3SIRT3 in DACs contributes to
neuroprotection via mitochondrial apoptotic pathway. (A) Protein
levels of Bax, Bcl-2, cleaved caspase-3, Mito-Cyt C and Cyto-Cyt C
in DACs from the SIRT3 group, Vector group, and Control group were
measured using western blotting. (B) Protein levels of Bax, Bcl-2,
cleaved caspase-3, Mito-Cyt C and Cyto-Cyt C in DACs from the
siSIRT3 group, Scrambled group, and Control group were determined
using western blotting. Representative western blot (left panel)
and quantification (right panel) of Bax, Bcl-2, Cyt C and cleaved
caspase-3 protein expression in DACs transfected with scrambled
siRNA or siSIRT3 for 48 h. The spectrophotometric method was used
to detect caspase-3 and caspase-9 enzyme activities in DACs
transfected with SIRT3 plasmid or empty vector (C), and scrambled
siRNA or SIRT3 siRNA (D) for 48 h. Results are normalized to those
of the Control group. Data are presented as mean ± S.D. n=4.
*P<0.05 vs. Control; #P<0.05 vs. Vector
or Scrambled. SIRT3, sirtuin 3; DAC, dopaminergic neuronal cell;
si/siRNA, small interfering RNA; Mito-Cyt C, mitochondrial
cytochrome c; Cyto-Cyt C, cytoplasmic cytochrome
c. |
In addition, SIRT3 overexpression significantly
reduced the enzymatic activities of caspase-3 and -9 (Fig. 3C), while SIRT3 knockdown
significantly increased them (Fig.
3D). These results demonstrated that SIRT3 in DACs attenuated
microglia activation-induced cell damage through the mitochondrial
signaling pathway, which included a decreased Bax protein level and
caspase-3/9 enzymatic activity, decreased mitochondrial Cyt C
release into the cytoplasm and increased Bcl-2 protein levels.
The mitophagy-NLRP3 pathway is
correlated with the neuroprotective effect of SIRT3 in DACs
In order to clarify whether the neuroprotection of
SIRT3 was associated with mitochondrial autophagy and NLRP3
inflammasome activation, the protein levels of the key molecules in
the mitophagy-NLRP3 inflammasome pathway were detected. As shown in
Fig. 4A, the protein levels of
LC3II/LC3I and Beclin-1 in the control group were significantly
reduced compared with the blank group, and those of NLRP3 and
caspase-1 were significantly increased. Compared with the control
group, SIRT3 overexpression increased the levels of LC3II/LC3I and
Beclin-1, and decreased those of NLRP3 and caspase-1. Conversely,
SIRT3 knockdown led to an opposite protein expression trend. The
results of RT-qPCR showed that microglia activation significantly
decreased the mRNA levels of LC3 and Parkin, and increased the mRNA
levels of p62 and mitochondrial marker COX-IV. SIRT3 knockdown
further exacerbated the changing trends of these genes expression,
except for the COX-IV gene. However, SIRT3 overexpression increased
LC3 and Parkin mRNA levels, and decreased those of p62 (Fig. 4B). Furthermore, compared with the
blank group, microglia activation significantly elevated the
protein levels of IL-1β and IL-18 in the control group. Compared
with the control group, SIRT3 knockdown further increased IL-1β and
IL-18 levels in siSIRT3-transfected DACs, but decreased them in
SIRT3-overexpressing DACs (Fig.
4C).
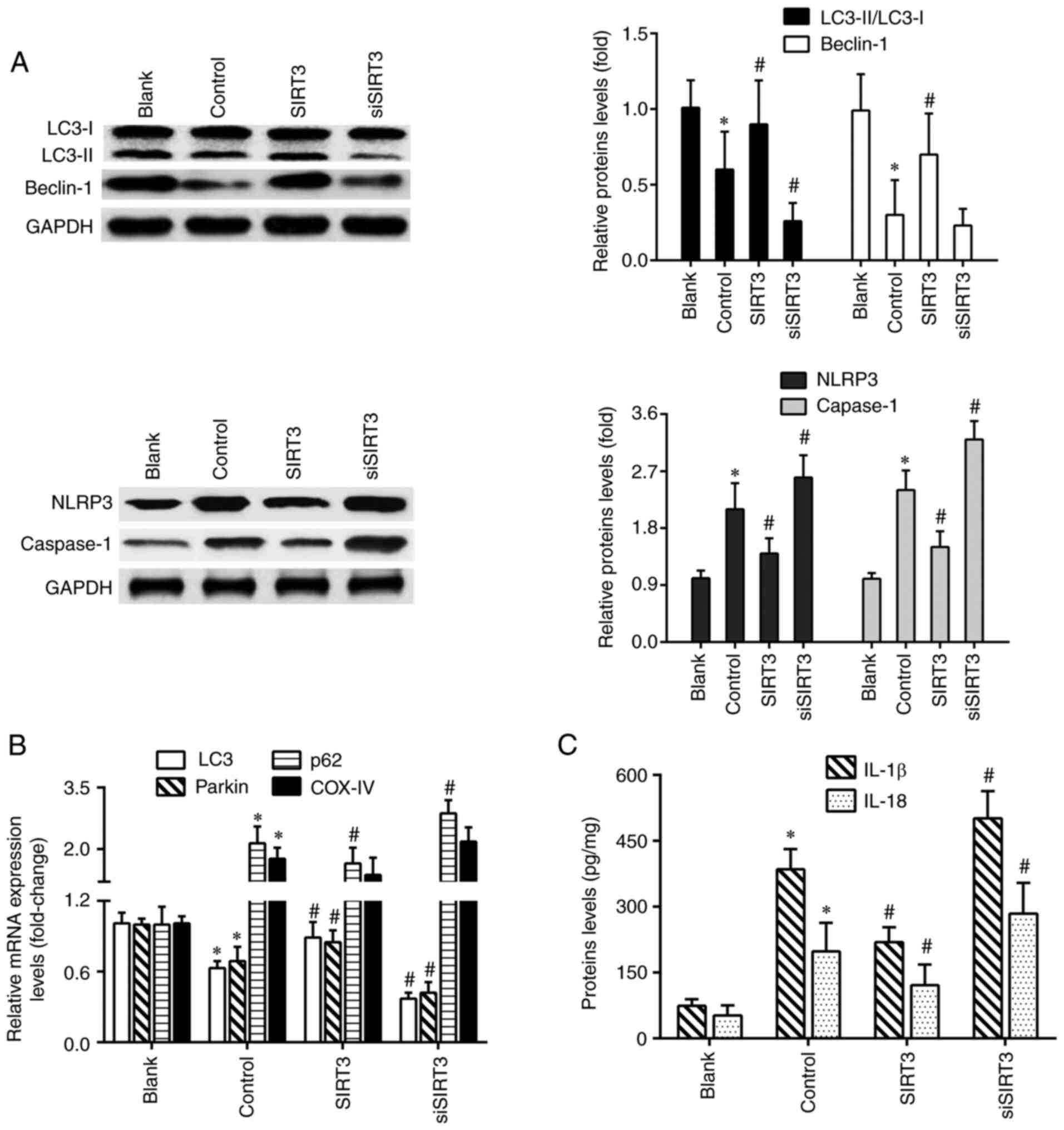 | Figure 4SIRT3 overexpression promotes
mitochondrial autophagy and inhibits NLRP3 inflammasome activation
in DACs. (A) Protein expression of LC3II/LC3I, Beclin-1, NLRP3 and
caspase-1 in DACs treated with 1 µg/ml lipopolysaccharide, SIRT3
plasmid or SIRT3 siRNA were analyzed using western blotting. (B)
The mRNA expression levels of LC3, Parkin, p62 and COX-IV in DACs
from the Blank, Control, SIRT3 and siSIRT3 group were detected
using reverse transcription-quantitative PCR. (C) IL-1β and IL-18
protein levels in DACs from the Blank, Control, SIRT3 and siSIRT3
group were measured using ELISA. *P<0.05 vs. blank;
#P<0.05 vs. control. SIRT3, sirtuin 3; DACs,
dopaminergic neuronal cells; siRNA, small interfering RNA; NLRP3,
nucleotide-binding oligomerization domain, leucine-rich repeat and
pyrin domain-containing protein 3. |
Discussion
In the present study, it was found that SIRT3
overexpression in dopaminergic neurons reduced microglia
activation-mediated apoptosis and promoted cell cycle progression,
which were conducive to the neuronal survival, whereas SIRT3
knockdown had the opposite effects in DACs upon exposure to
LPS-challenged microglia. Pharmacologically increasing the SIRT3
level could counteract α-synuclein-induced mitochondrial
dysfunction by decreasing α-synuclein oligomer formation and
normalizing mitochondrial bioenergetics in a rodent model of PD
(33). Xu et al (34) suggested that rat chondrocytes
underwent mitochondrial dysfunction, inflammation, cell apoptosis
and degeneration following IL-1β stimulation, which were inhibited
by SIRT3 overexpression and were enhanced in chondrocytes following
SIRT3 knockdown. Decreased NAD+ levels and SIRT3
activity are involved in the aging process and have been
pathologically associated with PD pathogenesis (35). SIRT3 chemical activators and
NAD+ precursors can upregulate SIRT3 activity to protect
against dopaminergic neuron degeneration in PD models (35). A decrease in SIRT3 expression via
the inhibitor nicotinamide or siRNA transfection results in the
accumulation of Cyt C oxidase 1 acetylation, increased cell
apoptosis and decreased ΔΨm in primary neuronal cells (36). Clinical and experimental studies
have demonstrated that the expression of peroxisome
proliferator-activated receptor coactivator 1α (PGC-1α) is
decreased in the brain tissue of patients with PD and animal models
(26). PGC-1α overexpression can
inhibit α-synuclein aggregation, and PGC-1α can also bind to the
SIRT3 gene promoter to increase SIRT3 transcription, SIRT3
activates SOD2 and ATP synthase to reduce ROS accumulation and ATP
consumption, respectively, thereby reducing neurotoxicity and
inhibiting the loss of dopaminergic neurons (26).
The results of the present study revealed that the
molecular mechanism underlying the protective effect of SIRT3
against inflammatory and oxidative damage in dopaminergic neurons
is linked to the mitochondrial apoptosis pathway. Specifically,
SIRT3 overexpression in dopaminergic neurons could maintain ΔΨm,
prevent mPTP opening and Cyt C release from the mitochondria to the
cytoplasm, inhibit caspase-3/9 activity, upregulate Bcl-2 protein
expression, and downregulate CypD and Bax protein expression. SIRT3
knockdown resulted in an opposite trend in these parameters. PL171
inhibits ΔΨm reduction and ROS generation induced by
Aβ42 oligomers in an SIRT3-dependent manner, and the
weakening of SIRT3 activity abolishes the protective actions of
PL171 in human neuronal cells (17). The neuroprotection of the sesamin
and sesamol compounds in H2O2-treated human
neuronal cells can be attributed to the activation of SIRT3-FOXO3A
expression, Bax inhibition and Bcl-2 upregulation (37). Ginsenoside Rb1 protects human
umbilical vein endothelial cells from high glucose-induced
apoptosis through the SIRT3 signaling pathway. Rb1 increases the
activities of antioxidant enzymes, decreases Cyt C release from the
mitochondria to the cytosol and maintains the ΔΨm; the action of
Rb1 against high glucose-induced mitochondria-related apoptosis is
inhibited by SIRT3 inhibitor 3-(1H-1,2,3-triazol-4-yl) pyridine
(3-TYP) (38). Yang et al
(39) reported that ischemic
injury resulted in cell apoptosis and mPTP opening by reducing
SIRT3, which helped identify a novel target for the treatment of
ischemic stroke. The conclusions of this study are in line with
those of previous reports.
The present study also determined that
LPS-stimulated microglia activation could reduce the levels of key
proteins in the mitophagy pathway and increase the expression of
important molecules in the NLRP3 inflammasome pathway in DACs.
SIRT3 silencing further aggravated mitophagy inhibition and NLRP3
inflammasome activation. Conversely, SIRT3 overexpression could
significantly reverse these phenomena. SIRT3 expression in
dopaminergic neurons relieved microglial activation-induced
cytotoxicity, which was associated with the mitophagy-NLRP3
inflammasome pathway. It has been reported that SIRT3 promotes
autophagy through the adenosine monophosphate-activated protein
kinase (AMPK)-mTOR signaling pathway, thereby reducing α-synuclein
aggregation, inhibiting oxidative stress and alleviating
mitochondrial dysfunction in a rotenone-induced PD cell model
(40). Moderate oxygen-glucose
deprivation can upregulate the expression of SIRT3 in neurons, and
SIRT3 also facilitates mitochondrial autophagy through the
AMPK-mTOR pathway, thus contributing to neuronal survival (41). SIRT3 overexpression can reverse
autophagy inhibition caused by IL-1β in rat chondrocytes (33). Furthermore, SIRT3 can bind to and
deacetylate PTEN-induced kinase 1 (PINK1) and Parkin to facilitate
mitochondrial autophagy. The level of mitophagy is markedly
increased in diabetic corneal epithelial cells when the
FOXO3A/PINK1-Parkin pathway is activated via SIRT3 overexpression
(28). SIRT3 knockdown results in
the downregulation of Beclin-1 and LC3II, suggesting that SIRT3 is
an important regulator of autophagy in Aβ1-42
oligomer-treated HT22 cells (12).
Mitochonic acid 5 effectively alleviates neuroinflammatory damage
by inhibiting mitochondrial dysfunction and enhancing cell
survival. The protective effect of mitochonic acid 5 on neuronal
damage can be attributed to the activation of AMPK-SIRT3 pathway
and Parkin-related mitophagy (42). Metformin suppresses IL-1β-mediated
oxidative and osteoarthritis-like inflammatory damage by activating
the SIRT3/PINK1/Parkin signaling pathway, and the SIRT3 inhibitor
3-TYP effectively inhibits mitophagy initiation and reduces the
LC3II/LC3I ratio (43). Cyperone
increases SIRT3 expression and decreased ROS production in the
hippocampus of depressed mice, NLRP3 inflammasome-related proteins
including NLRP3, caspase-1, IL-1β and IL-18 were downregulated
(44). The aforementioned results
and those of the present study are consistent.
Stilbene glycoside treatment facilitates mitophagy
and cell survival and reduces apoptosis; however, SIRT3 knockdown
reduces ΔΨm, cell viability, and LC3II/I and Bcl-2 levels, while
markedly increasing apoptosis, and Bax and caspase 3 protein
expression in ischemic PC12 cells (45). SIRT3-knockout macrophages exhibit
autophagy dysfunction, as well as enhanced NLRP3 inflammasome
expression and vascular inflammation, whereas SIRT3 overexpression
induces autophagosome maturation and inhibits NLRP3 inflammasome
activation (46). AEDC, a
cycloartane triterpenoid isolated from Actaea vaginata,
increased SIRT3 expression to reverse mitochondrial dysfunction and
activate AMPK, which together induced autophagy and in turn
suppressed the NLRP3 inflammasome activation in LPS-treated THP-1
macrophages (47). The SIRT3
agonist resveratrol inhibits NLRP3 inflammasome activation by
maintaining mitochondrial integrity and amplifying the effect of
autophagy (48). Resveratrol also
protects neuronal cells against tunicamycin-induced endoplasmic
reticulum stress damage by the SIRT3-mediated mitochondrial
autophagy pathway (49). The data
in the aforementioned reports are also in line with our findings.
SIRT3 in dopaminergic neurons plays a role in resisting
neuroinflammation through the mitophagy-NLRP3 inflammasome pathway.
However, the findings the present study are not sufficient to
support this conclusion, which needs to be verified by additional
experiments using specific inhibitors and gene silencing in the
future. Future experiments should include differentiation of Lund
human mesencephalic cells, an immortalized precursor cell line of
human dopaminergic neuron, to further validate the results obtained
in the present study.
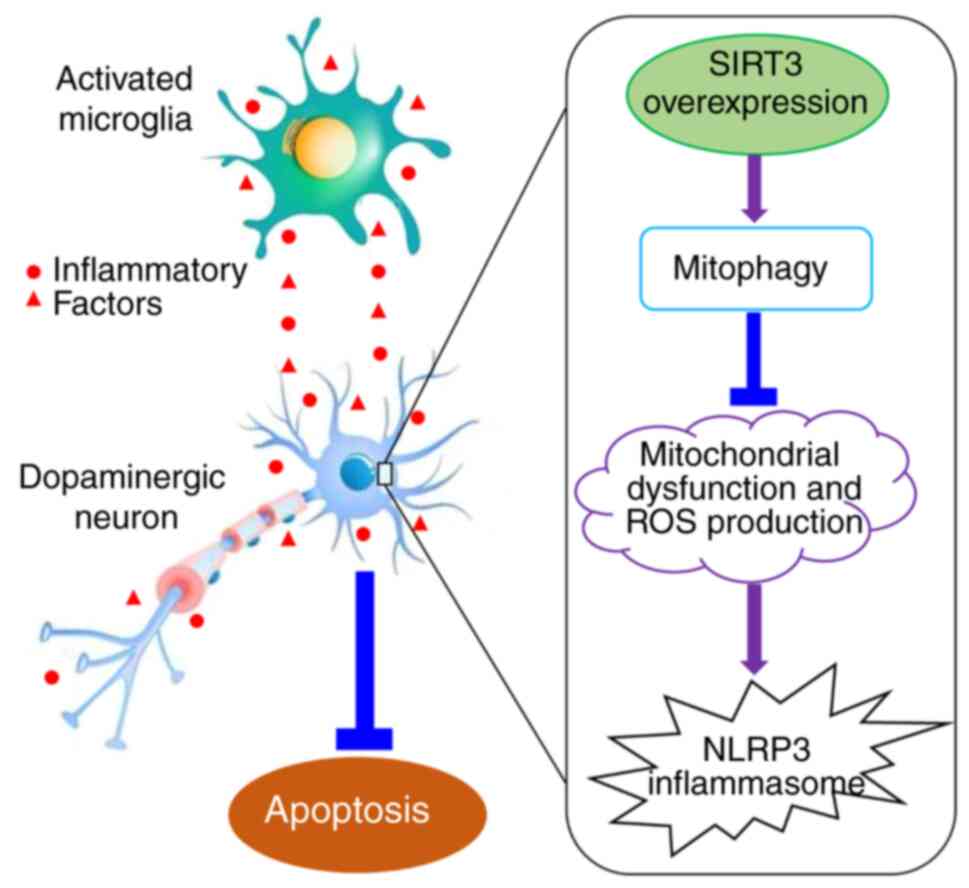
In conclusion, the results of the present study
indicated that mitochondrial SIRT3 in dopaminergic neurons can
improve mitochondrial dysfunction and decrease ROS generation,
thereby reducing the loss of dopaminergic neurons caused by
microglia activation, which plays a role in resisting
neuroinflammation and oxidative stress injury in PD. The molecular
mechanisms underlying this neuroprotection may be associated with
increased mitophagy, and reduced NLRP3 inflammasome activation
(Fig. 5). The exact association
between the mitophagy-NLRP3 pathway and the neuroprotective effect
of SIRT3 in DACs needs to be further investigated and verified in
the future. The findings of the present study elucidated the
mechanism of SIRT3 neuroprotection in PD, and identified new
intervention targets for the clinical prevention and treatment of
PD and the development of innovative drugs.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the Natural
Science Foundation of Guangxi Zhuang Autonomous Region of China
(grant nos. 2021GXNSFAA220001 and 2018GXNSFAA050002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DQJ designed and performed experiments, and drafted
manuscript. QMZ designed experiments. LLJ and CSL analyzed the
data. SHZ and LCX performed experiments. CSL and LCX reviewed and
edited the manuscript. DQJ and QMZ confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martínez-Menárguez JÁ, Martínez-Alonso E,
Cara-Esteban M and Tomás M: Focus on the small GTPase Rab1: A key
player in the pathogenesis of Parkinson's disease. Int J Mol Sci.
22(12087)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chu YT, Tai CH, Lin CH and Wu RM: Updates
on the genetics of Parkinson's disease: Clinical implications and
future treatment. Acta Neurol Taiwan. 30:83–93. 2021.PubMed/NCBI
|
|
3
|
Luo Y, Hoffer A, Hoffer B and Qi X:
Mitochondria: A therapeutic target for Parkinson's disease? Int J
Mol Sci. 16:20704–20730. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rasheed M, Liang J, Wang C, Deng Y and
Chen Z: Epigenetic regulation of neuroinflammation in Parkinson's
disease. Int J Mol Sci. 22(4956)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Badanjak K, Fixemer S, Smajić S, Skupin A
and Grünewald A: The contribution of microglia to neuroinflammation
in Parkinson's disease. Int J Mol Sci. 22(4676)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zheng T and Zhang Z: Activated microglia
facilitate the transmission of α-synuclein in Parkinson's disease.
Neurochem Int. 148(105094)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang DQ, Ma YJ, Wang Y, Lu HX, Mao SH and
Zhao SH: Microglia activation induces oxidative injury and
decreases SIRT3 expression in dopaminergic neuronal cells. J Neural
Transm (Vienna). 126:559–568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin W, Qian X, Yang LK, Zhu J, Wang D,
Hang CH, Wang Y and Chen T: Inhibition of miR-134-5p protects
against kainic acid-induced excitotoxicity through Sirt3-mediated
preservation of mitochondrial function. Epilepsy Res.
176(106722)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang W, Wang Y, Hao Y, Wang Z, Liu J and
Wang J: Piceatannol alleviate ROS-mediated PC-12 cells damage and
mitochondrial dysfunction through SIRT3/FOXO3a signaling pathway. J
Food Biochem. 46(e13820)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Anamika Khanna A, Acharjee P, Acharjee A
and Trigun SK: Mitochondrial SIRT3 and neurodegenerative brain
disorders. J Chem Neuroanat. 95:43–53. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu L, Peritore C, Ginsberg J, Kayhan M
and Donmez G: SIRT3 attenuates MPTP-induced nigrostriatal
degeneration via enhancing mitochondrial antioxidant capacity.
Neurochem Res. 40:600–608. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Z, Han K, Wang C, Sun C and Jia N:
Dioscin protects against Aβ1-42 oligomers-induced neurotoxicity via
the function of SIRT3 and autophagy. Chem Pharm Bull (Tokyo).
68:717–725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Naia L, Carmo C, Campesan S, Fão L, Cotton
VE, Valero J, Lopes C, Rosenstock TR, Giorgini F and Rego AC:
Mitochondrial SIRT3 confers neuroprotection in Huntington's disease
by regulation of oxidative challenges and mitochondrial dynamics.
Free Radic Biol Med. 163:163–179. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nissanka N and Moraes CT: Mitochondrial
DNA damage and reactive oxygen species in neurodegenerative
disease. FEBS Lett. 592:728–742. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shen Y, Wu Q, Shi J and Zhou S: Regulation
of SIRT3 on mitochondrial functions and oxidative stress in
Parkinson's disease. Biomed Pharmacother.
132(110928)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang DQ and Wang Y, Li MX, Ma YJ and Wang
Y: SIRT3 in neural stem cells attenuates microglia
activation-induced oxidative stress injury through mitochondrial
pathway. Front Cell Neurosci. 11(7)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Y, Lu J, Cao X, Zhao H, Gao L, Xia P
and Pei G: A newly synthesized rhamnoside derivative alleviates
Alzheimer's amyloid-β-induced oxidative stress, mitochondrial
dysfunction, and cell senescence through upregulating SIRT3. Oxid
Med Cell Longev. 2020(7698560)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng A, Wang J, Ghena N, Zhao Q, Perone
I, King TM, Veech RL, Gorospe M, Wan R and Mattson MP: SIRT3
Haploinsufficiency aggravates loss of GABAergic interneurons and
neuronal network hyperexcitability in an Alzheimer's disease model.
J Neurosci. 40:694–709. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang S, Ma Y and Feng J: Neuroprotective
mechanisms of ε-viniferin in a rotenone-induced cell model of
Parkinson's disease: Significance of SIRT3-mediated FOXO3
deacetylation. Neural Regen Res. 15:2143–2153. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hong C, Seo H, Kwak M, Jeon J, Jang J,
Jeong EM, Myeong J, Hwang YJ, Ha K, Kang MJ, et al: Increased TRPC5
glutathionylation contributes to striatal neuron loss in
Huntington's disease. Brain. 138:3030–3047. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Karuppagounder SS, Xu H, Shi Q, Chen LH,
Pedrini S, Pechman D, Baker H, Beal MF, Gandy SE and Gibson GE:
Thiamine deficiency induces oxidative stress and exacerbates the
plaque pathology in Alzheimer's mouse model. Neurobiol Aging.
30:1587–1600. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Y, Li H, Li Y, Yang M, Wang X and Peng
Y: Velvet antler methanol extracts ameliorate Parkinson's disease
by inhibiting oxidative stress and neuroinflammation: From C.
elegans to mice. Oxid Med Cell Longev. 2021(8864395)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee S, Jeon YM, Jo M and Kim HJ:
Overexpression of SIRT3 suppresses oxidative stress-induced
neurotoxicity and mitochondrial dysfunction in dopaminergic
neuronal cells. Exp Neurobiol. 30:341–355. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Cui XX, Li X, Dong SY, Guo YJ, Liu T and
Wu YC: SIRT3 deacetylated and increased citrate synthase activity
in PD model. Biochem Biophys Res Commun. 484:767–773.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Duan WJ, Liang L, Pan MH, Lu DH, Wang TM,
Li SB, Zhong HB, Yang XJ, Cheng Y, Liu B, et al: Theacrine, a
purine alkaloid from kucha, protects against Parkinson's disease
through SIRT3 activation. Phytomedicine. 77(153281)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang X, Ren X, Zhang Q, Li Z, Ma S, Bao
J, Li Z, Bai X, Zheng L, Zhang Z, et al: PGC-1α/ERRα-Sirt3 pathway
regulates DAergic neuronal death by directly deacetylating SOD2 and
ATP synthase β. Antioxid Redox Signal. 24:312–328. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guo Y, Jia X, Cui Y, Song Y, Wang S, Geng
Y, Li R, Gao W and Fu D: Sirt3-mediated mitophagy regulates
AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biol.
41(101915)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu J, Kan T and Hu X: Sirt3 regulates
mitophagy level to promote diabetic corneal epithelial wound
healing. Exp Eye Res. 181:223–231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu W, Gao B, Li N, Wang J, Qiu C, Zhang G,
Liu M, Zhang R, Li C, Ji G and Zhang Y: Sirt3 deficiency
exacerbates diabetic cardiac dysfunction: Role of
Foxo3A-Parkin-mediated mitophagy. Biochim Biophys Acta Mol Basis
Dis. 1863:1973–1983. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zheng K, Bai J, Li N, Li M, Sun H, Zhang
W, Ge G, Liang X, Tao H, Xue Y, et al: Protective effects of
sirtuin 3 on titanium particle-induced osteogenic inhibition by
regulating the NLRP3 inflammasome via the GSK-3β/β-catenin
signalling pathway. Bioact Mater. 6:3343–3357. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen ML, Zhu XH, Ran L, Lang HD, Yi L and
Mi MT: Trimethylamine-N-oxide induces vascular inflammation by
activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS
signaling pathway. J Am Heart Assoc. 6(e006347)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park JH, Burgess JD, Faroqi AH, DeMeo NN,
Fiesel FC, Springer W, Delenclos M and McLean PJ:
Alpha-synuclein-induced mitochondrial dysfunction is mediated via a
sirtuin 3-dependent pathway. Mol Neurodegener. 15(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu K, He Y, Moqbel SAA, Zhou X, Wu L and
Bao J: SIRT3 ameliorates osteoarthritis via regulating chondrocyte
autophagy and apoptosis through the PI3K/Akt/mTOR pathway. Int J
Biol Macromol. 175:351–360. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhou ZD and Tan EK: Oxidized nicotinamide
adenine dinucleotide-dependent mitochondrial deacetylase sirtuin-3
as a potential therapeutic target of Parkinson's disease. Ageing
Res Rev. 62(101107)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tu LF, Cao LF, Zhang YH, Guo YL, Zhou YF,
Lu WQ, Zhang TZ, Zhang T, Zhang GX, Kurihara H, et al:
Sirt3-dependent deacetylation of COX-1 counteracts oxidative
stress-induced cell apoptosis. FASEB J. 33:14118–14128.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ruankham W, Suwanjang W, Wongchitrat P,
Prachayasittikul V, Prachayasittikul S and Phopin K: Sesamin and
sesamol attenuate H2O2-induced oxidative
stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling
pathway. Nutr Neurosci. 24:90–101. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ke SY, Yu SJ, Liu DH, Shi GY, Wang M, Zhou
B, Wu L, Song ZM, Zhu JM, Wu CD and Qian XX: Ginsenoside Rb1
protects human umbilical vein endothelial cells against high
glucose-induced mitochondria-related apoptosis through activating
SIRT3 signalling pathway. Chin J Integr Med. 27:336–344.
2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang Y, Tian Y, Guo X, Li S, Wang W and
Shi J: Ischemia Injury induces mPTP opening by reducing Sirt3.
Neuroscience. 468:68–74. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang M, Deng YN, Zhang JY, Liu J, Li YB,
Su H and Qu QM: SIRT3 protects rotenone-induced injury in SH-SY5Y
cells by promoting autophagy through the LKB1-AMPK-mTOR pathway.
Aging Dis. 9:273–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dai SH, Chen T, Li X, Yue KY, Luo P, Yang
LK, Zhu J, Wang YH, Fei Z and Jiang XF: Sirt3 confers protection
against neuronal ischemia by inducing autophagy: Involvement of the
AMPK-mTOR pathway. Free Radic Biol Med. 108:345–353.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang D, Liu M and Jiang Y: Mitochonic
acid-5 attenuates TNF-α-mediated neuronal inflammation via
activating Parkin-related mitophagy and augmenting the AMPK-Sirt3
pathways. J Cell Physiol. 234:22172–22182. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang C, Yang Y, Zhang Y, Liu J, Yao Z and
Zhang C: Protective effects of metformin against osteoarthritis
through upregulation of SIRT3-mediated PINK1/Parkin-dependent
mitophagy in primary chondrocytes. Biosci Trends. 12:605–612.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xia B, Tong Y, Xia C, Chen C and Shan X:
α-Cyperone confers antidepressant-like effects in mice via
neuroplasticity enhancement by SIRT3/ROS mediated NLRP3
inflammasome deactivation. Front Pharmacol.
11(577062)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li Y, Hu K, Liang M, Yan Q, Huang M, Jin
L, Chen Y, Yang X and Li X: Stilbene glycoside upregulates
SIRT3/AMPK to promotes neuronal mitochondrial autophagy and inhibit
apoptosis in ischemic stroke. Adv Clin Exp Med. 30:139–146.
2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu P, Huang G, Wei T, Gao J, Huang C, Sun
M, Zhu L and Shen W: Sirtuin 3-induced macrophage autophagy in
regulating NLRP3 inflammasome activation. Biochim Biophys Acta Mol
Basis Dis. 1864:764–777. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang T, Fang Z, Linghu KG, Liu J, Gan L
and Lin L: Small molecule-driven SIRT3-autophagy-mediated NLRP3
inflammasome inhibition ameliorates inflammatory crosstalk between
macrophages and adipocytes. Br J Pharmacol. 177:4645–4665.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chang YP, Ka SM, Hsu WH, Chen A, Chao LK,
Lin CC, Hsieh CC, Chen MC, Chiu HW, Ho CL, et al: Resveratrol
inhibits NLRP3 inflammasome activation by preserving mitochondrial
integrity and augmenting autophagy. J Cell Physiol. 230:1567–1579.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yan WJ, Liu RB, Wang LK, Ma YB, Ding SL,
Deng F, Hu ZY and Wang DB: Sirt3-mediated autophagy contributes to
resveratrol-induced protection against ER stress in HT22 cells.
Front Neurosci. 12(116)2018.PubMed/NCBI View Article : Google Scholar
|