Introduction
Mixed tumor of the skin (MTS), also termed
‘chondroid syringoma,’ is a small benign tumor arising from the
sweat glands (1). This tumor is
uncommon, with an incidence of 0.01-0.098% among all primary skin
tumors, and demonstrates a predilection for males (2,3). MTS
commonly involves in the head and neck areas; however, a very small
number of cases of MTS of the lip have been reported (4-7).
It presents as asymptomatic slow growing, firm subcutaneous or
intradermal nodule (3,6). It is difficult for most clinicians to
clinically differentiate MTS from other lesions because of its
silent presentation and rarity (3,8,9).
Pathologically, MTS consists of epithelial and
mesenchymal stromal components, and shows neoplastic proliferation
of glandular cells embedded in a myxoid or chondroid stroma,
findings occasionally include osteoid stroma or adipocytes
(1,4,10).
The histological features of these tumors are recognized as two
variants: the eccrine cell type, which shows smaller lumens lined
by a single row of cuboidal epithelial cells, and the apocrine cell
type, which shows tubular and cystic branching lumina lined by
double-layered epithelial cells, dual epithelial cells and
myoepithelial cells (1,11). This tumor is considered as the
cutaneous analog to pleomorphic adenoma of the salivary glands, and
shares some features with pleomorphic adenomas (or salivary gland
mixed tumors) (1,9). Therefore, its diagnosis is dependent
on the tumor location, to exclude tumors originating in the
salivary glands (12).
Furthermore, the broad spectrum of metaplastic changes and
differentiation can sometimes lead to its misdiagnosis as other
adnexal or mesenchymal neoplasms (4).
In this report, we present a case of MTS with
abundant adipose tissue and large cystic structures in the upper
lip. This case is unique because of the clinical location of the
mass and the rare histopathological features. Awareness of the
various variants of MTS can contribute to making a correct
diagnosis and the provision of appropriate treatment.
Case report
A 47-year-old Japanese man was referred to the
Department of Oral and Maxillofacial Surgery at Kyushu University
Hospital (Fukuoka, Japan) with a 6-year history of a painless mass
in the right upper lip. Recently, the mass had been slowly growing
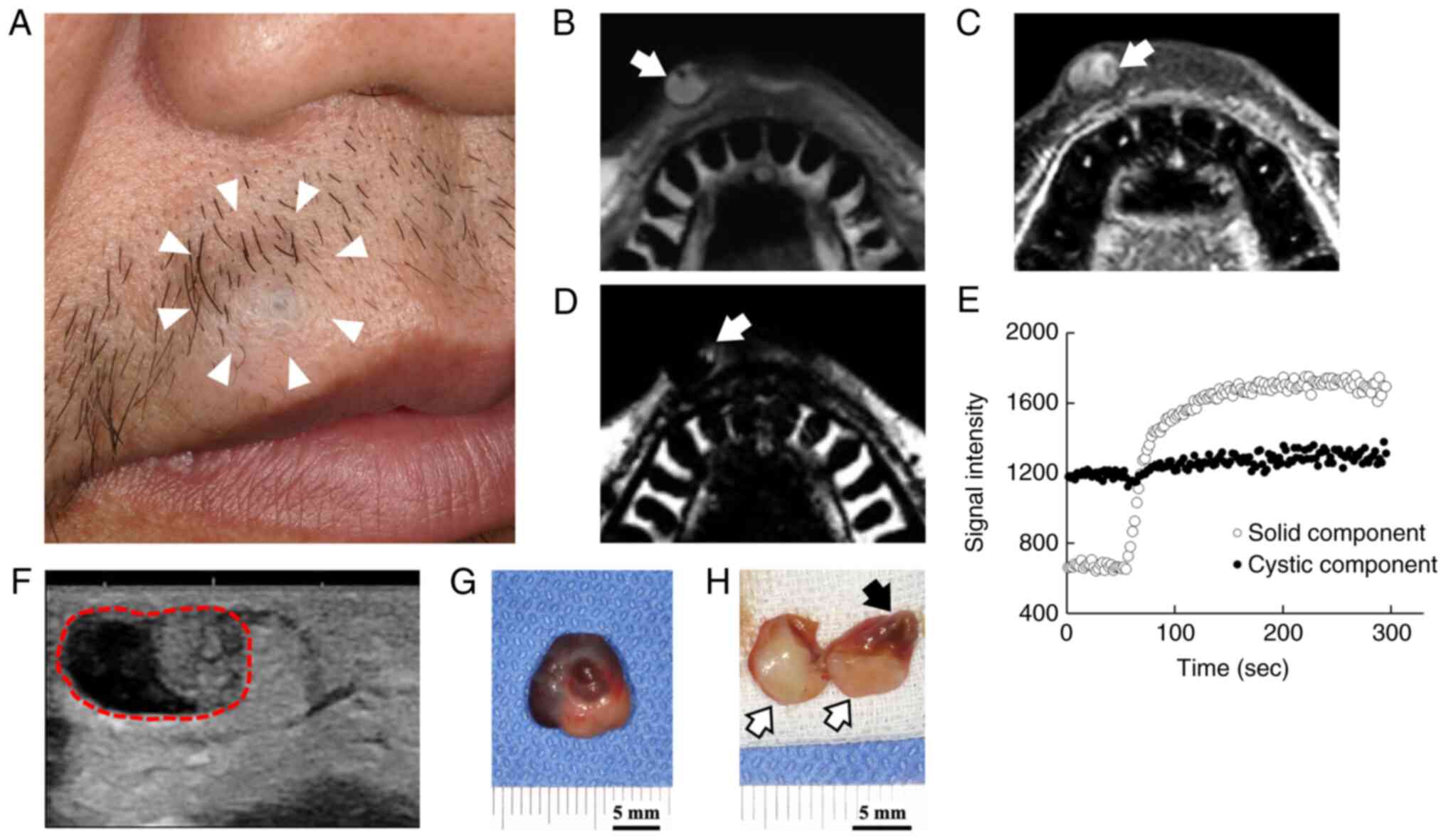
(Fig. 1A). He had no clinical
evidence of cervical lymphadenopathy and no medical history of any
significant diseases. On extra- and intra-oral examination, he had
14x12x10 mm round, soft-elastic, less movable, intradermal nodule
of the upper lip that appeared to be fixed to the skin. Both the
overlying skin-a part showed bluish-and the underlying intraoral
mucosa appeared normal. In addition, the patient was concerned
about the postoperative functional and aesthetic consequences.
Magnetic resonance imaging (MRI) demonstrated a
10x11x7 mm area of delimited soft tissue without apparent
attachment to the skin of the right upper lip. On T1-weighted
imaging, the mass was partially hyperintense (Fig. 1B). It had an inhomogeneous and
slightly high signal intensity on T2-weighted water-only imaging
(Fig. 1C). An area of the high
signal intensity was found in the proximal portion on T2-weighted
fat-only imaging, which suggested the presence of a fatty component
(Fig. 1D). A time intensity curve
showed gradual enhancement in proximal portion and non-enhancement
in distal portion (Fig. 1E). Taken
together, the proximal portion was a solid tumor with fatty
component, while the distal portion was cystic and possibly
contained highly viscous fluid. Ultrasonography showed a
well-circumscribed mass with two different components (Fig. 1F). The proximal solid portion was
hyperechoic, and the distal portion was hypoechoic. It was close to
the skin surface, but the direct contact was not clear. Based on
these findings, the tumorous mass was clinically diagnosed as a
non-malignant tumor.
An excisional biopsy was performed via an intraoral
approach by extracapsular dissection of the lesion under local
anesthesia for aesthetic reasons. The tumor was easily and bluntly
dissected and totally removed from the adjacent tissues. The mass
was well-encapsulated, and intracutaneously located between the
orbicularis oris muscle and the skin, without invading the
orbicularis oris muscle or skin. There was no obvious damage to the
nerves or blood vessels. Gross examination of the specimen revealed
a well-circumscribed tumor mass measuring of 12x14x10 mm in size.
The specimen had a dark-red colored area with a central convex part
and a yellowish white area. The cut surface showed a yellowish
white solid area and cystic structures in which the viscous liquid
content leaked out when the specimen was cut (Fig. 1G and H).
On histopathological examination, the lesion was
encapsulated with thin fibrous tissue and consisted of a solid
lesion and a few large cystic structures in the hematoxylin and
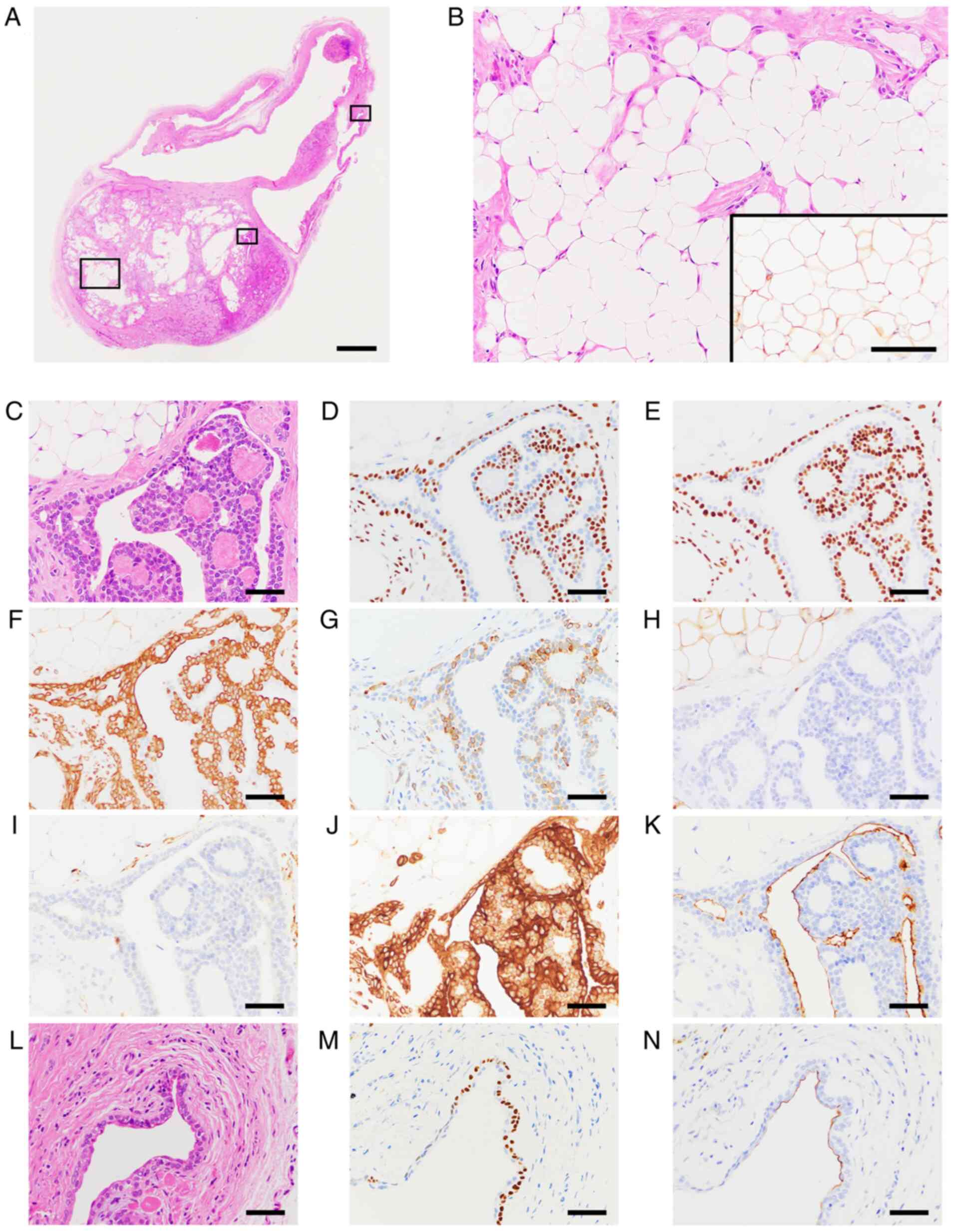
eosin-stained section (Fig. 2A).
The solid lesion showed a stromal component with abundant adipose
tissue and epithelial structures with elongated branched
ducts/tubules and sheet-like growth patterns. The stromal component
showed myxoid, hyaline and fibrous changes, and abundant mature
adipose tissue replaced stromal component, intermingled with the
epithelial structures and was observed to account for approximately
40% of the solid lesion (Fig. 2B).
The ductal/tubular structures were lined by double-layered cells;
the outer layer was composed of polygonal to flattened clear cells,
and the inner layer was formed by cuboidal/columnar ductal
epithelial type cells (Fig. 2C-K).
A few large cystic lesions were lined by thin double-layered
cuboidal and/or polygonal to flattened epithelial cells that were
associated with the tumor cells (Fig.
2L-N). Serous and mucous acinar cells were not evident in the
tumor. The following antibodies were used in immunohistochemical
staining for this case: epithelial markers, AE1/3 and EMA;
mesenchymal and myoepithelial markers, vimentin, S-100, αSMA, GFAP,
CK14, p40 and p63; and a cell proliferation marker, Ki-67.
Immunohistochemically, the epithelial tumor cells with elongated
branched ducts/tubules and sheet-like growth patterns were positive
for AE1/3 (Fig. 2J). The polygonal
to flattened clear cells in the outer layer were positive for p63
(Fig. 2D and M), p40 (Fig.
2E) and CK14 (Fig. 2F), and
the ductal epithelial type cells were positive for EMA (Fig. 2K and N). S100-positive cells were observed in
the limited epithelial area, in the stromal component and in the
lipomatous area (Fig. 2B inset,
Fig. 2H). Ki-67-positive cells
were scattered and the proliferative index was low (data not
shown). GFAP-positive signals were observed in some epithelial
cells of the outer layer (Fig.
2G). αSMA signals were almost negative in the epithelial areas
(Fig. 2I). Based on the
above-mentioned histopathological features and anatomical location
of the lesion, the tumor was diagnosed as lipomatous MTS with large
cystic formation affecting the upper lip. No local recurrence of
the tumor was observed during 5 months of follow-up.
Discussion
MTS, also known as chondroid syringoma, is a rare
benign skin appendageal tumor with an incidence of 0.01-0.098%
among all primary skin tumors (1-3).
This tumor presents as a slow growing, asymptomatic, intradermal or
subcutaneous nodule, commonly affecting the middle-aged and elderly
males, and generally involves the head and neck region (6). Stout and Gorman (6), Hirsch and Helwig (5), and Kazakov et al (4) reported that among cases of MTS, the
frequency of MTS in the head and neck region was 67.9% (91/134
cases), 79.8% (150/188 cases), and 75.0% (183/244 cases) in MTS,
respectively. We herein report a rare case of MTS with three
unusual clinicopathological features.
The first feature of the present case is the
location of the lesion. MTS most commonly occurs in the head and
neck region. Among cases of MTS, the frequency of MTS of the lip
reported by Stout and Gorman (6),
Hirsch and Helwig (5), and Kazakov
et al (4) was only 13.4%
(18/134 cases), 12.8% (24/188 cases), and 16.0% (39/244 cases),
respectively. The frequency among MTSs in the head and neck region
is similar to that in the nose and cheek skin regions (4-6).
The frequency at which MTS occurs in the lip is considered to be
very low in comparison to the frequency of all primary skin tumors
(8). In our search of the relevant
literatures, the number of cases of MTS of the lower lip was less
than one-eighth the number of cases of MTS of the upper lip. In
addition, the clinical diagnosis of MTS is quite difficult due to
the lack of specificity of the clinical manifestations, therefore
MTS is most often overlooked or mistaken for other lesions, such as
dermoid or sebaceous cysts or any other benign adnexal tumors
(8,9). Therefore, a histopathological
examination is considered very important for establishing a
definitive diagnosis in MTS (8,9).
MTS shows various histological findings, but mainly
exhibits as epithelial components embedded in a mucus-like,
cartilage-like, and/or fibrous matrixes, and consists of varying
proportions in each case. MTS can be mainly divided into two
variants, apocrine and eccrine types, based on the pattern of the
lumina observed in the MTS. The apocrine type is characterized by
tubular and cystic branching lumina lined by double layers of
epithelial cells of different types, whereas the rare eccrine type
is characterized by small tubular lumina lined by a single layer of
cuboidal epithelial cells (1,11).
It is well known that the apocrine type may show a broad spectrum
of differentiation and metaplastic changes (4). Sometimes these changes are so
pronounced that they can cause the clinicians to misdiagnose MTS as
other adnexal or mesenchymal neoplasms (4). Based on the findings mentioned above,
the present case was diagnosed as apocrine MTS with two unusual
histological features.
The second feature is that the fatty component was
prominent and constituted approximately 40% of the solid lesion.
Although lipomatous metaplasia is a frequent finding, being found
in 44% of apocrine MTSs, it is usually observed multifocally with
clusters of mature lipocytes (4).
MTSs with predominant and extensive lipomatous metaplasia are rare
and were found in only 3 cases of 244 apocrine MTS cases (1%)
(4). Only a few cases showing a
prominent adipocytic growth pattern has been reported. In those
cases, the proportion of the MTS that was filled with adipose
tissue ranged from 40% to more than 90% (13-18).
To date, there are no reported cases of MTS with extensive
lipomatous metaplasia occurring in the lip. MTS shares some
features with pleomorphic adenoma of the salivary gland (1,9). To
the best of our knowledge, 16 cases of pleomorphic adenoma with
predominant adipocytes in the salivary gland has been reported, and
the proportion of the tumor that was occupied by adipose tissue
ranged from 25% to more than 95% (19-21).
Thus a prominent adipocytic metaplastic pattern is rare in both MTS
and pleomorphic adenoma. Abundant mature adipose tissue prominently
replaced the stromal component and was intermingled with epithelial
structures, and could be confused with various other lesions. The
histopathological differential diagnosis included lipoma, atypical
and spindle cell lipoma, adenolipoma of the skin, eccrine
angiomatous hamartoma, eccrine and apocrine hidrocystoma,
fibroadenoma, hidradenoma, mucinous adenocarcinoma, and adenoid
cystic carcinoma (11,17,22).
In our case, cellular atypia and pleomorphism were not observed and
its histomorphological features differentiated it from malignant
tumors. These differentiatial diagnoses were ruled out mostly based
on clinical findings and/or histopathological findings (11,17,22).
The third feature was that the lesion included large
cystic components. As mentioned above, ultrasonography showed the
cystic structure as a hypoechoic region located at the distal
portion in the well-circumscribed mass. The region that appeared
hypoechoic on ultrasonography showed hyperintensity on T1-weighted
imaging, showed inhomogeneous and slightly high signal intensity on
T2-weighted water-only imaging, and was not enhanced by contrast
agent, which suggested that the cystic structure contained a
protein-rich fluid with high viscosity. The mass appeared to be
located anterior to the orbicularis oris muscle and close to the
skin; thus, cystic lesions and benign adnexal or mesenchymal
neoplasms on the skin would be included in the differential
diagnosis of this case. Although the clinical differential
diagnosis included dermal cyst, epidermoid cyst, sebaceous cyst,
trichilemmal cyst and benign adnexal or mesenchymal neoplasms, it
was possible to exclude these lesions because in the present case
both the hyperechoic and echo-free regions was observed in a single
capsule. The mechanism of cyst formation in MTS has been published
in the relevant literature. To consider the origin of cyst
formation, it may be helpful to refer to cases of pleomorphic
adenoma with large cystic formation (23,24).
The large cyst formation may originate from the followings:
squamous metaplasia of tumor cells; enlargement of ductal-like
structures by secretions from tumor cells or salivary gland tissue;
hemorrhagic infarction; and necrosis in the tumor (23,24).
In our case, the thin double-layered ductal epithelial cells and
polygonal to flattened cleat cells lining the large cystic
structures were immunohistochemically positive for EMA and p63,
respectively. Therefore, the large cyst formation in our case may
have originated from enlargement of ductal-like structures by
secretions from tumor cells.
The immunoprofile in our case demonstrated that the
polygonal to flattened clear cells in the outer layer were positive
for p63, p40 and CK14, and S100-/GFAP-positive cells were observed
in the limited epithelial area, but αSMA signals were almost
negative. The results suggest that the degree of myoepithelial
differentiation in this case was lower. Wan et al (9) reported that the outer epithelium and
epithelial nests of MTS express epithelial and mesenchymal markers,
and showed the high expressions of S-100, GFAP, αSMA, and p63,
verifying that myoepithelial differentiation was present, but that
the tumor was almost negative for desmin and actin. MTS is
considered to be the cutaneous analog to pleomorphic adenoma of the
salivary glands (1,4). Because neoplastic myoepithelial cells
in the tumors can show diverse morphological and cytological
features (spindle, polygonal, plasmacytoid, etc) (1,4,9), it
is possible that the neoplastic myoepithelial cells can exhibit
various steps in the combined epithelial and smooth muscle
immunoprofiles. These immunostaining results may be due to the
metaplastic features in the MTS, which can be caused by the
transformation of myoepithelial cells into stromal components,
including adipocytes.
The histopathological examination of the skin
lesions could establish a definitive diagnosis of the lesion as
MTS; however, there are histological similarities between MTS and
pleomorphic adenoma of the salivary glands. Consequently, the
points for the differential diagnosis between skin lesions of
salivary gland origin or skin origin should be considered. In the
report of Reddy et al (8),
histological features of MTS and pleomorphic adenoma were compared
and some contrasting features were listed, as follows. In MTS, the
tumor epithelial cells show differentiation towards adnexal
structures (i.e., hair follicle, sebaceous gland, apocrine sweat
gland and eccrine sweat gland). Epithelial cells arrange in
tubulocystic structures lined by single or double rows. Merkel
cells may be involved in differentiation towards adnexal structures
(25). Meanwhile, in pleomorphic
adenoma, serous and mucous acinar cells are evident. In this case,
the tumor epithelial structures mainly showed elongated branched
ducts/tubules lined by double rows and only a little sheet-like
growth pattern, and serous and mucous acinar cells were not
evident. These findings in the present case were suggestive of MTS;
however, differentiation towards adnexal structures and involvement
of Merkel cells were not apparent in the tumor. Although Merkel
cells may be an integral constituent of follicles in apocrine MTSs
with follicular differentiation, only 14% of apocrine MTSs were
reported to be associated with Merkel cells (25). Therefore, even if Merkel cells were
not found in the tumor tissue, it would not be considered a reason
to deny the diagnosis of MTS in this case. The differentiation into
adnexal structures that was found within the tumor would be
considered to be an important finding in differentiation between
MTS and pleomorphic adenoma of the salivary glands.
In addition, the genetic profile could be considered
as a means of differentiating MTS from pleomorphic adenoma of the
salivary glands; however, the difference in the genetic and protein
expression profiles of these two tumors has not yet been completely
revealed (7). For example, PLAG1
fusion gene is detected in approximately 58% of pleomorphic
adenomas (ranged 24-85%) (26). On
the other hand, according to the most recent report published in
2022, PLAG1 fusion genes are found in approximately 33% of MTSs
(27). There have also been
reports concerning other genes other than PLAG1, but no genes have
been found to be useful for differentiating between the two tumors,
and further research is warranted (28,29).
In the differential diagnosis, the anatomical
location of the tumor is one of the most important things to be
considered. Based on the clinical findings-including MRI and
ultrasonographic images-as well as the operative findings in this
case, this mass was well-encapsulated, existing intracutaneously
between the skin of the upper lip and the orbicularis oris muscle,
without invading into the adjacent structures. The sweat glands on
the facial skin comprise both eccrine and apocrine glands and the
apocrine glands are mainly distributed on the alae nasi, nasal
vestibule and ear canal (7).
Hence, the lesion was determined to be of skin origin.
In general, fine-needle aspiration cytology (FNAC),
a type of biopsy, is frequently performed to check abnormal area,
lump or swelling for the diagnosis of various diseases, including
cancers. For example in the thyroid, breast or lymph nodes, FNAC
can be used when the lesion is felt or touched as a palpable lump
or swelling and/or found as an abnormal growth or area by imaging
tests. FNAC also has great value in the diagnosis of lesions of the
head and neck region (30,31). Even if MTS is considered a rare
differential diagnosis of a lump or swelling in the head and neck
region, the diagnosis can be confirmed or ruled out by means of
FNAC when the histomorphological and cytomorphological features of
MTS are well understood by the cytologist (30,31).
Although FNAC was not performed in our case, further attention
should be required when diagnosing lesions (like the present case)
in which the imaging analysis reveals predominant fatty components
and large cystic structures. Therefore, it would be necessary to
make the diagnosis in FNAC based on the anatomical location and
clinical presentation of the lesion, histomorphological features
(1,4,6,9) and
previously reported cases (30,31),
together with consideration of the cytological features of rare
variant pleomorphic adenoma, cystic (24) and lipomatous pleomorphic adenoma
(32), as a reference.
There is little information concerning the variants
of MTS where more uncommon features predominate, which makes the
diagnosis and treatment more challenging. In this case, although
the predominance of adipocytes and cystic structures were notable
findings, the tubular/ductal structures in a fibromyxoid background
did not readily evoke the diagnosis of an MTS variant with distinct
features. However, these findings of the lesion led us to diagnose
this neoplasm as a unique, rare variant of a lipomatous MTS with
large cystic formation. To the best of our knowledge, this is the
first report of lipomatous MTS with cystic formation in the
lip.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by JSPS KAKENHI
(2020-2022; grant nos. JP20K09906 and JP20K10096).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RN contributed to conception of the study and
acquisition of the literature related to the study, drafted the
initial manuscript, performed the histopathological analysis, and
provided the associated images. TK contributed to conception and
design of the study, analysis and interpretation of data, made the
final histopathological diagnosis, performed the histopathological
and anatomical analyses, provided the associated images, reviewed
the literature, and drafted, reviewed and edited the manuscript.
MMK, YM, MM, SN, TC and KY made substantial contributions in the
acquisition of clinical and imaging data. MMK and MM performed the
clinical diagnosis, treatment and follow-up of the patient. SF and
HW made the histopathological diagnosis, performed pathological
examinations, analyzed the data and interpreted the data. TK, SF,
MM and TC confirmed the authenticity of all the raw data and
contributed to critical revisions of the intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Kyushu University (approval no. 29-392;
Fukuoka, Japan). Written informed consent was obtained from the
patient.
Patient consent for publication
The patient provided written informed consent for
the publication of the case and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sangüeza OP, Cassarino DS, Glusac EJ,
Kazakov DV, Requena L, Swanson PE and Vassallo C (eds): WHO
Classification of Skin Tmours Vol. 11. 4th edition. International
Agency for Research on Cancer. IARC, Lyon, 2018.
|
|
2
|
Kitazawa T, Hataya Y and Matsuo K:
Chondroid syringoma of the orbit. Ann Plast Surg. 42:100–102.
1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yavuzer R, Başterzi Y, Sari A, Bir F and
Sezer C: Chondroid syringoma: A diagnosis more frequent than
expected. Dermatol Surg. 29:179–181. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kazakov DV, Belousova IE, Bisceglia M,
Calonje E, Emberger M, Grayson W, Hantschke M, Kempf W, Kutzner H,
Michal M, et al: Apocrine mixed tumor of the skin (‘mixed tumor of
the folliculosebaceous-apocrine complex’). Spectrum of
differentiations and metaplastic changes in the epithelial,
myoepithelial, and stromal components based on a histopathologic
study of 244 cases. J Am Acad Dermatol. 57:467–483. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hirsch P and Helwig EB: Chondroid
syringoma: Mixed tumor of skin, salivary gland type. Arch Dermatol.
84:835–847. 1961.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stout AP and Gorman JG: Mixed tumors of
the skin of the salivary gland type. Cancer. 12:537–543.
1959.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gotoh S, Ntege HE, Nakasone T, Matayoshi
A, Miyamoto S, Shimizu Y and Nakamura H: Mixed tumour of the skin
of the lower lip: A case report and review of the literature. Mol
Clin Oncol. 16(69)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reddy PB, Nandini DB, Sreedevi R and
Deepak BS: Benign chondroid syringoma affecting the upper lip:
Report of a rare case and review of literature. J Oral Maxillofac
Pathol. 22:401–405. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wan H, Xu M and Xia T: Clinical and
pathological study on mixed tumors of the skin. Medicine
(Baltimore). 97(e12216)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ohata C and Hanada M: Lipomatous apocrine
mixed tumor of the skin. Am J Dermatopathol. 25:138–141.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Headington JT: Mixed tumors of skin:
Eccrine and apocrine types. Arch Dermatol. 84:989–996.
1961.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dubb M and Michelow P: Cytologic features
of chondroid syringoma in fine needle aspiration biopsies a report
of 3 cases. Acta Cytol. 54:183–186. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Miracco C, De Santi MM, Lalinga AV,
Pellegrino M, Schürfeld K, Sbano P and Miracco F: Lipomatous mixed
tumour of the skin: A histological, immunohistochemical and
ultrastructural study. Br J Dermatol. 146:899–903. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vicioso L, Gallego E and Sanz A: Cutaneous
mixed tumor with lipomatous stroma. J Cutan Pathol. 33 (Suppl
2):S35–S38. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Misago N and Narisawa Y: Lipomatous
apocrine mixed tumor of the skin associated with chondroid and
ossiferous stroma. J Dermatol. 33:380–382. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kasashima S, Hiroshi M, Toshinori M and
Yoshio O: Lipomatous mixed tumor with follicular differentiation of
the skin. J Cutan Pathol. 33:389–394. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nguyen CM and Cassarino DS: Local
recurrence of cutaneous mixed tumor (chondroid syringoma) as
malignant mixed tumor of the thumb 20 years after initial
diagnosis. J Cutan Pathol. 44:292–295. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Linda D, Christine P, Ashley E, Christina
K and Scott B: Rare case of lipomatous mixed tumor with follicular
differentiation. Am J Dermatopathol. 42:e26–e27. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Haskell HD, Butt KM and Woo SB:
Pleomorphic adenoma with extensive lipometaplasia: Report of three
cases. Am J Surg Pathol. 29:1389–1393. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Musayev J, Onal B, Hasanov A and
Farzaliyev I: Lipomatous pleomorphic adenoma in the hard palate:
Report of a rare case with cyto-histo correlation and review. J
Cytol. 31:36–39. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shah SS and Moustafa TZ: An unusual
variant of a common palatal salivary gland tumor: Case report of a
pleomorphic adenoma with significant lipomatous metaplasia. Case
Rep Dent. 2018(2052347)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Azari-Yam A and Abrishami M: Apocrine
mixed tumor of the eyelid: A case report. Diagn Pathol.
11(32)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abiko Y, Kaku T, Shimono M, Noma H and
Shigematsu T: Large cyst formation in pleomorphic adenoma. Bull
Tokyo Dent Coll. 34:9–14. 1993.PubMed/NCBI
|
|
24
|
Siddaraju N, Murugan P, Basu D and Verma
SK: Preoperative cytodiagnosis of cystic pleomorphic adenoma with
squamous metaplasia and cholesterol crystals: A case report. Acta
Cytol. 53:101–104. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Salama ME, Azam M, Ma CK, Ormsby A, Zarbo
RJ, Amin MB and Lee MW: Chondroid syringoma. Cytokeratin 20
immunolocalization of Merkel cells and reappraisal of apocrine
folliculo-sebaceous differentiation. Arch Pathol Lab Med.
128:986–990. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bubola J, MacMillan CM, Demicco EG, Chami
RA, Chung CT, Leong I, Marrano P, Onkal Z, Swanson D, Veremis BM,
et al: Targeted RNA sequencing in the routine clinical detection of
fusion genes in salivary gland tumors. Genes Chromosomes Cancer.
60:695–708. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Macagno N, Sohier P, Kervarrec T,
Pissaloux D, Jullie ML, Cribier B and Battistella M: Recent
advances on immunohistochemistry and molecular biology for the
diagnosis of adnexal sweat gland tumors. Cancers (Basel).
14(476)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Agaimy A, Ihrler S, Baněčková M, Martineau
VC, Mantsopoulos K, Hartmann A, Iro H, Stoehr R and Skálová A:
HMGA2-WIF1 rearrangements characterize a distinctive subset of
salivary pleomorphic adenomas with prominent trabecular
(Canalicular Adenoma-like) morphology. Am J Surg Pathol.
46:190–199. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Panagopoulos I, Gorunova L, Andersen K,
Lund-Iversen M, Lobmaier I, Micci F and Heim S: NDRG1-PLAG1
and TRPS1-PLAG1 fusion genes in chondroid syringoma. Cancer
Genomics Proteomics. 17:237–248. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kumar B: Chondroid syringoma diagnosed by
fine needle aspiration cytology. Diagn Cytopathol. 38:38–40.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Kapatia G and Dey P: Fine-needle
aspiration cytology of chondroid syringoma. Diagn Cytopathol.
47:1324–1325. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Siddaraju N, Singh N, Muniraj F,
Jothilingam P, Kumar S, Basu D and Saxena SK: Preoperative
cytodiagnosis of pleomorphic adenoma with extensive lipometaplasia:
A case report. Acta Cytol. 53:457–459. 2009.PubMed/NCBI View Article : Google Scholar
|