Introduction
Acute respiratory tract infections are the third
leading factor of morbidity and mortality worldwide, and constitute
an enormous economic burden and public health threat worldwide
(1). There are numerous and
diverse viruses, which cause co-infections with other causative
agents, such as fungus, atypical pathogens and other bacteria
(2). Respiratory viruses are
detected more frequently compared with bacteria in adults with
pneumonia (3). Multiple viruses
have been linked to acute respiratory viral infections (ARVI), such
as influenza virus, parainfluenza viruses, respiratory syncytial
virus, rhinovirus and coronaviruses (4). Influenza virus has caused several
pandemics worldwide, and has become one of the most widely
recognized viral infections. More recently, 2019 novel coronavirus
(SARS-CoV-2) has crossed the species barrier and become a global
pandemic. According to the World Health Organization report, Corona
Virus Disease 2019 (COVID-19) has infected >514 million patients
and resulted in >6 million deaths worldwide (as of May 6, 2022).
SARS-CoV-2 can affect multiple organs, which can lead to severe
disease in patients with underlying comorbidities (5). Given the rapid emergence and global
spread, reducing SARS-CoV-2 infection and increasing recovery rate
are critical.
Arbidol (ARB) was developed in Russia and has been
used for >10 years in China for prophylaxis and treatment of
influenza (6). Due to its high
consumption for the prevention and treatment of COVID-19 and some
other viral infections (7), it is
important to reappraise the effect in reducing the risk of
COVID-19(8). As the only available
antiviral drug that targets hemagglutinin (HA) (9), ARB has shown broad-spectrum antiviral
activity to inhibit the replication of multiple viruses (10), and has been reported to have
preventive and therapeutic effects against influenza, COVID-19 or
other ARVI (6).
ARB is recommended by Chinese guidelines (11) as a potential medication against
COVID-19. ARB monotherapy or in combination with other antiviral
drugs are suggested as potential strategies to combat SARS-CoV-2
(12,13). However, the effectiveness of ARB
remains controversial. To the best of our knowledge, systematic
reviews evaluating outcomes of clinical ARB application on ARVI are
lacking. Therefore, it is important to analyze the available data
on the efficacy of ARB and its therapeutic potential in COVID-19.
Herein, the present study conducted a systematic review and
meta-analysis of published studies and clinical trials to assess
the efficacy of ARB on ARVI in order to provide guidance for the
treatment of COVID-19.
Materials and methods
Search strategies
The present systematic review was based on the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
principles. The PubMed (www.ncbi.nlm.nih.gov/pubmed), MedLine, EmBase
(www.embase.com), Web of Science (www.webofknowledge.com), Foreign Medical Literature
Retrieval Service (FMRS) (fmrs.metstr.com) and mdRxiv (www.medrxiv.org) databases were systematically
searched for relevant studies published up to May 6, 2022. Search
terms used were as follows: Arbidol/umifenovir, respiratory viral
infection, novel coronavirus, COVID-19, influenza, SARS (severe
acute respiratory syndrome), Middle East respiratory syndrome and
related words. The references of selected articles were reviewed
for additional studies not retrieved by the initial search.
Inclusion and exclusion criteria
Studies conducted in humans describing the impact of
ARB treatment against RVI were included. In vitro and animal
studies, articles written in languages other than English, review
articles and studies focused on the mechanism of action of drugs
were excluded. Two investigators (JY and HD) independently screened
and extracted the relevant data from the included studies.
Data extraction
Information from selected studies was extracted and
tabulated. The extracted data included the first author, year of
publication, country, study type, characteristics of patients,
treatment plan and outcomes. The primary outcomes included PCR
negative rate on day 7 and 14, PCR negative conversion time, rate
of clinical improvement, time of clinical improvement, rate of
chest computed tomography (CT) absorption and duration of chest CT
absorption. The secondary outcomes included hospital length of
stay, duration of fever, rate of disease progression, adverse
events and mortality.
Risk of bias assessment
Two investigators (YS and XZ) independently assessed
the risks of bias in each included study. For observational studies
the Newcastle-Ottawa scale (NOS) (14) was used, which consists of three
domains: Selection, comparability and outcome. NOS scores of 1-4,
5-7 and 8-9 indicated low, moderate and high quality. Generally,
studies which earned ≥five points were included in the final
analysis. For randomized controlled trials (RCTs), the Cochrane
risk of bias (RoB) tool (15) was
used, which consists of five domains: Selection bias, performance
bias, attrition bias, reporting bias and other biases. The
potential bias was graded as low, unclear or high.
Statistical analysis
The analysis was conducted using the Review Manager
software (version 5.4; Cochrane) (https://training.cochrane.org/online-learning/core-software/revman).
Mean difference (MD) was used for continuous outcomes, and risk
ratio (RR) was used for dichotomous variables. A 95% confidence
interval (CI) was calculated for each study. Statistical
heterogeneity was evaluated using the I-square and χ2
tests, where I-square >50% or P<0.10 were considered to
indicate a statistically significant difference. The random-effects
model was used for studies with significant heterogeneity.
Otherwise, the fixed-effect model was used. Sensitivity analyses
was performed manually by single study elimination method in Review
Manager 5.4 software to verify conclusions of meta-analysis and
explore the possible reasons of heterogeneity. Publication bias was
evaluated using funnel plots. Review Manager software (version 5.3;
Cochrane) was used to statistically analyze all of the data.
Results
Search results
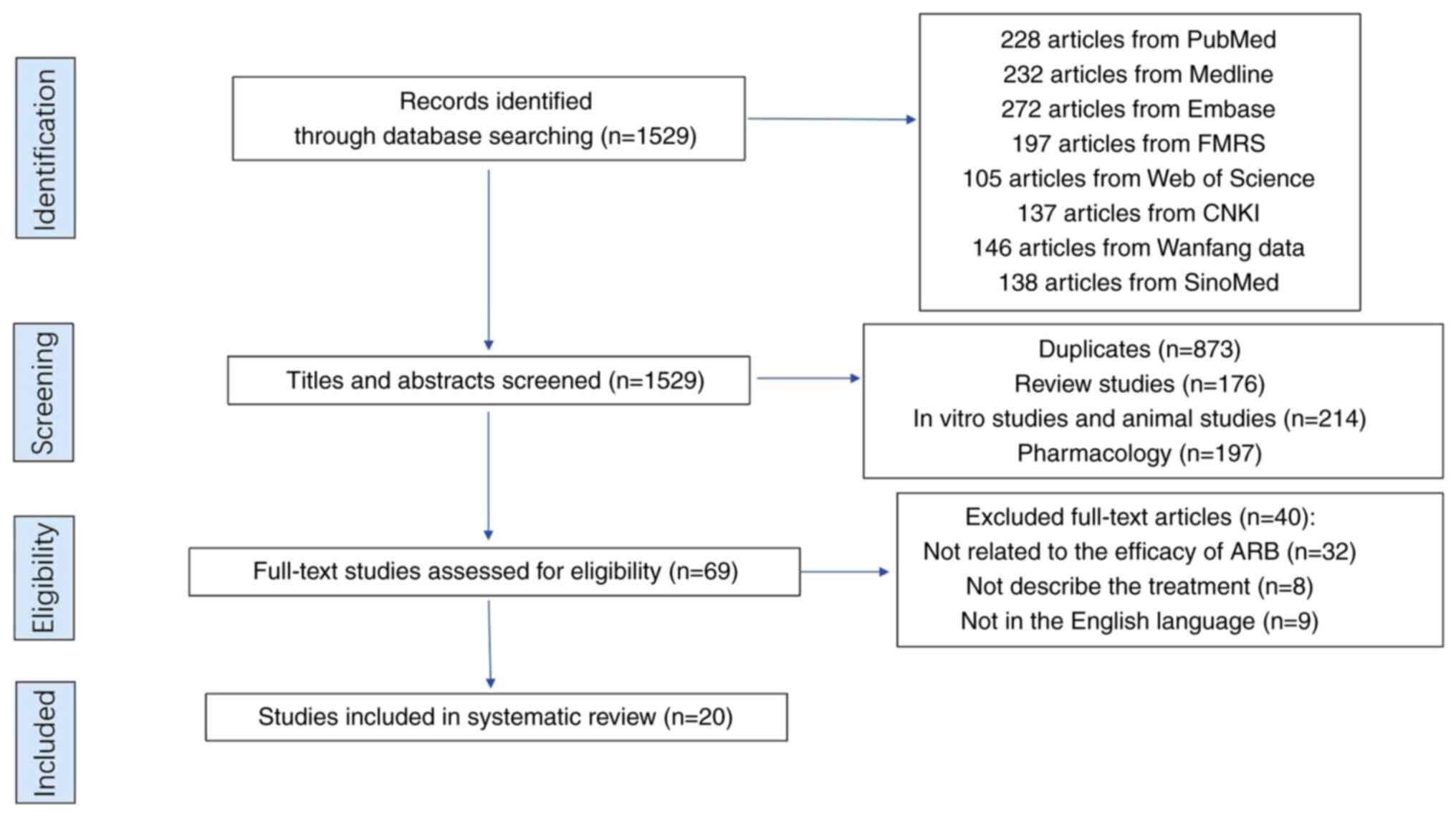
A total of 1,529 articles were retrieved from the
initial search of databases. Of those, 1,500 articles were excluded
due to the following reasons: Duplicates (n=873), review articles
(n=176), in vitro and animal studies (n=214), pharmacology
(n=197), unrelated to ARB (n=32) and were not written in the
English language (n=9) or describe the treatment (n=8). Therefore,
only 20 articles (16-36)
eventually met the inclusion and exclusion criteria, and were
included in the final analysis (Fig.
1). Overall, one study reported therapeutic effect among
patients with influenza during epidemic period, while 19 studies
reported on the therapeutic and prophylaxis effect among patients
with COVID-19. Characteristics of the 20 studies included in the
present review are summarized in Table
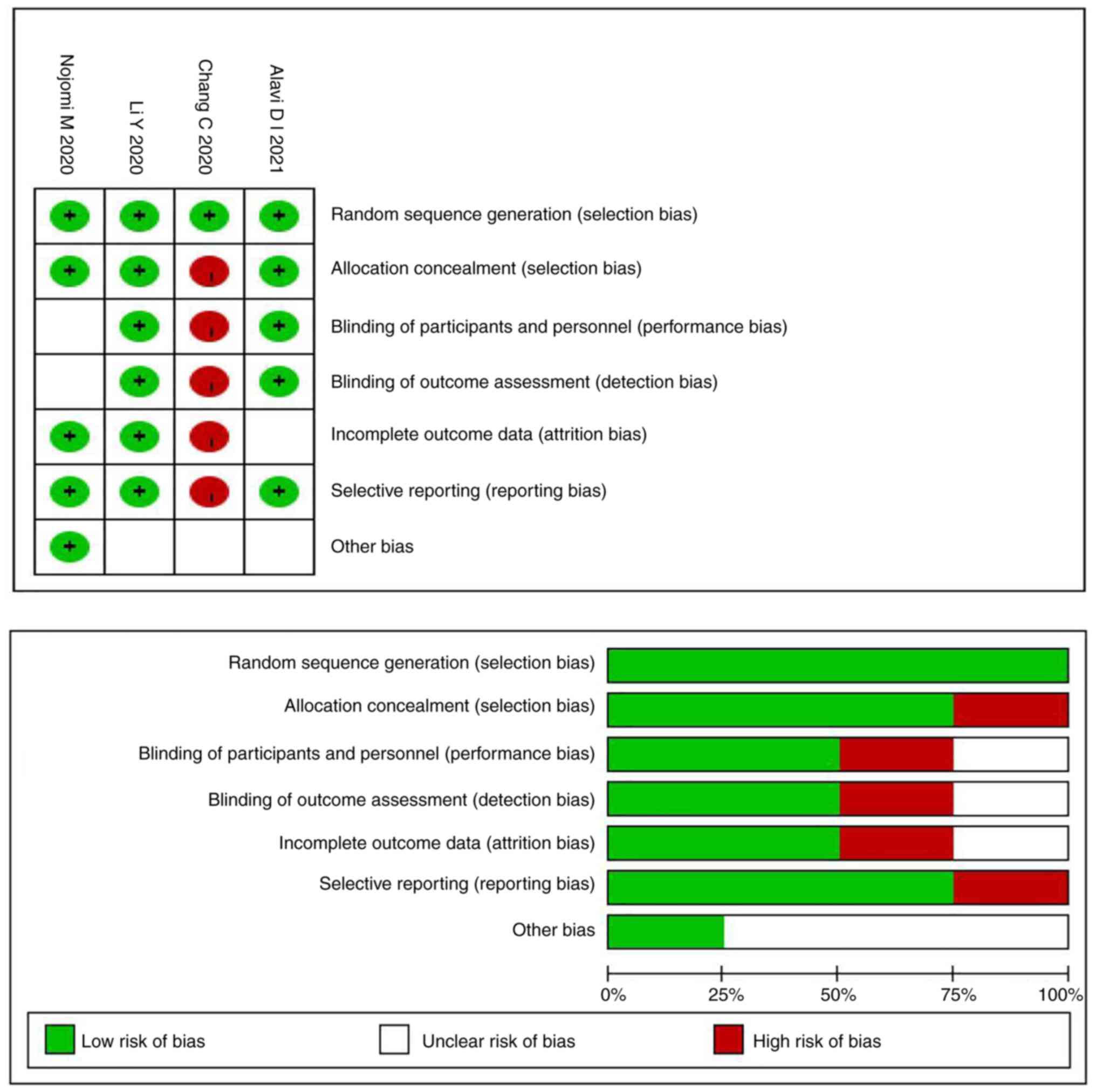
I. Assessment of quality of the results of RoB and NOS were
presented in Table I and Fig. 2. More than half of the
observational studies (n=10) were moderate quality, the main
reasons were being the lack of ascertainment of exposure and
adequacy of follow up of cohorts. The other six studies were high
quality (n=6). All of the included four RCTs had a low risk of bias
for random sequence generation. With regard to other risk of bias,
in the study by Chang et al (20), the staff knew the patient grouping
and did not acquire complete outcome data; therefore, the high risk
of bias was due to allocation concealment, insufficient blinding,
incomplete outcome and selective reporting.
 | Table ICharacteristics of studies included
in the systematic review. |
Table I
Characteristics of studies included
in the systematic review.
| Observational
studies |
|---|
| Author, year | Country | Patients | Treatment plan | Primary
outcome | NOS | (Refs.) |
|---|
| Leneva IA,
2016 | Russia | 442 patients with
influenza | Early antiviral
treatment: | The overall illness
duration | 6 | (16) |
| | | | 55 patients: ARB
(200 mg, qid, po) for 5 days | Duration of main
symptoms, including fever and catarrhal symptoms | | |
| | | | 55 patients:
Oseltamivir (75 mg, bid, po) for 5 days | | | |
| | | | 252 patients: Late
antiviral treatment | | | |
| | | | 48 patients: No
antiviral treatment | | | |
| Zhu Z, 2020 | China | 50 patients with
COVID-19 | 34 patients: LPV/r
(400 mg/100mg, bid, po) for a week | Duration of
fever | 7 | (17) |
| | | | 16 patients: ARB
(0.2 g, tid, po) | PCR negative
conversion time | | |
| Li M, 2021 | China | 62 patients with
COVID-19 | 42 patients: ARB
0.2 g tid po for 10 days | The PCR negative
rates | 8 | (18) |
| | | | 20 patients:
Chloroquine 500 mg bid for 10 days | The length of
hospital stay | | |
| Wang ZL, 2020 | China | 69 patients with
COVID-19 | 36 patients: ARB
(0.4 g, tid, po) for 9 days (median). | Discharge rate | 6 | (21) |
| | | | 33 patients:
Placebo | Mortality | | |
| Chen W, 2020 | China | 62 patients with
COVID-19 | 42 patients: ARB
(0.2 g, tid, po) + symptomatic treatment | The main symptom
improvement | 6 | (23) |
| | | | | The PCR negative
conversion time | | |
| | | | 20 patients:
Symptomatic treatment | Duration of
fever | | |
| | | | | The length of
hospital stay | | |
| Jie X, 2021 | China | 252 patients with
COVID-19 | 228 patients: ARB
200 mg tid po | The rate of
clinical improvement | 8 | (24) |
| | | | 24 patients: Did
not use ARB | | | |
| Gao W, 2020 | China | 220 patients with
COVID-19 | 90 patients: ARB
200 mg tid po for 4-8 days | The main symptom
improvement | 8 | (25) |
| | | | 40 patients: ARB
and other antiviral drugs | The length of
hospital stay | | |
| | | | 45 patients: No
antiviral drugs | The PCR negative
rates | | |
| | | | 45 patients: Other
antiviral drugs | | | |
| Chen N, 2021 | China | 140 patients with
COVID-19 | 79 patients: ARB
0.2 g tid po for 7-10 days | Duration of
fever | 6 | (26) |
| | | | 61 patients: Did
not use ARB | The PCR negative
rates | | |
| | | | | The PCR negative
conversion time | | |
| | | | | The main symptom
improvement | | |
| Deng L, 2020 | China | 33 patients with
COVID-19 | 16 patients: ARB
(200 mg, q8h, po) + LPV/r (400 mg/100 mg, q12h, po); | The PCR negative
rates | 9 | (27) |
| | | | | The rates of
improvement of chest CT | | |
| | | | 17 patients: LPV/r
(400 mg/100 mg, q12h, po); | | | |
| | | | All patients
received supportive therapy | | | |
| Xu P, 2020 | China | 141 patients with
COVID-19 | 71 patients: ARB
(200 mg, po, tid for 7-10 days) + IFN-α2b | The PCR negative
conversion time | 7 | (28) |
| | | | | The main symptom
improvement | | |
| | | | 70 patients:
IFN-α2b inhale, bid for 10-14 days) | Duration of CT
absorption | | |
| | | | | Duration of
fever | | |
| Wei S, 2021 | China | 132 patients with
COVID-19 | 72 patients: ARB
200 mg tid po for 10 days | The PCR negative
conversion time | 5 | (29) |
| | | | 82 patients: Did
not use ARB | Duration of CT
absorption | | |
| Lian N, 2020 | China | 81 patients with
COVID-19 | 45 patients: ARB
(0.2 g, tid, po) | Hospital length of
stay | 7 | (30) |
| | | | 36 patients:
Control | The PCR negative
rates | | |
| | | | | The PCR negative
conversion time | | |
| Lan X, 2020 | China | 73 patients with
COVID-19 | 34 cases: LPV/r 400
mg/100 mg, bid | Hospital length of
stay | 8 | (33) |
| | | | 39 cases: LPV/r 400
mg/100 mg bid + ARB 200 mg tid | Duration of
fever | | |
| | | | | The PCR negative
rates | | |
| | | | | The rate of disease
progression | | |
| | | | | Mortality | | |
| | | | | The main symptom
improvement | | |
| | | | | Duration of CT
absorption | | |
| Chen X, 2020 | China | 280 patients with
COVID-19 | 37 patients:
ARB | Hospital length of
stay | 9 | (34) |
| | | | 121 patients: Did
not use antiviral | The PCR negative
conversion time | | |
| | | | 17 patients:
Chloroquine | | | |
| | | | 13 patients:
Oseltamivir | | | |
| | | | 60 patients:
LPv/r | | | |
| | | | 16 patients: Lpv/r
+ ARB | | | |
| | | | 5 patients:
Chloroquine + ARB | | | |
| | | | 11 patients:
Oseltamivir + ARB | | | |
| Zhang JN, 2020 | China | 66 family members
and 124 health care workers had close exposure with COVID-19
patients | 1st cohort: | The infection risk
of the novel coronavirus in hospital and family settings | 7 | (35) |
| | | | 45 family members:
ARB PEP (0.2 g, po, tid for 5-14 days) | | | |
| | | | 21 family members:
Did not use ARB | | | |
| | | | 2nd cohort: | | | |
| | | | 55 health care
workers: ARB PEP (0.2 g, po, tid for 5.14 days) | | | |
| | | | | | | |
| | | | 69 health care
workers: did not use ARB | | | |
| Yang C, 2020 | China | 82 patients with
COVID-19 | 82 cases: Infected
group | The cumulative
uninfected rate | 6 | (36) |
| | | | 19 patients: ARB
600 mg qd po | The hospitalization
rate | | |
| | | | 82 cases:
Uninfected group | | | |
| | | | 48 patients: ARB
200 mg qd po | | | |
| Nojomi M, 2020 | China | 100 patients with
COVID-19 | 50 cases:
Hydroxychloroquine (400 mg bid) on first day followed by LPV/r
bid | The PCR negative
conversion time | RoB | (19) |
| | | | | The length of
hospital stay | | |
| | | | 50 cases:
Hydroxychloroquine (400 mg bid) on first day followed by ARB (200
mg tid) | Duration of
fever | | |
| | | | | Duration of CT
absorption | | |
| | | | | Mortality | | |
| Chang C, 2020 | China | 240 patients with
COVID-19 | 120 patients: ARB
(200 mg tid) | The rate of
clinical improvement | RoB | (20) |
| | | | 120 patients:
Favipiravir (1,600 mg bid first day followed by 600 mg bid) for 10
days | The rate of disease
progression | | |
| | | | | Mortality | | |
| Alavi DI, 2021 | Iran | 101 patients with
COVID-19 | 51 patients: LPV/r
(400 mg/100 mg bid for 10-14 days) + hydroxychloroquine (400 mg
single dose) + IFNβ1a (Subcutaneous injections of 44 µg (12,000 IU)
on days 1, 3, 5) + ARB (200 mg tid for 10 days) | Hospital length of
stay | RoB | (22) |
| | | | | The rate of disease
progression | | |
| | | | | Mortality | | |
| | | | | The main symptom
improvement | | |
| | | | 50 patients: LPV/r
(same dose) + hydroxychloroquine (same dose) + IFNβ1a (same
dose) | | | |
| Li Y, 2020 | China | 86 patients with
COVID-19 | 34 patients: LPV/r
400/100 mg bid po for 7-14d | The PCR negative
rates | RoB | (32) |
| | | | 35 patients: ARB
200 mg tid po for 7-14d | The PCR negative
conversion time | | |
| | | | 17 patients: No
antiviral therapy | The rate of disease
progression | | |
| | | | | The main symptom
improvement | | |
| | | | | Duration of CT
absorption | | |
Efficacy of ARB in influenza
According to the inclusion and exclusion criteria,
there was only one article among patients with influenza or acute
respiratory tract infection that was included in the final study.
In the retrospective study performed on 442 patients with influenza
(16), the patients treated with
oseltamivir or ARB had significantly lower chances (0 and 0.3%,
respectively) of developing pneumonia compared with patients who
did not receive antiviral therapy (23.7%; P<0.001).
Therapeutic effects of ARB in
COVID-19
Numerous studies have been conducted on ARB effect
against COVID-19. The 17 available (17-34)
articles were mostly from China. A total of four RCTs and 13
observational studies reported clinical outcome data of therapeutic
effects on ARB treatment. Overall, sixteen studies were from China
and one study was from Iran.
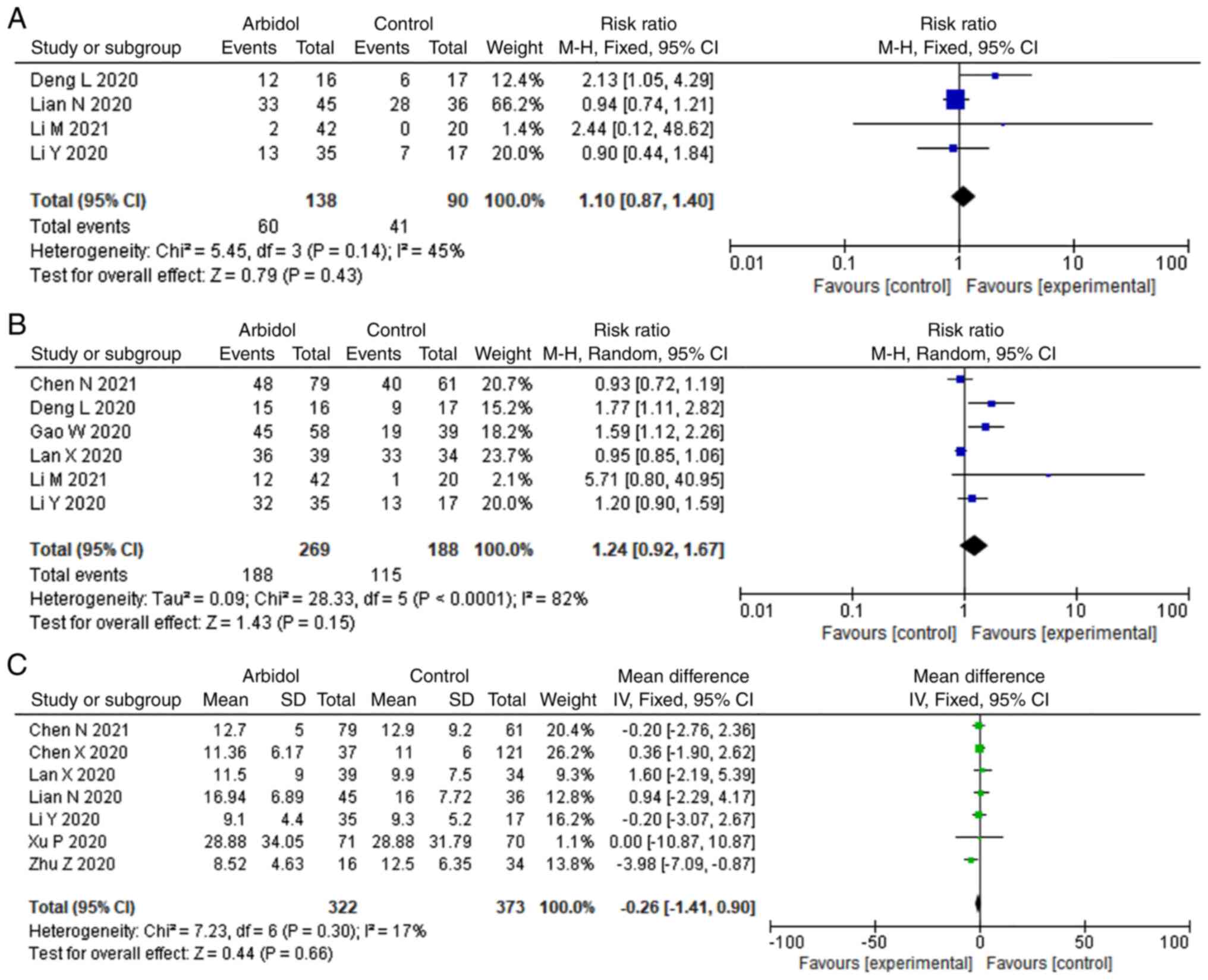
The PCR negative conversion
There were 4 and 6 studies that reported PCR
negative rate on days 7 and 14, respectively. As depicted in
Fig. 3, ARB was not significantly
associated with higher negative rate of PCR on day 7 (RR, 1.1; 95%
CI, 0.87-1.40; Fig. 3A) and day 14
(RR, 1.24; 95% CI, 0.92-1.67; Fig.
3B). A total of seven studies reported PCR negative conversion
time. No significant difference was observed for PCR negative
conversion time (MD, -0.26; 95% CI, -1.41-0.90; Fig. 3C).
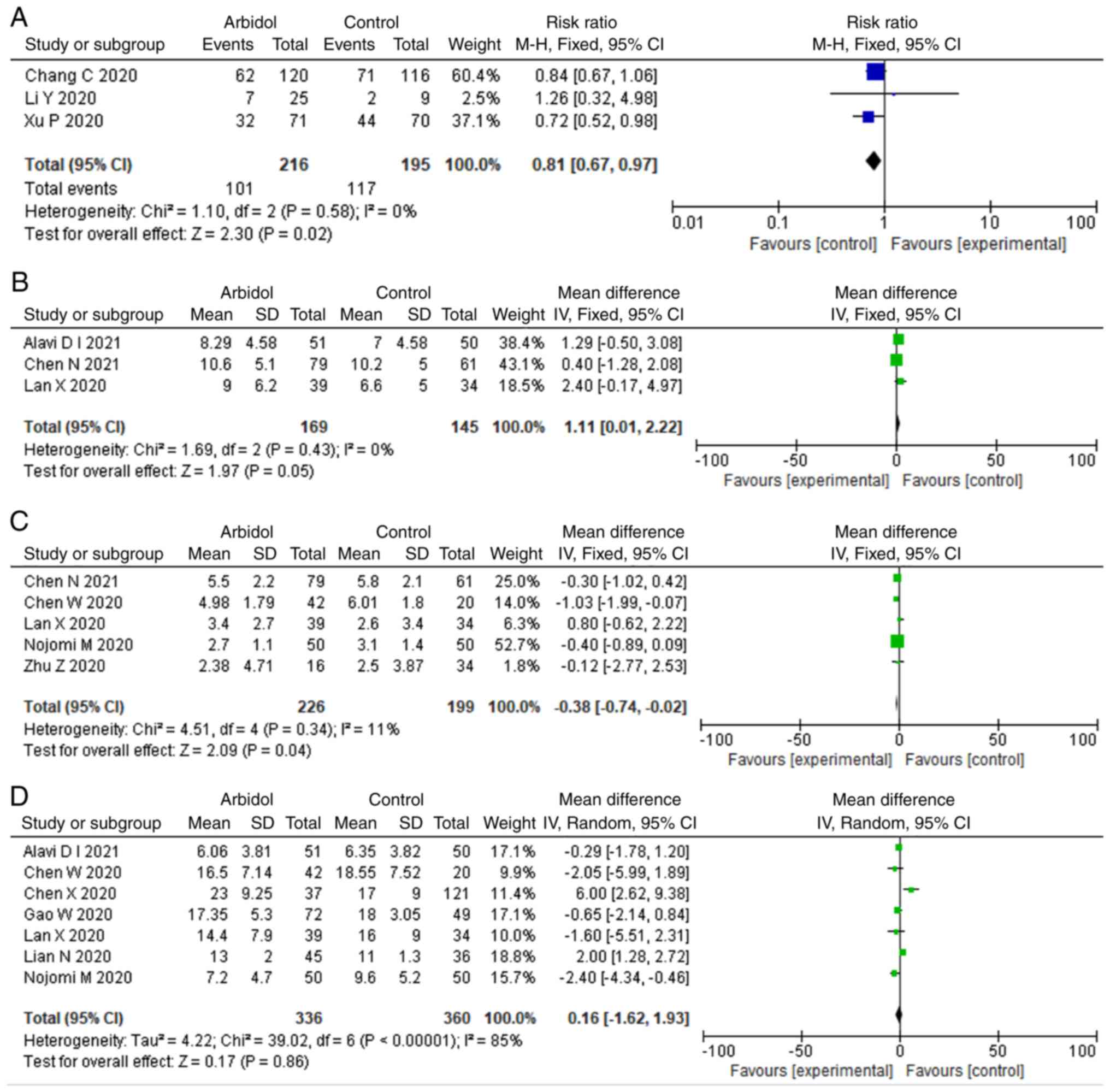
Main symptom improvement
There were three studies that reported the rate and
time of clinical improvement, respectively. ARB demonstrated
significant difference in the rate of clinical improvement (RR,
0.81; 95% CI, 0.67-0.97; Fig. 4A),
but this difference was not significant in the time of clinical
improvement (MD, 1.11; 95% CI, 0.01-2.22; Fig. 4B). A total of seven studies
reported the duration of fever that is the most representative main
symptom of COVID-19. ARB was associated with shorter duration of
fever (MD, -0.38; 95% CI, -0.74- -0.02; Fig. 4C). In addition, five studies
reported the hospital length of stay. ARB showed no significant
difference in terms of hospital stay (MD, 0.16; 95% CI, -1.62-1.93;
Fig. 4D).
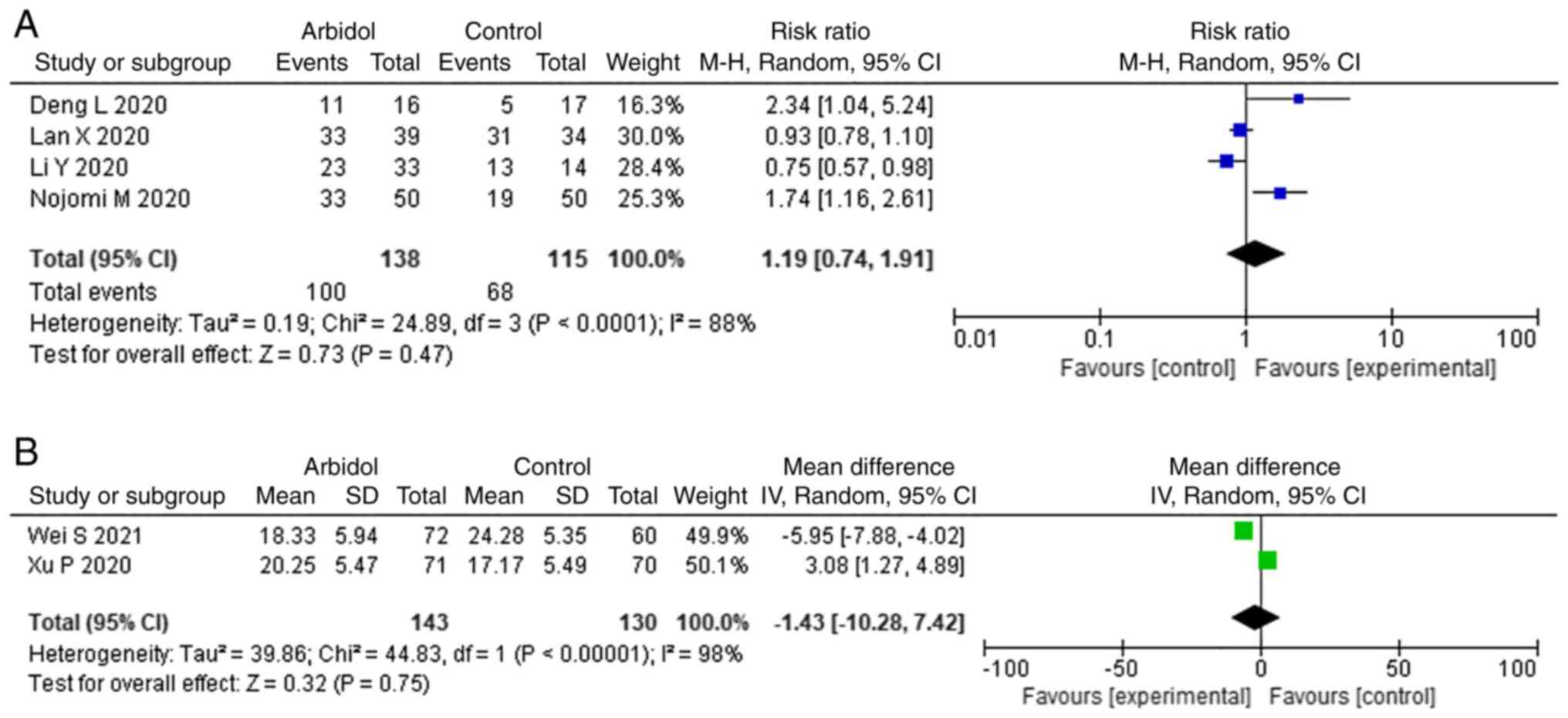
Chest CT absorption
There were four and two studies that reported the
rate and duration of chest CT absorption, respectively. No
significant difference was observed between ARB and non-ARB group
in rate of improvement on chest CT (RR, 1.19; 95% CI, 0.74-1.91;
Fig. 5A) and duration of CT
absorption (MD, -1.43; 95% CI, -10.28-7.42; Fig. 5B).
Preventive effects of ARB
In addition to therapeutic effect, clinical studies
recommended ARB for prophylaxis. The two studies (35,36)
reported prophylaxis effects on ARB treatment. The retrospective
study, conducted on 66 family members and 126 healthcare workers
who were exposed to confirmed patients with COVID-19, revealed that
ARB post-exposure prophylaxis was a protective factor against the
development of COVID-19 (P<0.01) (35). Another study (36) reported that the cumulative
uninfected rate of healthcare professionals in the ARB group was
significantly higher compared with that of individuals in the
non-ARB group; and the hospitalization rate was significantly
associated with age and oral ARB administration.
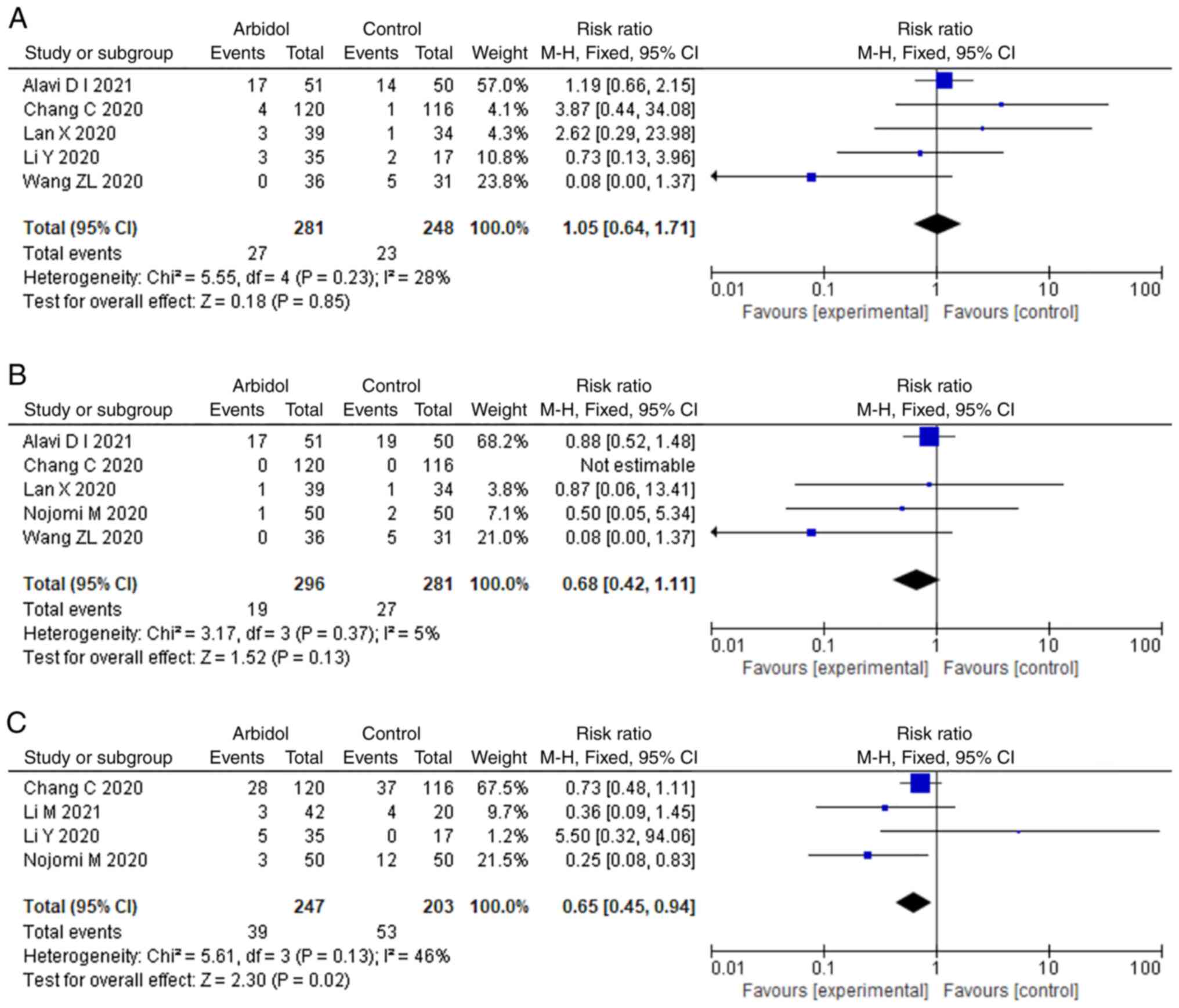
Safety of ARB
There were eight and five studies that reported the
rate of disease progression and mortality, respectively. As shown
in Fig. 6, no significant
difference was observed in terms of disease progression (RR, 1.05;
95% CI, 0.64-1.71; Fig. 6A) and
mortality (RR, 0.68; 95% CI, 0.42-1.11; Fig. 6B). A total of four studies reported
the adverse events. ARB showed significant difference in terms of
adverse events (RR, 0.65; 95% CI, 0.45-0.94; Fig. 6C).
Publication bias and sensitivity
analysis
Given that only a few studies (n<10) were
included in each outcome, funnel plots to evaluate publication bias
might have limited. Thus, publication bias was not analyzed
further. For outcomes with high heterogeneity, sensitivity analysis
was performed to verify conclusions of meta-analysis. After
excluding each single study, sensitivity analysis had similar
results (I2 67-91%; P>0.05) which did not change the
significance in outcomes. Generally, the conclusion of the present
study was relatively stable.
Discussion
The present study described and summarized the
available published literature on the outcomes of ARB on ARVI.
Although ARB has shown inhibitory activity against various viruses,
evidence for clinical beneficial effects on ARVI mainly focused on
patients suffering from influenza or COVID-19(6). The present study reviewed current
clinical studies on ARB and discussed whether patients would
benefit from this antiviral drug.
Since the emergence of the COVID-19 pandemic, the
antiviral treatments remain limited. Some FDA-approved drugs (even
originally non-antiviral) could have a potential benefit against
SARS-CoV-2. Jeon et al (37) and Weston et al (38) reported >20 potential antiviral
drug candidates inhibit SARS-CoV-2 in vitro. An RCT
(39) that enrolled 379 patients
with severe COVID-19 revealed that hydrocortisone has a 80-93%
probability of superiority compared with no hydrocortisone. Some
other drugs (Favipiravir, Remedsivir) have also been recommended,
although there is insufficient evidence to support their
effectiveness (40). Repurposing
and reappraising existing antiviral drugs is the most appropriate
recommendation, which deserves further consideration. ARB has
improved efficacy and advantages over other commonly used antiviral
drugs. M2 ion channel blockers (amantadine and rimantadine) may
lead to significant adverse events, and are not recommended for
treating influenza due to drug resistance (41). Neuraminidase inhibitors (NA)
(zanamivir and oseltamivir) are more expensive compared with ARB
(42) and ineffective in
inhibiting SARS-CoV-2(41). Given
the shortcomings of these currently approved compounds and the
potential risk of antiviral resistance, there is an urgent need for
developing new antiviral drugs (43). Leneva et al (16) reported that both ARB and
oseltamivir are efficient at reducing the duration of overall
illness and main influenza symptoms. Another study performed on
patients diagnosed with COVID-19(17) suggested that ARB monotherapy may be
superior to lopinavir/ritonavir (LPV/r). Therefore, ARB could be a
suitable candidate to combat COVID-19 and other respiratory viral
infections.
The majority of studies have revealed that ARB
possesses a dual pharmacological action, specific antiviral effect
and anti-inflammatory efficacy (6,44,45).
ARB has been regarded as the pioneer of HA-targeted drugs. By
inhibiting HA located on the surface of influenza virus, ARB can
specifically inhibit virus attachment to host cells, and block
viral fusion and viral replication. A recent study (10) demonstrated sequence and structural
similarities between influenza virus (H3N2) HA protein and
SARS-CoV-2 spike glycoprotein, which indicates how the influenza
virus drug ARB can be a potential drug for SARS-CoV-2 infections.
Another study (41) also revealed
that ARB interferes with SARS-CoV-2 binding and intracellular
vesicle trafficking. In addition, ARB inhibits the release of
several pro-inflammatory cytokines (IL-6, IL-8, IL-10 and TNF-a) in
serum induced by influenza (46).
The inhibitory effect of ARB on sudden cytokine storm in patients
with COVID-19 has also been suggested (47). Furthermore, ARB can influence
non-specific defense factors, induce interferon and specifically
activate phagocytes (48). A study
based on the dual pharmacological action, ARB could therefore
constitute an alternative drug for the prophylaxis and treatment of
influenza, COVID-19 and other respiratory viruses.
In the present study, available clinical data on the
use of ARB against SARS-CoV-2 were collected from limited studies.
The majority of studies reported that ARB treatment shows a
tendency to minimize the duration of symptoms, diminish SARS-CoV-2
replication, decrease the mortality rate and improve the discharge
rate (21,23,27).
Seasonal and post-exposure prophylaxis with ARB can reduce the
infection risk of SARS-CoV-2 and significantly prevent transmission
(35). Yang et al (36) also concluded that prophylactic oral
ARB is associated with a lower incidence of SARS-CoV-2 infection
but not hospitalization rate among healthcare professionals.
Although the treatment of ARB alone may be beneficial for numerous
viral infections, combination therapy with other drugs with
different antiviral mechanisms and resistance profiles may be able
to produce the desired results in the fight against
COVID-19(49). The most commonly
used combination is ARB and LPV/r. Two studies (23,27)
have demonstrated that patients with COVID-19 show significant
improvement in pneumonia-associated symptoms and apparent favorable
clinical response with ARB+LPV/r. Another study (28) infers that ARB+IFN-2b therapy can be
used as an effective method to improve COVID-19 pneumonia of mild
patients, although it could not accelerate viral clearance. Hence,
ARB may show an improved efficacy if combined with other antiviral
drugs. However, ARB effect on COVID-19 remains controversial.
Several studies (30,32) infer that ARB presents little
benefit for improving the symptoms of patients or reducing the
negative conversion time of SARS-CoV-2 nucleic acid. Furthermore, a
timely initiation of antiviral treatment is likely an important
factor that may be able to influence the prognosis of ARVI
(50). Early antiviral treatment
within 48 h of symptom onset shows a shorter overall illness
compared with delayed antiviral treatment (16). Another study also found that early
prescription of ARB in the acute stage of influenza can majorly
reduce the duration, severity of all symptoms and minimize the risk
of development of complications (35).
ARB is well tolerated and safe in the treatment of
influenza, COVID-19 and other ARVI (51). Most studies did not find apparent
side effects in the ARB treatment group (30,31).
The adverse events reported were gastrointestinal symptoms,
increased transaminase, moderate thirst and less sleep (23). Xu et al (28) reported that 18.8% of patients
treated with ARB demonstrate mild nausea and stomachache, but all
patients can tolerate this without giving up treatment. However,
not all studies have arrived at a consistent conclusion on ARB
safety. Deng et al (27)
observed that 68.7% of patients demonstrate elevated levels of
bilirubin and 43.7% patients demonstrate digestive issues, such as
mild diarrhea and nausea (P>0.05), but no premature
discontinuation secondary to adverse effects was observed. Jiang
et al (52) demonstrated
that LPV/r can significantly inhibit the metabolism of ARB, hence
the combination treatment of LPV/r and ARB was an independent risk
factor for liver injury. Another study (28) revealed that adverse reactions occur
more frequently in groups receiving LPV/r or ARB compared with the
control group (P<0.05). Therefore, the adverse reactions of the
antiviral medication should be carefully monitored.
The present review aimed to summarize all relevant
published clinical data updated until May 6, 2022. The results
suggested significant potential for using ARB in the prophylaxis
and treatment of influenza and the target of future COVID-19
studies. However, available clinical data on the ARB treatment for
COVID-19 comes from limited studies. The designs of the
observational studies were inconclusive; the RCT data was lacking,
and the quality evidence of the case series reports was very low.
These studies were heterogeneous and thus a definitive conclusion
that ARB was beneficial against COVID-19 could not be established.
Many of the patients underwent multiple concurrent and
comprehensive treatments; so it is not known whether the clinical
benefit was from ARB or other treatments.
In summary, the HA inhibitor ARB has been
extensively used to combat influenza. Potential associations were
investigated, and the majority of studies reported ARB efficacy.
Based on the experience from influenza therapy, ARB could be a
potential treatment for SARS-CoV-2. However, there is no consensus
on the ARB therapy in ARVI caused by coronaviruses. It is still
uncertain whether ARB improves clinical outcomes of COVID-19.
Hence, repurposing existing antiviral drugs against COVID-19
deserves further evaluation and clinical verification. High quality
evidence is needed to assess the benefits of ARB in treating
COVID-19 to improve clinical and programmatic decisions.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Science and Technology
Planning Project of Sichuan Province (grant no. 2019YFS0309) and
Cadre Health Care Project of Sichuan Province (grant no.
2018-224).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
TF, JG and RT were involved in the design, analysis
and manuscript writing. YS and XZ performed statistical analysis
and assessed the quality of the study. JY and HD extracted and
analyzed the data. TF and RT confirm the authenticity of all the
raw data. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Welte T and Köhnlein T: Global and local
epidemiology of community-acquired pneumonia: The experience of the
CAPNETZ network. Semin Respir Crit Care Med. 30:127–135.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu YG, Tang XD, Lu YT, Zhang J and Qu JM:
Contemporary situation of community-acquired pneumonia in China: A
systematic review. J Transl Int Med. 6:26–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jain S, Self WH, Wunderink RG, Fakhran S,
Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM,
et al: Community-acquired pneumonia requiring hospitalization among
U.S. Adults. N Engl J Med. 373:415–427. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shi T, Arnott A, Semogas I, Falsey AR,
Openshaw P, Wedzicha JA, Campbell H, Nair H and Investigators R:
The etiological role of common respiratory viruses in acute
respiratory infections in older adults: A systematic review and
meta-analysis. J Infect Dis. 222:S563–S569. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Armentano GM and Carneiro-Ramos MS: Effect
of COVID-19 on cardiorenal axis: Known or unknown universe? Braz J
Med Biol Res. 55(e11932)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Blaising J, Polyak SJ and Pécheur EI:
Arbidol as a broad-spectrum antiviral: An update. Antiviral Res.
107:84–94. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ul'yanovskii NV, Kosyakov DS, Sypalov SA,
Varsegov IS, Shavrina IS and Lebedev AT: Antiviral drug Umifenovir
(Arbidol) in municipal wastewater during the COVID-19 pandemic:
Estimated levels and transformation. Sci Total Environ.
805(150380)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McKee DL, Sternberg A, Stange U, Laufer S
and Naujokat C: Candidate drugs against SARS-CoV-2 and COVID-19.
Pharmacol Res. 157(104859)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kadam RU and Wilson IA: Structural basis
of influenza virus fusion inhibition by the antiviral drug Arbidol.
Proc Natl Acad Sci USA. 114:206–214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vankadari N: Arbidol: A potential
antiviral drug for the treatment of SARS-CoV-2 by blocking
trimerization of the spike glycoprotein. Int J Antimicrob Agents.
56(105998)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
National Health Commission of the People's
Republic of China. Diagnosis and treatment plan for COVID-19 (trial
version 8 revision). Chin J Clin Infect Dis. 14:81–88. 2021.
|
|
12
|
Abdelrahman Z, Liu Q, Jiang S, Li M, Sun
Q, Zhang Y and Wang X: Evaluation of the current therapeutic
approaches for COVID-19: A systematic review and a meta-analysis.
Front Pharmacol. 12(607408)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jomah S, Asdaq SMB and Al-Yamani MJ:
Clinical efficacy of antivirals against novel coronavirus
(COVID-19): A review. J Infect Public Health. 13:1187–1195.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Higgins JP, Altman DG, Gøtzsche PCM, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Leneva IA, Burtseva EI, Yatsyshina SB,
Fedyakina IT, Kirillova ES, Selkova EP, Osipova E and Maleev VV:
Virus susceptibility and clinical effectiveness of anti-influenza
drugs during the 2010-2011 influenza season in Russia. Int J Infect
Dis. 43:77–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T,
Lu J and Xue Y: Arbidol monotherapy is superior to
lopinavir/ritonavir in treating COVID-19. J Infect. 81:e21–e23.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li M, Yu T, Zhu J, Wang Y, Yang Y, Zhao K,
Yi Y and He J, Li C and He J: Comparison of the antiviral effect of
Arbidol and Chloroquine in treating COVID-19. Ann Palliat Med.
10:3307–3312. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nojomi M, Yassin Z, Keyvani H, Makiani MJ,
Roham M, Laali A, Dehghan N, Navaei M and Ranjbar M: Effect of
arbidol (Umifenovir) on COVID-19: A randomized controlled trial.
BMC Infect Dis. 20(954)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen C, Zhang YI, Huang J, Yin P, Cheng Z,
Wu J, Chen S, Zhang Y, Chen BO, Lu M, et al: Favipiravir versus
Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv
2020.03.17.20037432.
|
|
21
|
Wang ZL, Yang B, Li Q, Wen L and Zhang R:
Clinical features of 69 cases with coronavirus disease 2019 in
Wuhan, China. Clin Infect Dis. 71:769–777. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Darazam IA, Shokouhi S, Mardani M,
Pourhoseingholi MA, Rabiei MM, Hatami F, Shabani M, Moradi O,
Gharehbagh FJ, Irvani SSN, et al: Umifenovir in hospitalized
moderate to severe COVID-19 patients: A randomized clinical trial.
Int Immunopharmacol. 99(107969)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen W, Yao M, Fang Z, Lv X, Deng M and Wu
Z: A study on clinical effect of Arbidol combined with adjuvant
therapy on COVID-19. J Med Virol. 92:2702–2708. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jie X, Hongmei Y, Ping F, Kuikui Z, Bohan
Y and Rui M: Beneficial effect of Arbidol in the management of
COVID-19 infection. Aging (Albany NY). 13:9253–9264.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao W, Chen S, Wang K, Chen R, Guo Q, Lu
J, Wu X, He Y, Yan Q, Wang S, et al: Clinical features and efficacy
of antiviral drug, Arbidol in 220 nonemergency COVID-19 patients
from East-West-Lake Shelter Hospital in Wuhan: A retrospective case
series. Virol J. 17(162)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen N, Wang X, Zhang S, Lin R and Jiang
Y: Efficacy analysis of Arbidol treatment in patients with 2019
novel coronavirus pneumonia: A retrospective cohort study. Ann
Palliat Med. 10:10626–10632. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Deng L, Li C, Zeng Q, Liu X, Li X, Zhang
H, Hong Z and Xia J: Arbidol combined with LPV/r versus LPV/r alone
against corona virus disease 2019: A retrospective cohort study. J
Infect. 81:e1–e5. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu P, Huang J, Fan Z, Huang W, Qi M, Lin
X, Song W and Yi L: Arbidol/IFN-α2b therapy for patients with
corona virus disease 2019: A retrospective multicenter cohort
study. Microbes Infect. 22:200–205. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wei S, Xu S and Pan YH: Efficacy of
arbidol in COVID-19 patients: A retrospective study. World J Clin
Cases. 9:7350–7357. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lian N, Xie H, Lin S, Huang J, Zhao J and
Lin Q: Umifenovir treatment is not associated with improved
outcomes in patients with coronavirus disease 2019: A retrospective
study. Clin Microbiol Infect. 26:917–921. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu MY, Wang S, Yao WF, Wu HZ, Meng SN and
Wei MJ: Pharmacokinetic properties and bioequivalence of two
formulations of arbidol: An open-label, single-dose,
randomized-sequence, two-period crossover study in healthy Chinese
male volunteers. Clin Ther. 31:784–792. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y,
Mo X, Wang J, Wang Y, Peng P, et al: Efficacy and safety of
lopinavir/ritonavir or arbidol in adult patients with mild/moderate
COVID-19: An exploratory randomized controlled trial. Med (N Y).
1:105–113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lan X, Shao C, Zeng X, Wu Z and Xu Y:
Lopinavir-ritonavir alone or combined with arbidol in the treatment
of 73 hospitalized patients with COVID-19: A pilot retrospective
study. Int J Clin Pharmacol Ther. 59:378–385. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen X, Zhu B, Hong W, Zeng J, He X, Chen
J, Zheng H, Qiu S, Deng Y, Chan JCN, et al: Associations of
clinical characteristics and treatment regimens with the duration
of viral RNA shedding in patients with COVID-19. Int J Infect Dis.
98:252–260. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang JN, Wang WJ, Peng B, Peng W, Zhang
YS, Wang YL, Wan Y, Chang J, Mao L, Miao XP, et al: Potential of
arbidol for post-exposure prophylaxis of COVID-19 transmission: A
preliminary report of a retrospective cohort study. Curr Med Sci.
40:480–485. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang C, Ke C, Yue D, Li W, Hu Z, Liu W, Hu
S, Wang S and Liu J: Effectiveness of Arbidol for COVID-19
prevention in health professionals. Front Public Health.
8(249)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jeon S, Ko M, Lee J, Choi I, Byun SY, Park
S, Shum D and Kim S: Identification of antiviral drug candidates
against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents
Chemother. 64:e00819–e00820. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Weston S, Coleman CM, Haupt R, Logue J,
Matthews K, Li Y, Reyes HM, Weiss SR and Frieman MB: Broad
anti-coronavirus activity of food and drug administration-approved
drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J Virol.
94:e01218–e01220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Angus DC, Derde L, Al-Beidh F, Annane D,
Arabi Y, Beane A, van Bentum-Puijk W, Berry L, Bhimani Z, Bonten M,
et al: Effect of hydrocortisone on mortality and organ support in
patients with severe COVID-19: The REMAP-CAP COVID-19
corticosteroid domain randomized clinical trial. JAMA.
324:1317–1329. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Şimşek-Yavuz S and Çelikyurt FI: An update
of anti-viral treatment of COVID-19. Turk J Med Sci. 51:3372–3390.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H,
Li Y, Zhao L, Li W, Sun X, et al: The anti-influenza virus drug,
arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell
Discov. 6(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Boriskin YS, Leneva IA, Pécheur EI and
Polyak SJ: Arbidol: A broad-spectrum antiviral compound that blocks
viral fusion. Curr Med Chem. 15:997–1005. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Behzadi MA and Leyva-Grado VH: Overview of
current therapeutics and novel candidates against influenza,
respiratory syncytial virus, and middle east respiratory syndrome
coronavirus infections. Front Microbiol. 10(1327)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Q, Zhou YH and Yang ZQ: The cytokine
storm of severe influenza and development of immunomodulatory
therapy. Cell Mol Immunol. 13:3–10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang M, Wu T, Zuo Z, You Y, Yang X, Pan L,
Hu Y, Luo X, Jiang L, Xia Z and Deng M: Evaluation of current
medical approaches for COVID-19: A systematic review and
meta-analysis. BMJ Support Palliat Care. 11:45–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang Y, Ding Y, Yang C, Li R, Du Q, Hao Y,
Li Z, Jiang H, Zhao J, Chen Q, et al: Inhibition of the infectivity
and inflammatory response of influenza virus by Arbidol
hydrochloride in vitro and in vivo (mice and ferret). Biomed
Pharmacother. 91:393–401. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li H, Liu R, Zhang R, Zhang S, Wei Y,
Zhang L, Zhou H and Yang C: Protective effect of arbidol against
pulmonary fibrosis and sepsis in mice. Front Pharmacol.
11(607075)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Silin DS, Lyubomska OV, Ershov FI, Frolov
VM and Kutsyna GA: Synthetic and natural immunomodulators acting as
interferon inducers. Curr Pharm Des. 15:1238–1247. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Song Y, Zhang M, Yin L, Wang K, Zhou Y,
Zhou M and Lu Y: COVID-19 treatment: Close to a cure? A rapid
review of pharmacotherapies for the novel coronavirus (SARS-CoV-2).
Int J Antimicrob Agents. 56(106080)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis
RF, Chen JIP, Gutierrez RA, Gwee SXW, Chua PEY, Yang Q, et al:
Potential rapid diagnostics, vaccine and therapeutics for 2019
novel coronavirus (2019-nCoV): A systematic review. J Clin Med.
9(623)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Guo YZ, Xu KJ, Li YT, Fu JD, Xu M, Yu L,
Sheng JF and Zhu B: Safety of protease inhibitors and Arbidol for
SARS-CoV-2 pneumonia in Zhejiang Province, China. J Zhejiang Univ
Sci B. 21:948–954. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Jiang S, Wang R, Li L, Hong D, Ru R, Rao
Y, Miao J, Chen N, Wu X, Ye Z, et al: Liver injury in critically
Ill and non-critically Ill COVID-19 patients: A multicenter,
retrospective, observational study. Front Med (Lausanne).
7(347)2020.PubMed/NCBI View Article : Google Scholar
|