Diabetic retinopathy (DR) is a common microvascular
complication that presents at the late stages of diabetes.
Approximately 80% of diabetic patients experience DR 20 years
following onset, and its incidence is increasing worldwide
(1,2). It has become one of the most
important causes of blindness and visual impairment in working-age
individuals (3,4). The initial stage of DR does not
present with apparent symptoms; however, as the disease progresses,
patients may experience blurred vision or even blindness (5,6).
Early lesions in DR are characterized by loss of retinal capillary
pericytes, resulting in increased vascular permeability, the
presence of decellularized capillaries and microaneurysms, and
rupture of the blood-retinal barrier (BRB) (7). Progression of DR to its later stage
is followed by neocapillary proliferation, which significantly
increases the likelihood of visual loss (8,9).
Endothelial cell dysfunction (ED) is the key element to the
development of microvascular lesions. Certain studies have shown
that hyperglycemia-induced oxidative stress is increasing, which
stimulates the inflammatory pathways and promotes vascular
dysfunction of the retina leading to increased capillary
permeability and vascular leakage (10). In addition, mitochondrial
homeostasis is associated with ED (11). The enzyme, endothelial nitric oxide
synthase (eNOS), also plays a vital role in maintaining the
function of endothelial cells (ECs) (12).
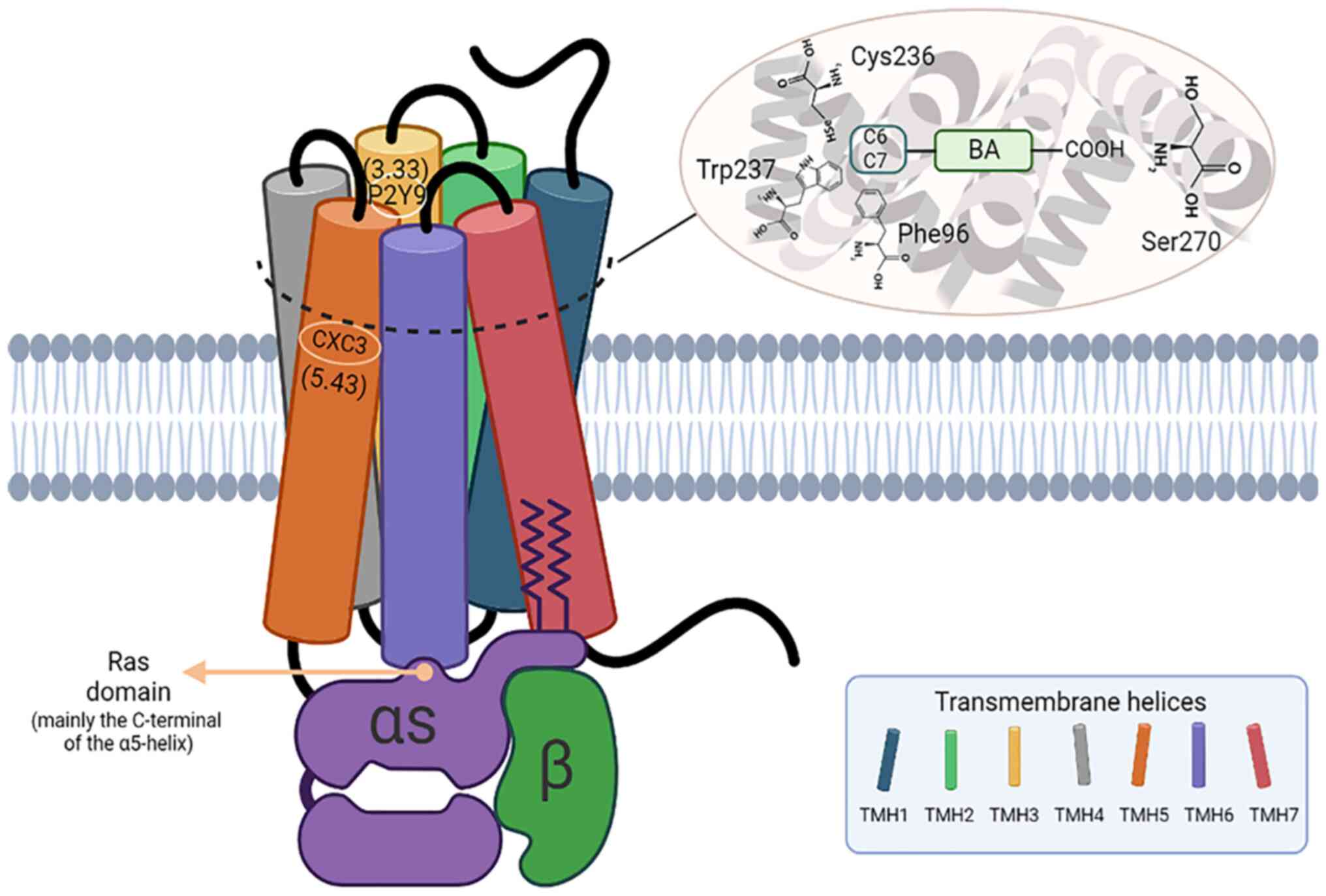
Bile acids (BAs) are a class of endogenous molecules
synthesized in the liver; they are present in the bile as ionic
salts derived from the metabolism of cholesterol (13). The main role of cholesterol is to
promote the digestion and absorption of lipids. In the case of
diabetes mellitus (DM), lithocholic acids and deoxycholic acids,
formed by the enterohepatic cycle of BAs, have a high affinity for
Takeda G protein-coupled receptor 5 (TGR5); the BA-induced
activation of TGR5 increases glucagon-like peptide-1 (GLP-1) and
insulin release (14). In recent
years, a high number of studies have shown that BAs can be used as
a signaling molecule to bind to the corresponding receptors and
participate in the regulation of various metabolic diseases
(15,16). TGR5 is a widely studied signaling
molecule involved in this process. TGR5 is expressed in a variety
of tissues and organs, such as the liver, kidney, brain, and heart
(17,18). It is also widely expressed in
almost all types of ECs and is involved in the regulation of
glucose and lipid metabolism processes present in various metabolic
diseases, such as obesity, non-alcoholic fatty liver disease, and
type 2 diabetes mellitus (T2DM) (19,20).
Currently, farnesoid X receptor (FXR) is mainly
reported to be related to the function of macrovessels, while TGR5
is less relevant (21). In
addition, as a small tissue in the eyeball, the blood vessels
distributed on the retina are mainly microvessels. From an
etiological point of view, the specific pathogenesis of DR remains
unelucidated, but the current view accepted widely by researchers
is that DR is a retinal microvascular complication induced by the
long-term hyperglycemic environment. Therefore, in the present
review, the role TGR5 plays in microvessels was investigated and an
attempt was made to elucidate the underlying possible
mechanisms.
To date, various studies on BAs and their receptors
have implicated their possible roles in the regulation of EC
function (22). Previously, it has
been revealed that TGR5 is highly expressed in retinal
microvascular ECs (23), which may
produce BAs through the ‘alternative’ pathway (24). It has also been found that
intermittent fasting increases the production of taurodeoxycholic
acid (TUDCA), a metabolite of BAs in the retina, and protects
retinal ECs to delay the progression of DR (23). A previous study has shown that TGR5
agonists are beneficial in diabetes and TGR5 has become a promising
target for the treatment of this disease (25). Therefore, it was hypothesized that
TGR5 activation may delay the progression of DR by improving ED,
which plays a protective role in the retina; however, the
underlying mechanism remains to be elucidated in further
studies.
In the present review, the role of TGR5 in delaying
the progression of DR was summarized by its effect on maintaining
mitochondrial homeostasis and counteracting inflammation to protect
ECs from damage. Therefore, the present study aimed to provide
possible evidence for the application of the targeted therapy of
DR.
ECs are a layer of squamous epithelial cells
covering the inner surface of blood vessels, which constitute a
barrier between blood vessels and tissues and control the transport
of substances between tissues and blood vessels. ECs act as a
metabolic interface between the blood and the tissues and are
important in maintaining the stability of the intravascular
environment (26). ED occurs when
ECs are unable to maintain homeostasis of the vascular environment.
It is a systemic pathological condition characterized by changes in
the phenotype of ECs, which leads to diminished vasoconstriction
and the formation of a proinflammatory and prothrombotic state
(27). ED forms the basis of the
chronic microvascular and macrovascular complications of diabetes.
In recent years, significant progress has been made in
understanding the mechanism of ED and its pathogenesis in patients
with type 1 diabetes mellitus (T1DM) and T2DM. Several factors that
cause ED have been identified and the common causes include
hypoxia, aging, hyperglycemia, hypercholesterolemia, and
hypertension (28). Previous
studies have shown that pathophysiological processes caused by a
high glucose environment found in diabetics, such as inflammation,
oxidative stress, and endoplasmic reticulum (ER) stress are
responsible for the continuous progression and aggravation of ED in
the course of the disease (29).
As a common microvascular complication in the late stage of
diabetes, the risk factors for the development of DR are mainly
related to the severity and exposure time of hyperglycemia,
hypertension, and hyperlipidemia (30). ED is the pathological basis of
diabetic microvascular complications and plays an important role in
the pathological progression of DR. Progressive dysfunction of ECs
will certainly lead to changes in morphological structures, such as
capillary basement membrane thickening, perivascular cell loss, BRB
damage, and neovascularization, which accelerates the progression
of DR (31,32).
The specific mechanisms leading to DR have not been
fully elucidated. However, disruption of mitochondrial homeostasis
and inflammation are considered to be closely related to the
pathogenesis of DR (33,34).
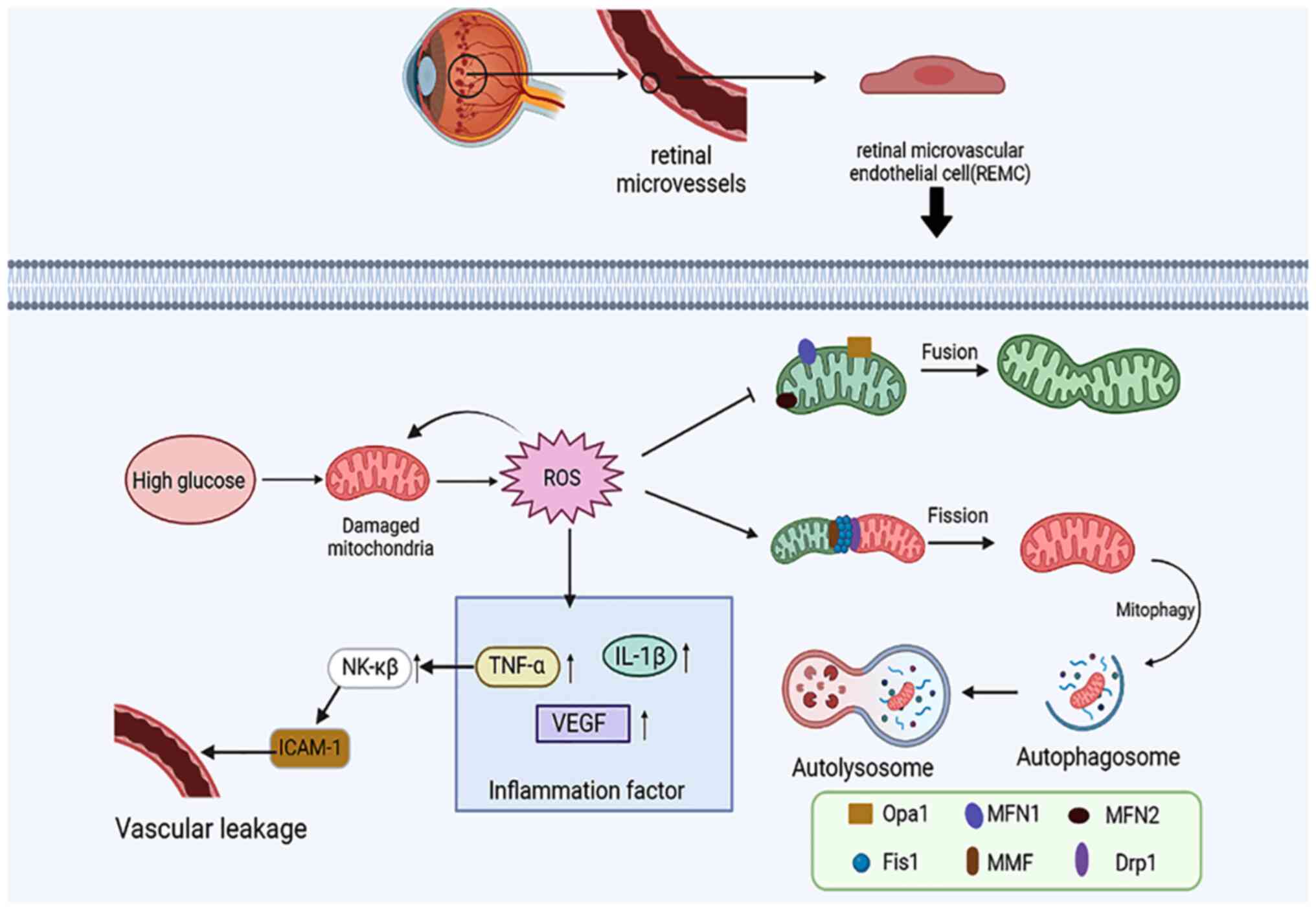
Diabetes can disturb mitochondrial dynamic
homeostasis, causing impaired mitochondrial function, which in turn
causes the development of related diseases (35,36).
Under high glucose conditions, the electron flux through the
electron transport chain increases, eventually leading to increased
reactive oxygen species (ROS) production, which in turn causes
retinal damage (37,38). The mitochondrial fusion division
mechanisms are also compromised in diabetes; swollen retinal
mitochondria decrease mitofusin 2 (Mfn2) expression and increase
dynamin-related protein 1 (Drp1) expression (39). Decreased mitosis and inflammasome
activation can be observed in the retina of diabetic patients
(40), which further leads to
deterioration of mitochondrial homeostasis.
In addition, several studies have implicated various
systemic and local inflammatory factors in DR (41,42).
Diabetes causes increased local and systemic expression of
inflammatory cytokines, chemokines, and growth factors, all of
which are involved in the development of DR (43-45).
It has been shown that fortified extracts of red berries, ginkgo
biloba leaves, and white willow bark containing carnosine and
α-lipoic acid can significantly reduce cytokine levels in the
retina and inhibit lipid peroxidation, which is associated with
diabetes (46). Another study has
demonstrated that curcumin can protect against high
glucose-mediated retinal pigment epithelial cell injury due to
induction of an anti-inflammatory pathway (47). Purinergic signaling has been shown
to be a key factor in regulating the inflammatory status in
different organ tissues. P2X purinergic receptor 7 (P2RX7) is a
common purinergic ionotropic receptor; its activation leads to the
release of proinflammatory mediators and the induction of cell
damage. This receptor is considered to be a target for restoring
BRB and reducing inflammation. It has been experimentally
demonstrated that the inhibition or downregulation of P2X7R
expression plays a protective role in inflammation-induced cell
damage (48-50).
TGR5 is a common membrane receptor during BA
metabolism and has been demonstrated to be expressed in a variety
of tissues and organs (54). The
role of TGR5 in regulating homeostatic metabolism is also well
documented. A previous study has shown that TGR5 can delay the
occurrence and development of portal hypertension by reversing ED
(56). It was also found that
activation of TGR5 could reverse the injury of liver sinusoidal ECs
in a mouse model of cirrhosis and could reverse cardiovascular
injury by reducing the secretion of inflammatory factors in aortic
intimal cells (56,57). These studies indicate that
activation of TGR5 may be a potential therapeutic strategy to delay
ED caused by DR.
Previous studies have confirmed a close association
between the damage of ECs and mitochondrial damage. Under damaged
conditions, mitochondria generate large amounts of ROS, such as
superoxide anion (O2-), hydrogen peroxide
(H2O2), peroxyl radical (ROO·), and reactive
hydroxyl radical (·OH), which are generally considered harmful to
cells (58,59). Concomitantly, oxidative stress can
cause changes in mitochondrial dynamics, such as activation of
mitochondrial fission, inhibition of mitochondrial fusion, and an
increase in the levels of mitophagy (60,61).
Mitochondrial fusion involves three proteins, namely Mfn1, Mfn2,
and optic atrophy 1 (OPA1), while mitochondrial fission is mediated
by Drp1 and its receptors, including mitochondrial fission factor
(MFF) and fission 1 (Fis1) (62,63).
High levels of ROS can generate the release of inflammatory
factors, such as vascular endothelial growth factor (VEGF), IL-1β,
and TNF-α. For example, TNF-α can cause leakage of blood vessels by
upregulating NK-κβ to activate ICAM-1(64) and induce EC damage. Numerous
experiments have demonstrated that the production of a series of
inflammatory cytokines and the changes in mitochondrial dynamics in
diabetic rats contribute to retinal microvascular endothelial cell
(RMEC) dysfunction in this animal model (65,66)
(Fig. 2).
Mitochondria are the main site of energy production
and play a crucial role in energy conversion and metabolism. In
addition, mitochondria perform various functions that are essential
for cell survival and have to maintain these processes and also
adapt to the changing cellular environment. Mitochondria, as highly
mobile double-membrane organelles, can form dynamic and extensive
cellular networks that maintain homeostasis through fusion,
fission, and mitophagy (62).
Normally, fusion and division of mitochondria exist in a dynamic
equilibrium. Mitochondrial dynamics are essential for the
regulation of mitochondrial function and mitochondrial
fragmentation has been shown to be involved in the induction of
pathological processes including DM (67-69).
Substantial evidence has demonstrated that therapies
that improve mitochondrial function can ameliorate damage to
retinal ECs. D-Arg-dimethylTyr-Lys-Phe-NH2 (SS-31) is a
mitochondria-targeted antioxidant peptide, which effectively
reverses the decreased visual acuity in a streptozotocin-induced
diabetic mouse model (70). Huang
et al (71) demonstrated
that diabetic rats treated with SS-31 exhibited improved retinal
ganglion cell structure, thinner capillary basement membrane, and
reduced inner BRB leakage. Therefore, the improvement of
mitochondrial damage may become a new strategy to treat DR.
ECs rely on glycolysis for energy supply, which may
be a misleading concept suggesting that adenosine triphosphate
(ATP) derived from mitochondria has no important role in ECs
(72). However, recent evidence
suggests that while the energy requirements between ECs are not as
large as those of cardiomyocytes and smooth muscle cells,
intracellular ATP may play an important role in mediating the
normal physiological functions of ECs (73). Mitochondrial oxidative
phosphorylation plays an integral role in energy stores and
mitochondrial dysfunction can enhance oxidative stress sensitivity
and lead to EC death (65).
Therefore, a decreased mitochondrial function also contributes to
ED. Recently, it has been shown that EC injury can be delayed by
reducing mitochondrial division and/or enhancing mitophagy via the
activation of TGR5.
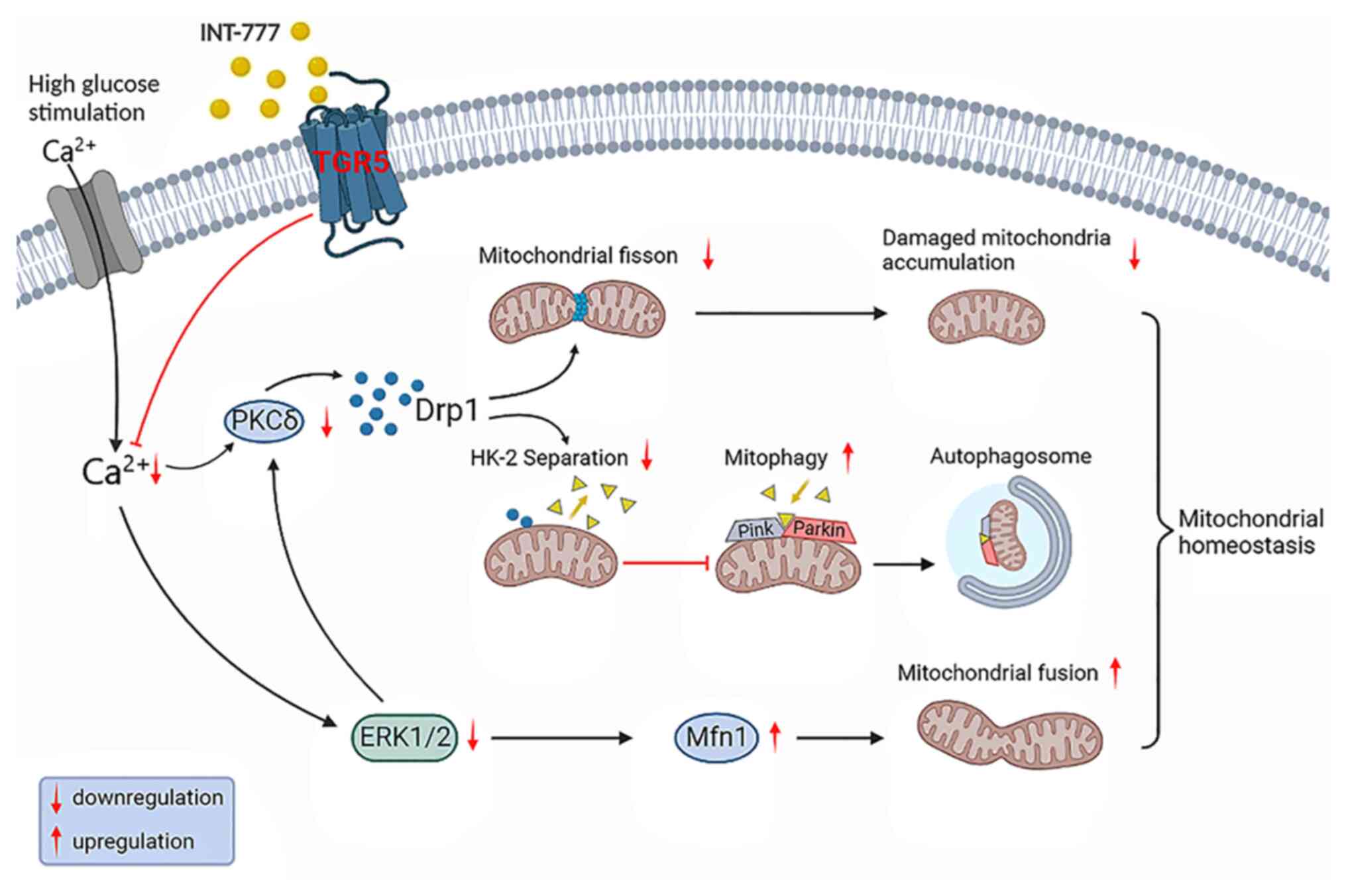
It has been shown that INT-777, an agonist of TGR5,
prevents mitochondrial division by decreasing calcium
concentration, attenuating protein kinase C (PKC) activation, and
inhibiting the Ca2+-PKCδ/Drp1 pathway (65). It has also been found that PKCδ can
lead to Drp1 phosphorylation and translocation of phosphorylated
Drp1 to mitochondria can promote mitochondrial division (74). Intracellular calcium can activate
calcineurin to promote mitochondrial division and induce activation
of Ca2+/calmodulin-dependent protein kinase II, which
can mediate p-S616 expression in Drp1 (75,76).
Activation of TGR5 can reduce intracellular Ca2+
concentrations by blocking the influx of extracellular
Ca2+, thereby inhibiting mitochondrial division.
Concomitantly, decreased intracellular Ca2+ inhibits the
extracellular regulated protein kinases (ERK1/2) signaling pathway,
which can cause a decrease in Drp1 expression; this, in turn
inhibits mitochondrial division and promotes Mfn1 oligomer
formation, thereby promoting mitochondrial fusion (77-79).
Physiologically, a low number of damaged
mitochondria are formed during mitochondrial fission, and damaged
mitochondria are degraded by mitochondria-targeted autophagy termed
mitophagy (80). TGR5 can activate
the PTEN-induced kinase (PINK)/Parkin pathway and inhibit the
PKCδ/Drp1-hexokinase (HK)2 pathway to enhance mitophagy. HK is a
positive modulator of Parkin recruitment and glycolysis (81,82).
As the major HK isoform in insulin-sensitive tissues including
retinopathy, HK2 binds to voltage-dependent anion channels and
localizes to the outer mitochondrial membrane. HK2 translocates
from the mitochondria into the cytosol in response to high glucose
conditions in diabetic mice. Treatment with INT-777 promotes
recruitment of HK2 to the mitochondria and further activation of
the PINK1/Parkin signaling pathway. The use of the Drp1 inhibitor
Mdivi-1 can promote, in a similar manner, the translocation of HK2
from the cytosol to the mitochondria. This suggests a role for TGR5
in enhancing mitophagy and inhibiting cell division in
vitro. In vivo, capillary degeneration and pericyte loss
has been shown to be milder in TGR5-knockdown rats injected with
the mitochondrial fission inhibitor Mdivi-1 or the mitophagy
agonist rapamycin compared with that noted in control animals
(65). In summary, TGR5 maintains
mitochondrial homeostasis by reducing mitochondrial division and
enhancing mitophagy, which in turn improves ED to delay the
progression of DR (Fig. 3).
DR is classified as a chronic low-level inflammatory
process and accumulating evidence has shown that minor inflammation
is responsible for the vasculopathy of DR. In the presence of
oxidative stress caused by hyperglycemia, the levels of
inflammatory factors, such as cytokines, VEGF, IL-1β, and TNF-α in
the serum and local microenvironment are increased. This increase
in inflammatory factors leads to retinopathy (83). Previous studies have demonstrated
that activation of the TGR5 receptor can delay the production of
IL-1, TNF-α, and other inflammatory factors by macrophages; it may
also reduce the production of proinflammatory factors by inhibiting
the Toll-like receptor (TLR)4/NK-κβ pathway and can play a role in
inhibiting the phosphorylation of STAT3 (21,84,85).
Among all inflammatory factors, TNF-α was first implicated in the
progression of insulin resistance, as well as in abnormal glucose
metabolism associated with T2DM. Therefore, the association between
inflammation and DR was assessed with regard to the contribution of
TNF-α.
It has been reported that TNF-α can activate various
caspases leading to apoptosis of inflammatory cells in the chronic
inflammatory response. TNF-α can also activate NK-κβ, thereby
causing an upregulation in the expression levels of related genes
involved in inflammation and resulting in increased intercellular
adhesion molecule 1 (ICAM-1) synthesis. A large amount of ICAM-1
will damage vascular ECs following binding to activated leukocytes,
resulting in vascular leakage at the corresponding site (64). Several lines of evidence suggest
that the elevation of TNF-α is significantly associated with
diabetic angiopathy in diabetic complications. The experiments
indicated higher TNF-α levels in the serum of diabetic rats than
those noted in normal mice in vivo. Following treatment with
apigenin and ramipril, the increase in the levels of TNF-α was
inhibited. Moreover, glomerular hypertrophy, fibrosis, and matrix
expansion were improved, and the degree of inflammation was reduced
in diabetic rats (86). In
vitro, it has been demonstrated that the mRNA expression and
secretion of TNF-α are markedly upregulated in human glomerular EC
cells (HRGECs) treated with high concentrations of glucose
(87). A recent study has also
demonstrated that TNF-α levels are elevated in the early stages of
retinopathy and remain high throughout the process; therefore, it
is speculated that TNF-α can be used as a marker to predict DR
(88).
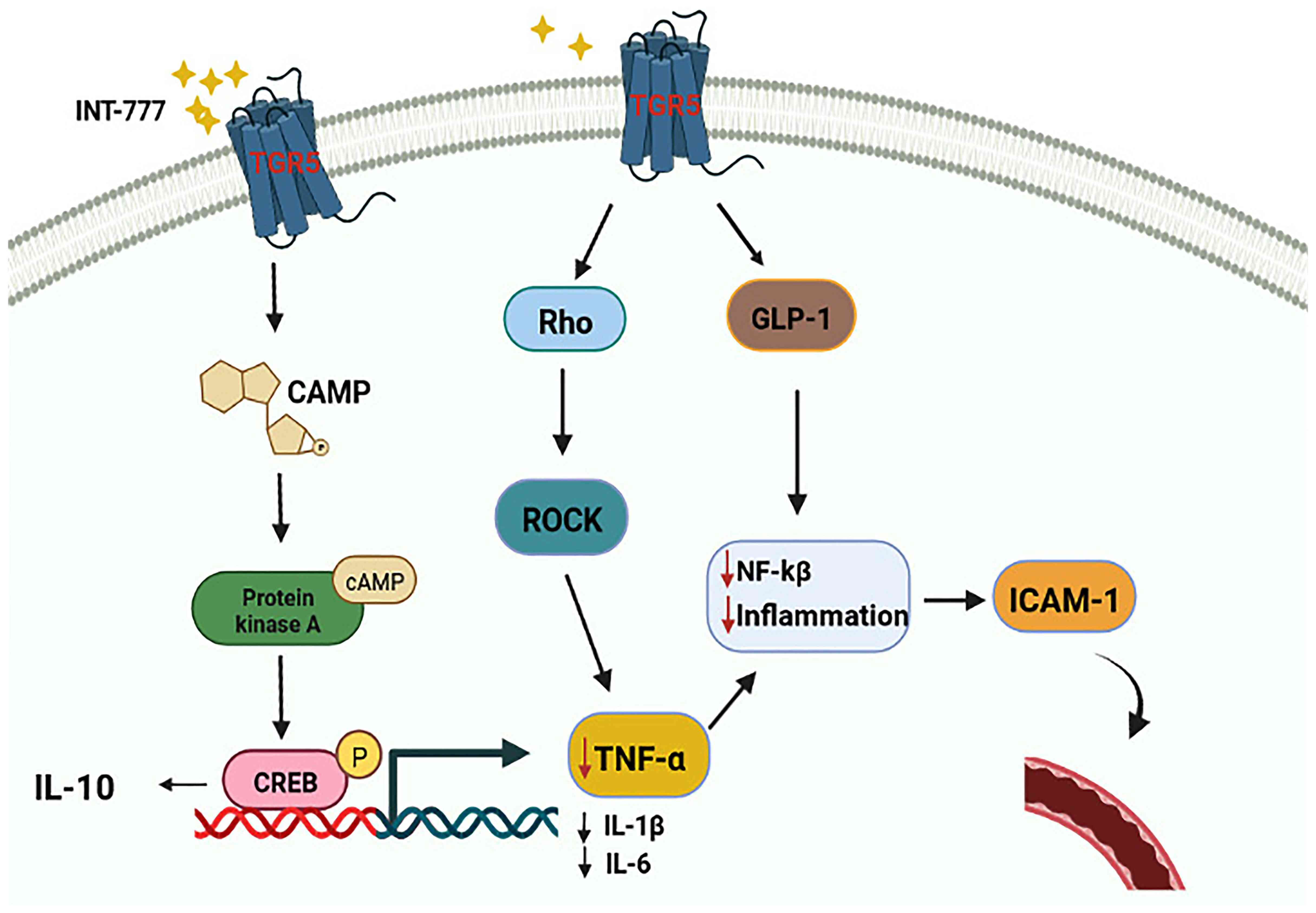
TGR5 has been found to regulate TNF-α by modulating
the Rho/Rho-associated protein kinase (ROCK) signaling pathway. As
an agonist of TGR5, INT-777 can block TNF-α-induced RMEC
proliferation and migration and inhibit the effect of TNF-α on
promoting vascular permeability (66,89).
In addition, previous evidence has shown that TGR5 agonists can
upregulate IL-10 expression to exert anti-inflammatory and
immunosuppressive effects and reduce the expression of the
proinflammatory cytokines IL-1β, IL-6, and TNF-α by activating the
TGR5-cAMP-protein kinase A (PKA) signaling pathway (90,91).
In addition to the two classical signaling pathways described
above, GLP-1, an intestinal hormone with a short half-life, has
been shown to inhibit inflammation and improve EC function
(92). A study has shown that
GLP-1 can delay the damage of ECs by inhibiting NF-κB, which in
turn inhibits the secretion of inflammatory factors, such as IL-1β,
IL-6, and TNF-α (93). While
various studies confirm that GLP-1 is one of the targets of TGR5;
its secretion is dependent on TGR5 (93,94).
Therefore, it was speculated that activation of TGR5 may be an
effective way to stimulate GLP-1 secretion and delay RMEC injury
(Fig. 4).
As previously mentioned, oxidative stress,
inflammation, and mitochondrial damage are important mechanisms of
DR that lead to EC damage. The activation of TGR5 can reduce TNF-α
expression via the Rho/ROCK pathway as well as promote the
secretion of GLP-1 (66,94). TGR5 affects microsomal kinetics by
inhibiting the Ca2+-PKCδ/Drp1 pathway, upregulating the
PINK/Parkin pathway, and regulating the PKCδ/Drp1-HK2 pathway to
alter DR. Therefore, the ability of TGR5 to ameliorate EC function
may be one of the potential mechanisms responsible for its
inhibitory effect on DR.
In addition to the aforementioned mechanisms,
activation of TGR5 stimulates vascular ECs to produce eNOS to
maintain vascular health and function; this mechanism has also the
potential to improve DR (95).
Previous studies have shown that activation of TGR5 can effectively
increase eNOS expression; and its expression level is increased
through the bile salt-TGR5-cAMP pathway and the
TGR5-GLP-1-PI3K-eNOS pathway. (17,27).
In addition, a study has also shown that taurolithocholic acids
(TLCAs), taurocholic acid (TCA), and taurochenodeoxycholic acid
(TCDCA) as agonists of eNOS has been shown to elevate eNOS
expression and Ser1177 phosphorylation of this enzyme, leading to
increased nitric oxide (NO) production (96). The increase in NO production can
effectively protect the vascular endothelium.
Exchange proteins directly activated by cAMP
(EPACs), consisting of Epac1 and Epac2, are cAMP mediators
independent of PKA. As a mediator of cAMP, Epacs take part in
numerous biological functions (97,98).
Increasing studies in recent years have demonstrated the role
cAMP/Epac signaling plays in endothelial cell barrier function
(99,100). It has been found that Epac-1
expression is significantly reduced in mouse models of DR,
suggesting that Epac-1 is a critical regulator of endothelial
function in diabetic microangiopathy involving endothelial
dysfunction associated with hypoxia. Activation of Epac-1 by
forskolin or the cAMP analog 8-pCPT reduces its sensitivity to
oxidative stress, restores the endothelial permeability barrier,
rescues NO production by eNOS and inhibits ROS formation (101), suggesting that Epac-1 may be a
potential target for the treatment of ED during DR. It has also
been revealed that activation of Epac inhibits VEGF receptor
signaling through the Ras/MEK/ERK pathway to improve BRB
permeability (102). In addition,
Epac has also been demonstrated to reduce inflammatory mediators in
retinal endothelial cells, potentially mediating anti-inflammatory
responses in endothelial cells (103). In the present review, it was
indicated that TGR5 can activate cAMP, from which it can be
speculated that TGR5, after activating cAMP, may improve retinal
endothelial cell function through cAMP/Epac signaling, thereby
delaying the progression of DR.
To date, insufficient evidence has been reported
supporting the notion that TGR5 can delay vascular endothelial
injury in DR through ER stress. Therefore, it is possible that a
new mechanism may be responsible for this process. Achieving a
relatively balanced state of mitochondria by maintaining the
homeostasis of the ER has the potential to be a novel mechanism by
which TGR5 delays EC injury. ER stress, such as interference in
Ca2+ homeostasis, redox imbalance, and defects in
protein folding can cause disorders in ECs (104). In ER stress, the unfolded protein
response (UPR) can be triggered and the UPR is an adaptive process
to restore ER stress. It has been shown that oral administration of
TUDCA can reduce ED triggered by hyperglycemia. Moreover,
activation of the UPR by the use of the chemical chaperone TUDCA
can alleviate the glucose-induced increase in inflammatory
cytokines and endothelin-1, as well as the decrease in NO levels
(105,106). It has been demonstrated that ER
stress is associated with TGR5 and that TGR5 mRNA levels are
upregulated in skeletal myotubes in response to the UPR inducers
thapsigargin (ER-specific Ca-ATPase inhibitor) and tunicamycin
(N-glycosylation inhibitor), demonstrating that TGR5 is a novel UPR
target gene (107). Additional
studies have demonstrated that TUDCA can reduce ER stress by
stimulating the TGR5 signaling pathway (108,109). It has been shown that
upregulation of TGR5 expression inhibits the TLR4/NF-κB pathway to
reduce oxidative stress, which may delay endothelial injury
(85). TGR5 may alleviate ER
stress through the protein kinase R (PKR)-like endoplasmic
reticulum kinase (PERK)/eukaryotic initiation factor 2(eIF2)/NF-κB
pathway and activating transcription factor
4-CCAAT-enhancer-binding protein homologous protein (ATF4-CHOP);
therefore, these targets have the potential to be used for delaying
necrosis of diabetic RMECs (104).
Since the activation of the TGR5 receptor improves
EC injury caused by mitochondrial injury, oxidative stress,
inflammatory factors, and ER stress, it may play a role in delaying
DR-induced ED. Therefore, the TGR5 receptor can be used as a new
target to ameliorate DR. Quinoa can cause an upregulation of the
expression of GLP-1 via increased expression levels of TGR5 and it
may be useful in the treatment of DR (85). Therefore, it is possible that
certain components in Chenopodium album may act as agonists
of TGR5. This application can be supported further by in
vivo studies and clinical trials in humans.
Not applicable.
Funding: The present review was supported by the Natural Science
Foundation of Liaoning Province (grant no. 2020-BS-189), the Basic
Scientific Research Projects of Liaoning Provincial Education
Department (grant no. LQ2017005) and the Liaoning Provincial
Program for Top Discipline of Basic Medical Sciences (grant nos.
2020-BS-189 and LQ2017005).
Not applicable.
MZ conceived and designed the article. MZ and ZD
prepared and wrote the manuscript. MZ, ZD and WD performed a
literature search, selected the studies to be included and drew the
figures. MZ, ZD, DZ and XR revised the manuscript. DZ retrieved and
analyzed relevant documents. Data authentication is not applicable.
All authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Williams R, Airey M, Baxter H, Forrester
J, Kennedy-Martin T and Girach A: Epidemiology of diabetic
retinopathy and macular oedema: A systematic review. Eye (Lond).
18:963–983. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Simó R and Hernández C: Prevention and
treatment of diabetic retinopathy: Evidence from large, randomized
trials. The emerging role of fenofibrate. Rev Recent Clin Trials.
7:71–80. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Singh R, Barden A, Mori T and Beilin L:
Advanced glycation end-products: A review. Diabetologia.
44:129–146. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pardue MT and Allen RS: Neuroprotective
strategies for retinal disease. Prog Retin Eye Res. 65:50–76.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kollias AN and Ulbig MW: Diabetic
retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl
Int. 107:75–83; quiz 84. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moreno A, Lozano M and Salinas P: Diabetic
retinopathy. Nutr Hosp. 28 (Suppl 2):S53–S56. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wong TY, Cheung CM, Larsen M, Sharma S and
Simó R: Diabetic retinopathy. Nat Rev Dis Primers.
2(16012)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Henriques J, Vaz-Pereira S, Nascimento J
and Rosa PC: Diabetic eye disease. Acta Med Port. 28:107–113.
2015.PubMed/NCBI(In Portuguese).
|
|
9
|
Lechner J, O'Leary OE and Stitt AW: The
pathology associated with diabetic retinopathy. Vision Res.
139:7–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shafabakhsh R, Aghadavod E, Mobini M,
Heidari-Soureshjani R and Asemi Z: Association between microRNAs
expression and signaling pathways of inflammatory markers in
diabetic retinopathy. J Cell Physiol. 234:7781–7787.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singh LP, Yumnamcha T and Devi TS:
Mitophagy, ferritinophagy and ferroptosis in retinal pigment
epithelial cells under high glucose conditions: Implications for
Diabetic retinopathy and age-related retinal diseases. JOJ
Ophthalmol. 8:77–85. 2021.PubMed/NCBI
|
|
12
|
Meza CA, La Favor JD, Kim DH and Hickner
RC: Endothelial dysfunction: Is There a Hyperglycemia-induced
imbalance of NOX and NOS? Int J Mol Sci. 20(3775)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Maldonado-Valderrama J, Wilde P,
Macierzanka A and Mackie A: The role of bile salts in digestion.
Adv Colloid Interface Sci. 165:36–46. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
van Nierop FS, Scheltema MJ, Eggink HM,
Pols TW, Sonne DP, Knop FK and Soeters MR: Clinical relevance of
the bile acid receptor TGR5 in metabolism. Lancet Diabetes
Endocrinol. 5:224–233. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
de Boer JF, Bloks VW, Verkade E,
Heiner-Fokkema MR and Kuipers F: New insights in the multiple roles
of bile acids and their signaling pathways in metabolic control.
Curr Opin Lipidol. 29:194–202. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Molinaro A, Wahlström A and Marschall HU:
Role of bile acids in metabolic control. Trends Endocrinol Metab.
29:31–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Thomas C, Gioiello A, Noriega L, Strehle
A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski
M, et al: TGR5-mediated bile acid sensing controls glucose
homeostasis. Cell Metab. 10:167–177. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li S, Qiu M, Kong Y, Zhao X, Choi HJ,
Reich M, Bunkelman BH, Liu Q, Hu S, Han M, et al: Bile Acid G
protein-coupled membrane receptor TGR5 Modulates Aquaporin
2-Mediated water homeostasis. J Am Soc Nephrol. 29:2658–2670.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li T and Chiang JY: Bile acid signaling in
metabolic disease and drug therapy. Pharmacol Rev. 66:948–983.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chávez-Talavera O, Tailleux A, Lefebvre P
and Staels B: Bile acid control of metabolism and inflammation in
obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty
liver disease. Gastroenterology. 152:1679–1694.e3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Voiosu A, Wiese S, Voiosu T, Bendtsen F
and Møller S: Bile acids and cardiovascular function in cirrhosis.
Liver Int. 37:1420–1430. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang XX, Wang D, Luo Y, Myakala K,
Dobrinskikh E, Rosenberg AZ, Levi J, Kopp JB, Field A, Hill A, et
al: FXR/TGR5 dual agonist prevents progression of nephropathy in
diabetes and obesity. J Am Soc Nephrol. 29:118–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Beli E, Yan Y, Moldovan L, Vieira CP, Gao
R, Duan Y, Prasad R, Bhatwadekar A, White FA, Townsend SD, et al:
Restructuring of the gut microbiome by intermittent fasting
prevents retinopathy and prolongs survival in db/db Mice. Diabetes.
67:1867–1879. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ren S, Hylemon P, Marques D, Hall E,
Redford K, Gil G and Pandak WM: Effect of increasing the expression
of cholesterol transporters (StAR, MLN64, and SCP-2) on bile acid
synthesis. J Lipid Res. 45:2123–2131. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pellicciari R, Gioiello A, Macchiarulo A,
Thomas C, Rosatelli E, Natalini B, Sardella R, Pruzanski M, Roda A,
Pastorini E, et al: Discovery of 6alpha-ethyl-23(S)-methylcholic
acid (S-EMCA, INT-777) as a potent and selective agonist for the
TGR5 receptor, a novel target for diabesity. J Med Chem.
52:7958–7961. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Galley HF and Webster NR: Physiology of
the endothelium. Br J Anaesth. 93:105–113. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cai Z, Yuan S, Zhong Y, Deng L, Li J, Tan
X and Feng J: Amelioration of Endothelial dysfunction in diabetes:
Role of takeda G protein-coupled receptor 5. Front Pharmacol.
12(637051)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Poredos P, Poredos AV and Gregoric I:
Endothelial dysfunction and its clinical implications. Angiology.
72:604–615. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Basha B, Samuel SM, Triggle CR and Ding H:
Endothelial dysfunction in diabetes mellitus: Possible involvement
of endoplasmic reticulum stress? Exp Diabetes Res.
2012(481840)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sorrentino FS, Matteini S, Bonifazzi C,
Sebastiani A and Parmeggiani F: Diabetic retinopathy and endothelin
system: Microangiopathy versus endothelial dysfunction. Eye (Lond).
32:1157–1163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625.
2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fu D, Yu JY, Yang S, Wu M, Hammad SM,
Connell AR, Du M, Chen J and Lyons TJ: Survival or death: A dual
role for autophagy in stress-induced pericyte loss in diabetic
retinopathy. Diabetologia. 59:2251–2261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kowluru RA: Mitochondrial stability in
diabetic retinopathy: Lessons learned from epigenetics. Diabetes.
68:241–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Forrester JV, Kuffova L and Delibegovic M:
The role of inflammation in diabetic retinopathy. Front Immunol.
11(583687)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liang Q and Kobayashi S: Mitochondrial
quality control in the diabetic heart. J Mol Cell Cardiol.
95:57–69. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Williams M and Caino MC: Mitochondrial
dynamics in type 2 diabetes and cancer. Front Endocrinol
(Lausanne). 9(211)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Madsen-Bouterse SA, Mohammad G, Kanwar M
and Kowluru RA: Role of mitochondrial DNA damage in the development
of diabetic retinopathy, and the metabolic memory phenomenon
associated with its progression. Antioxid Redox Signal. 13:797–805.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kowluru RA and Mishra M: Regulation of
matrix metalloproteinase in the pathogenesis of diabetic
retinopathy. Prog Mol Biol Transl Sci. 148:67–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhong Q and Kowluru RA: Diabetic
retinopathy and damage to mitochondrial structure and transport
machinery. Invest Ophthalmol Vis Sci. 52:8739–8746. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Singh LP, Devi TS and Yumnamcha T: The
role of txnip in mitophagy dysregulation and inflammasome
activation in diabetic retinopathy: A new perspective. JOJ
Ophthalmol. 4(10)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Noda K, Nakao S, Ishida S and Ishibashi T:
Leukocyte adhesion molecules in diabetic retinopathy. J Ophthalmol.
2012(279037)2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kaštelan S, Tomić M, Gverović Antunica A,
Salopek Rabatić J and Ljubić S: Inflammation and pharmacological
treatment in diabetic retinopathy. Mediators Inflamm.
2013(213130)2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tang J and Kern TS: Inflammation in
diabetic retinopathy. Prog Retin Eye Res. 30:343–358.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shabab T, Khanabdali R, Moghadamtousi SZ,
Kadir HA and Mohan G: Neuroinflammation pathways: A general review.
Int J Neurosci. 127:624–633. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rübsam A, Parikh S and Fort PE: Role of
inflammation in diabetic retinopathy. Int J Mol Sci.
19(942)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bucolo C, Marrazzo G, Platania CB, Drago
F, Leggio GM and Salomone S: Fortified extract of red berry, Ginkgo
biloba, and white willow bark in experimental early diabetic
retinopathy. J Diabetes Res. 2013(432695)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bucolo C, Drago F, Maisto R, Romano GL,
D'Agata V, Maugeri G and Giunta S: Curcumin prevents high glucose
damage in retinal pigment epithelial cells through ERK1/2-mediated
activation of the Nrf2/HO-1 pathway. J Cell Physiol.
234:17295–17304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Platania CBM, Lazzara F, Fidilio A, Fresta
CG, Conti F, Giurdanella G, Leggio GM, Salomone S, Drago F and
Bucolo C: Blood-retinal barrier protection against high glucose
damage: The role of P2X7 receptor. Biochem Pharmacol. 168:249–258.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tassetto M, Scialdone A, Solini A and Di
Virgilio F: The P2X7 receptor: A promising pharmacological target
in diabetic retinopathy. Int J Mol Sci. 22(7110)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Platania CBM, Drago F and Bucolo C: The
P2X7 receptor as a new pharmacological target for retinal diseases.
Biochem Pharmacol. 198(114942)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Maruyama T, Miyamoto Y, Nakamura T, Tamai
Y, Okada H, Sugiyama E, Nakamura T, Itadani H and Tanaka K:
Identification of membrane-type receptor for bile acids (M-BAR).
Biochem Biophys Res Commun. 298:714–719. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hov JR, Keitel V, Laerdahl JK, Spomer L,
Ellinghaus E, ElSharawy A, Melum E, Boberg KM, Manke T, Balschun T,
et al: Mutational characterization of the bile acid receptor TGR5
in primary sclerosing cholangitis. PLoS One.
5(e12403)2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Macchiarulo A, Gioiello A, Thomas C, Pols
TW, Nuti R, Ferrari C, Giacchè N, De Franco F, Pruzanski M, Auwerx
J, et al: Probing the binding site of bile acids in TGR5. ACS Med
Chem Lett. 4:1158–1162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Guo C, Chen WD and Wang YD: TGR5, not only
a metabolic regulator. Front Physiol. 7(646)2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen G, Wang X, Ge Y, Ma L, Chen Q, Liu H,
Du Y, Ye RD, Hu H and Ren R: Cryo-EM structure of activated bile
acids receptor TGR5 in complex with stimulatory G protein. Signal
Transduct Target Ther. 5(142)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Renga B, Cipriani S, Carino A, Simonetti
M, Zampella A and Fiorucci S: Reversal of Endothelial Dysfunction
by GPBAR1 agonism in portal hypertension involves a AKT/FOXOA1
dependent regulation of H2S generation and endothelin-1. PLoS One.
10(e0141082)2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Carino A, Marchianò S, Biagioli M, Bucci
M, Vellecco V, Brancaleone V, Fiorucci C, Zampella A, Monti MC,
Distrutti E and Fiorucci S: Agonism for the bile acid receptor
GPBAR1 reverses liver and vascular damage in a mouse model of
steatohepatitis. FASEB J. 33:2809–2822. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Poprac P, Jomova K, Simunkova M, Kollar V,
Rhodes CJ and Valko M: Targeting free radicals in oxidative
stress-related human diseases. Trends Pharmacol Sci. 38:592–607.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Shutt T, Geoffrion M, Milne R and McBride
HM: The intracellular redox state is a core determinant of
mitochondrial fusion. EMBO Rep. 13:909–915. 2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sabouny R, Fraunberger E, Geoffrion M, Ng
AC, Baird SD, Screaton RA, Milne R, McBride HM and Shutt TE: The
Keap1-Nrf2 stress response pathway promotes mitochondrial
hyperfusion through degradation of the mitochondrial fission
protein Drp1. Antioxid Redox Signal. 27:1447–1459. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ferrington DA, Fisher CR and Kowluru RA:
Mitochondrial defects drive degenerative retinal diseases. Trends
Mol Med. 26:105–118. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Sabouny R and Shutt TE: Reciprocal
regulation of mitochondrial fission and fusion. Trends Biochem Sci.
45:564–577. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Lutty GA: Effects of diabetes on the eye.
Invest Ophthalmol Vis Sci. 54:ORSF81–ORSF87. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang MY, Zhu L, Zheng X, Xie TH, Wang W,
Zou J, Li Y, Li HY, Cai J, Gu S, et al: TGR5 activation ameliorates
mitochondrial homeostasis via regulating the PKCδ/Drp1-HK2
signaling in diabetic retinopathy. Front Cell Dev Biol.
9(759421)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhu L, Wang W, Xie TH, Zou J, Nie X, Wang
X, Zhang MY, Wang ZY, Gu S, Zhuang M, et al: TGR5 receptor
activation attenuates diabetic retinopathy through suppression of
RhoA/ROCK signaling. FASEB J. 34:4189–4203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Mishra P and Chan DC: Metabolic regulation
of mitochondrial dynamics. J Cell Biol. 212:379–387.
2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Rovira-Llopis S, Bañuls C, Diaz-Morales N,
Hernandez-Mijares A, Rocha M and Victor VM: Mitochondrial dynamics
in type 2 diabetes: Pathophysiological implications. Redox Biol.
11:637–645. 2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Huang M, Wei R, Wang Y, Su T, Li P and
Chen X: The uremic toxin hippurate promotes endothelial dysfunction
via the activation of Drp1-mediated mitochondrial fission. Redox
Biol. 16:303–313. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Alam NM, Mills WC IV, Wong AA, Douglas RM,
Szeto HH and Prusky GT: A mitochondrial therapeutic reverses visual
decline in mouse models of diabetes. Dis Model Mech. 8:701–710.
2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Huang J, Li X, Li M, Li J, Xiao W, Ma W,
Chen X, Liang X, Tang S and Luo Y: Mitochondria-targeted
antioxidant peptide SS31 protects the retinas of diabetic rats.
Curr Mol Med. 13:935–945. 2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Dikalov SI, Nazarewicz RR, Bikineyeva A,
Hilenski L, Lassègue B, Griendling KK, Harrison DG and Dikalova AE:
Nox2-induced production of mitochondrial superoxide in angiotensin
II-mediated endothelial oxidative stress and hypertension. Antioxid
Redox Signal. 20:281–294. 2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Verónica Donoso M, Hernández F, Villalón
T, Acuña-Castillo C and Pablo Huidobro-Toro J: Pharmacological
dissection of the cellular mechanisms associated to the spontaneous
and the mechanically stimulated ATP release by mesentery
endothelial cells: Roles of thrombin and TRPV. Purinergic Signal.
14:121–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chen C, Huang J, Shen J and Bai Q:
Quercetin improves endothelial insulin sensitivity in obese mice by
inhibiting Drp1 phosphorylation at serine 616 and mitochondrial
fragmentation. Acta Biochim Biophys Sin (Shanghai). 51:1250–1257.
2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Slupe AM, Merrill RA, Flippo KH, Lobas MA,
Houtman JC and Strack S: A calcineurin docking motif (LXVP) in
dynamin-related protein 1 contributes to mitochondrial
fragmentation and ischemic neuronal injury. J Biol Chem.
288:12353–12365. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bo T, Yamamori T, Suzuki M, Sakai Y,
Yamamoto K and Inanami O: Calmodulin-dependent protein kinase II
(CaMKII) mediates radiation-induced mitochondrial fission by
regulating the phosphorylation of dynamin-related protein 1 (Drp1)
at serine 616. Biochem Biophys Res Commun. 495:1601–1607.
2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Cho B, Cho HM, Jo Y, Kim HD, Song M, Moon
C, Kim H, Kim K, Sesaki H, Rhyu IJ, et al: Constriction of the
mitochondrial inner compartment is a priming event for
mitochondrial division. Nat Commun. 8(15754)2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Cook SJ, Stuart K, Gilley R and Sale MJ:
Control of cell death and mitochondrial fission by ERK1/2 MAP
kinase signalling. FEBS J. 284:4177–4195. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Chakrabarti R, Ji WK, Stan RV, de Juan
Sanz J, Ryan TA and Higgs HN: INF2-mediated actin polymerization at
the ER stimulates mitochondrial calcium uptake, inner membrane
constriction, and division. J Cell Biol. 217:251–268.
2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Schmukler E, Solomon S, Simonovitch S,
Goldshmit Y, Wolfson E, Michaelson DM and Pinkas-Kramarski R:
Altered mitochondrial dynamics and function in APOE4-expressing
astrocytes. Cell Death Dis. 11(578)2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Xu S and Herschman HR: A Tumor agnostic
therapeutic strategy for hexokinase 1-Null/Hexokinase 2-positive
cancers. Cancer Res. 79:5907–5914. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Li M, Shao J, Guo Z, Jin C, Wang L, Wang
F, Jia Y, Zhu Z, Zhang Z, Zhang F, et al: Novel
mitochondrion-targeting copper(II) complex induces HK2 malfunction
and inhibits glycolysis via Drp1-mediating mitophagy in HCC. J Cell
Mol Med. 24:3091–3107. 2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Clausell N, Kalil P, Biolo A, Molossi S
and Azevedo M: Increased expression of tumor necrosis factor-alpha
in diabetic macrovasculopathy. Cardiovasc Pathol. 8:145–151.
1999.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Zhou X and Guan Z, Jin X, Zhao J, Chen G,
Ding J, Ren Y, Zhai X, Zhou Q and Guan Z: Reversal of alopecia
areata, osteoporosis follow treatment with activation of Tgr5 in
mice. Biosci Rep. 41(BSR20210609)2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Wang TY, Tao SY, Wu YX, An T, Lv BH, Liu
JX, Liu YT and Jiang GJ: Quinoa Reduces High-Fat diet-induced
obesity in mice via potential microbiota-gut-brain-liver
interaction mechanisms. Microbiol Spectr.
10(e0032922)2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Malik S, Suchal K, Khan SI, Bhatia J,
Kishore K, Dinda AK and Arya DS: Apigenin ameliorates
streptozotocin-induced diabetic nephropathy in rats via
MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am J Physiol
Renal Physiol. 313:F414–F422. 2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Xiang E, Han B, Zhang Q, Rao W, Wang Z,
Chang C, Zhang Y, Tu C, Li C and Wu D: Human umbilical cord-derived
mesenchymal stem cells prevent the progression of early diabetic
nephropathy through inhibiting inflammation and fibrosis. Stem Cell
Res Ther. 11(336)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Khaloo P, Qahremani R, Rabizadeh S, Omidi
M, Rajab A, Heidari F, Farahmand G, Bitaraf M, Mirmiranpour H,
Esteghamati A and Nakhjavani M: Nitric oxide and TNF-α are
correlates of diabetic retinopathy independent of hs-CRP and HbA1c.
Endocrine. 69:536–541. 2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Mikelis CM, Simaan M, Ando K, Fukuhara S,
Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T,
Adams RH, et al: RhoA and ROCK mediate histamine-induced vascular
leakage and anaphylactic shock. Nat Commun. 6(6725)2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Hu X, Yan J, Huang L, Araujo C, Peng J,
Gao L, Liu S, Tang J, Zuo G and Zhang JH: INT-777 attenuates
NLRP3-ASC inflammasome-mediated neuroinflammation via TGR5/cAMP/PKA
signaling pathway after subarachnoid hemorrhage in rats. Brain
Behav Immun. 91:587–600. 2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Haselow K, Bode JG, Wammers M, Ehlting C,
Keitel V, Kleinebrecht L, Schupp AK, Häussinger D and Graf D: Bile
acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and
reduce proinflammatory capability of human macrophages. J Leukoc
Biol. 94:1253–1264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Kolka CM and Bergman RN: The endothelium
in diabetes: Its role in insulin access and diabetic complications.
Rev Endocr Metab Disord. 14:13–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Sampedro J, Bogdanov P, Ramos H,
Solà-Adell C, Turch M, Valeri M, Simó-Servat O, Lagunas C, Simó R
and Hernández C: New insights into the mechanisms of action of
topical administration of GLP-1 in an experimental model of
diabetic retinopathy. J Clin Med. 8(339)2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wang LY, Cheng KC, Li Y, Niu CS, Cheng JT
and Niu HS: Glycyrrhizic acid increases glucagon like peptide-1
secretion via TGR5 activation in type 1-like diabetic rats. Biomed
Pharmacother. 95:599–604. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Claybaugh T, Decker S, McCall K, Slyvka Y,
Steimle J, Wood A, Schaefer M, Thuma J and Inman S: L-Arginine
supplementation in type II diabetic rats preserves renal function
and improves insulin sensitivity by altering the nitric oxide
pathway. Int J Endocrinol. 2014(171546)2014.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kida T, Tsubosaka Y, Hori M, Ozaki H and
Murata T: Bile acid receptor TGR5 agonism induces NO production and
reduces monocyte adhesion in vascular endothelial cells.
Arterioscler Thromb Vasc Biol. 33:1663–1669. 2013.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Gloerich M and Bos JL: Epac: Defining a
new mechanism for cAMP action. Annu Rev Pharmacol Toxicol.
50:355–375. 2010.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Lezoualc'h F, Fazal L, Laudette M and
Conte C: Cyclic AMP Sensor EPAC proteins and their role in
cardiovascular function and disease. Circ Res. 118:881–897.
2016.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Gündüz D, Troidl C, Tanislav C, Rohrbach
S, Hamm C and Aslam M: Role of PI3K/Akt and MEK/ERK Signalling in
cAMP/Epac-Mediated endothelial barrier stabilisation. Front
Physiol. 10(1387)2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Yuan Y, Engler AJ, Raredon MS, Le A,
Baevova P, Yoder MC and Niklason LE: Epac agonist improves barrier
function in iPSC-derived endothelial colony forming cells for whole
organ tissue engineering. Biomaterials. 200:25–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Garcia-Morales V, Friedrich J, Jorna LM,
Campos-Toimil M, Hammes HP, Schmidt M and Krenning G: The
microRNA-7-mediated reduction in EPAC-1 contributes to vascular
endothelial permeability and eNOS uncoupling in murine experimental
retinopathy. Acta Diabetol. 54:581–591. 2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Ramos CJ, Lin C, Liu X and Antonetti DA:
The EPAC-Rap1 pathway prevents and reverses cytokine-induced
retinal vascular permeability. J Biol Chem. 293:717–730.
2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Liu L, Jiang Y, Chahine A, Curtiss E and
Steinle JJ: Epac1 agonist decreased inflammatory proteins in
retinal endothelial cells, and loss of Epac1 increased inflammatory
proteins in the retinal vasculature of mice. Mol Vis. 23:1–7.
2017.PubMed/NCBI
|
|
104
|
Luchetti F, Crinelli R, Cesarini E,
Canonico B, Guidi L, Zerbinati C, Di Sario G, Zamai L, Magnani M,
Papa S and Iuliano L: Endothelial cells, endoplasmic reticulum
stress and oxysterols. Redox Biol. 13:581–587. 2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Song J, Li J, Hou F, Wang X and Liu B:
Mangiferin inhibits endoplasmic reticulum stress-associated
thioredoxin-interacting protein/NLRP3 inflammasome activation with
regulation of AMPK in endothelial cells. Metabolism. 64:428–437.
2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Fiorentino TV, Procopio T, Mancuso E,
Arcidiacono GP, Andreozzi F, Arturi F, Sciacqua A, Perticone F,
Hribal ML and Sesti G: SRT1720 counteracts glucosamine-induced
endoplasmic reticulum stress and endothelial dysfunction.
Cardiovasc Res. 107:295–306. 2015.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Sasaki T, Kuboyama A, Mita M, Murata S,
Shimizu M, Inoue J, Mori K and Sato R: The exercise-inducible bile
acid receptor Tgr5 improves skeletal muscle function in mice. J
Biol Chem. 293:10322–10332. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Dicks N, Gutierrez K, Currin L, de Macedo
MP, Glanzner WG, Mondadori RG, Michalak M, Agellon LB and Bordignon
V: Tauroursodeoxycholic acid/TGR5 signaling promotes survival and
early development of glucose-stressed porcine embryos†. Biol
Reprod. 105:76–86. 2021.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Dicks N, Gutierrez K, Currin L, Priotto de
Macedo M, Glanzner W, Michalak M, Agellon LB and Bordignon V:
Tauroursodeoxycholic acid acts via TGR5 receptor to facilitate DNA
damage repair and improve early porcine embryo development. Mol
Reprod Dev. 87:161–173. 2020.PubMed/NCBI View Article : Google Scholar
|