Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common malignancy worldwide and the most common oral
malignancy, accounting for ~90% of cases (1). High-risk factors include periodontal
diseases, viral infections, smoking and alcohol drinking habits, in
addition to betel quid chewing (2,3).
Despite developments in the treatment options over the past decade,
such as surgery, chemotherapy and radiation, the overall survival
rate remains poor (4). In
particular, ~50% patients diagnosed with OSCC will typically
succumb to this disease within 5 years (4). In addition, OSCC negatively impact
the patients' quality of life by impairing taste and speech
(5). Due to multidrug resistance,
currently used chemotherapeutic strategies (such as 5-FU and
cisplatin) for OSCC have low therapeutic efficacy and patients
frequently suffer from unforeseen treatment failure in the clinical
setting (6). Therefore, it remains
in demand to develop novel anticancer methods for the treatment of
OSCC.
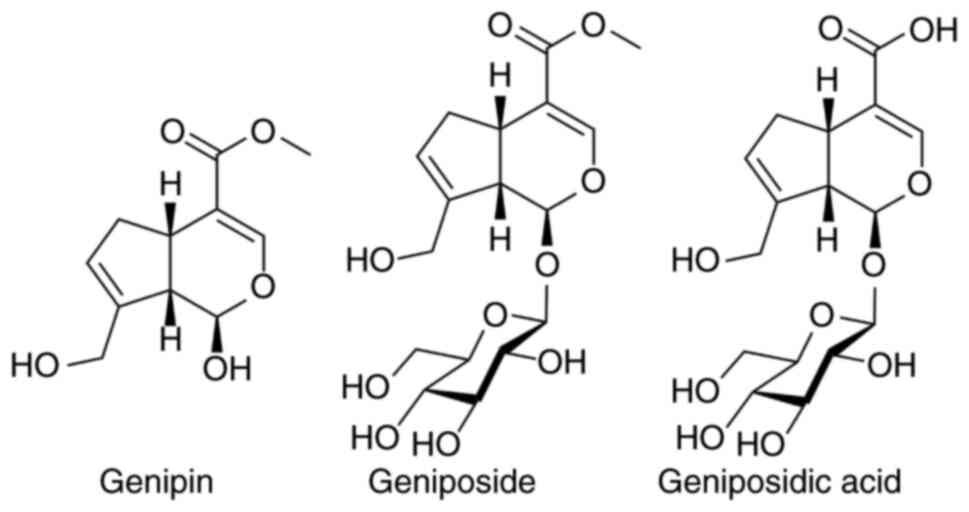
Geniposide (Fig. 1)
is a water-soluble iridoid glucoside that is mainly derived from
the fruits of the flowering plant Gardenia jasminoides Ellis
(Rubiaceae) and has been used as a traditional Chinese
medicine for centuries (7).
Several other sources of geniposide are known, including the small
tree Eucommia ulmoides Oliv (8). In particular, hydrolysis products of
geniposide, genipin is also found along with geniposide and several
other derivatives like geniposidic acid (Fig. 1) (9). Various biological activities and
pharmacological effects of geniposide, genipin and geniposidic acid
have been previously reported, including anti-inflammatory,
neuroprotective, antioxidative, anticancer, antidiabetic,
hepatoprotective and cholagogic effects (7,10-14).
In addition, previous studies have demonstrated that geniposide can
significantly inhibit the proliferation of several cancer cell
lines, such as diffuse large B-cell lymphoma cells (15), medulloblastoma cells (16) and gastric MKN45 cells (17). Lactobacillus and
Lactobacillus casei strain Shirota have also been shown to
enhance the antiproliferative effects of geniposide in the oral
squamous carcinoma cell line HSC-3 (18,19).
Furthermore, genipin has been reported to exert in vitro
anticancer effects against colon cancer (20), bladder cancer (21), hepatocellular carcinoma (22), OSCC (23), colon (24) and gastric cancer cell lines
(25,26). Geniposidic acid also has antitumor
and radioprotective effects; Hsu et al (27) reported that 500 mg/kg of
geniposidic acid significantly inhibited the growth of Erlich
ascitic xenograft tumors in vivo, and preinadiation with
geniposidic acid (500 mg/kg) showed positive effect on the recovery
of leukocytes after 4 Gy sublethal irradiation.
The present study aimed to explore the effects of
three iridoids on OSCC cell lines via CCK-8 assay, further
investigate the roles of geniposide in apoptosis, cell cycle, cell
migration and autophagy, as well as the underlying mechanisms via
flow cytometry, AO/EB staining, wound healing assay and Western
blot analysis. Finally, the regulation of geniposide on AMPK and
JNK signaling was also studies. Taken together, the findings of the
present study suggest that geniposide may be a promising lead
compound for the development of clinical candidate for the OSCC
treatment.
Materials and methods
Materials and chemical reagents
Genipin, geniposide and geniposidic acid were
purchased from Dalian Meilun Biology Technology Co., Ltd.
5-Fluorouracil (5-FU) was purchased from MilliporeSigma. The
chemical reagents were dissolved in DMSO to prepare stock solutions
(100 µM). Antibodies against cyclin-dependent kinase 2 (CDK2; cat.
no. ab32147), cyclin A2 (cat. no. ab181591), cleaved caspase-3
(cat. no. ab32042), cleaved poly-ADP ribose polymerase (PARP; cat.
no. ab32561), E-cadherin (cat. no. ab40772), MMP-2 (cat. no.
ab92536), Beclin-1 (cat. no. ab210498), light chain 3 (LC3; cat.
no. ab192890), 5'-AMP-activated protein kinase (AMPK; cat. no.
ab207442), phosphorylated (p)-JNK1/2/3 (cat. no. ab124956) and
JNK1/2/3 (cat. no. ab179461) were purchased from Abcam. Anti-p-AMPK
(cat. no. 2535) antibody was obtained from Cell Signaling
Technology, Inc. Anti-rabbit IgG (cat. no. KGAA35) and β-actin
(cat. no. KGAA006) were purchased from Jiangsu KeyGen Biotech Co.,
Ltd. SP600125 (cat. no. T3109) and BML-275 (cat. no. T1977) was
purchased from Shanghai Topscience Co., Ltd.
Cell culture
HSC-2, SCC-9 and A253 human OSCC cell lines were
obtained from Cobioer Biosciences Co., Ltd. HSC-2 cells (cat. no.
CBP60260) were cultured in minimum essential medium (MEM; cat. no.
KGM41500-500; Jiangsu KeyGen Biotech Co., Ltd.) at 37˚C with 5%
CO2. A253 cells (cat. no. CBP60662) were cultured in
RPMI-1640 medium (cat. no. KGM31800-500; Jiangsu KeyGen Biotech
Co., Ltd.) at 37˚C with 5% CO2. SCC-9 cells (cat. no.
CBP60428) were grown in DMEM/F-12 (cat. no. KGM12500-500; Jiangsu
KeyGen Biotech Co., Ltd.) supplemented with 10% heat-inactivated
FBS (cat. no. KGY008; Jiangsu KeyGen Biotech Co., Ltd.) and 1%
streptomycin/penicillin antibiotics at 37˚C with 5%
CO2.
Cell viability
HSC-2, SCC-9 and A253 cells (3.5x104
cells/well) were seeded into 96-well plates and then incubated with
0.1% DMSO or 100 µM test compounds (genipin, geniposide,
geniposidic acid and 5-FU) at 37˚C with 5% CO2 for 72 h.
Subsequently, 10 µl CCK-8 solution (cat. no. KGA317; Jiangsu KeyGen
Biotech Co., Ltd.) was added into each well before the cells were
incubated for 2 h at 37˚C. Following incubation, the absorbance of
each well was recorded at 450 nm using a microplate reader (Elx800;
BioTek Instruments, Inc.).
Dose-response of geniposide on SCC-9
cell viability
SCC-9 cells (3.5x104 cells/well) were
seeded into 96-well plates and then incubated with 0.1% DMSO or
geniposide (12.5, 25, 50 and 100 µM) at 37˚C with 5% CO2
for 48 h. Subsequently, 10 µl CCK-8 solution (cat. no. KGA317;
Jiangsu KeyGen Biotech Co., Ltd.) was added into each well before
the cells were incubated for 2 h at 37˚C. Following incubation, the
absorbance of each well was recorded at 450 nm using a microplate
reader (Elx800; BioTek Instruments, Inc.).
Cell cycle analysis
SCC-9 cells (3x105 cells/well) were
seeded into six-well plates and incubated with 0.1% DMSO or
geniposide (25, 50 and 100 µM) at 37˚C with 5% CO2 for
48 h. The cells were then detached with trypsin and washed twice
with PBS. Cells were then fixed with ice-cold 70% ethanol at 4˚C
overnight. Cells were then processed with a Cell Cycle Analysis Kit
(cat. no. KGA511; Jiangsu KeyGen Biotech Co., Ltd.). The cells
(5x105) were incubated with 100 µl RNase A at 37˚C for
30 min and then with 400 µl PI (both included in the kit) at 4˚C
for 30 min in the dark. The cell cycle distribution was then
analyzed using a flow cytometer (BD CellQuest Pro version 6.0; BD
Biosciences).
Apoptosis analysis
SCC-9 cells (3x105 cells/well) were
seeded in six-well plates and incubated with 0.1% DMSO or 25, 50
and 100 µM geniposide at 37˚C with 5% CO2 for 48 h. The
cells were then trypsinized, washed twice with PBS and collected by
centrifugation at 500 x g for 5 min at 20˚C. Cells
(5x105) were re-suspended in 500 µl binding buffer (cat.
no. KGF005; Jiangsu KeyGen Biotech Co., Ltd.) and stained with 5 µl
Annexin V-APC and 5 µl 7-AAD (cat. no. KGA1024; Annexin Apc V 7 AAD
Apoptosis Detection Kit; Jiangsu KeyGen Biotech Co., Ltd.) for 15
min in the dark. Subsequently, cells were analyzed using a flow
cytometry (BD FACSCalibur™; BD Biosciences) and data were analyzed
with BD CellQuest Pro version 6.0 (BD Biosciences). Dot-plots were
assessed for the percentage of cells considered to be live (lower
left quadrant), early apoptotic (lower right quadrant), late
apoptotic (upper right quadrant), and necrotic (upper left
quadrant). Apoptotic index=apoptotic cell number/total cell number
x100%.
Mitochondrial membrane potential (∆Ψm)
analysis
SCC-9 cells (3x105 cells/well) were
seeded into six-well plates and incubated with 0.1% DMSO or 25, 50
and 100 µM geniposide at 37˚C with 5% CO2 for 48 h. The
cells were then trypsinized, washed with PBS and collected by
centrifugation at 800 x g for 5 min at 20˚C. Cells
(1x106) were re-suspended in 500 µl incubation buffer
containing tetramethylrhodamine methyl ester staining (JC-1; cat.
no. KGA602; Jiangsu KeyGen Biotech Co., Ltd.) and incubated at 37˚C
for 15 min in a 5% CO2 incubator. The cells were
collected by centrifugation at 500 x g for 5 min at room
temperature, re-suspended and then analyzed using flow cytometry
(BD CellQuest Pro version 6.0; BD Biosciences). The relative
∆Ψm was expressed as the ratio of JC-1 red fluorescence of
normal mitochondrion detected in the red channel (FL2-H) (upper
right quadrant) to green fluorescence of low-potential mitochondria
detected in the green FITC channel (FL1-H) (lower right
quadrant).
Acridine orange/ethidium bromide
(AO/EB) staining
SCC-9 cells (1x105 cells/well) were
seeded into six-well plates and incubated with 0.1% DMSO or 25, 50
and 100 µM geniposide at 37˚C with 5% CO2 for 48 h. The
cells were then stained with 100 µl AO/EB dyemix (cat. no. KGA213;
Jiangsu KeyGen Biotech Co., Ltd.) at room temperature for 15 min in
the dark and then washed twice with PBS. The apoptotic cells were
visualized under a fluorescence microscope (IX51; Olympus
Corporation) at x100 magnification.
Wound-healing assay
SCC-9 cells (1x106 cells/well) were
seeded into six-well plates and grown to 80% confluence at 37˚C in
serum-free culture medium (DMEM/F12). The cells were then scratched
using a tip (1 ml) and washed with PBS. These SCC-9 cells were
incubated with 0.1% DMSO or 25, 50 and 100 µM geniposide at 37˚C
with 5% CO2 in serum-free culture medium (DMEM/F12) for
48 h. Images were captured at 0 and 48 h timepoints with a phase
contrast light microscope (magnification, x100; IX51; Olympus
Corporation) and the migration distance in each group was
determined.
Western blot analysis
After culturing, SCC-9 cells were treated with 0.1%
DMSO or 25, 50 and 100 µM geniposide at 37˚C with 5% CO2
for 48 h. For p-JNK1/2/3 or JNK1/2/3 or p-AMPK or AMPK, SCC-9 cells
were pre-treated for 1 h with/without 10 µM SP600125 or 10 µM
BML-275 before exposure to 100 µM geniposide at 37˚C with 5%
CO2 for 48 h. The cells were then collected by
trypsinization, washed with PBS and then lysed with ice-cold cell
lysis buffer for western and immunoprecipitation (cat. no. KGP701;
JiangsuNanjing KeyGen Biotech Co., Ltd). After centrifugation at
24,080 x g for 15 min at 4˚C, the protein samples were collected
and quantified using the Bradford assay. Equal amounts of proteins
(30 µg/lane) were separated by 10% SDS-PAGE, before the separated
proteins were transferred onto nitrocellulose membranes (cat. no.
66485; Pall Corporation). After blocking in 5% non-fat milk (Anchor
Corporation) at room temperature for 2 h, the membranes were washed
three times with tris-buffered saline containing Tween-20 (TBST;
cat. no. KGP109-T; Jiangsu KeyGen Biotech Corp., Ltd.) and
incubated overnight at 4˚C with primary antibodies against CDK2
(1:2,000 dilution), cyclin A2 (1:2,000), cleaved caspase-3 (1:500),
cleaved PARP (1:1,000), E-cadherin (1:10,000), MMP-2 (1:2,000),
Beclin-1 (1:1,000), LC3 (1:2,000), AMPK (1:1,000), p-JNK1/2/3
(1:2,000), JNK1/2/3 (1:1,000), p-AMPK (1:1,000) and β-actin
(1:1,000). Following primary antibody incubation, membranes were
washed three times with TBST and incubated with HRP-conjugated goat
anti-rabbit IgG secondary antibody (1:4,000) for 1 h at room
temperature. Immunolabeling was then visualized using an enhanced
chemiluminescence kit (cat. no. KGP116; Jiangsu KeyGen Biotech Co.,
Ltd.). Densitometric analysis of the protein bands was performed
with Gel-Pro Analyzer version 4.0 software (Media Cybernetics
Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Data were analyzed using GraphPad
Prism 8.3.0 (GraphPad Software, Inc.). Multiple comparisons were
performed using one-way ANOVA test with Geisser-Greenhouse
correction and Dunnett's post-hoc test. Comparison of each group
vs. every other group was performed by one-way ANOVA with
Geisser-Greenhouse correction and Tukey's multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
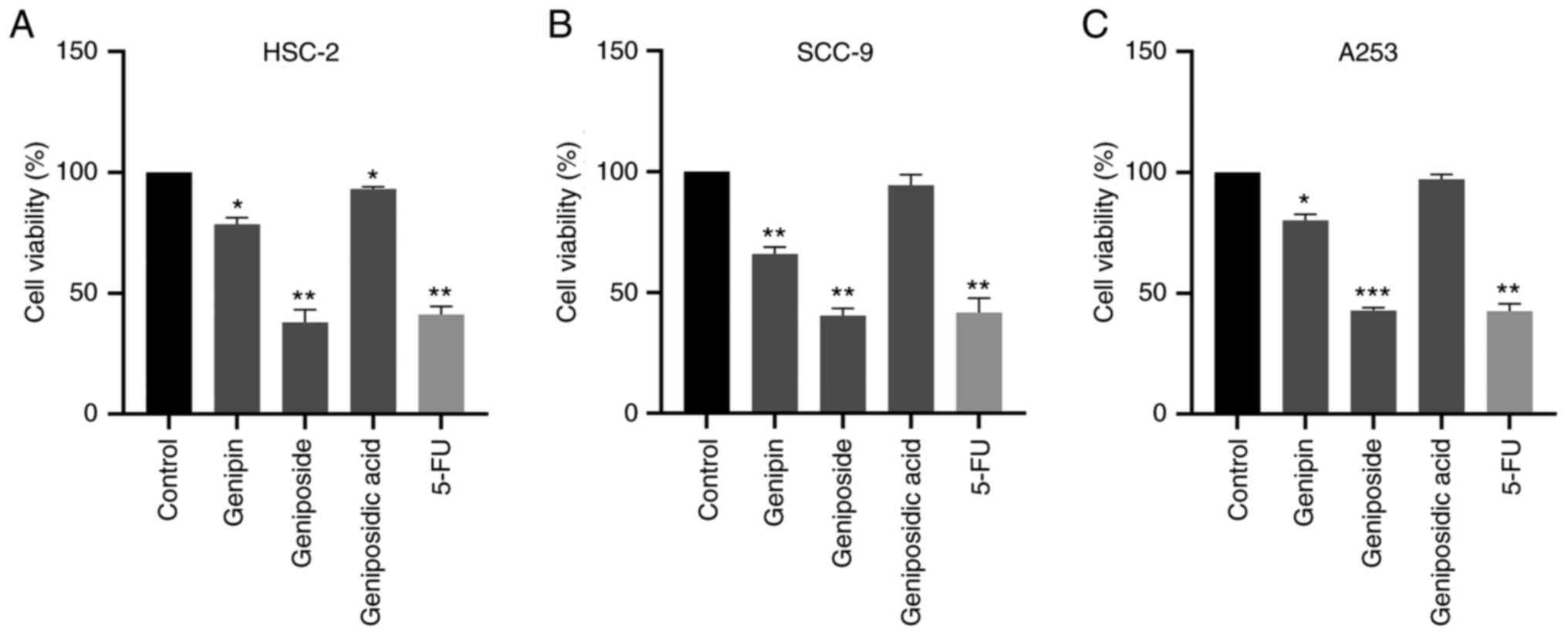
Cytotoxic effects of genipin,
geniposide and geniposidic acid on OSCC cells
The HSC-2, SCC-9 and A253 human OSCC cell lines were
incubated with genipin, geniposide and geniposidic acid (all 100
µM) for 72 h before cell viability was measured using CCK-8 assay.
Genipin and geniposidic acid slightly inhibited the viability of
HSC-2, SCC-9 and A253 cells (Fig.
2). Geniposide had the strongest antiproliferative activity in
all three OSCC lines (Fig. 2).
Treatment with geniposide significantly reduced cell viability by
>50% in HSC-2, SCC-9 cells and A253 cells, the extent of which
was comparable with that mediated by the clinical drug 5-FU in all
three cell lines (Fig. 2).
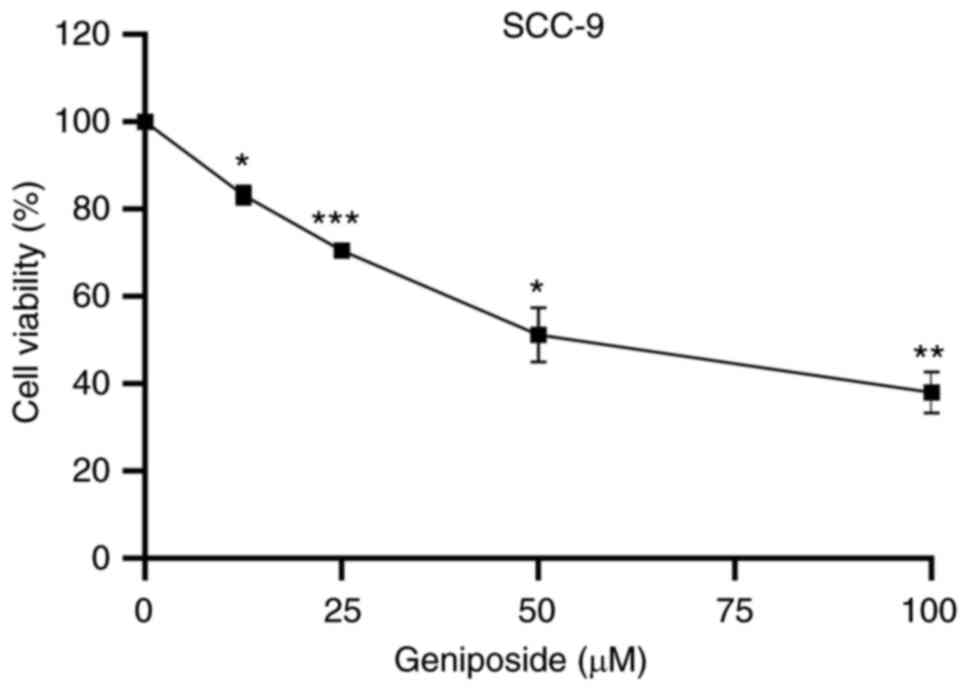
Geniposide suppresses the viability of
SCC-9 cells in a concentration-dependent manner
The possible effects of different concentrations of
geniposide (12.5, 25, 50 and 100 µM) on the viability of tongue
squamous cell carcinoma cell line SCC-9 were investigated further
using CCK-8 assay. Geniposide treatment significantly suppressed
the viability of SCC-9 cells in a concentration-dependent manner
(Fig. 3).
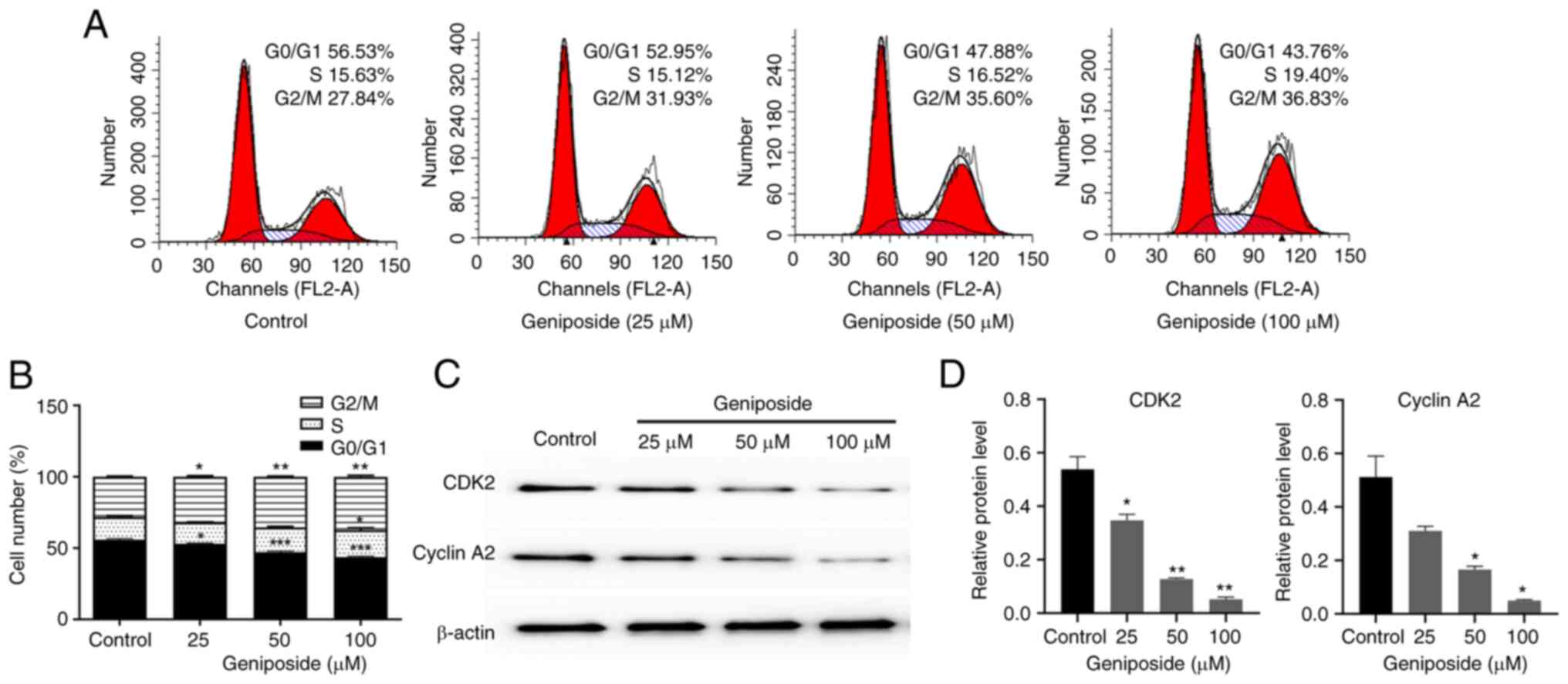
Geniposide induces G2/M
arrest and downregulates CDK2 and cyclin A2 expression in SCC-9
cells
To determine if the inhibitory effects of geniposide
on SCC-9 cell viability resulted from cell cycle arrest, the
effects of geniposide on cell cycle distribution was investigated
using flow cytometry after labeling with PI. The cells were
therefore incubated with 25, 50 and 100 µM geniposide for 48 h.
Treatment with geniposide induced cell cycle arrest at
G2/M phases and significantly reduced the number of
cells in the G0/G1 phase in a dose-dependent
manner, compared with those in the control group (Fig. 4A and B). In addition, the effect of geniposide
on the expression levels of cell cycle regulators in SCC-9 cells
was also evaluated. Cells were incubated with 25, 50 and 100 µM
geniposide for 48 h, before the expression levels of CDK2 and
Cyclin A2 were measured through western blot analysis. Geniposide
resulted in the downregulation of both CDK2 and CyclinA2 in a
dose-dependent manner, which may have been the underlying reason
for the geniposide-induced G2/M phase arrest observed
(Fig. 4C and D).
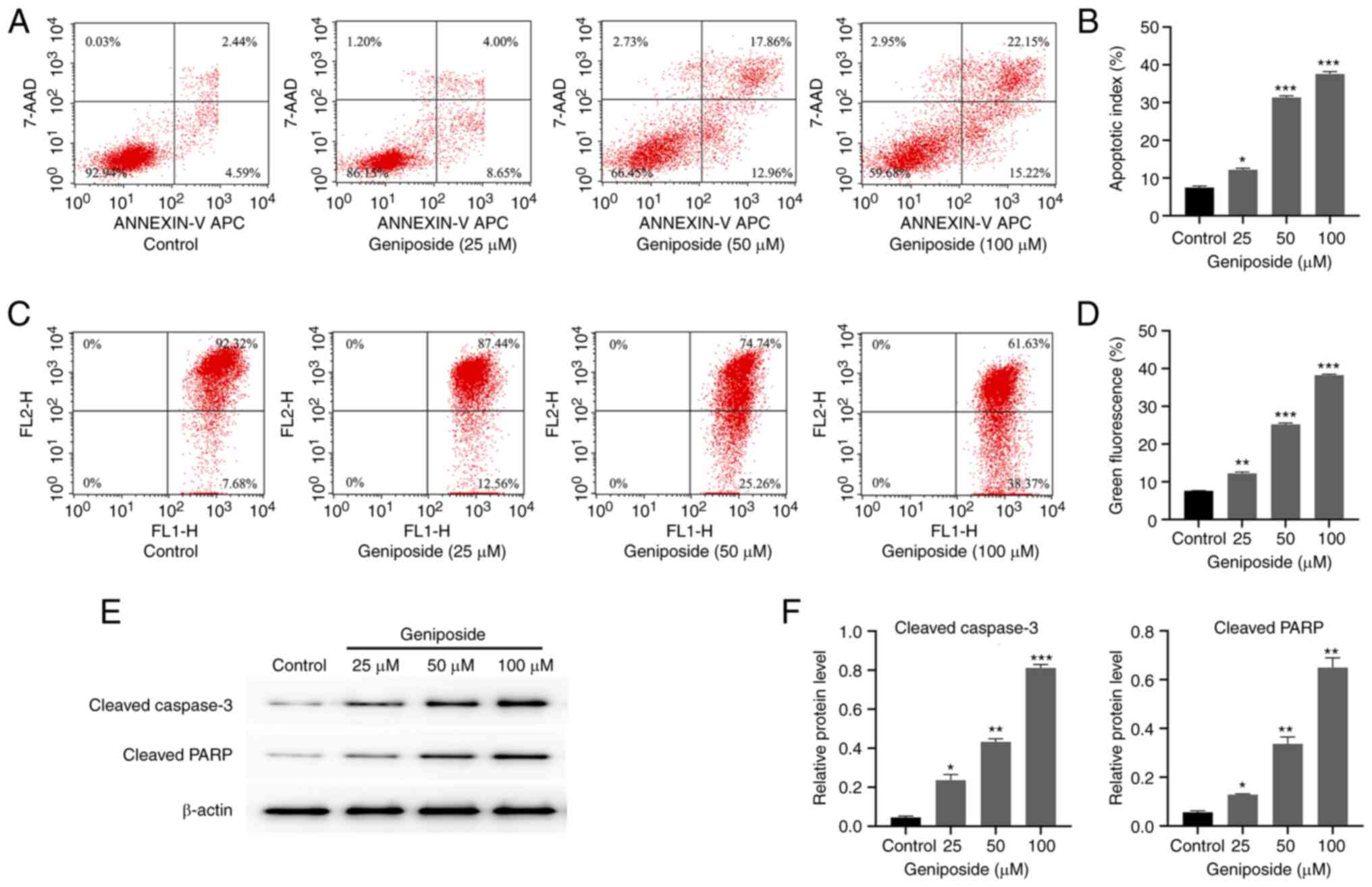
Geniposide induces apoptosis by
decreasing the mitochondrial membrane potential and increasing the
expression levels of cleaved caspase-3 and cleaved PARP in SCC-9
cells
To study the effects of geniposide on SCC-9 cell
apoptosis, flow cytometry and western blot analysis were performed.
Cells were incubated with 25, 50 and 100 µM geniposide for 48 h,
before the percentages of apoptotic cells were analyzed using flow
cytometry after labeling with Annexin V-FITC/7-AAD. Geniposide
promoted the apoptotic ratio of SCC-9 cells in a dose-dependent
manner. After treatment with 25, 50 and 100 µM geniposide, the
percentages of apoptotic cells were all significantly greater
compared with those in the control group (Fig. 5A and B). Following incubation with 25, 50 and
100 µM geniposide for 48 h and further staining with JC-1, the
mitochondrial membrane potential in SCC-9 cells was also
significantly decreased from 12.56 to 38.37% (Fig. 5C and D). In addition, geniposide significantly
increased the expression levels of cleaved caspase-3 and cleaved
PARP in a dose-dependent manner in SCC-9 cells (Fig. 5E and F). These results suggest that geniposide
can induce SCC-9 cell apoptosis by reducing the mitochondrial
membrane potential, in addition to upregulating the expression of
cleaved caspase-3 and cleaved PARP.
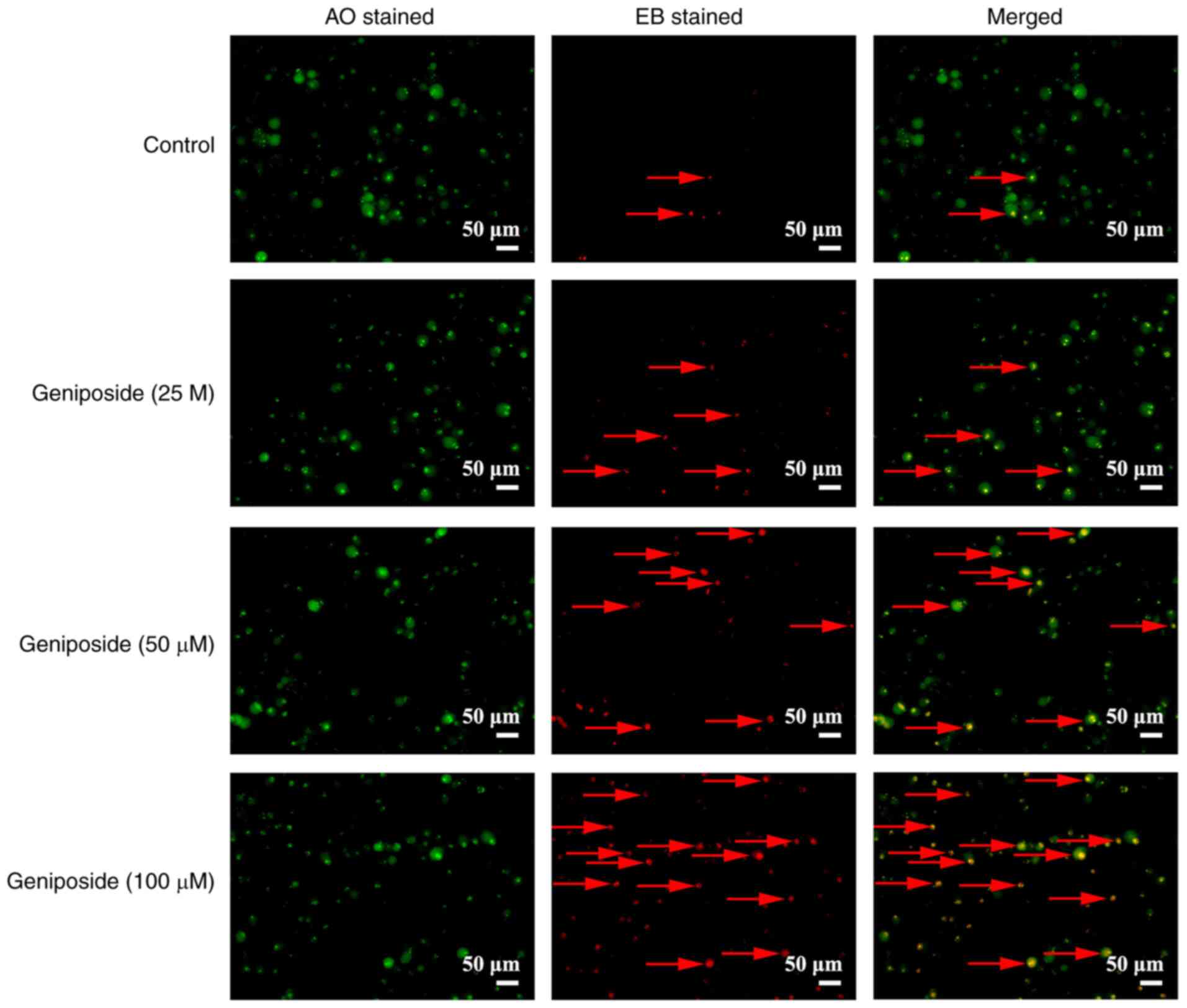
AO/EB staining observation of
apoptosis
Subsequently, the effects of geniposide on the
apoptosis of SCC-9 cells was visualized using AO/EB staining. Cells
exhibited normal morphology with uniform green staining in the
control group (Fig. 6). However,
light orange fluorescence, chromatin condensation and shrinkage
were observed in SCC-9 cells incubated with geniposide, suggesting
that geniposide can induce SCC-9 cell apoptosis (Fig. 6).
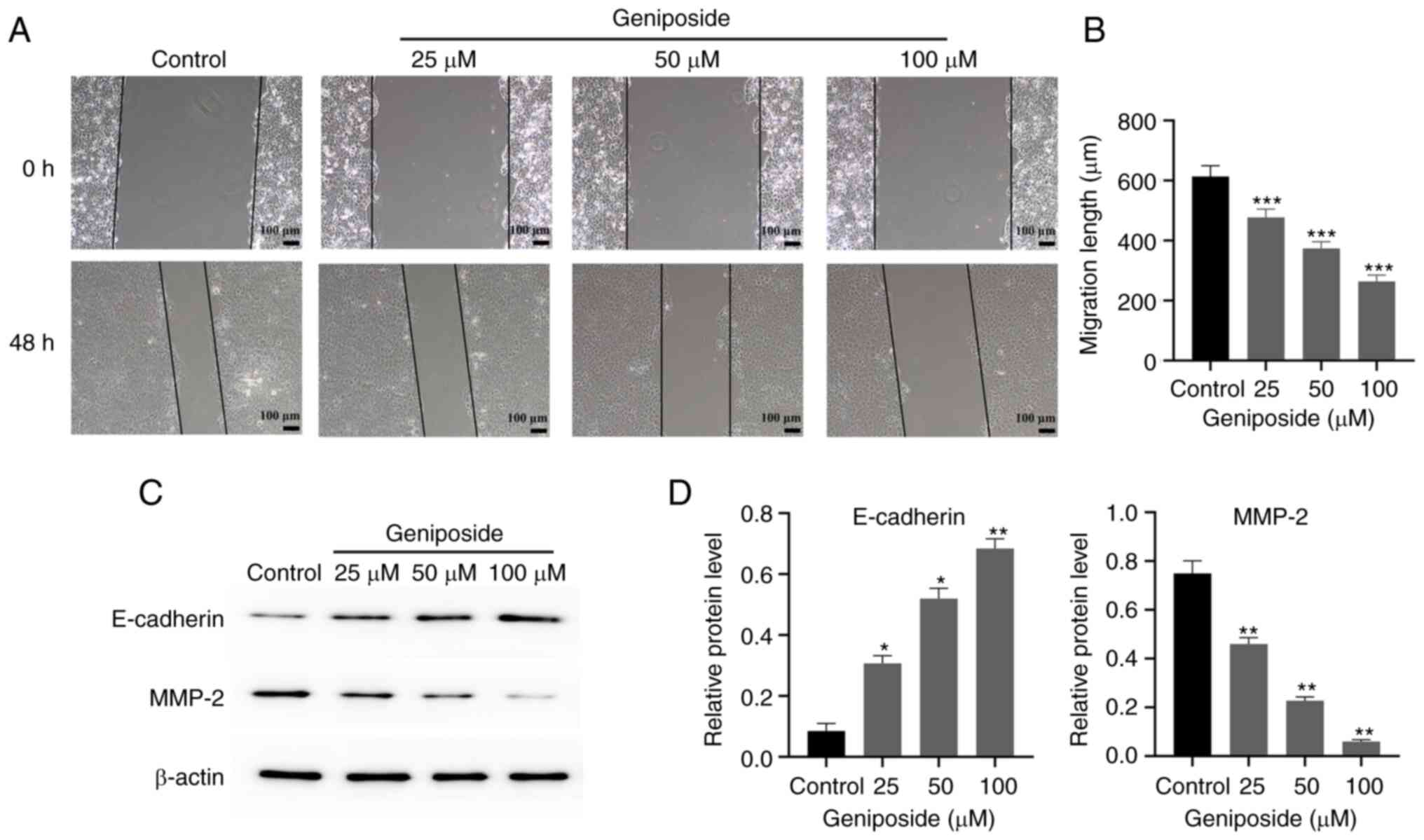
Geniposide inhibits the migration of
SCC-9 cells
The effects of geniposide on SCC-9 cell migration
was next evaluated using wound healing assay. Geniposide treatment
significantly decreased the migration of SCC-9 cells after 48 h in
a dose-dependent manner compared with that in the Control group
(Fig. 7A and B). Furthermore, geniposide significantly
increased the expression of E-cadherin, whilst significantly
suppressing the expression of MMP-2 in SCC-9 cells in a
dose-dependent manner compared with those in the Control group
(Fig. 7C and D).
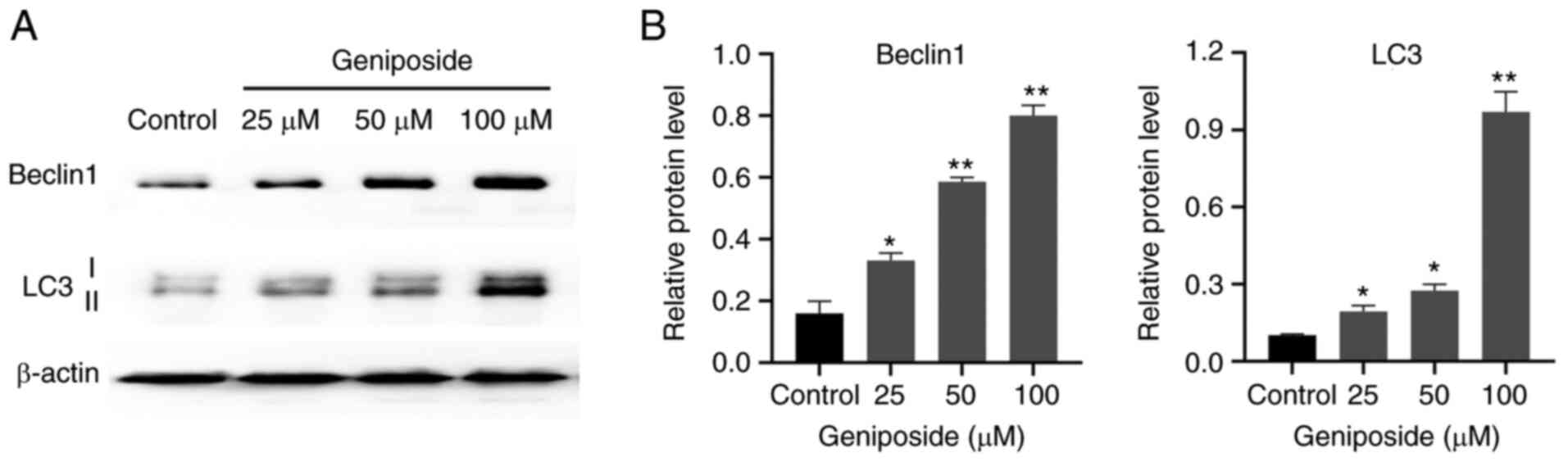
Effect of geniposide on the expression
autophagy markers in SCC-9 cells
Since autophagy is a crucial programmed cell death
process (28), it was next
investigated whether autophagy was involved in geniposide-reduced
SCC-9 cell death. The effect of geniposide on the expression levels
of autophagy markers Beclin1 and LC3 in SCC-9 cells was evaluated.
Treatment with geniposide significantly increased the expression
levels of Beclin1 and LC3, in particular increasing the generation
of LC3-II, compared with those in the Control group (Fig. 8). These findings suggest that
geniposide treatment enhanced SCC-9 cell autophagy.
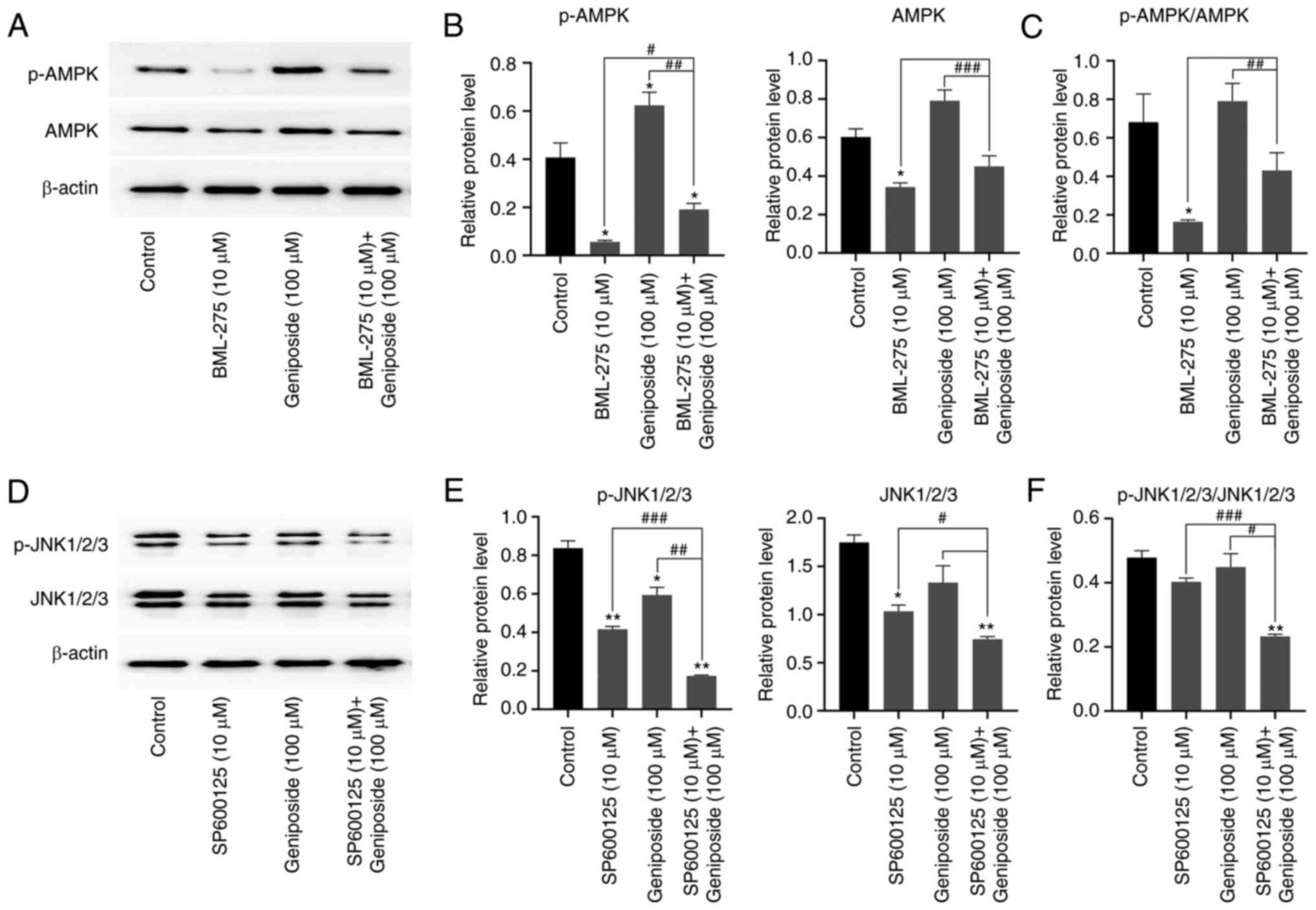
Effect of geniposide on AMPK and JNK
signaling in SCC-9 cells
A previous study reported that the AMPK and JNK
signaling pathways are key regulators of apoptosis and autophagy
(29). To assess the effect of
geniposide on the AMPK and JNK pathways in SCC-9 cells, western
blot analysis was performed to analyze the phosphorylation levels
of AMPK and JNK. Compared with those in the control group, the
levels of AMPK phosphorylation and expression were increased in the
geniposide group (Fig. 9A and
B). However, in Fig. 9C, geniposide was not observed to
affect the p-AMPK/AMPK ratio compared with that in the control
group. In addition, 10 µM AMPK inhibitor BML-275 significantly
reversed the upregulation of AMPK phosphorylation and AMPK
expression caused by geniposide treatment. Decreased JNK
phosphorylation and JNK expression were observed in the geniposide
group (Fig. 9D-E). By contrast,
the downregulation in the levels of JNK phosphorylation and JNK
expression originally caused by geniposide were significantly
potentiated after pre-treatment with 10 µM JNK inhibitor SP600125,
compared with those in the control group (Fig. 9D-E). While, in Fig. 9F, geniposide was not observed to
affect the p-JNK/JNK ratio, compared with the control group. These
data suggest that geniposide activated the AMPK signaling pathway
but inhibited the JNK signaling pathway in SCC-9 cells.
Discussion
Geniposide is a major active ingredient in the fruit
of Gardenia jasminoides Ellis (9). It has been previously demonstrated
that geniposide has various biological properties, including
anti-inflammatory, neuroprotective, anticancer, antidiabetic and
cholagogic effects (9). Previous
studies showed that geniposide can exert significant cytotoxicity
towards several cancer cell lines, such as diffuse large B-cell
lymphoma cells, medulloblastoma cells and gastric MKN45 cells
(15-17).
In addition, Cheng et al (18) demonstrated that
Lactobacillus can improve the in vitro antineoplastic
effects of geniposide on the human OSCC cell line HSC-3 through
β-glucosidase production to transform geniposide into genipin.
Similarly, Qian et al (19)
discovered that treatment with Lactobacillus casei strain
Shirota enhanced the in vitro anti-proliferative effects of
geniposide on HSC-3 cells. In the present study, geniposide
significantly decreased cell viability in three OSCC cell lines
tested. In addition, geniposide suppressed the viability of HSC-2,
SCC-9 and A253 cells by levels comparable to those mediated by the
clinic drug 5-FU. In particular, geniposide significantly inhibited
the viability of SCC-9 cells in a concentration-dependent
manner.
Cell cycle regulators CDK2 and cyclin A2 serve
important roles in regulating the cell cycle transitions,
specifically at progression through the S and G2 cell
cycle phases (30). Hwang et
al (31) previously
demonstrated that geniposide can increase DU145 cell accumulation
at the sub-G1 phase. The present study revealed that
geniposide induced G2/M phase arrest in SCC-9 cells by
downregulating the expression of CDK2 and cyclin A2.
Caspase-3 and PARP are involved in the regulation of
cell apoptosis, such that the presence of cleaved caspase-3 and
cleaved PARP are considered to be markers of apoptosis (32). In the present study, flow cytometry
and western blot analyses indicated that geniposide significantly
increased the apoptotic ratio in SCC-9 cells by increasing the
expression levels of cleaved caspase-3 and cleaved PARP. Recently,
Chen et al (16) reported
that geniposide treatment resulted in in vitro anticancer
activity in Daoy medulloblastoma cells by upregulating the
expression level of cleaved caspase 3.
Tumor metastasis is one of the main causes of
chemotherapy failure and neoplasm recurrence (33). Cell surface E-cadherin and MMP-2
are proteins associated with cancer cell migration and tumor
metastasis (34). In the present
study, wound healing assay results showed that geniposide decreased
SCC-9 cell migration, possibly by increasing E-cadherin expression
whilst decreasing MMP-2 expression. It has been previously reported
that treatment with geniposide inhibited Daoy cell migration by
suppressing MMP-2 expression (16).
Autophagy serves a key role in maintaining cell
homeostasis and survival under stressful conditions and has a
significant effect on health (cell aging and differentiation) and
disease (liver cirrhosis, myocardial infarction and heart failure)
(35). However, the role of
autophagy in cancer is dichotomous, since it has been found to both
inhibit tumor initiation and promote tumor progression (36). In the present study, western blot
analysis indicated that geniposide activated SCC-9 cell autophagy
by upregulating the expression of Beclin-1 and LC3-II. To the best
of our knowledge, the present study was the first to report that
the in vitro anticancer effects of geniposide is associated
with the regulation of autophagy.
AMPK is a key sensor of cellular energy homeostasis
in mammalian cells and serve an important regulatory role in the
metabolism of carbohydrates and fats (37). By contrast, JNK is a member of the
MAPK family that has been reported to regulate cell proliferation
and differentiation (38). AMPK
and JNK have been previously shown to regulate apoptosis and
autophagy (39). In the present
study, it was observed that geniposide could stimulate AMPK
signaling pathway whilst inhibiting the JNK signaling pathway in
SCC-9 cells.
In conclusion, results of the present study
indicated that geniposide can be an effective in vitro
antineoplastic agent against OSCC cells by acting through multiple
mechanisms. Potential mechanisms include cell cycle arrest at the
G2/M phase through downregulation of CDK2 and cyclin A2
expression, cell apoptosis by disturbing the mitochondrial membrane
potential and upregulating cleaved caspase-3 and cleaved PARP
expression, inhibition of cell migration by increasing the
E-cadherin/MMP-2 expression ratio, cell autophagy through
upregulation of Beclin-1 and LC3-II expression, in addition to the
regulation of AMPK and JNK signaling. Although geniposide exhibited
inhibitory effects against three OSCC cell lines similarly to the
clinical drug 5-FU, further investigations into the in vivo
effectiveness and efficiency of geniposide should be conducted,
with focus on the toxicity and pharmacokinetics of this agent.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 31870285), National Major
Project of Cultivating New Varieties of Genetically Modified
Organisms (grant no. 2016ZX08010003-009) and Guizhou Province
High-level Innovative Talent Training Program Project [grant no.
(2016)4003].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GB, BC, XX, YL, XL and DanZ performed the
experiments, collected data and wrote the manuscript. GB, LZ and
DegZ analyzed the data and wrote the manuscript. GB and LZ confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salahshourifar I, Vincent-Chong VK,
Kallarakkal TG and Zain RB: Genomic DNA copy number alterations
from precursor oral lesions to oral squamous cell carcinoma. Oral
Oncol. 50:404–412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wen CP, Tsai SP, Cheng TY, Chen CJ, Levy
DT, Yang HJ and Eriksen MP: Uncovering the relation between betel
quid chewing and cigarette smoking in Taiwan. Tob Control. 14
(Suppl 1):i16–i22. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nör JE and Gutkind JS: Head and neck
cancer in the new era of precision medicine. J Dent Res.
97:601–602. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
6
|
Tshering Vogel DW, Zbaeren P and Thoeny
HC: Cancer of the oral cavity and oropharynx. Cancer Imaging.
10:62–72. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Habtemariam S and Lentini G: Plant-derived
anticancer agents: Lessons from the pharmacology of geniposide and
its aglycone, genipin. Biomedicines. 6(39)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang CY, Tang L, He JW, Li J and Wang YZ:
Ethnobotany, phytochemistry and pharmacological properties of
Eucommia ulmoides: A review. Am J Chin Med. 47:259–300.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shan M, Yu S, Yan H, Guo S, Xiao W, Wang
Z, Zhang L, Ding A, Wu Q and Li SF: A review on the phytochemistry,
pharmacology, pharmacokinetics and toxicology of geniposide, a
natural product. Molecules. 22(1689)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Habtemariam S: Iridoids and other
monoterpenes in the alzheimer's brain: Recent development and
future prospects. Molecules. 23(117)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou YX, Zhang RQ, Rahman K, Cao ZX, Zhang
H and Peng C: Diverse pharmacological activities and potential
medicinal benefits of geniposide. Evid Based Complement Alternat
Med. 2019(4925682)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Neri-NumaMarina IA, Pessoa MG, Paulino BN
and Pastore GM: Genipin: A natural blue pigment for food and health
purposes. Trends Food Sci Tech. 67:271–279. 2017.
|
|
13
|
Shanmugam MK, Shen H, Tang FR, Arfuso F,
Rajesh M, Wang L, Kumar AP, Bian J, Goh BC, Bishayee A and Sethi G:
Potential role of genipin in cancer therapy. Pharmacol Res.
133:195–200. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ramos-de-la-Peña AM, Renard CM, Montañez
J, de la Luz Reyes-Vega M and Contreras-Esquivel JC: Phytochem Rev.
15:37–49. 2016.
|
|
15
|
Hu L, Zhao J, Liu Y, Liu X, Lu Q, Zeng Z,
Zhu L, Tong X and Xu Q: Geniposide inhibits proliferation and
induces apoptosis of diffuse large B-cell lymphoma cells by
inactivating the HCP5/miR-27b-3p/MET axis. Int J Med Sci.
17:2735–2743. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen Z, Liu W, Qin Z, Liang X and Tian G:
Geniposide exhibits anticancer activity to medulloblastoma cells by
downregulating microRNA-373. J Biochem Mol Toxicol.
34(e22471)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma J and Ding Y: Geniposide suppresses
growth, migration and invasion of MKN45 cells by down-regulation of
lncRNA HULC. Exp Mol Pathol. 105:252–259. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng Z, Xu H, Wang X and Liu Z:
Lactobacillus raises in vitro anticancer effect of geniposide in
HSC-3 human oral squamous cell carcinoma cells. Exp Ther Med.
14:4586–4594. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Qian Y, Song JL, Sun P, Yi R, Liu H, Feng
X, Park KY and Zhao X: Lactobacillus casei strain shirota enhances
the in vitro antiproliferative effect of geniposide in human oral
squamous carcinoma HSC-3 cells. Molecules. 23(1069)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ye J, Li J, Wang X and Li L: Medicinal
supplement genipin induces p53 and Bax-dependent apoptosis in colon
cancer cells. Oncol Lett. 16:2957–2964. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li Z, Zhang TB, Jia DH, Sun WQ, Wang CL,
Gu AZ and Yang XM: Genipin inhibits the growth of human bladder
cancer cells via inactivation of PI3K/Akt signaling. Oncol Lett.
15:2619–2624. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hong M, Lee S, Clayton J, Yake W and Li J:
Genipin suppression of growth and metastasis in hepatocellular
carcinoma through blocking activation of STAT-3. J Exp Clin Cancer
Res. 39(146)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wei M, Wu Y, Liu H and Xie C: Genipin
induces autophagy and suppresses cell growth of oral squamous cell
carcinoma via PI3K/AKT/MTOR pathway. Drug Des Devel Ther.
14:395–405. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee SY, Kim HJ, Oh SC and Lee DH: Genipin
inhibits the invasion and migration of colon cancer cells by the
suppression of HIF-1α accumulation and VEGF expression. Food Chem
Toxicol. 116:70–76. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ko H, Kim JM, Kim SJ, Shim SH, Ha CH and
Chang HI: Induction of apoptosis by genipin inhibits cell
proliferation in AGS human gastric cancer cells via Egr1/p21
signaling pathway. Bioorg Med Chem Lett. 25:4191–4196.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jo MJ, Jeong S, Yun HK, Kim DY, Kim BR,
Kim JL, Na YJ, Park SH, Jeong YA, Kim BG, et al: Genipin induces
mitochondrial dysfunction and apoptosis via downregulation of
Stat3/mcl-1 pathway in gastric cancer. BMC Cancer.
19(739)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hsu HY, Yang JJ, Lin SY and Lin CC:
Comparisons of geniposidic acid and geniposide on antitumor and
radioprotection after sublethal irradiation. Cancer Lett.
113:31–37. 1997.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mulcahy LJ and Thorburn A: Autophagy in
cancer: Moving from understanding mechanism to improving therapy
responses in patients. Cell Death Differ. 27:843–857.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kaminskyy VO and Zhivotovsky B: Free
radicals in cross talk between autophagy and apoptosis. Antioxid
Redox Signal. 21:86–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hochegger H, Takeda S and Hunt T:
Cyclin-dependent kinases and cell-cycle transitions: Does one fit
all? Nat Rev Mol Cell Biol. 9:910–916. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hwang H, Kim C, Kim SM, Kim WS, Choi SH,
Chang IM and Ahn KS: The hydrolyzed products of iridoid glycoside
with β-glucosidase treatment exert anti-proliferative effects
through suppression of STAT3 activation and STAT3-regulated gene
products in several human cancer cells. Pharm Biol. 50:8–17.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yuan J, Najafov A and Py BF: Roles of
caspases in necrotic cell death. Cell. 167:1693–1704.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sethi N and Kang Y: Unravelling the
complexity of metastasis-molecular understanding and targeted
therapies. Nat Rev Cancer. 11:735–748. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dikic I and Elazar Z: Mechanism and
medical implications of mammalian autophagy. Nat Rev Mol Cell Biol.
19:349–364. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Levy JM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Carling D: AMPK signalling in health and
disease. Curr Opin Cell Biol. 45:31–37. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhou YY, Li Y, Jiang WQ and Zhou LF:
MAPK/JNK signalling: A potential autophagy regulation pathway.
Biosci Rep. 35(e00199)2015.PubMed/NCBI View Article : Google Scholar
|