1. Introduction
Seizures and epilepsy are correlated with increased
morbidity and mortality (1) and
require a personalized approach because of their association with
other neurological disorders such as multiple sclerosis (MS)
(2).
MS, the most common inflammatory pathology of the
central nervous system (CNS), has registered a significant increase
in incidence and prevalence worldwide in recent years, being an
increasing burden for individuals and the healthcare systems
(3). MS is also one of the main
causes of disability in young individuals, having a major
socioeconomic impact (4). In
addition, MS is associated with significant comorbidities.
Autoimmune diseases such as systemic lupus erythematosus (5) and rheumatoid arthritis (6) and Crohn's disease (7), along with other neurological
disorders such as epilepsy, have a higher prevalence in patients
with MS.
There are several clinical-evolutive forms of MS,
with an accurate diagnosis being mandatory for the therapeutic
approach. Thus, the relapsing-remitting form of MS (RRMS) is the
most commonly encountered in the young population and is
characterized by clearly defined recurrent attacks followed by
periods of partial or complete recovery (8). According to different studies, RRMS
is the initial form of the disease in >70% of the patients
(9,10). RRMS is one of the most promising
neurological disorders in terms of therapeutic options, with a
myriad of disease-modifying therapies (DMTs) now available
(11). Besides the different types
of beta interferons (the first approved DMTs for MS treatment),
newer and more potent monoclonal antibodies such as Natalizumab and
Ocrelizumab are now available (12). During the natural history of RRMS,
the patient's clinical status may evolve towards the slow
accumulation of disability in the absence of relapses, this form
being known as secondary progressive MS (SPMS). Lastly, a minority
of cases are diagnosed with primary progressive MS (PPMS), where
the disability accumulation is slowly evolving from the beginning,
with no clearly defined exacerbations, but reduced therapeutic
possibilities (13). PPMS and SPMS
remain a challenge for the neurologist, as currently available
anti-CD20 medication and sphingosine-1-phosphate receptor
modulators insufficiently slow the neurodegenerative process
(14).
The correlation between MS and seizures was first
noticed over 30 years ago (15),
however remains to be elucidated. Seizures occurring in patients
with MS have multiple similarities in terms of pathophysiology and
treatment with seizures associated with other neurological
pathologies (such as trauma, infection, stroke and neoplasia)
(16), but particular
etiopathogenic aspects should also be considered, especially for
epileptic syndrome associated with MS. The exact prevalence of
MS-related epilepsy is largely unknown. One study indicates that,
after excluding alternative diagnoses, the exact numbers are lower
than previously assumed, suggesting a possible bias in previous
research (17). The widespread
development and use of new DMTs and modern antiseizure medications
(ASMs) open up new research directions related to possible indirect
potentiation mechanisms between these two classes. In addition,
there is still an unanswered question about the DMTs that are
currently administered to patients with MS according to the current
No Evidence of Disease Activity (NEDA) principles and their impact
on secondary seizures (18).
The currently existing literature (18-20)
only summarizes specific aspects of the MS-epilepsy association and
offers narrow therapeutic directions which are insufficient to
establish strong, internationally validated guidelines. Moreover,
to the best of the authors' knowledge, the most recent similar
publication to the present comprehensive approach dates back to
2008(21), with subsequent
research unable to answer the remaining therapeutic dilemmas, as
there are still a number of unknowns related to the optimal
diagnosis and treatment of patients with MS diagnosed additionally
with epilepsy. These aspects urgently need to be elucidated to
bring significant benefits to the patient's care.
Thus, the present review aimed to address in a
systematic manner the debated issues related to MS-seizures
association, first by presenting the most relevant epidemiological
and clinical data in the literature. After revealing the intricate
bidirectional correlation between the two entities, the present
review subsequently attempted to explain this association by
reviewing the most relevant involved pathophysiological mechanisms.
Given the importance of paraclinical investigations in both MS and
seizures, the present review also highlighted the contribution of
electroencephalography (EEG) and brain imaging [focusing on
magnetic resonance imaging (MRI)] in the diagnosis and monitoring
of the two pathologies. Finally, considerations on specific
therapeutical issues in patients with concomitant seizures and MS
are presented, including relevant information about DMTs and ASM
and their specific administration protocols in these patients. As
the terms ‘seizure’ and ‘epilepsy’ were used in an interchangeable
way (despite the evident difference) in some of the articles that
were included in this review, it was decided to mention explicitly
when data related to the MS-epilepsy association occurs.
2. Methodology and method
Search strategy and study
selection
Literature research was conducted covering three of
the most important online databases (PubMed, Embase and Google
Scholar), using relevant keywords for the present study depending
on the discussed topic. For the epidemiological facts, the
following terms were used: ‘Multiple sclerosis’, ‘seizure’,
‘epilepsy’, ‘epileptic seizures’, ‘convulsion’ and ‘epidemiology’.
Only research conducted on humans (double-blind, single-blind, and
unblinded trials), published in the last 20 years were included.
Abstract-only articles, letters to editors, non-English language
manuscripts, and studies on animal or cell models were excluded.
When referring to imagistic techniques, neurophysiological
investigations, and therapeutic options, Medical Subject Headings
(MeSH) terms such as ‘MRI’, ‘EEG’, ‘video-EEG’, ‘antiseizure
medication’, ‘antiseizure drugs’ were used associated with the
abovementioned keywords. The same study inclusion criteria were
applied. The final article selection was done by two independent
reviewers (T.G.S. and D.C.A.), and, in case of debates that did not
lead to a resolution, a third reviewer (B.E.I.) made the final
decision on the disagreements. Fig.
1 illustrates the whole protocol.
Diagnostic criteria
The results demonstrated great variability in
epidemiological, diagnostic and therapeutic data related to
MS-associated seizures or epilepsy. In fact, the first step in
conducting studies in this regard is the correct definition of
terms, which in clinical practice can often be misleading or
difficult.
According to the latest International League Against
Epilepsy (ILAE) definition, epilepsy is diagnosed when at least two
unprovoked seizures separated by a minimum of 24 h occur, an
epileptic syndrome is confirmed, or the presence of only one single
seizure was validated, but there is an additional risk of at least
60% for developing another seizure within the next 10 years
(22). In the case of an epileptic
seizure in an MS patient, the neurologist faces at least two
problems. First, it must be clarified whether the seizure was
provoked or not and, second, in the case of a single seizure,
whether the risk of recurrence is at least 60%, thus allowing the
diagnosis of epilepsy and initiation of appropriate treatment.
The first important fact related to MS diagnosis is
represented by determining the correct subtype, as seizures may
vary in type and frequency according to the MS subtype. There are
several possible ways MS can evolve in a patient; the presence or
absence of relapses together with the progressive course of the
disease determine the existence of the following subtypes:
relapsing-remitting multiple sclerosis (RRMS), primary progressive
multiple sclerosis (PPMS), and secondary progressive multiple
sclerosis (SPMS) (8). Another
relevant aspect is related to the employed diagnostic criteria. The
studies analyzed in the present review included patients with a
diagnosis of definite MS according to the McDonald (23) diagnostic criteria available at the
time of the research publication.
3. Epidemiological data revealing a
significant association between MS and seizures
Epilepsy, the fourth most common neurological
disease after migraine, stroke and Alzheimer's disease, has a
significant effect at the individual level (24). According to a recent meta-analysis,
there is a prevalence of 6.38 per 1,000 individuals for active
epilepsy and 7.60 per 1,000 individuals with epileptic lifelong
risk (25). Numerous factors
influence the incidence and prevalence of epilepsy, with notable
variations depending on the region. For example, a recent
systematic analysis showed a higher prevalence of epilepsy in
eastern, western, and southern sub-Saharan Africa regions, central
and south-east Asia and central Latin America compared with other
regions (26). Genetic,
environmental, and cultural differences and, finally, accessibility
to health services, can at least partially explain these
epidemiological differences.
MS incidence and prevalence are also
region-dependent. The latest data suggest an increased prevalence
of MS worldwide compared with the figures from the last decade
(27). Regarding the regional
variability, a higher incidence is reported in Europe (based on the
high rates from the north European countries), followed by the
Americas, while African countries have a very low prevalence rate
(27).
Available data regarding patients with MS, although
highly heterogeneous, show an increased prevalence and incidence of
seizures compared with the general population. An older systematic
review estimated the prevalence of seizures at 3.09% and the
incidence at 2.28% for patients with MS (28), being in line with more recent
figures which show a pooled prevalence of 2% for seizures and 3%
for epilepsy among patients with MS (29). Similar data were extracted from
other studies, but with greater inter-study variability. For
example, in a study conducted on a Swedish cohort, the cumulative
incidence of epilepsy was 3.5% for patients with MS, compared with
1.4% for the control group (30).
In a Norwegian study, Benjaminsen et al (2) found that the prevalence of focal
epilepsy in patients with MS was 3.2%, 4.5 times higher compared
with the general population. According to their results, epilepsy
was associated with an increased conversion risk from
relapsing-remitting multiple sclerosis (RRMS) to secondary
progressive multiple sclerosis (SPMS). Engelsen and Grønning
(31) report the prevalence of
epilepsy at 4% in patients with MS, almost four times higher than
the reported prevalence in the general population. In a study
conducted by Eriksson et al (32) in Sweden, the prevalence of epilepsy
in individuals diagnosed with MS was 3.5%, compared with 0.53-0.64%
in the general population. Another Swedish study reported the
10-year cumulative risk of epilepsy to be 51.4% in patients with MS
and 41.3% in the control population (33). An increased risk was observed for
SPMS (60%) compared with patients with RRMS (40%), a relevant
aspect also regarding the pathophysiological mechanisms. Krökki
et al (34) noted that
epilepsy is the most common comorbidity in MS, being found in 4.7%
of 491 patients with defined MS. Langenbruch et al (17) evaluated 4,078 patients with MS and
reported seizures at 1.5% and epilepsy at 0.9%. In a Japanese study
by Nakano et al (35), the
prevalence of epilepsy in patients with MS was twice as high as in
patients diagnosed with neuromyelitis optica spectrum disorders
(NMOSD). According to another study conducted by Koch et al
(36) on 19,804 patients, the
estimated prevalence of epileptic seizures ranged from 0.5 to 8.3%,
with an average of 2.3%. Table I
summarized the most relevant data from the abovementioned studies
and other significant research related to this topic that was
conducted during the last two decades (37-50).
 | Table IMost relevant epidemiologic studies
on epilepsy in patients with MS in the last 20 years. |
Table I
Most relevant epidemiologic studies
on epilepsy in patients with MS in the last 20 years.
| First author | Sample size | Number of patients
with epilepsy | Prevalence of
epilepsy (%) | (Refs.) |
|---|
| Nyquist P,
2001 | 5,715 | 85 | 1.5 | (37) |
| Sokic D, 2001 | 268 | 20 | 7.4 | (38) |
| Eriksson M,
2002 | 255 | 20 | 7.8 | (32) |
| Gambardella A,
2003 | 350 | 16 | 4.6 | (39) |
| Striano P,
2003 | 270 | 13 | 4.8 | (40) |
| Nicoletti A,
2003 | 195 | 5 | 2.6 | (41) |
| Martínez-Juárez I,
2009 | 122 | 8 | 6.55 | (42) |
| Viveiros C,
2010 | 160 | 5 | 3.1 | (43) |
| Nakano H, 2013 | 63 | 4 | 6.3 | (35) |
| Krökki O, 2014 | 491 | 23 | 4.7 | (34) |
| Lund C, 2014 | 332 | 24 | 6.6 | (44) |
| Simpson R,
2014 | 3,826 | 72 | 1.9 | (45) |
| Averianova L,
2017 | 1,850 | 48 | 2.59 | (46) |
| Burman J, 2017 | 14,545 | 502 | 1.7 | (30) |
| Laroni A, 2017 | 1,877 | 7 | 0.4 | (47) |
| Passarell M,
2017 | 5,548 | 109 | 1.96 | (48) |
| Mahamud Z,
2018 | 15,810 | 289 | 1.8 | (33) |
| Benjaminsen E,
2019 | 658 | 20 | 3.1 | (2) |
| Langenbruch L,
2019 | 4,078 | 38 | 1.5 | (17) |
| Schorner A,
2019 | 1,267 | 18 | 1.74 | (49) |
| Neuß F, 2021 | 2,285 | 59 | 2.5 | (50) |
Considering available data, there is a greater risk
of unprovoked seizures in patients with MS, the prevalence of
epileptic seizures being 2-3 times higher compared with the general
population (51). However, the
temporal characteristic of this association is still unclear. In
most cases, epilepsy is diagnosed after MS is diagnosed, with a
mean time of ~10 years between the two entities (52). One explanation for this long
latency period could be related to the MS evolution phase,
transition to SPMS seemingly increasing the seizure risk. Studies
have indeed shown an increased association between progressive MS
(PMS) and epilepsy (53). Another
potential interpretation of the existing figures may suggest that
epilepsy increases the risk of transition from RRMS to SPMS.
However, the relatively decreased prevalence of epilepsy compared
with other MS comorbidities can be partially explained by the early
administration of immunomodulatory/immunosuppressive treatment.
There is, additionally, the possibility for seizure
activity to be the inaugural manifestation of MS (54), including in childhood-onset MS
(55). Moreover, cumulative
seizure incidence is directly related to MS duration, reaching
almost 6% in patients with MS with a disease duration of >30
years (56). Finally, epileptic
seizures can also occur before MS is diagnosed, with different
percentages depending on the study group (50). However, it is still debatable if an
epileptic event without a clear cause should be considered a
retrospective relapse or an associated disorder.
4. Relevant aspects of seizures in patients
with MS
Seizure occurrence and their clinical manifestation
in patients with MS is a relevant aspect that seems to be dependent
on the MS form. In this context, several suggest that SPMS is
associated with an increased risk of seizures compared with RRMS
(2,42,50).
The risk of seizure recurrence is another topic of
interest, as establishing an optimal ASM therapy remains an
essential part of epilepsy management. In this regard, Langenbruch
et al (17) observe that
there are statistically significant differences depending on the
form of MS disease in terms of recurrence of seizures. As the
abovementioned epidemiological data suggest, PMS is associated with
higher seizure risk. Moreover, the primary progressive form of MS
(PPMS) shows a stronger correlation with the development of
recurrent seizures, compared with SPMS (16). The same study also suggests a link
between MS relapses and the occurrence of seizures. During MS
exacerbations, the recurrence risk of seizures is significantly
higher.
Another clinically relevant aspect for the correct
diagnosis and treatment of epilepsy is the type of seizure.
Although little data exists on this topic, according to Ooi et
al 2021(57), focal seizures
are the commonest, accounting for ≤80% of the total. This is in
line with other results, that suggest that the majority of these
patients (>75%) suffer a transformation from focal to bilateral
tonic-clonic seizures (49).
Moreover, bilateral tonic-clonic seizures, frequently of unknown
onset, are also considered common in patients with MS (58). By contrast, epileptic status is
rare in patients with MS (50). It
can be easily observed that the type of epileptic seizures in
patients with MS mirrors only partially the statistical general
trends in patients with epilepsy, in which bilateral tonic-clonic
seizures are the most commonly encountered (56).
Atypical forms of seizures, encountered in dysphasic
status and musicogenic epilepsy, have also been reported in
patients with MS (59). Other
(very) rare epilepsy types are also described in the literature,
with Epilepsia partialis continua a relevant example. Being first
described in patients with MS in 1990 by Hess and Sethi (60); only a few cases are known up to the
present. Autonomic seizures, including ictal vomiting, ictal
spitting, and ictal hypersalivation are rare manifestations by
default and can be frequently omitted because of their non-dominant
semiological features. The present study did not find any reports
linking these types of seizure semiology to MS. During the course
of the disease, other non-epileptic symptoms such as tonic spasms,
dizziness, and diplopia may occur, probably as an expression of the
axonal lesion. However, due to their origin in the spinal cord or
in the brainstem, these manifestations cannot be considered of
epileptic nature (61).
Last, epilepsy might be related to higher morbidity
and mortality in patients with MS. Morbidity is
disability-dependent, being quantified in the case of patients with
MS by the Expanded Disability Status Scale (EDSS) scale (62). The most recent results from the
literature suggest a correlation between increased disability
status and any type of epilepsy in patients with MS (62). Another study conducted on a cohort
of Swedish patients showed a correlation between an increased EDSS
score (≥7) and an increased prevalence of epilepsy compared with
patients with MS without disabilities (56). Finally, Grothe et al
(63) demonstrate in German
patients with MS that the concomitant diagnosis of epilepsy
correlates with a higher EDSS score at MS onset, a faster
progression rate, and an increased overall disability status
compared with patients with MS without epilepsy. It remains an open
question if these results are sustaining a causality relationship
between epilepsy and disability.
Regarding mortality, the study conducted by Mahamud
et al (64) shows higher
mortality in patients with MS with associated epilepsy, although
epilepsy was in very rare cases the primary cause of death. A
similar association between an increased mortality risk in patients
with MS with epilepsy has recently been demonstrated in a UK cohort
of patients (65). However, the
results remain heterogeneous, as other studies do not assess any
difference in mortality in patients with MS with and without
epilepsy (66).
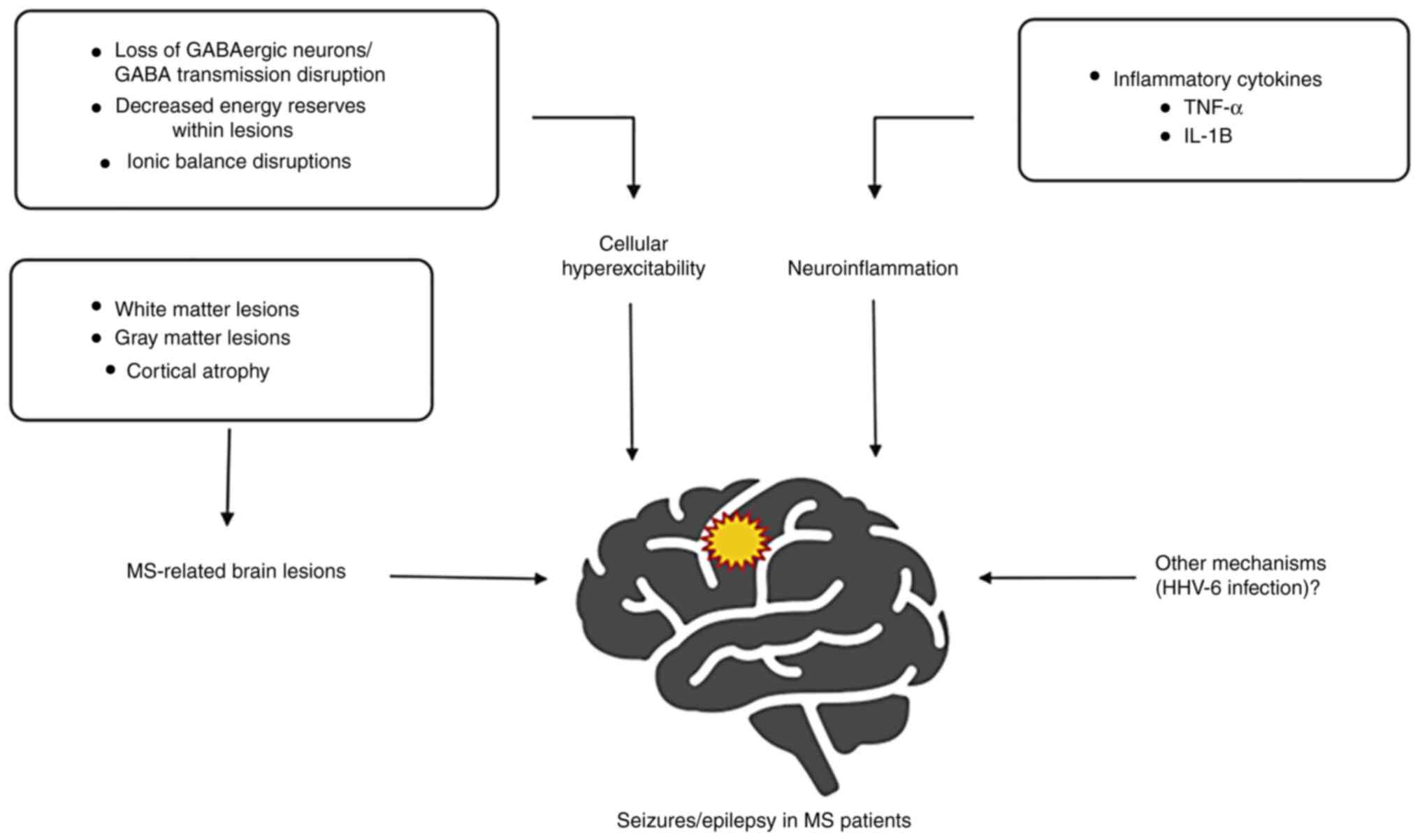
5. Pathophysiological mechanisms
(incompletely) explaining the MS-epilepsy association
Starting with the first case reports of
MS-associated epilepsy, neurologists have been searching for
explanatory pathophysiological mechanisms. Despite the fact that
this association has been studied from multiple perspectives,
including imaging and pathology studies, current data cannot yet
entirely explain it and future research is needed.
Pathological and radiological
evidence
Although MS lesions typically occur in white matter,
gray matter abnormalities have been long recognized in MS (67). Initially, active lesions (detected
by imagistic methods) were thought to be the origin of the clinical
and EEG-associated epileptic activity, but increased seizure risk
in SPMS suggests that there are also other potential epileptic foci
in the brain of patients with MS. Pathological anatomy first
assumed the role of altered gray matter in the pathophysiology of
epileptic seizures. In this regard, the older post-mortem studies
that have shown a significant number of lesions in the gray matter
or at the border between the cortical and subcortical parenchyma,
more commonly in the temporal, parietal and frontal lobes should be
mentioned (68).
Additional evidence to support common
pathophysiological mechanisms has been provided by imagistic
investigations. Thus, gray matter lesions and cerebral atrophy are
related to the formation of epileptic foci, as longitudinal MRI
studies demonstrate a correlation between a higher lesion load and
greater cortical atrophy on one side and a higher prevalence of
epilepsy on the other (69). It is
understood that not all patients with MS with gray matter lesions
and cortical atrophy develop seizures, lesion type and localization
presumably being critical to epileptogenesis. The presence of
lesions at the cortical or cortical-subcortical level has been
associated with an increased risk of seizures (70). However, another study suggests that
the location of lesions in the temporal lobe is a risk factor for
seizure development in patients with MS, with lesions in the
hippocampus, lateral temporal lobe, and cingulate lobe being most
frequently detected (71). The
relation between brain lesions and epilepsy has also been studied
in the other direction. In this sense, epileptic seizures, although
primarily causing changes in the gray matter, have been found to
favor the presence of (demyelinating) lesions in the white matter
as well (72). The association
between demyelinating lesions and epileptic seizures, more
precisely between relapses and the onset of new seizures, is
additional proof of the MS-epilepsy association.
GABA system and ions
Several recent hypotheses attempt to clarify the
molecular mechanisms connecting MS and epilepsy. Thus, on one hand,
the abnormalities of the γ-aminobutyric acid (GABAergic) system
play a major role in epilepsy (73) and on the other, SPMS has been
associated with a loss of parvalbumin-positive GABAergic
interneurons in the cortex (74).
Similarly, Cao et al (75)
demonstrate a low concentration of GABA in the posterior cingulate
cortex and left hippocampus in patients with RRMS, partially
explaining the loss of GABAergic neurons.
Ion and energy imbalance could be another
contributing cause of epileptic seizures in patients with MS.
Within demyelinating lesions, the potential decrease in ATP
production and disturbances of ionic balance (primarily
Ca2+), may lead to neuronal degeneration. Demyelination
may also have an impact on the activation of sodium ionic channels,
subsequently leading to neuronal hyperexcitability (76). Among cortical regions, the
hippocampus is more susceptible to decreased energy reserves, a
lesser amount of available ATP potentially leading to complex ionic
imbalances and a pathological activation of ion channels, that
would finally result in cellular hyperexcitability and abnormal
synchronized neuronal activity (77).
Neuroinflammation
Neuroinflammation is another common aspect of
epilepsy and MS (78,79). More specifically, glial cells
(astrocyte and activated microglia) and immune cells (T and B
cells) produce pro-inflammatory cytokines that play essential roles
in sustaining both pathological processes. For example, TNF-α
maintains chronic inflammation and apoptosis (leading to brain
atrophy) in MS by acting on the Tumor necrosis factor receptor
1(80); while in epilepsy, by
associative mechanisms (GABA receptor endocytosis, glutamate uptake
stimulation and upregulation of AMPA receptors), TNF-α supports and
facilitates epileptic activity (81). In MS, T lymphocytes produce IL-1B,
which acts on specific receptors. Subsequently, IL-1B activates the
NF-κB pathway and leads to the destruction of the blood-brain
barrier (BBB), both processes supporting the chronic inflammatory
status. In the case of epilepsy, IL-1B, via its direct action at
the astrocyte level, inhibits GABA activity in parallel with a
reduction of glutamate uptake, thus favoring an excess of
excitatory neurotransmitters (82).
Human herpesvirus 6A/6B
There are also other important, still incompletely
understood, molecular pathways, related to the abovementioned
mechanisms (Fig. 2). An example of
an interesting future direction for research is the dual role of β
subfamily herpesviruses such as human herpesvirus 6A and 6B
(HHV-6A, HHV-6B) in both MS and epilepsy (83). HHV-6 is considered to serve an
important role in triggering demyelination, being associated with
circulating IgM levels in patients with MS (84). Moreover, a study conducted on a
large cohort determined an association between seropositivity
against the HHV-6A antigen and an increased risk of developing MS
(85). Regarding epilepsy, HHV
viral DNA was detected in the hippocampal tissue of patients
diagnosed with mesial temporal lobe epilepsy, and HHV viral
proteins were detected in the astrocytes located in epileptic
tissue (86). By maintaining a
latent state in astrocytes, HHV-6 is able to alter the astrocyte's
functions, inducing neurotransmitter imbalances that might cause
epileptic seizures. HHV-6 is also suspected to affect the MAPK
kinase signaling pathway, an important molecular pathway shown to
be affected in status epilepticus (87). It remains to be determined whether
HHV-6 alone or in combination with other precipitating factors is
involved in inducing and sustaining epileptic activity in patients
with MS.
6. The role of imagistic techniques in
studying the MS-epilepsy association
According to the ILAE classification, structural
etiology is defined by visible neuroimaging anomalies
superimposable on the anatomic-electroclinical hypothesis as a
predisposing factor for epileptic seizures (88). In this context, the diagnosis of
structural epilepsy is established for a significant proportion of
patients with epilepsy despite a possible absence of clinical
findings. Although according to McDonald's criteria 2017(23), the diagnosis of MS relies heavily
on MRI-detected suggestive CNS lesions, the usual imaging
techniques occasionally lack precision in terms of
anatomical-clinical correlation. In early studies of epilepsy in
patients with MS, a relationship between electro-clinical
manifestations and pathological MRI findings was not clearly
demonstrated (31,38). A possible explanation could be the
fact that the MRI examination was performed during the interictal
period, where the probability to find epileptic clinical and
electrical markers is much lower (37). Detection of cortical lesions can be
difficult in routine imaging examinations, with determination of
the lesion-seizure onset areas correlation being only partially
possible (89). According to one
study, brain MRI identified >5 lesions in 88% of patients with
MS with epilepsy, but no specific lesion distribution was reported
(52). This raises the question of
properly assigning the etiology according to ILAE classification,
considering the fact that imagistic or electrophysiologic
techniques are rarely performed in the short-lived ictal period and
that new MRI lesions in MS relapses also have variable persistence
with a median timeframe of 6 weeks.
The distribution of MS lesions may be related to
cognitive impairment, recurrent seizures, or status epilepticus
(90). Cortical or juxtacortical
lesions have been found to be a precipitating factor for epileptic
seizures in patients with MS in multiple studies (91,92).
In these patients, continuous administration of ASMs due to the
increased risk of recurrence was required. In this regard, a new
entity called cortical MS is now recognized (93). Calabrese et al (94) report that intracortical lesions are
five times more common in patients with RRMS with concomitant
epileptic seizures. Another study shows that cortical and
juxtacortical lesions are independent predictors of seizures,
epilepsy being also related to brain lesion load and cerebral
parenchyma atrophy (29). The use
of newer imaging techniques for diagnosis and follow-up [such as
double inversion recovery (DIR), and high-resolution (3-7 Tesla)
MRI sequences] has improved the early detection of cortical lesions
(94). The currently accepted
sequences for early detection of new and/or epilepsy-related
demyelinating lesions are DIR, diffusion-weighted imaging (95), magnetization transfer ratio
(96), and gradient echo sequences
(GRE) (97). Neuroaxonal damage,
astrogliosis, and demyelination lead to dysfunctions in cortical
connectivity and can be quantified by myelin water fraction as well
as by magnetoencephalography (98).
Apparent normally-structured gray matter analysis by
unconventional quantitative MRI can stratify patients with MS at
risk for epilepsy. Thus, patients with MS and with an increased
rate of cortical atrophy progression are at a higher risk of
developing epilepsy (an additional explanation for the correlation
between SPMS and seizures) (70).
Moreover, an MRI-EEG correlation may be useful for an improved
understanding of the underlying pathophysiological mechanisms
behind this association. At present, the causal relationship is not
completely elucidated. The ‘edema effect’ of growing demyelinating
lesions may play an important role during the relapse, and it could
explain the reduction of seizures with focal onset in patients
treated with corticosteroids, as well as the reduced risk of
subsequent episodes in these subjects.
7. Neurophysiology in patients with MS with
epilepsy-in search of specific patterns
EEG anomalies show variability in time and space and
a low degree of specificity. In addition to detecting the
electro-clinical particularities of epileptic seizures, video-EEG
and activation techniques (hyperventilation, photic stimulation,
and sleep deprivation) are important tools in differentiating
veritable seizures from non-epileptic psychogenic and other
paroxistical events (99). To
highlight the importance of clinical-electrophysiological
correlation for the accuracy of the diagnosis and classification
processes, current data suggest that ≤70% of patients with epilepsy
had a false positive diagnosis in the general population (100). Early studies revealed EEG
abnormalities in 20-60% of patients with MS, dependent on the
location of the lesions, the duration, and the stage of the disease
and its progression (38,40,101). Frequent abnormalities consist of
diffuse asynchronous theta activity, slow rhythmic synchronous
activity, and occasionally, mainly during chronic-progressive
disease evolution, hypo-voltage, a potential result of the variable
degree of cortical atrophy (43).
Occasionally, slow focal waves or localized EEG suppression may be
found (52). The interictal
epileptiform activity appears to be quite rare, while an EEG
amelioration or impairment usually does not positively correlate
with the clinical condition (102). In addition, in patients with MS,
hyperventilation may worsen underlying EEG activity and may also
precipitate non-epileptic paroxysmal symptoms, such as focal
paresthesia or tonic spasms in the limbs (103).
According to research, EEG examination showed an
abnormal interictal pattern in approximately one-third of patients
with MS who suffered seizures before being diagnosed with MS, and
in more than 50% of patients with MS with onset of epileptic
seizures following MS diagnosis (55). In another series of cases, EEG was
considered pathological in >80% of patients (4,15).
In an attempt to delineate a correlation with an interictal
pattern, Dagiasi et al (52) demonstrated non-specific
electrophysiological changes such as focal slowing in 40% of cases
and epileptiform changes in 38% of the examined patients, with the
mention that 46% had a seizure-free one-year time interval. Moreau
et al (104) objectified
other relevant pathological EEG patterns, such as focal spikes,
focal slowing, and periodic lateralized epileptiform discharges
(PLEDs), with >50% of the above cases being diagnosed with
persistent seizures. In a number of cases, the observed
electrophysiological changes consisted of focal slowing with
isolated or grouped diffuse theta waves, with predominant bilateral
frontal-temporal localization (43). PLEDs are the result of
cortico-subcortical structures disconnections, being clinically
associated with a focal with impaired consciousness non-convulsive
status, especially in patients with longstanding MS (104). According to a reference study,
bi-PLEDs can be found in other pathological conditions apart from
MS, mainly related to anoxic encephalopathy and CNS infections,
such patients having increased mortality rates (105). Patients with MS with epilepsy had
significantly lower posterior dominant rhythm (PDR) frequency and
amplitude compared with controls, with 34% having a PDR frequency
of <8.5 Hz (106). The PDR
frequency was negatively associated with the functional level of
disability among patients. Slowing of the background rhythm and
epileptiform discharges suggest degeneration of the neuronal body
and may contribute to the prediction and follow-up of cortical
lesions and functional disabilities among patients with MS.
Therefore, electroencephalographic monitoring of the PDR spectrum
can serve as an alternative or complementary tool to other
detection and follow-up imaging techniques. Table II summarized the most relevant EEG
patterns found in patients with MS with epilepsy.
 | Table IIImportant EEG pathological patterns
in patients with MS with epilepsy. |
Table II
Important EEG pathological patterns
in patients with MS with epilepsy.
| First author | Study design | Relevant findings
related to EEG pathological patterns | (Refs.) |
|---|
| Salim A, 2021 | 50 patients with MS
with epilepsyvs. 50 controls | Lower posterior
dominant rhythm (PDR) frequency and amplitude; PDR frequency of
less than 8.5 Hz in 34% of cases | (106) |
| Dagiasi I,
2018 | Multicenter
retrospective study 62 patients with MS | Focal slowing in
40% of cases; epileptiform changes in 38% of cases | (52) |
| Viveiros C,
2010 | Case series 160
patients with MS (5 with concomitant epilepsy) | Focal slowing;
isolated or grouped diffuse theta waves; EEG anomalies located
predominantly bilateral frontal-temporal | (43) |
| Moreau T, 1998 | 402 patients with
MS (17 with concomitant epilepsy) | Focal spikes; focal
slowing; periodic lateralized epileptiform discharges (PLEDs); | (104) |
Complementary, a retrospective study has shown that
brainstem auditory evoked potentials and somatosensory potentials
of the upper limb are preferentially involved in patients with MS
and concomitant epilepsy (107).
According to currently available literature, the main cause for
this phenomenon seems to be the unilateral demyelinating lesion of
the substantia nigra. However, the exact cause-effect
interconnection with epilepsy is not fully determined, and future
prospective longitudinal studies are required.
8. Individualized ASMs treatment and
prognosis in patients with MS with epilepsy
The choice of the ASM is individualized, according
to the general recommendations that consider the type of seizure,
drug tolerability and related comorbidities (36). Although extensive research on the
etiology of epilepsy has been conducted, there are still a number
of knowledge gaps. It is also the case for patients with MS and
epilepsy. In their work, Dagiasi et al (52) made some assumptions regarding the
ASM treatment in MS patients, the most relevant being related to
the clinical features, the increased incidence of epileptic status,
and the sensitivity to the ASMs' adverse effects. There might be
also a bidirectional relationship between epilepsy treatment and
MS, thus explaining why only some ASMs were proven to be effective.
In this context, some of the currently employed ASMs with
demonstrated effectiveness and potential interaction with the
immune system are sodium valproate (inhibits NK cells),
carbamazepine, levetiracetam (decreases inflammatory mediators in
glial cell cultures), and vigabatrin (modulates humoral and
cellular response) (108,109). It has been observed that MS
relapse-associated seizures have a predominantly benign course,
similarly to symptomatic seizures that do not require chronic ASM
treatment, in contrast to seizures that occur apart from the MS
activity state and require more aggressive treatment (62). However, epileptogenesis is a
dynamic process that evolves over a significant period of time, and
the incomplete understanding of the phenomenon prompts for early
initiation of ASM treatment. With no standardized therapeutic
protocol currently available, extensive research on larger cohorts
is mandatory in order to establish valid guidelines.
According to a cohort study, monotherapy led to a
favorable outcome in 29 patients with MS with seizures (102). In another study, Nyquist et
al (37) found that from a
group of 51 patients with MS and concomitant epilepsy, 35 (78%) had
complete seizure remission under ASM therapy, five (11%) had
recurrent seizures with fluctuating seizure-free intervals despite
ASM administration, while another 11% developed persistent
seizures. The results are not surprising, considering the
heterogeneity of data available on patients with epilepsy under ASM
treatment. For example, one recent 30-year longitudinal cohort
study reported a 1-year seizure-free interval for >80% of the
included patients under new ASM monotherapy, a percentage similar
to that in the general epilepsy population (110).
Several other works reported mixed results, with a
number of reporting a favorable outcome of seizures in patients
with MS, such as the study conducted by Kinnunen and Wikström
(15) which report that epilepsy
had a spontaneous remission in almost half (10 out of 21) of the
patients with MS. Other research (conducted on 51 patients with MS
with epilepsy) reported that 3 out of 4 patients had persistent
focal seizures (37).
Dagiasi et al (52) revealed that 65% of the total
patients with MS included were on monotherapy with carbamazepine or
phenytoin as the first therapeutic option, although they are also
drugs that have significant interactions. Additionally, the authors
observed a low seizure remission rate (~44%) for the MS group
compared with 65% in the general population. Several explanations
for these results are proposed: i) The inclusion of tertiary
centers treating patients with high EDSS scores, suggesting a
biased selection; ii) increased sensitivity of patients to adverse
effects with limited adequate titration; iii) decreased level of
determination (lower therapeutical target) in seizure management in
(disabled) patients with MS compared with the general
population.
Regarding ASMs tolerance, Solaro et al
(111) demonstrate the adverse
effect profile of most commonly used ASMs in patients with MS.
Thus, 56% of patients treated with carbamazepine developed adverse
effects, predominantly ataxic/pyramidal syndromes. Relevant side
effects were also experienced by 19% of those treated with
gabapentin and by 22% of patients under lamotrigine therapy. The
therapeutic compliance of patients with MS to specific ASMs could
be partially related to their adverse effects, which might have
additive or synergistic values although there are no specific
studies addressing this issue. One study showed increased
therapeutic compliance to Na+ channel blockers, but
without a statistically significant difference regarding efficiency
(112). Finally, another relevant
aspect that might be taken into consideration is related to the
adverse effects of the employed ASMs that may mimic a relapse in
patients with MS, one example being ataxia (111).
According to a previous study, no positive
correlations were found between immunomodulatory treatments, mainly
β interferon, and epileptic seizures (36). There are, however, data suggesting
that DMTs might be a factor in seizure behavior in patients with
MS. Prophylactic administration of glatiramer acetate shows a
protective effect on the hippocampus and cortical myelination
(113). In separate studies,
Natalizumab treatment had a favorable effect on refractory epilepsy
by α 4 integrin-mediated migration of T-cells towards an inflamed
brain (114), while Fingolimod
administration could have additional anticonvulsant and
neuroprotective potential in temporal lobe drug-resistant epilepsy
through the S1P-signalling pathway in inflammation and blood-brain
disruption (115). These aspects
suggest the hypothesis of a persistent positive inflammatory
feedback loop in the MS-epilepsy interaction.
The aspects related to DMTs are also relevant for
patients with MS without epilepsy, as the current treatment
directions suggest a personalized approach. For example, the choice
for a certain DMT depends also on the MS type. The initial therapy
for mild forms of RRMS can be successfully conducted with
glatiramer acetate and interferons; however, the concomitant
presence of skin pathologies or hypercoagulable states impose the
use of oral medication. Severe RRMS forms can be treated from the
beginning with more potent therapies, Natalizumab being a valuable
option as both first and second-line DMT (116). When evolving to SPMS, the
patients initially with RRMS become candidates for Siponimod, the
latest DMT approved for this type of MS (117). PPMS remains a challenge from the
therapeutic point of view, with Ocrelizumab the first approved
medication, real-world results showing a stabilization of
disability progression in PPMS treated patients (118).
Symptomatic concomitant treatment of MS
comorbidities such as spasticity, depression and cognitive
impairment should be carefully considered, especially in patients
with MS and epilepsy, with the correct selection of ASM in this
context. The most commonly incriminated drugs are baclofen and
aminopyridines (such as fampridine for fatigue). On the other hand,
concomitant administration of melatonin and sodium valproate
produced a more potent anticonvulsant effect, also decreasing the
severity of audiogenic seizures in rat models (119).
Interactions between ASMs, in particular enzyme
inducers, and MS-related drugs have been reported (120). Carbamazepine, phenobarbital, and
phenytoin may lower plasma levels of cyclophosphamide (except for
phenobarbital), cyclosporine, dexamethasone, methotrexate,
methylprednisolone, and prednisolone (Table III). Dexamethasone may modulate
plasma phenytoin, oxcarbazepine may alter cyclosporine levels and
methotrexate may decrease plasma levels of valproic acid.
Fortunately, cyclosporine and methotrexate are rarely used in MS.
No interactions are reported between the new generation of ASMs and
the immunomodulators administered to patients with MS. Furthermore,
no significant interactions have been reported so far between new
ASMs and the currently available DMTs for RRMS, including
interferons, glatiramer acetate, teriflunomide, fingolimod,
dimethyl fumarate, alemtuzumab, mitoxantrone, and natalizumab while
the interactions were common with the old ASMs. Indeed, a new
concept is gaining ground, according to which epileptic
manifestations are relapses or worsening of the MS-related
inflammatory process. Epilepsy and seizures might be worth
integrating as a separate item into the EDSS scale, perhaps in the
cerebral functions category.
 | Table IIIRelevant characteristics of
antiseizure medication for patients with MS. |
Table III
Relevant characteristics of
antiseizure medication for patients with MS.
| Antiseizure
medication | Potential adverse
effects | Modulation of the
immune system | Drug
interactions |
|---|
| Carbamazepine | Ataxic syndrome
Pyramidal syndrome Gastrointestinal symptoms | Decrease of
inflammatory mediators in glial cell cultures | Lowers the plasma
levels of cyclophosphamide, cyclosporine, dexamethasone,
methotrexate, methylprednisolone, and prednisolone |
| Gabapentin | Blurred/double
vision Ataxic syndrome Tremor | Anti-inflammatory
effects by modulating the substance P-mediated neurokinin-1
receptor | No significant
interactions with DMTs or relapse acute treatment |
| Lamotrigine | Ataxic syndrome
Skin rash Headache Blurred/double vision | Anti-inflammatory
effects by inhibiting the production of IL-6, TNF-α, and IL-1β | No significant
interactions with DMTs |
| Levetiracetam | Headache Mood
changes Dizziness | Anti-inflammatory
effects by inhibiting the production of IL-1β | No significant
interactions with DMTs |
| Phenobarbital | Gastrointestinal
symptoms Headache | Hypersensitivity of
the immune system | Lowers the plasma
levels of cyclosporine, dexamethasone, methotrexate,
methylprednisolone, and prednisolone |
| Phenytoin | Headache Ataxic
syndrome | Decrease of
suppressor T cells Increase in the production of IL-6 and IL-8 | Lowers the plasma
levels of cyclosporine, dexamethasone, methotrexate,
methylprednisolone, and prednisolone |
| Sodium
valproate | Gastrointestinal
symptoms Headache Tremor | Inhibition of NK
cells | Decreased plasma
level by methotrexate |
| Vigabatrin | Blurred/double
vision Ataxic syndrome Tremor CNS depressant | Modulation of the
humoral and cellular immune response | Interferon
beta-increased risk of depression |
9. Conclusions
The complex association between epilepsy and MS,
although observed for a long time, possesses still a number of
unknowns. In recent years, research has brought new evidence that
strengthens this association between the two pathologies. First,
the results from epidemiological studies, although with significant
heterogeneity, show a clear increase in the prevalence of seizures
encountered in patients with MS. The present study considered that
the figures should however be cautiously interpreted because of the
cohort size variability and the influence of other well-known
external factors (latitude, climate) that predispose to biases in
MS diagnosis. Regarding the semiology of seizures, based on the
existing literature, it can be concluded that focal and bilateral
tonic-clonic seizures are the most frequently encountered in
patients with MS, with atypical seizures being rare.
Second, imagistic investigations bring additional
data that support a close association between MS and epileptic
seizures/epilepsy. White and gray matter demyelinating lesions are
both associated with an increased risk of seizures. It is
hypothesized that brain imaging could become an indirect tool to
assess epilepsy risk in patients with MS, with large cohort studies
currently missing.
Although there are several well-founded
pathophysiological hypotheses (excitatory-inhibitory
neurotransmitter imbalance, ionic imbalance that causes neuronal
hyperexcitability, and the role of chronic neuroinflammation),
further studies are needed to fully reveal the cellular and
molecular mechanisms linking the two diseases. The present study
also discussed the role of HHV-6 as a potential link between
epilepsy and MS, however, it must be admitted that the
bidirectional relationship between MS and epilepsy remains under
scrutiny, as etiological considerations for classification purposes
are still not well established.
EEG examination could become a reliable tool for the
optimal understanding of epileptic seizures in patients with MS. At
present, according to the findings, there is no specific EEG
pattern for seizures in patients with MS, with currently existing
scarce literature on this topic. With the discovery of new
pathological patterns specific to this category of patients, it is
hypothesized that EEG and video-EEG might provide clues for a more
personalized diagnosis and treatment.
Finally, choosing the optimal ASM in patients with
MS with concomitant epilepsy is still a challenge for the
neurologist. As illustrated above, some of the most important
questions are monotherapy vs. ASM associations, the potential
adverse effects, and the modulation of the immune system. With no
clear treatment directions, the establishment of therapeutic
protocols and proper guideline integration is mandatory, in order
to improve the clinical outcome and the quality of life of patients
with MS with epilepsy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DCA and TGS contributed to the study design and data
collection (search and selection of studies). DCA and IDC
contributed equally to data analysis and interpretation (final
selection and inclusion of the studies). DCA, TGS and TEC prepared
the first draft of the manuscript, while BEI, VSAA and IDC reviewed
the manuscript and wrote its final version. All authors read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh G and Sander JW: The global burden
of epilepsy report: Implications for low- and middle-income
countries. Epilepsy Behav. 105(106949)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Benjaminsen E, Myhr KM and Alstadhaug KB:
The prevalence and characteristics of epilepsy in patients with
multiple sclerosis in Nordland county, Norway. Seizure. 52:131–135.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dobson R and Giovannoni G: Multiple
sclerosis-a review. Eur J Neurol. 26:27–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gilmour H, Ramage-Morin PL and Wong SL:
Multiple sclerosis: Prevalence and impact. Health Rep. 29:3–8.
2018.PubMed/NCBI
|
|
5
|
Jácome Sánchez EC, García Castillo MA,
González VP, Guillén López F and Correa Díaz EP: Coexistence of
systemic lupus erythematosus and multiple sclerosis. A case report
and literature review. Mult Scler J Exp Transl Clin.
4(2055217318768330)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tseng CC, Chang SJ, Tsai WC, Ou TT, Wu CC,
Sung WY, Hsieh MC and Yen JH: Increased incidence of rheumatoid
arthritis in multiple sclerosis: A nationwide cohort study.
Medicine (Baltimore). 95(e3999)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kosmidou M, Katsanos AH, Katsanos KH,
Kyritsis AP, Tsivgoulis G, Christodoulou D and Giannopoulos S:
Multiple sclerosis and inflammatory bowel diseases: A systematic
review and meta-analysis. J Neurol. 264:254–259. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Klineova S and Lublin FD: Clinical course
of multiple sclerosis. Cold Spring Harb Perspect Med.
8(a028928)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cortesi PA, Cozzolino P, Cesana G, Capra R
and Mantovani LG: The prevalence and treatment status of different
multiple sclerosis phenotypes in a italian reference center. Value
Health. 20(PA720)2017.
|
|
10
|
Engelhard J, Oleske DM, Schmitting S,
Wells KE, Talapala S and Barbato LM: Multiple sclerosis by
phenotype in Germany. Mult Scler Relat Disord.
57(103326)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu Z, Liao Q, Wen H and Zhang Y: Disease
modifying therapies in relapsing-remitting multiple sclerosis: A
systematic review and network meta-analysis. Autoimmun Rev.
20(102826)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang JH, Rempe T, Whitmire N, Dunn-Pirio A
and Graves JS: Therapeutic advances in multiple sclerosis. Front
Neurol. 13(824926)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Manouchehri N, Salinas VH, Rabi Yeganeh N,
Pitt D, Hussain RZ and Stuve O: Efficacy of disease modifying
therapies in progressive MS and how immune senescence may explain
their failure. Front Neurol. 13(854390)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hollen CW, Paz Soldán MM, Rinker JR II and
Spain RI: The future of progressive multiple sclerosis therapies.
Fed Pract. 37 (Suppl 1):S43–S49. 2020.PubMed/NCBI
|
|
15
|
Kinnunen E and Wikstrom J: Prevalence and
prognosis of epilepsy in patients with multiple sclerosis.
Epilepsia. 27:729–733. 1986.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kelley BJ and Rodriguez M: Seizures in
patients with multiple sclerosis: Epidemiology, pathophysiology and
management. CNS Drugs. 23:805–815. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Langenbruch L, Krämer J, Güler S, Möddel
G, Geßner S, Melzer N, Elger CE, Wiendl H, Budde T, Meuth SG and
Kovac S: Seizures and epilepsy in multiple sclerosis: Epidemiology
and prognosis in a large tertiary referral center. J Neurol.
266:1789–1795. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
de Sa JC, Airas L, Bartholome E,
Grigoriadis N, Mattle H, Oreja-Guevara C, O'Riordan J, Sellebjerg
F, Stankoff B, Vass K, et al: Symptomatic therapy in multiple
sclerosis: A review for a multimodal approach in clinical practice.
Ther Adv Neurol Disord. 4:139–168. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kavčič A and Hofmann WE: Unprovoked
seizures in multiple sclerosis: Why are they rare? Brain Behav.
7(e00726)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Asadi-Pooya AA, Sahraian MA, Sina F,
Baghbanian SM, Habibabadi JM, Shaygannejad V, Asadollahi M, Karvigh
SA, Moghadasi AN, Nikseresht A and Motamedi M: Management of
seizures in patients with multiple sclerosis; an Iranian consensus.
Epilepsy Behav. 96:244–248. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Koch M, Uyttenboogaart M, Polman S and De
Keyser J: Seizures in multiple sclerosis. Epilepsia. 49:948–953.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fisher RS, Cross JH, French JA, Higurashi
N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez
E, et al: Operational classification of seizure types by the
international league against epilepsy: Position paper of the ILAE
commission for classification and terminology. Epilepsia.
58:522–530. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thompson AJ, Banwell BL, Barkhof F,
Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M,
Freedman MS, et al: Diagnosis of multiple sclerosis: 2017 revisions
of the McDonald criteria. Lancet Neurol. 17:162–173.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lai ST, Tan WY, Wo MC, Lim KS, Ahmad SB
and Tan CT: Burden in caregivers of adults with epilepsy in Asian
families. Seizure. 71:132–139. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fiest KM, Sauro KM, Wiebe S, Patten SB,
Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL and Jetté N:
Prevalence and incidence of epilepsy: A systematic review and
meta-analysis of international studies. Neurology. 88:296–303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
GBD 2016 Neurology Collaborators. Global,
regional, and national burden of neurological disorders, 1990-2016:
A systematic analysis for the global burden of disease study 2016.
Lancet Neurol. 18:459–480. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Walton C, King R, Rechtman L, Kaye W,
Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der
Mei I, et al: Rising prevalence of multiple sclerosis worldwide:
Insights from the Atlas of MS, third edition. Mult Scler.
26:1816–1821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marrie RA, Reider N, Cohen J, Trojano M,
Sorensen PS, Cutter G, Reingold S and Stuve O: A systematic review
of the incidence and prevalence of sleep disorders and seizure
disorders in multiple sclerosis. Mult Scler. 21:342–349.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mirmosayyeb O, Shaygannejad V, Nehzat N,
Mohammadi A and Ghajarzadeh M: Prevalence of seizure/epilepsy in
patients with multiple sclerosis: A systematic review and
meta-analysis. Int J Prev Med. 12(14)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Burman J and Zelano J: Epilepsy in
multiple sclerosis: A nationwide population-based register study.
Neurology. 89:2462–2468. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Engelsen BA and Grønning M: Epileptic
seizures in patients with multiple sclerosis. Is the prognosis of
epilepsy underestimated? Seizure. 6:377–382. 1997.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eriksson M, Ben-Menachem E and Andersen O:
Epileptic seizures, cranial neuralgias and paroxysmal symptoms in
remitting and progressive multiple sclerosis. Mult Scler.
8:495–499. 2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mahamud Z, Burman J and Zelano J: Risk of
epilepsy after a single seizure in multiple sclerosis. Eur J
Neurol. 25:854–860. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Krökki O, Bloigu R, Ansakorpi H, Reunanen
M and Remes AM: Neurological comorbidity and survival in multiple
sclerosis. Mult Scler Relat Disord. 3:72–77. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nakano H, Tanaka M, Kinoshita M, Tahara M,
Matsui M, Tanaka K and Konishi T: Epileptic seizures in Japanese
patients with multiple sclerosis and neuromyelitis optica. Epilepsy
Res. 104:175–180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Koch MW, Polman SK, Uyttenboogaart M and
De Keyser J: Treatment of seizures in multiple sclerosis. Cochrane
Database Syst Rev: Jul 8, 2009 (Epub ahead of print).
|
|
37
|
Nyquist PA, Cascino GD and Rodriguez M:
Seizures in patients with multiple sclerosis seen at Mayo Clinic,
Rochester, Minn, 1990-1998. Mayo Clin Proc. 76:983–986.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sokic DV, Stojsavljevic N, Drulovic J,
Dujmovic I, Mesaros S, Ercegovac M, Peric V, Dragutinovic G and
Levic Z: Seizures in multiple sclerosis. Epilepsia. 42:72–79.
2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gambardella A, Valentino P, Labate A,
Sibilia G, Ruscica F, Colosimo E, Nisticò R, Messina D, Zappia M
and Quattrone A: Temporal lobe epilepsy as a unique manifestation
of multiple sclerosis. Can J Neurol Sci. 30:228–232.
2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Striano P, Orefice G, Brescia Morra V,
Boccella P, Sarappa C, Lanzillo R, Vacca G and Striano S: Epileptic
seizures in multiple sclerosis: Clinical and EEG correlations.
Neurol Sci. 24:322–328. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nicoletti A, Sofia V, Biondi R, Lo Fermo
S, Reggio E, Patti F and Reggio A: Epilepsy and multiple sclerosis
in Sicily: A population-based study. Epilepsia. 44:1445–1448.
2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Martínez-Juárez IE, López-Meza E,
González-Aragón Mdel C, Ramírez-Bermúdez J and Corona T: Epilepsy
and multiple sclerosis: Increased risk among progressive forms.
Epilepsy Res. 84:250–253. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Viveiros CD and Alvarenga RM: Prevalence
of epilepsy in a case series of multiple sclerosis patients. Arq
Neuropsiquiatr. 68:731–736. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lund C, Nakken KO, Edland A and Celius EG:
Multiple sclerosis and seizures: Incidence and prevalence over 40
years. Acta Neurol Scand. 130:368–373. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Simpson RJ, McLean G, Guthrie B, Mair F
and Mercer SW: Physical and mental health comorbidity is common in
people with multiple sclerosis: Nationally representative
cross-sectional population database analysis. BMC Neurol.
14(128)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Averianova L, Shakirzianova S, Khaibullin
T, Khabirov F, Granatov E and Babicheva N: Epilepsy in multiple
sclerosis (MS): Clinical, electroencephalographic (EEG) and
magnetic resonance imaging (MRI) characteristics. Mult Scler J.
23(735)2017.
|
|
47
|
Laroni A, Signori A, Maniscalco GT,
Lanzillo R, Russo CV, Binello E, Lo Fermo S, Repice A, Annovazzi P,
Bonavita S, et al: Assessing association of comorbidities with
treatment choice and persistence in MS: A real-life multicenter
study. Neurology. 89:2222–2229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Passarell MA, Otero-Romero S, Bufill E,
Lopez-Jimenez T, Deniel J and Sastre-Garriga J: Excess of
neurological and psychiatric comorbidity in multiple sclerosis
patients as compared to the general population in Catalonia, Spain.
Mult Scler J. 23(169)2017.
|
|
49
|
Schorner A and Weissert R: Patients with
epileptic seizures and multiple sclerosis in a multiple sclerosis
center in Southern Germany between 2003-2015. Front Neurol.
10(613)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Neuß F, von Podewils F, Wang ZI, Süße M,
Zettl UK and Grothe M: Epileptic seizures in multiple sclerosis:
Prevalence, competing causes and diagnostic accuracy. J Neurol.
268:1721–1727. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gasparini S, Ferlazzo E, Ascoli M, Sueri
C, Cianci V, Russo C, Pisani LR, Striano P, Elia M, Beghi E, et al:
Risk factors for unprovoked epileptic seizures in multiple
sclerosis: A systematic review and meta-analysis. Neurol Sci.
38:399–406. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dagiasi I, Vall V, Kumlien E, Burman J and
Zelano J: Treatment of epilepsy in multiple sclerosis. Seizure.
58:47–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Catenoix H, Marignier R, Ritleng C, Dufour
M, Mauguière F, Confavreux C and Vukusic S: Multiple sclerosis and
epileptic seizures. Mult Scler J. 17:96–102. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Alroughani R and Boyko A: Pediatric
multiple sclerosis: A review. BMC Neurol. 18(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pack A: Is there a relationship between
multiple sclerosis and epilepsy? If so what does it tell us about
epileptogenesis? Epilepsy Curr. 18:95–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Sponsler JL and Kendrick-Adey AC: Seizures
as a manifestation of multiple sclerosis. Epileptic Disord.
13:401–410. 2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ooi S, Kalincik T, Perucca P and Monif M:
The prevalence of epileptic seizures in multiple sclerosis in a
large tertiary hospital in Australia. Mult Scler J Exp Transl Clin.
7(2055217321989767)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Atmaca MM and Gurses C: Status epilepticus
and multiple sclerosis: A case presentation and literature review.
Clin EEG Neurosci. 49:328–334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Spatt J, Goldenberg G and Mamoli B: Simple
dysphasic seizures as the sole manifestation of relapse in multiple
sclerosis. Epilepsia. 35:1342–1345. 1994.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hess DC and Sethi KD: Epilepsia partialis
continua in multiple sclerosis. Int J Neurosci. 50:109–111.
1990.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Spatt J, Chaix R and Mamoli B: Epileptic
and non-epileptic seizures in multiple sclerosis. J Neurol.
248:2–9. 2001.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lublin FD, Häring DA, Ganjgahi H, Ocampo
A, Hatami F, Čuklina J, Aarden P, Dahlke F, Arnold DL, Wiendl H, et
al: How patients with multiple sclerosis acquire disability. Brain.
(awac016)2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
63
|
Grothe M, Ellenberger D, von Podewils F,
Stahmann A, Rommer PS and Zettl UK: Epilepsy as a predictor of
disease progression in multiple sclerosis. Mult Scler. 28:942–949.
2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mahamud Z, Burman J and Zelano J:
Prognostic impact of epilepsy in multiple sclerosis. Mult Scler
Relat Disord. 38(101497)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chou IJ, Kuo CF, Tanasescu R, Tench CR,
Tiley CG, Constantinescu CS and Whitehouse WP: Epilepsy and
associated mortality in patients with multiple sclerosis. Eur J
Neurol. 26:342–e23. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Marrie RA, Elliott L, Marriott J, Cossoy
M, Blanchard J, Leung S and Yu N: Effect of comorbidity on
mortality in multiple sclerosis. Neurology. 85:240–247.
2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ontaneda D, Raza PC, Mahajan KR, Arnold
DL, Dwyer MG, Gauthier SA, Greve DN, Harrison DM, Henry RG, Li DKB,
et al: Deep grey matter injury in multiple sclerosis: A NAIMS
consensus statement. Brain. 144:1974–1984. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kidd D, Barkhof F, McConnell R, Algra PR,
Allen IV and Revesz T: Cortical lesions in multiple sclerosis.
Brain. 122:17–26. 1999.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Horakova D, Kalincik T, Dusankova JB and
Dolezal O: Clinical correlates of grey matter pathology in multiple
sclerosis. BMC Neurol. 12(10)2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Calabrese M, Grossi P, Favaretto A,
Romualdi C, Atzori M, Rinaldi F, Perini P, Saladini M and Gallo P:
Cortical pathology in multiple sclerosis patients with epilepsy: A
3 year longitudinal study. J Neurol Neurosurg Psychiatry. 83:49–54.
2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Calabrese M, Castellaro M, Bertoldo A, De
Luca A, Pizzini FB, Ricciardi GK, Pitteri M, Zimatore S, Magliozzi
R, Benedetti MD, et al: Epilepsy in multiple sclerosis: The role of
temporal lobe damage. Mult Scler J. 23:473–482. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Hatton SN, Huynh KH, Bonilha L, Abela E,
Alhusaini S, Altmann A, Alvim MKM, Balachandra AR, Bartolini E,
Bender B, et al: White matter abnormalities across different
epilepsy syndromes in adults: An ENIGMA-Epilepsy study. Brain.
143:2454–2473. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Briggs SW and Galanopoulou AS: Altered
GABA signaling in early life epilepsies. Neural Plast.
2011(527605)2011.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Uchida T, Furukawa T, Iwata S, Yanagawa Y
and Fukuda A: Selective loss of parvalbumin-positive GABAergic
interneurons in the cerebral cortex of maternally stressed
Gad1-heterozygous mouse offspring. Transl Psychiatry.
4(e371)2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Cao G, Edden RAE, Gao F, Li H, Gong T,
Chen W, Liu X, Wang G and Zhao B: Reduced GABA levels correlate
with cognitive impairment in patients with relapsing-remitting
multiple sclerosis. Eur Radiol. 28:1140–1148. 2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Waxman SG: Acquired channelopathies in
nerve injury and MS. Neurology. 56:1621–1627. 2001.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Rocca MA, Barkhof F, De Luca J, Frisén J,
Geurts JJG, Hulst HE, Sastre-Garriga J and Filippi M: MAGNIMS Study
Group. The hippocampus in multiple sclerosis. Lancet Neurol.
17:918–926. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Pracucci E, Pillai V, Lamers D, Parra R
and Landi S: Neuroinflammation: A signature or a cause of epilepsy?
Int J Mol Sci. 22(6981)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Vavasour IM, Sun P, Graf C, Yik JT, Kolind
SH, Li DK, Tam R, Sayao AL, Schabas A, Devonshire V, et al:
Characterization of multiple sclerosis neuroinflammation and
neurodegeneration with relaxation and diffusion basis spectrum
imaging. Mult Scler. 28:418–428. 2022.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zahid M, Busmail A, Penumetcha SS,
Ahluwalia S, Irfan R, Khan SA, Rohit Reddy S, Vasquez Lopez ME and
Mohammed L: Tumor necrosis factor alpha blockade and multiple
sclerosis: Exploring new avenues. Cureus. 13(e18847)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kamaşak T, Dilber B, Yaman SÖ, Durgut BD,
Kurt T, Çoban E, Arslan EA, Şahin S, Karahan SC and Cansu A:
HMGB-1, TLR4, IL-1R1, TNF-α, and IL-1β: Novel epilepsy markers?
Epileptic Disord. 22:183–193. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Akyuz E, Polat AK, Eroglu E, Kullu I,
Angelopoulou E and Paudel YN: Revisiting the role of
neurotransmitters in epilepsy: An updated review. Life Sci.
265(118826)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Dunn N, Kharlamova N and Fogdell-Hahn A:
The role of herpesvirus 6A and 6B in multiple sclerosis and
epilepsy. Scand J Immunol. 92(e12984)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Ortega-Madueño I, Garcia-Montojo M,
Dominguez-Mozo MI, Garcia-Martinez A, Arias-Leal AM, Casanova I,
Arroyo R and Alvarez-Lafuente R: Anti-human herpesvirus 6A/B IgG
correlates with relapses and progression in multiple sclerosis.
PLoS One. 9(e104836)2014.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Engdahl E, Gustafsson R, Huang J, Biström
M, Lima Bomfim I, Stridh P, Khademi M, Brenner N, Butt J, Michel A,
et al: Increased serological response against human herpesvirus 6A
Is associated with risk for multiple sclerosis. Front Immunol.
10(2715)2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Donati D, Akhyani N, Fogdell-Hahn A,
Cermelli C, Cassiani-Ingoni R, Vortmeyer A, Heiss JD, Cogen P,
Gaillard WD, Sato S, et al: Detection of human herpesvirus-6 in
mesial temporal lobe epilepsy surgical brain resections. Neurology.
61:1405–1411. 2003.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Shin YW: Understanding new-onset
refractory status epilepticus from an immunological point of view.
Encephalitis. 1:61–67. 2021.
|
|
88
|
Scheffer IE, Berkovic S, Capovilla G,
Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW,
Moshé SL, et al: ILAE classification of the epilepsies: Position
paper of the ILAE commission for classification and terminology.
Epilepsia. 58:512–521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Filippi M, Preziosa P, Banwell BL, Barkhof
F, Ciccarelli O, De Stefano N, Geurts JJG, Paul F, Reich DS, Toosy
AT, et al: Assessment of lesions on magnetic resonance imaging in
multiple sclerosis: Practical guidelines. Brain. 142:1858–1875.
2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Rayatpour A, Farhangi S, Verdaguer E,
Olloquequi J, Ureña J, Auladell C and Javan M: The cross talk
between underlying mechanisms of multiple sclerosis and epilepsy
may provide new insights for more efficient therapies.
Pharmaceuticals (Basel). 14(1031)2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Calabrese M, De Stefano N, Atzori M,
Bernardi V, Mattisi I, Barachino L, Rinaldi L, Morra A, McAuliffe
MM, Perini P, et al: Extensive cortical inflammation is associated
with epilepsy in multiple sclerosis. J Neurol. 255:581–586.
2008.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Cheng MY, Wai YY, Ro LS and Wu T: Seizures
and multiple sclerosis in Chinese patients: A clinical and magnetic
resonance imaging study. Epilepsy Res. 101:166–173. 2012.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zarei M, Chandran S, Compston A and Hodges
J: Cognitive presentation of multiple sclerosis: Evidence for a
cortical variant. J Neurol Neurosurg Psychiatry. 74:872–877.
2003.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Calabrese M, De Stefano N, Atzori M,
Bernardi V, Mattisi I, Barachino L, Morra A, Rinaldi L, Romualdi C,
Perini P, et al: Detection of cortical inflammatory lesions by
double inversion recovery magnetic resonance imaging in patients
with multiple sclerosis. Arch Neurol. 64:1416–1422. 2007.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Arashloo FT, Hanzaei FF, Sedighi B, Amjad
G and Younesi L: Efficacy of diffusion-weighted imaging in
symptomatic and asymptomatic multiple sclerotic plaques. J Family
Med Prim Care. 8:2409–2413. 2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Zheng Y, Lee JC, Rudick R and Fisher E:
Long-term magnetization transfer ratio evolution in multiple
sclerosis white matter lesions. J Neuroimaging. 28:191–198.
2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Sommer NN, Saam T, Coppenrath E, Kooijman
H, Kümpfel T, Patzig M, Beyer SE, Sommer WH, Reiser MF, Ertl-Wagner
B and Treitl KM: Multiple sclerosis: Improved detection of active
cerebral lesions with 3-dimensional T1 black-blood magnetic
resonance imaging compared with conventional 3-dimensional T1 GRE
imaging. Invest Radiol. 53:13–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Tewarie P, Steenwijk MD, Brookes MJ,
Uitdehaag BMJ, Geurts JJG, Stam CJ and Schoonheim MM: Explaining
the heterogeneity of functional connectivity findings in multiple
sclerosis: An empirically informed modeling study. Hum Brain Mapp.
39:2541–2548. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Dericioğlu N, Saygi S and Ciğer A: The
value of provocation methods in patients suspected of having
non-epileptic seizures. Seizure. 8:152–156. 1999.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Xu Y, Nguyen D, Mohamed A, Carcel C, Li Q,
Kutlubaev MA, Anderson CS and Hackett ML: Frequency of a false
positive diagnosis of epilepsy: A systematic review of
observational studies. Seizure. 41:167–174. 2016.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Poser CM and Brinar VV: Epilepsy and
multiple sclerosis. Epilepsy Behav. 4:6–12. 2003.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Shaygannejad V, Ashtari F, Zare M, Ghasemi
M, Norouzi R and Maghzi H: Seizure characteristics in multiple
sclerosis patients. J Res Med Sci. 18 (Suppl 1):S74–S77.
2013.PubMed/NCBI
|
|
103
|
Grabow JD: Optimal recordings techniques
and activation procedures: childrens and adults In: Clinical
neurophysiology of epilepsy. (EEG handbook, revised series, vol.
4). Wada JA and Ellingson RJ (eds). Elsevier Science, Amsterdam,
pp39-77, 1990.
|
|
104
|
Moreau T, Sochurkova D, Lemesle M,
Madinier G, Billiar T, Giroud M and Dumas R: Epilepsy in patients
with multiple sclerosis: Radiological-clinical correlations.
Epilepsia. 39:893–896. 1998.PubMed/NCBI View Article : Google Scholar
|
|
105
|
de la Paz D and Brenner RP: Bilateral
independent periodic lateralized epileptiform discharges. Clinical
significance. Arch Neurol. 38:713–715. 1981.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Salim AA, Ali SH, Hussain AM and Ibrahim
WN: Electroencephalographic evidence of gray matter lesions among
multiple sclerosis patients: A case-control study. Medicine
(Baltimore). 100(e27001)2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Papathanasiou ES, Pantzaris M,
Myrianthopoulou P, Kkolou E and Papacostas SS: Brainstem lesions
may be important in the development of epilepsy in multiple
sclerosis patients: An evoked potential study. Clin Neurophysiol.
121:2104–2110. 2010.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Stefanović S, Janković SM, Novaković M,
Milosavljević M and Folić M: Pharmacodynamics and common drug-drug
interactions of the third-generation antiepileptic drugs. Expert
Opin Drug Metab Toxicol. 14:153–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Perucca E: Antiepileptic drugs: Evolution
of our knowledge and changes in drug trials. Epileptic Disord.
21:319–329. 2019.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Chen Z, Brodie MJ, Liew D and Kwan P:
Treatment outcomes in patients with newly diagnosed epilepsy
treated with established and new antiepileptic drugs: A 30-year
longitudinal cohort study. JAMA Neurol. 75:279–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Solaro C, Brichetto G, Battaglia MA,
Messmer Uccelli M and Mancardi GL: Antiepileptic medications in
multiple sclerosis: Adverse effects in a three-year follow-up
study. Neurol Sci. 25:307–310. 2005.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Rajagopalan K and Lee LK: Association
between adherence to sodium channel blockers and patient-reported
outcomes: Analysis of US survey data among patients with epilepsy.
Epilepsy Behav. 99(106483)2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
You Y, Zhao Y, Bai H, Liu Z, Meng F, Zhang
H and Xu R: Glatiramer acetate, an anti-demyelination drug, reduced
rats' epileptic seizures induced by pentylenetetrazol via
protection of myelin sheath. Eur J Pharm Sci. 49:366–370.
2013.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Fabene PF, Laudanna C and Constantin G:
Leukocyte trafficking mechanisms in epilepsy. Mol Immunol.
55:100–104. 2013.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Leo A, Citraro R, Marra R, Palma E, Paola
EDD, Constanti A, De Sarro G and Russo E: The sphingosine
1Phosphate signaling pathway in epilepsy: A possible role for the
immunomodulator drug fingolimod in epilepsy treatment. CNS Neurol
Disord Drug Targets. 16:311–325. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Morrow SA, Clift F, Devonshire V, Lapointe
E, Schneider R, Stefanelli M and Vosoughi R: Use of natalizumab in
persons with multiple sclerosis: 2022 update. Mult Scler Relat
Disord. 65(103995)2022.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Scott LJ: Siponimod: A review in secondary
progressive multiple sclerosis. CNS Drugs. 34:1191–1200.
2020.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Daniels K, van der Nat PB, Frequin STFM,
van der Wees PJ, Biesma DH, Hoogervorst ELJ and van de Garde EMW:
Real-world results of ocrelizumab treatment for primary progressive
multiple sclerosis. Mult Scler Int. 2020(5463451)2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Zaccara G and Perucca E: Interactions
between antiepileptic drugs, and between antiepileptic drugs and
other drugs. Epileptic Disord. 16:409–431. 2014.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Beiske GA, Holmøy T, Beiske AG,
Johannessen SI and Johannessen Landmark C: Antiepileptic and
antidepressive polypharmacy in patients with multiple sclerosis.
Mult Scler Int. 2015(317859)2015.PubMed/NCBI View Article : Google Scholar
|