Introduction
High blood pressure can cause damage to several
organs, including the kidneys, this increases the possibility of
chronic kidney disease (CKD) in hypertensive patients (1). Endothelial cell injury and decreased
regeneration and repair ability have important consequences in
patients with renal function impairment. Endothelial progenitor
cells (EPCs) have been shown to promote endothelial repair
(2), regulate angiogenesis
(3) and have therapeutic effects
in patients with acute renal ischemia-reperfusion injury and
patients with kidney transplantation (4). Therefore, we hypothesized that EPCs
may have a protective effect on renal cells against hypertensive
nephropathy. Microvesicles (MVs) are secreted continuously by a
variety of cells in the body, such as epithelial, tumor and stem
cells, and exist in a variety of body fluids, including blood and
urine, where they mediate biological functions (5). There is evidence that the protective
effect of EPCs is closely related to the release of MVs (6,7).
However, the role and underlying mechanism of EPC-MVs in
hypertensive nephropathy are still unclear.
Angiotensin II (Ang II) induces vascular injury and
plays a key role in vascular diseases by inducing apoptosis,
increasing reactive oxygen species (ROS) levels and promoting
oxidative stress responses by inducing the secretion of
malondialdehyde (MDA) and decreasing that of glutathione (GSH) and
superoxide dismutase (SOD) (8-10).
Ang II also promotes the production of inflammatory cytokines, such
as IL-6, IL-1β and TNF-α (11-13).
Therefore, Ang II-induced damage to primary renal kidney cells
(PRKs) can be used to simulate hypertension in vitro
(14,15).
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
that are involved in the progression and treatment of a variety of
diseases (16). Among them,
miR-98-5p is a key regulator in the development of diabetic
nephropathy and can reverse renal fibrosis induced by high glucose
(17). miRNAs can suppress the
transcription and translation of mRNA by binding to the end of the
3'-untranslated region (3'-UTR) of the Mrna (18). New evidence shows that insulin-like
growth factor 1 receptor (IGF1R) is involved in the progression of
diabetes (19,20). miRNAs can also affect cell
viability, differentiation, migration, oxidative stress and
inflammation by regulating a series of downstream signaling
pathways (21-23).
The activation of endothelial nitric oxide synthase (eNOS) in colon
cells promotes the viability of endothelial cells and regulates
inflammation (24). The protective
effect of PI3K/Akt/eNOS on endothelial cells has been established
in numerous studies (25,26). Previous evidence has indicated that
miR-98-5p affects apoptosis, inflammation and oxidative stress by
regulating the PI3K/Akt signaling pathway (27-29).
Moreover, studies have shown that IGF1R can affect the development
of diabetes through the PI3K/Akt/eNOS axis (30-32).
In the present study, the potential application of
miR-98-5p-MVs in the treatment of hypertensive nephropathy was
explored by investigating the protective effect and mechanism of
miR-98-5p expressed in EPC-MVs on Ang II-induced PRK injury.
Materials and methods
Animals
A total of 12, 8-week-old male Wistar-Kyoto specific
pathogen-free rats weighing 80-120 g were obtained from Beijing
Vital River Laboratory Animal Technology Co. Ltd. Rats were placed
in a room with a 12-h light/dark cycle and a constant temperature
and humidity (temperature, 23±2˚C; humidity, 45±15%) with ad
libitum access to standard rat food and water in a polystyrene
cage. Animal experiments were approved by the Animal Care and Use
Committee of Hainan Medical University (Haikou, China; approval no.
HYLL-2021-053) and were conducted according to the National
Institutes of Health guidelines.
Isolation and culture of PRKs
Rats were euthanized by intraperitoneal injection of
pentobarbital sodium (200 mg/kg body weight). The kidneys were
removed aseptically and the cortical portion of the kidney was
excised. The renal cortex was cut into tissue fragments of <1
mm3, washed with phosphate-buffered saline (PBS) three
times and centrifuged at 1,000 x g at 25˚C for 5 min. The
supernatant was then discarded. Tissue fragments were added to
collagenase type I solution (Gibco; Thermo Fisher Scientific, Inc.)
at a final concentration of 1 g/l, and the tissue fragments were
digested at 37˚C for 30 min under oscillation. After filtration
through a 200-mesh stainless steel filter, the cells were separated
by Ficoll®-Paque PREMIUM (Cat. No. 17-5442-02; GE
Healthcare, Uppsala, Sweden) density gradient centrifugation
(33). The supernatant was
discarded after centrifugation at 1,000 x g at 25˚C for 2 min. The
precipitate was mixed with Dulbecco's Modified Eagle
Medium/Nutrient Mixture F-12 (DMEM/F12) (Gibco; Thermo Fisher
Scientific, Inc.), inoculated in a 6-well plate and cultured at
37˚C with 5% CO2. After 24 h, the supernatant was
replaced with fresh medium, and the unattached renal cells and
tissues were discarded. After 48 h, the cells were washed twice
with PBS and denoted as PRKs (34,35).
PRKs were cultured in DMEM/F12 with 10% Fetal bovine serum (FBS;
Gibco) for three generations and treated with Ang II (1 µmol) at
37˚C for 24 h to establish the PRK renal damage model. The
concentration used for Ang II was selected based on the study by
Nair et al (36).
Identification of PRKs
PRKs (5x104) were inoculated into a
6-well plate (on round glass coverslips; Corning, Inc.) for 24 h.
After twice rinses with PBS, Fix with 4% formaldehyde at 25˚C for
10 min. Then, Triton-X-100 (0.1%) was added at 4˚C for 2 h. PRKs
were incubated with 1% BSA (Beijing Solarbio Science &
Technology Co., Ltd.) for 30 min at 4˚C and then overnight with
anti-α-smooth muscle actin (α-SMA) (1:200; ab7817; Abcam) and
anti-vimentin (1:250; ab92547; Abcam) antibodies at 4˚C in the
dark. After rinsing with PBS three times, the cells were incubated
with Alexa Fluor® 488-(1:100; cat. no. ab150077; Abcam)
or 647-labeled (1:200; cat. no. ab150075; Abcam) secondary
antibodies at 37˚C for 1 h. Then, the cells were mounted with Gold
Antifade Mountant with DAPI (ProLong™; Thermo Fisher Scientific,
Inc.). Finally, images were captured using a fluorescence
microscope (magnification, x400; Leica Microsystems GmbH).
Culture and identification of
EPCs
The femur and tibia of the rats were separated, and
each bone marrow tube was rinsed with sterile PBS. The resulting
mixture was centrifuged (1,000 x g; 25˚C; 5 min), and the EPCs were
isolated by Ficoll density gradient centrifugation (1,000 x g;
25˚C; 20 min) and cultured (DMEM with 10% FBS) at 37˚C with 5%
CO2. After 4 days, the medium was exchanged with fresh
culture medium, and the adherent cells were cultured for another 3
days (37). According to the
manufacturer's instructions, Dil complex acetylated low-density
lipoprotein (Dil-Ac-LDL) staining (Cat. No. IL2140; Beijing
Solarbio Science & Technology Co., Ltd.) was used to identify
EPCs. The criterion for the suitability of isolated EPCs for
subsequent experiments was that the red fluorescence of Dil-Ac-LDL
and blue fluorescence (DAPI nuclear staining) of most cells
overlapped under a fluorescence microscope (magnification x400). In
the co-culture system, PRKs (1x104) were added to the
lower chamber of transwell plates (Corning, Inc.) and incubated at
37˚C for 24 h, after which Ang II (1 µM) and EPCs
(3x104) were added to the upper chamber. The medium in
both the upper and lower chambers was DMEM/F12 contained 10% FBS.
After incubation for 24 h at 37˚C, cells were used for subsequent
experiments. As for the co-culture of PRKs and EPC supernatant,
after the supernatant of EPCs (3x104) was collected,
cell debris was removed using a filter (Millipore) and then added
to PRKs for culture.
Preparation of EPC-MVs
EPCs were cultured for 7 days as aforementioned,
washed twice with PBS, and then serum starved for 12 h.
Subsequently, DMEM/F12 containing cultured EPCs was centrifuged at
4˚C for 15 min (1,000 x g), and the supernatant was further
centrifuged at 4˚C for 60 min (100,000 x g) for the collection of
secreted EPC-MVs. MVs were fixed with glutaraldehyde (2.5%; Beijing
Solarbio Science & Technology Co., Ltd.) in the dark at 4˚C for
1 hours, 10 µl was added dropwise to the copper stain at 25˚C for 1
min, and the suspension was removed by filter paper. Subsequently,
10 µl of phosphotungstic acid (1%; Beijing Solarbio Science &
Technology Co., Ltd.) was added dropwise to the copper stain at
25˚C for 1 min, and the suspension was removed by filter paper.
After drying at 25˚C for 30 min, transmission electron microscopy
(TEM) was performed at 80 kV for imaging. In the co-culture system,
50 µg/ml EPC-MVs (38) were added
to the top chamber of a Transwell assay plate, and PRKs were added
to the bottom chamber as aforementioned and incubated for 24 h.
Gene Expression Omnibus (GEO)
analysis
Raw data of GSE110231 dataset (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE110231)
were downloaded from the GEO website (https://www.ncbi.nlm.nih.gov/geo/) and analyzed. The
GSE110231 dataset included three healthy Sprague-Dawley rats
(subsample, GSM2983040-2983042) and three diabetic rats (subsample,
GSM2983037-2983039). Use Gene Expression Profiling Interactive
Analysis 2 (http://gepia2.cancer-pku.cn/#index) to analyze the
differential expression of miRNA, and the criteria for differential
expression are as follows: P<0.05, log2|fold
change|≥2. Kyoto Encyclopedia of Genes and Genomes (KEGG;
https://www.genome.jp/kegg/) was used to
analyze the influence of related signal transduction on diabetes
development in rats.
Cell transfection
Mimic (100 nM), inhibitor (100 nM), negative control
mimic (NC; 100 nM), NC inhibitor (100 nM), overexpression (ov)
IGF1R pCDNA3.1 plasmid (50 nM) and ov-NC plasmid (50 nM) (all from
Sangon Biotech Co., Ltd.) were transfected into PRKs or EPCs with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions,
and incubated in the dark for 4 h (37˚C, 5% CO2). After
24 h (for RT-qPCR analysis) or 48 h (for western blot analysis) of
the transfection medium replaced with fresh medium, cells and
supernatants were collected for further processing. miR-98-5p mimic
and inhibitor sequences are presented in Table I.
 | Table ISequences of miRNAs and reverse
transcription-quantitative PCR primers. |
Table I
Sequences of miRNAs and reverse
transcription-quantitative PCR primers.
| A, miRNA
sequences |
|---|
| miRNA | Sequence
(5'-3') |
|---|
| miR-98-5p
mimic |
UGAGGUAGUAAGUUGUAUUGUU |
| miR-98-5p mimic
NC |
UAUAGAUUGUUGGAGUUGUUAG |
| miR-98-5p
inhibitor |
AACAATACAACTTACTACCTCA |
| miR-98-5p inhibitor
NC |
CAGUACUUUUGUGUAGUACAA |
| B, Primer
sequences |
| Primer | Sequence
(5'-3') |
| miR-98-5p-F |
ACACTCCAGCTGGGTGAGGTAGT AAGTTGT |
| miR-98-5p-R |
CTCAACTGGTGTCGTGGA |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
| IGF1R-F |
CTCTAAGGCCAGAGGTGGAGAA TAA |
| IGF1R-R |
TGTGGACGAACTTGTTGGCA |
| GAPDH-F |
TGGGGCCAAAAGGGTCATCA |
| GAPDH-R |
GCAGGATGCATTGCTGACAA |
Reverse transcription-quantitative
(RT-q)PCR
According to the manufacturer's instructions, PRKs
were extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). After 10 min of centrifugation at 4˚C
(13,000 x g), the precipitate was adsorbed and dissolved with 15 µl
diethylpyrocarbonate-treated water and reverse-transcribed into
cDNA using a PrimeScript™ RT-PCR Kit (Takara Bio Inc.) according to
the manufacturer's protocol. SYBR® Premix Ex Taq™ II kit
(Takara Bio Inc.) was used for RT-qPCR analysis with the Applied
Biosystems® 7500 Real-Time PCR system (Thermo Fisher
Scientific, Inc.). qPCR was carried out under the following
conditions: 95˚C for 30 sec, followed by 40 cycles of 95˚C for 3
sec and 60˚C for 30 sec. The primer sequences used for RT-qPCR are
presented in Table I. MiR-98-5p
and IGF1R RNA levels were normalized to those of U6 or
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and calculated
using the 2-ΔΔCq method (39).
Western blotting
PRKs were lysed using RIPA lysis buffer (Elabscience
Biotechnology, Inc.). Lysate protein concentrations were determined
using a BCA protein assay kit (Beijing Solarbio Science &
Technology Co., Ltd.) and resolved denatured proteins (20 µg) using
10% SDS-PAGE (Elabscience Biotechnology, Inc.). Protein bands were
transferred onto a PVDF membrane at 60 V for 2 h at 4˚C. Then,
Ponceau S dye was used to stain the membrane at 25˚C for 5 min. The
membrane was blocked with 5% BSA at 25˚C for 2 h, incubated with
primary antibodies (anti-IGF1R, anti-PI3K, anti-AKT, anti-p-PI3K,
anti-p-AKT, anti-eNOS, anti-p-eNOS) overnight at 4˚C, rinsed with
TBS-0.05%Tween 20 buffer (Beijing Solarbio Science & Technology
Co., Ltd.) twice, for 10 min each time, and incubated with the
secondary antibody (Goat anti-rabbit; Horseradish peroxidase) for
1.5 h at 23±2˚C. Details of the antibodies are shown in Table II. Subsequently, ECL reagent
(Thermo Fisher Scientific, Inc.) was used for the chemiluminescence
reaction. Finally, the membrane was developed and fixed using a
Developer and Fixer Kit (Beyotime Institute of Biotechnology), and
the densitometry was quantified using ImageJ software (version
1.8.0; National Institutes of Health, Bethesda, MD, USA).
 | Table IIDetails of the antibodies used in
western blot analysis. |
Table II
Details of the antibodies used in
western blot analysis.
| Antibody | Dilution | Cat. no. | Manufacturer | Application |
|---|
| IGF1R | 1:1,000 | ab182408 | Abcam | Primary
antibody |
| PI3K | 1:1,000 | ab191606 | Abcam | Primary
antibody |
| AKT | 1:500 | ab8805 | Abcam | Primary
antibody |
| p-PI3K | 1:500 | ab182651 | Abcam | Primary
antibody |
| p-AKT | 1:500 | ab38449 | Abcam | Primary
antibody |
| eNOS | 1:1,000 | PA1-037 | Invitrogen; Thermo
Fisher Scientific, Inc. | Primary
antibody |
| p-eNOS | 1:500 | MA5-14957 | Invitrogen; Thermo
Fisher Scientific, Inc. | Primary
antibody |
| Goat
anti-rabbit | 1:20,000 | ab205718 | Abcam | Secondary
antibody |
| GAPDH | 1:5,000 | ab181602 | Abcam | Loading
control |
Cell viability assays
PRKs were digested with trypsin, inoculated in
96-well plates at a density of 0.5x104 cells/well, and
cultured for 24 h. The optical density at 490 nm was measured using
the Cell Counting Kit-8 reagent (CCK-8; 10 µl/well; Beijing
Solarbio Science & Technology Co., Ltd.) at 37˚C for 1 h, added
at 0 and 24 h according to the manufacturer's instructions, to
evaluate the cell viability.
EPC-MVs and PRK fusion
EPC-MVs were labeled with a lipid membrane-embedded
fluorescent dye (PKH26; Sigma-Aldrich; Merck KGaA) prior to
co-incubation with PRKs. Briefly, 50 g/ml EPC-MVs were mixed with 2
ml PKH26 (2x10-6 M) and incubated at 23±2˚C for 5 min.
The labeled mixture was added to 2 ml of 1% BSA and centrifuged at
4˚C for 60 min (120,000 x g), and the precipitate was rinsed with
PBS. The precipitate was then suspended in 2 ml DMEM/F12 in 6-well
plates and added to PRKs (2x105/ml) before incubation at
37˚C for 24 h. Finally, 1 µg/ml DAPI was added for nuclear staining
at 25˚C for 5 min. Cell images were acquired using a fluorescence
microscope (Leica Biosystems).
ROS measurements using flow cytometry
(FCM)
PRKs (2x105/ml) were incubated in 6-well
plates with 2',7'-dichlorodihydrofluorescein diacetate (1.0 µM;
Beijing Solarbio Science & Technology Co., Ltd.) at 37˚C for 15
min. Subsequently, PRKs were washed twice with PBS and analyzed by
FCM (FACSCanto II; BD FACSChorus™ software, version: 1.0; BD
Biosciences) to detect ROS using a 488-nm laser for excitation and
a 535-nm laser for detection.
Enzyme-linked immunosorbent assay
(ELISA)
PRKs cell supernatant of each subgroup was collected
to determine the levels of MDA, GSH, SOD, IL-6, IL-1β and TNF-α.
The following kits were used according to the manufacturer's
instructions: MDA Content Assay Kit (cat. no. BC0020; Beijing
Solarbio Science & Technology Co., Ltd.), Reduced GSH Content
Assay Kit (cat. no. BC1175; Beijing Solarbio Science &
Technology Co., Ltd.), SOD Activity Assay Kit (cat. no. BC0170;
Beijing Solarbio Science & Technology Co., Ltd.), Rat IL-1
beta/IL-1F2 Quantikine ELISA Kit (cat. no. RLB00; R&D Systems),
Rat IL-6 Quantikine ELISA kit (cat. no. R6000B; R&D Systems)
and Rat TNF-α Quantikine ELISA Kit (cat. no. RTA00; R&D
Systems).
Dual-luciferase reporter assay
TargetScan software v7.2 (https://www.targetscan.org/vert_72/) was used to
predict the binding sites of miRNA and mRNA. PRKs were transfected
with 500 ng each of miR-98-5p mimic or inhibitor and their NCs, 1
µg each of the psi-CHECK2 vector (Promega, Madison, WI, USA)
containing wild-type or mutant IGF1R 3'-UTR and 50 ng of the
pRL-SV40 reporter vector plasmid (Promega) Using
Lipofectamine® 2000. Transfected cells were incubated at
37˚C for 48 h, and luciferase activity was measured using the
Dual-Luciferase® Reporter Assay System (Promega
Corporation) according to the manufacturer's instructions, and the
ratio of firefly to Renilla activity was used to normalize
firefly luciferase values.
Statistical analysis
All experiments were repeated three times. Data are
expressed as the mean ± standard deviation. Differences between
multiple groups were assessed using one-way analysis of variance
and Bonferroni post hoc test. Student's t-test was used for
independent two-group analyses (unpaired). P<0.05 was considered
to indicate a statistically significant difference using Graphpad
prism (Version: 8.0; GraphPad Software Inc.).
Results
Identification of EPCs and PRKs
The isolated EPCs were identified using Dil-Ac-LDL
staining. We observed under the fluorescence microscope that most
of the cells expressed the superposition of highly positive red
fluorescence (Dil-Ac-LDL) and blue fluorescence (nuclear staining
with DAPI), which means that the isolated cells are suitable for
use in subsequent studies (Fig.
1A). Isolated PRKs were analyzed using immunofluorescence
staining (vimentin/α-SMA). The results revealed high expression of
vimentin and α-SMA in PRKs, which suggested the successful
isolation of PRKs (Fig. 1B).
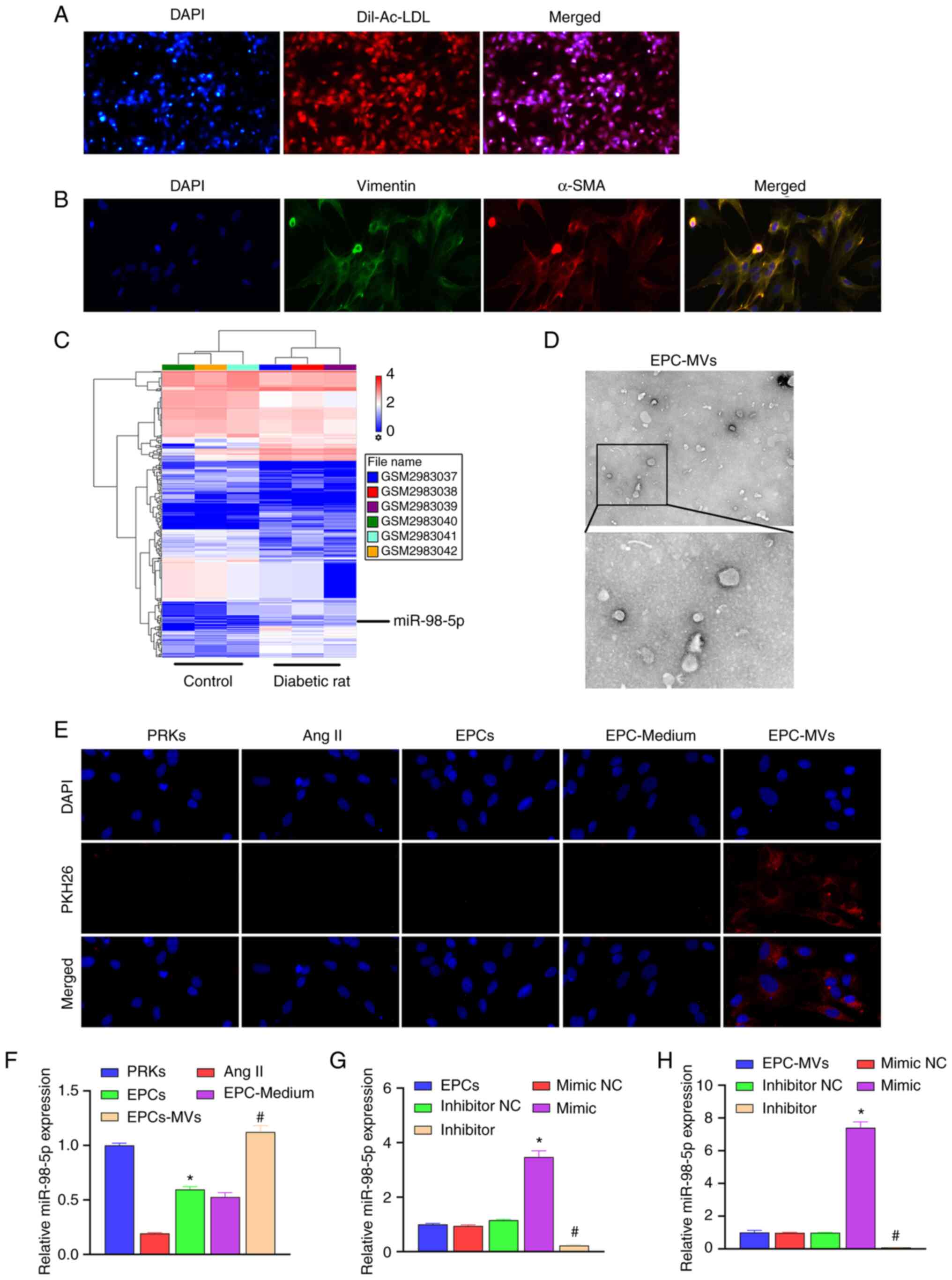 | Figure 1Recovery of Ang II-induced injury of
PRKs and co-culture with EPC-MVs. (A) Confirmation of the isolation
of EPCs using Dil-Ac-LDL staining (magnification, x100). (B)
Confirmation of the isolation of PRKs through immunofluorescence
assay (magnification, x400). (C) The GSE110231 dataset was analyzed
using Gene Expression Omnibus to screen miRNAs. This heat map shows
differentially expressed miRNAs in diabetic rats compared with
healthy rats. Blue bands represent low expression, and red bands
represent high expression. (D) Analysis of isolated MVs using
transmission electron microscopy (upper image magnification,
x15,000; lower image magnification, x40,000). (E) PKH26-labeled
EPC-MVs successfully fused with PRKs (magnification, x100). (F)
RT-qPCR analysis of the impact of Ang II, EPCs, EPC-Medium and
EPC-MVs on the expression of miR-98-5p in PRKs (n=3).
*P<0.05 vs. Ang II; #P<0.05 vs.
EPC-Medium. (G) RT-qPCR analysis of the impact of miR-98-5p
mimic/inhibitor transfection on the expression of miR-98-5p in EPCs
(n=3). *P<0.05 vs. mimic NC; #P<0.05
vs. inhibitor NC. (H) RT-qPCR analysis of the impact of miR-98-5p
mimic/inhibitor transfection on the expression of miR-98-5p in
EPC-MVs (n=3). *P<0.05 vs. mimic NC;
#P<0.05 vs. inhibitor NC. Dil-Ac-LDL, Dil complex
acetylated low-density lipoprotein; Ang II, Angiotensin II; EPCs,
endothelial progenitor cells; PRKs, primary renal kidney cells;
MVs, microvesicles; RT-qPCR, reverse transcription-quantitative
PCR; NC, negative control; α-SMA, α-smooth muscle actin; miR,
microRNA. |
Analysis and screening of target
miRNAs using GEO
To explore the protective mechanism of EPC-MVs
against PRK injury, GSE110231 dataset was analyzed using GEO. The
results revealed that 430 miRNAs were differentially expressed
between the control and the diabetic rat group (Fig. 1C). Among them, 13 miRNAs met the
differential expression criteria set in the present study, as shown
in Table III. A previous study
demonstrated that miR-98-5p expression is reduced in diabetic
nephropathy in mice (17).
However, the mechanism of miR-98-5p in rat PRKs has not been
confirmed. Therefore, miR-98-5p was selected as the target miRNA in
the present study.
 | Table IIIList of miRNAs that meet the criteria
of log2|FC|≥2 and P<0.05. |
Table III
List of miRNAs that meet the criteria
of log2|FC|≥2 and P<0.05.
| Transcript
identification (array design) | Diabetic rat
average (log2) | Control average
(log2) | Fold change | P-value |
|---|
| rno-miR-1-3p | 2.39 | 4.76 | -5.16 | 0.001 |
| rno-miR-9a-5p | 3.37 | 4.65 | -2.43 | 0.034 |
| rno-miR-98-5p | 5.18 | 6.30 | -2.18 | 0.017 |
| rno-miR-205 | 4.95 | 6.78 | -3.55 | <0.001 |
| rno-miR-206-3p | 5.99 | 7.18 | -2.29 | 0.010 |
|
rno-miR-216a-3p | 1.23 | 2.45 | -2.34 | 0.016 |
|
rno-miR-219a-2-3p | 0.26 | 1.83 | -2.97 | 0.007 |
| rno-miR-451-5p | 3.63 | 2.34 | 2.45 | 0.001 |
|
rno-miR-466b-5p | 1.83 | 3.12 | -2.44 | 0.023 |
| rno-miR-490-3p | 2.84 | 1.34 | 2.84 | 0.014 |
|
rno-miR-743b-3p | 3.37 | 2.33 | 2.06 | 0.003 |
| rno-miR-881-3p | 4.04 | 2.65 | 2.62 | 0.001 |
| rno-miR-1949 | 6.47 | 7.53 | -2.08 | <0.001 |
Transfection of miR-98-5p
mimic/inhibitor affects the expression of miR-98-5p in EPC-MVs
The MVs isolated from the culture supernatant of
EPCs were observed using TEM (Fig.
1D). Ang II was added to PRK cells to simulate an in
vitro renal cell injury model, and the protective mechanism of
EPCs on Ang II-induced damage of renal cells was further analyzed.
EPC-MVs were labeled with PKH26 and incubated with PRKs in the
presence of Ang II. PKH26 fluorescence was detected in the
cytoplasm of PRKs (Fig. 1E), which
indicated the fusion of EPC-MVs with PRKs. RT-qPCR results revealed
that Ang II induction reduced the expression of miR-98-5p compared
with the control group, which was consistent with the GEO data
analysis. The co-culture of PRKs with EPCs or EPC supernatant
increased the expression of miR-98-5p, while after treatment with
EPC-MVs the expression of miR-98-5p returned to the normal level
(Fig. 1F). Subsequently, miR-98-5p
mimic and inhibitor were transfected into EPCs. RT-qPCR results
revealed that the expression of miR-98-5p was upregulated in the
miR-98-5p mimic group, and downregulated in the miR-98-5p inhibitor
group compared with their respective controls, which suggested that
the transfection with miR-98-5p mimic and inhibitor was successful
(Fig. 1G). Additionally, the
supernatant of each group was collected and the MVs were extracted.
RT-qPCR results revealed that the expression of miR-98-5p in the
exosomes of each group was consistent with that in EPCs (Fig. 1H).
miR-98-5p mimic/inhibitor affects PRK
viability, oxidative stress and inflammation through EPC-MVs
The collected exosomes from each group were added to
the PRKs induced by Ang II. The results revealed that all groups
incubated with MVs showed red fluorescence, indicating that the MVs
and PRKs were successfully fused (Fig.
2A). CCK-8 analysis revealed that EPC-MVs increased viability
of PRKs compared with the Ang II group, and miR-98-5p mimic-MVs
enhanced the effect of EPC-MVs, whereas miR-98-5p inhibitor-MVs
inhibited the increased cell viability effect of EPC-MVs on PRKs
(Fig. 2B). EPC-MVs inhibited Ang
II-induced oxidative stress compared with Ang II group [ROS
(Fig. 2C, D) and MDA (Fig. 2E) levels decreased, whereas GSH
(Fig. 2F) and SOD (Fig. 2G) levels increased] and
inflammatory response [IL-1β (Fig.
2H), IL-6 (Fig. 2I) and TNF-α
(Fig. 2J)]. Furthermore,
co-treatment with miR-98-5p mimic-MVs enhanced the inhibitory
effect of EPC-MVs on oxidative stress and inflammation, whereas
co-treatment with miR-98-5p inhibitor-MVs had an opposite effect to
that of miR-98-5p mimic-MVs. These results indicated the successful
establishment of the Ang II-induced PRK injury model and the
injury-repair effect of miR-98-5p mimic-MVs. Therefore, the
mechanism of miR-98-5p on Ang II-induced PRK injury was further
examined.
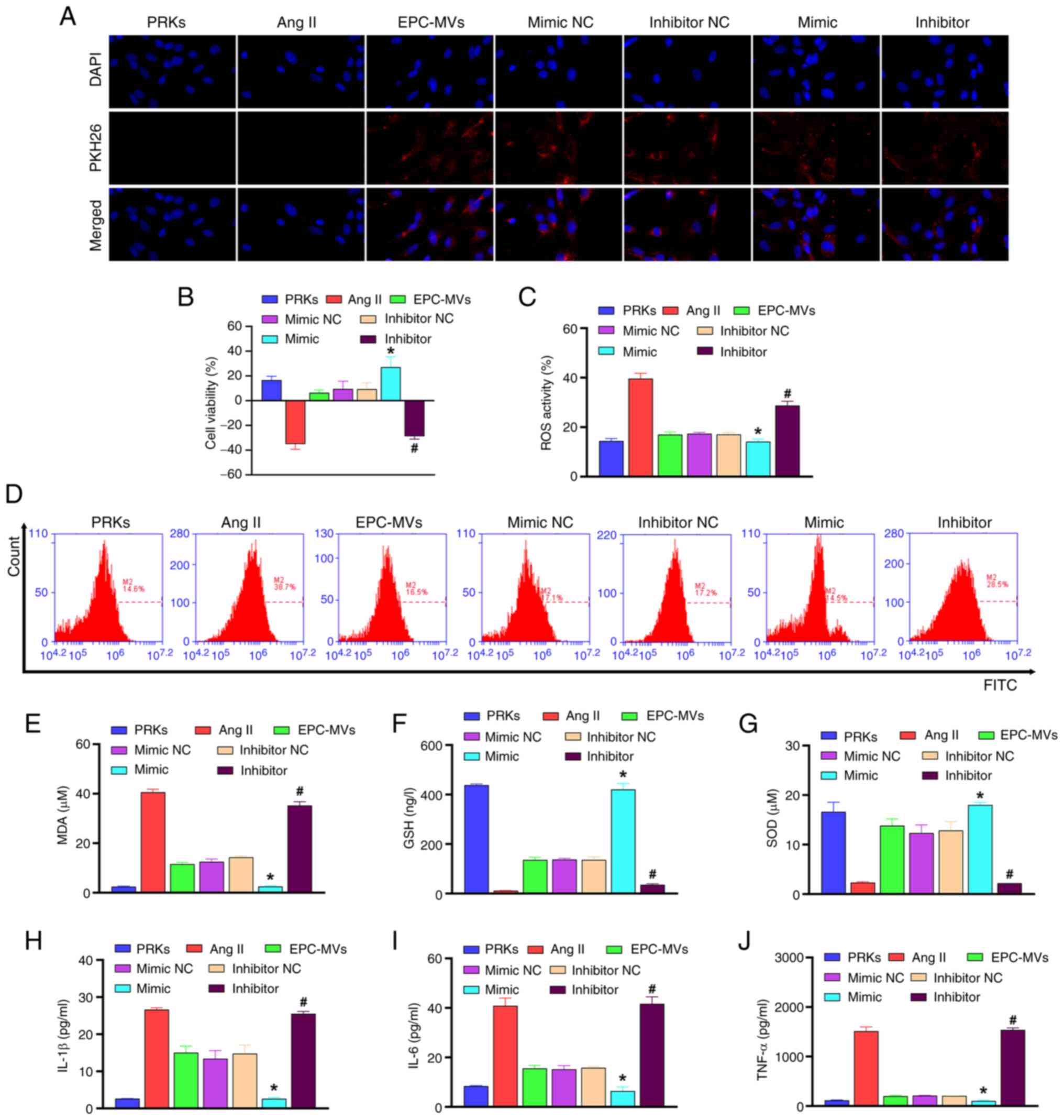 | Figure 2miR-98-5p-MVs inhibit Ang II-induced
oxidative stress and the secretion of inflammatory cytokines in
PRKs. (A) PKH26-labeled miR-98-5p-MVs successfully fused with PRKs
(magnification, x100). (B) Cell Counting Kit-8 analysis determined
the effects of miR-98-5p-MVs on the cell viability of PRKs treated
with Ang II (n=3). (C and D) Flow cytometry analysis determined the
effect of miR-98-5p-MVs on the levels of Ang II-induced ROS
production in PRKs (n=3). ELISA analysis determined the effect of
miR-98-5p-MVs on the levels of (E) MDA, (F) GSH and (G) SOD in PRKs
treated with Ang II (n=3). ELISA analysis determined the effect of
miR-98-5p-MVs on the levels of secreted (H) IL-1β, (I) IL-6 and (J)
TNF-α in PRKs treated with Ang II (n=3). *P<0.05 vs.
mimic NC; #P<0.05 vs. inhibitor NC. Ang II,
angiotensin II; ROS, reactive oxygen species; EPCs, endothelial
progenitor cells; PRKs, primary renal kidney cells; MVs,
microvesicles; MDA, malondialdehyde; GSH, glutathione; SOD,
superoxide dismutase; NC, negative control; miR, microRNA. |
Mechanism of miR-98-5p MVs against PRK
injury
The results of KEGG analysis indicated that the
PI3K-Akt signaling pathway is an important factor affecting the
progress of diabetes (Fig. 3A). In
the GSE110231 dataset, 16 target genes were identified as key
factors affecting the PI3K-Akt signaling pathway. A total of 16
mRNAs were compared with the downstream target genes of miR-98-5p
(predicted by the TargetScan database) and it was found that IGF1R
was shared between the two datasets (Fig. 3B). The subsequent dual-luciferase
assay result revealed that when wild-type IGF1R was co-transfected
with miR-98-5p mimic, the luciferase activity was significantly
lower than that of the group co-transfected with mimic NC. However,
when mutant IGF1R was co-transfected with miR-98-5p mimic, the
luciferase activity was not significantly different from that of
the mimic NC co-transfection group (Fig. 3C). Additionally, the overexpression
of IGF1R did not affect the expression of miR-98-5p (Fig. 3D). These results suggested that
miR-98-5p directly targets IGF1R. Moreover, the co-culture of PRKs
with EPCs or EPC supernatant reduced the expression of IGF1R
compared with Ang II group, and under the action of EPC-MVs the
expression of IGF1R was reduced compared with Ang II group
(Fig. 3E).
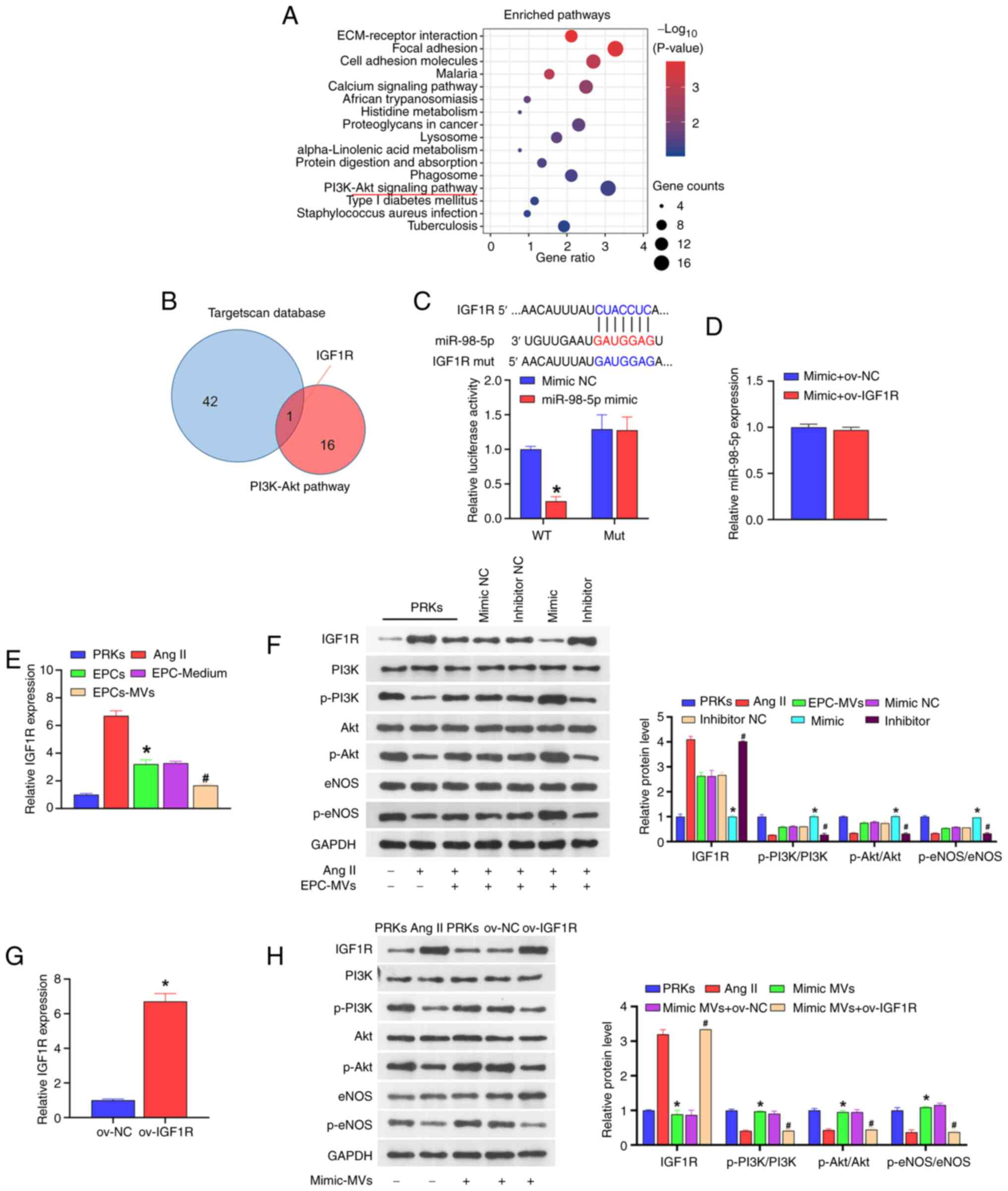 | Figure 3miR-98-5p targets IGF1R to regulate
the PI3K/Akt/eNOS signaling pathway. (A) KEGG analysis of the
signaling pathways associated with the progression of diabetes. (B)
Key factors affecting the PI3K-Akt signaling pathway (result of the
KEGG analysis) and TargetScan database were jointly used to screen
target genes. (C) Dual-luciferase analysis of the binding of
miR-98-5p and the putative target gene IGF1R (n=3)
*P<0.05 vs. mimic NC. (D) RT-qPCR analysis of the
impact of ov-IGF1R on the expression of miR-98-5p in PRKs (n=3).
(E) RT-qPCR analysis the impact of Ang II, EPCs, EPC-Medium and
EPC-MVs on the expression of IGF1R in PRKs (n=3).
*P<0.05 vs. Ang II; #P<0.05 vs.
EPC-Medium. (F) Western blot analysis of the impact of
miR-98-5p-MVs on the protein levels of IGF1R, PI3K, p-PI3K, Akt,
p-Akt, eNOS and p-eNOS (n=3). *P<0.05 vs. mimic NC;
#P<0.05 vs. inhibitor NC. (G) RT-qPCR analysis of
IGF1R expression in PRKs after transfection with ov-NC or ov-IGF1R
(n=3) *P<0.05 vs. ov-NC. (H) Western blot analysis of
the combined effects of miR-98-5p-MVs and IGF1R on the protein
levels of IGF1R, PI3K, p-PI3K, Akt, p-Akt, eNOS and p-eNOS (n=3).
*P<0.05 vs. Ang II; #P<0.05 vs.
mimic-MVs + ov-NC. IGF1R, insulin-like growth factor 1 receptor;
eNOS, endothelial nitric oxide synthase; RT-qPCR, reverse
transcription-quantitative PCR; Ang II, Angiotensin II; KEGG, Kyoto
Encyclopedia of Genes and Genomes; EPCs, endothelial progenitor
cells; PRKs, primary renal kidney cells; MVs, microvesicles; p,
phosphorylated; NC, negative control; ov, overexpression; WT,
wild-type; mut, mutant; miR, microRNA. |
miR-98-5p/IGF1R regulates the
PI3K/Akt/eNOS signaling pathway
Western blot analysis revealed that compared with
the Ang II group, EPC-MVs and miR-98-5p mimic-MVs increased the
phosphorylation levels of PI3K/Akt/eNOS and reduced the protein
level of IGF1R, while miR-98-5p inhibitor-MVs reduced the
phosphorylation levels of PI3K/Akt/eNOS and increased the protein
level of IGF1R (Fig. 3F). In order
to explore the mechanism of IGF1R in PRKs, a plasmid overexpressing
IGF1R was constructed and transfected into PRKs. RT-qPCR results
revealed that the expression of IGF1R in the ov-IGF1R group
increased compared with the ov-NC group (Fig. 3G), confirming the transfection
efficiency of the IGF1R plasmid. Subsequently, western blot
analysis showed that miR-98-5p mimic-MVs decreased the level of
IGF1R protein and increased the levels of phosphorylation of
PI3K/Akt/eNOS compared with the Ang II group. The effects were
reversed by ov-IGF1R (Fig. 3H).
Compared with the Ang II group, miR-98-5p mimic-MVs protected PRKs
from decreased viability (Fig.
4A), oxidative stress [ROS (Fig.
4B, C) and MDA (Fig. 4D) levels decreased, whereas GSH
(Fig. 4E) and SOD (Fig. 4F) levels increased] and
inflammatory response [IL-1β (Fig.
4G), IL-6 (Fig. 4H) and TNF-α
(Fig. 4I)] induced by Ang II.
However, overexpression of IGF1R reversed the protective effect of
miR-98-5p mimic-MVs on PRKs.
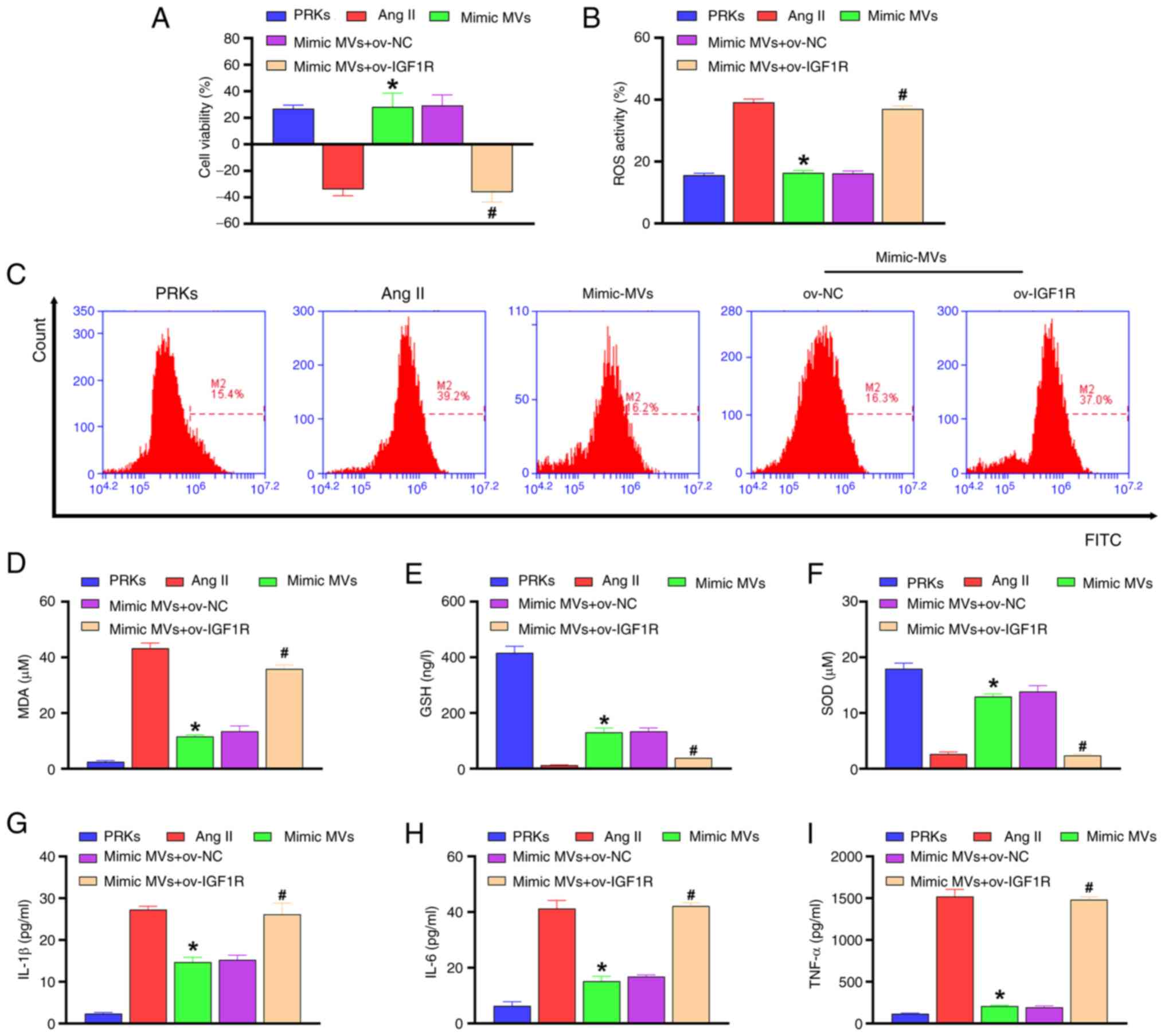 | Figure 4miR-98-5p/IGF1R axis regulates
oxidative stress and inflammation in PRKs induced by Ang II. (A)
Cell Counting Kit-8 analysis determined the combined effects of
miR-98-5p-MVs and IGF1R on the cell viability of PRKs treated with
Ang II (n=3). (B and C) Flow cytometry analysis determined the
combined effects of miR-98-5p-MVs and IGF1R on Ang II-induced ROS
generation in PRKs (n=3). ELISA analysis determined the combined
effect of miR-98-5p-MVs on the levels of (D) MDA, (E) GSH and (F)
SOD produced by PRKs treated with Ang II (n=3). ELISA analysis
determined the combined effects of miR-98-5p-MVs and IGF1R on the
levels of (G) IL-1β, (H) IL-6 and (I) TNF-α secreted by PRKs
treated with Ang II (n=3). *P<0.05 vs. Ang II;
#P<0.05 vs. mimic-MVs + ov-NC. IGF1R, insulin-like
growth factor 1 receptor; Ang II, Angiotensin II; ROS, reactive
oxygen species; EPCs, endothelial progenitor cells; PRKs, primary
renal kidney cells; MVs, microvesicles; MDA, malondialdehyde; GSH,
glutathione; SOD, superoxide dismutase; NC, negative control; ov,
overexpression; miR, microRNA. |
Discussion
In the present study, it was demonstrated for the
first time to the best of our knowledge, that EPC-MVs with high
levels of miR-98-5p can protect PRKs from Ang II-induced cell
damage.
EPCs can effectively protect from renal function
deterioration in CKD (40) and
play an important role in maintaining vascular integrity, repairing
endothelial injury and improving organ function (41). Recent evidence shows that EPCs may
play a protective role by secreting MVs (42). MVs are important mediators of
intercellular communication (43)
and are detached from the cell surface after activation, stress or
apoptosis (44). MVs have
anti-inflammatory, anticoagulant and angiogenic effects (45,46),
improve endothelial function and alleviate endothelial dysfunction
induced by oxidative stress (47).
As the damaged kidney can no longer effectively filter out the
metabolic waste in the blood, which eventually leads to the
occurrence of kidney disease, the GEO analysis in the current study
used diabetic rats based on the possibility of diabetic rats
suffering from kidney disease (48). One of the main conclusions of the
current study is that after the successful establishment of the Ang
II-induced PRK injury model, EPC-MVs suppressed the reduction of
PRK viability induced by Ang II, as well as the promotion of
oxidative stress and inflammation. This result is similar to those
in previous studies (49,50).
Dysregulated miR-98-5p expression has been reported
to play a key role in the progression of several diseases, such as
oral squamous cell carcinoma (51)
and bronchial asthma (52).
Kokkinopoulou et al (53)
demonstrated that low levels of miR-98-5p adversely affected the
treatment of patients with diabetes, and the results of the GEO
data analysis in the present study support this view that the
expression of miR-98-5p is downregulated in diabetic rats. In the
current study, it was found that miR-98-5p mimic enhanced
EPC-MVs-induced viability of PRKs, whereas miR-98-5p inhibitor
showed the opposite effect, which was consistent with the research
by Kokkinopoulou et al (53). In addition, miR-98-5p MVs also
reversed the Ang II-induced increase of ROS, MDA, IL-1β, IL-6 and
TNF-α levels, as well as the decrease of GSH and SOD levels.
Therefore, the potential mechanism of the protective effect of
miR-98-5p MVs on PRKs may involve the regulation of oxidative
stress and inflammation.
The PI3K/Akt/eNOS signaling pathway is closely
related to cell inflammation, viability, endothelial injury and
dysfunction (54-56).
Our previous study showed that EPC-MVs protect cardiomyocytes from
Ang II-induced apoptosis by activating the PI3K/Akt/eNOS signaling
pathway (38). In the current
study, the mechanism by which miR-98-5p regulates the PI3K/Akt/eNOS
signaling pathway was further explored. Through combined analysis
of the TargetScan database and KEGG, the IGF1R gene was identified
as a potential target gene for miR-98-5p. Subsequent results showed
that miR-98-5p directly targets IGF1R and regulates the
phosphorylation level of PI3K/Akt/eNOS through IGF1R. Consistently,
the overexpression of IGF1R reversed the promotive effect of
miR-98-5p mimic-MVs on PRK viability and its inhibitory effect on
oxidative stress and inflammation. This result indicates that
miR-98-5p regulates the IGF1R/PI3K/Akt/eNOS axis and inhibits the
effect induced by Ang II, thereby protecting PRKs.
A limitation of the present study is whether EPC-MVs
can protect against kidney injury in animal models of hypertensive
nephropathy, and therefore the mechanism of EPC-MVs needs to be
further elucidated. Secondly, changes in various signaling
pathways, especially inflammation or related pathways, have not
been further explored in the current study. Finally, the
intracellular distribution of miRNA could be further explored using
fluorescence in situ hybridization, which was not developed
in the present study. These limitations will be the focus of future
research.
In summary, miR-98-5p, which is present in high
levels in EPC-MVs, protected PRKs from Ang II-induced injury by
activating the PI3K/Akt/eNOS signaling pathway and inhibiting
oxidative stress and inflammation. Therefore, the current study
provides a solid theoretical basis for the potential treatment of
hypertensive nephropathy.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by the Finance Science
and Technology Projects of Hainan Province (grant no. ZDYF2019193),
the National Natural Science Foundation of China (grant no.
8156020231) and the CAMS Innovation Fund for Medical Sciences
(grant no. 2019-I2M-5-023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM, ZH and SG conceived and designed the study. XZ,
YuaZ, JC and MC performed the experiments. YunZ, YS, BW and YL
collected and analyzed experimental data. HM and ZH confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Animal Care
and Use Committee of Hainan Medical University (Haikou, China;
approval no. HYLL-2021-053).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hart PD and Bakris GL: Hypertensive
nephropathy: Prevention and treatment recommendations. Expert Opin
Pharmacother. 11:2675–2686. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hu H, Jiang C, Li R and Zhao J: Comparison
of endothelial cell- and endothelial progenitor cell-derived
exosomes in promoting vascular endothelial cell repair. Int J Clin
Exp Pathol. 12:2793–2800. 2019.PubMed/NCBI
|
|
3
|
Naito H, Iba T and Takakura N: Mechanisms
of new blood-vessel formation and proliferative heterogeneity of
endothelial cells. Int Immunol. 32:295–305. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Di Marco GS, Rustemeyer P, Brand M, Koch
R, Kentrup D, Grabner A, Greve B, Wittkowski W, Pavenstadt H,
Hausberg M, et al: Circulating endothelial progenitor cells in
kidney transplant patients. PLoS One. 6(e24046)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Panagiotou N, Davies RW, Selman C and
Shiels PG: Microvesicles as vehicles for tissue regeneration:
Changing of the guards. Curr Pathobiol Rep. 4:181–187.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ranghino A, Cantaluppi V, Grange C,
Vitillo L, Fop F, Biancone L, Deregibus MC, Tetta C, Segoloni GP
and Camussi G: Endothelial progenitor cell-derived microvesicles
improve neovascularization in a murine model of hindlimb ischemia.
Int J Immunopathol Pharmacol. 25:75–85. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang M, Malik AB and Rehman J:
Endothelial progenitor cells and vascular repair. Curr Opin
Hematol. 21:224–228. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
El-Shoura EAM, Messiha BAS, Sharkawi SMZ
and Hemeida RAM: Perindopril ameliorates lipopolysaccharide-induced
brain injury through modulation of angiotensin-II/angiotensin-1-7
and related signaling pathways. Eur J Pharmacol. 834:305–317.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jia N, Dong P, Ye Y, Qian C and Dai Q:
Allopurinol attenuates oxidative stress and cardiac fibrosis in
angiotensin II-induced cardiac diastolic dysfunction. Cardiovasc
Ther. 30:117–123. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang H, Zhang S, Jia L and Li H: MyD88
overexpression deteriorates Ang-II-induced ED via upregulating MPO
and COX2 and downregulating eNOS in the corpus cavernosum of rats.
J Cell Biochem. 28(10.1002/jcb.27987)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang L, Du J, Hu Z, Han G, Delafontaine
P, Garcia G and Mitch WE: IL-6 and serum amyloid A synergy mediates
angiotensin II-induced muscle wasting. J Am Soc Nephrol.
20:604–612. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang LL, Huang S, Ma XX, Zhang WY, Wang
D, Jin SY, Zhang YP, Li Y and Li X: Angiotensin(1-7) attenuated
angiotensin II-induced hepatocyte EMT by inhibiting NOX-derived
H2O2-activated NLRP3 inflammasome/IL-1beta/Smad circuit. Free Radic
Biol Med. 97:531–543. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu YS, Yang Q, Li S, Luo L, Liu HY, Li XY
and Gao ZN: Luteolin attenuates angiotensin IIinduced renal damage
in apolipoprotein Edeficient mice. Mol Med Rep.
23(157)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mohammed-Ali Z, Cruz GL, Lu C, Carlisle
RE, Werner KE, Ask K and Dickhout JG: Development of a model of
chronic kidney disease in the C57BL/6 mouse with properties of
progressive human CKD. Biomed Res Int. 2015(172302)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Souza ACP, Tsuji T, Baranova IN, Bocharov
AV, Wilkins KJ, Street JM, Alvarez-Prats A, Hu X, Eggerman T, Yuen
PST and Star RA: TLR4 mutant mice are protected from renal fibrosis
and chronic kidney disease progression. Physiol Rep.
3(12558)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Correia de Sousa M, Gjorgjieva M, Dolicka
D, Sobolewski C and Foti M: Deciphering miRNAs' action through
miRNA editing. Int J Mol Sci. 20(6249)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu Y, Xu J, Liang W, Li J, Feng L, Zheng
P, Ji T and Bai S: MiR-98-5p alleviated epithelial-to-mesenchymal
transition and renal fibrosis via targeting hmga2 in diabetic
nephropathy. Int J Endocrinol. 2019(4946181)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Y, Sun Y, Peng R, Liu H, He W, Zhang
L, Peng H and Zhang Z: The long noncoding rna 150Rik promotes
mesangial cell proliferation via miR-451/IGF1R/p38 MAPK signaling
in diabetic nephropathy. Cell Physiol Biochem. 51:1410–1428.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lan S and Albinsson S: Regulation of
IRS-1, insulin signaling and glucose uptake by miR-143/145 in
vascular smooth muscle cells. Biochem Biophys Res Commun.
529:119–125. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tiwari A, Mukherjee B and Dixit M:
MicroRNA key to angiogenesis regulation: MiRNA biology and therapy.
Curr Cancer Drug Targets. 18:266–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saliminejad K, Khorshid HR, Fard SS and
Ghaffari SH: An overview of microRNAs: Biology, functions,
therapeutics, and analysis methods. J Cell Physiol. 234:5451–5465.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Olejniczak M, Kotowska-Zimmer A and
Krzyzosiak W: Stress-induced changes in miRNA biogenesis and
functioning. Cell Mol Life Sci. 75:177–191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kolluru GK, Siamwala JH and Chatterjee S:
eNOS phosphorylation in health and disease. Biochimie.
92:1186–1198. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li CY, Wang LX, Dong SS, Hong Y, Zhou XH,
Zheng WW and Zheng C: Phlorizin exerts direct protective effects on
palmitic acid (PA)-induced endothelial dysfunction by activating
the PI3K/AKT/eNOS signaling pathway and increasing the levels of
nitric oxide (NO). Med Sci Monit Basic Res. 24:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xing Y, Lai J, Liu X, Zhang N, Ming J, Liu
H and Zhang X: Netrin-1 restores cell injury and impaired
angiogenesis in vascular endothelial cells upon high glucose by
PI3K/AKT-eNOS. J Mol Endocrinol. 58:167–177. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang Y, Zhang J, Su Y, Wang C, Zhang G,
Liu X, Chen Q, Lv M, Chang Y, Peng J, et al: MiRNA-98-5p targeting
IGF2BP1 induces mesenchymal stem cell apoptosis by modulating
PI3K/Akt and p53 in immune thrombocytopenia. Mol Ther Nucleic
Acids. 20:764–776. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang D, Mei L, Long R, Cui C, Sun Y, Wang
S and Xia Z: RiPerC attenuates cerebral ischemia injury through
regulation of miR-98/PIK3IP1/PI3K/AKT signaling pathway. Oxid Med
Cell Longev. 2020(6454281)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Van Dyken P and Lacoste B: Impact of
metabolic syndrome on neuroinflammation and the blood-brain
barrier. Front Neurosci. 12(930)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang P, Liang Y, Luo Y, Li Z, Wen Y, Shen
J, Li R, Zheng H, Gu HF and Xia N: Liraglutide ameliorates
nonalcoholic fatty liver disease in diabetic mice via the
IRS2/PI3K/Akt signaling pathway. Diabetes Metab Syndr Obes.
12:1013–1021. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu Q and Hu Y: Systematic evaluation of
the mechanisms of mulberry leaf (Morus alba Linne) acting on
diabetes based on network pharmacology and molecular docking. Comb
Chem High Throughput Screen. 24:668–682. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kobayashi T, Matsumoto T and Kamata K:
Possible involvement of IGF-1 receptor and IGF-binding protein in
insulin-induced enhancement of noradrenaline response in diabetic
rat aorta. Nihon Yakurigaku Zasshi. 122 (Suppl):40P–42P.
2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tan YS and Lei YL: Isolation of
tumor-infiltrating lymphocytes by ficoll-paque density gradient
centrifugation. Methods Mol Biol. 1960:93–99. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gyabaah K, Aboushwareb T, Souza NG,
Yamaleyeva L, Varner A, Wang HJ, Atala A and Yoo JJ: Controlled
regulation of erythropoietin by primary cultured renal cells for
renal failure induced anemia. J Urol. 188:2000–2006.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Elliget KA and Trump BF: Primary cultures
of normal rat kidney proximal tubule epithelial cells for studies
of renal cell injury. In Vitro Cell Dev Biol. 27A:739–748.
1991.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nair AR, Ebenezer PJ, Saini Y and Francis
J: Angiotensin II-induced hypertensive renal inflammation is
mediated through HMGB1-TLR4 signaling in rat tubulo-epithelial
cells. Exp Cell Res. 335:238–247. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Giles EM, Godbout C, Chi W, Glick MA, Lin
T, Li R, Schemitsch EH and Nauth A: Subtypes of endothelial
progenitor cells affect healing of segmental bone defects
differently. Int Orthop. 41:2337–2343. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gu S, Zhang W, Chen J, Ma R, Xiao X, Ma X,
Yao Z and Chen Y: EPC-derived microvesicles protect cardiomyocytes
from Ang II-induced hypertrophy and apoptosis. PLoS One.
9(e85396)2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sung PH, Chen KH, Li YC, Chiang JY, Lee MS
and Yip HK: Sitagliptin and shock wave-supported peripheral blood
derived endothelial progenitor cell therapy effectively preserves
residual renal function in chronic kidney disease in rat-role of
dipeptidyl peptidase 4 inhibition. Biomed Pharmacother.
111:1088–1102. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhou Y, Li P, Goodwin AJ, Cook JA,
Halushka PV, Chang E and Fan H: Exosomes from endothelial
progenitor cells improve the outcome of a murine model of sepsis.
Mol Ther. 26:1375–1384. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zeng W, Lei Q, Ma J, Gao S and Ju R:
Endothelial progenitor cell-derived microvesicles promote
angiogenesis in rat brain microvascular endothelial cells in vitro.
Front Cell Neurosci. 15(638351)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Soni S, Wilson MR, O'Dea KP, Yoshida M,
Katbeh U, Woods SJ and Takata M: Alveolar macrophage-derived
microvesicles mediate acute lung injury. Thorax. 71:1020–1029.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Watanabe K: Bacterial membrane vesicles
(MVs): Novel tools as nature- and nano-carriers for immunogenic
antigen, enzyme support, and drug delivery. Appl Microbiol
Biotechnol. 100:9837–9843. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jaimes Y, Naaldijk Y, Wenk K, Leovsky C
and Emmrich F: Mesenchymal stem cell-derived microvesicles modulate
lipopolysaccharides-induced inflammatory responses to microglia
cells. Stem Cells. 35:812–823. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang Y, Liu D, Chen X, Li J, Li L, Bian
Z, Sun F, Lu J, Yin Y, Cai X, et al: Secreted monocytic miR-150
enhances targeted endothelial cell migration. Mol Cell. 39:133–144.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zeng W, Lei Q, Ma J and Ju R: Effects of
hypoxic-ischemic pre-treatment on microvesicles derived from
endothelial progenitor cells. Exp Ther Med. 19:2171–2178.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tonneijck L, Muskiet MH, Smits MM, van
Bommel EJ, Heerspink HJ, van Raalte DH and Joles JA: Glomerular
hyperfiltration in diabetes: Mechanisms, clinical significance, and
treatment. J Am Soc Nephrol. 28:1023–1039. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Du Y, Han J, Zhang H, Xu J, Jiang L and Ge
W: Kaempferol prevents against ang II-induced cardiac remodeling
through attenuating ang II-induced inflammation and oxidative
stress. J Cardiovasc Pharmacol. 74:326–335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Singh MV, Cicha MZ, Meyerholz DK, Chapleau
MW and Abboud FM: Dual activation of TRIF and MyD88 adaptor
proteins by angiotensin II evokes opposing effects on pressure,
cardiac hypertrophy, and inflammatory gene expression.
Hypertension. 66:647–656. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Niu X, Yang B, Liu F and Fang Q: LncRNA
HOXA11-AS promotes OSCC progression by sponging miR-98-5p to
upregulate YBX2 expression. Biomed Pharmacother.
121(109623)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Du J, Wu H and Wu Y: MiR-98-5p may be a
biomarker for screening bronchial asthma in children by targeting
IL-13. Clin Lab. 65(10.7754)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kokkinopoulou I, Maratou E, Mitrou P,
Boutati E, Sideris DC, Fragoulis EG and Christodoulou MI: Decreased
expression of microRNAs targeting type-2 diabetes susceptibility
genes in peripheral blood of patients and predisposed individuals.
Endocrine. 66:226–239. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Duan MX, Zhou H, Wu QQ, Liu C, Xiao Y,
Deng W and Tang QZ: Andrographolide protects against HG-induced
inflammation, apoptosis, migration, and impairment of angiogenesis
via PI3K/AKT-eNOS signalling in HUVECs. Mediators Inflamm.
2019(6168340)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li JB, Wang HY, Yao Y, Sun QF, Liu ZH, Liu
SQ, Zhuang JL, Wang YP and Liu HY: Overexpression of microRNA-138
alleviates human coronary artery endothelial cell injury and
inflammatory response by inhibiting the PI3K/Akt/eNOS pathway. J
Cell Mol Med. 21:1482–1491. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang Z and Zhang D:
(-)-Epigallocatechin-3-gallate inhibits eNOS uncoupling and
alleviates high glucose-induced dysfunction and apoptosis of human
umbilical vein endothelial cells by PI3K/AKT/eNOS pathway. Diabetes
Metab Syndr Obes. 13:2495–2504. 2020.PubMed/NCBI View Article : Google Scholar
|


















