Introduction
Lung cancer is a common clinical malignant tumor,
and its morbidity and mortality ranks first among malignant tumors
(1). The cases of pulmonary are
also increasing year by year (2).
Histopathological specimens obtained by nodules lung biopsy are
considered the gold standard for the diagnosis of lung cancer
(3). The diagnostic accuracy of
percutaneous transthoracic needle biopsy (PTNB) is ~91.1% (4). However, the accuracy of detecting
pulmonary nodules with a diameter of ≤2.0 cm is usually low, owing
to the difficulty in puncturing.
Rapid on-site evaluation (ROSE) can guide the
operator in real time with regard to the direction of material
sampling and ensure a rapid decision on the adequacy of the
material obtained during the biopsy of certain lesions, which
effectively improves the diagnostic success rate of the biopsy.
ROSE is a safe method and has not been previously reported to
exhibit serious surgical complications (5).
Agarwal et al (6) proposed that ROSE could reduce the
inadequacy of biopsy specimens for bronchoscopy. Izumo et al
(7) suggested that the number of
punctures in endobronchial ultrasound with a guide sheath could be
reduced and the accuracy of pathological results could be improved
by the application of ROSE. However, the use of ROSE has not been
previously examined for PTNB of pulmonary nodules with a diameter
of ≤2.0 cm. Pulmonary nodules of >2.0 cm in diameter can be
examined more accurately by needle biopsy, owing to the larger
sizes of the lesions. When the size of the lesion is uncertain, the
use of ROSE may reduce the accuracy of needle biopsy. The novelty
of the present study was in the application of ROSE only for the
biopsy of pulmonary nodules with a diameter of ≤2.0 cm, which
increases its accuracy. The present study aimed to evaluate the
specimen adequacy rate, diagnostic accuracy, secondary biopsy rate,
complication rate and consistency with the final diagnosis of
pulmonary nodules (≤2.0 cm in diameter) using ROSE in CT-guided
PTNB. The effectiveness of ROSE was also analyzed.
Materials and methods
Study population
The present retrospective study was approved by the
Ethics Committee of Qiqihar Medical College (Qiqihar, China;
protocol no. 2021-193). The data used in the present study were
anonymous; therefore, the present study was exempt from informed
consent and was compliant with The Declaration of Helsinki. The
medical records of patients undergoing PTNB in the Second
Affiliated Hospital of Qiqihar Medical College, between June 2018
and June 2021, were retrospectively analyzed. Inclusion criteria
patients who received PTNB and were able to provide images and
reports. Exclusion criteria included lesions >2.0 cm and fine
needle aspiration biopsy. A total of 250 patients were included
(age, 26-85 years; 152 males and 98 female); of these, 167 patients
(age, 29-85 years; 101 males and 85 female) with malignant disease
and 83 patients (age, 26-84 years; 51 males and 32 female) with
benign disease. PTNB was selected for patients with lung lesions
that were unsuitable for or did not provide sufficient specimens
for transbronchial biopsy.
Procedure protocol and policies
All patients signed the preoperative informed
consent for PTNB (the patients were informed regarding the purpose
and possible risks of PTNB, as well as treatment options based on
available technology). Each patient did not receive treatment with
aspirin for >7 days, and their blood count and coagulation
function met the guidelines for interventional radiology (8). DXW and YGW had >7 years of
experience in CT-guided PTNB. The procedure was performed under the
guidance of a 64-slice spiral CT (Aquillion; Canon Medical Systems
Corporation) using an 18G semi-automatic biopsy needle with a
matching 17G coaxial needle (TSK Surecut; TSK Laboratory). The
patient position was determined by selecting the puncture route
that was the shortest to the lesion and that could be used to avoid
contact with the interlobar pleura, bullae and blood vessels. All
biopsies were performed with a coaxial technique. Following
insertion of the 17G coaxial needle into the edge of the lesion,
the 18G semi-automatic biopsy needle was then used for biopsy.
Biopsy procedures
The following steps were performed: i) The skin
puncture point, determined by a CT scan in an appropriate body
position, was selected according to the puncture route set prior to
the operation; ii) the lesion was punctured using a biopsy needle
following local disinfection using Iodophor (20 ml) and local
anesthesia (2% concentration of lidocaine hydrochloride injection;
5 ml); iii) when the biopsy needle reached the outside of the
pleura, a CT scan was conducted to determine the forward direction
and distance to the lesion (this step was not carried out in the
ROSE group); iv) the CT scan was performed again when the biopsy
needle reached the lesion, and the tissue specimens were obtained
by sampling the tumor, following correction of the needle
direction; and v) complications were immediately observed with the
CT scan. Chest X-ray was performed at the 3- and 6-h follow-up
periods to exclude the development of a pneumothorax.
ROSE technology
In the present study, on-site cytological evaluation
for the ROSE group was conducted by pathologists who had >5
years of work experience. It has been previously reported that the
accuracy of ROSE does not differ significantly when performed by a
pulmonologist (with 1 month of cytopathology training) or a
cytopathologist (9). An on-site
assessment has also previously been performed by an experienced
cytopathologist in the study by Anila et al (10). Therefore, the on-site cytological
evaluation for the ROSE group in the present study was deemed to be
unbiased. The specimens were smeared on the cytology slides to
reduce the loss of tissue. The specimens were stored in 10%
formalin fixative solution at room temperature for 12 h and sent to
the Department of Pathology for histopathological diagnosis. The
cytology slides were fixed at room temperature for 30-40s) with a
95% ethanol solution on site, air-dried quickly and mounted
immediately following rapid hematoxylin-eosin (H&E) staining
(Room temperature, 90s.). The biopsy specimen was considered
sufficient and the procedure was terminated if the cytoarchitecture
was found to be consistent with the clinical findings. In case of
inconsistency, a re-biopsy (change of the sampling site) was
performed using the coaxial needle. The number of samplings per
lesion was <5.
Pathological techniques
In the non-ROSE group, the identification of tissue
adequacy was performed as follows: i) A sufficient specimen was a
complete piece of tissue with a size of >10x1x1 mm3.
Each lesion was sampled <5 times. Sufficient specimens were
placed in 10% formalin at room temperature, 12 h) and sent for
histopathological diagnosis by two experienced pathologists; ii)
fixed tissue was dehydrated and embedded in petrolin. The paraffin
was cut into 3- to 5-µm thick slices, which were fixed to the
slides. The slices were baked (50-60˚C for 30-60 min) and sealed
for conventional H&E staining at room temperature for 6-8 min).
The sections were observed by two experienced histopathologists and
final histopathological diagnosis (10) was made following consultation; and
iii) immunohistochemical staining was performed if necessary. In
brief, after the paraffin sections were dewaxed in xylene (room
temperature, 45~70%, 10 min) and rehydrated in absolute, 95, 90%
ethanol, 80% ethanol, 70% ethanol and distilled water for 5 min
each, the tissues were washed with phosphate-buffered saline (PBS)
three times. According to the requirements of each antibody, the
tissue antigen was retrieved by boiling in the pressure cooker (The
reagent is composed of 5 L distilled water and 100 ml
immunohistochemical antigen retrieval buffer, heated to 99˚C for 3
min) and the tissues were again washed with PBS three times.
Endogenous peroxidase was blocked using a 3% hydrogen peroxide
methanol solution, and the tissues were again washed with PBS three
times. Subsequently, 4% sheep serum (Beijing Nakasugi Company) was
added for 30-50 min at room temperature to reduce non-specific
staining. Sheep serum was removed, and different primary antibodies
(cat. nos. TTF1/ZM-0250, NAPSIN/ZM-0473 or P40/ZM-0472,
P63/ZM-0406, CD56/ZM-0057 and Syn/ZM-0246; Beijing Nakasugi
Company) were added to samples at room temperature for 30-50 min.
Following washing with PBS three times, the polymeric chelate (cat.
no. UM-9002, Beijing Nakasugi Company, peroxidase) was added
dropwise and incubated for 30 min (Room temperature). The samples
were then washed with PBS three times and diaminobenzidine color
solution was added dropwise to each slice. The slices were washed
with water, re-stained with hematoxylin (Room temperature, 5 min),
dehydrated, transparentized and sealed. Histopathological diagnosis
was performed with a light microscope (Olympus BX41).
Measured variables
The patient demographics, nodule size, nodule
location (superior and middle lobes for upper lungs and inferior
lobes for lower lungs), nodule-to-pleura distance and pleura-needle
angle (acute angle between the needle and the tangent to the
pleura) were recorded. The documented complications included
hemoptysis, pneumothorax and chest tube placement. The final
diagnosis was made according to the observations from H&E
staining of paraffin-embedded histopathological sections and/or
following ≥6 months of follow-up. The tumor histopathological
findings (benign or malignant) (11) and secondary biopsy results were
also recorded.
Statistical analysis
SPSS 18.0 software (SPSS, Inc.) was used for
statistical analysis. The measured data are expressed as the mean ±
standard deviation and the differences were compared using an
unpaired t-test. The enumerated data are expressed as percentages,
and the differences were compared using χ2 test.
P<0.05 was considered to indicate a statistically significant
difference. The correlation of the ROSE results with the final
diagnosis was analyzed using Cohen's κ, with κ>0.75 indicating
optimal consistency.
Results
Comparison of patient
demographics
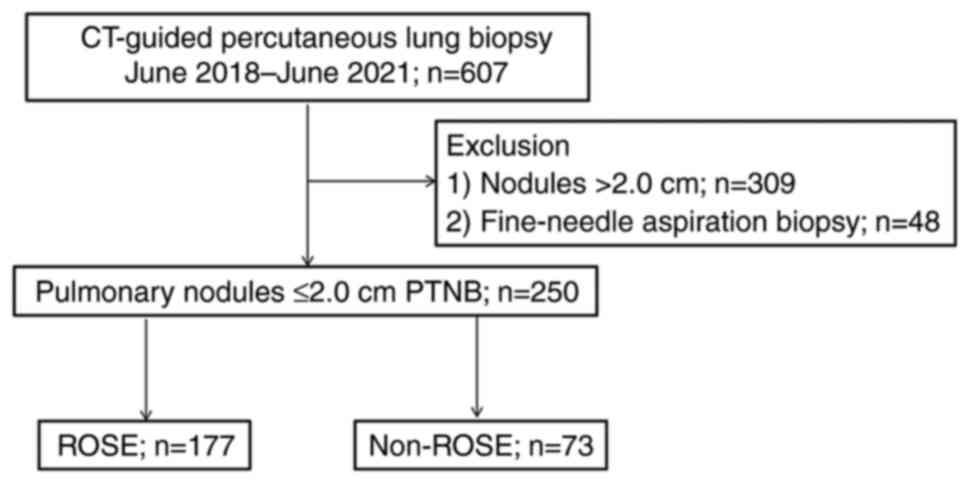
Between June 2018 and June 2021, 607 patients
underwent PTNB. Of the 607 patients, a total of 309 patients with
lesions >2.0 cm and 48 patients undergoing fine needle
aspiration biopsy were excluded. Finally, 250 patients (26-85
years) were identified as study subjects, of whom 177 patients with
ROSE (Fig. 1) were enrolled into
the ROSE group and 73 patients without ROSE were included in the
non-ROSE group (patients without ROSE were not available until
April 2019). The demographic data and baseline values of the
patients are presented in Table I.
The differences between the two groups were not considered
statistically significant (P>0.05).
 | Table IDemographics and baseline values of
the two groups. |
Table I
Demographics and baseline values of
the two groups.
| Characteristic | ROSE | Non-ROSE | χ2- or
t-value | P-value |
|---|
| Age,
yearsa | 62.79±8.61 | 64.15±8.15 | 1.26b | 0.248 |
| Sex, male/female | 105 (59.32)/72
(40.68) | 47 (64.38)/26
(35.62) | 0.56c | 0.456 |
| Nodule size,
cma | 1.72±0.84 | 1.63±0.69 | 1.61b | 0.109 |
| Nodule distribution,
upper lung/lower lung | 115 (64.97)/62
(35.03) | 45 (61.64)/28
(38.36) | 0.25c | 0.618 |
| Distance from nodule
to pleura, cma | 1.81±1.19 | 1.4.2±0.97 | 0.80b | 0.424 |
| Pleura-needle angle,
90˚/<90˚ | 71 (40.11)/106
(59.89) | 32 (43.84)/41
(56.16) | 0.30c | 0.587 |
Comparison of histopathological diagnosis results.
In the ROSE group, sufficient specimens (assessed by observation of
paraffin sections) from 165/177 patients (93.22%) were obtained,
whereas sufficient specimens from 153 patients were identified by
on-site cytology. Moreover, in 128 patients, the first examination
was sufficient. A total of 49 patients had insufficient specimens
for the first time, and 25 patients obtained valid specimens after
re-sampling. In addition, 101 patients presented with malignancies
by on-site cytology, which was consistent with the paraffin section
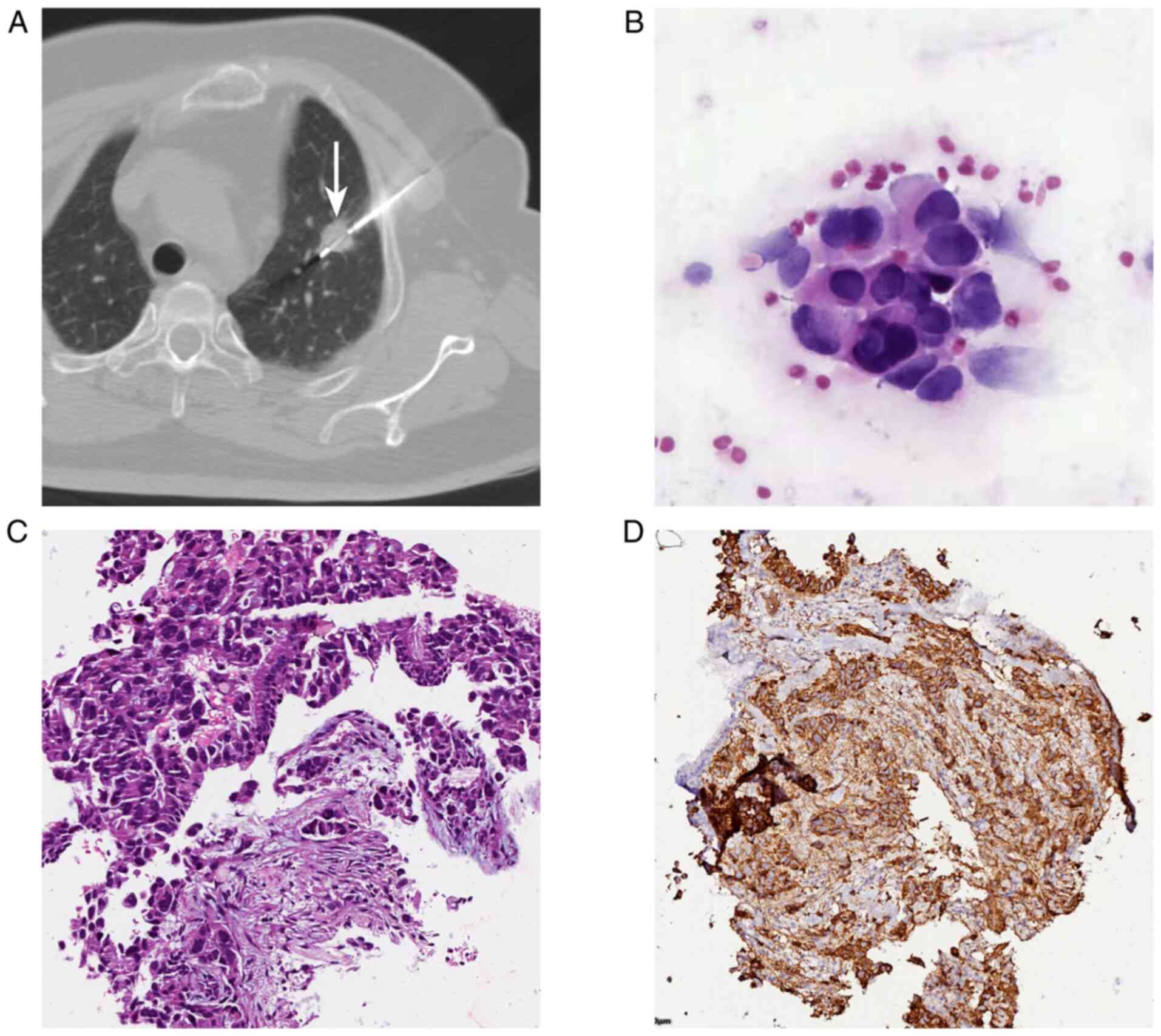
results (Fig. 2). A 71-year-old
female patient underwent CT-guided PTNB for a 15-mm nodule in the
left upper lobe (Fig. 2A). The
initial diagnosis of ROSE was lung adenocarcinoma (Fig. 2B), and the histopathological
diagnosis was lung adenocarcinoma (Fig. 2C). Immunohistochemistry was
positive for TTF-1 (Fig. 2D), and
the ROSE results in this case were consistent with
histopathological findings of lung adenocarcinoma. Furthermore, 52
patients exhibited benign lesions as determined by on-site
cytology, of whom 47 demonstrated absorption of pulmonary nodules
to different degrees or no change following >6 months of
follow-up; during the same period, 5 patients indicated an increase
in the tissue content compared with the previous evaluation.
Following surgical resection, the patients were finally diagnosed
as lung cancer cases. Specifically, 24 patients were assessed to
have insufficient specimens by on-site cytology, and 12 of them
were found to possess sufficient materials for the determination of
benign lesions using paraffin sections. The lesions were absorbed
following treatment. An additional 12 patients with paraffin
section specimens were classified as insufficient and underwent
secondary biopsies (9 were malignant and 3 were benign).
In the non-ROSE group, 52/73 patients (71.23%) were
classified as cases with sufficient material collection by H&E
staining of paraffin sections, including 35 patients who were
malignant cases and 17 patients who were benign cases. A total of
15 out of 52 patients indicated absorption of pulmonary nodules to
different degrees or had no change, whereas 2 patients exhibited
enlargement and were finally diagnosed as lung cancer cases
following surgical resection and 6 months of follow-up. The
specimens from 21 patients were characterized as insufficient (17
malignant and 4 benign specimens) and secondary biopsies were
performed. No significant differences were noted in the
histopathological types between the ROSE and the non-ROSE groups
(Table II).
 | Table IIComparison of specimen adequacy rate,
diagnostic accuracy rate, secondary biopsy rate and histopathology
between two groups of patients. |
Table II
Comparison of specimen adequacy rate,
diagnostic accuracy rate, secondary biopsy rate and histopathology
between two groups of patients.
| Characteristic | ROSE | Non-ROSE |
χ2-value | P-value |
|---|
| Sufficient
specimens | 165.00 (93.22) | 52 (71.23) | 21.81 | <0.001 |
| Accurate
diagnosis | 160.00 (90.40) | 50 (68.49) | 7.18 | 0.007 |
| Secondary biopsy | 9 (5.08) | 21 (28.77) | 27.45 | <0.001 |
| Malignant
diagnosis | 115 (64.97) | 52 (71.23) | 0.914 | 0.339 |
Comparison of patient specimen
adequacy rate, diagnostic accuracy rate, secondary biopsy rate and
histopathological type
The specimen adequacy rate [93.22% (165/177)] and
diagnostic accuracy [90.40% (160/177)] of the ROSE group were
significantly higher than those of the non-ROSE group [71.23%
(52/73) and 68.49% (50/73), respectively; both P<0.05]. The rate
of secondary biopsy [5.08% (9/177)] was significantly lower than
that of the non-ROSE group [28.77% (21/73); P<0.05]. The
comparison of the results between the two groups is shown in
Table II.
Comparison of patient complications
and procedure time
The comparison of complications and the procedure
time between the two groups are listed in Table III. No significant difference was
noted in the incidence of pneumothorax, thoracic tube placement,
hemoptysis or the time of procedure (P>0.05). No case of air
embolism or death was reported.
 | Table IIIComparison of complications and
procedure times between the two groups of patients. |
Table III
Comparison of complications and
procedure times between the two groups of patients.
| Characteristic | ROSE | Non-ROSE | χ2 or
t-value | P-value |
|---|
| Pneumothorax | 28 (15.82) | 11 (15.07) | 0.02a | 0.882 |
| Thoracic
catheterization | 9 (5.08) | 3 (4.11) | 0.11a | 0.743 |
| Hemoptysis | 12 (6.78) | 6 (8.22) | 0.16a | 0.689 |
| Time of procedure,
minb | 20.06±3.37 | 19.34±3.22 | 1.54c | 0.124 |
Comparison of ROSE results with final
diagnosis
A comparison of the ROSE results with the final
disease diagnosis is shown in Table
IV. The sensitivity and specificity of ROSE diagnosis were
95.28 and 100.00%, respectively. The coincidence rate between the
diagnosis of ROSE and the final pathological results was 96.73%,
indicating high consistency (κ=0.925, P<0.001).
 | Table IVComparison of ROSE results with final
diagnosis. |
Table IV
Comparison of ROSE results with final
diagnosis.
| | Final diagnostic
results | |
|---|
| ROSE results | Malignant | Benign | Total |
|---|
| Malignant | 101 | 0 | 101 |
| Benign | 5 | 47 | 52 |
| Total | 106 | 47 | 153 |
Discussion
PTNB is one of the traditional methods used to
obtain lung pathological specimens and exhibits high safety
(9). However, the diagnosis of
pulmonary nodules with a diameter of ≤2.0 cm is relatively
difficult and prone to produce false-negative results. To improve
the accuracy of PTNB in pulmonary nodules with a diameter of ≤2.0
cm, 250 patients undergoing biopsy of pulmonary nodules (≤2.0 cm)
were evaluated. The results indicated that ROSE could significantly
improve the diagnostic rate of PTNB. The incidence of pneumothorax
and thoracic tube placement was higher in the ROSE group than in
the non-ROSE group; however, only non-significant differences were
noted.
No significant differences were noted in the
demographic data or baseline values between the two groups. The
specimen adequacy rate and diagnostic accuracy of the ROSE group
were significantly higher than those of the non-ROSE group. Xu
et al (12) demonstrated
that the diagnostic accuracy of patients in the ROSE group was
85.7%, which is similar to the results of the present study. The
secondary biopsy rate was reduced from 28.77 to 5.08% by the
application of ROSE in the current study. In a recent meta-analysis
of biopsies of pulmonary nodules with a diameter of ≤2.0 cm, Liu
et al (13) indicated a
higher rate of secondary biopsy, ranging from 14.4 to 31.2%. ROSE
is used to assess specimen quality and provides the operator with
real-time guidance. It can also significantly reduce the rate of
secondary biopsy, notably in the biopsy of pulmonary nodules with a
diameter of ≤2.0 cm. The accuracy of CT-guided PTNB is associated
not only with the proficiency of the operator, but also with the
lesion size; notably, in the small nodules of the lower lungs, the
operation may be complicated due to respiratory movement (14). Yarmus et al (15) suggested that ROSE does not decrease
the rate of secondary biopsies. This suggestion is different from
the conclusion of the current findings, possibly due to lack of
differentiation between the pulmonary nodule sizes reported by
Yarmus et al (15). It is
suggested that the pulmonary nodules with a diameter of ≤2.0 cm
benefit more from the use of ROSE in CT-guided PTNB. Based on the
present study, ROSE can accurately assess whether the specimen is
sufficient and improve the diagnostic accuracy of the method used.
ROSE can also guide the location of the material during the
puncture process and thereby enhance the technical skill of the
medical practitioner.
Pneumothorax and hemoptysis are common complications
of PTNB (16). The incidence of
pneumothorax ranges from 5.3 to 37% (17,18).
It has been reported that between 1.4 and 16.7% of these patients
require chest tube placement (19,20).
Hemoptysis is often self-limiting, with an incidence of 1-13%
(21,22). These findings are in accordance
with the present study suggestion that the incidence rates of the
complications were not significantly different between the two
groups. This evidence suggests that the incidence of common
complications of PTNB will not be increased by the use of ROSE.
Moreover, no serious complications, such as air embolism and death,
occurred in the current study. Furthermore, ROSE did not prolong
the time of the puncture procedure and was safe and reliable.
In addition, the sensitivity and specificity of ROSE
for the diagnosis of pulmonary nodules with a diameter of ≤2 cm
were 95.28 and 100.00%, respectively. These results are highly
consistent with the final pathological findings and aid the
confirmation of the initial diagnosis of emergency cases.
Similarly, Anila et al (10) indicated that the sensitivity and
specificity of ROSE in 50 patients with pulmonary nodules were
92.00 and 100.00%, respectively.
The present study contains certain limitations.
Firstly, this is not a multicenter study and it therefore may
include certain biases. Secondly, this is not a prospective study
and may be subjected to certain confounding factors (for example:
incomplete data). Finally, the sample size used was fairly small.
Although ROSE was used, 5 malignant tumors were missed. The use of
ROSE can reduce the incidence of missed diagnoses; however, it
requires regular patient follow-up. It is suggested that the use of
ROSE should be implemented only in the puncture of small nodules of
the lung. The use of puncture in other organs could also be
beneficial, which will be investigated in future studies.
In conclusion, the current study indicated that when
CT-guided PTNB was performed for pulmonary nodules with a diameter
of ≤2.0 cm, ROSE not only ensured the adequacy of sampling, but
also improved the diagnostic accuracy and significantly reduced the
secondary biopsy rate. ROSE ensures high consistency between
diagnosis and the final pathological results, without increasing
the incidence of PTNB complications.
Acknowledgements
Not applicable.
Funding
Funding: This study received funding from the Qiqihar Science
and Technology Plan Joint Guidance Project (grant no.
LHYD-202045).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW was responsible for the conception and design of
the present study. QZ and YW participated in study design and data
collection. DW, WD, GD, QW, YH, YD and BL analyzed and interpreted
the data. DW drafted the manuscript. QZ and DW critically revised
the manuscript for intellectual content. All authors have read and
approved the final manuscript. QZ and YW confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The Ethics Committee of Qiqihar Medical College
(Qiqihar, China; protocol no. 2021-193) provided ethical approval
for the present study. As it is a retrospective study, the ethics
committee has exempted the patients' right of informed consent, but
the patient information should remain anonymized.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsai PC, Yeh YC, Hsu PK, Chen CK, Chou TY
and Wu YC: CT-Guided core biopsy for peripheral sub-solid pulmonary
nodules to predict predominant histological and aggressive subtypes
of lung adenocarcinoma. Ann Surg Oncol. 27:4405–4412.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wattanasatesiri T, Puntu W and
Vithitsuvanakul N: Influencing factors of pneumothorax and
parenchymal haemorrhage after CT-guided transthoracic needle
biopsy: Single-institution experience. Pol J Radiol. 83:e379–e388.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee KH, Lim KY, Suh YJ, Hur J, Han DH,
Kang MJ, Choo JY, Kim C, Kim JI, Yoon SH, et al: Diagnostic
accuracy of percutaneous transthoracic needle lung biopsies: A
multicenter study. Korean J Radiol. 20:1300–1310. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu C, Wang Y, Wang W, Yuan Q, Hu HD and Li
L: Improved diagnostic yield of transbronchial lung biopsy in
peripheral pulmonary lesions using a combination of endobronchial
ultrasound and rapid on-site evaluation. J Int Med Res.
49(300060521999535)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Agarwal P, Toi PC, Subramaniam H and
Apoorva Lakshmi S: Prospective comparison of cytological specimen
adequacy assessment by different rapid staining techniques for
rapid on-site evaluation in fine needle aspiration cytology and
their cost-effectiveness. Diagn Cytopathol. 47:469–474.
2019.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Izumo T, Matsumoto Y, Sasada S, Chavez C,
Nakai T and Tsuchida T: Utility of rapid on-site cytologic
evaluation during endobronchial ultrasound with a guide sheath for
peripheral pulmonary lesions. Jpn J Clin Oncol. 47:221–225.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Patel IJ, Davidson JC, Nikolic B, Salazar
GM, Schwartzberg MS, Walker TG and Saad WA: Standards of Practice
Committee with Cardiovascular and Interventional Radiological
Society of Europe (CIRSE) Endorsement. Consensus guidelines for
periprocedural management of coagulation status and hemostasis risk
in percutaneous image-guided interventions. J Vasc Interv Radiol.
23:727–736. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Umeda Y, Otsuka M, Nishikiori H, Ikeda K,
Mori Y, Kobayashi T, Asai Y, Takahashi Y, Sudo Y, Kodama K, et al:
Feasibility of rapid on-site cytological evaluation of lung cancer
by a trained pulmonologist during bronchoscopy examination.
Cytopathology. 30:628–633. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Anila KR, Nayak N, Venugopal M and
Jayasree K: Role of rapid On-site evaluation in CT-guided fine
needle aspiration cytology of lung nodules. J Cytol. 35:229–232.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu H, Li S, Yang S and Wu Z:
(99)Tc(m)N-NOET dual-phase SPECT in differential diagnosis of
benign and malignant lung tumors. Zhonghua Zhong Liu Za Zhi.
36:48–52. 2014.PubMed/NCBI(In Chinese).
|
|
12
|
Xu C, Wang W, Yuan Q, Hu H, Li L and Yang
R: Rapid on-site evaluation during radial endobronchial
ultrasound-guided transbronchial lung biopsy for the diagnosis of
peripheral pulmonary lesions. Technol Cancer Res Treat.
19(1533033820947482)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu GS, Wang SQ, Liu HL, Liu Y, Fu YF and
Shi YB: Computed tomography-guided biopsy for small (≤20 mm) lung
nodules: A meta-analysis. J Comput Assist Tomogr. 44:841–846.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li W, He XF, Wei YT, Zhang X, Zhang XB, Li
J, Li J, Yang J, Xue XD and Xiao YY: Clinical application of
CT-guided radiofrequency ablation combined with biopsy
synchronously to multiple small nodules of lung metastatic tumors.
Zhonghua Yi Xue Za Zhi. 98:2189–2193. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
15
|
Yarmus L, Van der Kloot T, Lechtzin N,
Napier M, Dressel D and Feller-Kopman D: A randomized prospective
trial of the utility of rapid on-site evaluation of transbronchial
needle aspirate specimens. J Bronchology Interv Pulmonol.
18:121–127. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Heerink WJ, de Bock GH, de Jonge GJ, Groen
HJ, Vliegenthart R and Oudkerk M: Complication rates of CT-guided
transthoracic lung biopsy: Meta-analysis. Eur Radiol. 27:138–148.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huo YR, Chan MV, Habib AR, Lui I and
Ridley L: Pneumothorax rates in CT-Guided lung biopsies: A
comprehensive systematic review and meta-analysis of risk factors.
Br J Radiol. 93(20190866)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rong E, Hirschl DA, Zalta B, Shmukler A,
Krausz S, Levsky JM, Lin J, Haramati LB and Gohari A: A
Retrospective multi-site academic center analysis of pneumothorax
and associated risk factors after CT-Guided percutaneous lung
biopsy. Lung. 199:299–305. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sabatino V, Russo U, D'Amuri F, Bevilacqua
A, Pagnini F, Milanese G, Gentili F, Nizzoli R, Tiseo M, Pedrazzi G
and De Filippo M: Pneumothorax and pulmonary hemorrhage after
CT-guided lung biopsy: Incidence, clinical significance and
correlation. Radiol Med. 126:170–177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ruud EA, Stavem K, Geitung JT, Borthne A,
Søyseth V and Ashraf H: Predictors of pneumothorax and chest
drainage after percutaneous CT-guided lung biopsy: A prospective
study. Eur Radiol. 31:4243–4252. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Andrade JR, Rocha RD, Falsarella PM, Rahal
Junior A, Santos RSD, Franceschini JP, Fernando HC and Garcia RG:
CT-guided percutaneous core needle biopsy of pulmonary nodules
smaller than 2 cm: Technical aspects and factors influencing
accuracy. J Bras Pneumol. 44:307–314. 2018.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
22
|
Mills M, Choi J, El-Haddad G, Sweeney J,
Biebel B, Robinson L, Antonia S, Kumar A and Kis B: Retrospective
analysis of technical success rate and procedure-related
complications of 867 percutaneous CT-guided needle biopsies of lung
lesions. Clin Radiol. 72:1038–1046. 2017.PubMed/NCBI View Article : Google Scholar
|