Introduction
Deep angiomyxoma (DAM), which was first described by
Steeper and Rossi in 1983, is a rare, infiltrative,
hormone-dependent, benign and mesenchymal neoplasm that typically
occurs in the deep soft tissues in the perineal region (1,2).
Clinically, DAM mimics more common gynecological conditions, such
as Bartholin's cyst, lipoma and hernia, rendering it frequently
misdiagnosed (3). In addition, DAM
is histologically a hypocellular and hypervascular tumor with a
mucinous stroma, containing cytologically pale stellate or
spindle-shaped cells with consistent nuclear immunoreactivity for
estrogen receptors (ER) and progesterone receptors (PgR) (4). High mobility group AT-hook 2
immunohistochemical staining is also a useful auxiliary marker, but
it lacks specificity (5). The
standard treatment method for DAM is normally surgical resection
followed by histological diagnosis, which relieves the mass effect
(4). However, the relatively high
recurrence rate, ranging from 30 to 40%, remains an unsolved
problem (4). Furthermore, the
radicality of surgery (negative or positive surgical margins) has
no statistically significant impact on the progression-free
interval (6).
Therefore, hormone therapy is frequently used in
combination with surgery to prevent recurrence (7). The majority of the drugs used for
DAMs are gonadotropin-releasing hormone agonist (GnRHa)
preparations, though there have been various reports of the use of
aromatase inhibitors or selective ER modulators (SERMs) (7). In addition, since DAM tends to be
more common in premenopausal females, recurrence after the
completion of GnRHa therapy has been frequently reported (7). However, the role of prophylactic
oophorectomy has remained poorly understood (8).
The present report doucments a case of a
premenopausal woman who underwent DAM tumor resection and
prophylactic oophorectomy followed by adjuvant hormonal therapy
with an aromatase inhibitor to prevent recurrence.
Case report
A 42-year-old Japanese female (gravida 4, para 2)
first visited the Plastic Surgery Department of Oita University
Hospital (Oita, Japan) in January 2021, complaining of a refractory
Bartholin's cyst persisting for 2 months. The patient had a medical
history of lumbar disc hernia (timing of disease was unclear). On
clinical examination, the patient's left labium majus was
determined to be enlarged and saddled. Subcutaneous tissue
hyperplasia due to chronic inflammation was suspected after
examination by transperineal ultrasonography (the ARIETTA 50
ultrasound machine; Hitachi, Ltd.; 2-10 Hz resolution transvaginal
ultrasound probe). Unenhanced pelvic CT (Aquilion ONE/PRISM
Edition; Canon Medical Systems; tube voltage, 120 kilovolt peak;
field of view, 398.44 mm2; scan speed, 0.5 rotation/sec;
pitch factor, 0.813; helical pitch, 65.0; reconstruction software
algorithm, Advanced intelligent Clear-IQ Engine Body sharp mild;
slice thickness, 1.0 mm; dose index, 8.6 mGy) indicated a
low-density, irregular pelvic mass, suggesting DAM or
angiofibroblastoma. Pelvic MRI (3.0T MRI MAGNETOM Skyra VE11C;
Siemens AG; scan time, 2:42; repetition time, 4,000 msec; echo
time, 90 msec; turbo factor, 15; band width, 302 Hz/px; slice
thickness, 3 mm; field of view, 25x25 cm; matrix size, 0.6x0.6 mm;
Parallel Imaging, GRAPPA 2) indicated a nodular, structured tumor
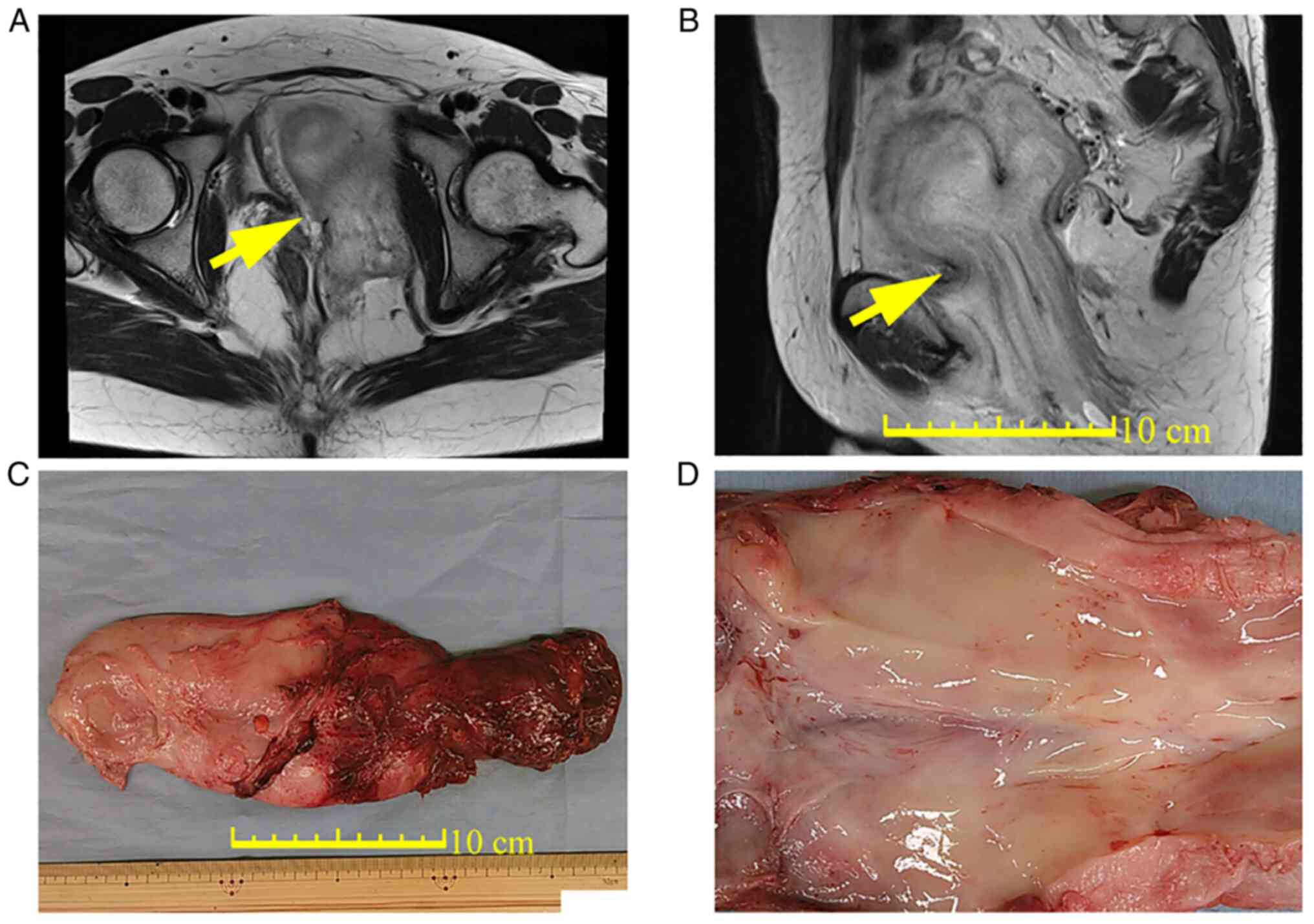
with a maximum diameter of 14 cm (Fig.
1A). The tumor, which had a swirled and layered pattern
according to T2-weighted MRI, extended from the left vulva
subcutaneously to the paravesical space (Fig. 1B). DAM was therefore suspected. The
patient was thereby referred to the Gynecology department, where a
transperineal tumor biopsy was performed. Pathological examination
results strongly suggested DAM. Therefore, considering all clinical
findings, DAM was finally diagnosed. Contrast-enhanced CT of the
chest and pelvis revealed no metastatic lesions.
The patient underwent bilateral ureteral stent
placement, followed by transabdominal and transperineal tumor
resection and bilateral salpingo-oophorectomy (BSO), performed by
trained gynecological oncologists and urologists. Intraoperatively,
the tumor extended from the left vulva to the anterior surface of
the bladder and the retroperitoneal space on the left side of the
uterus. The tumor was resected en bloc without any adjunct
organ injuries and weighed 480 g (Fig.
1C). Macroscopically, the resected tumor was not well-defined,
elastoplastic and soft with a glistening and gelatinous surface
(Fig. 1D). The excised specimens
were fixed using neutral-buffered 10% formalin, dehydrated in a
series of ethanols and embedded in paraffin. Serial sections of
4-µm thickness were made and then subjected to hematoxylin-eosin
staining. Immunohistochemical staining was performed with a Ventana
automated immunostainer (Ventana Medical Systems, Inc.) using an
UltraView Universal DAB Detection Kit (Ventana Medical Systems,
Inc.). Pathological diagnosis indicated DAM against a background of
sparse connective tissue with a mucous matrix (Fig. 2A) and mildly atypical
spindle-shaped cell proliferation (Fig. 2B). The tumor cells exhibited
diffuse positive immunoreactivity for ER (cat. no. 790-4325;
dilution, 1:1; Ventana Medical Systems, Inc.; Fig. 2C), PgR (cat. no. 790-4296;
dilution, 1:1; Ventana Medical Systems, Inc.; Fig. 2D) and desmin (cat. no. PA0033;
dilution, 1:1; Leica Microsystems GmbH; Fig. 2E). The tumor was partially positive
for α-smooth muscle actin (cat. no. PA0943; dilution, 1:1; Leica
Microsystems GmbH; Fig. 2F) and
CD34 (cat. no. PA0212; dilution, 1:1; Leica Microsystems GmbH;
Fig. 2G). However, it was negative
for cyclin-dependent kinase 4 staining (cat. no. AHZ0202; dilution,
1:40; Invitrogen; Thermo Fisher Scientific, Inc.). The patient's
postoperative course was uneventful. At 1 month postoperatively, a
follow-up MRI confirmed no residual tumor. The patient started
receiving oral letrozole treatment (2.5 mg/day), an aromatase
inhibitor, 1 month after surgery. A follow-up CT scan after 1 year
confirmed no recurrent lesion. Currently (June 2022, 1 year after
surgery), the patient is continuing with letrozole treatment. Mild
menopausal symptoms (hot flashes and tiredness) associated with
oophorectomy and aromatase inhibitor therapy were observed, but the
general condition was good.
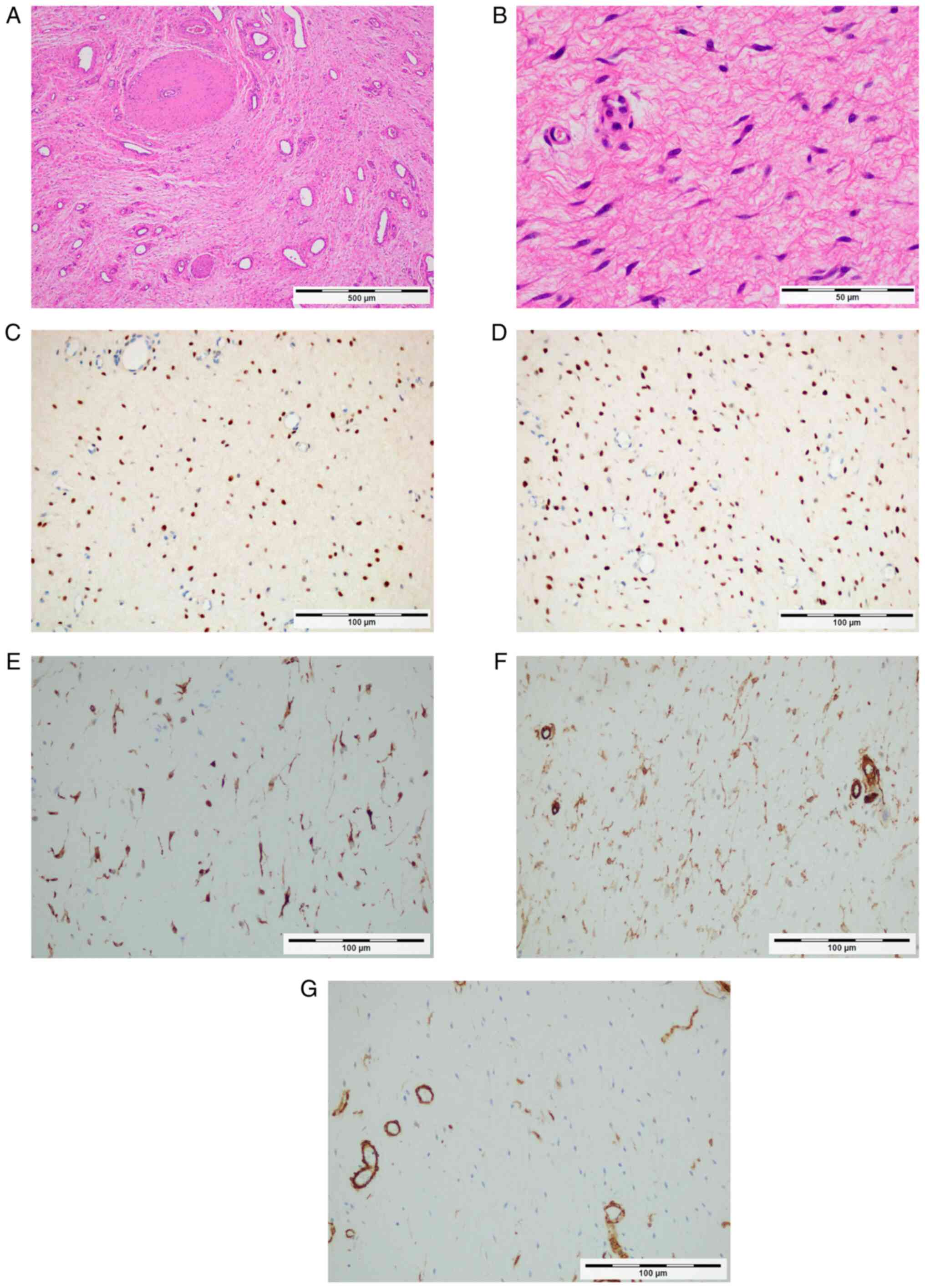 | Figure 2Tumor pathology and hormone receptor
expression. (A) The tumor is comprised loose fibromyxoid stroma,
fine collagen fibers and prominent vessels, according to H&E
staining (original magnification, x4; scale bar, 500 µm). (B)
High-power photomicrograph indicates the proliferation of mildly
atypical spindle-shaped cells according to H&E staining
(original magnification, x40; scale bar, 500 µm). Immunoreactivity
for (C) estrogen receptor (D) progesterone receptor is positive,
(E) desmin, (F) α-smooth muscle actin and (G) CD34 (original
magnification, x20; scale bar, 100 µm). |
Written informed consent was obtained from the
patient to publish anonymized data in the present case report. In
addition, the patient was informed and consented to all the
benefits and risks of this new treatment (prophylactic oophorectomy
and aromatase inhibitor) in advance.
Discussion
The present case demonstrates two important clinical
issues. First, a multidisciplinary team approach enabled the wide
local resection of the DAM without permanent sequelae. Furthermore,
prophylactic oophorectomy was performed for premenopausal DAM and
an aromatase inhibitor was administered as postoperative adjuvant
therapy.
The standard treatment strategy for DAM is surgery
with R0 resection (negative resection margin) (6). However, R0 resection is technically
difficult because DAM frequently infiltrates adjacent soft tissues
and organs and is poorly circumscribed (6). Therefore, R1 resection or fractional
resection is acceptable when a high risk of morbidity due to
extensive surgery is anticipated (9).
Whether surgical radicality affects clinical
outcomes in DAM remains controversial. Chan et al (6) previously reviewed 73 reported cases
of patients with DAM who underwent surgery and observed that 34
(47%) had recurrence. In addition, there was no significant
difference in the recurrence rate between the patients with
positive and negative resection margins. It should be noted that
there was no information in this previous report (6) regarding hormone manipulation therapy.
Furthermore, Zou et al (10) reviewed the data of 27 patients who
underwent surgery performed by a single surgeon at a single
university hospital over 15 years. They determined that a clear
surgical margin was an independent prognostic factor for the
disease-free interval (10). It
may be speculated that a radical multidisciplinary surgical
approach with greater invasiveness performed by skilled surgeons
may contribute to more favorable outcomes in patients with DAM.
Given that DAM occurs predominantly during
premenopausal periods or the fourth decade of life (11), reported rapid growth during
pregnancy (12) and stains
positive immunohistochemically for ER and PgR, it is highly likely
that DAM is hormone-sensitive (13). Therefore, hormonal therapy with
ovarian-derived estrogen, progesterone and non-ovarian-derived
estrogen, is frequently used in combination with surgical
resection.
Hormonal therapy for DAM has been used in both
primary and recurrent settings with agents, such as GnRHa,
aromatase inhibitors and SERMs (7). In particular, GnRHa has been actively
used as an adjuvant therapy for premenopausal DAM. However, in
premenopausal women, recurrence and enlargement of the lesions
after completion of hormone therapy has been frequently observed
due to the residual ovaries (7).
Artificial menopause with prophylactic oophorectomy may have a
longer-lasting effect compared with GnRHa for preventing
recurrence, since it permanently depletes ovarian-derived estrogen
and progesterone. Table I
summarizes seven premenopausal patients with DAM who underwent
prophylactic oophorectomy and radical resection. None of the
patients had any recurrence after bilateral salpingo-oophorectomy
was performed.
 | Table IReported cases of prophylactic
oophorectomy for premenopausal deep angiomyxoma. |
Table I
Reported cases of prophylactic
oophorectomy for premenopausal deep angiomyxoma.
| First author,
year | Age, Years | Relapses, n | History/neoadjuvant
therapy | Concurrent surgeries
with radical resection | Adjuvant therapy | Follow-up,
months | Outcome | (Refs.) |
|---|
| Fetsch, 1996 | 35 | 1 | Local resection | TAH + BSO | Radiation | 91 | NED | (11) |
| Lourenço, 2013 | 47 | 2 | Two local
resections | TAH + BSO | None | 12 | NED | (9) |
| Sirasagi, 2014 | 45 | 0 | None | TAH + BSO | None | NA | NA | (22) |
| Beuran, 2017 | 45 | 5 | Four local
resections | TAH + BSO + Ureter
resection | None | 12 | NED | (23) |
| Song, 2017 | 49 | 0 | Fulvestrant +
goserelin | TAH + BSO + Anterior
exenteration | None | 15 | NED | (24) |
| Gaurav, 2020 | 45 | 0 | None | Lap-BSO | None | NA | NA | (25) |
| Tonai,
2022a | 42 | 0 | None | Abdominal BSO | Letrozole | 12 | NED | |
DAM causing death is a rare phenomenon (14). The present study also focused on
non-ovarian-derived estrogen to minimize the recurrence risk of
DAM. Non-ovarian-derived estrogen is synthesized by aromatase from
androstenedione derived from adipose tissue and adrenal glands
(15). In postmenopausal females
(whether natural or artificial), non-ovarian-derived estrogen
requires strict control. Aromatase inhibitors and SERMs inhibit
estrogen synthesis and receptors, respectively, to reduce the
recurrence of aggressive angiomyomas more effectively compared with
GnRHa. Fucà et al (7)
previously reported that hormonal therapy with aromatase inhibitors
and SERMs tended to result in longer progression-free survival.
Reported cases of patients receiving aromatase inhibitors or SERMs
are summarized in Table II. Of
the 12 patients, including one male patient, who underwent surgery
and anti-estrogen therapy (including GnRHa), three had stable
disease, six had partial response and three had a complete
response. The effects of aromatase inhibitors and SERMs were
independent of sex, age, menopausal status and surgical treatment
(7). Therefore, it was necessary
to use aromatase inhibitors or SERMs to suppress the levels of
non-ovarian derived estrogen. Since SERMs act as agonists or
antagonists of estrogen on an organ-by-organ basis, an aromatase
inhibitor was selected in the present report. Furthermore, similar
treatment options may be effective for other hormone-sensitive soft
tissue tumors, such as leiomyoma and adenomyosis. Mizoguchi et
al (16) and Nasu et al
(17) reported that a combination
of prophylactic oophorectomy and adjuvant aromatase inhibitors is
effective for premenopausal patients with intravenous
leiomyomatosis and benign metastasizing leiomyoma, respectively. In
addition, combined ovarian ablation and aromatase inhibition were
effective for metastatic breast cancer in premenopausal women
(18). Oophorectomy also improved
primary cancer incidence and mortality in women with BRCA
mutations (19,20). Advantages of BSO include preventing
recurrence due to permanent hormone deficiency and avoiding
long-term GnRH administration (7).
By contrast, disadvantages include the possibility of hormone
deficiency symptoms such as menopause and osteoporosis. The
advantage of aromatase inhibitors is that they can inhibit
recurrence by suppressing non-ovarian derived estrogen, whilst
disadvantages include the requirement for long-term medication and
possible side effects, such as menopausal symptoms, liver
dysfunction, osteoporosis and lipid metabolism abnormalities
(21).
 | Table IIClinical outcomes of patients treated
with aromatase inhibitors or selective estrogen receptor
modulators. |
Table II
Clinical outcomes of patients treated
with aromatase inhibitors or selective estrogen receptor
modulators.
| First author,
year | Sex | Age, years | Tumor resection | BSO | Type of hormone
therapy | Therapy response | (Refs.) |
|---|
| Fucà, 2019 | Female | 61 | Yes | No | Anastrozole | SD | (7) |
| Fucà, 2019 | Male | 63 | Yes | No | Letrozole | SD | (7) |
| Fucà, 2019 | Female | 40 | No | No | Tamoxifen | PR | (7) |
| Fucà, 2019 | Female | 35 | Yes | No | Raloxifen | PR | (7) |
| Fucà, 2019 | Female | 45 | Yes | No | Leuprorelin +
tamoxifen | CR | (7) |
| Fucà, 2019 | Female | 43 | Yes | No | Triptorelin +
tamoxifen | PR | (7) |
| Fucà, 2019 | Female | 54 | Yes | No | Anastrozole | SD | (7) |
| Fucà, 2019 | Female | 37 | Yes | No | Triptorelin +
letrozole | CR | (7) |
| Fucà, 2019 | Female | 48 | Yes | No | Triptorelin +
letrozole | PR | (7) |
| Lee, 2019 | Female | 44 | No | No | Leuprolide +
anastrozole | PR | (20) |
| Giles, 2008 | Female | 78 | No | No | Exemestane | PR | (21) |
| Tonai, 2022 | Female | 42 | Yes | Yes | Letrozole | CR | Present case |
A literature search was conducted in MEDLINE/PubMed
(https://pubmed.ncbi.nlm.nih.gov/) and
Google Scholar (https://scholar.google.co.jp/schhp?hl=ja) for cases of
prophylactic oophorectomy with the administration of aromatase
inhibitors as a potential treatment for DAM. The English-language
literature was searched using the terms ‘aggressive
angiomyoplasty’, ‘deep angiomyoplasty’ and ‘oophorectomy’, with no
publication date filter. Cases in which therapeutic oophorectomy
was performed were excluded. To the best of our knowledge, the
present study was the first to report prophylactic oophorectomy
followed by treatment using an aromatase inhibitor as a strategy
for DAM. A limitation of the present report is the short follow-up
period. In addition, the criteria for cases that should receive
hormone therapy remain controversial. Since DAM has a high
postoperative recurrence rate (30-50%) (4), it may be suggested that hormone
therapy (prophylactic oophorectomy or an aromatase inhibitor)
should be actively introduced. Case accumulation and long-term
follow-up on this treatment strategy for DAM is required in the
future.
In conclusion, surgical resection with minimal
invasiveness is preferred for premenopausal women with DAM, but
radical surgery with greater invasiveness should be used if
necessary. Prophylactic oophorectomy and adjuvant hormone therapy
with aromatase inhibitors, as well as GnRH agonists, may be
promising treatment options to optimize the outcome of surgical
treatment. Furthermore, this treatment strategy may also apply to
hormone-sensitive mesenchymal tumors. Long-term follow-up is
required to confirm late and local recurrence of DAM, along with
the side effects of adjuvant hormonal therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NT and MY drafted the manuscript and made
substantial contributions to the conception and design of the
study, as well as the acquisition, analysis, interpretation of
data. NT and MY confirm the authenticity of all the raw data. MS,
KK and MN made contributions to the acquisition, analysis and
interpretation of data. KN and YK made contributions to the
analysis and interpretation of data, as well as reviewing and
editing of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
participant for participation in the study and the publication of
the data. The patient consented to the images being taken for
research and also consented to their publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO Classification of Tumours Editorial
Board (ed.): Female genital tumours: WHO Classification of Tumours
(Medicine). Vol 4. 5th edition. International Agency for Research
on Cancer, Lyon France, 2020.
|
|
2
|
Steeper TA and Rosai J: Aggressive
angiomyxoma of the female pelvis and perineum. Report of nine cases
of a distinctive type of gynecologic soft-tissue neoplasm. Am J
Surg Pathol. 7:463–475. 1983.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Smith HO, Worrell RV, Smith AY, Dorin MH,
Rosenberg RD and Bartow SA: Aggressive angiomyxoma of the female
pelvis and perineum: Review of the literature. Gynecol Oncol.
42:79–85. 1991.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chapel DB, Cipriani NA and Bennett JA:
Mesenchymal lesions of the vulva. Semin Diagn Pathol. 38:85–98.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Harkness R and McCluggage WG: HMGA2 is a
useful marker of vulvovaginal aggressive angiomyxoma but may be
positive in other mesenchymal lesions at this site. Int J Gynecol
Pathol. 40:185–189. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chan YM, Hon E, Ngai SW, Ng TY and Wong
LC: Aggressive angiomyxoma in females: Is radical resection the
only option? Acta Obstet Gynecol Scand. 79:216–220. 2000.PubMed/NCBI
|
|
7
|
Fucà G, Hindi N, Ray-Coquard I, Colia V,
Dei Tos AP, Martin-Broto J, Brahmi M, Collini P, Lorusso D,
Raspagliesi F, et al: Treatment outcomes and sensitivity to hormone
therapy of aggressive angiomyxoma: A multicenter, International,
retrospective study. Oncologist. 24:e536–e541. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dahiya K, Jain S, Duhan N, Nanda S and
Kundu P: Aggressive angiomyxoma of vulva and vagina: A series of
three cases and review of literature. Arch Gynecol Obstet.
283:1145–1148. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lourenço C, Oliveira N, Ramos F, Ferreira
I and Oliveira M: Aggressive angiomyxoma of the vagina: A case
report. Rev Bras Ginecol Obstet. 35:575–582. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zou R, Xu H, Shi Y, Wang J, Wang S and Zhu
L: Retrospective analysis of clinicopathological features and
prognosis for aggressive angiomyxoma of 27 cases in a tertiary
center: A 14-year survey and related literature review. Arch
Gynecol Obstet. 302:219–229. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fetsch JF, Laskin WB, Lefkowitz M,
Kindblom LG and Meis-Kindblom JM: Aggressive angiomyxoma: A
clinicopathologic study of 29 female patients. Cancer. 78:79–90.
1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Orfanelli T, Kim CS, Vitez SF, Van Gurp J
and Misra N: A case report of aggressive angiomyxoma in pregnancy:
Do hormones play a role? Case Rep Obstet Gynecol.
2016(6810368)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kooy J, Carlson V, Šačiragić L, Sawhney S
and Nelson G: A case series of aggressive angiomyxoma: Using
morphologic type and hormonal modification to tailor treatment.
Gynecol Oncol Rep. 36(100765)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Blandamura S, Cruz J, Faure Vergara L,
Machado Puerto I and Ninfo V: Aggressive angiomyxoma: A second case
of metastasis with patient's death. Hum Pathol. 34:1072–1074.
2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dellapasqua S and Colleoni M: Letrozole.
Expert Opin Drug Metab Toxicol. 6:251–259. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mizoguchi C, Matsumoto H, Nasu K, Arakane
M, Kai K and Narahara H: Intravenous leiomyomatosis treated with
radical hysterectomy and adjuvant aromatase inhibitor therapy. J
Obstet Gynaecol Res. 42:1405–1408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nasu K, Tsuno A, Takai N and Narahara H: A
case of benign metastasizing leiomyoma treated by surgical
castration followed by an aromatase inhibitor, anastrozole. Arch
Gynecol Obstet. 279:255–257. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
El-Saghir NS, El-Hajj II, Makarem JA and
Otrock ZK: Combined ovarian ablation and aromatase inhibition as
first-line therapy for hormone receptor-positive metastatic breast
cancer in premenopausal women: Report of three cases. Anticancer
Drugs. 17:999–1002. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Metcalfe K, Lynch HT, Foulkes WD, Tung N,
Kim-Sing C, Olopade OI, Eisen A, Rosen B, Snyder C, Gershman S, et
al: Effect of oophorectomy on survival after breast cancer in BRCA1
and BRCA2 mutation carriers. JAMA Oncol. 1:306–313. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Finch AP, Lubinski J, Møller P, Singer CF,
Karlan B, Senter L, Rosen B, Maehle L, Ghadirian P, Cybulski C, et
al: Impact of oophorectomy on cancer incidence and mortality in
women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 32:1547–1553.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen D, Reierstad S, Lu M, Lin Z, Ishikawa
H and Bulun SE: Regulation of breast cancer-associated aromatase
promoters. Cancer Lett. 273:15–27. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sirasagi A and Arakeri S: Deep aggressive
angiomyxoma of pelvic soft tissue: A rare case report. J Obstet
Gynaecol India. 64:438–439. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Beuran M, Ciubotaru C, Runcanu A, Enache V
and Negoi I: Surgical resection of retroperitoneal aggressive
angiomyxoma: Case report and review of the literature. Cureus.
9(e1485)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song M, Glasgow M, Murugan P and Rivard C:
Aggressive angiomyxoma of the vulva and bladder. Obstet Gynecol.
130:885–888. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gaurav A, Gill P, Khoiwal K, Chowdhuri S,
Kapoor D and Chaturvedi J: Aggressive angiomyxoma of the vulva-a
rare entity: Case report and review of literature. Int J Reprod
Contracept Obstet Gynecol. 9:2605–2609. 2020.
|
|
26
|
Lee MY, da Silva B, Ramirez DC and Maki
RG: Novel HMGA2-YAP1 fusion gene in aggressive angiomyxoma. BMJ
Case Rep. 12(e227475)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Giles DL, Liu PT, Lidner TK and Magtibay
PM: Treatment of aggressive angiomyxoma with aromatase inhibitor
prior to surgical resection. Int J Gynecol Cancer. 18:375–379.
2008.PubMed/NCBI View Article : Google Scholar
|