Introduction
Inverted hyperplastic polyp (IHP) was first reported
by Kamata et al (1). This
disease is very rare and is characterized by the downward growth of
hyperplastic mucosa into the submucosa, forming an inverted
histological morphology that presents an overall vase-like
appearance under low magnification, whereas hyperplastic polyps
generally grow to the mucosal surface and into the gastric cavity
(2). The polyp consists of
foveolar-type cells, pyloric gland-like cells, gastric gland cells
and smooth muscle cells (3,4) and
a few cases have been accompanied by canceration (1). IHP was considered heterotopic or
hamartomatous until the 1990s and various terms have been coined
for the lesions, including ‘solitary polypoid hamartoma,’ ‘unusual
heterotopia of pyloric glands,’ and ‘submucosal unusual polyps’
(5). IHP is often misdiagnosed as
other tumor types and correct diagnosis usually requires
pathological assessment. The present study reported a case of IHP
diagnosed on admission due to abdominal pain and treated by
endoscopic mucosal resection (EMR).
Case report
Case presentation
A 75-year-old man presented with abdominal pain and
constipation, which he had experienced for one and a half years.
The patient originally developed abdominal distension and pain
(upper abdomen dominant) without obvious reasons, accompanied by
defecation once every 3-4 days, laborious defecation and dry stool.
The patient took trimebutine and probiotics after symptom onset to
help with defecation, but the therapeutic effect was not
significant. The month before he presented for care, he experienced
unexplained diarrhea followed by alternating episodes of diarrhea
and constipation. The patient denied having other illnesses.
Laboratory examination and routine blood and fecal tests were
normal and showed no Helicobacter pylori infection.
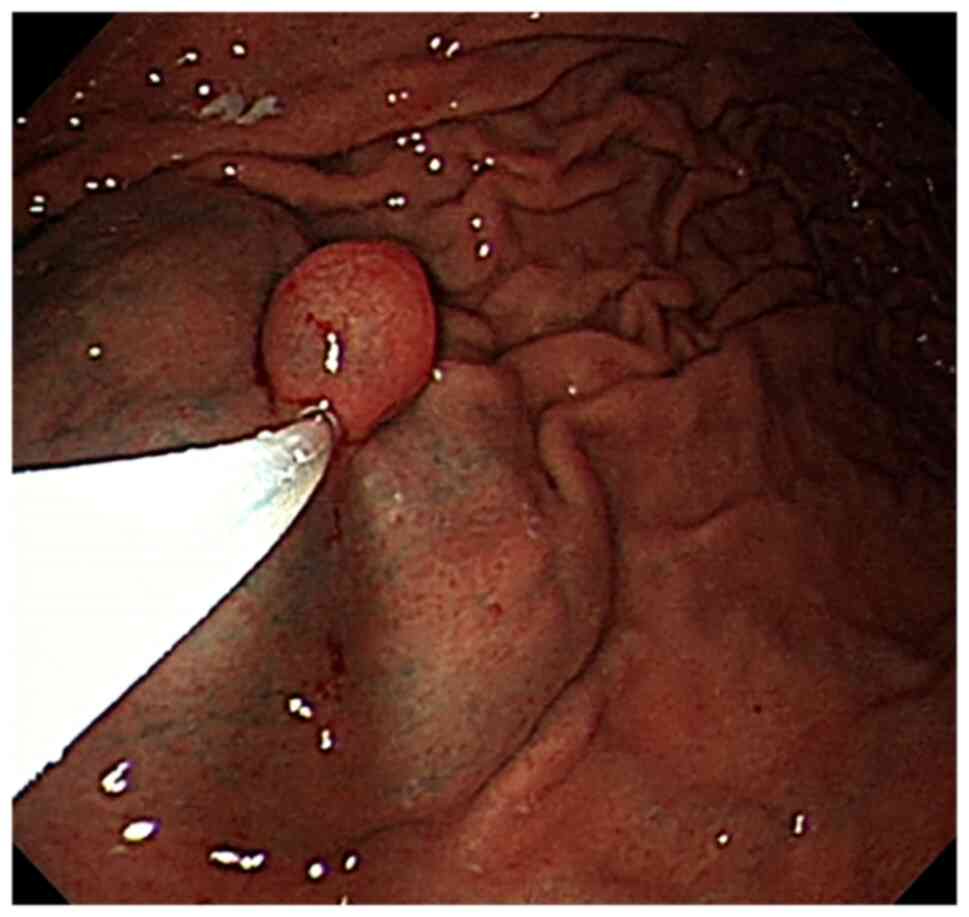
Endoscopy showed a 15-mm submucosal eminence with a
smooth surface in the greater curvature of the stomach. After
normal saline (with epinephrine and indigo carmine) was injected
into the base to lift the tumor, the tumor was completely excised
with a high-frequency electrical snare and the wound surface was
clamped with metal for hemostasis (Figs. 1 and 2). Base on that results of the endoscopy,
neuroendocrine tumors, heterotopic pancreatic and gastrointestinal
stromal tumors were considered. and the final diagnosis awaited
pathological examination. The present study was approved by the
Ethics Committee of Sunshine Union Hospital (approval no.
2022-11-0028).
Pathological findings.
Macro-examination
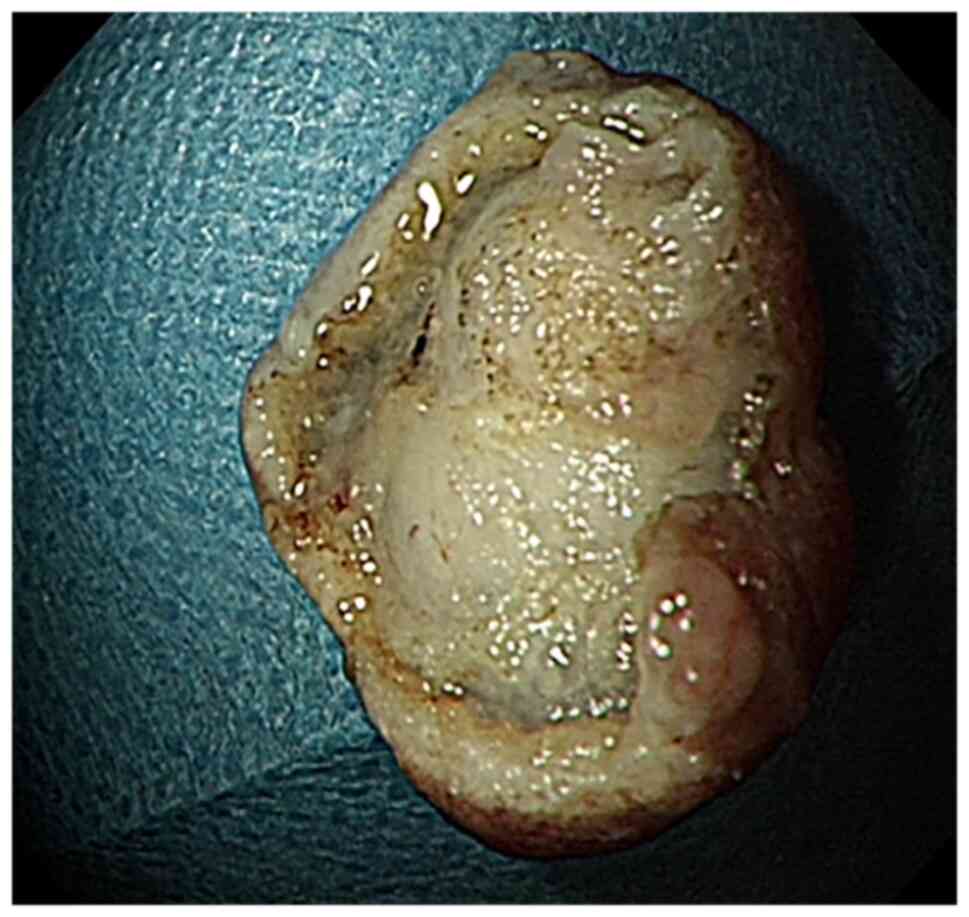
A gray mass measuring 1.5x1.4x1 cm was excised. The
tissue was fixed with 4% neutral formalin at room temperature for
48 h, dehydrated with alcohol and xylene, embedded in paraffin at
62˚C and then cooled. The 4-µm serial sections were prepared and
then stained with hematoxylin for 5 min and eosin for 2 min at room
temperature.
Microscopic observation
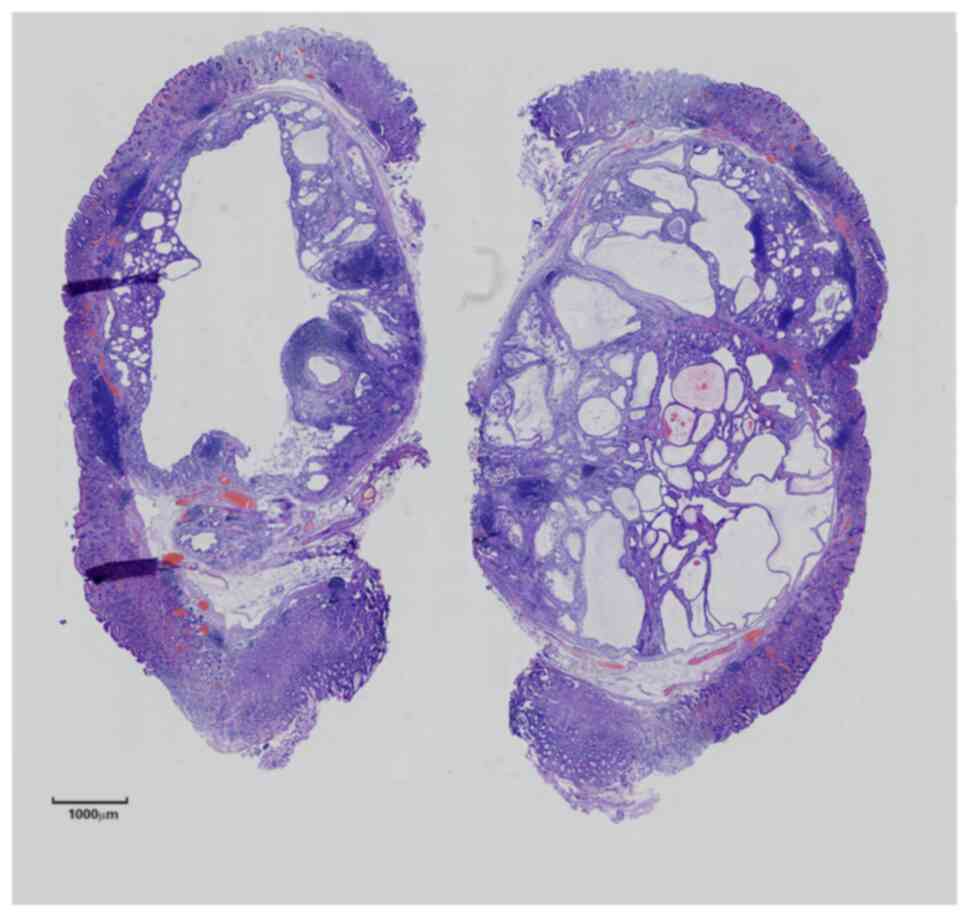
Histopathological examination showed that the fundic
gland and gastric pit of the inherent gastric mucosa were normal in
shape, with mild atrophy but no enterolization. Submucosal
‘inverted’ glands were seen as nodular, lobulated hyperplasia with
thin, smooth muscle bundles encircling the ‘nodules’ or ‘lobules’
or surrounding a single gland. Some glands were similar in size to
the normal intrinsic glands and some were saccular-dilated with
visible eosinophilic secretions in the glandular cavity. Similar to
the chief cells, the cells of the normal-sized glands were located
in the basement and had a short columnar shape, round nuclei and
basophilic cytoplasm. The cells of the dilated glands were similar
to the surface mucous cells of the gastric pit as they were located
in the base with a high columnar shape, oval nuclei and pale,
stained cytoplasm that could be flattened due to the extrusion of
secretions. None of the above cells had obvious atypia or mitotic
figures. Scattered infiltration of lymphocytes or formation of
lymphoid follicles were seen in the interstitium (Figs. 3 and 4). Immunohistochemical staining were
incubated with primary antibodies mucin-5AC (MUC-5AC; working
solution; cat. no. MAB-0079; Fuzhou Maixin Biotechnology
Development Co., Ltd.), mucin-6 (MUC-6; working solution; cat. no.
ZM-0396; OriGene Technologies, Inc.), smooth muscle actin (SMA;
working solution; cat. no. ZM-0003; OriGene Technologies, Inc.),
Desmin (working solution, cat. no. Kit-0023; Fuzhou Maixin
Biotechnology Development Co., Ltd.), CD10 (working solution, cat.
no. MAB-068; Fuzhou Maixin Biotechnology Development Co., Ltd.),
caudal-related homeobox transcription factor 2 (CDX-2; working
solution; cat. no. MAB-0216; Fuzhou Maixin Biotechnology
Development Co., Ltd.), mucin-2 (MUC-2; working solution; cat. no.
MAB-0075; Fuzhou Maixin Biotechnology Development Co., Ltd.) and
Ki-67 (working solution, cat. no. ZM-0166; OriGene Technologies,
Inc.) overnight at 4˚C from EnVision Systems (Agilent Technologies,
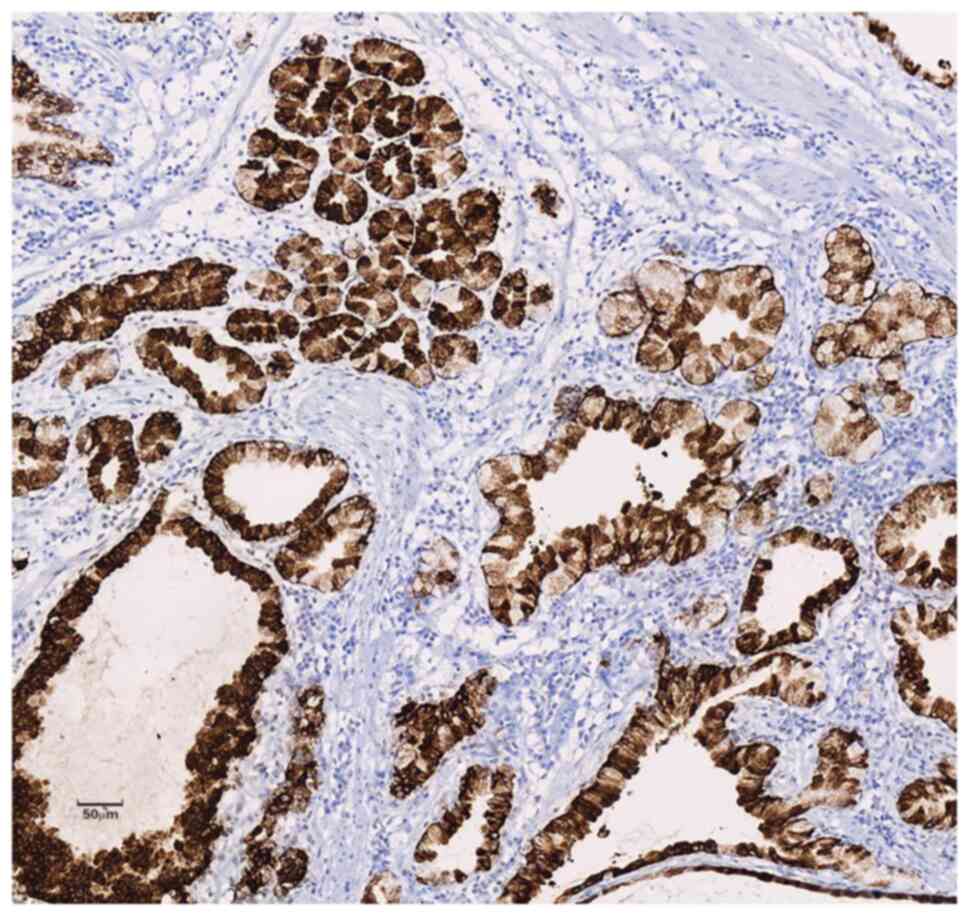
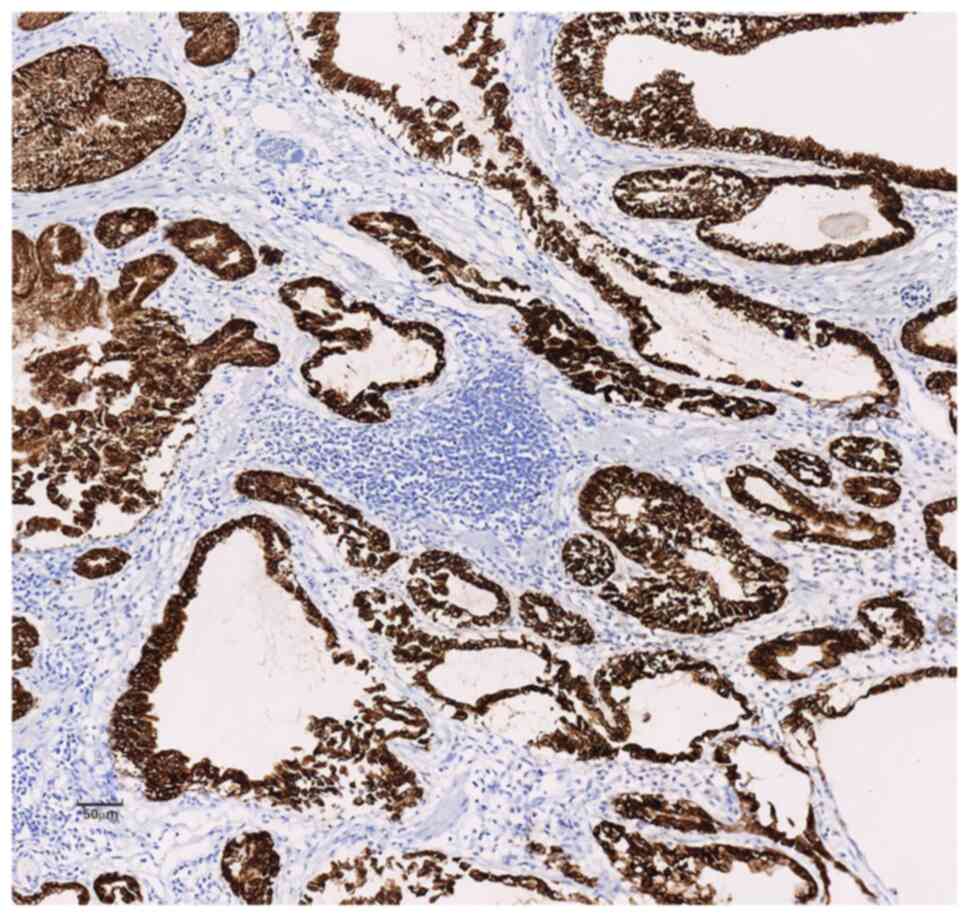
Inc.) showed diffuse strong positive expression of MUC-5AC and
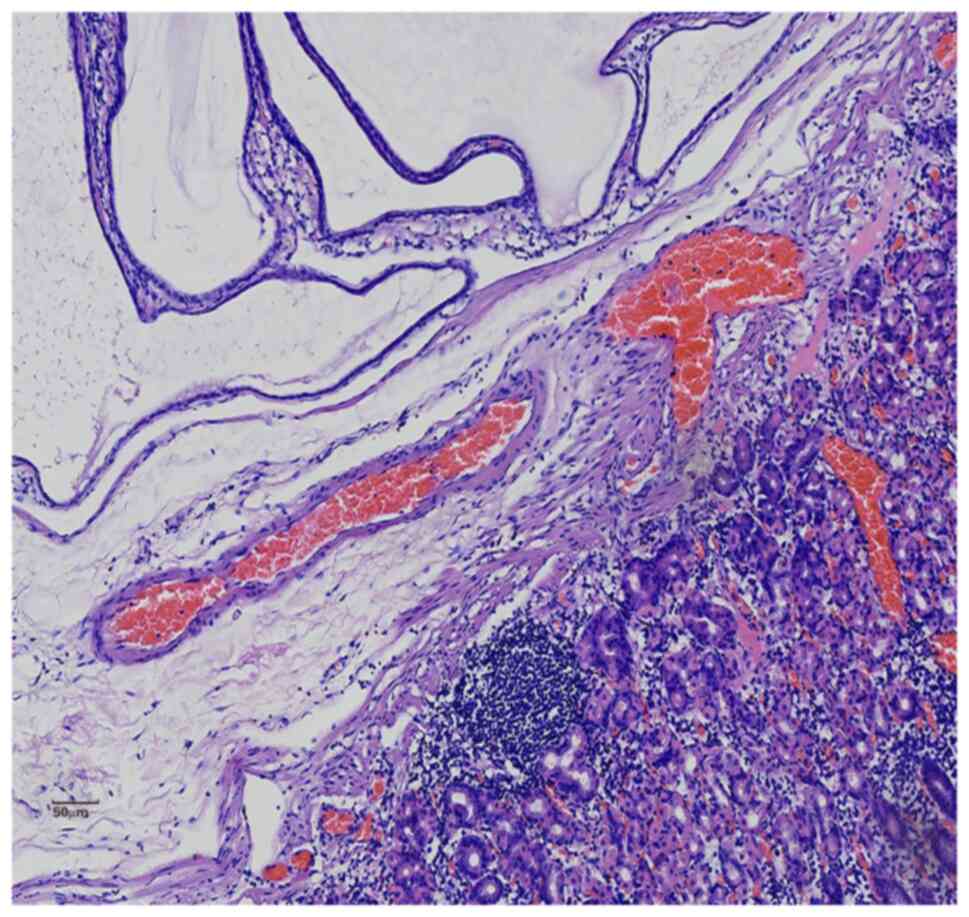
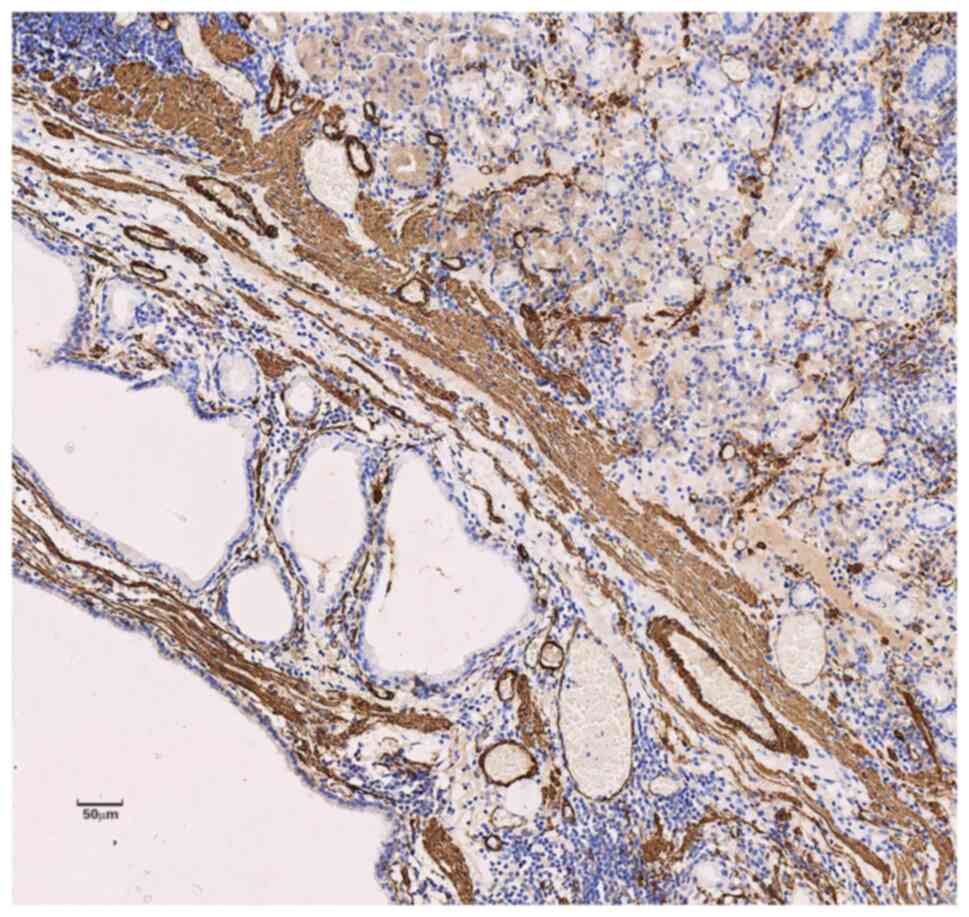
MUC-6 in the cytoplasm of the polyp glands (Figs. 5 and 6) and positive expression of SMA and
Desmin in the muscular layer of mucosa and the smooth muscle bundle
of interstitial tissue of the polyps (Fig. 7). The staining further showed
negative expression of CD10, CDX-2 and MUC-2 and the Ki-67
proliferating index was less than 2% of the glands in the
polyps.
Pathological diagnosis
The patient was diagnosed with (greater curvature of
stomach) IHP.
Treatment and follow-up
The tumor was completely removed by EMR and
postoperative recovery was good. There was no recurrence during the
6-week follow-up.
Discussion
Gastric hyperplastic polyps usually present with
multiple types of benign lesions above the gastric mucosa; inverted
growth of polyps into the gastric mucosa is rare. Gastric IHP is
characterized by inverted growth of hyperplastic mucosa under
normal mucosa and it has been reported that IHP can be pedicled
(5). Kim et al (6) classified gastric inverted polyps into
three types according to the characteristics of the lesion's
connection with mucosal surfaces, smooth muscle boundaries and
tissues. Type 1 is characterized by a central mucosal communicating
structure and clear smooth muscle borders and has a typical shape
of a round vase under low magnification. Half of type 1 may be
accompanied by simultaneous cancer transformation. Type 2 is
similar to type 1 but has no central communicating structure. Type
3 is mainly histologically characterized by lobular tissue composed
of cystic or hyperplastic glands and smooth muscle formation,
without a mucosal communicating structure or smooth muscle
boundary. According to this classification method, the present case
can be classified as type 1 IHP. Kono et al (7) showed that IHP could be found in
individuals of any age, with an mean age of 55.6 years, and without
significant gender difference. They further showed that IHP can
occur at the fundus of the stomach, body of the stomach and gastric
antrum with a maximum diameter of 11 cm. The pathogenesis of IHP
remains unclear at present. It may be caused by i) congenital
malformations of gastrointestinal submucosal glands developed on
the basis of embryo remnants or ii) repeated acquired inflammation
and ulcer cause laceration of the muscularis mucosae and invasion
of mucosal epithelial cells into the submucosal layer (2,7). As
the first does not explain why IHP is more common in older adults,
the second explanation is more compelling (2,8). In
combination with the above, the occurrence of IHP in our patient
may be related to gastric injury caused by prolonged gastric
discomfort. IHP is reportedly accompanied by canceration, so it is
of great significance to make a clear diagnosis of IHP.
IHP has no specific symptoms; abdominal discomfort
is most common and some patients may experience anemia. If IHP
occurs in the intestine, it may present symptoms of intestinal
obstruction. When epigastric discomfort occurs, clinicians may
first consider gastric ulcer, duodenal ulcer, or even malignant
diseases, such as gastric cancer or liver cancer; few will consider
IHP. At this time, gastrointestinal endoscopy provides an effective
method of differential diagnosis. Under the endoscope, IHP is often
misdiagnosed as other submucosal lesions. Diagnosis usually relies
on histopathology distinguishing it from other diseases, such as
gastritis cystica profunda (GCP), gastrointestinal stromal tumors,
neuroendocrine tumors and ectopic pancreas. It is most difficult to
distinguish IHP from GCP as the development processes of IHP and
GCP are currently considered to be similar and GCP may be a
precursor lesion of IHP (2). IHP
and GCP are especially difficult to differentiate if the lesion has
submucosal glandular ectopic sites (9). IHP often presents with a vase-like
shape and ‘opened’ on the mucosal surface, with edge tissues
turning inward and an increased number of internal glands, which
are relatively densely arranged and irregular in shape. GCP often
occurs at the anastomotic site after gastric surgery, with a single
gland, small number and relatively loose arrangement (2).
Most IHP are benign lesions, but because IHP can
become cancerous and the location of the canceration may be deep or
shallow (8), biopsy cannot rule
out the possibility of malignant change. Moreover, biopsy often
faces incomplete pathological sampling of the remaining masses,
thus reducing the diagnostic accuracy. Complete endoscopic excision
should be performed, regardless of whether the mass is benign or
malignant, to reduce the risk of surgical metastasis of the mass as
well as the physical and financial burden on the patient. The
currently recommended surgical methods are endoscopic sub-mucosal
section and EMR and the former is preferred for patients with
tumors >2 cm (4). When combined
malignant transformation is found, it is important to examine the
depth of infiltration of malignant components, vascular invasion
and the incised edge to determine whether additional surgery is
required. Regular follow-up examinations should be performed
postoperatively to prevent recurrence.
In summary, IHP is a rare submucosal lesion with
unique morphological features. As it can become cancerous, its
misdiagnosis should be vigilantly avoided. Endoscopic treatment is
preferred, including complete resection of the tumor and close
follow-up to prevent recurrence.
Acknowledgements
The authors thank Mr Jun Gao in the Department of
Gastroenterology of Sunshine Union Hospital (Weifang, China) for
his meticulous operation on the patient and for providing the
tissue.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QM and XY drafted the manuscript and conceived the
study. QM, LG, NS, YC, LL, LML and WG performed the research and
analyzed the data. QM wrote the manuscript. XY and NS revised the
manuscript. XY and WG confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Sunshine Union Hospital (approval no.
2022-11-0028).
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamata Y, Kurotaki H, Onodera T and
Nishida N: An unusual heterotopia of pyloric glands of the stomach
with inverted downgrowth. Acta Pathol Jpn. 43:192–197.
1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hua HJ, Wu J, Li KD, Song GX and Li H:
Analysis of clinicopathological characteristics of gastric-type
inverted hyperplastic polyps. Zhonghua Bing Li Xue Za Zhi.
51:749–751. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
3
|
Lee SJ, Park JK, Seo HI, Han KH, Kim YD,
Jeong WJ, Cheon GJ and Eom DW: A case of gastric inverted
hyperplastic polyp found with gastritis cystica profunda and early
gastric cancer. Clin Endosc. 46:568–571. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim HS, Hwang EJ, Jang JY, Lee J and Kim
YW: Multifocal adenocarcinomas arising within a gastric inverted
hyperplastic polyp. Korean J Pathol. 46:387–391. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Itoh K, Tsuchigame T, Matsukawa T,
Takahashi M, Honma K and Ishimaru Y: Unusual gastric polyp showing
submucosal proliferation of glands: Case report and literature
review. J Gastroenterol. 33:720–723. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim JY, Ahn S, Kim KM, Chang SH, Kim HS,
Lee JH, Kim JJ, Sohn TS, Kang HJ and Joo M: Gastric inverted
polyps-distinctive subepithelial lesions of the stomach:
Clinicopathologic analysis of 12 cases with an emphasis on
neoplastic potential. Am J Surg Pathol. 45:680–689. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kono T, Imai Y, Ichihara T, Miyagawa K,
Kanemitsu K, Ajiki T, Kawasaki K, Kamigaki T, Ikuta H, Ohbayashi C,
et al: Adenocarcinoma arising in gastric inverted hyperplastic
polyp: A case report and review of the literature. Pathol Res
Pract. 203:53–56. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yun JT, Lee SW, Kim DP, Choi SH, Kim SH,
Park JK, Jang SH, Park YJ, Sung YG and Sul HJ: Gastric inverted
hyperplastic polyp: A rare cause of iron deficiency anemia. World J
Gastroenterol. 22:4066–4070. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Franzin G and Novelli P: Gastritis cystica
profunda. Histopathology. 5:535–547. 1981.PubMed/NCBI View Article : Google Scholar
|