Introduction
Diabetic nephropathy (DN) is a serious microvascular
complication of diabetes, which will contribute to severe renal
failure in the advanced stage, posing a great threat to the life
and health of patients. Some studies have found that China has the
largest diabetes population, with a prevalence rate of ~10.9% among
adults, among which the prevalence rate of DN is as high as 33.6%
(1,2). The pathogenesis of DN is complex and
is closely related to genetic metabolic factors, inflammatory
response, oxidative stress, glucose and lipid metabolism, and blood
flow (3). At present, symptomatic
treatments are available for DN, but they cannot fundamentally
reverse the progression of the disease.
Sodium/glucose cotransporter 2 (SGLT2) inhibitors
are a new class of oral hypoglycemic agents, which can reduce blood
glucose levels, prevent renal injuries, and repress glucose
reabsorption in proximal convoluted tubules. In patients with poor
blood glucose control, significant hypoglycemic effects have been
observed by administering SGLT-2 inhibitors because of the
increasing glucose excretion in urine and improving renal
hyperfiltration (4). Studies have
revealed that (5) renal
dysfunction can be alleviated by the SGLT-2 inhibitor by inhibiting
the expression level of renal tubular damage markers and neutrophil
gelatinase-associated lipocalin, which further represses renal
tubular interstitial fibrosis. Furthermore, a reduction of
high-glucose-induced o-junction n-acetyl glucosamine
glycosylation will be triggered by SGLT-2 inhibitors through the
HIF pathway, which further modulates renal tubular response to
hypoxia. In recent years, SGLT-2 inhibitors have shown renal
protection by improving the high filtration condition, reducing
proteinuria, ameliorating renal hypoxia, decreasing weight,
suppressing blood pressure, reducing uric acid levels, and
alleviating inflammation and oxidative stress, which have been used
in the clinical practice. At present, the SGLT2 inhibitors approved
in China primarily include empagliflozin, canagliflozin, and
dapagliflozin. A large number of clinical tracking data,
represented by CANVAS, EMPA-Reg, and DAPA-HF trial, have proven
that blood glucose levels, body weights, and blood pressure levels
of patients can be significantly repressed by the administration of
empagliflozin, canagliflozin, and dapagliflozin (6,7).
Furthermore, benazepril is widely reported for the treatment of
diabetes. Xue et al (8)
found that benazepril hydrochloride could alleviate DN by reducing
the expression of ANGPTL-4, which is considered as an effective
drug to treat DN and reduce proteinuria. Benazepril can also
improve renal function and reduce renal injury in DN rats (9).
In the present study, DN was established in rats by
high-fat diet and injecting streptozotocin (STZ) through the tail
vein, followed by observation on the pathological changes in renal
tissues. The difference in the expression level of fibrosis and
inflammation-related proteins was also determined to compare the
therapeutic effect of SGLT2 inhibitor and benazepril on DN
rats.
Materials and methods
Establishment of DN model in rats
Thirty-eight-week-old rats were purchased from
Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China), and
divided into the control and DN model groups. Animals in the
control group were fed with normal diets. Rats in the DN model
group were fed with high-fat diets for 8 weeks, followed by
intraperitoneal injection with 1% STZ solution. Blood glucose was
detected using a glucometer (Roche,) 3, 7 and 14 days after
injection. If the blood glucose was all higher than 16.7 mmol/l,
then the DN model was successfully established in rats. For in
vivo experiments, rats were divided into four groups. Animals
in the control and DN groups were intraperitoneally injected with
normal saline. Rats in the SGLT2 inhibitor and benazepril groups
were dosed orally with 1 mg/kg/day of SGLT2 inhibitor (10) and 62.5 mg/kg/day of benazepril for
4 weeks, respectively. Four weeks later, rats were sacrificed by
intraperitoneal injection of 1% pentobarbital sodium (45 mg/kg) for
follow-up detection. No breathing, no heartbeat, and pupils dilated
were used to confirm death following euthanasia with sodium
barbital. Clinical symptoms, such as unmitigable severe pain and
incapable of maintaining normal activities or eating on their own,
were humane endpoints used in order to determine when the animals
should be sacrificed to minimize suffering.
Hematoxylin and eosin (HE)
staining
After collecting renal tissues from each animal,
they were washed and dehydrated with 70, 80 and 90% ethanol
solution, followed by incubation with equal quality of ethanol and
xylene for 15 min. Subsequently, the samples were incubated with
equal quality of xylene for another 15 min, followed by repeat
incubation until the tissues looked transparent. Then, the tissues
were embedded in paraffin, sectioned, and stained with HE staining,
followed by randomly selecting the images from five fields at 100x
magnification, and all phases of follicles and corpora lutea were
counted by using an inverted microscope (Olympus).
Masson staining
Sections were stained with Weigert hematoxylin iron
for 10 min, followed by differentiation for 5-15 sec using acid
ethanol. Slides were stained with Masson blue solution for 5 min,
followed by washing and staining with Ponceau dye for 8 min.
Sections were washed using a weak acid working solution and then
directly stained in aniline blue for 2 min. After quick dehydration
using 95% ethanol and 100% ethanol successively, the images were
collected using an inverted microscope (Olympus,).
Periodic acid-Schiff (PAS)
staining
Sections were mixed with oxidants for 15-20 min,
washed three times, and added with Schiff reagents, followed
exhaust dyeing in the dark for 10-20 min. After two washes using
the sodium sulfite solution, sections were stained with
Mayer-hematoxylin dyes for 1-2 min. Finally, images were taken
using an inverted microscope (Olympus).
Transmission electron microscopy
(TEM)
Renal tissues were collected and washed using PBS
buffer and then fixed in 4.0% glutaraldehyde in PBS overnight.
Subsequently, samples were embedded in epoxy resin, and ultrathin
sections (50-70 nm) were collected on copper grids. After
counterstaining with aqueous uranyl acetate for 1 h,
phosphotungstic acid for 1 h, and Reynolds' lead citrate for 20 min
successively, the samples were examined using a transmission
electron microscope (JEOL).
The sections prepared from tissues were fixed in
2.5% glutaraldehyde PBS (v/v, pH 7.2), post-fixed in 1% osmium
tetroxide (v/v), and stained with 4.8% uranyl acetate after
dehydration. Subsequently, the sections were washed in propylene
oxide and impregnated with epoxy resins and then contrasted with
uranyl acetate and lead citrate. Finally, the sections were
observed under a transmission electron microscope (JEOL).
Immunohistochemical analysis
Renal tissues isolated from animals were fixed in 2%
formaldehyde solution and embedded in paraffin and then cut into
4-µm-thick sections. The sections were deparaffinated and
rehydrated, which were further incubated with 3%
H2O2 for 15 min. Subsequently, the sections
were blocked with 5% BSA in TBST for 30 min, followed by incubating
with primary antibody against ET-1 (1:100, Affinity), VWF (1:200,
Abcam), col-I (1:200, Proteintech), or α-AMA (1:200, Abcam). After
washing, the sections were incubated with HRP-conjugated secondary
antibody (CST), and then images were taken using a light microscope
(Olympus). Based on the images taken under a microscope, Image J
was used to read the IOD (gray value) and area (Area), and finally
the IOD/area ratio was used to make histogram display (11).
Western blot assay
After isolating the total proteins from renal
tissues, proteins were quantified using a BCA kit (ZIKER), and ~40
µg of proteins was loaded onto the SDS PAGE, followed by separation
for 2 h. Subsequently, the proteins were transferred to the PVDF
membrane (Invitrogen; Thermo Fisher Scientific, Inc.), and the
membrane was further incubated with 5% BSA. Then, the membrane was
incubated with the primary antibody against NT-proBNP (1:100,
Affinity), TGF-β (1:1,000, Affinity), MMP-9 (1:1,000, Proteintech),
and GAPDH (1:1,000, Affinity), followed by incubation using a
secondary antibody (1:2,000, Affinity). Finally, the membrane was
exposed to ECL solution, and the bands were visualized and
quantified using Image J.
Statistical analysis
The data in the present study were presented by mean
± SD and analyzed using GraphPad (GraphPad Software, Inc.). The
difference among groups was analyzed using one-way ANOVA and
Tukey's test. P<0.05 was considered as a significant
difference.
All animal experiments involved in this manuscript
were approved by the ethical committee of The Third Affiliated
Hospital of Nanchang University and carried out in accordance with
the guidelines for care and use of laboratory animals and the
principles of laboratory animal care and protection.
Results
Establishment of the DN model in
rats
After modeling, blood glucose was detected. As shown
in Table I, the level of blood
glucose in DN rats was significantly elevated compared with control
(*P<0.05 vs. control).
 | Table ILevel of blood glucose in rats. |
Table I
Level of blood glucose in rats.
| Groups | N | Time point | Blood glucose
(mmol/l) |
|---|
| Control | 4 | 3 weeks after STZ
injection | 6.28±0.38 |
| | | Before sacrifice | 8.27±2.35 |
| Model | 12 | 3 weeks after STZ
injection |
24.23±2.71a |
| | | Before
sacrifice |
23.45±3.71a |
Impacts of SGLT2 inhibitor on 24-h
urine protein in DN rats
As shown in Table
II and Fig. 1, the 24-h urine
protein in DN rats was significantly elevated compared with
control, which was dramatically suppressed by the administration of
SGLT2 inhibitor or benazepril (*P<0.05 vs.
control, #P<0.05 vs. model). In addition,
compared with the SGLT2 inhibitor group, significantly higher 24-h
urine protein was observed in the benazepril group
(ΔP<0.05 vs. SGLT2 inhibitor).
 | Table IILevel of 24-h urine protein in
rats. |
Table II
Level of 24-h urine protein in
rats.
| Groups | N | Urine protein
(mg/24 h) |
|---|
| Control | 4 | 5.68±1.58 |
| Model | 4 |
38.30±7.49a |
| SGLT2
Inhibitor | 4 |
12.04±2.38b |
| Benazepril | 4 |
19.49±2.74b,c |
SGLT2 inhibitor significantly
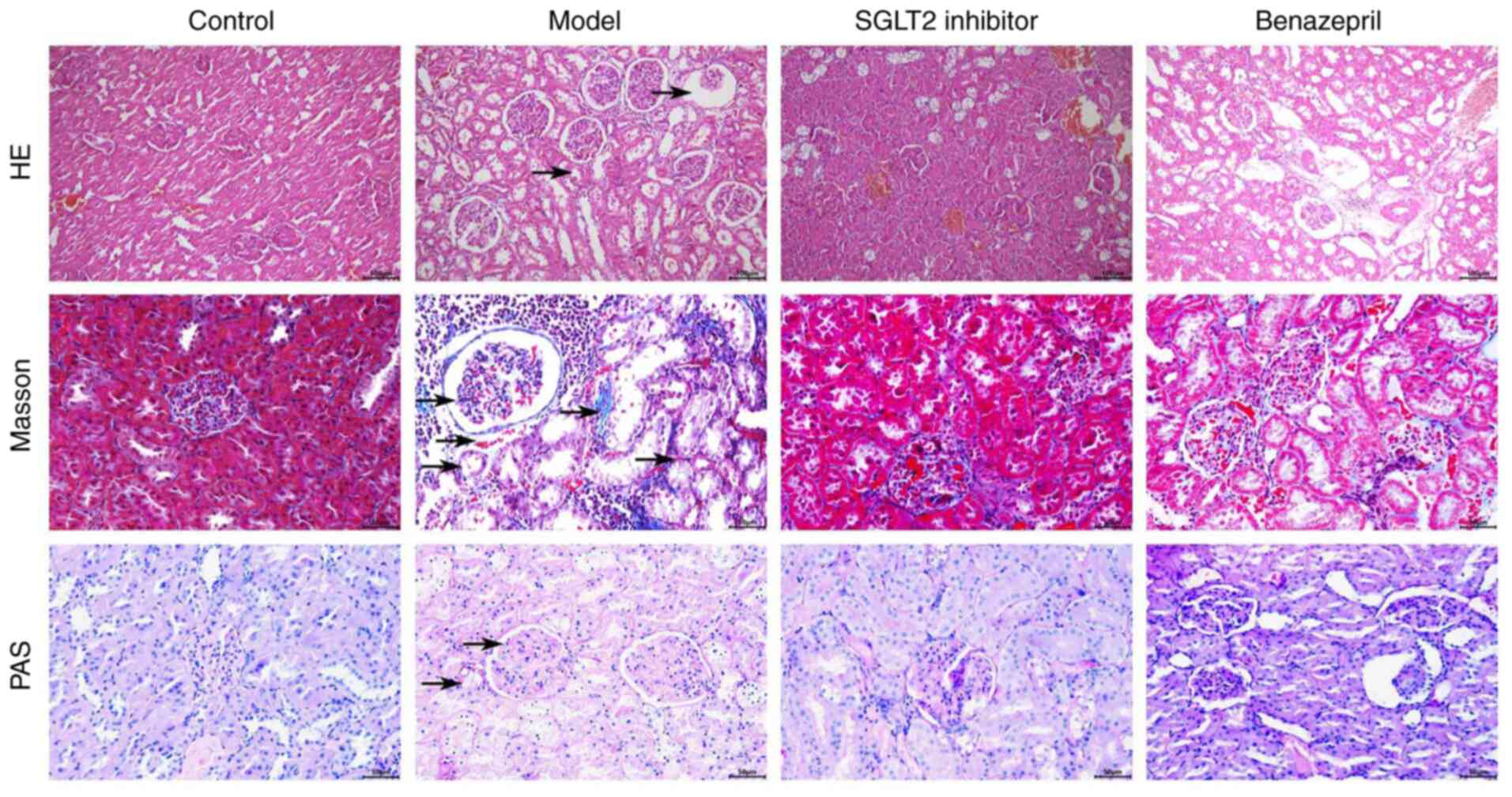
ameliorated the pathological state of renal tissues in DN rats
Based on the images taken in the HE, Masson, and PAS
staining assay, compared with control, the glomerular basement
membrane and mesangial membrane thickened; a protein tube was
formed, and renal tubular hypertrophy, lumen dilation, diffuse
monocytes, and infiltrated cells were observed in DN rats,
accompanied with infiltration of focal inflammatory cells in the
renal interstitial area, vacuolar degeneration or exfoliation of
renal tubular epithelial cells, significant increase of collagen
fibers, and positive glycogen reaction (Fig. 2). After the treatment of SGLT2
inhibitor, the lumen and renal tubules became smaller, and the
number of protein tubules decreased remarkably. However, in the
benazepril group, weak alleviatory effects on the renal injury were
observed, accompanied with small amounts of glomerular basement
membranes and infiltration of inflammatory factors.
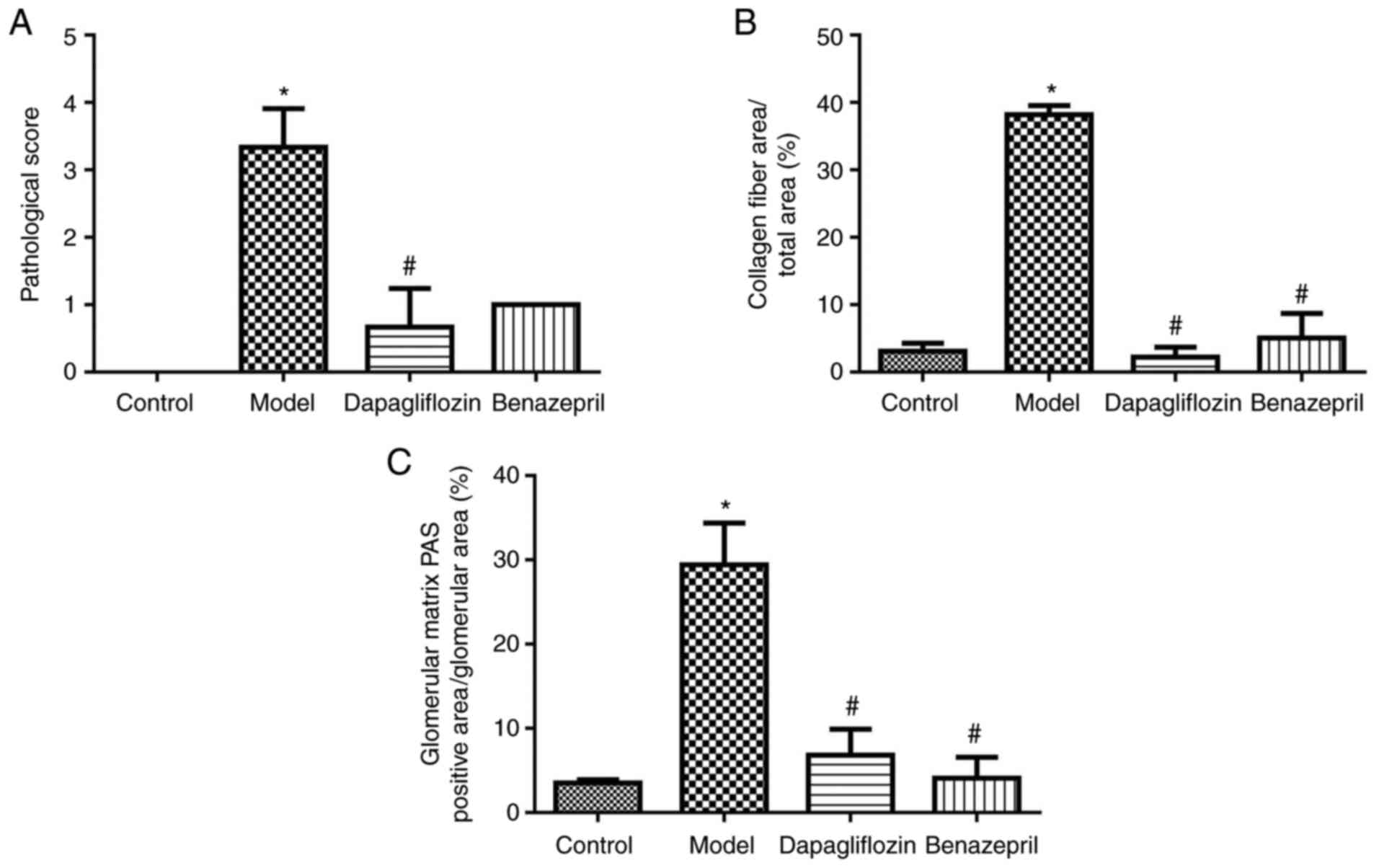
In addition, pathological analysis was performed,
and the results are shown in Fig.
2. Scoring was determined in accordance with the following
rules: score 0, no specific variation under a light microscope and
normal basement membrane under an electron microscope; score 1,
slight non-specific changes under a light microscope and thickening
of basement membrane under an electron microscope; score 2, higher
than 25% of mesangial lengthening and less mesangial growth area
compared with capillary area; score 3, at least one nodular
sclerosis; score 4, 50% of glomerular progressive diabetic
glomerular sclerosis. As shown in Fig.
3A, the HE score in the DN model group increased significantly
compared with the control group, which was greatly repressed by the
addition of SGLT2 inhibitor. As shown in Fig. 3B, the proportion of collagen fiber
area in the DN model group increased significantly compared with
the control group, which was greatly reversed by the SGLT2
inhibitor and benazepril. As shown in Fig. 3C, the proportion of PAS-positive
area and glomerular area in the glomerular matrix of the DN model
group increased significantly compared with the control group,
which were dramatically reversed by the SGLT2 inhibitor and
benazepril (*P<0.05 vs. control;
#P<0.05 vs. model).
Impact of SGLT2 inhibitor on the
morphology of renal tissues in DN rats
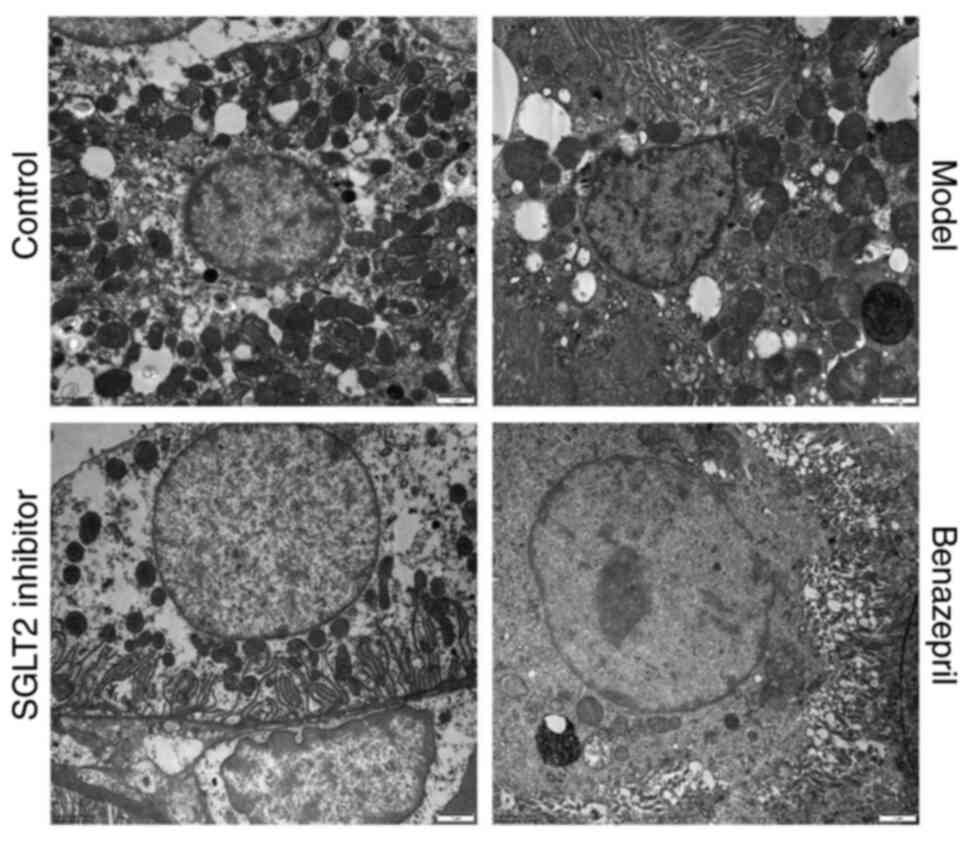
The ultrastructure of renal tissues is shown in
Fig. 4. Compared with control, the
epithelial cells of renal tubules in DN rats were damaged; the
deposition of lumps of electron density in endothelial cells and
basal membrane was observed, and the capillary lumen in the nephron
was blocked. In addition, the severe injury on the mitochondria was
induced, which was characterized by swelling, deformation,
vacuolation, disorder, and blurred or disappeared mitochondrial
crest. The increased endoplasmic reticulum phagocytes, enlarged
filtration membrane space, and deciduous basal membrane and
endothelial cells were also observed. After the treatment of SGLT2
inhibitor, the pathological state was significantly alleviated, and
the deposition in the basal membranes and endothelial cells
disappeared. In addition, the deformation and vacuolation in the
mitochondria were not observed. However, after the treatment of
benazepril, the alleviated effect was not significant.
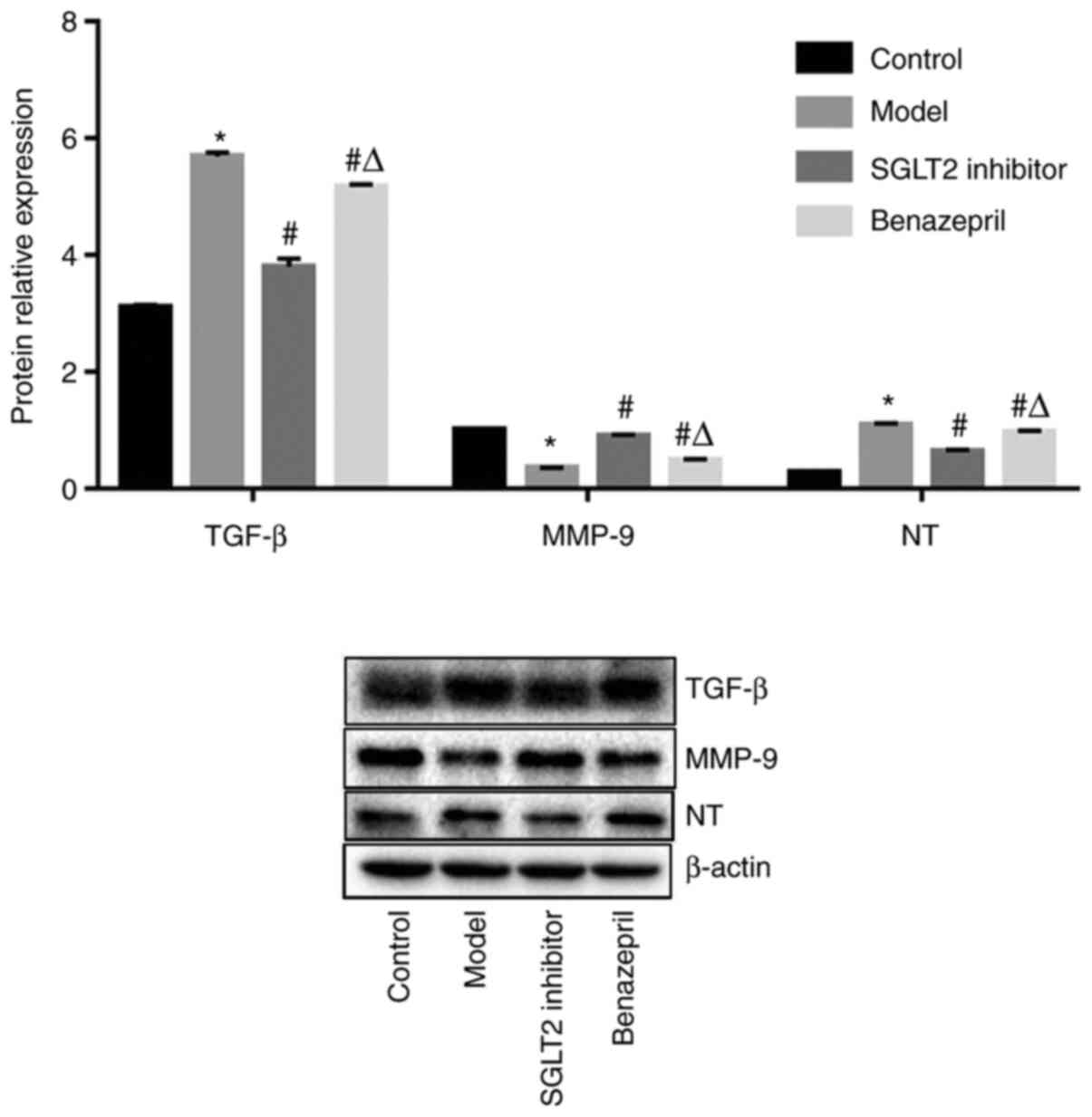
Impact of SGLT2 inhibitor on the
expression level of TGF-β, NT-proBNP, and MMP-9 in renal tissues of
DN rats
As shown in Fig. 5,
the expression level of TGF-β and NT-proBNP was significantly
promoted, and the expression level of MMP-9 remarkably declined in
DN rats compared with control (*P<0.05 vs. control).
After the treatment of SGLT2 inhibitor and benazepril, TGF-β and
NT-proBNP were significantly downregulated, and MMP-9 was
significantly upregulated (#P<0.05 vs. model).
However, compared with SGLT2 inhibitor, the impact of benazepril on
the expression level of TGF-β, NT-proBNP, and MMP-9 was less
significant (ΔP<0.05 vs. SGLT2 inhibitor).
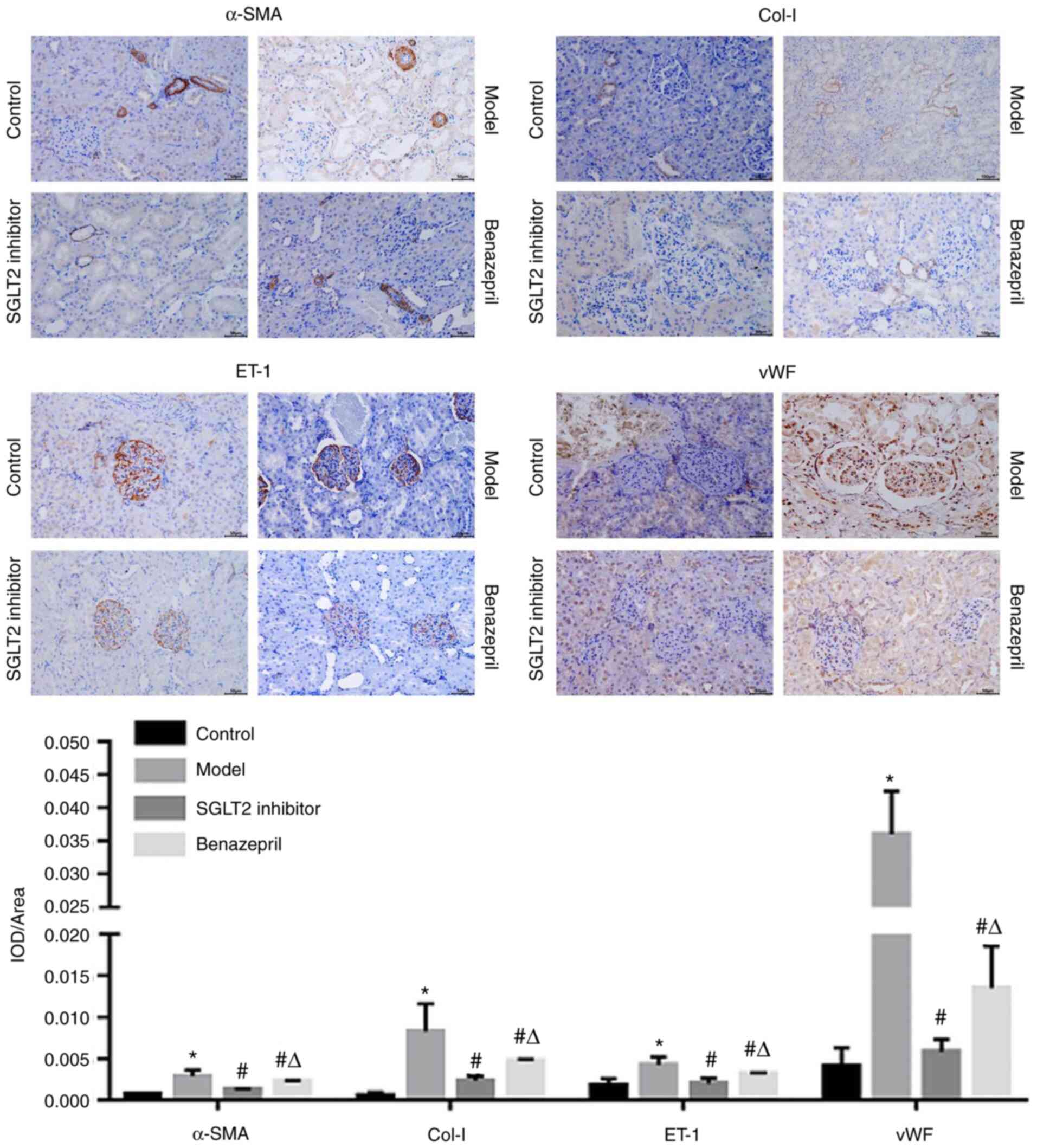
SGLT2 inhibitor significantly
downregulated the expression level of ET-1, vWF, col-I, and
α-SMA
As shown in Fig. 6,
ET-1 was distributed in the glomeruli and tubules. However, α-SMA
and col-I were primarily expressed in the basal membrane of tubular
cells and partly distributed in the mesangial area of the
glomerulus. In addition, vWF was highly expressed in renal tubular
epithelial cells and in the renal interstitium and partly
distributed in endothelial cells and mesangial cells of the
glomerulus. Compared with control, ET-1, vWF, col-I, and α-SMA were
significantly upregulated in DN rats, which were dramatically
downregulated by the treatment of SGLT2 inhibitor or benazepril
(*P<0.05 vs. control, #P<0.05 vs.
model). However, compared with the SGLT2 inhibitor group,
significantly high expression levels of ET-1, vWF, col-I, and α-SMA
were observed in the benazepril group (ΔP<0.05 vs.
SGLT2 inhibitor).
Discussion
Similar to other diabetic microvascular
complications, the pathogenesis and process of DN have not been
fully elucidated, whereas hyperglycemia is the key factor leading
to the onset of DN. At present, DN animal models are established on
the basis of a diabetes model. With disease progression, the
established diabetes model will gradually develop into an
appropriate DN model, and the establishment of an appropriate DN
model is the basis for studying the pathogenesis of DN. At present,
the commonly used method to establish a DM model is the injection
of STZ. The standards for DN modeling using STZ are different. In
the present study, the methods for modeling and identification are
referred to the description reported previously (12). Animals were fed with high-fat diets
for 8 weeks, followed by intraperitoneal injection with 1% STZ
solution to establish the DN model. The results indicated that the
average blood glucose level within 3 weeks after the injection of
STZ was higher than 16.7 mmol/l, and the 24 h urine protein level
was higher than 30 mg, which indicated that the DN model was
successfully established.
DN is a common complication of diabetes. In the
early stage of DN, the development of proteinuria will be induced
by renal enlargement and damage of glomerular filtration function
(13). Some studies have shown
that SGLT2 inhibitors can improve the glomerular filtration rate of
patients with type 2 diabetes, thereby reducing the occurrence of
glomerular toxicity (14). In the
present study, 24 h urinary protein levels decreased significantly
after treatment with SGLT2 inhibitors and benazepril.
DN is a common complication of diabetes, which is
primarily characterized by microcirculation disturbance,
micro-hemangioma formation, and microvascular basement membrane
thickening. At the early stage, the primary renal pathological
changes of DN include renal hypertrophy, mesangial area dilation,
and glomerular basement membrane thickening. Wang reported that
(15) 1 week after STZ injection,
increased tubular lumen and mild necrosis of tubular epithelial
cells were observed in the DN group using the HE staining assay
because of significant renal toxicity. Extensive tubular
vacuolation was observed at the 12th week. In the present study, we
found that the glomerular volume in DN rats was significantly
larger than that in normal rats, accompanied by the thickened basal
membrane of glomerular capillaries, widened mesangial area,
increased collagen fibers, and positive PAS staining on the
mesangial matrix. These observations indicated that a certain renal
damage was induced in DN rats. In addition, based on the TEM
results, in renal tissues of DN rats, epithelial cells in renal
tubules were damaged; endothelial cells and basal membrane were
deposited; the capillary lumen in renal unit was blocked, and the
basal membrane and endothelial cells were shed. After the treatment
of SGLT2 inhibitor and benazepril, the pathological state in DN
rats was significantly alleviated. Moreover, the therapeutic effect
of SGLT2 inhibitor was superior to that of benazepril, which
provided a reliable pathological basis for replacing benazepril
with SGLT2 inhibitor for the clinical treatment of DN.
In the mechanism study of DN, significantly induced
macrophage accumulation and activation could be induced by high
glucose levels, which further trigger the glomerular immune complex
deposition and increase the production of chemokines and
inflammatory factors. Furthermore, excessive release of ROS will be
induced by renal hemodynamics, disordered renal metabolic pathway,
and oxidative stress, which further stimulates the opening of
related signaling pathways, resulting in declined degradation of
the extracellular matrix (ECM) and a large accumulation of ECM in
the kidney. Consequently, renal fibrosis and renal dysfunction will
be induced (16).
TGF-β1 is involved in glomerular and
tubulointerstitial damage during the development of DN by inducing
the synthesis of ECM in epithelial cells, mesangial cells, proximal
convoluted renal tubular epithelial cells, and fibroblasts. In the
early stage of DN, the expression level of TGF-β1 is significantly
elevated. The blood glucose and expression level of TGF-β1 is
positively correlated (17). In
the present study, the upregulation of TGF-β1 was accompanied by
the elevation of blood glucose levels. In addition, the development
of renal fibrosis and DN is mediated by TGF-β1(18). Compared with DN rats, TGF-β1 was
significantly downregulated by the treatment of SGLT inhibitor,
indicating that the SGLT inhibitor might alleviate renal fibrosis
by regulating TGF-β1, which finally contributed to the alleviation
of DN symptoms.
Proliferation will be facilitated, and the trans
differentiation of mesangial cells, renal tubular epithelial cells,
fibroblasts, and pericellular cells into myofibroblasts (MFB) will
be stimulated. In addition, the expression of α-SMA will be induced
by TGF-β1, the expression level of which is used to indirectly
reflect the degree of renal fibrosis. Pathological secretion of
collagens (primarily Col I and Col III) can be observed in MFB,
which further contributes to the excessive deposition of ECM.
Studies have found that the renal cell phenotypic transformation,
secretion of ECM, and inflammatory response induced by high glucose
levels can be repressed by salvianolic acid B, which further
alleviate renal blood flow (18).
In the present study, based on the results of the
immunohistochemical assay, α-SMA and Col I were highly expressed in
the mesangial area of the glomerulus in DN rats, particularly in
vessels. After treatment of SGLT2 inhibitor and benazepril, the
expression of α-SMA and Col I was significantly decreased. Matrix
metalloproteinase (MMP) is a zinc- and calcium-dependent
proteolytic enzyme that degrades ECM. The expression level of the
tissue inhibitor of metalloproteinase and plasminogen activator
inhibitor will be facilitated by TGF-β1 by inhibiting the
degradation of ECM against MMPs. In the MMP family, MMP-9 is an
important proteolytic enzyme (19). In the present study, deposition was
found in endothelial cells and basal membranes, and collagen fibers
were significantly increased in DN rats. The expression level of
MMP-9 was significantly declined. After the treatment of SGLT2
inhibitor and benazepril, MMP-9 was significantly upregulated, and
the number of collagen fibers was decreased, indicating that the
therapeutic effect of SGLT2 inhibitor and benazepril on DN might be
related to the degradation of ECM mediated by MMP-9.
NT-proBNP is a neuroendocrine hormone secreted by
ventricular muscles primarily metabolized by the kidney. In
addition, NT-proBNP is closely associated with the development of
microangiopathy. DN is an important complication of microangiopathy
during the development of diabetes (20,21).
Studies have shown that NT-proBNP is involved in kidney diseases.
NT-proBNP expands vascular diuretic sodium discharge and inhibits
the sympathetic nerve and renin-angiotensin-aldosterone system,
which is a potential indicator of the glomerular overpass rate by
alleviating the declined glomerular overpass rate to delay the
progression of chronic kidney disease (22). In the present study, we found that
NT-proBNP was significantly upregulated in DN rats, which was
consistent with previous reports (23).
Endothelin-1 (ET-1), encoded by the EDN1 gene, is
primarily expressed in glomerular endothelial cells (GENs), and it
plays an important role in DN (24). ET-1 acts by binding to endothelin
receptor subtypes, endothelin A receptor (ETAR), and endothelin B
receptor (ETBR). In ETBR−/− GENs
exposed to high glucose levels, suppression of ET-1 binding to ETBR
activated the NF-κB pathway to secrete large amount of ET-1. Given
the communication between GENs and mesangial cells in diabetes,
ET-1 binding to ETAR in mesangial cells promoted the RhoA/ROCK
pathway, thereby accelerating mesangial cell proliferation and ECM
accumulation (25). Studies have
shown that vascular endothelial injury plays an important role in
the development of DN (26). Von
Willebrand factor (vWF) is a glycoprotein that plays an important
role in platelet formation. The lack of vWF will lead to von
Willebrand disease and result in bleeding, whereas the increased
expression level of vWF will create an environment that promotes
thrombosis. Increased serum vWF level indicates the vascular
endothelial cell injury and blood hypercoagulability (27). Some studies have found that
increased vWF in patients is related to DN-related arteriosclerosis
and blood hypercoagulability, and reducing the vWF level
significantly improves patients' renal function (28). In the present study, in DN rats,
significant injuries in endothelial cells and renal tubular
epithelial cells were observed. Compared with control, the
expression level of vWF in DN rats was significantly promoted.
After the treatment of SGLT2 inhibitor and benazepril, the
expression level of vWF was significantly suppressed, indicating
that the therapeutic effect of SGLT2 inhibitor and benazepril on DN
might be related to the downregulation of vWF.
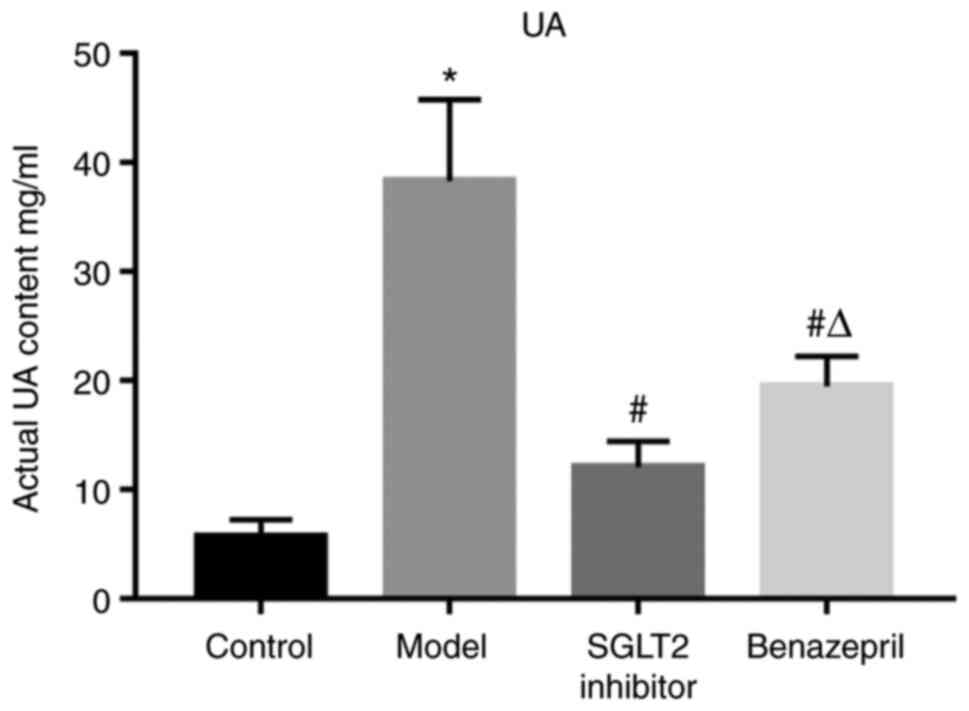
To confirm whether side effects could be induced in
normal mice by the administration of SGLT2 inhibitor. UA content in
24 h and HE staining were performed between the control rats and
SGLT2 inhibitor treated normal rats. As shown in Fig. S1A, no significant difference on
the actual UA content was observed between the control and the
SGLT2 inhibitor treated normal rats. Furthermore, in SGLT2
inhibitor treated normal rats, the glomerular basement membrane was
normal and the structure of the renal tubule was regular, with rare
infiltration of inflammatory cells (Fig. S1B), indicating that SGLT2
inhibitor did not induce any pathological changes on renal tissues
of normal rats. In addition, it is widely reported (29-31)
that the risk of hypoglycemia cannot be induced by the treatment of
SGLT2 inhibitor.
In the present study, DN was successfully
established in rats by STZ. Western Blot and immunohistochemistry
experiments showed that SGLT2 inhibitor and benazepril reduced the
expression of α-SMA and Col I by inhibiting TGF-β1. In addition,
the expression of MMP-9 was upregulated, which inhibited the
accumulation of ECM and improved renal fibrosis and interstitial
fibrosis. Furthermore, the vascular endothelial injury was
repaired, and blood flow dynamics was regulated by SGLT2 inhibitors
and benazepril by reducing the protein expression levels of ET-1,
vWF, and NT-probNP, which finally achieved the effect of improving
vascular lesions. As a SGLT2 inhibitor, dapagliflozin improved
renal pathology and morphology of rats by reducing urinary protein
content. The pathogenesis of DN is complex. Therefore, an in-depth
study on the renal protective mechanism of SGLT2 inhibitors will
provide a solid foundation for personalized drug usage and
precision therapy. Collectively, SGLT2 inhibitors have demonstrated
renal protective ability in clinical and basic studies, and their
application will change the treatment of DN, whereas studying the
renal protective mechanism of SGLT2 inhibitors will provide the
possibility for the wide application of SGLT2 inhibitors in other
nephropathy. The present study provides preliminary findings on the
molecular mechanism of SGLT2 inhibitors, particularly
dapagliflozin, in the treatment of DN.
Supplementary Material
(A) Level of 24 h urine protein in
rats. (B) Pathological analysis on rat renal tissues was evaluated
using hematoxylin and eosin staining (left magnification, x200;
right magnification, x400). No significant pathological changes
were observed in normal mice after the treatment of SGLT2
inhibitor. UA, urea; SGLT2, sodium/glucose cotransporter 2.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LH and FL confirm the authenticity of all the raw
data. LH conceived and designed the study. YaZ and FL performed the
experiments. JH and HC analyzed and interpreted the data. CC, DX
and YiZ performed the statistical analysis. LH drafted the
manuscript. FL revised the manuscript for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments involved in this manuscript
were approved by the Ethical Committee of The Third Affiliated
Hospital of Nanchang University (approval no. 2020031701) and
carried out in accordance with the guidelines for care and use of
laboratory animals and the principles of laboratory animal care and
protection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weng JP and Bi Y: Epidemiological status
of chronic diabetic complications in China. Chin Med J (Engl).
128:3267–3269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang L, Gao P, Zhang M, Huang Z, Zhang D,
Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al: Prevalence and ethnic
pattern of diabetes and prediabetes in China in 2013. JAMA.
317:2515–2523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cho NH, Shaw JE, Karuranga S, Huang Y, da
Rocha Fernandes JD, Ohlrogge AW and Malanda B: IDF diabetes Atlas:
Global estimates of diabetes prevalence for 2017 and projections
for 2045. Diabetes Res Clin Pract. 138:271–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zaccardi F, Webb DR, Htike ZZ, Youssef D,
Khunti K and Davies MJ: Efficacy and safety of sodium-glucose
co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic
review and network meta-analysis. Diabetes Obes Metab. 18:783–794.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hodrea J, Balogh DB, Hosszu A, Lenart L,
Besztercei B, Koszegi S, Sparding N, Genovese F, Wagner LJ, Szabo
AJ and Fekete A: Reduced O-GlcNAcylation and tubular hypoxia
contribute to the antifibrotic effect of SGLT2 inhibitor
dapagliflozin in the diabetic kidney. Am J Physiol Renal Physiol.
318:F1017–F1029. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rådholm K, Figtree G, Perkovic V, Solomon
SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai
M, et al: Canagliflozin and heart failure in type 2 diabetes
mellitus: Results from the CANVAS program. Circulation.
138:458–468. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sattar N, McLaren J, Kristensen SL, Preiss
D and McMurray JJ: SGLT2 inhibition and cardiovascular events: Why
did EMPA-REG outcomes surprise and what were the likely mechanisms?
Diabetologia. 59:1333–1339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xue L, Feng X, Wang C, Zhang X, Sun W and
Yu K: Benazepril hydrochloride improves diabetic nephropathy and
decreases proteinuria by decreasing ANGPTL-4 expression. BMC
Nephrol. 18(307)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li H, Wang Y, Zhou Z, Tian F, Yang H and
Yan J: Combination of leflunomide and benazepril reduces renal
injury of diabetic nephropathy rats and inhibits high-glucose
induced cell apoptosis through regulation of NF-κB, TGF-β and
TRPC6. Ren Fail. 41:899–906. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arab HH, Al-Shorbagy MY and Saad MA:
Activation of autophagy and suppression of apoptosis by
dapagliflozin attenuates experimental inflammatory bowel disease in
rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem
Biol Interact. 335(109368)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lin L, Hou G, Han D, Yin Y, Kang J and
Wang Q: Ursolic acid alleviates airway-vessel remodeling and muscle
consumption in cigarette smoke-induced emphysema rats. BMC Pulm
Med. 19(103)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang Y, Zhang Z, Su K, Chen Z and Huang S:
Methodological study of streptozotocin-induced diabetic nephropathy
model in rats. West China Medicine. 20:299–300. 2005.
|
|
13
|
Chang TT and Chen JW: The role of
chemokines and chemokine receptors in diabetic nephropathy. Int J
Mol Sci. 21(3172)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Scheen AJ: SGLT2 inhibitor empagliflozin
reduces renal outcomes and dampens the progressive reduction in
glomerular filtration rate in patients with type 2 diabetes and
antecedents of cardiovascular disease. Evid Based Med. 22:69–70.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang W, Li Z, Chen Y, Wu H, Zhang S and
Chen X: Prediction value of serum NGAL in the diagnosis and
prognosis of experimental acute and chronic kidney injuries.
Biomolecules. 10(981)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sifuentes-Franco S, Padilla-Tejeda DE,
Carrillo-Ibarra S and Miranda-Díaz AG: Oxidative stress, apoptosis,
and mitochondrial function in diabetic nephropathy. Int J
Endocrinol. 2018(1875870)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garcia FA, Rebouças JF, Balbino TQ, da
Silva TG, de Carvalho-Júnior CH, Cerqueira GS, Brito GA and Viana
GS: Pentoxifylline reduces the inflammatory process in diabetic
rats: Relationship with decreases of pro-inflammatory cytokines and
inducible nitric oxide synthase. J Inflamm (Lond).
12(33)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Navarro-González JF, Mora-Fernández C,
Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, Macía M, del
Castillo N, Rivero A, Getino MA, et al: Effect of pentoxifylline on
renal function and urinary albumin excretion in patients with
diabetic kidney disease: The PREDIAN trial. J Am Soc Nephrol.
26:220–229. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dimas GG, Didangelos TP and Grekas DM:
Matrix gelatinases in atherosclerosis and diabetic nephropathy:
Progress and challenges. Curr Vasc Pharmacol. 15:557–565.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu R and Liang G: Changes and significance
of serum homocysteine, uric acid and blood lipid levels in patients
with chronic heart failure. Hebei Med J. 39:2113–2116. 2017.
|
|
21
|
Zhao Huihui XD: The value of NT proBNP in
the evaluation of curative effect and prognosis of patients with
heart failure. Modern Instruments Med. 21:101–102. 2015.
|
|
22
|
Chang LH, Hwu CM, Chu CH, Lin YC, Huang
CC, You JY, Chen HS and Lin LY: The combination of soluble tumor
necrosis factor receptor type 1 and fibroblast growth factor 21
exhibits better prediction of renal outcomes in patients with type
2 diabetes mellitus. J Endocrinol Invest. 44:2609–2619.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Svensson M, Gorst-Rasmussen A, Schmidt EB,
Jorgensen KA and Christensen JH: NT-pro-BNP is an independent
predictor of mortality in patients with end-stage renal disease.
Clin Nephrol. 71:380–386. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Gagliardini E, Zoja C and Benigni A: Et
and diabetic nephropathy: Preclinical and clinical studies. Semin
Nephrol. 35:188–196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zou HH, Wang L, Zheng XX, Xu GS and Shen
Y: Endothelial cells secreted endothelin-1 augments diabetic
nephropathy via inducing extracellular matrix accumulation of
mesangial cells in ETBR(-/-) mice. Aging (Albany NY). 11:1804–1820.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Karasek D, Spurna J, Kubickova V,
Krystynik O, Cibickova L, Schovanek J and Goldmannova D:
Association of pigment epithelium derived factor with von
Willebrand factor and plasminogen activator inhibitor 1 in patients
with type 2 diabetes. Physiol Res. 68:409–418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Favaloro EJ, Henry BM and Lippi G:
Increased VWF and decreased ADAMTS-13 in COVID-19: Creating a
milieu for (micro)thrombosis. Semin Thromb Hemost. 47:400–418.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tang S, Wang X, Deng T, Ge H and Xiao X:
Identification of C3 as a therapeutic target for diabetic
nephropathy by bioinformatics analysis. Sci Rep.
10(13468)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ferrannini E, Seman L, Seewaldt-Becker E,
Hantel S, Pinnetti S and Woerle HJ: A phase IIb, randomized,
placebo-controlled study of the SGLT2 inhibitor empagliflozin in
patients with type 2 diabetes. Diabetes Obes Metab. 15:721–728.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Stenlöf K, Cefalu WT, Kim KA, Alba M,
Usiskin K, Tong C, Canovatchel W and Meininger G: Efficacy and
safety of canagliflozin monotherapy in subjects with type 2
diabetes mellitus inadequately controlled with diet and exercise.
Diabetes Obes Metab. 15:372–382. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ferrannini E, Ramos SJ, Salsali A, Tang W
and List JF: Dapagliflozin monotherapy in type 2 diabetic patients
with inadequate glycemic control by diet and exercise: A
randomized, double-blind, placebo-controlled, phase 3 trial.
Diabetes Care. 33:2217–2224. 2010.PubMed/NCBI View Article : Google Scholar
|