Introduction
The three most common cancers worldwide are breast,
lung and colon, of which, breast cancer is the most prevalent
malignant tumor in women (1). In
2012, ~1.7 million individuals were diagnosed with breast cancer
worldwide, and ~500,000 individuals succumbed to the disease
(2). Global cancer statistics in
2018 showed ~2.1 million new female breast cancer cases worldwide,
accounting for nearly a quarter of the total number of women with
cancer (3). However, the
mechanisms underlying breast cancer cell migration, invasion and
metastasis are still poorly understood. Targeted therapy has become
a popular research field, and has demonstrated efficacy in breast
cancer treatment; for example, trastuzumab combined with paclitaxel
after doxorubicin and cyclophosphamide treatment improves outcomes
among women with surgically removed HER2-positive breast cancer
(4). Therefore, it is imperative
to seek novel molecular targeted therapies of breast cancer at the
genomic level and to dissect the mechanisms underlying breast
cancer cell invasion and metastasis.
Prenylated rab acceptor 1 domain family member 2
(PRAF2), originally known as JM4, is considered to be a novel
endoplasmic reticulum (ER) protein involved in protein transport
and vesicle transport from ER to Golgi (5-7).
The PRAF2 gene encodes a 178-residue protein, with a molecular
weight of 19.3 kDa, an isoelectric point of 9.21 and a net charge
of +7.4 at pH 7.0(8). The PRAF2
protein has four transmembrane domains, including a large PRA1
domain. The three members of the PRAF family, PRAF1, PRAF2 and
PRAF3, are functionally and structurally related proteins,
representing a new family of membrane transport-related proteins
(8). PRAF2 was found to be
expressed in most human tissues, showing strong expression in the
brain, small intestine, lung, spleen and pancreas, but not detected
in testicular tissues (8). A
previous study has reported that PRAF1 regulates colon cancer cell
proliferation and tumor progression by regulating T-cell factor
(TCF)/β-catenin signaling pathway (9). Multiple research groups have
demonstrated that PRAF3 acts as a tumor suppressor and regulates
the migration and angiogenesis of gastric cancer, melanoma and
cervical cancer cells through a variety of downstream signalling
pathways (10-12).
Downregulation of PRAF3 expression can be used as a poor prognostic
indicator for a variety of cancers.
Existing studies have shown that PRAF2 is closely
associated with the clinical pathology and poor prognosis of
neuroblastoma, malignant glioma and liver cancer, and greatly
promotes tumor cell proliferation, migration and metastasis. Geerts
et al (13) found that the
mRNA expression of PRAF2 was significantly upregulated in
neuroblastoma, which is associated with many genetic and clinical
features, such as age, survival, International Neuroblastoma
Staging System stage and MYCN (neuroblastoma derived homolog gene)
amplification of patients. The amplification of genomic MYCN plays
a key oncogenic role in the pathogenesis of neuroblastoma (14). In in vitro experiments,
researchers have found that silencing PRAF2 in SK-N-SH cells (human
neuroblastoma cells) inhibits PRAF2 protein expression, resulting
in a significant decrease in cell proliferation, migration and cell
matrix adhesion capacity (15).
Borsics et al (16)
highlighted the prevalent PRAF2 expression in brain tissues, while
PRAF2 expression was significantly higher in glioma tissue samples
than in normal adjacent brain tissues. PRAF2 may render malignant
gliomas highly aggressive through its involvement in vesicle
transport or its interaction with chemokine receptors. In addition,
Wang et al demonstrated that PRAF2 is an independent factor
that negatively affects the overall survival of patients with
hepatocellular carcinoma based on a study of 518 individuals. PRAF2
may also serve an oncogenic role in hepatocellular carcinoma
progression (17). Another study
has shown that the mRNA expression of PRAF2 is significantly
increased in esophageal squamous cell carcinoma (ESCC) tissues
compared with non-tumor tissues (18). Survival analysis results revealed
that elevated expression of PRAF2 was associated with poor overall
survival in patients with ESCC. Downregulation of PRAF2 expression
inhibited the proliferation, cell cycle progression and invasion of
ESCC cells, and induced cell apoptosis. Results suggest that PRAF2
may serve as a potential prognostic biomarker and therapeutic
target for ESCC (18).
The aforementioned data have suggested the
involvement of PRAF2 in the progression of multiple tumors.
However, the underlying mechanisms of PRAF2 in tumor development
and progression have, to the best of our knowledge, not yet been
reported. In addition, PRAF3 has been confirmed to be an important
regulatory protein of the p38 signaling pathway in breast cancer
MDA-MB-231 cells (19). It can
also inhibit the migration and invasion of breast cancer cells by
downregulating the expression of C-X-C chemokine receptor type
4(20). Therefore, we hypothesized
that PRAF2 may also play a role as an oncogene in the development
of breast cancer. The present study aimed to confirm that PRAF2 can
act as a potential prognostic biomarker and theraptic target for
patients with breast cancer.
Materials and methods
Cell culture and transfection
The breast cancer cell line MDA-MB-231 and the human
normal mammary epithelial cell line MCF-10A were purchased from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences, while MCF-7 (cat. no. CL-0149) was obtained from Procell
Life Science & Technology Co., Ltd. All cell lines were
cultured with high-glucose DMEM (cat. no. SH30022.01; Cytiva)
supplemented with 10% FBS (cat. no. S711-001S; Shanghai Shuangru
Biotechnology Co., Ltd.) at 37˚C and 5% CO2. Small
interfering RNA (siRNA) sequences were designed and synthesized by
TsingKe Biotechnology Co., Ltd., comprising three siRNA sequences
targeting PRAF2 and a negative control (NC) sequence: siRNA1,
siRNA2, siRNA3 and siRNA-NC. The siRNA sequences are provided in
Table I. Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was used for cell
transfection. The MDA-MB-231, MCF-10A and MCF-7 cells were seeded
in 24-well plates at an optimized concentration of
~1x105 cells/well, 24 h before transfection. On the
following day, when cell confluence had reached 60-70%, PRAF2 siRNA
(10 µl/well) or siRNA-NC (10 µl/well) were transfected using 1 µl
Lipofectamine 2000 reagent at room temperature for 48 h at a final
concentration of 100 nM. Complete media was added to each well 6 h
after transfection as per the manufacturer's protocol. Subsequent
cellular function assays were performed on cells transfected with
the siRNA that possessed the highest transfection efficiency, which
was determined by PRAF2 protein expression analysis 48 h after
transfection.
 | Table IsiRNA sequences. |
Table I
siRNA sequences.
| Product cat.
no. | Product name | Position | Target sequence
(5'-3') |
|---|
| stB0009893A | siRNA1 | 471 |
AAGAUCGAGAGCAUUGGUCUCdTdT |
| stB0009893B | siRNA2 | 532 |
AAGAGCAGGAGGCUGGAUCCUdTdT |
| stB0009893C | siRNA3 | 916 |
AAGGCACUCUCAAAUCUUGAAdTdT |
| stB0009893D | siRNA-NC | 0 |
GCCUGUGCGAAGGAAUCUUAAdTdT |
Breast cancer samples
The present study enrolled 37 patients diagnosed
with breast cancer and also receiving modified radical mastectomy
at the Department of Thyroid and Breast Surgery, Yijishan Hospital
of Wannan Medical College (Wuhu, China) from October 2017 to
October 2019. All patients had not received neoadjuvant
chemotherapy, radiotherapy or endocrine treatment before surgery.
Patients were female, aged between 45-76 years, with a median age
of 58 years. Cases of invasive ductal carcinoma, intraductal
carcinoma and other types of carcinoma were included in the present
study. Cases with multiple primary cancers and a postoperative
positive margin were excluded. The postoperative
immunohistochemical staining results of the patients for the
markers estrogen receptor, progesterone receptor, HER2 and Ki67
were recorded, and clinical and pathological data, including age,
histopathological grade, number of axillary lymph node dissections
and sites of metastasis, were also collected. The patient
characteristics are presented in Table II. Once the breast tissue
specimens were surgically resected, the fresh breast cancer tissues
and normal adjacent tissues were separated, quickly frozen in
liquid nitrogen tanks and then stored in a -80˚C freezer. All
patients were informed in writing and signed an informed consent
form before sample collection. The study protocol was approved by
the Ethics Committee of the Yijishan Hospital of Wannan Medical
College (Wuhu, China).
 | Table IIClinicopathological patient
characteristics. |
Table II
Clinicopathological patient
characteristics.
| Patients
characteristics (n=37) | Absolute
number | Ratio (%) |
|---|
| Age, years | | |
|
≤60 | 25 | 67.57 |
|
>60 | 12 | 32.43 |
| Tumor size, cm | | |
|
≤2 | 23 | 62.17 |
|
>2 | 14 | 37.83 |
| Lymph node
status | | |
|
No
metastasis (N0) | 29 | 78.38 |
|
Metastasis
(N1-3) | 8 | 21.62 |
| Histological
type | | |
|
Invasive | 32 | 86.30 |
|
DCIS | 2 | 5.60 |
|
Others | 3 | 8.10 |
| Estrogen receptor
status | | |
|
Negative | 8 | 21.62 |
|
Positive | 29 | 78.38 |
| Progesterone
receptor status | | |
|
Negative | 14 | 37.83 |
|
Positive | 23 | 62.17 |
| HER-2 status | | |
|
Negative | 16 | 43.24 |
|
Equivocal | 16 | 43.24 |
|
Positive | 5 | 13.52 |
| PRAF2 status | | |
|
Negative | 9 | 24.32 |
|
Positive | 28 | 75.68 |
Reverse transcription-quantitative PCR
(RT-qPCR)
QuantiNova™ SYBR Green PCR kit was used for this
assay (cat. no. 208252; Qiagen AB). The primers for PRAF2 and GAPDH
genes were synthesized by Guangzhou RiboBio Co. Ltd., and their
sequences are detailed in Table
III.
 | Table IIIReverse transcription-qPCR primer
sequences. |
Table III
Reverse transcription-qPCR primer
sequences.
| Product cat.
no. | Product name | Primer sequence
(5'-3') |
|---|
| GQP0005157 | geneDETECTTM
h-PRAF2_qPCR_91bp_F1 |
CCCAGGTCAAGACATTGCC |
| GQP0005158 | geneDETECTTM
h-PRAF2_qPCR_91bp_R1 |
GGTCCAACAGTCAGGATACCC |
| ssD1021 | GAPDH_2_forward
primer (h,m,r) |
GAACGGGAAGCTCACTGG |
| ssD1022 | GAPDH_2_reverse
primer (h,m,r) |
GCCTGCTTCACCACCTTCT |
| B6612 | TCF4 forward
primer |
CCTGGCTATGCAGGAATGTT |
| B6612 | TCF4 reverse
primer |
CAGGAGGCGTACAGGAAGAG |
| B6611 | β-catenin forward
primer |
AACAGGGTCTGGGACATTAGTC |
| B6611 | β-catenin reverse
primer |
CGAAAGCCAATCAAACACAAAC |
Total RNA from tissue samples and transfected cells
was extracted by using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), following the manufacturer's
protocol. RNA was reverse transcribed into cDNA using PrimeScript™
RT reagent Kit with gDNA Eraser (cat. no. RR047A; Takara Bio,
Inc.), according to the manufacturer's protocols. qPCR was
performed with the QuantiNova™ SYBR Green PCR kit (cat. no. 208252;
Qiagen AB) according to the manufacturer's protocols, using the ABI
PRISM 7000 fluorescent quantitative PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used: 1 cycle at 94˚C for 3 min; followed by 35
cycles at 94˚C for 30 sec, 58˚C for 30 sec and 72˚C for 45 sec.
Relative expression was calculated based on the
2-ΔΔCq method (21). GAPDH served as the internal
control. This experiment was repeated three times.
Western blotting
MCF-7 cells were collected, washed with cold PBS,
and lysed with PhosphoSafe™ Extraction Reagent (cat. no. P0013;
Beyotime Institute of Biotechnology). The culture medium was
aspirated from cells, before cells were rinsed once with PBS. The
recommended amount of Phospho Safe™ Extraction Reagent was added,
cultures were incubated at room temperature for 5 min, cells were
dislodged using a cell scraper, and the lysate was transferred to a
1.5 ml tube before centrifugation for 5 min at 16,000 x g at 4˚C.
Finally, the supernatant was transferred to a new tube and protein
content was quantified using BCA assay (cat. no. AS1086; Wuhan
Aspen Biotechnology Co., Ltd.). The total protein loading of each
sample was normalized to 30 µg, then samples were electrophoresed
using 15% SDS-PAGE and transferred to a PVDF membrane. Non-specific
binding in membranes was blocked using PBS containing 5% non-fat
milk for 2 h at room temperature. Subsequently, the membranes were
incubated with primary antibodies at 4˚C overnight. The membranes
were then incubated with an HRP-conjugated goat anti-rabbit IgG
[1:5,000; cat. no. BL103A; Micro Biotechnology (Shanghai) Co.,
Ltd.] secondary antibody for 2 h at room temperature. Finally,
protein bands were detected using an enhanced chemiluminescence kit
(cat. no. A38555; Thermo Fisher Scientific, Inc.). The following
antibodies were used in the present study: β-actin (1:5,000; cat.
no. AF7018; Affinity Biosciences, Ltd.), GAPDH (1:5,000; cat. no.
ab37168; Abcam), PRAF2 (1:3,000; cat. no. ab230420; Abcam),
β-catenin (1:3,000; cat. no. ab223075; Abcam) and TCF4 (1:3,000;
cat. no. ab217668; Abcam). β-actin and GAPDH were used for
normalization. ImageJ software (version 4.1; National Institutes of
Health) was used to evaluate and quantify the gray value of protein
bands. This experiment was repeated three times.
Cell function assays
The following three groups were set for the
subsequent experiments in MCF-7 cell lines: NC group (with complete
medium only), siRNA-NC group and siRNA-PRAF2 group.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Beyotime Institute of
Biotechnology) was used to determine the cell proliferation,
according to the manufacturer's instructions. Cells in the
logarithmic growth phase (100 µl) were seeded on a 96-well plate
(103 cells/well), then the culture medium was discarded
after the cells had adhered. The cells were subsequently incubated
with 10 µl CCK-8 reagent for 72 h. Culture medium without cells was
used as the negative control, which was then incubated with CCK-8
reagent. The absorbtion of each well at 450 nm was measured using a
microplate reader to calculate an average value of each group of
cells and the negative controls, which allowed the calculation of
the MCF-7 cell proliferation rate. MCF-7 cell proliferation rate
(%)=Value of each experimental group/NC control group x100. This
experiment was repeated three times.
Colony formation assay
For each experimental group, cells were resuspended
with 1 ml complete culture medium and diluted to
1x104/ml for counting. Each well of a 6-well plate was
seeded with 300 cells, with each group seeded in triplicate. The
6-well plate was incubated at 37˚C with 5% CO2 for 2
weeks. After being fixed with 4% paraformaldehyde for 30 min at
25˚C, the cell colonies were stained for 30 min at room temperature
using 0.01% crystal violet solution. To observe stained colonies
(>50 cells), samples were imaged under a light microscope (IX71;
Olympus Corporation). The average value of the number of colonies
for MCF-7 cells in each group was then calculated manually. The
experiment was repeated three times.
Scratch wound healing assay
Using a marker pen, evenly spaced parallel lines,
~0.5 cm apart, were drawn on the bottom of a 6-well plate, with 5
lines per well. In each well, ~5x105 cells suspended in
2 ml high-glucose DMEM (cat. no. SH30022.01; Cytiva) supplemented
with 10% FBS (cat. no. S711-001S; Shanghai Shuangru Biotechnology
Co., Ltd.). Once the cells reached 100% confluence, a pipette tip
was used to mark the cell monolayer perpendicular to the horizontal
lines created by the marker pen. The scratched cells were rinsed
gently with PBS, before adding fresh culture medium. Scratched
cultures were imaged under a light microscope, and this was
recorded as the 0 h time point. siRNA solution was prepared by
adding 10 µl of the stock solution to 250 µl of Opti-MEM serum-free
medium (cat. no. SH30022.01; Cytiva). Once siRNA solutions were
added, the plate was left at room temperature for 5 min before it
was returned to the incubator. After 24 h of incubation at 37˚C,
each well was imaged at the same location under a light microscope,
which was recorded as the 24 h time point. Calculation of the
scratch healing rate used the following formula: Scratch healing
rate (%)=(0 h scratch width-24 h scratch width)/0 h scratch width
x100. This experiment was repeated three times.
Transwell invasion assay
The Transwell chamber (Corning, Inc.) with inserts
was prepared by diluting 50 µl of Matrigel solution with complete
culture medium at a dilution ratio of 1:3. The dilution was then
added to the Transwell chamber and allowed to dry at 37˚C for 2 h.
To each Transwell chamber, 200 µl of cell suspension at
105 cells/ml in serum-free culture medium was added,
before each chamber was inserted into the wells of a 24-well plate
with 500 µl high-glucose DMEM (cat. no. SH30022.01; Cytiva)
supplemented with 10% FBS (cat. no. S711-001S; Shanghai Shuangru
Biotechnology Co., Ltd.) per well in the lower chamber. The plate
was then incubated at 37˚C for 48 h. The Transwell chambers were
removed from the wells and the culture medium was discarded. Using
a cotton swab, the upper portion of the chamber was wiped clean to
remove any non-migratory cells. The remaining cells on the bottom
side of the chamber were then stained with 0.01% crystal violet
stain solution in PBS at room temperature for 10 min. The chambers
were rinsed to remove excess crystal violet stain, before being
imaged using an inverted light microscope. To quantify the invasion
in each chamber, three regions of interest were randomly identified
at x40 magnification, before capturing corresponding triplicate
x100 magnification images. From these images, the average number of
invaded cells was calculated manually. This experiment was repeated
three times.
The Cancer Genome Atlas (TCGA)
database validation
The University of Alambama in Birmingham Cancer Data
Analysis Portal (UALCAN; http://ualcan.path.uab.edu/index.html) is an effective
website for online analysis and mining of cancer data, mainly based
on relevant cancer data from TCGA database (22). Through this tool the expression of
PRAF2 in breast cancer samples in TCGA database was verified. The
database of Genotypes and Phenotypes accession no. for the analyzed
data is phs000178(23).
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.) and GraphPad Prism 7.0 (GraphPad Software,
Inc.) Data are presented as the mean ± SD. Comparisons between two
groups were made using paired or unpaired Student's t-test, while
comparisons among multiple groups were performed by one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PRAF2 expression in clinical samples
measured by RT-qPCR and western blotting
In the present study, PRAF2 gene expression was
firstly analyzed in patients with breast cancer in TCGA database
using the cancer data accessed through UALCAN. PRAF2 was found to
be upregulated in breast cancer tissue samples compared with normal
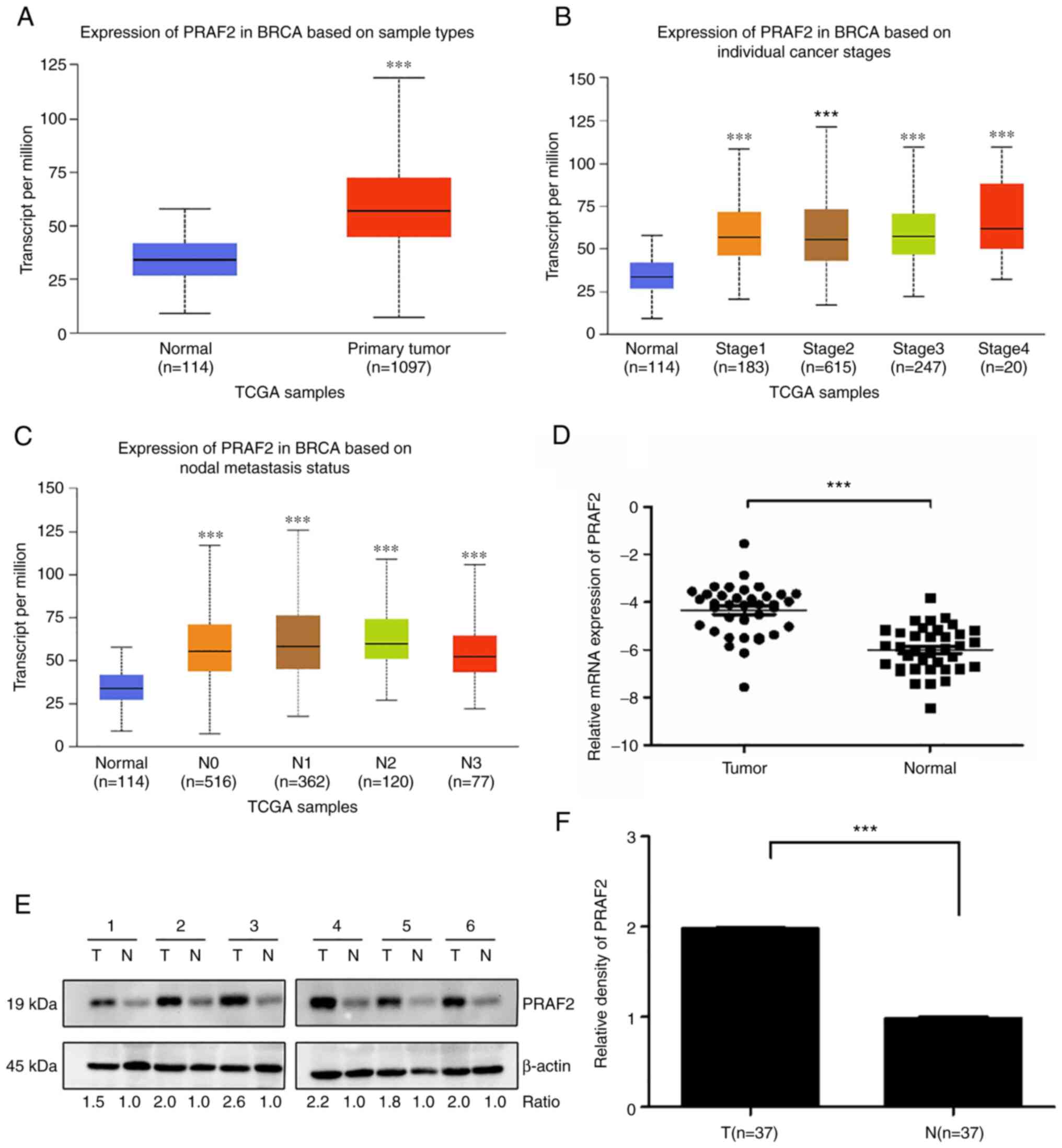
tissue samples (Fig. 1A).
Additionally, with the increase of the clinical stage of tumors,
PRAF2 expression in breast cancer tissues showed an upward trend
(Fig. 1B). The most common form of
metastasis in breast cancer is lymphatic metastasis (24). The data showed that the high
expression of PRAF2 in breast cancer samples was closely associated
with metastasis to the regional lymph nodes in each stage (Fig. 1C). Subsequently, PRAF2 expression
in cancer and normal adjacent tissues from 37 patients with breast
cancer was examined by RT-qPCR. The results showed that the mRNA
expression of PRAF2 was increased in cancer tissues compared with
normal adjacent tissues (Fig. 1D).
Based on the results of western blot analysis, the protein
expression of PRAF2 was also increased in breast cancer tissues of
the 37 patients. Gray value analysis of the PRAF2 and β-actin bands
showed that the relative expression intensity of PRAF2 protein in
cancer tissues of 37 patients with breast cancer (1.9750±0.0103)
was higher than that in normal adjacent tissues (0.9818±0.0140);
the expression levels were determined in all tissue samples and six
representative samples are shown (Fig.
1E and F).
PRAF2 expression in breast cancer cell
lines and silencing in MCF-7 cells through siRNA transfection
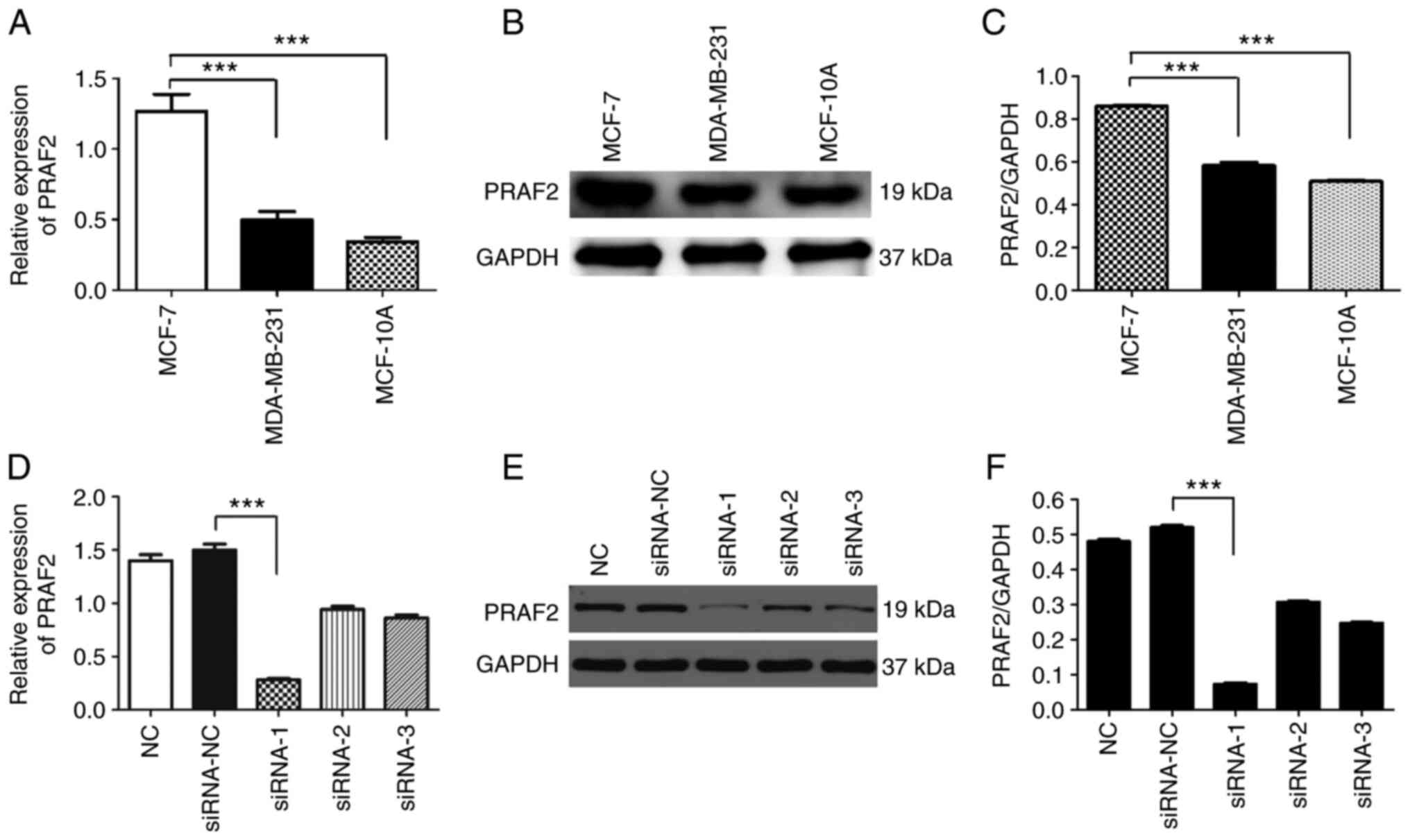
The expression of PRAF2 in breast cancer cell lines
MCF-7 and MDA-MB-231 and the human normal mammary epithelial cell
line MCF-10A was detected by RT-qPCR (Fig. 2A) and western blotting (Fig. 2B and 2C). The results showed significantly
higher PRAF2 mRNA and protein expression in MCF-7 cells compared
with MDA-MB-231 and MCF-10A cells. MCF-7 cells were subsequently
used for siRNA transfection to knock down the expression of PRAF2
protein. The following three groups were used: NC group, siRNA-NC
group and siRNA-PRAF2 group, divided into siRNA-1, siRNA-2 and
siRNA-3. Total protein from cells of each group was extracted after
transfection, and the gene knockdown rate in the control group was
detected by RT-qPCR (Fig. 2D) and
western blotting (Fig. 2E and
2F). The results showed that PRAF2
expression was decreased in MCF-7 cells transfected with
siRNA-PRAF2 compared with siRNA-NC, with siRNA-1 showing superior
knockdown efficiency. Therefore, siRNA-1 was selected for
subsequent experiments.
Effects of PRAF2 on the proliferation
of MCF-7 cells. Effect of PRAF2 on the colony formation capacity of
MCF-7 cells
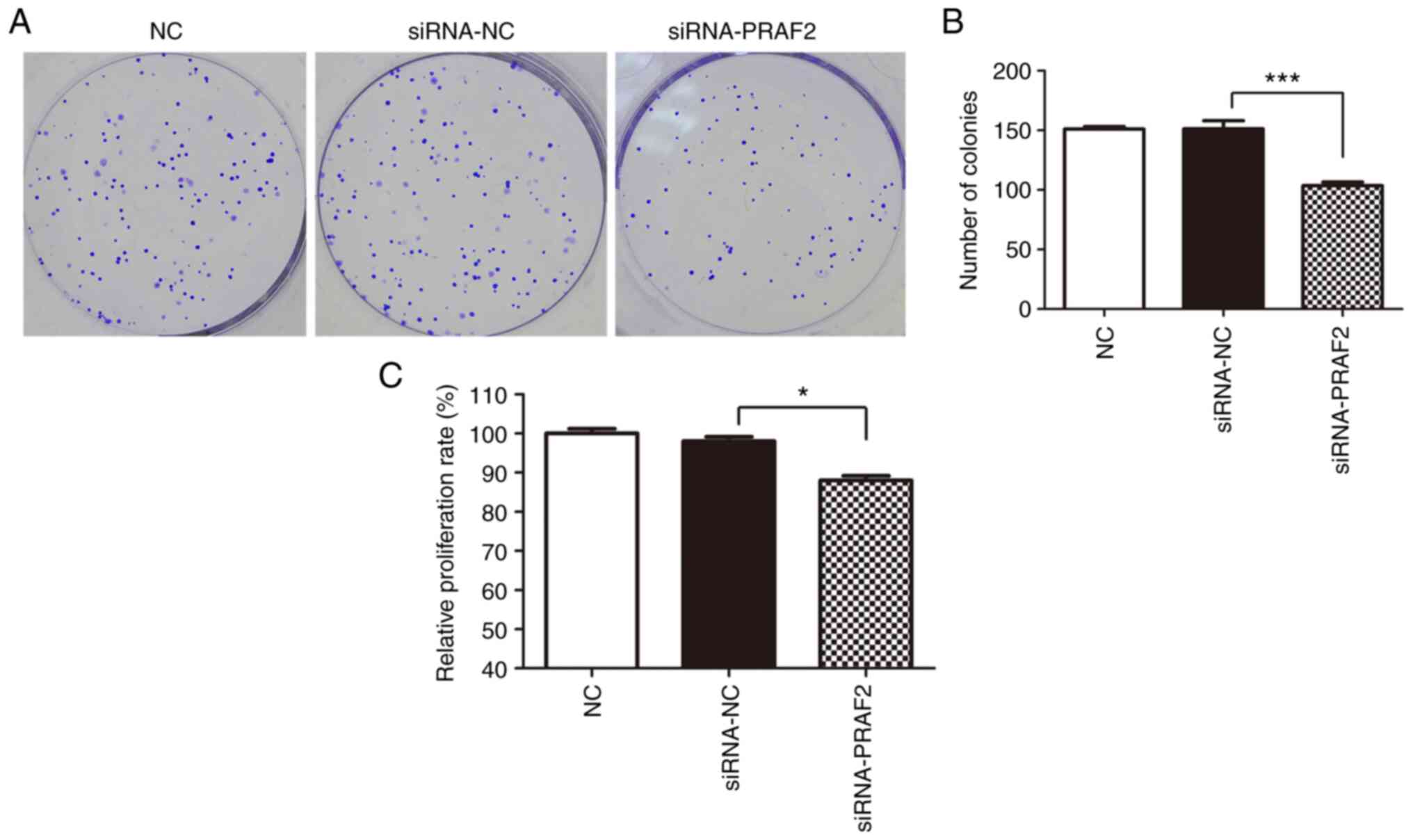
The tumorigenic ability of MCF-7 cells was examined
by colony formation assay. The results indicated that transfection
of siRNA-PRAF2 reduced the number of colonies in MCF-7 cells
compared with the siRNA-NC group (Fig.
3A and B), indicating that
decrease of PRAF2 can reduce the colony formation of breast cancer
cells.
Effect of PRAF2 on the viability of MCF-7 cells
as examined by CCK-8 assay. The effect of PRAF2 on the
viability of MCF-7 cells was examined using the CCK-8 assay. The
results showed that transfection of siRNA-PRAF2 led to lower
proliferation rate of MCF-7 cells after 72 h compared with siRNA-NC
cells (Fig. 3C). This suggested
that decrease of PRAF2 expression can diminish the viability of
MCF-7 cells.
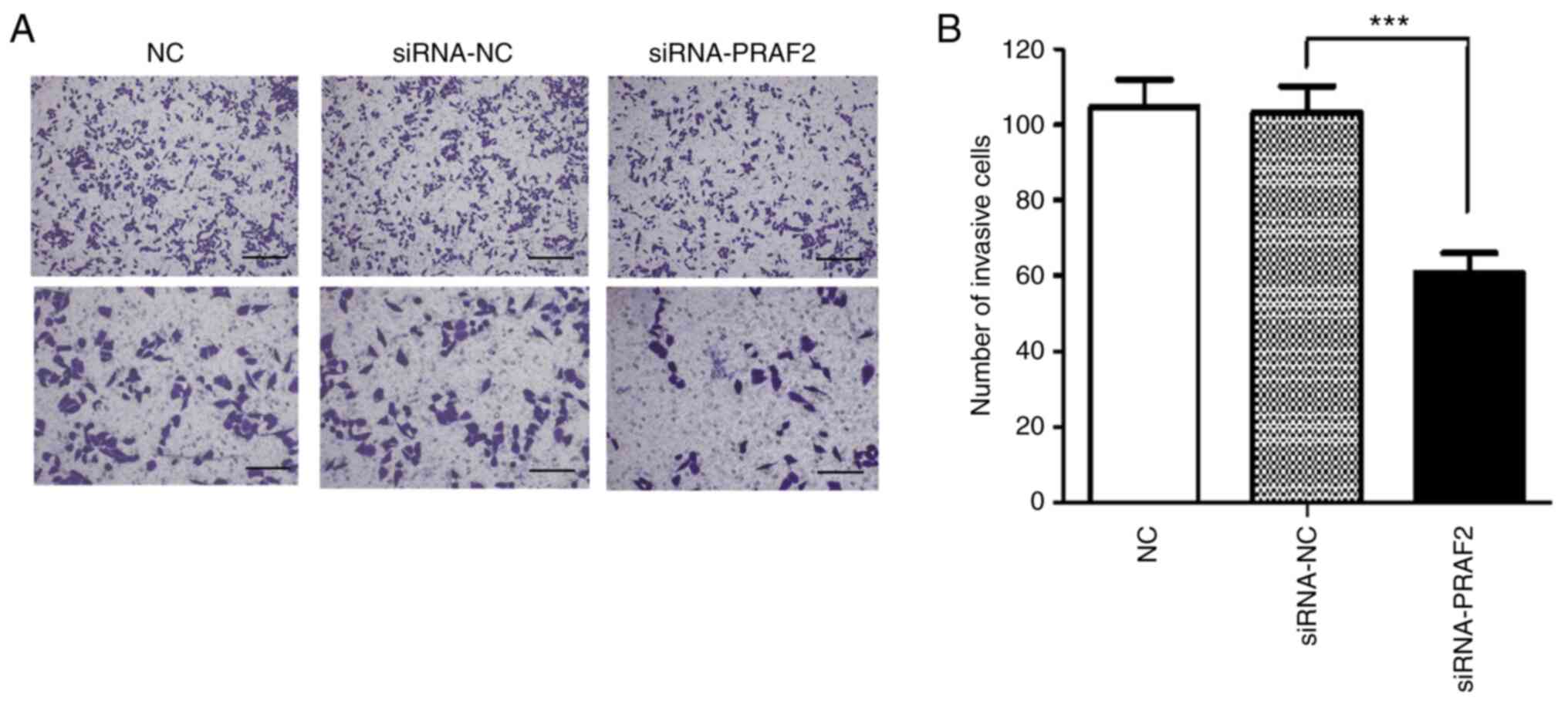
Invasion of MCF-7 cells assessed by
Transwell assay
The Transwell invasion assay was used to investigate
the effect of PRAF2 on the invasive ability of breast cancer cells.
The results showed that the number of cells crossing the basement
membrane, counted at x100 magnification, was 104.67±7.14 cells/high
power (HP) in the NC group, 103.33±6.84 cells/HP in the siRNA-NC
group and 60.89±5.06 cells/HP in the siRNA-PRAF2 group. There was
no significant difference in the number of cells crossing the
basement membrane between the siRNA-NC group and the NC group.
However, invasion of MCF-7 cells was reduced in the siRNA-PRAF2
group when compared with the siRNA-NC and NC groups (Fig. 4A and B). These data showed that decrease of
PRAF2 in MCF-7 cells significantly reduced cell invasion in
vitro.
Effect of PRAF2 on the migration of
MCF-7 cells
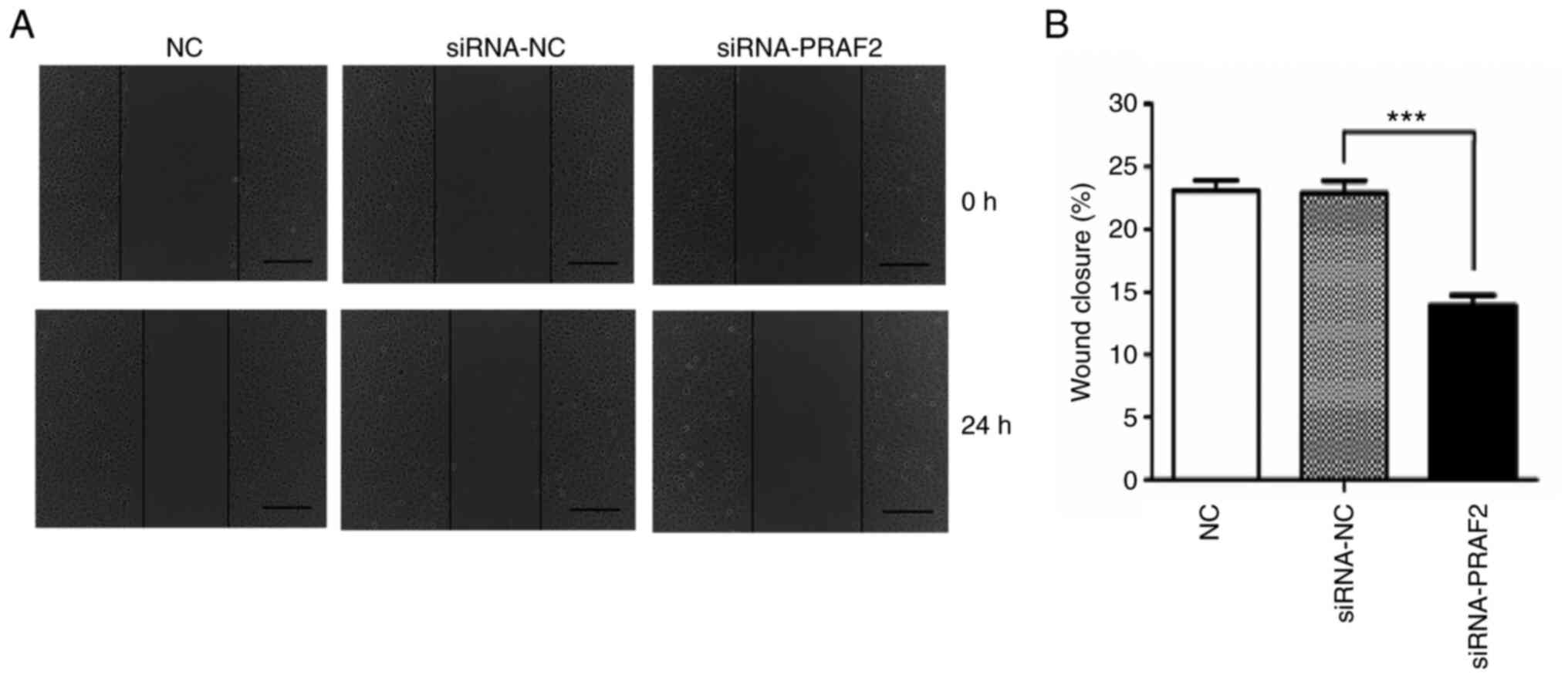
A scratch test was used to investigate the effect of
PRAF2 on the migration of breast cancer cells. The results revealed
that the healing rate (%) of MCF-7 cells after 24 h was 23.09±0.78
in the NC group, 22.90±0.96 in the siRNA-NC group and 13.94±0.79 in
the siRNA-PRAF2 group. No significant difference was found between
the siRNA-NC group and the NC group; however, the migration of the
siRNA-PRAF2 group was significantly reduced compared with that of
the siRNA-NC group (Fig. 5A and
B). This indicated that
downregulation of PRAF2 in MCF-7 cells significantly suppressed
cell migration in vitro.
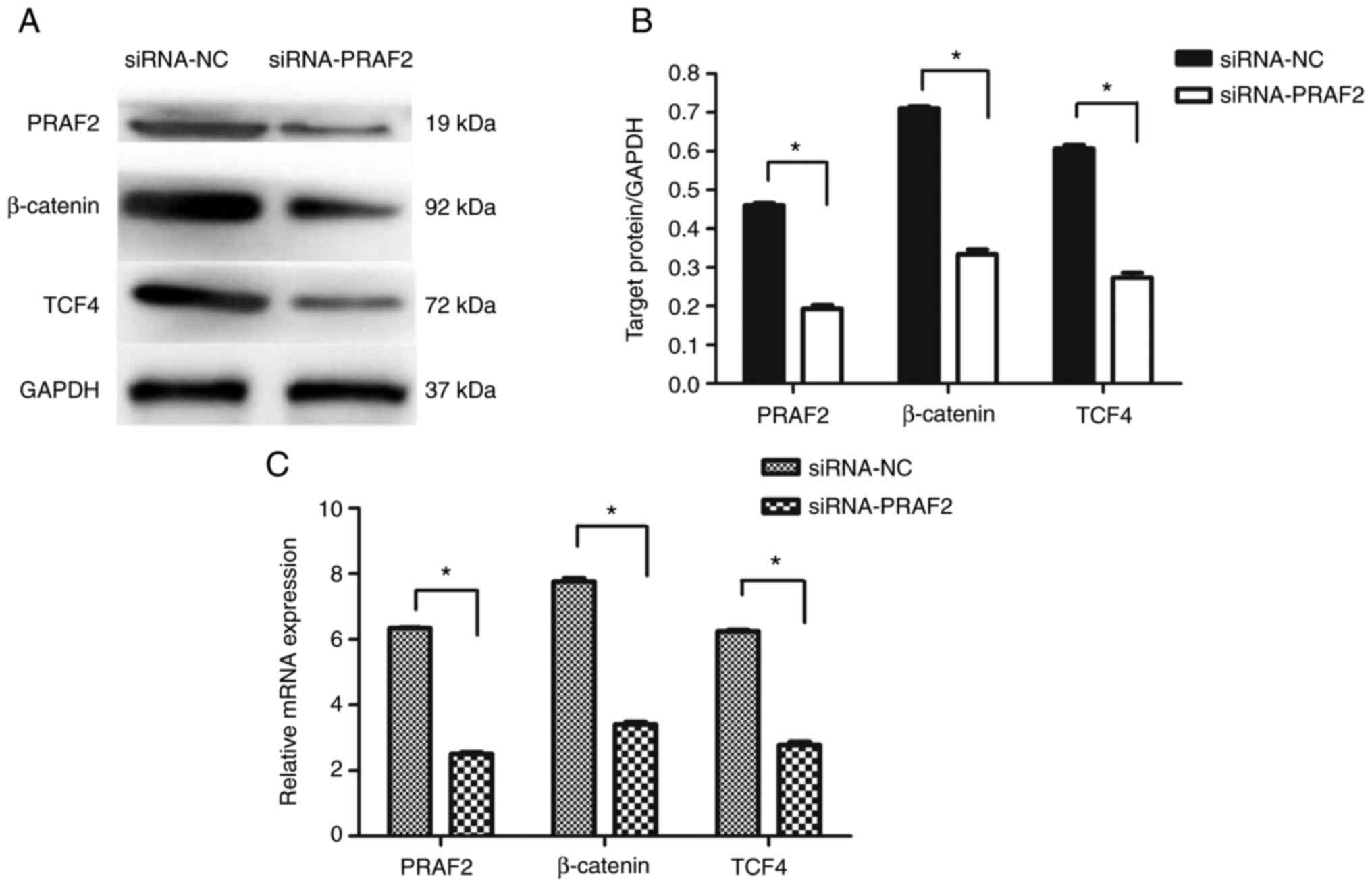
Western blot and RT-qPCR analysis
determines the mRNA and protein expression levels of β-catenin and
TCF4
MCF-7 cells were transfected with either siRNA-NC or
siRNA-PRAF2. Cells transfected with siRNA-PRAF2 showed decreased
β-catenin and TCF4 expression compared with those transfected with
siRNA-NC (Fig. 6A-C). This
indicated that downregulation of PRAF2 in MCF-7 cells may affect
the Wnt/β-catenin signalling pathway.
Discussion
Breast cancer is the most common cancer in women
worldwide, seriously threatening their health (1-3).
The current major treatment options for breast cancer include
surgery, radiotherapy, endocrine therapy, targeted therapy and
chemotherapy (25). Among them,
targeted therapy has achieved remarkable efficacy in the treatment
of HER-2-positive breast cancer, and has become the basic treatment
regimen for this type of breast cancer (4). In recent years, targeted therapy has
become a popular field of research, with targeted drug therapy and
clinical treatment developing rapidly. The current perspective of
diagnosis and treatment indicates that early breast cancer can be
cured. However, due to the invasion and metastasis of breast cancer
cells, current treatment methods fail to cure the patients with
advanced breast cancer, who have developed metastases to bone,
lung, liver and other organs (26). The primary goal of treatment for
such patients is to prolong the survival and maintain the quality
of life. Therefore, the early detection, diagnosis and treatment of
patients with breast cancer has become even more important
(27). Obvious genomic and genetic
regulatory abnormalities occur in breast cancer cells, which may
play an important role in development of breast cancer. For
example, in carriers of BRCA1 or BRCA2 mutations, the risk of
developing breast cancer by 80 years of age is as high as 70%,
compared with a 10% risk for women in the general population
(28). Therefore, the present
study aimed to investigate the occurrence and development of breast
cancer at the molecular level by analyzing gene expression
differences in breast cancer tissues and cells with a view to
provide earlier and more accurate diagnosis and targeted treatment
for patients with breast cancer, in addition to effectively
evaluating patient prognosis to develop better personalized
treatment methods. Moreover, the investigation of the invasion and
metastasis of breast cancer cells also aimed to exploit new
anti-metastatic therapeutic targets.
Firstly, the PRAF2 gene phenotype in patients with
breast cancer was analyzed in TCGA database using the cancer data
online analysis website UALCAN. It was found that PRAF2 expression
was significantly upregulated in breast cancer tissue samples when
compared with normal tissue samples. It was also demonstrated that
PRAF2 was strongly expressed in tumor tissues of the breast, colon,
lung and ovary, as well as in tissues immediately adjacent to the
corresponding tumor tissue, while expression of PRAF2 in
corresponding normal tissues was significantly weaker (8). However, there are few reports on the
PRAF2 expression in breast cancer cells and its effects on the
proliferation, invasion and migration (29). Therefore, in order to clarify the
expression of PRAF2 in breast cancer tissues and its impact on
cellular functions, breast cancer tissue samples and corresponding
normal adjacent tissues were collected from 37 patients with breast
cancer undergoing modified radical mastectomy. The expression of
PRAF2 in fresh breast cancer tissues was determined by RT-qPCR and
western blot analysis. The results revealed that the mRNA
expression of PRAF2 was increased in the cancer tissues compared
with normal adjacent tissues. Similarly, the protein expression of
PRAF2 was also found to be upregulated in cancer tissues of the 37
patients with breast cancer compared with normal adjacent tissues,
consistent with the outcome of RT-qPCR. These results indicated
that PRAF2 expression is significantly upregulated in breast cancer
tissues, suggesting that high PRAF2 expression may be associated
with the occurrence and development of breast cancer. During in
vitro cell functional experiments, the expression of PRAF2 was
firstly examined in the breast cancer cell lines MCF-7 and
MDA-MB-231 by RT-qPCR, and it was found that PRAF2 gene expression
was significantly higher in MCF-7 cells than in MDA-MB-231 cells.
Therefore, MCF-7 cells were used in further in vitro cell
functional experiments.
A key mechanism of tumorigenesis is the imbalance
between cell proliferation and apoptosis (30). PRAF2 has been reported to be
involved in the development of multiple cancer types, such as
hepatocellular carcinoma and esophageal carcinoma (17,18).
In the present study, the results of colony formation and CCK-8
assays demonstrated that silencing of PRAF2 in MCF-7 cells
suppressed the proliferation of MCF-7 cells, which is consistent
with relevant findings on PRAF2 in hepatocellular carcinoma and
neuroblastoma (15,17).
Tumor metastasis is a complex cascade of events
involving interactions between cancer cells and the surrounding
microenvironment, including mesenchymal cells, immune cells and the
extracellular matrix (31). The
first stage of breast cancer metastasis is the invasion of primary
tumor cells into the basement membrane and the subsequent
development of disseminated tumor cells (32). These cells consequently promote
abnormal angiogenesis, enter the circulatory or lymphatic system,
migrate to distant organs and form secondary tumors, ultimately
leading to poor prognosis in patients with metastasized breast
cancer (33). TCGA database
analysis revealed that high PRAF2 expression in breast cancer was
closely associated with the the TNM clinical stage as well as
metastasis to regional lymph nodes at each stage, suggesting that
PRAF2 is critical for breast cancer progression. Subsequent results
of scratch and Transwell assays indicated that the decrease of
PRAF2 significantly reduced the invasion and migration of MCF-7
cells. A previous study has shown that PRAF2 interacts with C-C
chemokine receptor type 5 (CCR5) (34), with increased CCR5 expression being
involved in brain tumorigenesis, especially in glioblastoma
progression (29). This may
partially explain the mechanism of metastasis associated with
PRAF2. Another member of the PRAF family, PRAF3, acts as a tumor
suppressor to regulate cancer cell migration, apoptosis and
angiogenesis through a variety of downstream signaling pathways,
such as the MAPK signalling cascade, integrin-linked kinase,
integrin αvβ3 pathway and SP1/MMP2 signaling pathway (10-12,35).
Therefore, we hypothesized that PRAF2 may reversely regulate these
pathways and this should be analyzed in further studies in the
future. Previous research has successively revealed that PRAF3 is
an important regulatory protein of p38 signaling pathway in
MDA-MB-231 cells (19), and can
inhibit cancer cell migration and invasion by downregulating the
expression of C-X-C chemokine recptor type 4(20).
β-catenin signalling has been broadly implicated in
human cancer. Aberrant activation of β-catenin signalling has been
implicated in malignant progression and poor patient prognosis
(36). The β-catenin pathway
involves the nuclear translocation of β-catenin and activation of
target genes via TCF/LEF transcription factors, which control cell
proliferation and migration (37).
In the present study, inhibition of PRAF2 expression could reduce
β-catenin and TCF4 expression. The study first found that PRAF2
regulated the β-catenin pathway in MCF7 cells. Results indicated
that PRAF2 may enhance the activation of the β-catenin signaling
pathway to promote breast cancer progression. He et al
(38) revealed that PRAF2
overexpression could increase the secretion of colon cancer cell
exosomes, and promote the migration and invasion of tumor cells
through Notch signal transduction. Therefore, PRAF2 expression may
also affect the malignant behavior of breast cancer through other
signalling pathways. The present study only investigated the
β-catenin signalling pathway and more in-depth research on the
specific mechanisms is required.
The present studiy provided solid evidence for
further investigation of the mechanisms through which PRAF2
regulates the proliferation, invasion and migration of breast
cancer. In conclusion, PRAF2 expression was significantly higher in
breast cancer tissues compared with normal adjacent tissues, and
was closely associated with TNM stage and regional lymph node
metastasis in breast cancer. Furthermore, PRAF2 was indicated to
function as an oncogene capable of promoting breast cancer cell
growth and invasion, providing novel insights into the metastasis
in breast cancer. However, the effect of PRAF2 on the apoptosis and
cell cycle of MCF-7 cells and the mechanism of PRAF2 regulation of
the proliferation, invasion and migration of MCF-7 cells have not
been investigated in the present study, and additional studies are
needed to validate the findings of these investigations and expand
the translational potential of this direction. In the current
study, the effect of PRAF2 was investigated in only one breast
cancer cell line, MCF-7, which has certain limitations, as it is a
luminal A subtype breast cancer cell line. In conclusion, PRAF2 may
be a potential prognostic factor in patients with breast cancer and
may become a potential target for the prevention and treatment of
breast cancer metastasis. PRAF2 may promote breast cancer cell
proliferation and invasion through activation of the β-catenin
signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Science and Technology
Achievement Transformation Project of Wuhu Science and Technology
Bureau (grant nos. 2021cg35 and 2021cg15), Key Scientific Research
Fund Project of Wannan Medical College (grant nos. WK2021ZF23 and
WK2022ZF17) and Research Foundation for Talents of Yijishan
Hospital of Wannan Medical College (grant no.YR202205).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, ZW, ZY and BC substantially contributed to the
conception and the design of the study. ZZ, HB, WJ and ZB performed
the experiments. ZZ, WJ, ZY, WZ, BD and BC analyzed and interpreted
the data. ZW, HB, ZB, BD and WZ revised the article. YW, ZW and BC
supervised the present study and wrote the manuscript. YW and ZZ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of the Yijishan Hospital of Wannan Medical College (Wuhu,
China) and written informed consent was obtained from all patients
before sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ruggiero AM, Liu Y, Vidensky S, Maier S,
Jung E, Farhan H, Robinson MB, Sitte HH and Rothstein JD: The
endoplasmic reticulum exit of glutamate transporter is regulated by
the inducible mammalian Yip6b/GTRAP3-18 protein. J Biol Chem.
283:6175–6183. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Doly S and Marullo S: Gatekeepers
controlling GPCR export and function. Trends Pharmacol Sci.
36:636–644. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Doly S, Shirvani H, Gäta G, Meye FJ,
Emerit MB, Enslen H, Achour L, Pardo-Lopez L, Yang SK, Armand V, et
al: GABAB receptor cell-surface export is controlled by an
endoplasmic reticulum gatekeeper. Mol Psychiatry. 21:480–490.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fo CS, Coleman CS, Wallick CJ, Vine AL and
Bachmann AS: Genomic organization, expression profile, and
characterization of the new protein PRA1 domain family, member 2
(PRAF2). Gene. 371:154–165. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim JT, Cho MY, Choi SC, Kim JW, Chae SK,
Yoon DY, Kim JW and Lim JS: Prenylated Rab acceptor 1 (PRA1)
inhibits TCF/beta-catenin signaling by binding to beta-catenin.
Biochem Biophys Res Commun. 349:200–208. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen Y, Huang Y, Huang Y, Xia X, Zhang J,
Zhou Y, Tan Y, He S, Qiang F, Li A, et al: JWA suppresses tumor
angiogenesis via Sp1-activated matrix metalloproteinase-2 and its
prognostic significance in human gastric cancer. Carcinogenesis.
35:442–451. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bai J, Zhang J, Wu J, Shen L, Zeng J, Ding
J, Wu Y, Gong Z, Li A, Xu S, et al: JWA regulates melanoma
metastasis by integrin alphaVbeta3 signaling. Oncogene.
29:1227–1237. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen H, Bai J, Ye J, Liu Z, Chen R, Mao W,
Li A and Zhou J: JWA as a functional molecule to regulate cancer
cells migration via MAPK cascades and F-actin cytoskeleton. Cell
Signal. 19:1315–1327. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Geerts D, Wallick CJ, Koomoa DL, Koster J,
Versteeg R, Go RC and Bachmann AS: Expression of prenylated Rab
acceptor 1 domain family, member 2 (PRAF2) in neuroblastoma:
Correlation with clinical features, cellular localization, and
cerulenin-mediated apoptosis regulation. Clin Cancer Res.
13:6312–6319. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brodeur GM, Seeger RC, Schwab M, Varmus HE
and Bishop JM: Amplification of N-myc in untreated human
neuroblastomas correlates with advanced disease stage. Science.
224:1121–1124. 1984.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yco LP, Geerts D, Koster J and Bachmann
AS: PRAF2 stimulates cell proliferation and migration and predicts
poor prognosis in neuroblastoma. Int J Oncol. 42:1408–1416.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Borsics T, Lundberg E, Geerts D, Koomoa
DL, Koster J, Wester K and Bachmann AS: Subcellular distribution
and expression of prenylated Rab acceptor 1 domain family, member 2
(PRAF2) in malignant glioma: Influence on cell survival and
migration. Cancer Sci. 101:1624–1631. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang CH, Liu LL, Liao DZ, Zhang MF, Fu J,
Lu SX, Chen SL, Wang H, Cai SH, Zhang CZ, et al: PRAF2 expression
indicates unfavorable clinical outcome in hepatocellular carcinoma.
Cancer Manag Res. 10:2241–2248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qian Z, Wei B, Zhou Y, Wang Q, Wang J, Sun
Y, Gao Y and Chen X: PRAF2 overexpression predicts poor prognosis
and promotes tumorigenesis in esophageal squamous cell carcinoma.
BMC Cancer. 19(585)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen X, Feng J, Ge Z, Chen H, Ding W, Zhu
W, Tang X, Chen Y, Tan Y and Ma T: Effects of the JWA gene in the
regulation of human breast cancer cells. Mol Med Rep. 11:3848–3853.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu L, Cheng L, Yang F, Pei B, Liu X, Zhou
J, Zhu Y and Wang S: JWA suppresses the invasion of human breast
carcinoma cells by downregulating the expression of CXCR4. Mol Med
Rep. 17:8137–8144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cancer Genome Atlas Research Network.
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Grebić D, Pirjavec A, Kustić D and Klarica
Gembić T: Surgical treatment for breast cancer and axillary
metastases: Historical perspective. Acta Med Hist Adriat.
19:125–136. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maughan KL, Lutterbie MA and Ham PS:
Treatment of breast cancer. Am Fam Physician. 81:1339–1346.
2010.PubMed/NCBI
|
|
26
|
Pagani O, Senkus E, Wood W, Colleoni M,
Cufer T, Kyriakides S, Costa A, Winer EP and Cardoso F: ESO-MBC
Task Force. International guidelines for management of metastatic
breast cancer: Can metastatic breast cancer be cured? J Natl Cancer
Inst. 102:456–463. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cardoso F, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S and Senkus E:
ESMO Guidelines Committee. Early breast cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 30:1194–1220. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kuchenbaecker KB, Hopper JL, Barnes DR,
Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, van Leeuwen FE,
Milne RL, Andrieu N, et al: Risks of breast, ovarian, and
contralateral breast cancer for BRCA1 and BRCA2 mutation carriers.
JAMA. 317:2402–2416. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kouno J, Nagai H, Nagahata T, Onda M,
Yamaguchi H, Adachi K, Takahashi H, Teramoto A and Emi M:
Up-regulation of CC chemokine, CCL3L1, and receptors, CCR3, CCR5 in
human glioblastoma that promotes cell growth. J Neurooncol.
70:301–307. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Roos WP, Thomas AD and Kaina B: DNA damage
and the balance between survival and death in cancer biology. Nat
Rev Cancer. 16:20–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Spill F, Reynolds DS, Kamm RD and Zaman
MH: Impact of the physical microenvironment on tumor progression
and metastasis. Curr Opin Biotechnol. 40:41–48. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wan L, Pantel K and Kang Y: Tumor
metastasis: Moving new biological insights into the clinic. Nat
Med. 19:1450–1464. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kozłowski J, Kozłowska A and Kocki J:
Breast cancer metastasis-insight into selected molecular mechanisms
of the phenomenon. Postepy Hig Med Dosw (Online). 69:447–451.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schweneker M, Bachmann AS and Moelling K:
JM4 is a four-transmembrane protein binding to the CCR5 receptor.
FEBS Lett. 579:1751–1758. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lu J, Tang Y, Farshidpour M, Cheng Y,
Zhang G, Jafarnejad SM, Yip A, Martinka M, Dong Z, Zhou J, et al:
JWA inhibits melanoma angiogenesis by suppressing ILK signaling and
is an independent prognostic biomarker for melanoma.
Carcinogenesis. 34:2778–2788. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu
C, Wang C and Ye L: Wnt/β-catenin signaling in cancers and targeted
therapies. Signal Transduct Target Ther. 6(307)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7(3)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
He W, Tang J, Li W, Li Y, Mei Y, He L,
Zhong K and Xu R: Mutual regulation of JAG2 and PRAF2 promotes
migration and invasion of colorectal cancer cells uncoupled from
epithelial-mesenchymal transition. Cancer Cell Int.
19(160)2019.PubMed/NCBI View Article : Google Scholar
|